Abstract
The term cerebral small vessel disease (SVD) refers to a group of pathologic processes with various etiologies that affect small arteries, arterioles, venules, and capillaries of the brain. Magnetic resonance imaging (MRI) correlates of SVD are lacunes, recent small subcortical infarcts, white-matter hyperintensities, enlarged perivascular spaces, microbleeds, and brain atrophy. Endothelial dysfunction is thought to have a role in the mechanisms leading to SVD-related brain changes, and the study of endothelial dysfunction has been proposed as an important step for a better comprehension of cerebral SVD. Among available methods to assess endothelial function in vivo, measurement of molecules of endothelial origin in peripheral blood is currently receiving selective attention. These molecules include products of endothelial cells that change when the endothelium is activated, as well as molecules that reflect endothelial damage and repair. This review examines the main molecular factors involved in both endothelial function and dysfunction, and the evidence linking endothelial dysfunction with cerebral SVD, and gives an overview of clinical studies that have investigated the possible association between endothelial circulating biomarkers and SVD-related brain changes.
Keywords: Cerebral small vessel disease, endothelium, inflammation, lacunar infarcts, white-mater hyperintensities
The term cerebral small vessel disease (SVD) refers to a group of pathologic processes with various etiologies that affect the small arteries, arterioles, venules, and capillaries of the brain.1 Age/hypertension-related SVDs and cerebral amyloid angiopathy are the most common sporadic forms of SVD. Among a few genetic forms of SVD, CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy), caused by NOTCH3 gene mutations, is the most frequent one. It is a systemic arteriopathy although the clinical symptoms are those caused by brain dysfunction. The effects of both sporadic and inherited SVD on the brain parenchyma are represented by lesions mainly located in the subcortical structures, and include lacunar infarcts, ischemic white-matter lesions, and intracerebral hemorrhage. An international working group has recently published the STRIVE (STandards for ReportIng Vascular changes on nEuroimaging) to provide definitions and imaging standards for markers and consequences of SVD.2 According to this consensus, changes currently seen on neuroimaging related to SVD include lacunes, recent small subcortical infarcts, white-matter hyperintensities (WMH), perivascular spaces (PVS), microbleeds (MB), and brain atrophy.
Over the last few decades, evidence has being accumulated regarding prevalence, clinical significance, and prognostic value of each of these changes. It is now accepted that they are strongly associated with stroke, cognitive decline, psychiatric and motor disorders, disability, and death. Overall, they are considered as a marker of poor prognosis.1–5 Mechanisms linking SVD with parenchyma damage, either ischemic or hemorrhagic, are heterogeneous and not completely understood. Vessel wall changes may in fact be responsible for the rupture of the vessel, thus manifesting as hemorrhagic SVD, or for structural restriction of the vessel lumen, or for its functional dysregulation, leading to a state of chronic hypoperfusion that is responsible for incomplete infarct or acute focal necrosis (lacunar infarct).1 Besides to and together with these mechanisms, conceptualization about the role of endothelial dysfunction, purported by some opinion leaders, leads to expect that it has a prominent role, and that the study of it may be an essential step for expanding knowledge of cerebral SVD.6 Endothelial functioning can be assessed in vivo using instrumental tests able to reflect functional properties of normal and activated endothelium.7 The assessment of circulating molecules of endothelial origin in blood may provide the opportunity of a wider appreciation of the various functions of the endothelium. These molecules include direct products of endothelial cells that change when the endothelium is activated, as well as molecules that reflect endothelial damage or repair. Many of these circulating biomarkers are difficult and expensive to measure, and are currently used only in the research setting.
The aim of this review is to analyze available evidence of biologic circulating markers of endothelial dysfunction in cerebral SVD. The first part of the review examines the main pathways and molecular factors involved in the physiologic function and dysfunction of the endothelium with a special focus on the peculiar situation in the central nervous system. Available evidence linking endothelial dysfunction with cerebral SVD is summarized. In the second part, we review clinical studies that have investigated potential associations between various endothelial biomarkers and the different manifestations of SVD.
Endothelial Functions and Dysfunctions
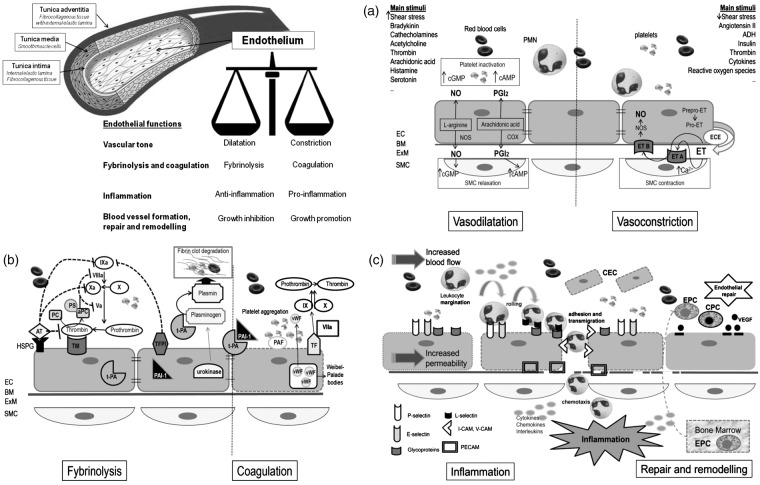
Endothelium is a monolayer of cells covering the inner surface of blood vessels, with an estimated total area in humans of about 350 m2.8 The endothelium is a dynamic organ that serves as a functional and structural barrier between the blood and the vessel wall, and has a wide variety of critical roles in the control of vascular function and, as a consequence of its dysfunction, in many mechanisms underlying vascular disorders. Figure 1 schematically depicts the structure of vessel wall, with endothelial cells and their main functions. Endothelial cells are the main regulator of vascular homeostasis due to their interaction with both the circulating cells and those present in the vascular wall, mainly the smooth muscle cells.9 They modulate blood flow, control permeability to plasma components, and influence adhesion and aggregation of platelets and leukocytes. The main molecules and pathways involved in the four main endothelial functions are schematically depicted in parts A, B, and C of Figure 1 (see figure legends for details).
-
(1)
Regulation of vascular tone which is obtained through the balanced production of vasodilators and vasoconstrictors in response to a variety of stimuli.9,10 Figure 1A shows the main mechanisms involved in vasodilatation and vasoconstriction.
-
(2)
Regulation of fibrinolysis and coagulation pathways. The intimal surface of healthy endothelium has both anticoagulant and antithrombotic properties: endothelial cells secrete a variety of molecules important for the regulation of blood coagulation and platelet functions.9–11 Vessel damage or exposure to certain cytokines or proinflammatory stimuli shift the balance toward a procoagulant/prothrombotic phenotype of the endothelial cells. Figure 1B shows the main molecules involved in this pathway.
-
(3)
Participation in inflammation (Figure 1C). Endothelial cells, together with leukocytes, mast cells, and platelets, are key cellular players of inflammatory reactions and strictly interact with the soluble factors involved in the regulation of each of the different steps of the inflammatory reaction.12,13 Left side of Figure 1C depicts the main implicated molecules.
-
(4)
Blood vessel formation, repair and remodeling. Angiogenesis is essential for several physiologic processes, such as reproduction, development, and tissue repair, as well as in certain diseases, including inflammation and malignancies. Also on this occasion, the final outcome depends on the balance or imbalance between angiogenic mediators and inhibitors.14,15,16 Main factors involved in endothelial repair are shown in the right side of Figure 1C.
Figure 1.
Schematic representation of blood vessel structure and main endothelial functions. (A) EC and regulation of vascular tone. The regulation of vascular tone is obtained through the balanced production of vasodilators and vasoconstrictors in response to a variety of stimuli.9,10 The left side of (A) shows the main mechanisms involved in vasodilatation: Nitric oxide (NO) is the predominant mediator of normal vascular function and is generated from L-arginine through endothelial NO synthase (NOS). When released by the endothelium, NO diffuses in the vessel wall, to the vascular SMC, and activates guanylate cyclase causing SMC relaxation. Another endothelium-derived vasodilator is prostacyclin (PGI2), generated by cyclooxygenase (COX) and arachidonic acid metabolism. PGI2 activates adenylate cyclase with subsequent generation of cAMP and final relaxation of the underlying smooth muscle cells. NO and PGI2 have also important antiplatelet effects, as they limit aggregation; both of them are involved in the inhibition of coagulation, inflammation, and smooth muscle cells proliferation. The right side of (A) shows the main mechanisms involved in vasoconstriction. The main vasoconstrictive factor is Endothelin (ET), an endothelium-derived 21-amino-acid peptide (the ET family consists of three structurally related peptides, ET-1, ET-2, and ET-3). The product of ET transcription is prepro-ET, which is cleaved by a neutral endopeptidase to form the active precursor pro-ET. Pro-ET may be released from the non-luminal surface of the endothelial cells and converted to mature ET by the membrane-bound metalloproteinase endothelin-converting enzyme (ECE). The two ET receptors, both coupled to G proteins, are endothelin A (ETA) receptor, situated on the vascular SMC, and endothelin B (ETB) receptor, located on endothelial cells. Binding to ETA receptor stimulates phospholipase C initiating in turn events leading eventually to SMC contraction. The result of ET binding to ETB receptor on endothelial cells is the release of NO and PGI2, which opposes the vasoconstricting action of ET. ET also exert mitogenic activity on SMC (not shown in picture). Many factors stimulating ET synthesis, such as thrombin and angiotensin II, also cause the release of vasodilatator PG12 and/or NO, which oppose the vasoconstricting action of ET. (B) Endothelial cells and coagulation and fybrinolysis. The major antiplatelet agents secreted by endothelial cells are PGI2 and NO. In the quiescent state, endothelial cells maintain blood fluidity by promoting the activity of numerous anticoagulant pathways, the most important being the protein C/protein S pathway (PC/PS), which is initiated when thrombin interacts with the endothelial cell receptor thrombomodulin (TM), facilitating activation of protein C. Activated protein C (aPC) inactivates two cofactors essential for blood coagulation: factors VIIIa and Va. To be effective, protein C must form a complex with protein S, which is synthesized by endothelial cells. Complex formation between thrombin and thrombomodulin also prevents thrombin from being able to clot fibrinogen or to activate platelets. Moreover, the endothelial cell surface is rich in heparin-like glycosaminoglycans (HSPG) to which antithrombin (AT) is bound thus providing the main site for inactivation of active thrombin. Endothelial cells also synthesize tissue factor (TF) pathway inhibitor and participates in fibrinolysis by releasing tissue-type plasminogen activator (t-PA) and urokinase, allowing the transformation of plasminogen into plasmin, which degrades thrombi by digesting fibrin network. t-PA is constitutively released while urokinase is only synthesized by activated endothelial cells. The natural inhibitor of t-PA, plasminogen activator inhibitor type 1 (PAI-1) is also constitutively secreted by endothelial cells. The balance of t-PA and PAI-1, which is normally in favor of PAI-1 is also altered by (continued) Figure 1. Continued. cytokines, again in a way that is procoagulant. After activation, the balance of endothelial properties can be tipped to favor platelet aggregation and clot formation through coordinate induction of procoagulant/prothrombotic factors and suppression of anticoagulant mechanisms. Operating in coordination, these changes can allow fibrin formation and platelet activation to proceed in an inflamed focus. At least two mediators released by activated endothelial cells favor platelet activation. The first one is the lipid mediator platelet-activating factor (PAF), synthesized by endothelial cells stimulated by thrombin, histamine, or cytokines. Most of the produced PAF remains membrane associated. PAF is a potent platelet activator and can promote platelet adhesion to endothelial cells. The second is the von Willebrand factor (vWF) of which endothelial cells are the major source. vWF is constitutively secreted into the plasma and the subendothelial matrix. It is also stored in high amount in intracellular Weibel–Palade bodies, which can be mobilized rapidly in response to agonists like thrombin. Endothelial cells do not normally express the primary trigger of the coagulation system, TF. However, when exposed to thrombin, cytokines, or lipopolysaccharides (LPS), endothelial cells synthesize and express TF at their surface. Once coagulation has been initiated, endothelial cells promote thrombi activation since the activity of factor Xa (generated by TF plus factor VII) is locally enhanced by promotion of binding with its cofactor Va at adjacent Xa and Va receptors on the endothelial cell surface. Thrombin is the main effector protease of the coagulation cascade. Factor Xa, activated by factor VIIa and TF, converts prothrombin into active thrombin. This protease converts circulating fibrinogen into fibrin monomer, which polymerizes to form fibrin, the fibrous matrix of blood clot. In endothelial cells, thrombin causes vWF release, the appearance of P-selectin at the plasma membrane, production of PAF and chemokines. Prothrombin fragment 1 + 2 (F1 + 2) and thrombin–antithrombin (TAT) complexes may be measured to assess thrombin activation. Endothelial cells also change shape and show increased permeability. All together, these changes initiate a proinflammatory and procoagulation situation. Thrombin similarly activates platelets. (C) Endothelial cells, inflammation, repair, and remodeling. Endothelial factors involved in the phase of increased vascular permeability include NO, prostaglandins, and platelet activating factor (PAF). Endothelial cells are also the ‘gatekeepers’ of the cell recruitment in inflammation. (C) Left side depicts the main events involved in the recruitment of leukocytes from the circulation into the extravascular space, critical for inflammatory responses, and repair of tissue injury. Once activated, endothelial cells express adhesion molecules on their surfaces thus allowing the binding to reciprocal molecules on the surfaces of circulating leukocytes. Finally, chemotactic factors attract leukocytes along a chemical gradient to the site of injury. Adhesion molecules involved in leukocyte recruitment include four molecular families which are selectins, addressins, integrins, and immunoglobulins. P-selectin is preformed and stored within Weibel-Palade bodies of endothelial cells and α-granules of platelets, allowing the rapid transportation to the cell surface, upon stimulation and thus the rapid adhesive interaction between endothelial cells and leukocytes. E-selectin is not expressed on quiescent endothelial cell surfaces but is induced by inflammatory mediators, such as cytokines or bacterial LPS. L-selectin is mainly expressed on leukocytes. Vascular addressins are mucin-like glycoproteins expressed on the surface of endothelial cells as well as leukocytes. They possess sialyl-Lewis X, which binds the lectin domain of selectins. Their functions include regulation of localization of leukocytes and are involved in lymphocyte activation. Vascular cell adhesion molecule (VCAM-1), together with intercellular adhesion molecules 1 and 2 (ICAM-1, ICAM-2), are adhesion molecules belonging to the immunoglobulin (Ig) superfamily. Platelet/endothelial cell adhesion molecule-1 (PECAM-1) is a member of the Ig superfamily expressed on the surface, at intercellular junctions, of unstimulated endothelial cells, as well as on other types of cells including circulating leukocytes, which is important for the passage of leukocyte across the endothelium. The interaction between endothelial and leukocyte PECAM-1 is fundamental for transmigration. The right side of (C) shows main mechanisms implicated in endothelial repair. Mediators of angiogenesis are growth factors, among which vascular endothelial growth factor (VEGF) is the most specific for endothelium, cytokines, chemokines, matrix metalloproteinases (MMPs), and extracellular matrix macromolecules, which may influence endothelial proliferation directly or indirectly by means of the stimulation and the production of other angiogenic factors. Circulating endothelial progenitor cells (EPC) are hematopoietic stem cells with limited pluripotent potential, mainly involved in formation of new vessels after ischemia and in repairing damaged endothelium. Circulating progenitors cells (CPCs) are more undifferentiated bone marrow-derived cells, involved in damaged brain repair. Circulating endothelial cells (CEC) are mature endothelial cells released from the intima after vascular damage. In pathologic conditions with endothelial dysfunction, the number of EPC and CPC is reduced, while CEC levels are increased. BM, basal membrane; EC, endothelial cells; ExM, extracellular matrix; PMN, polymorphonuclear leukocytes; SMC, smooth muscle cells.
Although schematically described separately, the four different endothelial functions, with related pathways, are strictly inter-related. The term endothelial dysfunction is applied to identify the shift from a normal endothelium to a damaged one that may express with a proinflammatory, provasoconstriction, proliferative, and procoagulation phenotype. Abnormal endothelial function has been documented in different other pathologic conditions, mainly atherosclerosis, diabetes, hypertension, hyperlipidemia, and chronic kidney disease. Finally, the role of some circulating factors as important cardiovascular risk factors has been explained by their main harmful effects on endothelial cells. In this sense, homocysteine (HCY), C-reactive protein (CRP), and asymmetric dimethylarginine (ADMA) may all be considered as endothelial toxins.17–19
Cerebral Endothelium: the Blood–Brain Barrier and the Neurovascular Unit
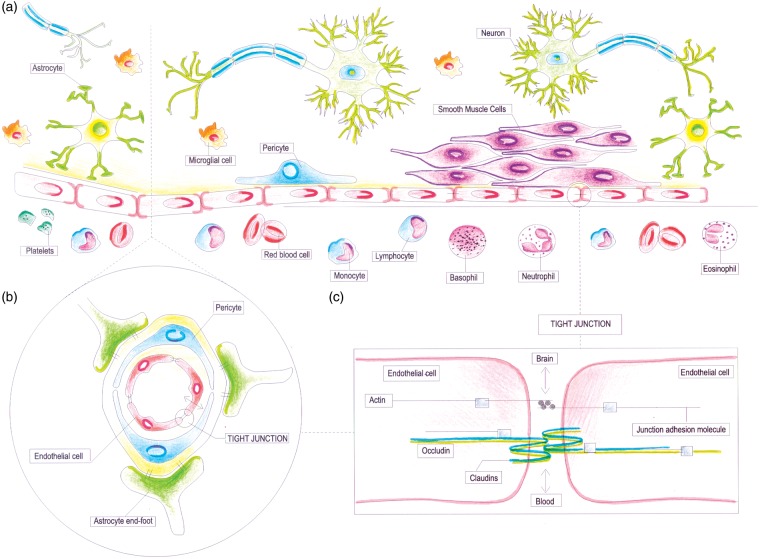
Within the vascular tree, endothelium presents a high degree of structural and functional heterogeneity. In the central nervous system, the ‘neurovascular unit (NVU)’ refers to the conceptual framework that links microvessels and neuron function and the responses of these compartments to injury.20 The ‘NVU’ consists of microvessels [endothelial cells–basal lamina matrix–astrocyte end-feet (and pericytes)], astrocytes, neurons and their axons, and other supporting cells that are likely to modulate the function of the ‘unit’ (Figure 2A).21 Endothelial cells in the cerebral microvessels are a part of the blood–brain barrier (BBB) (Figure 2B), where they exhibit a specialized phenotype with no fenestrations, extensive tight junctions, and sparse pinocytotic vesicular transport (Figure 2C). This barrier allows a strict control of exchange of solutes and circulating cells between the plasma and the interstitial space. Cerebral endothelial cells in most brain regions are connected by tight junctions that function as a ‘physical barrier’ preventing molecular traffic between blood and the brain.22 Blood–brain barrier also act as a ‘transport barrier’, given to the presence of specific transport systems regulating the transcellular traffic of small hydrophilic molecules, as well as a ‘metabolic barrier’, given the presence of a combination of intracellular and extracellular enzymes.23
Figure 2.
Schematic representation of the cerebral endothelium as integral part of the neurovascular unit (NVU) and blood–brain barrier (BBB). (A) Schematic representation of the NVU, which consists of the monolayer of endothelial cell, connected by tight junctions and resting on the basal lamina, pericytes, smooth muscle cells, astrocytic end-feet, microglia, and neuronal terminals. Circulating blood cells, such as polymorphonuclear (PMN) cells, lymphocytes, and monocytes, are also part of the unit, given the close interaction with the luminal surface of endothelial cells and their role in immune surveillance. (B) Schematic representation of the BBB, which is an integral part of the NVU. The BBB is formed by highly specialized endothelial cells, astrocytic end-feet, and pericyte that together constitute the dynamic interface separating the brain from the circulatory system. (C) Schematic representation of tight junctions, which consist of three main groups of proteins. They are transmembrane proteins (claudins, occludin), cytoskeletal proteins (actin), and accessory proteins. Structurally, they all interact to form a continuous network of transmembrane and cytoplasmic proteins linked with the actin-based cytoskeleton, allowing the tight junction to form a barrier while remaining capable of rapid modulation and regulation.
When dealing with cerebral SVD, also taking into account the different types of associated brain lesions, it is fundamental to bear in mind that all the above-mentioned components are strictly inter-related, and that presumably endothelial dysfunction is one of the major determinants of the structural and functional brain-vessel alterations.6
Evidence of Endothelial Dysfunction/Damage in Small Vessel Disease
Endothelial dysfunction is nowadays considered as one of the pivotal mechanisms of the structural and functional brain-vessel alterations in SVD, and the underpinned evidence comes from different settings.6
First of all, in SVD patients there is evidence suggesting the presence of reduced cerebral blood flow, and impaired cerebral blood flow autoregulation.24 Cerebral blood flow studies using a variety of techniques, including positron emission tomography and magnetic resonance imaging (MRI), have shown hypoperfusion. Using endogenous contrast MRI perfusion, perfusion is reduced in the white matter but not in the gray matter,25 and, within the white matter, it is reduced not only within regions of radiologic WMH but also in normal appearing white matter, although to a lesser extent.26 Endothelium might have a role in this sense, given the fact that nitric oxide signaling is an important factor in local cerebral blood flow regulation, and that it has been used as a marker to show endothelial dysfunction and decreased vasodilation in response to external stimuli such as hypercapnia or salbutamol in patients with lacunar infarction, compared with controls.27,28
Second, it has been hypothesized that cerebral SVD may be considered as a systemic condition resulting from dysfunction of arteriolar perfusion.29 Interestingly, there is now evidence pointing to a systemic endothelial dysfunction in patients with cerebral SVD, as indicated by several studies in which endothelial changes have been measured in other vascular beds other than the brain. This association has been documented with the kidney,30,31 with the skin,32 and the sublingual microvasculature.33 Impairment of endothelial function in systemic vessels, investigated by means of flow-mediated dilatation in the brachial artery, has also been associated with lacunar stroke.34
A third body of evidence comes from pathology studies. Available pathological studies on WMH show infarct-like areas of white-matter loss, and/or area of myelin attenuation and pallor. The glial pathology includes astrogliosis, changes in astrocytes, apoptosis of oligodendrocytes and astrocytes, and axonal loss.35 Tissue pathology changes seen postmortem are frequently interpreted as ‘ischemic’, although some of the changes also support the concept of endothelial dysfunction. An Australian study showed that reduced endothelial integrity was independently associated with increasing WMH severity, and with a significant decrease in endothelial and BBB integrity in areas of WMH compared with normal white matter.36 In the Medical Research Council Cognitive Function and Ageing Study, a prospective population-based neuropathologic study of elderly people in the United Kingdom, postmortem MRI was used to sample lesioned and nonlesioned white matter.35 Controls in these studies have further been divided into those from cases with lesions (controls—lesional) and those without lesions (controls—nonlesional). White-matter lesions were characterized by the expression of hypoxia-related molecules and by the expression of endothelial markers such as intracellular adhesion molecule 1 (ICAM-1), thus supporting the role of endothelial dysfunction.37 There is evidence of BBB leakage, as identified by albumin extravasation, which is widespread in the aging brain and increased in severe white-matter lesions.38 Together with BBB, there is evidence of the involvement of other components of the NVU. Microglial activation, identified by MHC class II upregulation, has been found to be increased in control white matter from cases with lesions compared with control white matter from individuals without white-matter lesions.39 Very recently, it was shown that oxidative DNA damage, identified by immunohistochemistry for 8-hydroxydeoxyguanosine, is also increased not only in white matter lesions but also in control white matter from lesional cases.40 Taken together, these data support the fact that normal-appearing white matter from cases with lesions more closely resembles the lesions themselves than it does white matter from nonlesional cases, and this suggests that lesions in the white matter exist or develop in white matter that shows a ‘field effect’ of diffuse abnormality.35
Finally, another hypothesized mechanism by which endothelial dysfunction may contribute to brain parenchyma lesions is increased BBB permeability, with consequent leakage of plasma components into the vessel wall and surrounding parenchyma.41 Permeability of the BBB augments with advancing age and in the presence of cerebral SVD. Increased cerebrospinal fluid/serum albumin ratio, a marker of BBB breakdown, has been documented in vascular dementia,42,43 and in patients with white-matter changes on neuroimaging.44 More recently, contrast-enhanced MRI has been used to study BBB permeability, documenting an increased permeability in the white matter of patients with lacunar stroke compared with patients with cortical stroke.45 Topakian et al.46 found increased BBB permeability in normal appearing white matter in patients affected by SVD compared with controls, and white-matter lesion severity was an independent predictor of these permeability-related signal changes.
From what reported so far, endothelial dysfunction might thus be per se involved as a cause of SVD-related symptoms but also the first step on which other conditions may superimpose. This might be the case, for example, of local thrombosis with subsequent arteriolar occlusion, leading to tissue ischemia through failure of vasodilatation in response to increased neuronal activity.47
Studies Investigating Potential Associations Between Circulating Biomarkers of Endothelial Dysfunction and Small Vessel Disease
Methods
Search Strategy
The literature search was performed using MEDLINE (Pubmed), and was restricted to the period January 2000 to September 2014 because we wanted to restrict the search to the most recent studies in which SVD-related changes had been assessed using current MRI technologies. Search strategy was as follows: (1) endothelial AND each of the following terms: lacunar, subcortical infarcts, silent brain infarcts, white-matter hyperintensities, white matter changes, leukoaraiosis, and microbleeds; (2) tPA, PAI, Fibrinogen, selectine, d-dimer, homocysteine, ICAM, VCAM, c-reactive protein, interleukin, metalloproteinase, Von Willebrand and each of the following terms: lacunar, subcortical infarcts, silent brain infarcts, white-matter hyperintensities, white-matter changes, leukoaraiosis, and microbleeds; (3) endothelial, tPA, PAI, Fibrinogen, selectine, d-dimer, homocysteine, ICAM, VCAM, c-reactive protein, interleukin, metalloproteinase, Von Willebrand, and CADASIL (Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy).
We included only English language written papers reporting studies that dealt with human cohorts, and considered both population- and hospital-based studies. These latter were defined as studies in which patients were recruited in hospital wards or outpatient settings. Hospital-based studies were excluded when stroke or other cardiovascular events had occurred less than 2 months before biomarkers assessment, to exclude changes consequent to the acute phase. We considered only studies regarding circulating biomarkers. Given the distinct pathophysiologic features of the arteriopathy, we report the results for sporadic SVD and CADASIL in separate tables.
Results
Sporadic Small Vessel Disease and Endothelial Biomarkers
Thirty-eight articles were selected and fully evaluated. Tables 1 and 2 summarize the main characteristics and results of these studies, with a focus on results dealing with the association between SVD and different circulating biomarkers of endothelial dysfunction or inflammation. Seventeen studies were hospital-based and twenty were population-based studies. Thirty-five studies performed only cross-sectional analyses, one longitudinal analyses,85 and two both.60,61
Table 1.
Studies investigating the cross-sectional association between biologic endothelial markers and chronic SVD.
| Ref. | Circulating markers | Study design | MRI markers | Investigated associations | Results |
|---|---|---|---|---|---|
| Population-based cohort studies | |||||
| Aono et al.48 | Fibrinogen, CRP | Japanese population without history of stroke: n = 958 - SVD (defined as the presence of LI or WMH of any degree, and LI + WMH): n = 466 (mean age 68 ± 5 years) - Controls: n = 492 (mean age 64 ± 5 years) | WMH LI | Plasma fibrinogen or CRP versus WMH or LI | (1) Fibrinogen levels>in SVD pts than in controls (2) Fibrinogen independently associated with WMH and LI (adjusting for demographic and vascular risk factors) |
| Aribisala et al.49 | CRP, IL-6, Fibrinogen | Elderly subjects (no medical exclusion criteria) born in 1936: n = 634 They create a variable ‘Inflammation’, which was composed by the three biologic markers (CRP, IL-6, Fibrinogen) | PVS WMH (PV and subcortical, volume) | Inflammatory markers versus WMH or PVS | (1) Centrum semiovale PVS significantly associated with CRP (2) ‘Inflammation’ slightly associated with PVS after multivariate structural equation modeling |
| Dufouil et al.50 | HCY | Participants n = 841 (no particular exclusion criteria; mean age 67 years) | WMH MRI performed 2 years after blood sample | HCY versus WMH | Moderate to severe WMH increasing slightly by level of HCY |
| Fornage et al.51 | IL-6, CRP | Healthy volunteers, no institutionalized: - White: n = 3,073 (mean age 74.5 years) - Black: n = 571 (mean age 73.9 years) | WMH SBI | IL-6 or CRP with WMH or SBI | (1) IL6 levels associated with WMH in both races (2) CRP levels associated with WMH only in white (3) IL-6 associated with SBI only in white (adjusting for demographics and vascular RF) |
| Gottesmann et al.52 | vWF, TPA, PAI-1, Plasminogen, DD, β-TG, CRP, TM | From the Atherosclerosis Risk in Communities cohort (no history of stroke; age 45–64): - Pts with presence of SLI: n = 196 - Controls from the same cohort without SLI: n = 214 | SLI | Hemostatic factors with SLI | (1) Levels of vWF, fibrinogen, DD, and β-TG>in cases versus control (2) vWF and DD associated with SLI (adjusting for demographics, vascular RF, and left ventricular hypertrophy) |
| Kloppenborg et al.53 | HCY | Pts with symptomatic atherosclerosis (Coronary, CV, peripheral artery disease, AAA): n = 1,223 (mean age: 59 years) SMART study | WMH LI | HCY levels with WMH and LI | (1) HCY levels and hypermocysteinemia independently associated with WMH (2) HCY levels, but not hypermocysteinemia associated with SLI (adjusting for vascular RF, coronary, and peripheral artery disease, AAA and IMT) |
| Notsu et al.54 | ADMA, SDMA, L-Arg/ ADMA, HCY | Consecutive participants of health examination: n = 712 - SVD (LI + moderate/severe WMH) pts: n = 146 - Controls: n = 566 | WMH LI | ADMA, SDMA, L-Arg/ADMA, and HCY levels versus SVD | (1) ADMA, SDMA, L-Arg/ADMA differing between SVD pts and controls (2) L-Arg/ADMA associated with SVD (adjusting for age and hypertension) |
| Pikula et al.55 | ADMA, SDMA | Framingham Offspring Study. Participants: n = 2,013 (mean age 58 ± 9.5 years; subject free of stroke, dementia, or other neurologic illness) | SBI WMH | ADMA and SDMA levels versus SBI and WMH | ADMA levels independently associated with SBI (adjusting for demographics, stroke RF, and time to MRI) |
| Pikula et al.56 | CRP, DD, PAI, HCY | Framingham Offspring Study Participants: n = 1,901(mean age 58 ± 10 years) | SBI, WMH volume, total cerebral brain volume | Selective biomarkers and the presence of SBI, WMH volume, and total cerebral brain volume | CRP, DD, HCY independently associated with TBCV (adjusting for age, sex, time to MRI, and stroke RF) |
| Pikula et al.57 | VEGF | Framingham study Participants: n = 1,863 (mean age: 61 ± 9 years; without history of stroke and TIA) | WMH (0.6 year from MRI and blood sample) | VEGF and BDNF levels and WMH | BDNF levels independently associated with lower WMH volume (adjusting for age, sex, time to MRI, and traditional stroke risk factors) |
| Romero et al.58 | Lp-PLA2 | Framingham Offspring study Participants: n = 819 (mean age: 73 years; subject free of stroke, dementia, or other neurologic illness) - No MB: n = 713 - MB pts: n = 106 | MB (Median time between brain MRI and blood sample is 6 years) | Lp-PLA2 levels and MB, with possible regional specificity | No significant association was found between MB and LpPLA2 |
| Sachdev et al.59 | HCY | Individuals selected randomly from the community: n = 385 (aged 60–64 years) | WMH (deep and PV), Brain atrophy | HCY levels and WMH | HCY levels associated with deep WMH (adjusting for sociodemographics, vascular RF, B12, folate, creatinine levels) |
| Satizabal et al.60 | IL-6, CRP | Community-dwelling elderly free of dementia: n = 1,841 (mean age: 72.5 years) | WMH (divided into total, PV, and deep), SBI, Brain volume | IL-6 and CRP levels and WMH, SBI and brain volumes | (1) Il-6 and CRP independently associated with higher total and periventricular WMH volume. (2) CRP associated with SBI (adjusting for age, gender, vascular RF, and total intracranial volume or white-atter mask volume) |
| Van Dijk et al.61 | CRP | Rotterdam Scan Study Participants at baseline: n = 1,033 (nondemented 60–90 years) | WMH (PV and subcortical) LI | CRP levels and WMH, LI | CRP levels associated with periventricular and subcortical WMH (adjusting for age, sex, cardiovascular RF, carotid plaques and IMT) |
| Vermeer et al.62 | HCY | Rotterdam Scan study Nondemented pts: n = 1,077 (60–90 years) Pts with WMH, SBI, or both: n = 378 | SBI WMH | HTY levels and SLI, WMH | Levels of HCY associated with both presence and extension of SBI and WMH (adjusting for demographics, vascular RF, and IMT) |
| Wada et al.63 | CRP | Elderly Japanese subjects: n = 689 (age 61–72 years) LI pts: n = 196 No LI pts: n = 493 | WMH LI | CRP levels and MRI features of SVD | (1) No correlation between CRP levels WMH or number of lacunes (2) No association between CRP and WMH or lacunes (adjusting for demographics, vascular RF, and IMT) |
| Wersching et al.64 | CRP | Community-dwelling, stroke and dementia-free individuals: n = 447 - High-field MRI performed: n = 321 | FA WMH, brain volume | CRP levels and WMH, brain volumes, and FA | CRP values independently associated with whole brain reduced FA (particularly frontal lobe) |
| Wright et al.65 | HCY | Northern Manhattan Study Multiethnic stroke-free population: n = 259 | WMH | HCY levels and WMH | Higher levels of HCY associated with WMH (adjusting for sociodemographics and vascular RF) |
| Wright et al.66 | Lp-PLA2, MPO, and hsCRP | Northern Manhattan Study. Multiethnic stroke-free population: n = 527 (mean age 71.3 years) | WMH | Lp-PLA2, MPO, hsCRP levels and WMH | (1) Levels of Lp-PLA2, MPO, and hsCRP in the fourth quartile associated with more severe WMH (2) Lp-PLA2, MPO associated with WMH (adjusting for demographics, vascular RF, and the other 2 biomarkers) |
| Yoshida et al.67 | Acrolein, CRP, IL-6, MMP-9 | Japanese elderly volunteers: n = 97 (no history of stroke and dementia; aged 65.3 ± 8.6 years) - Normal subjects: n = 53 - SBI pts: n = 44 | SBI | Acrolein, CRP, IL-6 levels and SBI | IL-6 and CRP levels>in pts with SBI than in controls |
| Hospital-based cohort studies | |||||
| Asymptomatic patients | |||||
| Han et al.68 | ICAM-1 | Consecutive asymptomatic subjects referred to a neurology outpatient clinic: n = 175 (no history of CV disease and dementia) | WMH (PV and deep) | ICAM-1 level versus WMH | ICAM-1 independently associated with periventricular and deep WMH. (adjusting for demographics and vascular RF) |
| Hoshi et al.69 | hsCRP, IL-6 | Neurologically asymptomatic patients: n = 194 - SBI pts: n = 40 - No SBI pts: n = 154 | SBI | hsCRP or Il-6 versus SBI | (1) hsCRP and IL-6 levels higher in SBI patients than in those without. (2) hsCRP and IL-6 levels independently associated with SBI (adjusting for demographics, vascular RF and IMT) |
| Kim et al.70 | IL-6, PAI-1, MMP-9 | Stroke-free subject: n = 137 - No WMH: n = 82 - WMH: n = 55 | WMH | IL-6, TNF-a, PAI-1, MMP-9 and WMH | MMP-9 level were >in pts with WMH versus those without |
| Miralbell et al.71 | CRP, PAI-1, Lp(a) | Pts:n = 86 Subjects (50–65 years old, free from dementia and without history of vascular disease) | FA, WMH, gray-matter volume | CRP, PAI-1, and Lp(a) levels versus WMH and FA | CRP and PAI resulted iassociated with FA (adjusting for demographics and vascular RF) |
| Miwa et al.72 | hs-CRP, IL-6, IL-18 | Neurologic pts: n = 431 (neurology out-patient; stroke/ TIA pts were excluded; MB pts: n = 65; No MB pts n = 366) | MB WMH SLI | hs-CRP, IL-6, IL-18 levels versus the presence of MB | hs-CRP, IL-6, IL-18 associated with MB (adjusting for demographics, vascular RF, IMT, WMH, and SLI) |
| Tomimoto et al.73 | TAT, F1 + 2, DD, Fibrinogen | Neurology outpatient clinic: n = 90 patients divided into: - WMH group (moderate/severe): n = 47 WMH without dementia: n = 18 Stable BD: n = 11 Deteriorating BD (BD symptoms in the last 3 months): n = 18 - No WMH group: n = 43 LI: n = 19 Other neurologic disease (excluded CV disease): n = 24 | WMH LI | TAT, F1 + 2, DD, Fibrinogen and different manifestations of SVD | (1) Levels of TAT and DD>in deteriorating BD versus pts with other neurologic diseases (2) WMH associated with TAT (adjusting for demographics, cognitive measures, and vascular RF) |
| Hypertensive patients | |||||
| Kario et al.74 | PAI-1, t-PA–PAI-1, PIC, TAT, F1 + 2, DD, vWF | Out-patient asymptomatic hypertensive subjects: n = 123 - No infarct (no SLI): n = 43 - Total infarct group (SLI): n = 80 (divided into multiple LI > 2: n = 48 and few LI⩽2: n = 32) | SLI | Hemostatic abnormalities versus SLI | (1) Levels of vWF, PAI-1, F1 + 2, TAT and DD>in infarct group versus non infarct group. (2) vWF, PAI-1, and F1 + 2 independently associated with multiple SLI (adjusting for demographics and vascular risk factors) |
| Rouhl et al.75 | putative EPC, Tang | Hypertensive pts: n = 61 (free of any symptomatic ischemic or vascular disease) - NonSVD: n = 29, - SVD: n = 32 (defined as the presence of at least one SVD feature: LI; WMH, MB) | LI WMH MB | Tang and EPC and cerebral SVD | (1) Tang and putative EPC<in SVD pts (2) Tang associated with SVD (adjusting for T-cell count and blood pressure) |
| Stroke patients | |||||
| Hassan et al.76 | ICAM-1, TM, TF, TFP | SU outpatient clinic: - SVD pts: n = 110 (clinical lacunar syndrome and compatible lesion on CT/MRI) (⩾2 months after stroke event) - iLI pts (one focal lesion and a WMH absent or mild): n = 47 - Ischemic WMH pts (one focal lesion and WMH moderate/severe): n = 63 - controls: n = 50 (free of clinical CV disease; no imaging available) | WMH LI 17 pts with CT | (1) Levels of endothelial dysfunction markers versus SVD subtypes (2) Endothelial activation markers versus grade of LI or extent of WMH | (1) ICAM1, TM, and TFPI higher in cerebral SVD pts compared with controls (adjusting for demographics and vascular RF) (2) ICAM1 and TM higher in both iLI and WMH versus controls; TFPI higher only in iLI versus WMH and controls (not adjusted) (3) TM associated with grade of LI, while TF/TFPI, TF associated with the extent of WMH (independent of demographics and vascular RF) |
| Hassan et al.77 | HCY | SU out-patient clinic SVD pts: n = 172 (clinical lacunar syndrome with a compatible lesion on CT/MRI) (⩾2 months after stroke event) - isolated LI pts: n = 90 (one focal lesion and a WMH absent or mild) - Ischemic WMH pts: n = 52 (one focal lesion and WMH moderate/severe) Controls: n = 50 (similar for demographics, vascular RF; no imaging available) | WMH LI 30 pts with CT | (1) HCY levels versus SVD (2) HCY levels and SVD subtypes: iLI and ischemic WMH | (1) HCY levels higher in patients with SVD versus controls (adjusting for demographics and vascular RF) (2) HCY levels higher among pts with WMH versus iLI and controls (adjusting for demographics and vascular RF) (3) The association between HCY and SVD no longer significant after correction for ICAM1 and TM |
| Khan et al.78 | ADMA, HCY | Lacunar stroke pts: n = 47 (⩾3 months after stroke event) Controls pts: n = 38 (community subjects free of symptomatic CV disease, no imaging available) | WMH LI (6 pts with CT) | (1) ADMA levels versus lacunar stroke pts (2) ADMA levels versus WMH and LI | ADMA independently associated with SVD and correlated with WMH severity (adjusting for age, gender, vascular RF, and creatinine clearance) |
| Knottnerus et al.79 | tPA, PAI-1, tPA–PAI, vWF, TF, TM, fVIII | First-ever LS population: (⩾3 months after stroke event) - Isolated LS pts: n = 43 - LS + extensive WMH (eWMH) pts: 53 - LS + asymptomatic LI + no WMH pts: 53 | LI SLI WMH | Levels of endothelial activation markers and different MRI features of SVD | (1) tPA>in eWMH pts and PAI-1< eWMH pts. (92) PAI-1 independently associated with WMH (adjusting for demographics and vascular RF) |
| Knottnerus et al.80 | TFPI | LS pts: n = 149 (⩾3 months after stroke) - ILA: isolated lacunar stroke (no SLI or WMH) - SIL: silent ischemic lesions (SLI and/or extensive WMH) Control pts: n = 42 (presenting at neurology out-patient clinic for myogenic back pain or entrapment neuropathies) | WMH LI | (1) TFPI levels versus lacunar stroke pts (2) TFPI versus different features of SVD among lacunar stroke patients | (1) TFPI<lacunar stroke pts versus control (2) TFPI>in SIL versus ILA pts (adjusting for age and vascular RF; no associations after controlling for total and LDL cholesterol) |
| Naka et al.81 | HCY | Stroke outpatients pts: n = 102 (all types of stroke) (⩾2 months after stroke event) | WMH MB | HCY levels versus WMH and MB | HCY levels associated with WMH (adjusting for demographics, vascular RF and MB) |
| Rouhl et al.82 | EPC, EPC cluster formation (EPC-CF) | LS patients: n = 42 (2 years after the event; silent ischemic lesion (SIL) pts: n = 29 (WMH and/or silent infarcts) Controls pts: n = 18 (presenting at neurology out-patient clinic for myogenic back pain or entrapment neuropathies) | LI WMH SLI | EPC and EPC-CF numbers and LS pts and SIL pts | EPC-CF<in LS and SIL pts versus controls |
| Stroke patients | |||||
| Rouhl et al.83 | IgG and IgM oxidized LDL (MDA, HOCl-modified oxidized LDL) | Lacunar stroke pts: n = 158 (⩾3 months after stroke) Hypertensive pts: n = 158 (free of any symptomatic ischemic or vascular disease) Controls pts: n = 43 (presenting at neurology out-patient clinic for myogenic back pain or entrapment neuropathies) | LI WMH PVS | (1) Antibodies against oxLDL levels and SVD (2) Antibodies against oxLDL levels and different SVD MRI features | (1) IgG-HOCl-oxLDL and IgM-MDA-oxLDL>in SVD pts versus controls (2) PVS associated with IgG-HOCl-oxLDL levels (adjusting for age, sex, hypertension, patient category, coronary and peripheral artery disease) |
| Rouhl et al.84 | ICAM-1, VCAM-1, E-selectin, P-selectin, neopterin, CRP | Lacunar stroke pts: n = 163 (⩾3 months after stroke event) Hypertensive pts: n = 93 (free of any symptomatic ischemic or vascular disease) Controls pts: n = 43 (presenting at neurology out-patient clinic for myogenic back pain or entrapment neuropathies) | SLI MB WMH PVS | (1) Inflammatory marker levels and the presence of LI, WMH, and WMH + LI (2) Inflammatory markers levels and SVD MRI features | (1) Neopterin, P-selectin, ICAM-1, and VCAM >in pts with SLI versus those without - neopterin and VCAM were >in pts with WMH versus those without - neopterin, ICAM-1, VCAM- 1 were>in pts with WMH + LI versus those without (2) Neopterin associated with PVS in BG and E-selectine with MB (adjusting for demographics and vascular RF) |
Abbreviations: AAA, abdominal aortic aneurysm; ADMA, asymmetric dymethil arginine; β-TG, Beta-thromboglobulin; BDNF, brain-derived neurotrophic factor; BD, Binswanger disease; BG, basal ganglia; CRP, C-reactive protein; CV, cerebrovascular; DD, D-dimer; EPC, endothelial progenitor cells; F1 + 2, prothrombin fragment 1 + 2; FA, fractional anisotropy; HCY, homocysteine; hsCRP, high sensivity C-reactive protein; HOCl, hypochlorite; ICAM-1, intercellular adhesion molecule-1; IMT, intima media thickness; L-Arg, L-arginine; iLI, isolated lacunar infarct; LI, lacunar infarcts; Lp(a), A Lipoprotein; Lp-PLA2, lipoprotein phospholipase-A2; LS, lacunar stroke; MB, microbleeds; MDA, malondialdehyde; MMPs, matrix metalloproteinases; MPO, myeloperoxidase; PAI-1, plasminogen activator inhibitor type 1; PIC, plasmin-alpha2-plasmin inhibitor complex; Pts, patients; PV, periventricular; PVS, perivascular spaces; RF, risk factor; SBI, subcortical brain infarcts; SDMA, symmetric dymethil arginine; SLI, silent lacunar infarct; SU, Stroke Unit; SVD, small vessel disease; TAT, thrombin–antithrombin complex; TF, tissue factor; TFPI, tissue factor pathways inhibitor; TIA, transient ischemic attack; TM, thrombomodulin; tPA, tissue plasminogen activator; VCAM-1, vascular adhesion molecule-1; VEGF, vascular endothelial growth factor; vWF, Von willebrand factor; WMH, white-matter hyperintensities. Studies are grouped according to types of cohort under investigation.
Table 2.
Longitudinal studies investigating the association between biologic endothelial markers and chronic SVD.
| Ref. | Circulating markers | Study design | MRI markers | Investigated associations | Results |
|---|---|---|---|---|---|
| Markus et al.85 | ICAM-1, TM, TFPI, F 1 + 2, DD | Population based. Austrian Stroke prevention study. Individuals randomly selected from a community register: n = 267 (free of stroke and dementia; Mean age: 60 years) 3 and 6 years MRI available | WMH | Plasma markers of endothelial function and WMH progression | ICAM associated with WMH lesion progression at both 3 and 6 years (adjusting for demographics, and vascular RF) |
| Satizabal et al.60 | IL-6, CRP | Population based. 3C Dijon study. Community-dwelling elderly free of dementia: n = 1,841 (mean age 72.5 years) Subjects with new MRI after 4 years: n = 1,341 | WMH (volume) divided in WMH total, PV and deep, SBI, brain volume | IL-6 and CRP levels and progression of SVD MRI markers | No significant associations between baseline IL-6 and CRP levels and MRI changes progression over 4 years |
| Van Dijk et al.61 | CRP | Population based. Rotterdam Scan Study At baseline nondemented pts: n = 1,033 (mean age 72 years) Subjects with new MRI: n = 636 (mean age 71 years; Mean follow-up period from 1st to 2nd: 3.3 years) | WMH progression (PV and deep) LI | CRP levels and progression of WMH volume and incidence of new LI | Progression of periventricular WMH greater among subjects with high CRP levels compared with subjects with low CRP levels (adjusting for demographics and vascular RF, carotid plaques and IMT). |
Abbreviations: CRP, C-reactive protein; DD, D-dimer; F1 + 2, prothrombin fragment 1 + 2; ICAM-1, intercellular adhesion molecule-1; IMT, intima media thickness; LI, lacunar infarct; pts, patients; PV, periventricular; SBI, subcortical brain infarct; TFPI, tissue factor pathway inhibitor; TM, thrombomodulin; WMH, white-matter hyperintensities.
Small vessel disease and coagulation/fibrinolysis
Ten studies evaluated markers of coagulation and fibrinolysis in relation to MRI changes consequent to SVD. Plasminogen activator inhibitor resulted independently associated with the presence of WMH and/or lacunar infarcts and reduced fractional anisotropy on diffusion tensor imaging sequences in three studies.71,74,79 Four studies investigated the association between D-dimer and markers of SVD. Gottesmann et al.52 found an independent association between D-dimer levels and subclinical lacunar infarcts in the Atherosclerosis Risk in Communities cohort. Data from the Framingham study showed an independent association between D-dimer and total cerebral brain volume but not with WMH or silent brain infarcts.56 In the remaining two studies, characterized by smaller sample sizes, D-dimer proved to be increased in SVD, i.e. lacunar infarcts and clinically manifest Binswanger disease (defined by the presence of WMH and evidence of subcortical vascular dysfunction such as gait disorders, incontinence, and parkinsonism), but the relation was not confirmed after multivariate analyses.73,74 Regarding tissue factor pathway inhibitor (TFPI), discordant results were found in two studies. Hassan et al.76 found higher TFPI values in lacunar infarct patients compared with patients presenting both lacunar infarcts and moderate/severe WMH and with healthy volunteers adjusting for possible confounders. In Knottnerus et al.'s study, TFPI values were lower in patients with lacunar stroke compared with controls; the highest values were found among patients with lacunar stroke and WMH, compared with patients with only lacunar stroke.80 These two studies examined also tissue factor (TF), which resulted independently associated with the extent of WMH only in Hassan’s et al. study.76 Regarding thrombomodulin studies, only Hassan et al. found higher values in SVD patients in comparison with controls and a positive association with lacunar infarcts, while in the remaining two studies no association was found.52,79 Thrombin–antithrombin (TAT) was independently associated with the presence of WMH only in a small-sized study.74 Two studies determined F1 + 2 levels in SVD, documenting an independent association with multiple subcortical lacunar infarcts.73,74 Finally, von Willebrand factors proved to be independently associated with SVD MRI markers in two studies.52,74
Small vessel disease and hyperhomocysteinemia
In the majority of the population-based cohorts, abnormally increased HCY levels were found independently associated with WMH and/or silent lacunar infarcts.53,59,62,65 In the Framingham Offspring cohort,56 a significant association with SVD was appraised only with total brain cortical volume, while in the French cohort of the Epidemiology of Vascular Ageing study only a nonsignificant trend between HCY levels and WMH severity was found.50 Hospital-based studies have reported less homogenous results.77,78,81
Small vessel disease and inflammation
Several studies reported on the association between inflammatory markers and SVD. One of the most widely studied biomarker was CRP. In population-based studies, results were contrasting; in three large cohorts (including overall 5,947 subjects), CRP resulted significantly associated with WMH,51,60,61 while in the remaining six cohorts, examining a total of 5,156 patients, the association was not demonstrated.49,56,63,64,65 Discrepant results were also apparent from the two longitudinal studies reporting on CRP and progression of WMH: subjects with high baseline CRP levels had significant more progression of periventricular WMH than people with low CRP levels in the Rotterdam Scan Study, while no significant association was observed between baseline CRP levels and the evolution of silent brain infarcts and WMH in the 3C Dijon study.60,61 Interestingly, the two studies that investigated the association between CRP and a more sensitive MRI marker of white-matter changes, i.e. the microstructural damage as assessed using diffusion tensor imaging, found a positive association.64,71 An independent relation between CRP and MB or CRP and periventricular spaces was reported by two different studies.49,72 Silent brain infarcts proved to be associated with higher level of CRP in three studies,60,67,69 a result that was not confirmed by Gottesmann et al.’s study.52 Data concerning IL-6 are more consistent because an independent association between IL-6 and either WMH or silent brain infarcts was shown by the majority of population and hospital-based studies.51,60,67,69 Nevertheless, no association with the progression of MRI SVD markers was found for IL-6 in the 3C-Dijon study.60 Only one study reported an association between IL-6, IL-18, and MB.72
Three studies evaluated the association between fibrinogen and WMH, lacunar infarcts, PVS, and the most severe SVD-related clinical picture defined as Binswanger’s disease. In the largest of the three studies,48 SVD patients compared with controls had higher levels of fibrinogen, a finding that was not confirmed in the two other cohorts.49,73 In all the studies in which ADMA levels were measured, an independent association with both lacunar infarcts and WMH was reported.54,55,78 Matrix metalloproteinase 9 (MMP9) was measured in a total of 234 patients, but the association with WMH was significant only at univariate analyses.69,70 The Northern Manhattan Study found an independent association of both MPO and Lp-PLA2 with WMH, but the result for Lp-PLA2 was not confirmed in the Framingham Offspring Study.66,58 Finally, one study evaluated the role of autoantibodies against oxidized-LDL finding an independent association with PVS.83
Considering adhesion molecules, the association between ICAM-1 levels and SVD markers was investigated in three studies. Roulh et al.68 reported an association with WMH that was confirmed after multivariate analyses in a further study cohort. Higher levels of ICAM-1 were observed in patients with lacunar infarcts and WMH compared with controls.76 Interestingly, in the Austrian Stroke Prevention Study, ICAM-1 proved to be associated with a robust outcome measure, i.e., WMH progression at both 3 and 6 years.85 Rouhl et al.84 studied several endothelial biomarkers finding higher levels of E-selectine, neopterin, and VCAM-1 in patients with lacunar infarcts and/or WMH. Considering MRI markers of SVD, PVS resulted independently associated with neopterin and MB with E-selectine.
SVD and repair and remodeling
Only two studies evaluated the association between SVD and peripheral circulating cells documenting that immature EPC, and Angiogenic T cells (Tang) were significantly reduced in SVD patients. The association remained significant in multivariate analyses only for Tang.75,82
Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy and Endothelial Biomarkers
Five studies investigated circulating markers of endothelial dysfunction in CADASIL patients (Table 3). ADMA levels were significantly increased in 14 CADASIL patients compared with 22 healthy controls.86 Similarly, in 59 CADASIL there were higher HCY levels in comparison with 14 non-CADASIL patients with stroke or transient ischemic attack, both in the fasting state and after methionine challenge. Median differences between HCY levels before and after methionine challenge were greater among CADASIL patients than in controls.87 However, among 15 mildly symptomatic CADASIL patients, compared with 16 controls, HCY levels were not increased in one study in which higher levels of antioxidant molecules (blood reduced glutathione) and lower levels of oxidant mediators (plasma reduced cysteine) were found, suggesting a protective action against free radical formation at an early stage of clinical symptoms.89 In a larger cohort of 127 CADASIL patients, HCY levels were higher in CADASIL patients with migraine compared with non-migraine CADASIL patients, and elevated HCY levels were independently associated with an earlier age of onset of migraine itself.88 It was hypothesized that hyperhomocysteinemia could act by exacerbating the vascular injury, which presumably leads to migraine in CADASIL. Alternatively, hyperhomocysteinemia could increase susceptibility to oxidative injury and excitotoxicity, leading to mitochondrial dysfunction. Finally, in a study performed by our group CADASIL patients had a reduced number of EPC in comparison with controls.90 We also found that CADASIL patients with a more severe clinical picture (i.e., history of stroke and dementia) presented with lower circulating progenitor cell (CPC) levels than patients with a milder clinical picture (i.e., no history of stroke or dementia). Moreover, lower CPC levels were associated with worse performances at neuropsychologic, functional, and motor tests.
Table 3.
Studies investigating the cross-sectional association between circulating endothelial markers and CADASIL.
| Ref. | Circulating markers under investigation | Patient sample | SVD markers on MRI | Investigated associations | Results |
|---|---|---|---|---|---|
| Rufa et al.86 | ADMA, L-Arg Arg/ADMA | Case control CADASIL pts: n = 16 (mean age 49.1 years) healthy controls: n = 22 (mean age 47.9 years) | WM lesion volume | Plasma levels of ADMA, L-Arg, L-Arg/ADMA in CADASIL versus controls and correlation with WM lesion volume | ADMA levels>in CADASIL pts than in controls. In CADASIL pts, an inverse significant correlation was found between L-Arg/ADMA WM lesion volume |
| Flemming et al.87 | HCY | Case control CADASIL pts n = 59 (mean age 48.8 years) NonCADASIL pts with TIA or stroke: n = 14 (mean age 46.5 years) | No one | Fasting and 6 hours after oral loading with methionine HCT levels in CADASIL pts versus nonCADASIL pts | CADASIL pts>HCY levels compared with nonCADASIL pts. Median difference between HCY levels before and after methionine load was greater in the CADASIL group |
| Singhal et al.88 | HCY | CADASIL patients: n = 127 - Asymptomatic: n = 8 (mean age 31 years) - symptomatic: n = 119 (mean age 48 years) | WM lesion volume | Association between HCY, clinical picture and WM lesion volume | Higher HCY levels in migraineurs. HCY associated with earlier age of onset of migraine (adjusting for age, gender, and vascular RF) |
| Campolo et al.89 | HCY, cysteine glutathione, cysteinylglycine, 3-nitrotyrosine | Case control CADASIL pts: n = 15 (mean age 42 years) Controls: n = 16 (mean age 44 years) defined as subjects without CV and/or cardiovascular disease | No one | Oxidative stress markers in CADASIL patients versus healthy controls | CADASIL pts<reduced cysteine level and>reduced glutathione concentrations than in controls. No differences regarding HCY levels between CADASIL and controls. |
| Pescini et al.90 | EPC, CPC | Case control CADASIL patients: n = 29 (mean age 54.5 years) Controls: n = 29 (mean age 54.1 years defined as subjects without history of CV and cardiovascular diseases, peripheral arteriopathy, cancer, or dementia) | No one | EPC and CPC levels in CADASIL patients compared with healthy controls. Correlation between EPC and CPC levels and disease severity | EPC levels<in CADASIL pts than in controls (adjusting for age, sex, and statin use). CPC levels<in CADASIL pts with a more severe clinical picture (stroke or dementia) than CADASIL pts without CPC levels correlated with cognitive and motor performances |
Abbreviations: ADMA, asymmetric dymethil aginine; CADASIL, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; CPC, circulating progenitor cells; CV, cerebrovascular; EPC, endothelial progenitor cells; HCY, homocysteine; L-Arg, L-arginine; pts, patients; RF, risk factor; TIA, transient ischemic attack; WM, white matter.
Discussion
Evidence from the above considered rather large number of published studies, in which the association of several circulating endothelial biomarkers with SVD was scrutinized, looks largely heterogeneous and inconclusive concerning the identification, or even the suspicion, of a pivotal pathogenic role exerted by one or more distinct molecular factors or pathways in cerebral SVD.40 The most consistent associations in sporadic SVD regard PAI, ICAM-1, ADMA, and IL-6. Noteworthy, these molecules are involved at different places of the endothelial dysfunction/coagulation system/inflammation cascade. Another marker of endothelial dysfunction, for which significant results seem to emerge in both sporadic SVD patients and in CADASIL cases in comparison with controls, is the level of EPC. Moreover, in CADASIL, CPC levels were associated with clinical/functional indicators of disease severity, suggesting that these cells might have a role in the determination of the final phenotypic expression of the disease.90
Data heterogeneity may be explained by a number of differences in studies design. A first major concern relates to the different cohorts under investigation: some studies were conducted in the framework of population-based surveys of elderly individuals, while others investigated hospital cohorts without age limits. It is presumably logical to think that the brain lesion load related to SVD is lower in the population-based compared with the hospital-based cohorts, especially in stroke cohorts. Also sample sizes were largely variable, ranging from over three thousands in the largest population-based study, to less than a hundred in a number of hospital-based studies. Moreover, among the hospital-based cohorts, the clinical status of the enrolled patients was largely variable across the different studies, ranging from asymptomatic subjects, to hypertensive patients, and finally to previous symptomatic lacunar stroke patients. Concerning this latter category, it is fundamental to note that only few studies distinguished between isolated lacunar infarcts and multiple lacunar infarcts associated or not with WMH, thus recalling the concept, first introduced by Fisher, that the pathogenesis of small subcortical infarcts may be distinct: arteriolosclerosis, lipohyalinosis on one side, and (micro)atheroma on the other.91 When this distinction has been made, results support the hypothesis of a distinct pathogenesis.76,77,79,80 Considering stroke cohorts, in some studies, infarcts cannot be defined conventionally and univocally as lacunar: in fact, while the inferior diameter was precisely defined (generally 3 mm), there was no cutoff value reported for the superior one. For this reason, even if the infarcts were subcortical, it cannot be excluded that, in some cases, they were not related to SVD.
Among the different SVD-related MRI changes selected for investigating the effect of circulating biomarkers, WMH (visual grading or volume) and lacunar infarcts were the most frequently studied, especially in the older studies. Only few recent studies considered novel MRI markers of SVD such as MB, enlarged PVS, and microstructural WM damage measured on diffusion tensor imaging. Very few studies used cellular biomarkers of endothelial dysfunction. Finally, with the exception of a few studies,52,66,74,79,84 only selective biomarkers involved in different points of the complex pathways linking together endothelial dysfunction and coagulation system/inflammation processes were analyzed. It should be underlined that the assessment of individual biomarkers cannot provide information on relative contributions by the distinct pathways. Conceptually, only the combined use of multiple circulating endothelial biomarkers may provide a comprehensive understanding of the role of selective endothelial pathways related to the variable pathologic or clinical expressions of brain SVD. In fact, the panel of biomarkers related to each single pathway was often heterogeneously studied across studies. Different methodologies can be applied to measure circulating biomarkers, thus leading to a limited reproducibility of the results obtained in different laboratories. Finally, for several circulating biomarkers investigated in many of the considered studies no definite cutoff values were reported.
Conclusion and Future Directions
The vascular endothelium, located at the interface of blood and tissue, is essential for vascular homeostasis, as it is capable of sensing changes in the hemodynamic forces and blood borne factors, and to respond by releasing different kind of substances involved in the different pathways of the above-summarized endothelial functions. The net balance between endothelial-derived factors involved in the regulation of vascular tone, of coagulation and fybrinolysis, of pro- and anti-inflammation, and factors associated with growth inhibition and promotion, is essential for the maintenance of vascular homeostasis. Any disruption of this balance may have a detrimental role in the overall pathophysiology of SVD or in the production of the final tissue changes consequent to it. Research in the field of cerebral SVD in relation to endothelial circulating biomarkers appears to be in a very preliminary stage. A major problem relates to the fact that what is measured from blood samples reflects systemic endothelial function, which does not necessarily correspond to what happens at the level of brain endothelium. As such, the study of endothelial circulating biomarkers needs to be strictly correlated with other measures specific for brain endothelial function, i.e., advanced MRI technologies with intravenous gadolinium contrast agent combined with perfusion imaging.
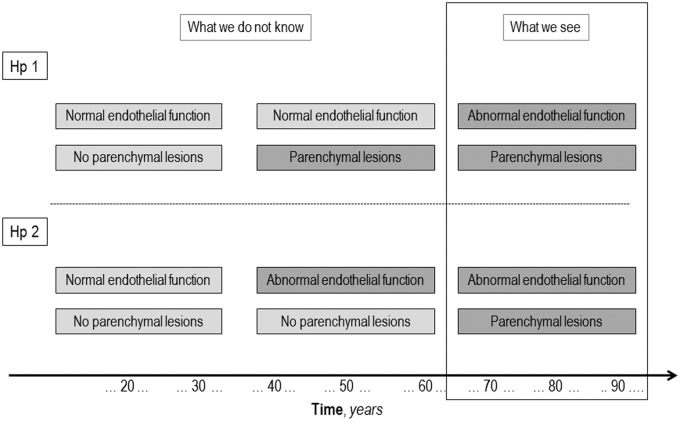
Finally, the issue of causality or possible reverse causality seems critical. Figure 3 is an attempt to schematically represent the possible relationship between endothelial dysfunction and brain parenchyma lesions related to SVD. We have evidence that, generally in elderly subjects, cerebral SVD-related lesions and endothelial dysfunction co-exist, but what we do not know is whether the association is causal, i.e., what happens before their coexistence. Support for a causal rather than a secondary role may come from longitudinal studies, as is the case of the three studies reported in Table 2, in which the predictive effect of the baseline circulating biomarkers levels is looked at on SVD progression.
Figure 3.
Conceptualization of the possible relationship between endothelial dysfunction and cerebral small vessel disease (SVD).
Another way of addressing the issue of causality may come from candidate gene association studies. As an example, HCY levels are known to be associated with SVD, particularly with WMH,77 and this could either be causal or could be secondary to established disease. Examination of a genetic variant associated with increased HCY levels throughout life allows causality to be explored, an approach referred to as ‘Mendelian randomization’.92 The methylenetetrahydrofolate reductase C677T polymorphism is associated with elevated HCY levels. A number of studies have shown no or only weak associations between a common methylenetetrahydrofolate reductase polymorphism and stroke as a whole. In contrast, an association was found in a well-phenotyped group of patients with lacunar stroke and, interestingly, this association was present only in the ischemic leukoaraiosis subtype.64 This supports a causal role for HCY in this specific subtype.
In conclusion, considerable evidence suggests that endothelial dysfunction may have an important role in cerebral SVD, although much molecular details still need to be clarified. The use of comprehensive panels of circulating endothelial biomarkers exploring functioning of the different biologic pathways may be useful in this regard, and it might overcome limitations associated with individual assays. The final translational goal is obviously to provide a more robust pathophysiologic background for the design of experimental research finalized to reduce the burden of cerebral SVD.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The VascMCI-Tuscany study is funded by Tuscany region.
Declaration of conflicting interests
DI serves as a member in the editorial board of Stroke, and is an associate editor of Neurological Sciences Journal. He has received grants for research by Bayer Italy, and fees for conferences by Boheringer Italy and Bayer Italy. LP is a member of the editorial boards of Cerebrovascular Diseases and Acta Neurologica Scandinavica and section editor (Vascular Cognitive Impairment) of Stroke.
Authors’ contributions
AP, MP, and FP made a substantial contribution to conception, design, acquisition of data, and drafting the article. LP and DI made substantial contribution to conception, design, drafting, and revising the manuscript.
References
- 1.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010; 9: 689–701. [DOI] [PubMed] [Google Scholar]
- 2.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. STandards for ReportIng Vascular changes on nEuroimaging (STRIVE v1). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poggesi A, Pantoni L, Inzitari D, Fazekas F, Ferro J, et al. The LADIS Study Group. 2001–2011: A Decade of the LADIS (Leukoaraiosis And DISability) Study: what have we learned about white matter changes and small-vessel disease? Cerebrovasc Dis 2011; 32: 577–588. [DOI] [PubMed] [Google Scholar]
- 4.Poggesi A, Pantoni L, Inzitari D. Consequences of cerebral small vessel disease: disability, mortality, and prognosis. In: Pantoni L, Gorelick PB. (eds). Cerebral small vessel disease, Cambridge University Press: Cambridge, UK, 2014, pp. 273–382. [Google Scholar]
- 5.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2010; 26: c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol 2013; 12: 483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation 2007; 115: 1285–1295. [DOI] [PubMed] [Google Scholar]
- 8.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch 2000; 440: 653–666. [DOI] [PubMed] [Google Scholar]
- 9.Michiels C. Endothelial cell functions. J Cell Physiol 2003; 196: 430–443. [DOI] [PubMed] [Google Scholar]
- 10.Pearson JD. Endothelial cell function and thrombosis. Baillieres Best Pract Res Clin Haematol 1999; 12: 329–341. [DOI] [PubMed] [Google Scholar]
- 11.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 1998; 91: 3527–3561. [PubMed] [Google Scholar]
- 12.Murphey HS. Inflammation. In: Streyer Rubin. (ed). Rubin's pathology: Clinicopathologic foundations of medicine, Lippincott Williams and Wilkins: Philadelphia, PA, 2012, pp. 23–44. [Google Scholar]
- 13.Nathan C. Points of control in inflammation. Nature 2002; 420: 846–852. [DOI] [PubMed] [Google Scholar]
- 14.Pescini F, Abbate R. Markers of endothelial dysfunction, oxidative stress, and inflammation in cerebral small vessel disease. In: Gorelick Pantoni. (ed). Cerebral small vessel disease, Cambridge University Press: Cambridge, UK, 2014, pp. 192–199. [Google Scholar]
- 15.Burger D, Touyz RM. Cellular biomarkers of endothelial health: microparticles, endothelial progenitor cells, and circulating endothelial cells. J Am Soc Hypertens 2012; 6: 85–99. [DOI] [PubMed] [Google Scholar]
- 16.Beck H, Voswinckel R, Wagner S, Ziegelhoeffer T, Heil M, Helisch A, et al. Participation of bone marrow-derived cells in long-term repair processes after experimental stroke. J Cereb Blood Flow Metab 2003; 23: 709–717. [DOI] [PubMed] [Google Scholar]
- 17.Cheng Z, Yang X, Wang H. Hyperhomocysteinemia and endothelial dysfunction. Curr Hypertens Rev 2009; 5: 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szmitko PE, Wang CH, Weisel RD, de Almeida JR, Anderson TJ, Verma S. New markers of inflammation and endothelial cell activation: Part I. Circulation 2003; 108: 1917–1923. [DOI] [PubMed] [Google Scholar]
- 19.Franceschelli S, Ferrone A, Pesce M, Riccioni G, Speranza L. Biological functional relevance of asymmetric dimethylarginine (ADMA) in cardiovascular disease. Int J Mol Sci 2013; 14: 24412–24421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neuwelt EA, Bauer B, Fahlke C, Fricker G, Iadecola C, Janigro D, et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci 2011; 12: 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.del Zoppo GJ. Aging and the neurovascular unit. Ann NY Acad Sci 2012; 1268: 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iadecola C. The pathobiology of vascular dementia. Neuron 2013; 80: 844–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006; 7: 41–53. [DOI] [PubMed] [Google Scholar]
- 24.Markus HS, Allan CL, Ebmeier KP. Cerebral hemodynamics in cerebral small vessel disease. In: Pantoni L, Gorelick PB. (eds). Cerebral small vessel disease, Cambridge University Press: Cambridge, UK, 2014, pp. 180–191. [Google Scholar]
- 25.Markus HS, Lythgoe DJ, Ostegaard L, O'Sullivan M, Williams SC. Reduced cerebral blood flow in white matter in ischaemic leukoaraiosis demonstrated using quantitative exogenous contrast based perfusion MRI. J Neurol Neurosurg Psychiatry 2000; 69: 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Sullivan M, Lythgoe DJ, Pereira AC, Summers PE, Jarosz JM, Williams SC, Markus HS. Patterns of cerebral blood flow reduction in patients with ischemic leukoaraiosis. Neurology 2002; 59: 321–326. [DOI] [PubMed] [Google Scholar]
- 27.Gunarathne A, Patel JV, Kausar S, Gammon B, Hughes EA, Lip GY. Glycemic status underlies increased arterial stiffness and impaired endothelial function in migrant South Asian stroke survivors compared to European Caucasians pathophysiological insights from the West Birmingham Stroke Project. Stroke 2009; 40: 2298–2306. [DOI] [PubMed] [Google Scholar]
- 28.Deplanque D, Lavallee PC, Labreuche J, Gongora-Rivera F, Jaramillo A, Brenner D, et al. Lacunar-BICHAT Investigators. Cerebral and extracerebral vasoreactivity in symptomatic lacunar stroke patients: a case-control study. Int J Stroke 2013; 8: 413–421. [DOI] [PubMed] [Google Scholar]
- 29.Thompson CS, Hakim AM. Living beyond our physiological means: small vessel disease of the brain is an expression of a systemic failure in arteriolar function: a unifying hypothesis. Stroke 2009; 40: e322–e330. [DOI] [PubMed] [Google Scholar]
- 30.Wada M, Nagasawa H, Iseki C, Takahashi Y, Sato H, Arawaka S, et al. Cerebral small vessel disease and chronic kidney disease (CKD): results of a cross-sectional study in community-based Japanese elderly. J Neurol Sci 2008; 272: 36–42. [DOI] [PubMed] [Google Scholar]
- 31.Wada M, Nagasawa H, Kurita K, Koyama S, Arawaka S, Kawanami T, et al. Microalbuminuria is a risk factor for cerebral small vessel disease in community-based elderly subjects. J Neurol Sci 2007; 255: 27–34. [DOI] [PubMed] [Google Scholar]
- 32.Ruchoux MM, Brulin P, Leteurtre E, Maurage CA. Skin biopsy value and leukoaraiosis. Ann NY Acad Sci 2000; 903: 285–292. [DOI] [PubMed] [Google Scholar]
- 33.Martens RJ, Vink H, van Oostenbrugge RJ, Staals J. Sublingual microvascular glycocalyx dimensions in lacunar stroke patients. Cerebrovasc Dis 2013; 35: 451–454. [DOI] [PubMed] [Google Scholar]
- 34.Pretnar-Oblak J, Sabovic M, Pogacnik T, Sebestjen M, Zaletel M. Flow-mediated dilatation and intima-media thickness in patients with lacunar infarctions. Acta Neurol Scand 2006; 113: 273–277. [DOI] [PubMed] [Google Scholar]
- 35.Wharton SB, Simpson JE, Brayne C, Ince PG. Age-associated white matter lesions: the MRC Cognitive Function and Ageing Study. Brain Pathol 2015; 25: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young VG, Halliday GM, Kril JJ. Neuropathologic correlates of white matter hyperintensities. Neurology 2008; 71: 804–811. [DOI] [PubMed] [Google Scholar]
- 37.Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R, et al. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke 2006; 37: 1391–1398. [DOI] [PubMed] [Google Scholar]
- 38.Simpson JE, Fernando MS, Clark L, Ince PG, Matthews F, Forster G, et al. White matter lesions in an unselected cohort of the elderly: astrocytic, microglial and oligodendrocyte precursor cell responses. Neuropathol Appl Neurobiol 2007; 33: 410–419. [DOI] [PubMed] [Google Scholar]
- 39.Simpson JE, Ince PG, Higham CE, Gelsthorpe CH, Fernando MS, Matthews F, et al. MRC Cognitive Function and Ageing Neuropathology Study Group. Microglial activation in white matter lesions and nonlesional white matter of ageing brains. Neuropathol Appl Neurobiol 2007; 33: 670–683. [DOI] [PubMed] [Google Scholar]
- 40.Al-Mashhadi S, Simpson JE, Heath PR, Dickman M, Forster G, Matthews FE, et al. Medical Research Council Cognitive Function and Ageing Study. Oxidative glial cell damage associated with white matter lesions in the aging human brain. Brain Pathol. advance online publication, 14 October 2014; doi:10.1111/bpa.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wardlaw JM, Sandercock PA, Dennis MS, Starr J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke 2003; 34: 806–812. [DOI] [PubMed] [Google Scholar]
- 42.Wallin A, Blennow K, Fredman P, Gottfries CG, Karlsson I, Svennerholm L. Blood brain barrier function in vascular dementia. Acta Neurol Scand 1990; 81: 318–322. [DOI] [PubMed] [Google Scholar]
- 43.Skoog I, Wallin A, Fredman P, Hesse C, Aevarsson O, Karlsson I, et al. A population study on blood-brain barrier function in 85-year-olds: relation to Alzheimer's disease and vascular dementia. Neurology 1998; 50: 966–971. [DOI] [PubMed] [Google Scholar]
- 44.Pantoni L, Inzitari D, Pracucci G, Lolli F, Giordano G, Bracco L, Amaducci L. Cerebrospinal fluid proteins in patients with leucoaraiosis: possible abnormalities in blood-brain barrier function. J Neurol Sci 1993; 115: 125–1231. [DOI] [PubMed] [Google Scholar]
- 45.Wardlaw JM, Farrall A, Armitage PA, Carpenter T, Chappell F, Doubal F, et al. Changes in background blood-brain barrier integrity between lacunar and cortical ischemic stroke subtypes. Stroke 2008; 39: 1327–1332. [DOI] [PubMed] [Google Scholar]
- 46.Topakian R, Barrick TR, Howe FA, Markus HS. Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. J Neurol Neurosurg Psychiatry 2010; 81: 192–197. [DOI] [PubMed] [Google Scholar]
- 47.Wardlaw JM, Pantoni L. Sporadic small vessel disease: pathogenic aspects. In: Pantoni L, Gorelick PB. (eds). Cerebral small vessel disease, Cambridge University Press: Cambridge, UK, 2014, pp. 52–63. [Google Scholar]
- 48.Aono Y, Ohkubo T, Kikuya M, Hara A, Kondo T, Obara T, et al. Plasma fibrinogen, ambulatory blood pressure, and silent cerebrovascular lesions: the Ohasama study. Arterioscler Thromb Vasc Biol 2007; 27: 963–968. [DOI] [PubMed] [Google Scholar]
- 49.Aribisala BS, Wiseman S, Morris Z, Valdés-Hernández MC, Royle NA, Maniega SM, et al. Circulating inflammatory markers are associated with magnetic resonance imaging-visible perivascular spaces but not directly with white matter hyperintensities. Stroke 2014; 45: 605–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dufouil C, Alpérovitch A, Ducros V, Tzourio C. Homocysteine white matter hyperintensities, and cognition in healthy elderly people. Ann Neurol 2003; 53: 214–221. [DOI] [PubMed] [Google Scholar]
- 51.Fornage M, Chiang YA, O'Meara ES, Psaty BM, Reiner AP, Siscovick DS, et al. Biomarkers of inflammation and MRI-defined small vessel disease of the brain: the Cardiovascular Health Study. Stroke 2008; 39: 1952–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gottesman RF, Cummiskey C, Chambless L, Wu KK, Aleksic N, Folsom AR, et al. Hemostatic factors and subclinical brain infarction in a community-based sample: the ARIC study. Cerebrovasc Dis 2009; 28: 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kloppenborg RP, Nederkoorn PJ, van der Graaf Y, Geerlings MI. Homocysteine and cerebral small vessel disease in patients with symptomatic atherosclerotic disease. The SMART-MR study. Atherosclerosis 2011; 216: 461–466. [DOI] [PubMed] [Google Scholar]
- 54.Notsu Y, Nabika T, Bokura H, Suyama Y, Kobayashi S, Yamaguchi S, et al. Evaluation of asymmetric dimethyalarginine and homocysteine in microangiopathy-related cerebral damage. Am J Hypertens 2009; 22: 257–262. [DOI] [PubMed] [Google Scholar]
- 55.Pikula A, Böger RH, Beiser AS, Maas R, DeCarli C, Schwedhelm E, et al. Association of plasma ADMA levels with MRI markers of vascular brain injury: Framingham offspring study. Stroke 2009; 40: 2959–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pikula A, Beiser AS, DeCarli C, Himali JJ, Debette S, Au R, et al. Multiple biomarkers and risk of clinical and subclinical vascular brain injury: the Framingham Offspring Study. Circulation 2012; 125: 2100–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pikula A, Beiser AS, Chen TC, Preis SR, Vorgias D, DeCarli C, et al. Serum brain-derived neurotrophic factor and vascular endothelial growth factor levels are associated with risk of stroke and vascular brain injury: Framingham Study. Stroke 2013; 44: 2768–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Romero JR, Preis SR, Beiser AS, DeCarli C, Lee DY, Viswanathan A, et al. Lipoprotein phospholipase A2 and cerebral microbleeds in the Framingham Heart Study. Stroke 2012; 43: 3091–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sachdev P, Parslow R, Salonikas C, Lux O, Wen W, Kumar R, et al. Homocysteine and the brain in midadult life: evidence for an increased risk of leukoaraiosis in men. Arch Neurol 2004; 61: 1369–1376. [DOI] [PubMed] [Google Scholar]
- 60.Satizabal CL, Zhu YC, Mazoyer B, Dufouil C, Tzourio C. Circulating IL-6 and CRP are associated with MRI findings in the elderly: the 3C-Dijon Study. Neurology 2012; 78: 720–727. [DOI] [PubMed] [Google Scholar]
- 61.van Dijk EJ, Prins ND, Vermeer SE, Vrooman HA, Hofman A, Koudstaal PJ, et al. C-reactive protein and cerebral small-vessel disease: the Rotterdam Scan Study. Circulation 2005; 112: 900–905. [DOI] [PubMed] [Google Scholar]
- 62.Vermeer SE, van Dijk EJ, Koudstaal PJ, Oudkerk M, Hofman A, Clarke R, et al. Homocysteine, silent brain infarcts, and white matter lesions: the Rotterdam Scan Study. Ann Neurol 2002; 51: 285–289. [DOI] [PubMed] [Google Scholar]
- 63.Wada M, Nagasawa H, Kurita K, Koyama S, Arawaka S, Kawanami T, et al. Cerebral small vessel disease and C-reactive protein: results of a cross-sectional study in community-based Japanese elderly. J Neurol Sci 2008; 15: 43–49. [DOI] [PubMed] [Google Scholar]
- 64.Wersching H, Duning T, Lohmann H, Mohammadi S, Stehling C, Fobker M, et al. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology 2010; 30: 1022–1029. [DOI] [PubMed] [Google Scholar]
- 65.Wright CB, Paik MC, Brown TR, Stabler SP, Allen RH, Sacco RL, et al. Total homocysteine is associated with white matter hyperintensity volume: the Northern Manhattan Study. Stroke 2005; 36: 1207–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wright CB, Moon Y, Paik MC, Brown TR, Rabbani L, Yoshita M, et al. Inflammatory biomarkers of vascular risk as correlates of leukoariosis. Stroke 2009; 40: 3466–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoshida M, Tomitori H, Machi Y, Katagiri D, Ueda S, Horiguchi K, et al. Acrolein, IL-6 and CRP as markers of silent brain infarction. Atherosclerosis 2009; 203: 557–562. [DOI] [PubMed] [Google Scholar]
- 68.Han JH, Wong KS, Wang YY, Fu JH, Ding D, Hong Z. Plasma level of sICAM-1 is associated with the extent of white matter lesion among asymptomatic elderly subjects. Clin Neurol Neurosurg 2009; 111: 847–851. [DOI] [PubMed] [Google Scholar]
- 69.Hoshi T, Kitagawa K, Yamagami H, Furukado S, Hougaku H, Hori M. Relations of serum high-sensitivity C-reactive protein and interleukin-6 levels with silent brain infarction. Stroke 2005; 36: 768–772. [DOI] [PubMed] [Google Scholar]
- 70.Kim Y, Kim YK, Kim NK, Kim SH, Kim OJ, Oh SH. Circulating matrix metalloproteinase-9 level is associated with cerebral white matter hyperintensities in non-stroke individuals. Eur Neurol 2014; 72: 234–240. [DOI] [PubMed] [Google Scholar]
- 71.Miralbell J, Soriano JJ, Spulber G, López-Cancio E, Arenillas JF, Bargalló N, et al. Structural brain changes and cognition in relation to markers of vascular dysfunction. Neurobiol Aging 2012; 33: e9–17. [DOI] [PubMed] [Google Scholar]
- 72.Miwa K, Tanaka M, Okazaki S, Furukado S, Sakaguchi M, Kitagawa K. Relations of blood inflammatory marker levels with cerebral microbleeds. Stroke 2011; 42: 3202–3206. [DOI] [PubMed] [Google Scholar]
- 73.Tomimoto H, Akiguchi I, Ohtani R, Yagi H, Kanda M, Shibasaki H, Yamamoto Y. The coagulation-fibrinolysis system in patients with leukoaraiosis and Binswanger disease. Arch Neurol 2001; 58: 1620–1625. [DOI] [PubMed] [Google Scholar]
- 74.Kario K, Matsuo T, Kobayashi H, Hoshide S, Shimada K. Hyperinsulinemia and hemostatic abnormalities are associated with silent lacunar cerebral infarcts in elderly hypertensive subjects. J Am Coll Cardiol 2001; 37: 871–877. [DOI] [PubMed] [Google Scholar]
- 75.Rouhl RP, Mertens AE, van Oostenbrugge RJ, Damoiseaux JG, Debrus-Palmans LL, Henskens LH, et al. Angiogenic T-cells and putative endothelial progenitor cells in hypertension-related cerebral small vessel disease. Stroke 2012; 43: 256–258. [DOI] [PubMed] [Google Scholar]
- 76.Hassan A, Hunt BJ, O'Sullivan M, Parmar K, Bamford JM, Briley D, Brown MM, et al. Markers of endothelial dysfunction in lacunar infarction and ischaemic leukoaraiosis. Brain 2003; 126: 424–432. [DOI] [PubMed] [Google Scholar]
- 77.Hassan A, Hunt BJ, O'Sullivan M, Bell R, D'Souza R, Jeffery S, et al. Homocysteine is a risk factor for cerebral small vessel disease, acting via endothelial dysfunction. Brain 2004; 127: 212–219. [DOI] [PubMed] [Google Scholar]
- 78.Khan U, Hassan A, Vallance P, Markus HS. Asymmetric dimethylarginine in cerebral small vessel disease. Stroke 2007; 38: 411–413. [DOI] [PubMed] [Google Scholar]
- 79.Knottnerus IL, Govers-Riemslag JW, Hamulyak K, Rouhl RP, Staals J, Spronk HM, et al. Endothelial activation in lacunar stroke subtypes. Stroke 2010; 78: 493–498. [DOI] [PubMed] [Google Scholar]
- 80.Knottnerus IL, Winckers K, Ten Cate H, Hackeng TM, Lodder J, Rouhl RP, et al. Levels of heparin-releasable TFPI are increased in first-ever lacunar stroke patients. Neurology 2012; 78: 493–498. [DOI] [PubMed] [Google Scholar]
- 81.Naka H, Nomura E, Takahashi T, Wakabayashi S, Kajikawa H, Kohriyama T, et al. Plasma total homocysteine levels are associated with advanced leukoaraiosis but not with asymptomatic microbleeds on T2*-weighted MRI in patients with stroke. Eur J Neurol 2006; 13: 261–265. [DOI] [PubMed] [Google Scholar]
- 82.Rouhl RP, van Oostenbrugge RJ, Damoiseaux JG, Debrus-Palmans LL, Theunissen RO, Knottnerus IL, et al. Haptoglobin phenotype may alter endothelial progenitor cell cluster formation in cerebral small vessel disease. Curr Neurovasc Res 2009; 6: 32–41. [DOI] [PubMed] [Google Scholar]
- 83.Rouhl RP, van Oostenbrugge RJ, Theunissen RO, Knottnerus IL, Staals J, Henskens LH, et al. Autoantibodies against oxidized low-density lipoprotein in cerebral small vessel disease. Stroke 2010; 41: 2687–2689. [DOI] [PubMed] [Google Scholar]
- 84.Rouhl RP, Damoiseaux JG, Lodder J, Theunissen RO, Knottnerus IL, Staals J, et al. Vascular inflammation in cerebral small vessel disease. Neurobiol Aging 2012; 33: 1800–1806. [DOI] [PubMed] [Google Scholar]
- 85.Markus HS, Hunt B, Palmer K, Enzinger C, Schmidt H, Schmidt R. Markers of endothelial and hemostatic activation and progression of cerebral white matter hyperintensities: longitudinal results of the Austrian Stroke Prevention Study. Stroke 2005; 36: 1410–1414. [DOI] [PubMed] [Google Scholar]
- 86.Rufa A, Blardi P, De Lalla A, Cevenini G, De Stefano N, Zicari E, et al. Plasma levels of asymmetric dimethylarginine in cerebral autosomal dominant arteriopathy with subcortical infarct and leukoencephalopathy. Cerebrovasc Dis 2008; 26: 636–640. [DOI] [PubMed] [Google Scholar]
- 87.Flemming KD, Nguyen TT, Abu-Lebdeh HS, Parisi JE, Wiebers DO, Sicks JD, et al. Hyperhomocysteinemia in patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). Mayo Clin Proc 2001; 76: 1213–1218. [DOI] [PubMed] [Google Scholar]
- 88.Singhal S, Bevan S, Barrick T, Rich P, Markus HS. The influence of genetic and cardiovascular risk factors on the CADASIL phenotype. Brain 2004; 127: 2031–2038. [DOI] [PubMed] [Google Scholar]
- 89.Campolo J, De Maria R, Frontali M, Taroni F, Inzitari D, Federico A, et al. Impaired vasoreactivity in mildly disabled CADASIL patients. J Neurol Neurosurg Psychiatry 2012; 83: 268–274. [DOI] [PubMed] [Google Scholar]
- 90.Pescini F, Cesari F, Giusti B, Sarti C, Zicari e, Bianchi S, et al. Bone marrow-derived progenitor cells in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke 2010; 41: 218–223. [DOI] [PubMed] [Google Scholar]
- 91.Del Bene A, Makin SD, Doubal FN, Inzitari D, Wardlaw JM. Variation in risk factors for recent small subcortical infarcts with infarct size, shape, and location. Stroke 2013; 44: 3000–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Markus HS. Genes, endothelial function and cerebral small vessel disease in man. Exp Physiol 2008; 93: 121–127. [DOI] [PubMed] [Google Scholar]