Abstract
Anticipatory postural adjustments (APAs) prior to gait initiation have been largely studied in in traditional, laboratory settings using force plates under the feet to characterize the displacement of the center of pressure. However clinical trials and clinical practice would benefit from a portable, inexpensive method for characterizing APAs. Therefore, the main objectives of this study were: 1) to develop a novel, automatic IMU-based method to detect and characterize APAs during gait initiation and 2) to measure its test-retest reliability. Experiment I was carried out in the laboratory to determine the validity of the IMU-based method in ten subjects with PD (OFF medication) and 12 control subjects. Experiment II was carried out in the clinic, to determine test-retest reliability of the IMU-based method in a different set of 17 early-to-moderate, treated subjects with PD (tested ON medication) and 17 age-matched control subjects.
Results showed that gait initiation characteristics (both APAs and 1st step) detected with our novel method were significantly correlated to the characteristics calculated with a force plate and motion analysis system. The size of APAs measured with either inertial sensors or force plate were significantly smaller in subjects with PD than in control subjects (p<0.05). Test-retest reliability for the gait initiation characteristics measured with inertial sensors was moderate-to-excellent (.56<ICC<.82) for both groups.
Our findings support the feasibility of automatically characterizing postural preparation and gait initiation with body-worn inertial sensors that would be practical for unsupervised clinical and home settings.
Keywords: Gait Initiation, Inertial sensors, Force plates, Parkinson’s disease
Introduction
Gait initiation involves the correct sequencing of movement preparation and movement execution. Specifically, anticipatory postural adjustments (APAs) precede the onset of voluntary movements such as gait initiation [1, 2]. The function of APAs is to reduce the effect of the forthcoming body perturbation with anticipatory corrections [3, 4]. In the case of gait initiation, APAs act to accelerate the center of body mass (COM) forward and laterally toward the stance foot by shifting the center of pressure (COP) posteriorly and toward the stepping leg [4, 5].
Impaired APAs are considered a major pathophysiological mechanism underlying difficulties in gait initiation in Parkinson’s disease (PD) [6–11], and are responsible for severe balance and mobility problems as summarized in a recent review [12]. The COP displacements during the APA phase of step initiation are reduced and often absent in PD, with prolonged delays between APA onset and step execution [6, 7, 9–13]. Abnormal coupling between APAs and step execution has been also associated with freezing of gait [14].
For decades, APAs have been extensively characterized using electromyography (EMG), force plates (COP displacements), and camera-based systems (body COM displacements) in gait analysis laboratories. Recently, with the advancement of microelectromechanical systems (MEMS), inertial sensors (accelerometers and/or gyroscopes) embedded in miniaturized inertial measurement units (IMUs) have introduced an interesting alternative to obtrusive and expensive laboratories for gait and balance assessment [15–17], including the analysis of gait initiation [18, 19]. This technological advancement offers novel possibilities for an ambulatory measure of APAs that would benefit clinical practice and clinical trials with portable tools to monitor effects of disease progression, therapies, interventions, or external cues on gait initiation.
By analyzing acceleration signals sensed by a waist-worn IMU our research group demonstrated the sensitivity of trunk acceleration, recorded during APAs prior to a step, in differentiating de novo, untreated PD not receiving L-dopa and age-matched controls [18]. In the same study, we also showed a significant correlation between magnitude of APAs measured from peak COP displacements and accelerations. However, a limitation of the aforementioned study was that APAs in the trunk acceleration signal were detected by an automated, threshold-based algorithm running on the COP displacement signals. Later, Martinez-Mendez et al. [19] proposed an algorithm to detect APAs prior to gait initiation exclusively based on inertial sensors attached to the lower back and to the lateral side of the dominant leg, at the ankle level. Despite showing good results on inter-subject repeatability and good agreement with the COP gold standard, test-retest reliability was not investigated. Moreover, the algorithm was only developed and tested in young healthy subjects, and not tested in elderly or neurological patients, for which APAs are known to be significantly smaller (and hence harder to detect) or even absent [6, 9, 12].
Therefore, the main objectives of this study were to: 1) develop an IMU-based method to automatically detect and characterize APAs, and 2) assess the test-retest reliability of this method in a group of healthy elderly and subjects with PD.
Methods
The study consists of two experiments. In Experiment I, persons with PD (OFF medication) and age-matched control subjects were tested in a laboratory setting to develop a novel method for detecting APAs and characterizing the execution of the first step during gait initiation. Validity of the method was determined by comparing: 1) APA onset, duration and magnitude measured with a waist-worn IMU with those measured by a force plate, and 2) first step length and velocity estimated by two ankle-worn IMUs with those measured by a camera-based motion analysis system. In Experiment II, another group of persons with PD (ON medication) and age-matched control subjects were tested in an ambulatory setting to evaluate the test-retest reliability of the method validated in experiment I.
In both experiments, subjects were excluded if they presented with any neurological disorders other than PD or if they had any other condition that could affect their balance. Persons with PD were clinically rated by a trained examiner on the Motor Section (III) of the UPDRS immediately before the experimental sessions. The UPDRS Part III consists of 23 items related to bradykinesia, rigidity, tremor, and posture and gait signs of PD, rated on a 4-point scale (ranging from 0 – no disability – to 4 – total disability). All participants provided informed consent according to the Oregon Health & Science University Institutional Review Board.
Experiment I: Validity Analysis
Ten persons with mild-to-moderate idiopathic PD (UPDRS III: 27.5±9, Hoehn&Yahr: 2.5±0.5, age: 67.2±5, 8 male, 2 female, disease duration: 8.5±3) and 12 healthy control subjects of similar age (68±5, 9 male, 3 female) were recruited for the validity study in the laboratory. The PD and control groups showed no significant difference in age or BMI. The diagnosis of PD was made by a movement disorders expert. Persons with PD were tested, in this experiment, in their practical OFF state, after a washout of antiparkinsonian medication of at least 12 hours.
Participants stood with each foot on separate, side-by-side custom force plates with feet externally rotated and heel-to-heel distance fixed at 10cm [20]. They performed three gait initiation trials following the instructions to voluntarily start walking at their normal, comfortable pace, starting with their most affected leg. Initial feet position was made consistent from trial to trial by tracing feet outlines on the force plate.
Data were collected from 3 IMUs (Opals by APDM Inc.) worn on the posterior trunk at the level of L5, and on the right and left shank at a sampling frequency of 128Hz. Data from the force platform were acquired at 480 Hz and low-pass filtered at 50 Hz. Vertical forces under each force plate were used to calculate the position of the COP. Feet kinematics data (reflective markers placed on the lateral malleolus and fifth metatarsus of both limbs) were collected with infrared cameras (Motion Analysis Inc., Santa Rosa, CA, USA) at 60 Hz to calculate first step characteristics. We resampled inertial sensors data, kinetics and kinematics data at a common frequency of 50Hz, as we previously reported in [18].
Force plate and camera-based measures
APA characteristics were extracted from the COP displacements as previously described in [13, 18]. The APA magnitude was measured both by the peak of the COP excursion in the backward direction (Peak AP, COP) and by the peak of lateral COP excursion towards the swing foot (Peak ML, COP). APA duration was measured as the time between the onset and end of the AP and ML COP displacement. Length and velocity of the first step were measured from the 3-D trajectory of the markers on the lateral malleoulus and fifth metatarsus. Specifically, the step onset was defined as the first observable increase (from baseline) in the vertical position trace of the lateral malleoulus followed by an increase in the vertical position trace of the fifth metatarsus, and the end was defined when the vertical position trace of the fifth metatarsus returned to the baseline value. 1st Step Length was defined as the root mean square of the 3D-coordinate of the malleoulus marker during the first step, and 1st Step Velocity as the 1st Step Length divided by the first step duration.
IMU-based measures
The algorithm uses the 3-D acceleration signals (ACC) from the IMU worn on the trunk to quantify the APA and from the two shank angular velocities to quantify the first step characteristics. As shown in the algorithm flowchart in Figure 1, the ACC data are resampled at 50Hz, transformed to a horizontal-vertical coordinate system, and filtered with a 3.5Hz cut-off, zero-phase, low-pass Butterworth filter, as in [18]. Subsequently, the algorithm automatically identifies the maximum peak ACC ML and identifies candidate APA periods when a threshold is exceeded (20% of max ACC ML, see figure 1). In parallel, the angular velocities of the stepping limb are sued to characterize the mid-swing and heel-strike events of the steps, as previously described in [21]. The APA is then identified among the different candidates as the one immediately preceding the 1st mid-swing. The APA measures computed from the lumbar accelerations are: 1) peak acceleration toward the stance foot from baseline (Peak ML, ACC), 2) forward trunk peak acceleration from baseline (Peak AP, ACC), and 3) APA duration. The measures describing first step length and velocity computed from the shank angular velocity are: 1) 1st Step Duration (defined as the time-to-peak angular velocity from APA end to heel-strike), and 2) the range of motion (RoM) of the swing phase (defined as the integrated angular velocity of the shank from APA end to heel-strike, 1st Step RoM).
Figure 1.
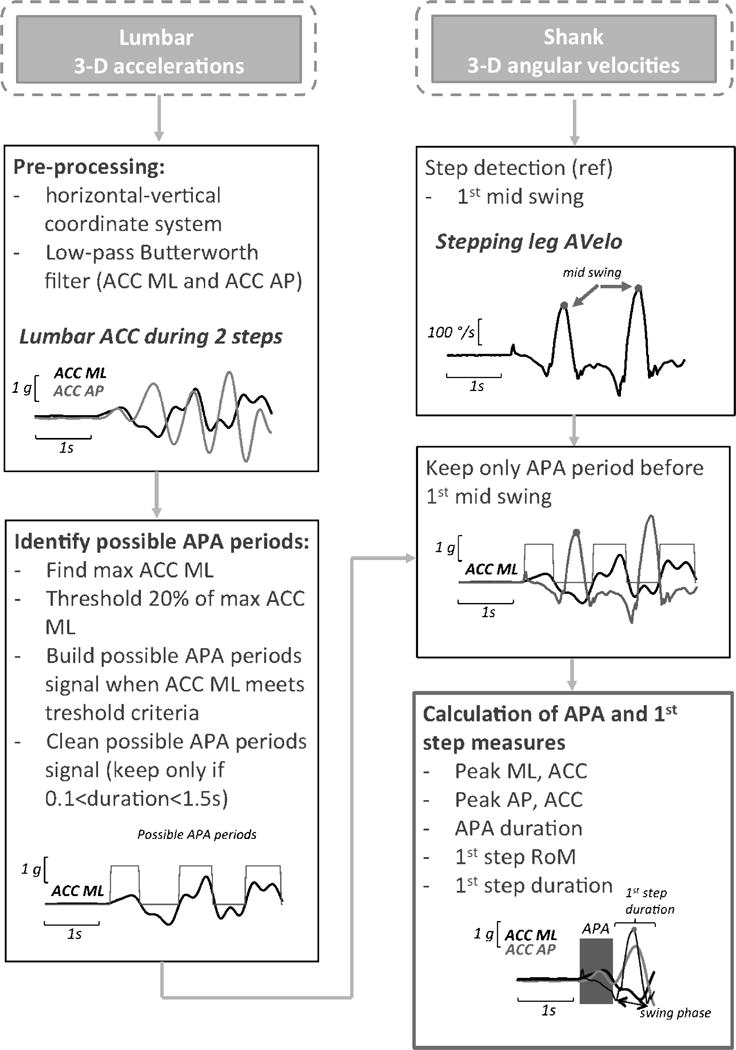
IMU-based gait initiation algorithm flowchart from lumbar and two shank sensors. ACC: acceleration, Avelo: angular velocity, RoM: range of motion, ML: medial-lateral, AP: anterior-posterior
Experiment II: Reliability Analysis
To assess test-retest reliability of the IMU-based method to detect and characterize APAs and first step measures, a different group of 17 PD (UPDRS III: 25.7±11, Hoehn&Yahr: 2.2±0.8, age: 67.1±7) and 17 age-matched control subjects (67.9±6) were tested in the neurology clinic by a research assistant. The PD and control groups showed no significant difference in age or BMI, but the PD group had more males (12) than the control group (6). Patients with PD were tested in their ON medication state at least 1h after taking their usual dose of dopaminergic medication.
Data were collected from 3 IMUs (MTX Xsens, different from Experiment I) worn as in Experiment I on the posterior trunk at the level of L5, and on the right and left shank, at a sampling frequency of 50Hz. To ensure a consistent foot position, a wedge with 10cm between the heels 30 degrees of external rotation was placed momentarily between the feet before each trial. Three trials of gait initiation were collected starting with their most affected leg.
The IMUs (MTX Xsens) were then removed after the third trial. After 30 min, the protocol was repeated. The same examiner put back the same IMUs following the same procedure and tested the subject for the second time. We assumed that the subjects’ performances remained the same within this relatively short time period.
To collect streaming data in the clinic with a laptop, a custom MATLAB (MathWorks Inc, Nantick, MA) graphical interface was built to acquire, store and analyze gait initiation.
Statistics
Algorithms for signal analysis and statistical evaluation of the outcomes were written in MATLAB. The mean of the three available trials was used for statistical analyses of each gait initiation measure. A Pearson Product moment correlation and Bland-Altman analysis were used to assess the relationship between the force plate-based and the IMU-based gait initiation measures. Intra-Class Correlation (ICC) was used for Test-retest reliability of the IMU-based gait initiation measures [22]. Since the same subjects and same device were used for Experiment II, ICC (1,1) was selected as the appropriate measure of reliability. The ICC and 95% confidence intervals were reported. A Pearson Product moment correlation was used to investigate the relationship between IMU-based gait initiation measures and the clinical score (UPDRS III). Student’s t-tests were used in both experiments to identify differences in gait initiation measures between PD and control subjects.
Results
Consistent with our previous findings [13, 18], the trunk ACC showed a medial-forward excursion during the APA, corresponding to the lateral-backward displacement of the COP. Out of 30 gait initiation trials in PD OFF (3 for each subject), our automatic method identified 4 trials without APAs from 3 different patients (see examples in Figure 2). Those trials were excluded from the analyses. Figure 2 shows representative examples of APAs detected in a control subject and in a subject with PD. Figure 2 also shows examples where APAs are smaller and when they are so small, they could not be detected.
Figure 2.
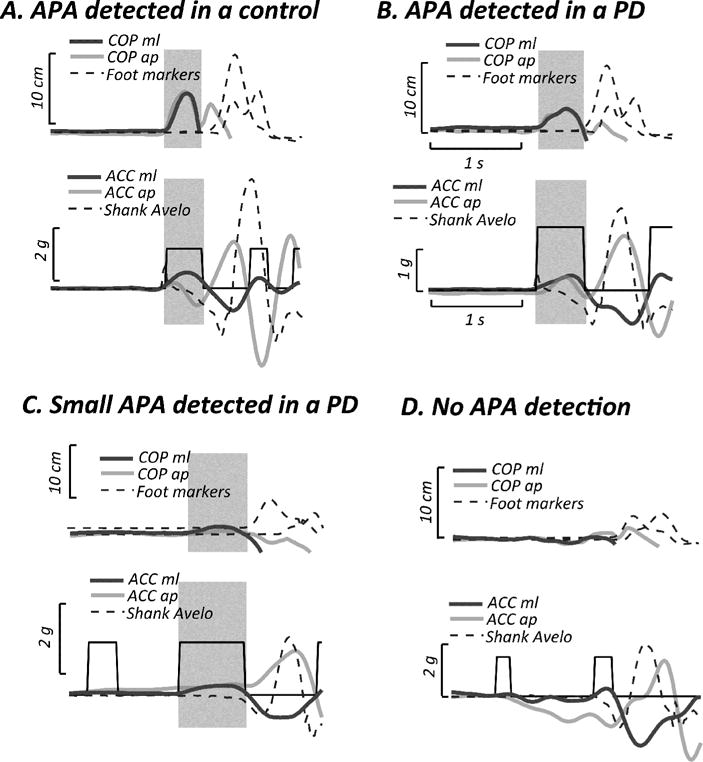
Representative APAs observed during gait initiation in Experiment I. A. Normal APA detected in a control subject. B. Small APA detected in a subject with PD. C. Feeble APA detected in a subject with PD. D. Feeble or absent, undetected APA in a subject with PD. Upper panel: COP displacements in the medio-lateral (ML) and antero-posterior (AP) directions, and trajectories of the malleolus and fifth metatarsal markers. Lower panel: trunk acceleration (ACC) in the medio-lateral (ML) and antero-posterior (AP) directions, and shank angular velocity. Shaded areas represent the APA phase.
Experiment I: Validity
Both APAs and first step measures detected with IMUs were significantly or nearly significantly related to those measured with the force plate (Peak ML, COP: r=0.76, p<0.001; Peak AP, COP: r=0.42, p=0.06) or with cameras (first step length: r=0.79, p<0.001; first step velocity: r= −0.64, p=0.001), see Figure 3. In addition, a Bland-Altman shows no obvious relation between the difference and the mean APA duration measured with the two different systems, although a slight bias towards longer APA durations was measured by the force plate (Figure 3.C).
Figure 3.
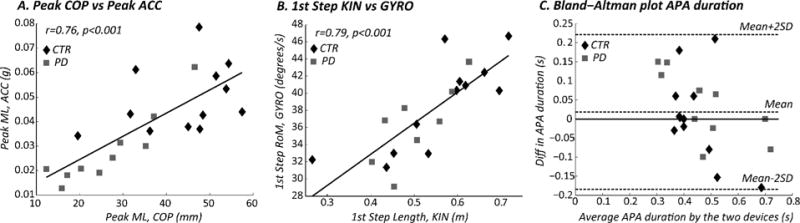
Comparison between APAs and first step characteristics calculated with two different systems in Experiment I. A. Linear correlation between Peak ML, calculated from COP and trunk acceleration; B. Linear correlation between first step length, calculated from foot markers, and first step range of motion (RoM), calculated from the angular velocity; C. Bland-Altman plot comparing APA duration calculated from the COP and from the ACC. The central dotted line represents the mean difference between the devices, while the upper and lower lines represents the limits of agreement. CTR: control subjects, PD: subjects with Parkinson’s Disease (OFF).
APA peaks, measured both with COP or ACC, were significantly smaller in PD OFF compared to controls (details in Table 1), whereas first step measures were similar between the two groups.
Table 1.
Gait initiation characteristics measured in the gait analysis laboratory with the two systems: comparison between healthy subjects (CTR) and subjects with Parkinson’s disease tested OFF medication (PD OFF). Values are mean (STD). T-tests p-values are reported.
| MEASURES | CTR | PD OFF | ||||
|---|---|---|---|---|---|---|
| Mean | STD | Mean | STD | p-value | ||
| COP and KIN | APA duration (s) | 0.45 | 0.08 | 0.49 | 0.12 | 0.3 |
| Peak ML (mm) | 43.8 | 11.3 | 26.6 | 10.8 | 0.002 | |
| Peak AP (mm) | 47.5 | 14.7 | 32.0 | 16.1 | 0.03 | |
| 1st Step Length (m) | 0.56 | 0.13 | 0.50 | 0.08 | 0.2 | |
| 1st Step Velocity (m/s) | 0.90 | 0.23 | 0.78 | 0.16 | 0.2 | |
| ACC & GYRO | APA duration (s) | 0.44 | 0.13 | 0.46 | 0.18 | 0.8 |
| Peak ML (g) | 0.049 | 0.014 | 0.028 | 0.015 | 0.002 | |
| Peak AP (g) | 0.044 | 0.031 | 0.015 | 0.015 | 0.01 | |
| 1st Step ROM (degree) | 38.7 | 5.4 | 36.4 | 4.6 | 0.3 | |
| 1st Step duration (s) | 0.40 | 0.18 | 0.53 | 0.23 | 0.1 | |
Experiment II: Reliability
Table 2 summarizes the test-retest reliability of the APAs and first step measures measured in the clinic in controls and PD subjects. Spatial measures (APA peak and first step RoM) showed higher test-retest reliability compared to temporal measures (APA duration and first step duration).
Table 2.
Test-retest reliability of APAs and first step characteristics measured with inertial sensors in healthy subjects and subjects with PD tested ON medication (PD ON) in Experiment II.
|
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
CTR
|
PD ON
|
|||||||||||||
| Test I | Test II | ICC(1-1) | 95 CI interval | Test I | Test II | ICC(1-1) | 95 CI interval | |||||||
| Mean | SEM | Mean | SEM | lower | upper | Mean | SEM | Mean | SEM | lower | upper | |||
|
|
||||||||||||||
| APA duration (s) | 0.371 | 0.019 | 0.386 | 0.019 | 0.56 | 0.12 | 0.82 | 0.34 | 0.03 | 0.35 | 0.04 | 0.63 | 0.17 | 0.87 |
|
| ||||||||||||||
| Peak ML (g) | 0.052 | 0.003 | 0.05 | 0.003 | 0.81 | 0.55 | 0.93 | 0.04 | 0.01 | 0.04 | 0.01 | 0.82 | 0.51 | 0.94 |
|
| ||||||||||||||
| Peak AP (g) | 0.053 | 0.004 | 0.058 | 0.005 | 0.52 | 0.06 | 0.8 | 0.03 | 0.01 | 0.04 | 0.01 | 0.63 | 0.17 | 0.87 |
|
| ||||||||||||||
| 1st Step ROM (degrees) | 39.911 | 2.072 | 41.524 | 1.708 | 0.78 | 0.5 | 0.92 | 30.44 | 3.61 | 28.51 | 3.92 | 0.68 | 0.25 | 0.89 |
|
| ||||||||||||||
| 1st Step duration (s) | 0.351 | 0.052 | 0.398 | 0.043 | 0.79 | 0.51 | 0.91 | 0.58 | 0.13 | 0.55 | 0.08 | 0.42 | −0.13 | 0.77 |
No significant correlations were found between the UDPRS III and any of the APA or first step measures.
Discussion
The automatic characterization of gait initiation with IMUs is an innovative approach that allows clinicians to objectively measure the postural preparation prior to gait initiation and the characteristics of the first step in a clinical setting. Smaller APAs or shorter steps, as in subjects with Parkinson’s disease, are not readily observable to the naked eye, therefore this approach might find promising applications in clinical practice or clinical trials. Because emotions, attention, external triggers, dopaminergic drugs, and Deep Brain Stimulation [7, 23–27] can all modify APAs, it could be helpful to measure gait initiation outside a sophisticated gait analysis laboratory.
In this study, we investigated the validity (Experiment I) and test-retest reliability (Experiment II) of our novel method to detect and measure APAs and first step in both subjects with PD and elderly control. The findings of Experiment I demonstrated that APAs prior to gait initiation, when present, can be detected and characterized with IMUs, similar to laboratory measures, ([18], [19], [28]). In addition, our novel, automatic method uses the acceleration of the trunk to characterize APAs and the angular velocity of the stepping leg to measure the main features of the first step, first step duration and first step range of motion. Preparation for gait initiation has traditionally been characterized as APA magnitude and duration via lateral and backward COP displacements and as execution of the first step length and velocity via motion analysis systems [6, 29, 30]. The present findings confirm significant correlations between force plate-based and IMU-based measures of APA magnitude and between camera-based and IMU-based measures characterizing first step amplitude and velocity. APA duration measured by the two systems also showed good agreement. The inclusion of a neurological population known to show feeble postural preparation for gait initiation such subjects with PD, strengthened our results. In fact, subjects with PD OFF medication usually show smaller APA amplitudes that could challenge APA detection. Our novel method identified APAs in both control and subjects with PD and, in agreement with force plate signals, four out of 30 trials were identified as gait initiation without APAs.
The findings of Experiment II showed a moderate (APA duration and Peak AP and first step RoM and duration) to excellent (for APA Peak ML) test-retest reliability for the IMU-based measures obtained by the novel algorithm. Generally, APA measures showed a higher test-retest reliability in PD compared to healthy controls, while we found the opposite for the first step measures. A possible explanation might be that APAs are more stereotyped in subjects with PD than controls and that impaired step preparation results in to variable first step execution in PD.
As expected, APA ML and AP amplitude were significantly smaller in PD OFF compared to controls, both when using COP and ACC signals to quantify the peaks. In contrast, APA duration was similar in PD OFF compared to controls, although other studies showed a longer APA duration in patients [7, 10, 13, 26, 27]. The first step velocity and duration were also similar in PD OFF and controls, unlike previous studies [6, 7, 10, 13, 26]. This discrepancy may be due to a less severe group of people with PD in the present study compared to previous studies. Overall, the present findings are similar to our previous results in untreated PD [18] leading us to hypothesize that a disruption of APA amplitude might occur early in the disease and before the first step execution is compromised. Longer APAs may try to compensate for impaired step execution later in the disease.
No significant correlations were found between the UDPRS III and any of the APA or first step measures. This is in contrast with a previous paper published by Rocchi et al. [27], in which a significant correlation between the APA peak and UPDRS III was presented in 21 patients with PD before undergoing DBS surgery, such discrepancy might be due to our smaller and less severe PD population.
In summary, we developed a novel IMU-based method to detect and characterize APAs and first step characteristics. We demonstrate the algorithm is feasible (tested in a laboratory as well as in a clinical setting), valid (compared to laboratory standard) and reliable in both a neurological and healthy population. The peak of the medio-lateral acceleration during APAs (Peak ML, ACC) resulted the measure most sensitive to the disease and with the best test-retest reliability.
However, further studies are needed to overcome some of the limitations of this study. First, Experiment I and II have been carried out with a set of similar, but not identical IMUs; although we confirmed the interchangeability of the two systems with a concurrent evaluation (data unpublished) this is a limitation of the study. A larger, and more diverse, population is needed, including patients with other neurological disorders. In addition, the method needs to be trained in a larger and more severe PD population, in which the percentage of trials with feeble or absent APAs is higher, and where multiple APAs could even occur (for instance in subjects with PD experiencing freezing of gait).
Highlights.
An IMU-based method to detect APAs was validated in healthy and subjects with PD.
The size of APAs was smaller in subjects with PD compared to healthy subjects.
Test-retest reliability for the IMU-based method was moderate-to-excellent.
Acknowledgments
We thank Kelsey Priest for scheduling and helping with data collection.
This publication was made possible with support from NIH RC1 NS068678 and R42 HD071760-03.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest disclosure
OHSU, Drs. Horak and Holmstrom have a significant financial interest in, and are employees of ADPM, a company that have a commercial interest in the results of this research and technology. This potential institutional and individual conflict has been reviewed and managed by OHSU.
Contributor Information
Martina Mancini, Email: mancinim@ohsu.edu.
Lorenzo Chiari, Email: lorenzo.chiari@unibo.it.
Lars Holmstrom, Email: lars@apdm.com.
Arash Salarian, Email: arash.salarian@gmail.com.
Fay B. Horak, Email: horakf@ohsu.edu.
References
- 1.Bouisset S, Do MC. Posture, dynamic stability, and voluntary movement. Neurophysiologie clinique = Clinical neurophysiology. 2008 Dec;38(6):345–62. doi: 10.1016/j.neucli.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Breniere Y, Do MC. When and how does steady state gait movement induced from upright posture begin? Journal of biomechanics. 1986;19(12):1035–40. doi: 10.1016/0021-9290(86)90120-x. [DOI] [PubMed] [Google Scholar]
- 3.Aruin AS, Latash ML. The role of motor action in anticipatory postural adjustments studied with self-induced and externally triggered perturbations. Experimental brain research. 1995;106(2):291–300. doi: 10.1007/BF00241125. [DOI] [PubMed] [Google Scholar]
- 4.Massion J. Movement, posture and equilibrium: interaction and coordination. Progress in neurobiology. 1992;38(1):35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- 5.Winter DA. ABC of balance during standing walking. Waterloo: 1995. [Google Scholar]
- 6.Halliday SE, Winter DA, Frank JS, Patla AE, Prince F. The initiation of gait in young, elderly, and Parkinson’s disease subjects. Gait & posture. 1998 Aug 1;8(1):8–14. doi: 10.1016/s0966-6362(98)00020-4. [DOI] [PubMed] [Google Scholar]
- 7.Burleigh-Jacobs A, Horak FB, Nutt JG, Obeso JA. Step initiation in Parkinson’s disease: influence of levodopa and external sensory triggers. Movement disorders: official journal of the Movement Disorder Society. 1997 Mar;12(2):206–15. doi: 10.1002/mds.870120211. [DOI] [PubMed] [Google Scholar]
- 8.Gantchev N, Viallet F, Aurenty R, Massion J. Impairment of posturo-kinetic coordination during initiation of forward oriented stepping movements in parkinsonian patients. Electroencephalography and clinical neurophysiology. 1996 Apr;101(2):110–20. doi: 10.1016/0924-980x(95)00253-h. [DOI] [PubMed] [Google Scholar]
- 9.Hass CJ, Waddell DE, Fleming RP, Juncos JL, Gregor RJ. Gait initiation and dynamic balance control in Parkinson’s disease. Archives of physical medicine and rehabilitation. 2005 Nov;86(11):2172–6. doi: 10.1016/j.apmr.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Latash ML, Aruin AS, Neyman I, Nicholas JJ. Anticipatory postural adjustments during self inflicted and predictable perturbations in Parkinson’s disease. Journal of neurology, neurosurgery, and psychiatry. 1995 Mar;58(3):326–34. doi: 10.1136/jnnp.58.3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roemmich RT, Nocera JR, Vallabhajosula S, et al. Spatiotemporal variability during gait initiation in Parkinson’s disease. Gait & posture. 2012 Jul;36(3):340–3. doi: 10.1016/j.gaitpost.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delval A, Tard C, Defebvre L. Why we should study gait initiation in Parkinson’s disease. Neurophysiologie clinique = Clinical neurophysiology. 2014 Jan;44(1):69–76. doi: 10.1016/j.neucli.2013.10.127. [DOI] [PubMed] [Google Scholar]
- 13.Rocchi L, Chiari L, Mancini M, Carlson-Kuhta P, Gross A, Horak FB. Step initiation in Parkinson’s disease: influence of initial stance conditions. Neuroscience letters. 2006 Oct 2;406(1–2):128–32. doi: 10.1016/j.neulet.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs JV, Nutt JG, Carlson-Kuhta P, Stephens M, Horak FB. Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Experimental neurology. 2009 Feb;215(2):334–41. doi: 10.1016/j.expneurol.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horak FB, Mancini M. Objective biomarkers of balance and gait for Parkinson’s disease using body-worn sensors. Movement disorders: official journal of the Movement Disorder Society. 2013 Sep 15;28(11):1544–51. doi: 10.1002/mds.25684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maetzler W, Domingos J, Srulijes K, Ferreira JJ, Bloem BR. Quantitative wearable sensors for objective assessment of Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society. 2013 Oct;28(12):1628–37. doi: 10.1002/mds.25628. [DOI] [PubMed] [Google Scholar]
- 17.Yang S, Li Q. Inertial sensor-based methods in walking speed estimation: a systematic review. Sensors (Basel) 2012;12(5):6102–16. doi: 10.3390/s120506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mancini M, Zampieri C, Carlson-Kuhta P, Chiari L, Horak FB. Anticipatory postural adjustments prior to step initiation are hypometric in untreated Parkinson’s disease: an accelerometer-based approach. European journal of neurology: the official journal of the European Federation of Neurological Societies. 2009 Sep;16(9):1028–34. doi: 10.1111/j.1468-1331.2009.02641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez-Mendez R, Sekine M, Tamura T. Detection of anticipatory postural adjustments prior to gait initiation using inertial wearable sensors. Journal of neuroengineering and rehabilitation. 2011;8:17. doi: 10.1186/1743-0003-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maki BE, Holliday PJ, Fernie GR. Aging and postural control. A comparison of spontaneous- and induced-sway balance tests. J Am Geriatr Soc. 1990 Jan;38(1):1–9. doi: 10.1111/j.1532-5415.1990.tb01588.x. [DOI] [PubMed] [Google Scholar]
- 21.Salarian A, Russmann H, Vingerhoets FJ, et al. Gait assessment in Parkinson’s disease: toward an ambulatory system for long-term monitoring. IEEE transactions on biomedical engineering. 2004 Aug;51(8):1434–43. doi: 10.1109/TBME.2004.827933. [DOI] [PubMed] [Google Scholar]
- 22.McGraw KO, Wong SP. Forming Inferences About Some Intraclass Correlation Coefficients. Psychological Methods. 1996;1(1):30–46. [Google Scholar]
- 23.Creath RA, Prettyman M, Shulman L, et al. Self-triggered assistive stimulus training improves step initiation in persons with Parkinson’s disease. Journal of neuroengineering and rehabilitation. 2013;10:11. doi: 10.1186/1743-0003-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hass CJ, Buckley TA, Pitsikoulis C, Barthelemy EJ. Progressive resistance training improves gait initiation in individuals with Parkinson’s disease. Gait & posture. 2012 Apr;35(4):669–73. doi: 10.1016/j.gaitpost.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 25.Jiang Y, Norman KE. Effects of visual and auditory cues on gait initiation in people with Parkinson’s disease. Clinical rehabilitation. 2006 Jan;20(1):36–45. doi: 10.1191/0269215506cr925oa. [DOI] [PubMed] [Google Scholar]
- 26.Liu W, McIntire K, Kim SH, et al. Bilateral subthalamic stimulation improves gait initiation in patients with Parkinson’s disease. Gait & posture. 2006 Jun;23(4):492–8. doi: 10.1016/j.gaitpost.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Rocchi L, Carlson-Kuhta P, Chiari L, Burchiel KJ, Hogarth P, Horak FB. Effects of deep brain stimulation in the subthalamic nucleus or globus pallidus internus on step initiation in Parkinson disease: laboratory investigation. Journal of neurosurgery. 2012 Dec;117(6):1141–9. doi: 10.3171/2012.8.JNS112006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breniere Y, Dietrich G. Heel-off perturbation during gait initiation: biomechanical analysis using triaxial accelerometry and a force plate. Journal of biomechanics. 1992 Feb;25(2):121–7. doi: 10.1016/0021-9290(92)90269-7. [DOI] [PubMed] [Google Scholar]
- 29.Crenna P, Frigo C. A motor programme for the initiation of forward-oriented movements in humans. The Journal of physiology. 1991 Jun;437:635–53. doi: 10.1113/jphysiol.1991.sp018616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elble RJ, Moody C, Leffler K, Sinha R. The initiation of normal walking. Movement disorders: official journal of the Movement Disorder Society. 1994 Mar;9(2):139–46. doi: 10.1002/mds.870090203. [DOI] [PubMed] [Google Scholar]


