Abstract
Several potential vascular risk factors exist for the development and accumulation of subcortical white matter disease in older people. We have reported that in older people followed for up to 4 years white matter hyperintensity (WMH) lesions on magnetic resonance imaging nearly doubled in volume and were associated with alterations in mobility and cognitive function. Herein we review the genetic, metabolic, and vascular risk factors that have been evaluated in association with the development and pathogenesis of WMH in older persons. Our research efforts have focused on systemic hypertension, particularly in the out-of-office setting as 24-hour ambulatory blood pressure (BP) has proven to be a stronger indicator of the progression of WMH in older people and the associated functional decline than doctor’s office BP. Based on relations between 24-hour systolic BP levels, the accrual of WMH, and functional decline, we have designed the INFINITY trial, the first interventional study to use ambulatory BP to guide antihypertensive therapy to address this problem in the geriatric population.
Keywords: Ambulatory blood pressure, cerebral small vessel disease (SVD), cognitive function, mobility, systolic hypertension, white matter hyperintensity lesions
Introduction
White Matter Hyperintensity Lesions and Functional Decline
White matter hyperintensities that represent small vessel brain disease are associated with vascular risk factors, including hypertension, in older people.1,2 The postmortem histopathology of white matter hyperintensity (WMH) shows nonspecific brain changes with gliosis, loss of myelin and axons from arteriosclerosis, tissue rarefaction and lipohyalinosis.3,4 Although the pathophysiology of WMH remains unclear, there are several proposed mechanisms including hypoxia, hypoperfusion because of altered cerebrovascular autoregulation, blood–brain barrier leakage, inflammation, degeneration, and amyloid angiopathy.5 Marstrand et al.6 demonstrated that cerebral blood flow and cerebrovascular reactivity were reduced in areas of WMH making tissue damage more likely during hypoperfusion states.
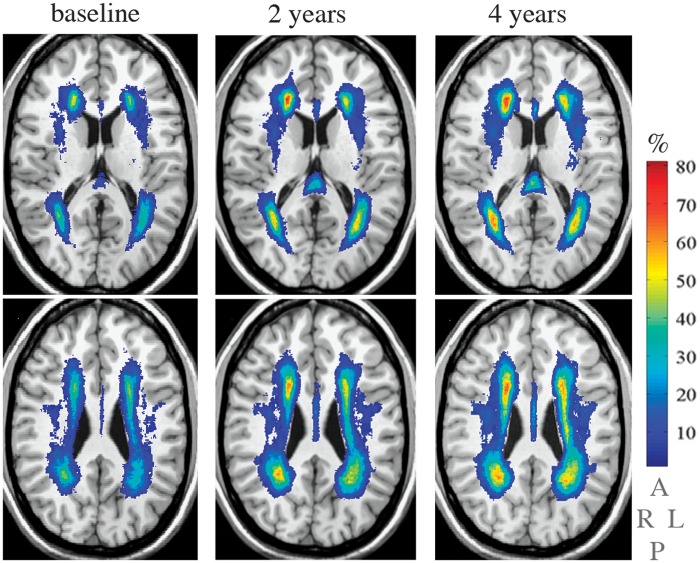
In most instances, WMH lesions are bilateral and symmetrical on T2-weighted magnetic resonance imaging (MRI). They are distributed in the periventricular and deep white matter regions and less frequently in the infratentorial areas of the brain. On computed tomography scan, WMH lesions appear as hypodensities.1,7 They are commonly assessed using visual rating scales, such as Fazekas scale and Scheltens scale.5 Another approach uses semiautomated, computerized analyses of the MRIs providing a quantitative distribution of cerebral small vessel brain disease lesions suitable for longitudinal numerical comparisons and regional localizations (Figure 1).8
Figure 1.
The figure depicts the location and frequency of white matter hyperintensities captured over three time points during 4 years of prospective evaluation (baseline (left column), 2 years (center column), and 4 years (right column) in 67 older study participants. The white matter hyperintensities are overlaid on the grayscale slice (0.87 m thickness) of the common anatomical brain (International Consortium of Brain Mapping). Columns show two slices separated by 12.2 mm. The vertical color bar represents the frequency (%) of white matter hyperintensities (e.g., color corresponding to 70% indicates the percent of participants with the white matter hyperintensities in that brain area. The lettering below the color bar indicates right (R), left (L), anterior (A), and posterior (P) brain aspects. From Wolfson et al.8 with permission from Oxford University Press on behalf of The Gerontological Society of America.
Cognitive function
Higher white matter disease burden is associated with impaired cognitive function, mobility impairment, depression, and impaired urinary function.1,9,10 The frequency of falls even in the absence of obvious neurologic deficits is more common in people with WMH lesions.8
Cognitive impairment has been studied extensively in persons with WMH lesions on MRI.9,11–18 Perceived cognitive dysfunction as measured by Cognitive Difficulties Scale was found to be worse with higher WMH burden and the annual rate of decline on the mini-mental status examination was 0.035 points per standard deviation increase in periventricular WMH.19 In addition, steeper declines in performance are found on measures of speed of processing and executive functioning, such as the Stroop Color Word test (P = 0.04) and the Symbol-Digit Substitution Test (P < 0.01) are seen with WMH lesions, whereas performance on memory tests such as the 15-word verbal learning test are not affected.9,19 Over the period of a decade, the European Leukoaraiosis and DISability Study showed steeper declines in the Stroop test, Trail Making A test, verbal fluency, and mini-mental status examination among patients with cerebral small vessel disease and the group found that these abnormalities predicted a doubling of risk for dementia and transition from an autonomous to dependent state.17
Importantly, among patients with a history of stroke and/or transient ischemic attack in the Perindopril Protection Against Recurrent Stroke Study, the dementia risk during a median follow-up period of 3.9 years was found to be 7.7 times higher in patients with white matter disease than those without it at the time of study enrolment.16 A meta-analysis performed by Debette and Markus20 also showed a significant association between white matter disease and risk of dementia (odds ratio (OR) 1.9, 95% confidence intervals (CIs), 1.3–2.8, P = 0.002) as well as faster declines in global cognitive performance, executive function, and processing speeds.
Mobility and balance
In addition to the aforementioned relationships between WMH burden and cognitive function, small vessel disease of the brain is also associated with impaired gait and balance in the older population.8 Furthermore, severe WMH burden has been linked to increased risk of falls (relative risk = 1.63, 95% CI, 1.11–2.40).10 In Leukoaraiosis and DISability Study, walking speed correlated with the white matter disease burden (1.24 ± 0.28 m/s for mild, 1.18 ± 0.32 m/s for moderate, 1.09 ± 0.31 m/s for severe categorizations respectively; P < 0.001).21 Patients with mild white matter disease burden performed better with single leg stance when compared with those with moderate and severe disease (P < 0.001). These findings have also been found in other studies correlating cerebral small vessel disease and mobility in the elderly and will be the subject of discussion later in this paper.22
Genomics of Vascular Risk Factors Associated with White Matter Lesions
White matter hyperintensities volumes are highly correlated in monozygotic twins and greater than in dizygotic twins. In a study involving US twins aged 68–79 years, the concordance rate for white matter disease (WMH > 0.5% of total intracranial volume) is 61% in monozygotic twins and 38% in dizygotic twins.23 These data are suggestive of a genetic component in the etiology of cerebral small vessel disease.
Mutations in the NOTCH3 gene result in a syndrome of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, which has clinicopathologic features similar to age-related cerebral small vessel disease.24 Schmidt et al.25 found four common single-nucleotide polymorphisms (SNPs) (minor allele frequency ≥5%)—rs1043994, rs10404382, rs10423702, and rs1043997—scattered over the NOTCH3 gene that were significantly related to the presence and progression of WMH among patients with hypertension (OR 2.1–3.4). In contrast, nine rare common SNPs (minor allele frequency ≤5%) were detected in patients with severe white matter lesions but without cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy features. Nearly all the patients with the rare SNPs were older than 65 years and had hypertension. These findings strongly suggest that genetic mutations are associated with WMH in the presence of hypertension.25
In the Ohasama study in Japan, the homozygous deletion (DD) polymorphism of adrenomedullin 2 (also known as intermedin or IMD) gene is associated with increased prevalence of WMH (66.7%) compared with 39.4% and 43.4% with homozygous insertion (II) and heterozygous carriers (ID), respectively (P = 0.001).26 Adrenomedullin 2 stimulates cyclic AMP production and has a potent vasodilator action.27,28 After adjusting for age, sex, body mass index, ambulatory blood pressure (BP), use of antihypertensive medications, presence of diabetes mellitus, chronic kidney and cardiac disease, smoking, and alcohol intake, the OR for WMH lesions in DD carriers was 2.7 (95% CI, 1.4–5.4, P = 0.003) and in ID carriers was 1.0 (95% CI, 0.7–1.4, P = 0.9). The DD carriers had significantly higher mean 24-hour ambulatory BP than II and ID carriers (systolic BPs of 127.4 versus 122.0 and 122.9 mmHg, respectively, P = 0.009; and diastolic BPs of 74.8 versus 71.3 and 72.5 mmHg, respectively, P = 0.002).26 These SNPs were found to be a risk factor for WMH independent of cardiovascular risks.
Using targeted genome-wide association analyses, French et al. demonstrated that a forkhead transcription factor (FOXC1) mutation adjacent to a cerebral small vessel disease locus resulted in the development of WMH lesions. Cerebral small vessel disease was evident with this gene mutation in individuals as young as 1 year. Mutations of the FOXC1-interacting transcription factor PITX2 gene were associated with phenotypes that develop WMH.29 In another analysis, SNPs for the gene encoding angiotensinogen (AGT rs699) was found to be correlated with the development and progression of WMH, particularly in the deep white matter, and independent of hypertension.30 Patients with such genetic variations have been reported to have high levels of angiotensinogen.30,31
Recently, the presence of haptoglobin 1 allele (Hp 1-1) in patients with Type 1 diabetes mellitus from the participants of the Pittsburgh Epidemiology of Diabetes Complication study was found to increase WMH burden but not lacunar infarcts and gray matter atrophy.32 Associations were strongest for the interhemispheric connecting fibers of the corpus callosum. The Hp 1-1 genotype is also associated with development of stroke in Type 1 diabetes.33
Metabolic Factors Related to Cerebral Small Vessel Disease
A number of metabolic biomarkers have been studied in small caliber vascular disease in the brain, including individuals with diabetes mellitus, dyslipidemia, homocystinemia, and the metabolic syndrome. A strong association between metabolic syndrome and the presence of subcortical (OR = 1.99; 95% CI, 1.28–3.10; P = 0.002) and periventricular WMH lesions (OR = 2.23, 95% CI, 1.09–4.58; P = 0.03) was observed in a cross-sectional study in 1,151 healthy older Japanese subjects.34 Fasting hyperglycemia was correlated with periventricular WMH (OR = 2.72 (95% CI, 1.54–4.82) P = 0.0006) and dyslipidemia with subcortical WMH (OR = 1.48 (95% CI, 1.06–2.07), P = 0.02). The body mass index did not show a strong association with WMH. Of note, of the various components of the metabolic syndrome, only hypertension correlated with the severity of the white matter disease. In another cross-sectional study of 308 healthy old people in France, the metabolic syndrome substantially increased the odds (OR 2.74, 95% CI, 1.25–6.03) of tempoparietal WMH disease.35
Findings relating glycemic status, WMH lesions, and/or cognitive function are variable in the literature. For example, in a population of patients with Type 1 diabetes mellitus aged 25–40 years (mean hemoglobin A1c of 7.8), there was no difference in WMH lesions compared with nondiabetic control group and in 3,300 elderly people. The Cardiovascular Health Study did not find any correlation between fasting blood glucose and white matter disease.36 In contrast, in a study of healthy old people, a linear positive association was found between fasting glucose and subclinical cerebrovascular disease (P = 0.011) and among patients aged 54–83 years old, the fasting glucose accounted for 3.7% of the variance associated with the cerebrovascular disease.37 Additionally, in a cross-sectional study of 134 participants in the Netherlands, deep WMH lesions were found to be significantly greater in patients with diabetes (with or without hypertension) compared with subjects without diabetes (P < 0.05 for both groups), whereas periventricular WMH volume was similar.38 Furthermore, the duration of the diabetes correlated with periventricular WMH (R2 = 0.24, P = 0.03), deep WMH (R2 = 0.28, P = 0.01), and total WMH (R2 = 0.29, P = 0.008). In a separate study using transcranial Doppler and quantitative MRI, WMH volume correlated negatively with cerebral blood flow velocity in patients with Type 2 diabetes mellitus, a finding that might explain part of the underlying mechanism of the WMH development.39
Nonglycemic biomarkers and white matter lesions
Homocysteine, an important inflammatory marker associated with atherosclerosis and large artery vascular disease, has also been found to be strongly associated with the development of dementia, including Alzheimer’s disease, in a sample from the Framingham study.40 In addition, an analysis of 259 stroke-free participants from the Northern Manhattan Study found that total homocysteine levels >1 s.d. above the mean (>11.9 μmol/L) was associated with WMH volume independent of other vascular risk factors.41 In a separate, controlled cohort study of 172 patients with cerebral small vessel disease,42 mean homocysteine levels were significantly higher than in the controls, particularly those with WMH lesions (OR, 2.02, 95% CI, 1.31–3.1, P = 0.0001), but inclusion of the endothelial markers intercellular adhesion molecule-1 (ICAM-1) and thrombomodulin in a logistic regression model resulted in the association between homocysteine and cerebral small vessel disease no longer being significant.
Other studies have also suggested a relationship between endothelial dysfunction, inflammatory endothelial activation, and the extent of white matter lesions in older people. In a study from Hong Kong in 175 older people without neurologic deficits, the measured plasma concentration of soluble ICAM-1 was associated with the severity of WMH, independent of age, and hypertension.43 In fact, patients in the highest quartile of plasma sICAM-1 had fourfold increased risk of developing WMH. In the population-based Rotterdam Scan study, strong associations between the presence and progression at 3.3 years of WMH lesions and serum C-reactive protein level (OR, 3.1 and 5.6 for periventricular WM and subcortical white matter, respectively) were observed in 636 participants, including after adjustments for conventional cardiovascular risk factors and the presence of carotid atherosclerosis.4 In contrast, among a Japanese community based study of 689 older people, the serum C-reactive protein level was lower than that observed in the Rotterdam study and was not associated with presence of WMH lesions after controlling for other vascular risk factors and carotid disease.44 Hence, the importance of C-reactive protein in association with WMH lesions may be heterogeneous depending on the ethnicity and race. Other inflammatory and prothrombotic markers that have been correlated with WMH lesions in smaller studies include plasma nitric oxide metabolites and urinary 8-iso-prostaglandin F2α and prothrombin factors, von Willebrand Factor, and plasminogen activator inhibitor-1 (PAI-1).45,46
Lipoproteins
Similar to the glycemic data noted above, studies have shown heterogeneous findings relating lipid abnormalities to small vessel disease of the brain. Fukuda and Kitani47 reported no correlation between lipid abnormalities (defined as total cholesterol > 220 mg/dL, triglycerides > 150 mg/dL, high-density lipoprotein < 40 mg/dL, low-density lipoprotein (LDL) > 130 mg/dL) and WMH lesions among 253 patients (mean age = 66 years) receiving no medical therapy for dyslipidemia. Population-based data from the 3C-Dojon Study (n = 1,842) and Epidemiology of Vascular Aging Study (n = 766) in France showed that a higher serum triglyceride level was associated with larger WMH volume (β = 0.0936; P = 0.0002) independent of age and other vascular risk factors.48 Surprisingly, there was a trend toward a negative correlation between low-density lipoprotein cholesterol levels and WMH volume and this reached statistical significance in the meta-analysis of the two studies. No association was found with high-density lipoprotein cholesterol level and white matter disease. An inverse correlation between low-density lipoprotein level and WMH grade was also shown in the Cardiovascular Health Study.49
In a separate study of patients who had sustained an acute ischemic stroke, Jimenez-Conde et al.50 reported that hyperlipidemia (defined as prestroke recorded serum cholesterol concentration >220 mg/dL or serum triglyceride concentration >150 mg/dL, and use of medication prescribed to control hyperlipidemia) may have a protective role in cerebral small vessel disease among patients with acute ischemic stroke (P = 0.02 and 0.06). Although this finding is poorly understood, it is thought that cholesterol may have a fundamental role in central nervous system development and in the creation and maintenance of new synapses which may improve response to chronic cerebral injury such as WMH progression.51,52
The Importance of Blood Pressure as a Risk Factor for White Matter Lesions and Functional Decline in Older Persons
White matter hyperintensities and its progression, present in the MRIs of older people have been associated with hypertension and evidence suggests that WMHs occur as a result of arteriosclerosis within the wall of the arteriole.53–56 Large arterial and small vessel disease of the cerebral circulation share risk factors, (e.g., hypertension, diabetes) and may coexist in individuals as noted above. Although given the differences noted in Table 1, it is unclear if they both produce white matter tissue damage through similar mechanisms.57,58
Table 1.
Comparison of some characteristics of stroke and white matter hyperintensity lesionsa.
| Characteristic | Stroke (large artery) | White matter hyperintensities |
|---|---|---|
| Onset/progression | Sudden/brief if any | Ill-Defined/gradual over years |
| Manifestations | Focal neurologic deficit | Functional limitations |
| Location | Vascular distribution | Grow from head/tail-lateral ventricles |
| Size | Stroke (cm)→lacune (mm) | <1 mm |
| Vessel | Large-to-small artery | Arteriolar |
| Pathophysiology | Ischemic | Unclear |
Note: there may be infarction because of small vessel disease that has onset/progression and manifestations that are similar to large artery stroke.
Modified from White et al.68 with permission of Elsevier.
White matter lesions have also been associated with deterioration of mobility, urinary control, and cognition.59–64 Evidence of WMHs within brain pathways known to support mobility, cognition or voiding confirms this association.63,64 Details seen on MRI of the brain have allowed localization and quantification of the disseminated WMHs.65 Cross-sectional and prospective cohort studies have documented the relationships among WMHs and neurologic function in older people and the distinctive nature of the distribution and volume of brain WMHs that are responsible for deterioration of these functions, particularly in older groups. Approximately two-thirds of individuals over 75 years of age have detectable WMH using MRI of the brain.54 The lower limit of detection of WMH by experts in neuroradiology is ∼0.2–0.3% of intracranial contents and 0.5% is easily visible to the naked eye based on our experience.8,54,65 Our cohort studies have demonstrated an increase of WMH volume from 0.99 to 1.47 to 1.7% of the intracranial contents volume from baseline to 2 and 4 years, respectively.8,54 This increase was even present in participants with normal mobility throughout the study period.
We have also observed that progression of WMH over time was strongly linked to the initial presence of WMH.8,66 We have observed that accumulation of WMH often occurred by expansion of preexisting periventricular lesions in a stereotyped manner. Regional analysis of the distribution of WMH lesions demonstrated a robust link to expansion of lesions in the splenium of the corpus callosum, a posterior periventricular structure important for the integration of cortical sensorimotor function.66
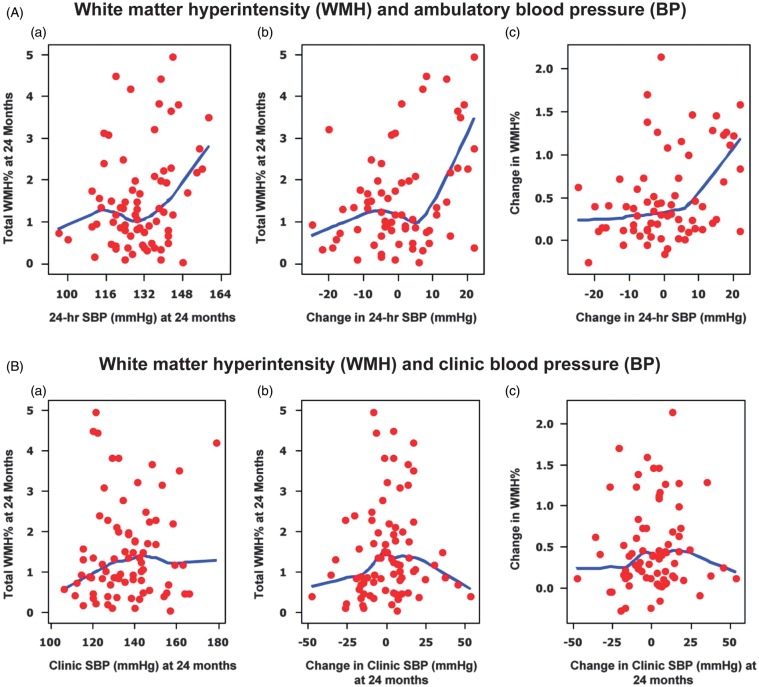
Blood pressure and other cardiovascular risk factors noted above have been related to brain WMH although predictors of quantitative WMH progression and their effect on the function of older persons have not been well understood. In our past work in this area, we have evaluated the progression of WMH over 2 and 4 years in a cohort of 99 patients 75–90 years (mean baseline age, 82 years) who had office and ambulatory BP and volumetric MRI.54,65 Neither clinic (doctor’s office) BP nor changes in clinic BP predicted progression of WMH, while the 24-hour ambulatory BP and changes in ambulatory BP significantly correlated with both WMH volume (P < 0.04) and changes in WMH (P < 0.003) (Figure 2).54 Further analyses demonstrated associations for WMH and mobility indices with level of systolic BP based on tertiles of the cohort—those in the higher (24-hour systolic BP = 144 mmHg) ambulatory BP group showed increases in WMH and slower mobility compared with the middle tertile (ambulatory systolic BP = 130 mmHg) (Table 2). Furthermore, gait speed in the higher ambulatory BP group decreased 0.15 m/s more than that in the low BP group and while this difference appears small, it represents a between group change after only 2 years of observation.54 Mobility limitation linked to WMH occurs gradually so that this decrement may be part of a long-term process which compromises gait velocity over 10 or more years. These data have suggested that an intervention using mean 24-hour systolic BP as the target could reduce progression of small vessel disease in the elderly and thus favorably impact function.
Figure 2.
(A) White matter hyperintensity and ambulatory blood pressure. Locally weighted scatterplot smoother plots of 24-hour average systolic blood pressure and white matter hyperintensity lesions (as percent of total intracranial volume); (a) change in 24-hour systolic blood pressure and white matter hyperintensity (%); (b) and change in 24-hour systolic blood pressure and change in white matter hyperintensity (%) at 24 months (c). (B) White matter hyperintensity and clinic blood pressure. Locally weighted scatterplot smoother plots of clinical systolic blood pressure and WMH lesions (as percent of total intracranial volume); (a) change in clinical systolic blood pressure and white matter hyperintensity (%); (b) and change in clinical systolic blood pressure and change in white matter hyperintensity (%) at 24 months (c). From White et al.54 with permission of the American Heart Association.
Table 2.
Functional parameters at 24 months of observation according to clinic and ambulatory blood pressure.
| Parameter | Low (n = 25) | Middle (n = 25) | High (n = 24) | P-value |
|---|---|---|---|---|
| Clinic blood pressure tertile | ||||
| Clinic systolic BP (mm Hg) | 120.7 ± 1.2 | 135.9 ± 0.8 | 152.8 ± 1.8 | |
| Total WMH (%) | 1.5 ± 0.3 | 1.4 ± 0.2 | 1.5 ± 0.2 | 0.935 |
| Mobility assessments | ||||
| Tinetti Gait | 11.2 ± 0.2 | 11.6 ± 0.2 | 10.9 ± 0.3 | 0.207 |
| Stair descent time (second) | 6.7 ± 0.6 | 5.8 ± 0.4 | 6.6 ± 0.5 | 0.393 |
| Maximal gait velocity (m/s) | 0.69 ± 0.04 | 0.76 ± 0.03 | 0.67 ± 0.03 | 0.168 |
| Walk time (second) | 3.4 ± 0.2 | 3.0 ± 0.1 | 3.2 ± 0.1 | 0.136 |
| 24-Hour ambulatory blood pressure tertile | ||||
| 24-Hour systolic BP (mm Hg) | 116.7 ± 1.4 | 130.4 ± 0.7 | 144.1 ± 1.3 | |
| Total WMH (%) | 1.4 ± 0.2 | 1.2 ± 0.2 | 2.03 ± 0.3 | 0.034 |
| Mobility assessments | ||||
| Tinetti Gait | 11.2 ± 0.3 | 11.8 ± 0.1 | 10.8 ± 0.3 | 0.025 |
| Stair descent time (second) | 6.4 ± 0.8 | 5.1 ± 0.4 | 7.1 ± 0.4 | 0.016 |
| Maximal gait velocity (m/s) | 0.71 ± 0.04 | 0.78 ± 0.03 | 0.65 ± 0.03 | 0.045 |
| Walk time (second) | 3.1 ± 0.1 | 2.8 ± 0.1 | 3.6 ± 0.2 | 0.002 |
Abbreviations: m/s, Meters/second; WMH, white matter hyperintensity lesions.
Significant values are italic typeface.
Unpublished data from our cohort study.53
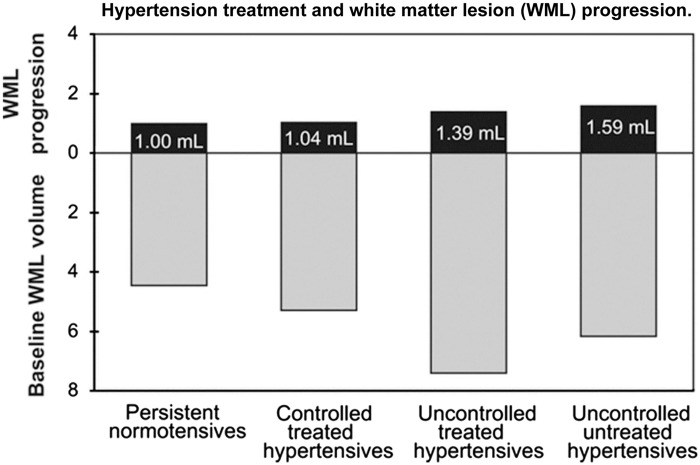
The results of our cohort studies have been supported by a larger longitudinal population-based study of 665 persons from the Rotterdam study.55 Over a 5-year period, clinical BP and WMH lesion progression was measured 3.5 years apart and after adjusting for baseline WMH, only systolic BP was significantly associated with progression (0.05 mL/year of s.d. increase). Of interest, people with uncontrolled and untreated hypertension had significantly greater white matter lesion progression than people with uncontrolled but treated hypertension (Figure 3). These studies suggest that antihypertensive treatment could reduce white matter lesion progression in uncontrolled hypertension. However, guiding therapy through the use of ambulatory BP monitoring could make this process more precise since ambulatory BP is more reproducible than clinical BP in older people67 and ambulatory BP recordings would provide a means to target BP elevations during sleep, a period which is strongly related to WMH and cerebrovascular disease.54,68
Figure 3.
Mean white matter hyperintensity progression in mL (95% confidence interval; black bars) on top of the baseline white matter lesion volume (gray bars) for four blood pressure categories. Categories were defined as follows based on their mean blood pressure and medication use in the 5 years before the first scan: (1) normotensives: normal mean blood pressure and receiving no antihypertensive medication (n = 255); (2) controlled treated hypertensives: normal mean blood pressure and receiving antihypertensive medication (n = 83); (3) uncontrolled treated hypertensives: hypertensive mean blood pressure and receiving antihypertensive medication (n = 155); and (4) uncontrolled untreated hypertensives: hypertensive mean blood pressure and receiving no antihypertensive medication (n = 172). Hypertensive mean blood pressure was defined as diastolic blood pressure ≥90 mm Hg or systolic blood pressure ≥140 mm Hg. A statistically significant difference in white matter lesion progression was observed between the uncontrolled untreated hypertensive group and the uncontrolled treated hypertensive group, after adjusting age, sex, intracranial volume, time between scans, and the baseline white matter lesion load (P < 0.05). From Verhaaren et al.55 with permission from the American Heart Association.
The INFINITY study
The Intensive versus Standard Ambulatory Blood Pressure Lowering to Prevent Functional DecliNe in The ElderlY (INFINITY) study69 has been designed to evaluate the functional impact of a clinically relevant separation in 24-hour mean ambulatory systolic BP in an older population (that is, <130 mmHg versus <145 mmHg. The study has been designed to evaluate these two levels of ambulatory BP control in hypertensive individuals 75 years or older with normal or mildly impaired mobility and cognition who already have detectable cerebrovascular disease (≥0.5% WMH fraction of intracranial volume). The key outcomes monitored over the 3 years of the trial are white matter lesion progression and measures of mobility and cognition. INFINITY is a prospective, randomized, open-label trial with blinded end points that will evaluate the changes from baseline in mobility and cognitive function and accumulation of WMH volume and changes in diffusion tensor imaging.
Our objective is to achieve a 24-hour systolic BP of ≤130 mmHg in an intensively treated group or standard goal of 24-hour systolic BP of ≤145 mmHg for a total of 36 months using similar classes of antihypertensive therapies. Data from the Hypertension in the Very Elderly Trial demonstrated that antihypertensive therapy decreases stroke mortality even in patients in their mid-80s.70 In Hypertension in the Very Elderly Trial, the goal of therapy was to reduce systolic BP to <150 mmHg and this intervention did result in a 39% reduction in stroke mortality that was related to a 15 mmHg difference in systolic BP between the active treatment and placebo groups. To our knowledge, there is no other information on level of systolic BP and cardiovascular outcomes in a hypertensive population over the age of 80 years. Hence, the goal of the standard of care systolic BP in the clinic in this age group is ∼150 mmHg, while the ambulatory BP goal is 145 mmHg. Furthermore, no clinical trial in hypertensive patients has used ambulatory BP to guide therapy and to assess cerebrovascular outcomes. The primary outcome of INFINITY is based on mobility parameters. A regression of gait velocity >8 feet/s on our covariates of age, gender, and 24-hour systolic BP at baseline in our prior cohort study8 showed an R2 of 0.127. If similar results are observed in the intervention study, 140 participants (70 in each group) will give us 85% power to observe an increase in R2 of 0.053 to 0.180. To cover screen failures and dropouts post randomization because of a variety of issues that affect this age group, more than 300 participants have been screened and/or enrolled. The INFINITY trial will be the first study to guide antihypertensive therapy using ambulatory BP monitoring rather than clinical BP to reduce cerebrovascular disease. Initiated in 2012, we project that the study will be completed in 2018.69
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study has been supported by National Institutes of Health R01AG022092 and R01DA024667, and The Lowell P Weicker, Jr. Clinical Research Center, University of Connecticut Health Center, Farmington.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Longstreth WT, Jr, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke 1996; 27: 1274–1282. [DOI] [PubMed] [Google Scholar]
- 2.Yikoski A, Erkinjuntti T, Raininko R, Sarna S, Sulkava R, Tilvis R. White matter hyperintensities on MRI in the neurologically nondiseased elderly. Analysis of cohorts of consecutive subjects aged 55 to 85 years living at home. Stroke 1995; 26: 1171–1177. [DOI] [PubMed] [Google Scholar]
- 3.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010; 9: 689–701. [DOI] [PubMed] [Google Scholar]
- 4.van Dijk EJ, Prins ND, Vermeer SE, Vrooman HA, Hofman A, Koudstaal PJ, et al. C-reactive protein and cerebral small-vessel disease: the Rotterdam Scan Study. Circulation 2005; 112: 900–905. [DOI] [PubMed] [Google Scholar]
- 5.Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, et al. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry 2011; 82: 126–135. [DOI] [PubMed] [Google Scholar]
- 6.Marstrand JR, Garde E, Rostrup E, Ring P, Rosenbaum S, Mortensen EL, et al. Cerebral perfusion and cerebrovascular reactivity are reduced in white matter hyperintensities. Stroke 2002; 33: 972–976. [DOI] [PubMed] [Google Scholar]
- 7.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolfson L, Wakefield DB, Moscufo N, Kaplan RF, Hall CB, Schmidt JA, et al. Rapid buildup of brain white matter hyperintensities over 4 years linked to ambulatory blood pressure, mobility, cognition, and depression in old persons. J Gerontol A Biol Sci Med Sci 2013; 68: 1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Jolles J, Koudstaal PJ, et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain 2005; 128: 2034–2041. [DOI] [PubMed] [Google Scholar]
- 10.Zheng JJ, Lord SR, Close JC, Sachdev PS, Wen W, Brodaty H, et al. Brain white matter hyperintensities, executive dysfunction, instability, and falls in older people: a prospective cohort study. J Gerontol A Biol Sci Med Sci 2012; 67: 1085–1091. [DOI] [PubMed] [Google Scholar]
- 11.Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke 2010; 41: 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuller LH, Shemanski L, Manolio T, Haan M, Fried L, Bryan N, et al. Relationship between ApoE, MRI findings, and cognitive function in the Cardiovascular Health Study. Stroke 1998; 29: 388–398. [DOI] [PubMed] [Google Scholar]
- 13.Smith EE, Egorova S, Blacker D, Killiany RJ, Muzikansky A, Dickerson BC, et al. Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Arch Neurol 2008; 65: 94–100. [DOI] [PubMed] [Google Scholar]
- 14.Silvert LC, Howieson DB, Dodge H, Kaye JA. Cognitive impairment risk: white matter hyperintensity progression matters. Neurology 2009; 73: 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith EE, Gurol ME, Eng JA, Engel CR, Nguyen TN, Rosand J. White matter lesions, cognition, and recurrent hemorrahage in lobar intracerebral hemorrhage. Neurology 2004; 63: 1606–1612. [DOI] [PubMed] [Google Scholar]
- 16.Dufouil C, Godin O, Chalmers J, Coskun O, MacMahon S, Tzourio-Mazoyer N, et al. Severe cerebral white matter hyperintensities predict severe cognitive decline in patients with cerebrovascular disease history. Stroke 2009; 40: 2219–2221. [DOI] [PubMed] [Google Scholar]
- 17.Jokinen H, Kalska H, Ylikoski R, Madureira S, Verdelho A, van der Flier WM, et al. Longitudinal cognitive decline in subcortical ischemic vascular disease—the LADIS Study. Cerebrovasc Dis 2009; 27: 384–391. [DOI] [PubMed] [Google Scholar]
- 18.Debette S, Bombois S, Bruandet A, Delbeuck X, Lepoittevin S, Delmaire C, et al. Subcortical hyperintensities are associated with cognitive decline in patients with mild cognitive impairment. Stroke 2007; 38: 2924–2930. [DOI] [PubMed] [Google Scholar]
- 19.Haley AP, Hoth KF, Gunstad J, Paul RH, Jefferson AL, Tate DF, et al. Subjective cognitive complaints relate to white matter hyperintensities and future cognitive decline in patients with cardiovascular disease. Am J Geriatr Psychiatry 2009; 17: 976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2010; 341: c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baezner H, Blahak C, Poggesi A, Pantoni L, Inzitari D, Chabriat H, et al. Association of gait and balance disorders with age-related white matter changes: the LADIS study. Neurology 2008; 70: 935–942. [DOI] [PubMed] [Google Scholar]
- 22.Soumaré A, Elbaz A, Zhu Y, Maillard P, Crivello F, Tavernier B, et al. White matter lesions volume and motor performances in the elderly. Ann Neurol 2009; 65: 706–715. [DOI] [PubMed] [Google Scholar]
- 23.Carmelli D, DeCarli C, Swan GE, Jack LM, Reed T, Wolf PA, et al. Evidence for genetic variance in white matter hyperintensity volume in normal elderly male twins. Stroke 1998; 29: 1177–1181. [DOI] [PubMed] [Google Scholar]
- 24.Adib-Samii P, Brice G, Martin RJ, Markus HS. Clinical spectrum of CADASIL and the effect of cardiovascular risk factors on phenotype: study in 200 consecutively recruited individuals. Stroke 2010; 41: 630–634. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt H, Zeginigg M, Wiltgen M, Freudenberger P, Petrovic K, Cavalieri M, et al. Genetic variants of the NOTCH3 gene in the elderly and magnetic resonance imaging correlates of age-related cerebral small vessel disease. Brain 2011; 134: 3384–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirose T, Totsune K, Nakashige Y, Metoki H, Kikuya M, Ohkubo T, et al. Influence of adrenomedullin 2/intermedin gene polymorphism on blood pressure, renal function and silent cerebrovascular lesions in Japanese: the Ohasama study. Hypertens Res 2011; 34: 1327–1332. [DOI] [PubMed] [Google Scholar]
- 27.Lah JJ, Frishman WH. Adrenomedullin: a vasoactive and natriuretic peptide with therapeutic potential. Heart Dis 2000; 2: 259–265. [PubMed] [Google Scholar]
- 28.Roh J, Chang CL, Bhalla A, Klein C, Hsu SY. Intermedin is a calcitonin/calcitonin gene-related peptide family peptide acting through the calcitonin receptor-like receptor/receptor activity-modifying protein receptor complex. J Biol Chem 2004; 279: 7264–7274. [DOI] [PubMed] [Google Scholar]
- 29.French CR, Seshadri S, Destefano AL, Fornage M, Arnold CR, Gage PJ, et al. Mutation of FOXC1 and PITX2 induces cerebral small-vessel disease. J Clin Invest 2014; 124: 4877–4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Assareh AA, Mather KA, Crawford JD, Wen W, Anstey KJ, Easteal S, et al. Renin-angiotensin system genetic polymorphisms and brain white matter lesions in older Australians. Am J Hypertens 2014; 27: 1191–1198. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt R, Schmidt H, Fazekas F, Launer LJ, Niederkorn K, Kapeller P, et al. Angiotensinogen polymorphism M235T, carotid atherosclerosis, and small-vessel disease-related cerebral abnormalities. Hypertension 2001; 38: 110–115. [DOI] [PubMed] [Google Scholar]
- 32.Rosenstock J, Cefalu WT, Lapuerta P, Zambrowicz B, Ogbaa I, Banks P, et al. Greater dose-ranging effects on A1C levels than on glucosuria with LX4211, a dual inhibitor of sodium glucose transporters SGLT1 and SGLT2, in Type 2 diabetes on metformin monotherapy. Diabetes Care 2014; 38: 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costacou T, Secrest AM, Ferrell RE, Orchard TJ. Haptoglobin genotype and cerebrovascular disease incidence in type 1 diabetes. Diab Vasc Dis Res 2014; 11: 335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bokura H, Yamaguchi S, Iijima K, Nagai A, Oguro H. Metabolic syndrome is associated with silent ischemic brain lesions. Stroke 2008; 39: 1607–1609. [DOI] [PubMed] [Google Scholar]
- 35.Portet F, Brickman AM, Stern Y, Scarmeas N, Muraskin J, Provenzano FA, et al. Metabolic syndrome and localization of white matter hyperintensities in the elderly population. Alzheimers Dement 2012; 8: S88–S95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weinger K, Jacobson AM, Musen G, Lyoo IK, Ryan CM, Jimerson DC, et al. The effects of type 1 diabetes on cerebral white matter. Diabetologia 2008; 51: 417–425. [DOI] [PubMed] [Google Scholar]
- 37.Sims RC, Katzel LI, Lefkowitz DM, Siegel EL, Rosenberger WF, Manukyan Z, et al. Association of fasting glucose with subclinical cerebrovascular disease in older adults without Type 2 diabetes. Diabet Med 2014; 31: 691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Harten B, Oosterman JM, Potter van Loon BJ, Scheltens P, Weinstein HC. Brain lesions on MRI in elderly patients with type 2 diabetes mellitus. Eur Neurol 2007; 57: 70–74. [DOI] [PubMed] [Google Scholar]
- 39.Novak V, Last D, Alsop DC, Abduljalil AM, Hu K, Lepicovsky L, et al. Cerebral blood flow velocity and periventricular white matter hyperintensities in type 2 diabetes. Diabetes Care 2006; 29: 1529–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D'Agostino RB, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med 2002; 346: 476–483. [DOI] [PubMed] [Google Scholar]
- 41.Wright CB, Paik MC, Brown TR, Stabler SP, Allen RH, Sacco RL, et al. Total homocysteine is associated with white matter hyperintensity volume: the Northern Manhattan Study. Stroke 2005; 36: 1207–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hassan A, Hunt BJ, O’Sullivan M, Bell R, D’Souza R, Jeffrey S, et al. Homocysteine is a risk factor for cerebral small vessel disease, acting via endothelial dysfunction. Brain 2004; 127: 212–219. [DOI] [PubMed] [Google Scholar]
- 43.Han JH, Wong KS, Wang YY, Fu JH, Ding D, Hong Z. Plasma level of sICAM-1 associated with extent of white matter lesion among asymptomatic elderly subjects. Clin Neurol Neurosurg 2009; 111: 847–851. [DOI] [PubMed] [Google Scholar]
- 44.Wada M, Nagasawa H, Kurita K, Koyama S, Arawaka S, Kawanami T, et al. Cerebral small vessel disease and C-reactive protein: results of a cross-sectional study in community-based Japanese elderly. J Neurol Sci 2008; 264: 43–49. [DOI] [PubMed] [Google Scholar]
- 45.Shibata H, Nabika T, Moriyama H, Masuda J, Kobayashi S. Correlation of NO metabolites and 8-iso-prostaglandin F2a with periventricular hyperintensity severity. Arterioscler Thromb Vasc Biol 2004; 24: 1659–1663. [DOI] [PubMed] [Google Scholar]
- 46.Nagai M, Hoshide S, Kario K. Association of prothrombotic status with markers of cerebral small vessel disease in elderly hypertensive patients. Am J Hypertens 2012; 25: 1088–1094. [DOI] [PubMed] [Google Scholar]
- 47.Fukuda H, Kitani M. Cigarette smoking is correlated with the periventricular hyperintensity grade of brain magnetic resonance imaging. Stroke 1996; 27: 645–649. [DOI] [PubMed] [Google Scholar]
- 48.Schilling S, Tzourio C, Dufouil C, Zhu Y, Berr C, Alpérovitch A, et al. Plasma lipids and cerebral small vessel disease. Neurology 2014; 83: 1844–1852. [DOI] [PubMed] [Google Scholar]
- 49.Manolio TA, Kronmal RA, Burke GL, Poirier V, O'Leary DH, Gardin JM, et al. Magnetic resonance abnormalities and cardiovascular disease in older adults. The Cardiovascular Health Study. Stroke 1994; 25: 318–327. [DOI] [PubMed] [Google Scholar]
- 50.Jimenez-Conde J, Biffi A, Rahman R, Kanakis A, Butler C, Sonni S, et al. Hyperlipidemia and reduced white matter hyperintensity volume in patients with ischemic stroke. Stroke 2010; 41: 437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr Opin Lipidol 2001; 12: 105–112. [DOI] [PubMed] [Google Scholar]
- 52.Mauch DH, Nägler K, Schumacher S, Göritz C, Müller EC, Otto A, et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science 2001; 294: 1354–1357. [DOI] [PubMed] [Google Scholar]
- 53.de Leeuw FE, de Groot JC, Oudkerk M, Witteman JC, Hofman A, van Gijn J, et al. A follow-up study of blood pressure and cerebral white matter lesions. Ann Neurol 1999; 46: 827–833. [DOI] [PubMed] [Google Scholar]
- 54.White WB, Wolfson L, Wakefield DB, Hall CB, Campbell P, Moscufo N, et al. Average daily blood pressure, not office blood pressure, is associated with progression of cerebrovascular disease and cognitive decline in older people. Circulation 2011; 124: 2312–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verhaaren BF, Vernooij MW, de Boer R, Hofman A, Niessen WJ, van der Lugt A, et al. High blood pressure and cerebral white matter lesion progression in the general population. Hypertension 2013; 61: 1354–1359. [DOI] [PubMed] [Google Scholar]
- 56.Wolfson L. Microalbuminurea as an index of brain microvascular dysfunction. J Neurol Sci 2008; 272: 34–35. [DOI] [PubMed] [Google Scholar]
- 57.Kuller LH, Longstreth WT, Jr, Arnold AM, Bernick C, Bryan RN, Beauchamp NJ, Jr, et al. White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke 2004; 35: 1821–1825. [DOI] [PubMed] [Google Scholar]
- 58.Rosenberg GA. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol 2009; 8: 205–216. [DOI] [PubMed] [Google Scholar]
- 59.Benson RR, Guttmann CR, Wei X, Warfield SK, Hall C, Schmidt JA, et al. Older people with impaired mobility have specific loci of periventricular abnormality on MRI. Neurology 2002; 58: 48–55. [DOI] [PubMed] [Google Scholar]
- 60.Baloh RW, Yue Q, Socotch TM, Jacobson KM. White matter lesions and disequilibrium in older people. I. Case-control comparison. Arch Neurol 1995; 52: 970–974. [DOI] [PubMed] [Google Scholar]
- 61.Sakakibara R, Hattori T, Uchiyama T, Yamanishi T. Urinary function in elderly people with and without leukoaraiosis: relation to cognitive and gait function. J Neurol Neurosurg Psychiatry 1999; 67: 658–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Junqué C, Pujol J, Vendrell P, Bruna O, Jódar M, Ribas JC, et al. Leuko-araiosis on magnetic resonance imaging and speed of mental processing. Arch Neurol 1990; 47: 151–156. [DOI] [PubMed] [Google Scholar]
- 63.Kuchel GA, Moscufo N, Guttmann CR, Zeevi N, Wakefield D, Schmidt J, et al. Localization of brain white matter hyperintensities and urinary incontinence in community-dwelling older adults. J Gerontol A Giol Sci Med Sci 2009; 64: 902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaplan RF, Cohen RA, Moscufo N, Guttmann C, Chasman J, Buttaro M, et al. Demographic and biological influences on cognitive reserve. J Clin Exp Neuropsychol 2009; 31: 868–876. [DOI] [PubMed] [Google Scholar]
- 65.Moscufo N, Guttmann CR, Meier D, Csapo I, Hildenbrand PG, Healy BC, et al. Brain regional lesion burden and impaired mobility in the elderly. Neurobiol Aging 2011; 32: 646–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moscufo N, Wolfson L, Meier D, Liguori M, Hildenbrand PG, Wakefield D, et al. Mobility decline in the elderly relates to lesion accrual in the splenium of the corpus callosum. Age (Dordr) 2012; 34: 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Campbell P, Ghuman N, Wakefield D, Wolfson L, White WB. Long-term reproducibility of ambulatory blood pressure is superior to office blood pressure in the very elderly. J Hum Hypertens 2010; 24: 749–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.White WB, Mansoor GA, Tendler BE, Anwar YA. Nocturnal blood pressure epidemiology, determinants, and effects of antihypertensive therapy. Blood Press Monit 1998; 3: 43–51. [PubMed] [Google Scholar]
- 69.White WB, Marfatia R, Schmidt J, Wakefield DB, Kaplan RW, Bohannon CB, et al. Intensive versus standard ambulatory blood pressure lowering to prevent functional decline in the elderly (INFINITY). Am Heart J 2013; 165: 258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358: 1887–1898. [DOI] [PubMed] [Google Scholar]