Abstract
Background
OBSERVE-5 was a 5-year FDA-mandated surveillance registry of psoriasis patients.
Objective
To assess long-term etanercept safety and effectiveness.
Methods
Patients with moderate to severe psoriasis enrolled; a single baseline dose of etanercept was required. Key outcome measures included serious adverse events (SAEs), serious infectious events (SIEs), events of medical interest, psoriasis-affected body surface area, physician global assessment, and Dermatology Life Quality Index. Safety outcomes were assessed relative to data from the MarketScan database.
Results
For 2,510 patients, 5-year cumulative incidence (95% confidence interval [CI]) was 22.2% (20.3%, 24.2%) for SAEs; 6.5% (5.4%, 7.7%) for SIEs; 3.2% (2.3%, 4.1%) for malignancies excluding nonmelanoma skin cancer (NMSC); 3.6% (2.7%, 4.5%) for NMSC; 2.8% (2.0%, 3.6%) for coronary artery disease; 0.7% (0.3%, 1.2%) for psoriasis worsening; 0.2% (0.0%, 0.4%) for CNS demyelinating disorder; 0.1% (0.0%, 0.3%) for lymphoma and for tuberculosis; 0.1% (0.0%, 0.2%) for opportunistic infection and for lupus; 55 fatal events were reported. Rates of malignancies, lymphomas, NMSC, and hospitalization-associated infections were not higher than expected relative to administrative claims data. The percentage of patients rated as clear/almost clear was 12% at baseline, which increased to 51% at month 6 and remained relatively stable throughout 5 years.
Limitations
No internal comparator group was included; rare events may not have been detected.
Conclusion
No new safety signals were observed with long-term, real-world etanercept use.
Keywords: etanercept, plaque psoriasis, surveillance registry, safety, adverse events, infections, malignancy
INTRODUCTION
Etanercept is a tumor necrosis factor (TNF) blocker indicated for the treatment of adults with chronic moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy, and for treatment of rheumatoid arthritis (RA), polyarticular juvenile idiopathic arthritis, psoriatic arthritis, and ankylosing spondylitis.1 An analysis of long-term safety in 506 patients with psoriasis who initiated etanercept in two phase 3 clinical trials showed a favorable safety profile, with no cumulative toxicity noted for up to 4 years of treatment.2
Although short-term clinical trials provide important information on the efficacy and safety of a drug, long-term registry studies are also needed to detect rare adverse events in a broader patient population. OBSERVE-5 was a 5-year observational registry that enrolled 2,510 patients with plaque psoriasis who received etanercept.3 We now report the final analysis of OBSERVE-5 data representing long-term, real-world experience with etanercept therapy.
METHODS
Study design
OBSERVE-5 was a phase 4, prospective, multicenter, observational, surveillance registry and has been previously described.4 Briefly, etanercept was self-administered at the dose and regimen determined by the investigator and patients were evaluated at 6-month intervals for up to 5 years. Patients could have discontinued etanercept, switched to another antipsoriatic therapy, used etanercept in combination with other antipsoriatic therapies, or discontinued any or all antipsoriatic treatments during the study. The study was approved by institutional review boards at all study sites. Written informed consent was provided by all patients before initiation of any study-related procedures. This study was registered under ClinicalTrials.gov identifier NCT00322439.
Patients
As previously described,4 patients with plaque psoriasis for whom etanercept therapy was indicated per prescribing information and for whom the treating physician decided to initiate, reinitiate, or continue etanercept therapy according to usual care were eligible. Initially, patients were etanercept-naïve but a protocol amendment allowed patients with prior etanercept exposure to enroll (capped at 50%). Patients were ineligible if they were contraindicated for etanercept treatment according to the prescribing information,1 had been treated with other TNF blockers or with commercial etanercept before April 2004 in the US or December 2005 in Canada (when etanercept was approved for psoriasis), or had participated in previous etanercept clinical studies.
Outcome measures
Serious adverse events (SAEs), serious infectious events (SIEs), which included infectious events requiring hospitalization, and events of medical interest (EMIs) were reported and assessed throughout the study and for 30 days after the study. An event was considered to be an SAE if it was fatal, life-threatening, required hospitalization, resulted in disability/incapacity, was a congenital anomaly/birth defect, or other significant medical hazard. EMIs included malignancies (including basal cell and squamous cell carcinomas); tuberculosis; opportunistic infections treated with intravenous therapy; histoplasmosis and coccidioidomycosis infections treated with oral antibiotics; central nervous system (CNS) demyelinating disorders; lupus disease; coronary artery disease; and worsening of psoriasis (change in psoriasis morphology and withdrawal of therapy). An EMI was considered to be an SAE if additional criteria such as death or hospitalization occurred. Effectiveness outcomes included changes in psoriasis-affected body surface area (BSA), physician global assessment (PGA),5 and Dermatology Life Quality Index (DLQI).6
External context analysis
Incidence rates of all malignancies (excluding nonmelanoma skin cancer [NMSC]), lymphoma, NMSC, and infectious events leading to hospitalization were assessed in relation to corresponding rates from the Truven Health MarketScan® Commercial Claims and Encounters and MarketScan® Medicare Supplemental databases using incidence rates based on person-time of observation for malignancy outcomes and person-time of exposure for the hospitalized infections. MarketScan databases collect enrollment data, medical claims, and laboratory and prescription data. For this analysis, MarketScan patients were ≥18 and ≤90 years of age, met the qualifying criteria between 1/1/2006 and 12/31/2006 (enrollment window), and had 12 months of continuous enrollment before their index date to describe their medical and treatment history (baseline period). The primary comparator analysis was with patients in MarketScan who received nonbiologic systemic therapies (methotrexate, cyclosporine); additional analyses were conducted with patients receiving etanercept, phototherapy, other biologic therapies, methotrexate only, all patients with psoriasis, and the general population. Incidence rates from MarketScan were calculated and age- and sex-standardized to the OBSERVE-5 population for each outcome of interest. Standardized incidence ratios (SIRs) with 95% confidence intervals (CIs) were calculated using MarketScan rates as the reference.
Statistical considerations
A study size of 2,500 patients was required by the FDA. No statistical hypothesis was tested in this observational study. Descriptive statistics are provided for baseline characteristics and outcome measures. The primary analysis of safety endpoints was performed using Kaplan-Meier methodology. Cumulative incidences were calculated using 2 methods: first, by including all time from first dose of etanercept to start date of the first event occurrence, regardless of the pattern of etanercept exposure (observation time) and second, by excluding time intervals and corresponding events when the patient was not on etanercept (exposure time). For EMIs, survival estimates were adjusted using left truncation methodology to address potential survival bias introduced by the inclusion of patients with prior etanercept exposure. It was assumed that patients with prior etanercept exposure who had experienced rare events or malignancies would have permanently discontinued treatment and would not have qualified to enter the study. The left truncation technique accounts for this conditional sampling. For example, a patient with 2 years of prior exposure before entering the registry would begin follow-up in his or her third year since becoming exposed; for such a patient, an event in the second year of being followed in the registry would be counted as an event in the fourth year since starting exposure.
RESULTS
Patients
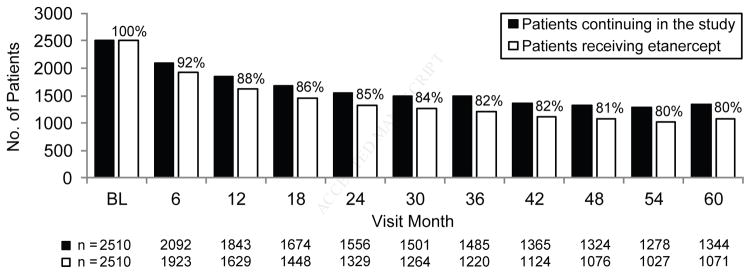
This study was conducted at 375 sites (37 Canada, 338 US). A total of 2,511 patients enrolled in OBSERVE-5 of which 2,510 received ≥1 dose of etanercept; 1,326 enrolled after the amendment allowing prior etanercept exposure. Six hundred sixty-four (26%) patients had prior etanercept exposure before enrolling in OBSERVE-5 and 1,846 (74%) patients were etanercept-naïve on enrollment. Baseline characteristics have been previously described.4 Of all patients, 1,455 (58.0%) continued in the study until the end of follow-up, 1,042 (41.5%) discontinued before the end of follow-up, and 13 patients did not have discontinuation status because of early site closure. At each study visit, ≥80% of patients who continued on study also continued on etanercept (Figure 1). A total of 164 patients received etanercept continuously without interruption throughout the study, 182 received etanercept throughout the study with 1–30 day gaps (mean 16 total gap days) in etanercept therapy, and 63 received etanercept throughout the study with 31–60 day gaps (mean 58 total gap days) in etanercept therapy. Of the patients who experienced gaps in etanercept therapy, most had only 1 or 2 treatment gaps.
Figure 1.
Plaque psoriasis. Etanercept status by visit. The number of patients remaining in the study (black bars) and those who remained on etanercept therapy (white bars) throughout the 5-year study is shown. BL, baseline.
SAEs
A total of 418 patients, on and off etanercept, reported an SAE during the study (Table I). The most commonly reported noninfectious SAEs were myocardial infarction (0.7% of patients) and coronary artery disease (0.6%), and osteoarthritis (0.6%) and the most common SIEs were pneumonia (1.2%) and cellulitis (0.9%). Fifty-five patients had a fatal event; of these, 17 were of unknown cause. Four deaths were considered by the investigator to be related to etanercept: brain cancer and lung cancer, heart failure, osteomyelitis and sepsis, and idiopathic pulmonary fibrosis. The 5-year Kaplan-Meier cumulative incidence (95% CI) of SAEs based on observation time was 22.2% (20.3%, 24.2%) and the incremental yearly incidence (data not shown) decreased during the study (Table II).
Table I.
| All Patients (N = 2510) | |
|---|---|
| SAEs, patients reporting event (%) | 418 (16.7) |
| Most common treatment-emergent SAEs, n (%) | |
| Pneumonia | 30 (1.2) |
| Cellulitis | 22 (0.9) |
| Myocardial infarction | 17 (0.7) |
| Coronary artery disease | 14 (0.6) |
| Osteoarthritis | 14 (0.6) |
| Diverticulitis | 11 (0.4) |
| Most common treatment-related SAEs, n (%) | |
| Pneumonia | 8 (0.3) |
| Cellulitis | 8 (0.3) |
| SIEs, patients reporting event, n (%) | 120 (4.8) |
| Most common treatment-emergent SIEs, n (%) | |
| Pneumonia | 30 (1.2) |
| Cellulitis | 22 (0.9) |
| Diverticulitis | 11 (0.4) |
| Staphylococcal infection | 7 (0.3) |
| Sepsis | 5 (0.2) |
| Appendicitis | 4 (0.2) |
| Bronchitis | 4 (0.2) |
| Herpes zoster | 4 (0.2) |
| SIEs leading to hospitalization, n (%) | 94 (3.7) |
| Pneumonia | 28 (1.1) |
| Cellulitis | 19 (0.8) |
| Diverticulitis | 8 (0.3) |
| Sepsis | 5 (0.2) |
| Appendicitis | 4 (0.2) |
| Gastroenteritis | 3 (0.1) |
| Staphylococcal infection | 3 (0.1) |
| EMIs, patients reporting event, n (%) | 604 (24.1) |
| Select EMIs, n (%) | |
| Malignancy | 122 (4.9) |
| NMSC | 66 (2.6) |
| Coronary artery disease | 49 (2.0) |
| Worsening of psoriasis | 13 (0.5) |
| CNS demyelinating disorder | 3 (0.1) |
| Lymphoma | 2 (0.1) |
| Tuberculosis | 2 (0.1) |
| Lupus disease | 1 (< 0.1) |
| Opportunistic infection | 1 (< 0.1) |
| Coccidioidomycosis | 0 |
| Histoplasmosis | 0 |
Includes events counted beyond year 5 due to left truncation adjustment in Kaplan-Meier analyses.
Includes patients on and off etanercept
SAEs, serious adverse events; SIEs, serious infectious events; EMIs, events of medical interest; NMSC, nonmelanoma skin cancer; CNS, central nervous system.
Table II.
Kaplan-Meier incidence proportions of SAEs and SIEs
| Registry Year | Based on Observation Time* | Based on Exposure Time† | ||
|---|---|---|---|---|
|
| ||||
| Patients With Event, n | Cumulative Incidence, Q (95% CI) | Patients With Event, n | Cumulative Incidence, Q (95% CI) | |
| SAEs | ||||
| First year | 139 | 0.0633 (0.0531, 0.0735) | 121 | 0.0581 (0.0480, 0.0682) |
| Second year | 103 | 0.1163 (0.1024, 0.1301) | 75 | 0.1070 (0.0926, 0.1215) |
| Third year | 70 | 0.1557 (0.1397, 0.1718) | 37 | 0.1386 (0.1215, 0.1558) |
| Fourth year | 53 | 0.1878 (0.1703, 0.2054) | 35 | 0.1771 (0.1565, 0.1977) |
| Fifth year | 51 | 0.2224 (0.2031, 0.2417) | 19 | 0.2029 (0.1799, 0.2260 |
| SIEs | ||||
| First year | 45 | 0.0204 (0.0145, 0.0263) | 39 | 0.0184 (0.0127, 0.0242) |
| Second year | 25 | 0.0334 (0.0257, 0.0411) | 18 | 0.0301 (0.0222, 0.0379) |
| Third year | 17 | 0.0432 (0.0343, 0.0521) | 12 | 0.0408 (0.0310, 0.0506) |
| Fourth year | 13 | 0.0514 (0.0415, 0.0613) | 5 | 0.0467 (0.0356, 0.0577) |
| Fifth year | 20 | 0.0650 (0.0536, 0.0765) | 8 | 0.0581 (0.0447, 0.0716) |
Includes all events and exposure days that occurred since the first day of treatment with etanercept.
Includes only events that occurred during etanercept exposure plus a 30-day risk window after each dosing interval.
SAEs, serious adverse events; SIEs, serious infectious events; CI, confidence interval.
SIEs
A total of 120 patients reported ≥1 SIE and 94 patients reported an SIE leading to hospitalization (Table I). The 5-year Kaplan-Meier cumulative incidence (95% CI) for SIEs based on observation time was 6.5% (5.4%, 7.7%) and the incremental yearly incidence (data not shown) generally decreased over time (Table II). The 5-year Kaplan-Meier cumulative incidence (95% CI) for SIEs requiring hospitalization was 5.2% (4.1%, 6.2%).
EMIs
A total of 604 patients had ≥1 EMI (Table I), including 159 patients with an EMI that was considered by the investigator to be related to etanercept. The 5-year Kaplan-Meier cumulative incidences (95% CI) based on observation time were 0.1% (0.0%, 0.3%) for tuberculosis (n=2); 0.2% (0.0%, 0.4%) for CNS demyelinating disorder (n=3); 0.1% (0.0%, 0.2%) for lupus disease and for opportunistic infections (n=1 each); 2.8% (2.0%, 3.6%) for coronary artery disease (n=46); and 0.7% (0.3%, 1.2%) for worsening of psoriasis (n=12). No case of histoplasmosis or coccidioidomycosis was reported. The 5-year Kaplan-Meier cumulative incidences (95% CI) based on observation time were 3.21% (2.34%, 4.08%) for malignancy excluding NMSC; 3.60% (2.69%, 4.50%) for NMSC; and 0.12% (0.0%, 0.29%) for lymphoma (Table III).
Table III.
Kaplan-Meier incidence proportions of malignancies*
| Registry Year | Based on Observation Time† | Based on Exposure Time‡ | ||
|---|---|---|---|---|
|
| ||||
| Patients With Event, n | Cumulative Incidence, Q (95% CI) | Patients With Event, n | Cumulative Incidence, Q (95% CI) | |
| All Malignancies | ||||
| Excluding NMSC | ||||
| First year | 7 | 0.0041 (0.0011, 0.0072) | 7 | 0.0033 (0.0004, 0.0061) |
| Second year | 12 | 0.0115 (0.0064, 0.0167) | 8 | 0.0090 (0.0039, 0.0141) |
| Third year | 15 | 0.0208 (0.0139, 0.0277) | 10 | 0.0156 (0.0085, 0.0226) |
| Fourth year | 6 | 0.0244 (0.0169, 0.0319) | 7 | 0.0230 (0.0142, 0.0319) |
| Fifth year | 11 | 0.0321 (0.0234, 0.0408) | 5 | 0.0304 (0.0195, 0.0413) |
| Sixth year | 7 | 0.0475 (0.0332, 0.0617) | 0 | 0.0304 (0.0195, 0.0413) |
| Seventh year | 1 | 0.0509 (0.0352, 0.0666) | 0 | 0.0304 (0.0195, 0.0413) |
| Eighth year | 1 | 0.0574 (0.0373, 0.0775) | 0 | 0.0304 (0.0195, 0.0413) |
| Lymphoma | ||||
| First year | 1 | 0.0006 (0.0000, 0.0018) | 1 | 0.0007 (0.0000, 0.0020) |
| Second year | 0 | 0.0006 (0.0000, 0.0018) | 0 | 0.0007 (0.0000, 0.0020) |
| Third year | 1 | 0.0012 (0.0000, 0.0029) | 1 | 0.0016 (0.0000, 0.0040) |
| Fourth year | 0 | 0.0012 (0.0000, 0.0029) | 0 | 0.0016 (0.0000, 0.0040) |
| Fifth year | 0 | 0.0012 (0.0000, 0.0029) | 0 | 0.0016 (0.0000, 0.0040) |
| Sixth year | 0 | 0.0012 (0.0000, 0.0029) | 0 | 0.0016 (0.0000, 0.0040) |
| Seventh year | 0 | 0.0012 (0.0000, 0.0029) | 0 | 0.0016 (0.0000, 0.0040) |
| NMSC | ||||
| First year | 13 | 0.0075 (0.0035, 0.0116) | 20 | 0.0069 (0.0028, 0.0109) |
| Second year | 14 | 0.0162 (0.0101, 0.0222) | 15 | 0.0158 (0.0092, 0.0223) |
| Third year | 10 | 0.0223 (0.0152, 0.0295) | 12 | 0.0254 (0.0165, 0.0342) |
| Fourth year | 18 | 0.0333 (0.0246, 0.0419) | 9 | 0.0352 (0.0244, 0.0460) |
| Fifth year | 4 | 0.0360 (0.0269, 0.0450) | 0 | 0.0352 (0.0244, 0.0460) |
| Sixth year | 4 | 0.0447 (0.0321, 0.0573) | 0 | 0.0352 (0.0244, 0.0460) |
| Seventh year | 3 | 0.0551 (0.0380, 0.0723) | 0 | 0.0352 (0.0244, 0.0460) |
Kaplan-Meier analyses of malignancies included left truncation adjustment for patients with prior etanercept exposure and therefore include follow-up time from etanercept exposure before the registry.
Includes all events and exposure days that occurred since the first day of treatment with etanercept.
Includes only events that occurred during etanercept exposure plus a 30-day risk window after each dosing interval.
NMSC, nonmelanoma skin cancer; CI, confidence interval.
External context
For the outcomes of malignancies excluding NMSC, lymphoma, and NMSC, incidence rates for registry patients were not higher than the rates of the psoriasis population using nonbiologic systemic therapies (primary comparator) or those receiving etanercept in the MarketScan database (Table IV). Incidence rates of SIEs requiring hospitalization in OBSERVE-5 were not higher than the rates from the primary external comparator group based on patient-years of etanercept exposure (SIR=0.34; 95% CI: 0.26, 0.43).
Table IV.
SIRs based on observed rates from OBSERVE-5 and expected rates from MarketScan*
| MarketScan Comparator Group, SIR (95% CI) | Malignancy Excluding NMSC† | Lymphoma† | NMSC† | Infectious Event Requiring Hospitalization‡ |
|---|---|---|---|---|
| Patients receiving nonbiologic systemic therapies | 0.78 (0.59, 1.00) | 0.26 (0.03, 0.95) | 0.54 (0.42, 0.69) | 0.34 (0.26, 0.43) |
| Patients receiving etanercept | 0.89 (0.68, 1.15) | 0.50 (0.06, 1.80) | 0.58 (0.45, 0.74) | 0.51 (0.39, 0.66) |
| Patients receiving phototherapy | 0.74 (0.57, 0.96) | 0.14 (0.02, 0.52) | 0.54 (0.42, 0.69) | 0.41 (0.32, 0.53) |
| Patients receiving other biologic therapies | 0.97 (0.74, 1.25) | 0.36 (0.04, 1.29) | 0.46 (0.36, 0.58) | 0.29 (0.22, 0.37) |
| Patients receiving methotrexate | 0.78 (0.60, 1.00) | 0.28 (0.03, 1.00) | 0.55 (0.43, 0.70) | 0.36 (0.27, 0.46) |
| Patients with psoriasis | 0.81 (0.61, 1.04) | 0.28 (0.03, 1.02) | 0.62 (0.48, 0.79) | NA |
| General MarketScan population§ | 0.95 (0.72, 1.22) | 0.46 (0.06, 1.67) | 0.95 (0.73, 1.20) | NA |
Incidence rates from MarketScan were age- and sex-standardized to the OBSERVE-5 population.
Analysis based on patient-years of observation.
Analysis based on patient-years of exposure.
The general MarketScan population comprised all insured individuals who met the eligibility criteria.
SIRs, standardized incidence ratios; CI, confidence interval; NMSC, nonmelanoma skin cancer; NA, not applicable.
Effectiveness outcomes
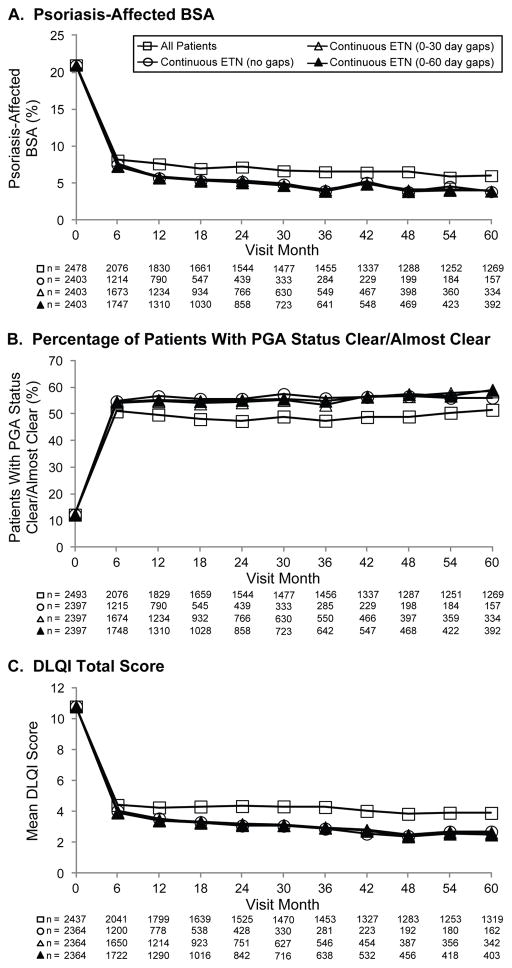
Mean baseline psoriasis-affected BSA was 12% and 24% for patients receiving prior etanercept and etanercept-naïve patients, respectively. Mean psoriasis-affected BSA improved from 21% at baseline to 8% at month 6, and remained stable throughout 5 years (Figure 2A). At baseline, 37% and 3% of patients who had received prior etanercept and etanercept-naïve patients, respectively, had PGA status of clear/almost clear (score of 0 or 1). The percentage of all patients with PGA status of clear/almost clear was 12% at baseline, which increased to 51% at month 6 and remained relatively stable throughout 5 years (Figure 2B). Mean (standard deviation [SD]) DLQI scores at baseline were 6.1 (6.3) and 12.5 (6.9) for patients who had received prior etanercept and etanercept-naïve patients, respectively. The mean (SD) score for all patients at baseline was 10.8 (7.3), which improved to 4.4 (5.1) at month 6 and remained stable throughout 5 years (Figure 2C).
Figure 2.
Plaque psoriasis. Effectiveness outcomes. (A) The percentage of psoriasis-affected BSA, (B) percentage of patients with PGA status of clear/almost clear (score 0/1), and (C) mean DLQI total score for all patients (squares), patients on continuous etanercept (circles), patients on etanercept who had a 0- to 30-day gap in therapy (open triangles), and patients on etanercept who had a 0- to 60-day gap in therapy (closed triangles) are shown. Data are presented as observed without imputation for missing data. BSA, body surface area; PGA, physician global assessment; DLQI, Dermatology Life Quality Index.
DISCUSSION
This assessment of long-term safety of etanercept in patients with plaque psoriasis found that Kaplan-Meier cumulative incidences of SAEs, SIEs, and SIEs requiring hospitalization were low and incremental yearly incidences decreased during the 5 years of the registry. Using MarketScan data to provide age- and sex-standardized expected incidence rates, observed rates of malignancies, lymphomas, NMSC, and infections requiring hospitalization in OBSERVE-5 were not higher than expected. Patients who had received prior etanercept had generally better baseline mean scores for BSA, PGA, and DLQI assessments compared with patients who were etanercept-naïve at study entry. Both groups had improvements from baseline in these assessments, and by month 6, the 2 groups were similar. The proportion of patients with PGA status of clear/almost clear after month 6 was approximately 45%–50% in patients with and without prior etanercept exposure, which was higher than the rate seen with etanercept monotherapy in a large, real-world, cross-sectional study (34%).7 Patients with up to 60-day gaps in etanercept therapy demonstrated similar effectiveness as patients who remained on continuous etanercept therapy.
The results for SAEs and SIEs in OBSERVE-5 were similar to those reported in an integrated analysis of safety, which examined data from 7 clinical trials that lasted up to 2 years.8 In that analysis, rates of SAEs and SIEs were comparable between placebo and etanercept groups in short-term studies, and no dose-related increases in these events were observed in both short- and long-term analyses. Additionally, cumulative event rates for SIEs did not differ importantly across dose groups and over time. In an analysis of 49 clinical trials across etanercept indications, rates of SIEs were similar between patients receiving etanercept and controls. In that analysis, 49 SIEs were reported in 4,361 psoriasis patients for an exposure-adjusted rate of 1.24 events per 100 patient-years.9 A meta-analysis of 20 randomized, controlled trials in patients with psoriatic diseases (including 14 trials in psoriasis patients) reported an odds ratio (OR) of 0.78 (95% CI: 0.38, 1.58) for serious infections in psoriasis patients treated with a TNF blocker vs placebo and an OR (95% CI) of 1.14 (0.92, 1.40) for overall infections in patients treated with etanercept vs control.10 In the 3-year interim analysis of OBSERVE-54 and the final 5-year results reported here, greater etanercept exposure was not associated with increased rates of SAEs or SIEs. Although SIEs are rare events in psoriasis patients, physicians should screen patients for infections before initiating etanercept and monitor them during therapy.11
Prior studies have suggested that patients with psoriasis may be at increased risk of malignancy,12 particularly NMSC12 and lymphoma,13 although the overall risk remains low and the magnitude of association is modest.13 Psoriasis severity is proportional to the risk of developing NMSC.12 In an integrated analysis of safety of 7 clinical trials of etanercept, there was no increase in overall malignancies with etanercept therapy compared with the psoriasis population.8 In an analysis of 49 clinical trials across indications, the rate ratio for NMSC comparing etanercept to placebo in psoriasis patients was 2.77 (95% CI: 0.59, 25.97).9 The SIR for lymphoma in these trials was 2.01 (95% CI: 0.24, 7.27) in psoriasis patients compared with Surveillance Epidemiology and End Results (SEER) data.9 Additionally, psoriasis patients who received etanercept appeared to have higher risk of squamous cell carcinomas.9 The meta-analysis of randomized, controlled trials in patients with psoriasis or psoriatic arthritis receiving TNF blockers showed no increased risk of malignancy.10 In OBSERVE-5, rates of malignancies, NMSCs, and lymphomas were not higher than expected relative to data from MarketScan.
In contrast to previous phase 3 studies, which had stringent eligibility criteria and excluded patients with medically significant underlying diseases and/or were concomitantly taking certain antipsoriatic medications, enrollment in OBSERVE-5 was based on the clinical decision by investigators to continue, initiate, or resume treatment with etanercept. Therefore, this surveillance registry did not exclude patients for comorbidities or concomitant medications, representing real-world use of etanercept.
A limitation of the study was lack of an internal comparator. In the absence of a placebo arm, conclusions about efficacy are less reliable; however, in large placebo-controlled clinical studies of similar populations, usually less than 10% of subjects achieve a status of clear or almost clear with placebo alone. Additionally, the size and duration of the study may not have been sufficient to detect extremely rare adverse events that may be related to treatment. Interpretation of SIRs should consider differences in data collection methods, sampling designs, and study populations in a prospective registry vs an administrative claims database compiled for billing purposes. Interpretation of safety and effectiveness-related outcomes should take into account the 42% drop-out rate, which may lead to an underestimation of safety events and an overestimation of effectiveness if discontinuation was related to these outcomes.
In summary, no new or unexpected safety signal was observed in this 5-year observational study. Rates of malignancies, lymphomas, NMSC, and infections requiring hospitalization in OBSERVE-5 were not higher than expected relative to data from MarketScan.
Acknowledgments
Funding: This study was funded by Immunex, a wholly owned subsidiary of Amgen Inc. and by Wyeth, which was acquired by Pfizer in October 2009.
Medical Writing Support: Julia R. Gage, PhD (on behalf of Amgen Inc.) and Dikran Toroser, PhD (Amgen Inc.) assisted with writing and editing the manuscript.
ABBREVIATIONS
- BSA
body surface area
- CI
confidence interval
- CNS
central nervous system
- DLQI
Dermatology Life Quality Index
- EMI
event of medical interest
- FDA
Food & Drug Administration
- NMSC
nonmelanoma skin cancer
- OR
odds ratio
- PGA
physician global assessment
- RA
rheumatoid arthritis
- SAE
serious adverse event
- SD
standard deviation
- SEER
Surveillance Epidemiology and End Results
- SIE
serious infectious event
- SIR
standardized incidence ratio
- TNF
tumor necrosis factor
Footnotes
Author Disclosures: A. Kimball is a consultant for AbbVie Inc., Amgen Inc., Merck, Janssen-Ortho Inc., and Pfizer Inc.; is an investigator for Janssen-Ortho Inc., and serves on an advisory board for Vascular Biogenics. K.J. Rothman is an employee of RTI International, an independent nonprofit research organization that does work for government agencies and pharmaceutical companies. G. Kricorian, G. Aras, N.A. Accortt, M. Hooper, and K.C. Rice are employees and shareholders of Amgen Inc. D. Pariser is a consultant for Abbott Laboratories, Amgen Inc., Astellas Pharma US, Inc., Bickel Biotechnology, Celgene Corporation, Dermira, DUSA Pharmaceuticals, Inc., LEO Pharma, US, MelaSciences, Novartis Pharmaceuticals Corp., Procter & Gamble Company, and Valeant Pharmaceuticals International; is an investigator for Abbott Laboratories, Amgen Inc., Astellas Pharma US, Inc., Asubio Pharmaceuticals, Inc., Basliea, Celgene Corporation, Eli Lilly and Company, Galderma Laboratories, L.P., Graceway Pharmaceuticals, LLC, Intendis, Inc., Johnson & Johnson Consumer Products Company, LEO Pharma, US, Novartis Pharmaceuticals Corp., Novo Nordisk A/S, Ortho Dermatologics, Peplin Inc., Pfizer Inc., Photocure ASA, Stiefel a GSK Company, and Valeant Pharmaceuticals International; and serves on advisory boards for Galderma Laboratories, L.P., Genentech, Inc., Janssen-Ortho Inc., Medicis Pharmaceutical Corporation, Ortho Dermatologics, Pfizer Inc., and Stiefel a GSK Company. P.S. Yamauchi is a consultant for AbbVie Inc., Amgen Inc., Baxter Healthcare Corp., Janssen-Ortho Inc., Novartis Pharmaceuticals Corp., and Pfizer Inc.; an investigator for Amgen Inc., Celgene Corporation, Galderma USA, Janssen-Ortho Inc., LEO Pharma Inc., Lilly ICOS LLC, and Pfizer Inc.; serves on speakers bureaus for AbbVie Inc., Amgen Inc., Galderma USA, Janssen-Ortho Inc., LEO Pharma Inc., and Novartis Pharmaceuticals Corp.; and serves on an advisory board for Lilly ICOS LLC. A. Menter serves on advisory boards for AbbVie Inc., Allergan Inc., Amgen Inc., Boehringer Ingelheim GmbH, Genentech, Inc., Janssen Pharmaceuticals, Inc., LEO Pharma Inc., and Pfizer; is a consultant for AbbVie Inc., Allergan Inc., Amgen Inc., Convoy Therapeutics, Inc., Eli Lilly and Co., Janssen Pharmaceuticals, Inc., LEO Pharma Inc., Novartis AG, Pfizer, Syntrix Biosystems, Inc., Wyeth, and XenoPort, Inc.; is an investigator for AbbVie Inc., Allergan Inc., Amgen Inc., ApoPharma Inc., Boehringer Ingelheim GmbH, Celgene Corporation, Convoy Therapeutics, Inc., Eli Lilly and Co., Genentech, Inc., Janssen Pharmaceuticals, Inc., LEO Pharma Inc., Merck & Co., Inc., Novartis AG, Pfizer, SymBio Pharmaceuticals/Maruho Co., Ltd., Syntrix Biosystems, Inc., and Wyeth; and serves on speakers bureaus for AbbVie Inc., Amgen Inc., Janssen Pharmaceuticals, Inc., LEO Pharma Inc., and Wyeth. C.F. Teller is a consultant for Amgen Inc., AbbVie Inc., Janssen Pharmaceuticals, Inc., and Celgene, and is an investigator for Amgen Inc. J.M. Gelfand is a consultant for Pfizer Inc., Janssen Pharmaceuticals, Inc., and Merck & Co., Inc., and a consultant and investigator for Amgen Inc., Eli Lilly and Co., and AbbVie Inc.
Prior presentation: Data from this study have been presented at the Fall Dermatology Conference, Las Vegas, NV; October 17–20, 2013 and the 72nd Annual Meeting of the American Academy of Dermatology, Denver, CO, March 21–25, 2014.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Enbrel® (etanercept) prescribing information. Immunex Corporation; Thousand Oaks, CA: 2013. [Google Scholar]
- 2.Papp KA, Poulin Y, Bissonnette R, Bourcier M, Toth D, Rosoph L, et al. Assessment of the long-term safety and effectiveness of etanercept for the treatment of psoriasis in an adult population. J Am Acad Dermatol. 2012;66:e33–45. doi: 10.1016/j.jaad.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 3.Kimball AB, Pariser D, Yamauchi PS, Menter A, Teller CF, Shi Y, et al. OBSERVE-5, an observational post-marketing safety surveillance registry of etanercept for the treatment of psoriasis: a simple model for studying new psoriasis therapies. Psoriasis Forum. 2010;16:3–7. [Google Scholar]
- 4.Kimball AB, Pariser D, Yamauchi PS, Menter A, Teller CF, Shi Y, et al. OBSERVE-5 interim analysis: an observational postmarketing safety registry of etanercept for the treatment of psoriasis. J Am Acad Dermatol. 2013;68:756–64. doi: 10.1016/j.jaad.2012.10.055. [DOI] [PubMed] [Google Scholar]
- 5.Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis. 2005;64(Suppl 2):ii65–8. doi: 10.1136/ard.2004.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–6. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 7.Gelfand JM, Wan J, Callis Duffin K, Krueger GG, Kalb RE, Weisman JD, et al. Comparative effectiveness of commonly used systemic treatments or phototherapy for moderate to severe plaque psoriasis in the clinical practice setting. Arch Dermatol. 2012;148:487–94. doi: 10.1001/archdermatol.2012.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pariser DM, Leonardi CL, Gordon K, Gottlieb AB, Tyring S, Papp KA, et al. Integrated safety analysis: short- and long-term safety profiles of etanercept in patients with psoriasis. J Am Acad Dermatol. 2012;67:245–56. doi: 10.1016/j.jaad.2011.07.040. [DOI] [PubMed] [Google Scholar]
- 9.Gottlieb AB, Gordon K, Giannini EH, Mease P, Li J, Chon Y, et al. Clinical trial safety and mortality analyses in patients receiving etanercept across approved indications. J Drugs Dermatol. 2011;10:289–300. [PubMed] [Google Scholar]
- 10.Dommasch ED, Abuabara K, Shin DB, Nguyen J, Troxel AB, Gelfand JM. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035–50. doi: 10.1016/j.jaad.2010.09.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papp KA, Dekoven J, Parsons L, Pirzada S, Robern M, Robertson L, et al. Biologic therapy in psoriasis: perspectives on associated risks and patient management. J Cutan Med Surg. 2012;16:153–68. doi: 10.1177/120347541201600305. [DOI] [PubMed] [Google Scholar]
- 12.Margolis D, Bilker W, Hennessy S, Vittorio C, Santanna J, Strom BL. The risk of malignancy associated with psoriasis. Arch Dermatol. 2001;137:778–83. [PubMed] [Google Scholar]
- 13.Gelfand JM, Shin DB, Neimann AL, Wang X, Margolis DJ, Troxel AB. The risk of lymphoma in patients with psoriasis. J Invest Dermatol. 2006;126:2194–201. doi: 10.1038/sj.jid.5700410. [DOI] [PubMed] [Google Scholar]