Abstract
There is a need for increased nosological knowledge to enable rational trials in Alzheimer’s disease (AD) and related disorders. The ongoing Gothenburg mild cognitive impairment (MCI) study is an attempt to conduct longitudinal in-depth phenotyping of patients with different forms and degrees of cognitive impairment using neuropsychological, neuroimaging, and neurochemical tools. Particular attention is paid to the interplay between AD and subcortical vascular disease, the latter representing a disease entity that may cause or contribute to cognitive impairment with an effect size that may be comparable to AD. Of 664 patients enrolled between 1999 and 2013, 195 were diagnosed with subjective cognitive impairment (SCI), 274 with mild cognitive impairment (MCI), and 195 with dementia, at baseline. Of the 195 (29%) patients with dementia at baseline, 81 (42%) had AD, 27 (14%) SVD, 41 (21%) mixed type dementia (=AD + SVD = MixD), and 46 (23%) other etiologies. After 6 years, 292 SCI/MCI patients were eligible for follow-up. Of these 292, 69 (24%) had converted to dementia (29 (42%) AD, 16 (23%) SVD, 15 (22%) MixD, 9 (13%) other etiologies). The study has shown that it is possible to identify not only AD but also incipient and manifest MixD/SVD in a memory clinic setting. These conditions should be taken into account in clinical trials.
Keywords: Alzheimer’s disease, cerebrovascular disease, cognitive impairment, subcortical vascular dementia, white matter changes
Background
The Gothenburg mild cognitive impairment (MCI) study, that started in 1999, is an ongoing single-center clinical-pathophysiologic study concerned with investigating early and manifest phases of Alzheimer’s disease (AD) and vascular dementia (VaD) in patients seeking medical care at a memory clinic. One of the central rationales of the study is to create a better foundation for rational intervention by enhancing the nosological knowledge of disease processes that may cause cognitive impairment, using multimodal markers. The agendas of for instance the Alzheimer’s Disease Neuroimaging Initiative and the Australian Imaging, Biomarkers and Lifestyle studies are similar, but these studies have not explicitly focused on cerebrovascular disease as in the Gothenburg MCI study.1,2 Another rationale for the Gothenburg MCI study is to examine the phenomenology of MCI.
Alzheimer's disease and VaD are the two most common dementia disorders.3 Although several attempts have been made to counteract the effects of amyloid beta (Aβ) mismetabolism, which is assumed to be one of the key pathogenic events in AD, there is not yet any effective treatment for the disorder.4,5 Possible reasons for the failures may be that non-homogeneous groups of patients have been included in the trials, the treatment has been administered too late in the course of the disease or has been too short, and/or that the Aβ aggregation is not the key event in AD. In spite of the knowledge that vascular disease gives rise to cognitive impairment, only few trials have been performed focusing on the disease mechanisms involved in cerebrovascular dysfunction and there is no registered treatment for the disease.6 Most of the trials in VaD have been performed in patients with stroke-related cognitive impairment, which is a heterogeneous group of patients, while in the fairly homogeneous entity of subcortical vascular dementia (SVD), which comprise about half of the patients with VaD,7 only few trials have been conducted. The border between AD and VaD/SVD is not clear-cut and many patients exhibit signs of both AD and VaD. The reported prevalence rate for the mixed form is highly variable ranging from 2% to 60% depending on the criteria used.8
Mild cognitive impairment is referred to as a circumscribed cognitive syndrome focused on memory loss9 or a comprehensive cognitive syndrome irrespective of the cognitive domains involved.10 While the cognitive impairment in MCI is objectively measurable it should not constrain daily life. The MCI entity has been used mainly in the context of AD due to an increased risk to develop the disease but it has not yet been clarified which of the circumscribed or the comprehensive forms of MCI that is characteristic for early AD. Cerebrovascular disease, systemic, and other neurodegenerative disorders may also cause MCI at early stages although there are hitherto comparatively few studies in vascular and other non-AD forms of MCI.
Subjective cognitive impairment (SCI) is very common in the aging population, and may exist in both the absence and presence of objective cognitive impairment, such as MCI. Recent SCI research deals primarily with possible implications of having SCI in the absence of objective cognitive decline, i.e., SCI as a very early sign. Several studies have reported higher risk to develop MCI and dementia, especially AD, among persons with SCI. For AD, it has been suggested that the SCI phase precedes MCI by 15 years.11 The knowledge is still insufficient about the implication of SCI for AD as well as cerebrovascular disease.
The aim of the Gothenburg MCI study is to improve the characterization of the early and manifest phases as well as the courses of pure AD, SVD, and the overlap between them, i.e., mixed type dementia (MixD). For this purpose, single and multimodal measurements from the clinical-, neuropsychological-, genetic-, biochemistry-, neurochemistry, and neuroimaging fields are employed. As repeated longitudinal assessments of putative disease markers are performed, etiological factors may be determined. In addition, factors that characterize regressive and stable cognitive impairment are under study. Methodological developments and the development of simplified (low technology) measures for identification of cognitive impairment are other tasks of the study.
The aim of this paper is to present the design of the study and report basic demographic data with particular focus on the distributions of the clinical syndromes SCI, MCI, and dementia, as well as AD, MixD, SVD, and other etiologies at baseline and after 2 and 6 years; furthermore, to present an overview of the methods used in the Gothenburg MCI study and the results from method development. In a sister publication, we present results from the Gothenburg MCI study including results from our own dementia studies that precede the Gothenburg MCI study (Wallin et al., p. 95, this issue).
Materials
Participants and Setting
The Gothenburg MCI study consists of patients with a wide range of cognitive impairment from very mild complaints to manifest dementia. Healthy (cognitively normal) controls are also included. The patients are referred to the Memory clinic at the Sahlgrenska University Hospital in Mölndal by other caregivers or directly seeking medical counseling themselves (less than 10% are self-referrals). The Memory clinic is the only clinic in the Gothenburg metropolitan area focusing on cognitive impairment and dementia. The Sahlgrenska University Hospital is Sweden’s largest hospital, with a catchment area that spans over 1 million inhabitants.
The patients are selected for further examination at the Memory clinic by a referral team with at least one of the Memory clinic’s physicians. The Memory clinic’s physicians all attend referral meetings on a rotating schedule. Patients selected by the referral team are then distributed to one of several diagnostic teams. All diagnostic teams are led by a physician (who may, or may not, be the physician who assessed the initial referral). The team physician assesses the patient according to the guidelines described below and decides whether the patient should be included in the Gothenburg MCI study.
Middle aged to young elderly individuals seeking help for self-observed or informant-reported cognitive decline assessed by the physician as significant and without an obvious underlying cause such as brain tumor, subdural hematoma, or major stroke, are eligible for inclusion in the study. Guidelines for inclusion of patients are age between 50 and 79 years, mini mental state examination (MMSE) > 18, self- or informant-reported cognitive decline, and duration of cognitive decline ≥6 months. Patients with a systemic or other somatic disease that may cause cognitive impairment such as subdural hemorrhage, brain tumor, hypothyroid state, encephalitis, unstable heart disease, and psychiatric disorders such as major affective disorder, schizophrenia, substance abuse, and confusion are excluded. The presence of minor depressive disorder does not lead to exclusion.
To obtain an overview of the sample selection of the Gothenburg MCI study, the ratio between clinical patient flow and study inclusion was assessed. During the period 2010 to 2012, 1,030 patients were admitted to the clinic and evaluated as study patients. If they were not included, the reason for exclusion was reported (N = 825; 80%). About 1/3 of the patients (37%) were excluded as they violated the age limits, declined participation, were cognitively healthy, or had a too pronounced cognitive impairment. In addition, 23% were excluded due to psychiatric or somatic illnesses. Data were missing for the reason of exclusion in 28%. Two percent of the patients were not included due to insufficient Swedish language proficiency. The remaining 10% comprised patients that fulfilled the inclusion criteria and were therefore included.
Healthy controls are primarily recruited through senior citizen organizations, e.g., at information meetings on dementia, and a small proportion are relatives of patients. To be regarded as healthy, the controls should not experience or exhibit any cognitive decline at the time of inclusion in the study and should also be ≥50 years and ≤79 years old and have a MMSE score of >26. Exclusion criteria for controls are the same as for the patients.
Ethics
The Gothenburg MCI study was approved by the local ethics committee (diary number: L091-99 15 March 1999/T479-11 8 June 2011), and is conducted in accordance with the Declaration of Helsinki of 1975 and 1983. Written informed consent is obtained from all participants in the study.
Schedule of Assessments
Baseline examinations are performed over eight different study visits, to date performed within an average timespan of 168 days, with a standard deviation of 117 days. Year 2 examinations are performed across eight visits, year 4 one visit, year 6 six visits, and year 10 four visits. The various methods of assessment at the different visits are presented in Table 1.
Table 1.
Schedule of assessments.
| Year |
0 |
2 |
4 |
6 |
10 |
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visit | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 |
| Study information | x | ||||||||||||||||||||||||||
| Informed consent | x | ||||||||||||||||||||||||||
| Demographya | x | x | x | x | x | ||||||||||||||||||||||
| Medical history | x | x | x | x | x | ||||||||||||||||||||||
| Concomitant medication review | x | x | x | x | x | ||||||||||||||||||||||
| Anthropometryb | x | x | x | ||||||||||||||||||||||||
| Psychic status | x | x | x | x | x | ||||||||||||||||||||||
| Cognitive status | x | x | x | x | x | ||||||||||||||||||||||
| Blood pressure | x | x | x | ||||||||||||||||||||||||
| MMSE | x | x | x | x | x | ||||||||||||||||||||||
| I-FLEX | x | x | x | x | x | ||||||||||||||||||||||
| GDS20 | x | x | x | ||||||||||||||||||||||||
| Neuropsychological exam 1 | x | x | x | x | |||||||||||||||||||||||
| Neuropsychological exam 2 | x | x | x | x | |||||||||||||||||||||||
| EEG | x | x | |||||||||||||||||||||||||
| SPECT | x | x | |||||||||||||||||||||||||
| MRI | x | x | x | ||||||||||||||||||||||||
| ECG | x | x | x | ||||||||||||||||||||||||
| Somatic status | x | x | x | ||||||||||||||||||||||||
| Neurologic status | x | x | x | ||||||||||||||||||||||||
| Blood sample, fasting | x | x | x | ||||||||||||||||||||||||
| Blood sample, non-fasting | x | x | x | ||||||||||||||||||||||||
| LP, fasting | x | x | |||||||||||||||||||||||||
| LP, non-fasting | x | ||||||||||||||||||||||||||
| Return visit | x | x | x | x | |||||||||||||||||||||||
ECG, electrocardiography; EEG, electroencephalography; GDS20, geriatric depression scale; I-FLEX, investigation of flexibility; LP, lumbar puncture; MMSE, mini mental state examination; SPECT, single-photon emission computed tomography; MRI, magnetic resonance imaging.
Age, years of education, sex, marital status, smoking, drinking, and drug habits.
Height, weight, sagittal abdominal diameter, waist circumference, and hip circumference.
Diagnostic Procedures
The diagnostic procedure contains three parts: (1) assessing the degree of cognitive impairment; (2) diagnostics of specific dementia diseases, and (3) classifying patients with cognitive impairment without dementia by their vascular burden. For full procedure and list of criteria, see Figure 1.
Figure 1.
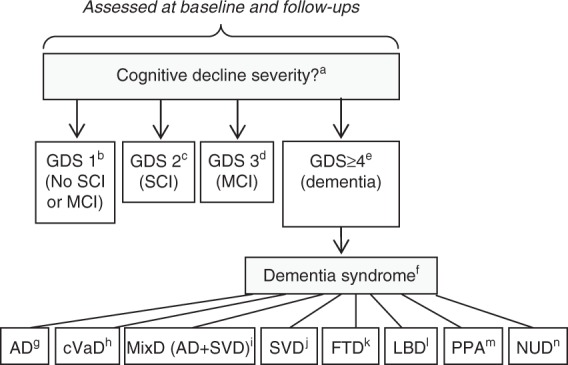
Diagnostic procedures and list of criteria in the Gothenburg mild cognitive impairment (MCI) study. (a) Cognitive decline severity is assessed at baseline and follow-ups, using stages 1 to 4 from the Global Deterioration Scale (GDS).12 (b) GDS stage 1 corresponds to no subjective or objective cognitive decline. Algorithm: STEP = 0, I-FLEX = 0, CDR≤0.5, MMSE≥29. (c) GDS stage 2 corresponds to subjective cognitive impairment. Algorithm: STEP = 0, I-FLEX < 3, CDR≤0.5, MMSE≥28 + subjective cognitive complaints reported in clinical interview (mandatory). (d) GDS stage 3 corresponds to MCI. Algorithm: STEP≤1, I-FLEX≤3, CDR > 0.5, MMSE≥26. (e) GDS stage ≥4 corresponds to probable mild dementia. Algorithm: STEP > 1, I-FLEX > 3, CDR > 1.0, MMSE≤25. (f) The presence of the dementia syndrome. Specific dementia disease diagnostics were operationalized using the Fazekas scale for WMC,17 and results on clinical assessment of brain regional symptoms. (g) The National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria for AD (1984)18 and no or mild WMC + parieto-temporal syndrome. (h) The National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherché et l'Enseignement en Neurosciences criteria for VaD (1993).23 (i) MixD. Either (1) AD + moderate or severe WMC or (2) AD + WMC + dysexecutive syndrome. (j) Research criteria for SVD22 with mild, moderate, or severe WMC + dysexecutive syndrome. (k) FTD: a consensus on clinical diagnostic criteria (1998).19 (l) Consortium on LBD (1999).20 (m) Classification of PPA (2011).21 (n) NUD. The presence of dementia syndrome without hallmarks for specific etiology. AD, Alzheimer’s disease; CDR, clinical dementia rating; cVaD, cortical vascular dementia; FTD, frontotemporal dementia; GDS, global deterioration scale; I-FLEX, investigation of flexibility, short form of the executive interview EXIT; LBD, Lewy-body dementia; MixD, mixed type dementia (=AD + subcortical vascular dementia (SVD)); MMSE, mini mental state examination; NUD, nonultra descriptum; PPA, primary progressive aphasia; STEP, stepwise comparative status analysis; VaD, vascular dementia; WMC, white matter changes.
Staging of Cognitive Impairment
The assessment of cognitive impairment results in four categories: cognitively healthy, SCI, MCI, and dementia. The assessment is performed at baseline and at follow-up examinations by assigning one of the Global Deterioration Scale (GDS)12 levels. GDS level 1 represents cognitively healthy; level 2 SCI; level 3 MCI; and level ≥4 dementia. The GDS assignment procedure is presented in Figure 1 and comprises the following instruments: MMSE,13 Clinical Dementia Rating (CDR),14 Stepwise Comparative Status Analysis (STEP),15 and Investigation of Flexibility (I-FLEX), which is a short form of the executive interview EXIT.16 A physician and/or registered nurse administer the cognitive instruments when the patients visit the Memory clinic. The GDS assignment is conducted by a registered nurse together with either a physician or a psychologist. All involved in the assignment are specially trained to ensure assessment reliability. Ambiguous results that do not fit with the functions in Figure 1 (e.g., STEP = 1, I-FLEX = 0, CDR = 0.5, and MMSE = 30) are classified according to consensus decision after discussion among senior physicians at the clinic. In these cases, results on STEP and I-FLEX are given priority in the assignment procedure, while MMSE and CDR scores are consulted secondarily. The presence of subjective cognitive complaints is ascertained through clinical interview.
Diagnostics of Phenotypically Specific Dementia Diseases
A specially trained physician considers specific diagnostics of all patients that are categorized as GDS level ≥4. Anamnestic and clinical symptomatology and the presence of cerebral white matter changes (WMC) determined by a modified version of the Fazekas scale are taken into account in the diagnostic procedure.17 To avoid circularity, neuropsychological test results, magnetic resonance imaging (MRI) results, and cerebrospinal fluid (CSF) markers are not used at this stage. In other words, the specialist physician is blinded to psychometric, CSF, and imaging results (except assessment of WMC). If the diagnosis cannot be unambiguously determined, then it is further discussed and established in a clinical consensus meeting, still blinded to psychometric, imaging, and CSF results. The specific diagnostic assessment procedure for each etiological dementia form is listed in Figure 1. A non ultra descriptum (NUD) diagnosis is set if the patient fulfills criteria for clinical dementia, but not for any specific dementia diagnosis.
Alzheimer’s disease is diagnosed using the 1984 National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria for AD.18 More specifically: for an AD diagnosis, the patient must have no or mild WMC, and predominant parietotemporal lobe symptoms. Frontotemporal dementia is diagnosed according to Neary et al.;19 Lewy-body dementia according to McKeith et al.;20 and primary progressive aphasia (PPA) according to Gorno-Tempini et al.21
Vascular dementia forms are either SVD or cortical vascular dementia (cVaD). Subcortical vascular dementia is diagnosed using the Erkinjuntti criteria (2000),22 and cVaD using the National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherché et l'Enseignement en Neurosciences criteria.23 More specifically: for SVD, the patient must have WMC (mild, moderate, or severe according to Fazekas classification)17 and predominant frontal lobe symptoms. If WMC are only mild, then SVD is set only if parietotemporal lobe syndromes are not marked (in which case MixD is indicated). A diagnosis of cVaD is set if dementia onset is stroke related (single- or multi-infarct).
A MixD diagnosis in the Gothenburg MCI study might be either a combination of AD/SVD or AD/cVaD, although the latter has been rare (one patient with AD/cVaD and two with AD and both cVaD and SVD at baseline, none among the converters). In both cases, the patient must also fulfill clinical AD symptomatology according to established criteria (i.e., parietotemporal lobe syndrome). Additionally, WMC must be either (1) moderate/severe, with no predominant frontal lobe syndrome or (2) mild, and in combination with a marked frontal lobe syndrome (in addition to the parietal lobe syndrome).
Vascular Burden
At baseline all patients are assessed for vascular burden using the three categories (1) vascular etiology, (2) mixed etiology, and (3) nonvascular (or in other words primary degenerative etiology). An explanation of the categories can be found in Table 2. This classification method has been used in some papers on SCI/MCI but not when diagnosing the etiology of the specific dementia diseases.
Table 2.
Assessment of vascular burden.
| Category | Definition |
|---|---|
| Vascular | • Two (or more) vascular risk factorsa and widespread WMC/several lacunae and/or brain imaging signs of infarction(s) |
| • One vascular risk factor with apparent brain influence (in practice TIA/stroke) and WMC/lacunae and/or signs of infarction as an expression of the cerebrovascular disease | |
| Mixed (vascular + nonvascular) | • Two vascular risk factorsa alternatively one vascular risk factor with long lasting complications and less severe but not insignificant WMC (or lacunae) or signs of infarction. Any infarction deemed contributing but not the main critical factor behind the progression of the cognitive impairment |
| Nonvascular | • One vascular risk factora without complications and insignificant WMC/few lacunae or the absence of cerebrovascular influence |
WMC, white matter changes.
Arterial hypertension, arterial hypotension (symptomatic), congestive heart failure, angina pectoris, cardiac dysrhythmia, myocardial infarction (also silent), transient ischemic attack (TIA), stroke (also silent), hyperlipidemia, diabetes mellitus, and peripheral vascular disease (e.g., claudicatio intermittens).
Methods
The Gothenburg MCI study uses variables from three different classes of markers for outcome evaluation; neuropsychological tests, imaging and brain physiology markers (MRI, SPECT, EEG), and CSF markers. To avoid circularity, these markers are not used for GDS classification. The results from the markers presented below are presented in the accompanying article (Wallin et al., this issue).
Neuropsychological Tests
The tests are administered by a licensed psychologist alternatively a psychologist-in-training supervised by a licensed psychologist. The administration of tests is standardized and divided into two sessions of 1 to 2 hours. Verbal tests are varied with nonverbal in each session and the test order is based on the risk of contamination on the memory tests, i.e., tasks between immediate and delayed recall would influence performance on delayed recall. The comprehensive neuropsychological examination comprises tests within the cognitive domains speed and attention, learning and episodic memory, visuospatial-, language-, and executive functions. To approximate a complete picture of the participants’ cognitive status, several cognitive functions are assessed within each cognitive domain.
Speed and attention
Digit Symbol from Wechsler’s Adult Intelligence Scale Revised (WAIS-R and -III)24,26 is a test of speed and alternating attention. Trail making A and B27 are frequently used tests when assessing visual scanning and complex attention. Digit Span24 is a test of attention span and working memory.
Learning and episodic memory
Rey Auditory Verbal Learning Test is a well-validated word recall test28 and Wechsler’s Logical Memory25 is a story recall test. Rey Complex Figure recall is a visual memory test.29
Visuospatial functions
Visual Object and Space Perception Silhouettes subtest is a visuoperceptual test.30 Rey Complex Figure copy29 is a visuoconstructive test sometimes used for dementia screening.30 Block Design is a spatial construction subtest of WAIS-R and -III.24,26
Language
Token Test, subtest V, is a test of syntax comprehension.31 Boston Naming Test is a confrontation naming test.32 Similarities is another WAIS-R and -III subtest, considered to assess verbal abstraction.24,26 Word fluency F-A-S is a test in generative verbal ability often used when assessing possible dementia.33
Executive functions
In Parallel Serial Mental Operations, the subject is asked to recite the alphabet, stating the number after each letter, i.e., A-1-B-2-C-3…; it is a measure of mental control and working memory. The task is presented in Lezak et al.34 Dual Task is a test of divided attention.35 Wisconsin Card Sorting Test is a test of several aspects of executive function, such as conceptual reasoning and set shifting, the computerized short version (CV64) is used.36 Stroop Test, Victoria version, is a short form of this classical executive test of distractability.37 Cognitive Estimation Test is a test of judgment and calculation.38 Letter number sequencing is a working memory subtest from WAIS-III.26 For an estimate of general cognitive capacity, Matrix reasoning from WAIS-III was used.26
The Gothenburg MCI study is a clinical study, thus neuropsychological test updates are unavoidable, as are exclusions of outdated tests. The aim has, however, been to retain a core of tests in as similar versions as possible. The test battery has undergone two major revisions, one in 2001 and the second in 2006. In 2001 Wechsler’s Logical Memory, Wisconsin Card Sorting Test, Stroop, and Cognitive Estimation Test were added to the battery. In 2006, WAIS-R versions of Digit Symbol, Block Design, and Similarities were replaced with the WAIS-III versions, Letter number sequencing and Matrix reasoning were added, and Dual Task was excluded.
Imaging and Brain Physiology
Magnetic resonance imaging scanners
Two MRI scanners have been used in the Gothenburg MCI study. A 0.5-T scanner (Philips NT5, Eindhoven, The Netherlands) was used from year 1999 to 2004 and a 1.5 T scanner (Siemens Symphony, Siemens Medical Systems, Erlangen, Germany) since 2005.39,40
Manual hippocampal segmentation
T1-weighted images from the 0.5-T scanner are used for manual volumetry of the hippocampus. The volumetry is performed by manual segmentation on an interactive Wacom screen using an in-house developed software with a segmentation protocol similar to the protocol in Convit (1997; http://www.hippocampal-protocol.net/SOPs/LINK_PAGE/Groups_Protocol/convit-protocol.pdf),41 but in accordance with Maller the whole tail is included in the segmentation.42 The hippocampal volumes are normalized by linear regression to intracranial volume with a residual method.39,43 Intracranial volume is estimated using the ellipsoid method measuring a total of four slices in three orthogonal orientations.39
Freesurfer segmentation
T1-weighted images from the 1.5-T scanner are analyzed using the software suite FreeSurfer.43,44 FreeSurfer is an automatic tool that estimates a number of regional brain volumes (e.g., hippocampus, amygdala, and thalamus) from T1-weighted MRI. In papers published from 2008 to 2014, estimates of hippocampal volume and WMC volume from the FreeSurfer version 4.0.5 were used. (https://surfer.nmr.mgh.harvard.edu/fswiki/ReleaseNotes).
Estimation of white matter changes
Estimation of WMC in the Gothenburg MCI study is performed using rating scales, FreeSurfer estimation, and manual volumetry.
A modified version of Fazekas scale is employed to rate WMC, where grade 1 includes both none and mild WMC. Briefly, T2-weighted images are compared with template images where the grade of WMC is defined. For each participant series, the image with the largest visible WMC is used for the comparison with the template images. No metrical measures are used during the ratings.
Manual WMC volumetry on FLAIR images is also performed in the Gothenburg MCI study using the software MRIcron, employing a modified method used by Holland et al.45 To consistently analyze each participant series, a grayscale mapping of the brain tissue is performed in the image containing the quadrigeminal plate. After the manual segmentation, an image intensity thresholding is applied and the overlap between the manually segmented regions and the intensity thresholding is used as estimates of WMC volume. The segmentations include all supratentorial regions with WMC.
Single-photon emission computed tomography imaging
The SPECT imaging is performed on patients using 99 m Tc-HMPAO SPECT. Controls are not examined due to ethical reasons. About 1,000 MBq of 99 m Tc-HMPAO is administered intravenously after a 15- to 20-minute period of resting in a quiet and dimly lit room. Imaging acquisitions are performed with a 3-headed Picker Irix-camera (Philips Medical Systems Inc., Hamburg, Germany) with high-resolution collimators about 45 minutes after the injection. A 128 × 128 pixel matrix is used and 90 projections are registered with an acquisition time of 45 seconds each. Besides quantitative analysis we used a software for semiquantitative analysis (EXINI brain; EXINI diagnostics AB, Lund, Sweden).46
Electroencephalography
The EEG examinations are perfomed during late mornings and the subjects are asked not to drink coffee or take any medication during the 12 hours before the examination to avoid unwanted influences on the alertness level. The recordings are performed with the patients in a semi-recumbent position with their eyes closed. Immediately before the recording is started, a 15-second photic stimulation is performed to ensure full alertness. Afterwards, the patient is left undisturbed, with no noise or movement in the examination room during the first 5 minutes, when the signal is stored for off-line analysis. The examination is then continued in a routine manner with eye openings and hyperventilation. The stored recordings consist of the activities from 16 EEG derivations (with a common reference), digitized with a sampling frequency of 200 Hz.
Cerebrospinal Fluid Markers
Lumbar puncture
Lumbar puncture (LP) is performed at baseline and at follow-ups, at a standardized time during the day (between 0800 and 1200 h) to avoid fluctuations in biomarker levels due to diurnal variations. Before the LP, the patient and the caregiver are informed about the procedure and about the risk for post-LP headache and how to handle it with supine rest and mild analgesics (paracetamol) if it should occur. The CSF sample is withdrawn by inserting the needle into the L3/L4 or L4/L5 interspace. An aseptic technique is applied and a local anesthetic (Lidocaine) is often administered. A standard Quincke cutting edge needle (0.7 mm/22 gauge) is used and the needle is inserted with the bevel parallel to the dura fibers. In all, 20 mL CSF is collected in polypropylene tubes. The first portion is discarded to avoid blood contamination of the CSF sample. Thereafter, 20 mL CSF is collected in polypropylene tubes. After the procedure, the patient is allowed to rest for 30 to 60 minutes. The CSF is sent to the Clinical Neurochemistry Laboratory at Sahlgrenska University Hospital in Mölndal. At the laboratory, the CSF is gently mixed by inverting the tube and a small portion is pipetted off for cell count and other routine analyses including the CSF/serum albumin ratio, the IgG and IgM index and analysis of oligoclonal IgG and IgM bands. The CSF is then centrifuged at room temperature at 2,000 × g for 10 minutes to eliminate cells and cellular debris. Smaller portions of CSF are aliquoted directly from this tube into smaller polypropylene tubes with screw caps. The tubes are labelled with date and patient ID and CSF for the AD core biomarker analysis is stored at −20°C pending analyses, while the remaining CSF is stored in a −80°C freezer with continuous temperature monitoring and an alarm system awaiting future analyses. The LP procedure and subsequent pre-analytical handling of the CSF such as centrifugation, aliquoted volumes, and choice of tubes are standardized, as described previously in detail.47 All laboratory analyses are accredited by the Swedish Board for Accreditation and Conformity Assessment (SWEDAC).
From all subjects whole blood is collected, and prepared into serum and plasma. All blood samples are aliquoted and stored at −80°C pending analyses. After the first visit, apolipoprotein E (APOE) genotype is performed by minisequencing as described previously in detail.48
Biochemical methods
Core CSF biomarker analyses, total tau protein (T-tau), phosphorylated tau (P-tau181), and amyloid beta (Aβ1–42) are performed after the CSF samples have been frozen once at −20°C for a maximum of 1 week. The CSF biomarker analyses are performed by board-certified laboratory technicians using a standard laboratory quality control program to assure quality, as described previously.49 The core biomarker levels are mainly evaluated by commercially available enzyme-linked immunosorbent assays (INNOTEST, Fujirebio, Gent, Belgium).50,51,52 Internal quality controls, two or more internal control CSF samples (aliquots of pooled CSF), are analyzed each run, to minimize between-assay variability.
Other CSF biomarkers that have been evaluated for different purposes in various substudies are described more in detail in the individual articles that are referred to in a sister publication (Wallin et al., p. 95, this issue).
Results
Here we present demography and distributions of syndromes and etiological diagnoses at baseline, 2 and 6 years for subjects qualified for the 6-year follow-up examination, and our results of method development. Review of the Gothenburg MCI study papers on neuropsychology, brain imaging/physiologic, biochemistry, multimodal prediction, and course of the disease/cognitive reserve is presented in a sister publication (Wallin et al., p. 95, this issue).
Characterization of Patients and Healthy Controls at Baseline
Between 1999 and 2013, 664 patients (mean age 64.8 ± 7.9, 58.6% female) from the Memory clinic at the Sahlgrenska university hospital and 115 healthy controls (mean age 64.4 ± 6.4, 61.7% female) were enrolled in the study (Table 3). Baseline characteristics for all included patients are presented in Table 4.
Table 3.
Demographic characteristics of the total sample.
| Baseline | All patients | Controls | P-value |
|---|---|---|---|
| N | 664 | 115 | |
| Sex (male/female) | 275/389 | 44/71 | 0.525a |
| Age | 64.8 ± 7.9 | 64.4 ± 6.4 | 0.591b |
| Education (years) | 12.1 ± 3.7 | 11.9 ± 3.0 | 0.866b |
| MMSE | 27.4 ± 2.5 | 29.3 ± 0.9 | <0.000b |
MMSE, mini mental state examination. Age, education years, and MMSE scores are given as mean ± s.d.
Chi-square test.
T-test.
Table 4.
Baseline patient demographics and diagnoses.
| SCI | MCI | Dementia | All patients | MCI versus SCI | Dementia versus SCI | Dementia versus MCI | |
|---|---|---|---|---|---|---|---|
| N | 195 | 274 | 195 | 664 | |||
| Sex (male/female) | 82/113 | 110/164 | 83/112 | 275/389 | P = 0.679a | P = 0.918a | P = 0.600a |
| Age | 61.8 ± 7.6 | 65.2 ± 7.7 | 67.3 ± 7.4 | 64.8 ± 7.9 | P < 0.001b | P < 0.001b | P = 0.004b |
| Education (years) | 13.6 ± 3.6 | 11.8 ± 3.5 | 11.0 ± 3.5 | 12.1 ± 3.7 | P < 0.001b | P < 0.001b | P = 0.042b |
| MMSE | 29.1 ± 0.9 | 28.0 ± 1.5 | 24.8 ± 2.7 | 27.4 ± 2.5 | P < 0.001b | P < 0.001b | P < 0.001b |
| AD | 81 (42%) | ||||||
| SVD | 27 (14%) | ||||||
| MixD | 41 (21%) | ||||||
| Other | 46 (24%) |
AD, Alzheimer’s disease; MCI, mild cognitive impairment; MixD, mixed type dementia (=AD + SVD); MMSE, mini mental state examination; SCI, Subjective cognitive impairment; SVD, subcortical vascular dementia. Age, education years, and MMSE scores are given as mean ± s.d.
Chi-square test.
T-test.
Baseline Distribution of Diagnoses
Of the 664 patients enrolled in the study between 1999 and 2013, at baseline 195 were diagnosed with SCI, 274 with MCI, and 195 with dementia. The distribution of specific etiological dementia diagnoses was as follows: 81 AD, 27 SVD, 41 MixD, and 46 other (26 NUD, 8 FTD, 1 mixed AD/FTD, 2 PPA, and 1 Lewy-body dementia) (Table 4). After an independent reexamination by an experienced physician, eight patients were recommended for exclusion according to inclusion/exclusion criteria (three with severe psychiatric disorders, two with severe somatic disorders, one with too severe dementia, one because of incomplete examination, and one patient was too young).
Follow-up Distribution of Diagnoses
Follow-up status year 2 and year 6 for all participants with SCI or MCI at baseline is reported in Tables 5 and 6. Only participants who had been eligible for their 6-year follow-up in 2013 were selected to the 6-year follow-up data set, i.e., those included between 1999 and 2006 allowing for 1-year follow-up delay. A total of 292 participants were selected (SCI 113 and MCI 179). The baseline characteristics of the groups are presented in Table 7.
Table 5.
Follow-up status for patients after 2 years.
| Follow-up status | Total, n | % | SCI at baseline, n | % | MCI at baseline, n | % |
|---|---|---|---|---|---|---|
| Conversion to dementia | ||||||
| AD | 21 | 7 | 0 | 0 | 21 | 12 |
| MixD | 12 | 4 | 0 | 0 | 12 | 7 |
| SVD | 10 | 3 | 2 | 2 | 8 | 5 |
| Dementia, other | 5 | 2 | 0 | 0 | 5 | 3 |
| Nonconverting | ||||||
| Stable MCI | 68 | 23 | 0 | 0 | 68 | 38 |
| Stable SCI | 69 | 24 | 69 | 61 | 0 | 0 |
| SCI–MCI | 21 | 7 | 21 | 19 | 0 | 0 |
| MCI–SCI | 38 | 13 | 0 | 0 | 38 | 21 |
| MCI–healthy | 3 | 1 | 0 | 0 | 3 | 2 |
| No follow-up data | ||||||
| Round skipped | 3 | 1 | 2 | 2 | 1 | 1 |
| Deceased | 1 | <1 | 0 | 0 | 1 | 1 |
| Somatic disease | 2 | 1 | 1 | 1 | 1 | 1 |
| Examination declined | 35 | 12 | 16 | 14 | 19 | 11 |
| No information | 4 | 1 | 2 | 2 | 2 | 1 |
| Total, n | 292 | 113 | 179 | |||
AD, Alzheimer’s disease; MCI, mild cognitive impairment; MixD, mixed type dementia (=AD + SVD); SCI, subjective cognitive impairment; SVD, subcortical vascular dementia. AD = conversion from Global deterioration scale (GDS) 2 or 3 to GDS 4 + fulfillment of AD criteria. SVD = conversion from GDS 2 or 3 to GDS 4 + fulfillment of SVD criteria. MixD = conversion from GDS 2 or 3 to GDS 4 + fulfillment of MixD criteria. Dementia, other = conversion from GDS 2 or 3 to GDS 4 (after 2 years 4 nonultra descriptum (NUD) and 1 primary progressive aphasia (PPA), after 6 years 3 additional NUD. Stable MCI = first and current examination GDS 3. Stable SCI = first and current examination GDS 2. SCI–MCI = first examination GDS 2 and current examination GDS 3. MCI–SCI = first examination GDS 3 and current examination GDS 2. MCI–healthy = first examination GDS 3 and current examination GDS 1. Round skipped = current round skipped, next examination carried out. Missing follow-up: deceased. Somatic disease = not followed up due to somatic disease. Examination declined = further examination declined. No information = not followed up, no information available.
Table 6.
Follow-up status for patients after 6 years.
| Follow-up status | Total, n | % | SCI at baseline, n | % | MCI at baseline, n | % |
|---|---|---|---|---|---|---|
| Conversion to dementia | ||||||
| AD | 29 | 10 | 2 | 2 | 27 | 15 |
| MixD | 15 | 5 | 0 | 0 | 15 | 8 |
| SVD | 16 | 6 | 4 | 4 | 12 | 7 |
| Dementia, other | 8 | 3 | 0 | 0 | 8 | 5 |
| Nonconverting | ||||||
| Stable MCI | 10 | 3 | 3 | 3 | 7 | 4 |
| Stable SCI | 35 | 12 | 29 | 26 | 6 | 3 |
| SCI–MCI | 13 | 5 | 9 | 8 | 4 | 2 |
| MCI–SCI | 23 | 8 | 8 | 7 | 15 | 8 |
| MCI–healthy | 3 | 1 | 0 | 0 | 3 | 2 |
| SCI–healthy | 9 | 3 | 2 | 2 | 7 | 4 |
| Stable healthy | 1 | <1 | 0 | 0 | 1 | 1 |
| No follow-up data | ||||||
| Deceased | 5 | 2 | 3 | 3 | 2 | 1 |
| Somatic disease | 7 | 2 | 2 | 2 | 5 | 3 |
| Severe dementia | 1 | <1 | 0 | 0 | 1 | 1 |
| Examination declined | 75 | 26 | 32 | 28 | 43 | 24 |
| No information | 42 | 14 | 19 | 17 | 23 | 13 |
| Total, n | 292 | 113 | 179 | |||
AD, Alzheimer’s disease; MCI, mild cognitive impairment; MixD, mixed type dementia (=AD + SVD); SCI, subjective cognitive impairment; SVD, subcortical vascular dementia. AD = conversion from Global deterioration scale (GDS) 2 or 3 to GDS 4 + fulfillment of AD criteria. SVD = conversion from GDS 2 or 3 to GDS 4 + fulfillment of SVD criteria. MixD = conversion from GDS 2 or 3 to GDS 4 + fulfillment of MixD criteria. Dementia, other = conversion from GDS 2 or 3 to GDS 4 (after 2 years 4 nonultra descriptum (NUD) and 1 primary progressive aphasia (PPA), after 6 years 3 additional NUD. SCI–healthy = first examination GDS 2 and current examination GDS 1. Stable MCI = first and current examination GDS 3. Stable SCI = first and current examination GDS 2. SCI–MCI = first examination GDS 2 and current examination GDS 3. MCI–SCI = first examination GDS 3 and current examination GDS 2. MCI–healthy = first examination GDS 3 and current examination GDS 1. Round skipped = current round skipped, next examination carried out. Missing follow-up: deceased. Somatic disease = not followed up due to somatic disease. Examination declined = further examination declined. No information = not followed up, no information available.
Table 7.
Demographic characteristics of participants eligible for 6 years follow-up examination.
| SCI | P-value versus HC | MCI | P-value versus HC | SCI + MCI | HC | |
|---|---|---|---|---|---|---|
| N | 113 | 179 | 292 | 115 | ||
| Sex (male/female) | 49/64 | 0.512a | 73/106 | 0.729a | 122/170 | 44/71 |
| Age | 61.7 ± 7.3 | 0.001b | 64.2 ± 7.7 | 0.311b | 63.2 ± 7.7 | 64.4 ± 6.4 |
| Education (years) | 13.2 ± 3.6 | < 0.000b | 11.4 ± 3.4 | 0.418b | 12.1 ± 3.6 | 11.9 ± 3.0 |
| MMSE | 29.2 ± 0.9 | 0.074b | 28.1 ± 1.5 | <0.000b | 28.5 ± 1.4 | 29.3 ± 0.9 |
HC, healthy (cognitively normal) controls; MCI, mild cognitive impairment; MMSE, mini mental state examination; SCI, subjective cognitive impairment. Age, education years, and MMSE scores are given as mean ± s.d.
Chi-square test.
T-test.
After 2 years, a total of 48 of 292 participants (16%) had converted to dementia (21 AD, 12 MixD, 10 SVD, and 5 other). After 6 years, a total of 69 of 292 participants (24%) had converted to dementia, 13 of which had converted after 4 years (6 AD, 2 MixD, 3 SVD, and 2 other). The distribution of specific dementia diagnoses was 29 AD, 15 MixD, 16 SVD, and 8 other. In the 4-year follow-up participants who had SCI after 2 years were not examined, further 4-year data are not reported in this paper.
After 6 years, 129 participants (44%) had dropped out for various reasons (75 declined further examinations, 7 due to somatic disease, 5 were deceased, and for 42 participants there was no information available). See Table 6 for details.
Among the 75 participants who at year 6 had declined further participation in the study, the mean age at baseline was 62.9 (standard deviation = 7.5), years of education 12.2 (3.5), and MMSE score 28.6 (1.1). For the 42 participants where no information was available at year 6 the mean baseline age was 62.1 (7.3), years of education 12.2 (4.1), and MMSE score 28.9 (1.2). The baseline means for all participants with SCI or MCI, excluding the above participants, were age 63.6 (7.8), years of education 12.0 (3.6), and MMSE score 28.4 (1.6). No group differences were significant.
Among controls enrolled between 1999 and 2006 (N = 79), two were deceased and one had converted to dementia (AD) by 2013.
Method Development in the Gothenburg Mild Cognitive Impairment Study
The STEP was constructed as a clinical instrument for assessment of symptoms that may be referred to major brain regions.15 The interrater reliability of the STEP protocol has been found to be satisfactory.53 In the Gothenburg MCI study, symptom items 13 to 20 of STEP have been used for identification of the various levels of severities of the GDS.
The Cognitive Impairment Questionnaire, constructed on the principle of STEP, is an instrument based on the information obtained by key informants to identify symptoms of dementia and dementia-like disorders reflecting disturbances in various brain regions. It was found to have high reliability and validity in patients with MCI and dementia.54 The questionnaire consists of three subscales reflecting impairment in parietal-temporal, frontal, and subcortical brain regions.
A Swedish version of the National Adult Reading Test, a test for assessment of premorbid IQ, has been constructed and tested for its validity and reliability in healthy controls and patients with mild AD.55 It was found to have satisfactory psychometric properties.
A short cognitive test battery, the Cognitive Assessment Battery (CAB) has been composed and was found to differentiate between controls and MCI as well as MCI of different severities and MCI and dementia with good sensitivity and specificity.56 Furthermore, CAB was recently found to differentiate between MCI with and without vascular disease, in terms of both overall performance and cognitive profile.57
A questionnaire specifically developed to examine subjects with SCI, the Sahlgrenska Academy Self-reported Cognitive Impairment Questionnaire, was developed and found to discriminate between SCI patients and healthy controls.58
In a recent reliability study of WMC estimation, a visual rating scale, a manual volumetric, and an automatic method were compared.59 All analyses showed significantly lower reliability for low WMC volumes possessed by over 3/4 of all the patients in spite of excellent overall reliability numbers. The main finding was hence that more thoroughgoing reliability analysis is needed when evaluating WMC estimation methods in distributions common in MCI and dementia research.
Various studies have been aimed at standardizing and improving the quality of the biomarker measurements60,61,62 and the Clinical Neurochemistry Laboratory is part of the International Federation of Clinical Chemistry and Laboratory Medicine Working Group on CSF proteins and the Alzheimer’s Association QC Program for CSF Biomarkers and the Global Biomarker Standardization Consortium.63
For an overview of methodological papers related to the Gothenburg MCI study, see Table 8.
Table 8.
Development of methods.
| Subjects | Aim | Results | Ref. no. |
|---|---|---|---|
| Cognition | |||
| Healthy controls, AD | To construct a Swedish version of the National Adult Reading Test (NART-SWE) and to investigate its validity and reliability | The Swedish NART was found to assess premorbid intelligence, even in mild AD | Rolstad et al.55 |
| Healthy controls, MCI, dementia | To evaluate and validate the Cognitive Assessment Battery (CAB) in a specialist clinic setting | CAB was found to differentiate between controls and MCI as well as MCI and dementia with good sensitivity and specificity | Nordlund et al.56 |
| Healthy controls, MCI | To evaluate cognitive profiles in MCI patients with and without vascular burden | CAB found different profiles in MCI with and without vascular burden | Nyström et al.57 |
| Healthy controls MCI, dementia | To examine the reliability and validity of the Cognitive Impairment Questionnaire (CIMP-QUEST) in a specialist clinic setting | CIMP-QUEST was found to have high test–retest reliability and high validity in comparison with psychometric test results | Åstrand et al.54 |
| Healthy controls, SCI | To develop and validate the Sahlgrenska Academy Self-reported Cognitive Impairment Questionnaire (SASCI-Q) in a specialist clinic setting | SASCI-Q was found to discriminate between SCI patients and healthy controls | Eckerström et al.58 |
| Dementia | To develop and validate the Stepwise Comparative Status Analysis (STEP) for identification of symptoms related to brain regions | The STEP instrument was found to identify symptom patterns related to brain regions | Wallin et al.15 |
| Dementia | To investigate the interrater reliability of the STEP protocol | For the majority of variables, the agreement was excellent or moderately good | Edman et al.53 |
| Imaging | |||
| Healthy controls, MCI, dementia | To evaluate three methods (Fazekas rating scale, FreeSurfer, manual volumetry) for the assessment of WMC | Reliability was generally acceptable for all methods but lower in the assessment of small WMC volumes | Olsson et al.59 |
| Neurochemistry | |||
| Healthy controls, AD | To standardize and improve quality of biomarker measurements | Stable quantification obtained for Amyloid beta 1 to 42, phosphorylated tau, and total tau measurements, implementation for clinical routine | Olsson et al.,61 Bjerke et al.,62 Vanderstichele et al.60 |
AD, Alzheimer’s disease; MCI, mild cognitive impairment; SCI, subjective cognitive impairment; WMC, white matter changes.
Discussion
The Gothenburg MCI study is an ongoing clinical study about phenotypes and symptoms of MCI and differentiating/overlapping features of AD, SVD, and related disorders in their pre- and mild-dementia phases. The results of the study (and its predecessors) so far have been reviewed by Wallin et al. (see p. 95, this issue). In this paper, we have presented the design of the study, diagnostic procedures, methodological developments and distributions of etiological diagnoses, and rates of progression/regression for those eligible for the 6-year follow-up examination.
The design of the study is an example of how hospital-based clinical research may be conducted and integrated into clinical practice. Clinical research consists not only of pharmacological trials, but also includes observational research which is a prerequisite for rational trials. In patients with chronic disorders such as AD and SVD, with partly unclear nosology, thorough hospital-based observational research is needed to find out what characterizes the disorders during various phases of the course as well as overlapping features and comorbidities.
In contrast to the approach taken by the Alzheimer’s Disease Neuroimaging Initiative and Australian Imaging, Biomarkers and Lifestyle studies,1,2 one of the main aims of the Gothenburg MCI study is on the vascular contribution to cognitive impairment of different severities. Research on vascular processes in AD and related disorders has been rated as a high priority research field by the US National Alzheimer’s Project Act.64 Vascular involvement was present in 80% of over 4,500 brains from patients with neuropathologically verified AD.65 The actual mechanisms linking vascular injury to neurodegeneration and clinical manifestation need to be defined. Well-defined clinical phenotypes of the kind that are presented by the Gothenburg MCI study may facilitate that development. A similar approach characterizes the Vascular Mild Cognitive Impairment Tuscany Study.66
In clinical-pathologic studies, the prevalence rate of MixD is highly variable between 2% and 60%8 indicating lack of clarifying knowledge about the relationship between vascular lesions, neurodegeneration, and the clinical manifestations. Almost 1/3 of the included patients exhibited mild dementia at baseline, where 42% had AD, 21% MixD, and 14% SVD. These figures are in agreement with the distributions found in an extensive neuropathologic study from Vienna;67 however, the proportion of vascular cases was slightly higher in our study. In the Hisayama neuropathologic dementia study, MixD was comparatively uncommon but the combined MixD and vascular group was as large as our combined group and the proportion of AD corresponded to our findings.68 However, in the Honolulu-Asia Aging study AD lesions were found only in 20% of the cases, whereas combined MixD and vascular lesions appeared in 69%.87 The variation between the studies indicates that the nosological classifications need to be clarified.
According to Brun’s pioneering clinical-neuropathologic study, vascular lesions contributed to the development of dementia in over 2/3 of dementia cases, while pure AD was as common as SVD (1/6 of the cases).69 Interestingly, the proportion of SVD cases corresponded to that of the Gothenburg MCI study. Both Brun’s study and Gothenburg MCI study focused on AD and vascular nosology and found from various viewpoints, despite selected cases, the same proportion of SVD cases. The agreement between the study results strengthens the validity of the SVD entity.
In the present study, we used the McKhann criteria from 198418 plus the presence of parietotemporal symptoms to identify manifest AD. Furthermore, if the patients displayed obvious signs of subcortical vascular disease they were not classified as AD patients. Interestingly, this more specific diagnostic approach is in line with the recently updated McKhann criteria for clinical AD.70 For patients with subcortical vascular disease, we used the Erkinjuntti criteria for SVD,22 which are only occasionally used although they correspond to a fairly homogeneous phenotype, with characteristic neuropsychological features.71 In this study, we found a large group of patients fulfilling the Erkinjuntti criteria for incipient or manifest SVD. Our results imply that vascular cognitive impairment of nonstroke type should be considered among patients seeking help for memory complaints in the health-care system.
Contrary to the new McKhann criteria there is also a trend toward being more unspecific about diagnostic issues and including several other neurodegenerative diseases under the Alzheimer umbrella. These studies focus on pathogenic events assumed to be the core features of AD such as amyloid disturbances. Consequently, it has been suggested that white matter involvement may be an early manifestation of AD.72,73 This way of thinking connects to the idea that AD is primarily a vascular disorder65 and is supported by epidemiologic studies demonstrating that vascular risk factors are of importance for the development of AD. As amyloid disturbances are not specific for AD and may, e.g., appear as a result of acute vascular events74 it is a risky way to go but may be beneficial for increasing the knowledge about the processes that take place in AD and related disorders. However, when the target is AD in clinical practice, the need for specific criteria cannot be underestimated. One possible cause of the lack of efficient treatment options in AD, in spite of several attempts, could be that heterogeneous groups of patients have been included in the treatment trials. One way of increasing the specificity is to rule out patients with subcortical vascular disease. There is also reason to design specific trials for patients with subcortical vascular disease.75
At baseline approximately 30% of the patients displayed SCI and 40% MCI. In the aggregated SCI/MCI group, 24% developed dementia during the period between baseline and 6 years follow-up visit corresponding to an annual conversion rate of 4%. The figure is low and even lower than that in community settings,76 probably due to the relatively low mean age of the sample and that a large fraction of the patients had very mild impairments as previously reported, the SCI phase may be as long as 15 years.11 Among the patients with MCI at baseline, 35% had developed dementia at the 6-year follow-up visit corresponding to an annual conversion rate of 5.8%, which is lower than in a meta-analysis that found an annual conversion rate of 9.6% among clinical patients with MCI.76 This could mean that our low conversion rates are not just a consequence of inclusion of a large portion of patients with very mild impairment but that several of our patients exhibit cognitive impairment due to other factors than incipient dementia disease, such as being sensitive to health conditions.
The conversion rate in the Gothenburg MCI study has not been consistent over the years. In the first reports on 2-year follow-up, the conversion rates in patients with SCI/MCI were 22% and 25% in respective study.77,78,79 These findings are much more in agreement with the reported annual rates of 10% to 15%. It seems that a larger proportion of the patients included early in the study were in the incipient phases of dementia. A possible explanation for the diminishing conversion rate is that an increasing number of patients in very early phases of a cognitive decline choose to seek medical care.
The majority of the MCI patients did not develop dementia during the follow-up period. This result is in line with that of other studies.76 After 2 years just under 50% of the patients were stable, whereas only around 15% were stable after 6 years indicating the presence of progressive and regressive movements in cognitive function outside the dementia domain. Despite the benign course of the majority of patients in the study, others have found that the risk of converting to dementia is increased even in those MCI patients who have reverted to normal cognitive functioning.79
The various severities of cognitive impairment, e.g., MCI, were identified using the GDS method. Global Deterioration Scale is a predecessor to the current psychometric MCI method used in several other studies. By the approach of operationalizing the GDS stages 2, 3, and ≥4 using well-validated and simplified clinical instruments, it was possible to identify various degrees of cognitive impairment. Thus, our findings indicate that GDS is useful in the context of MCI. The GDS procedure applied in our study is fairly well in agreement with previously published clinical approaches to MCI10 and the DSM-5 approach to classify mild neurocognitive disorder.80
A distinctive feature of the Gothenburg MCI study is that detailed psychometric test methods have not been used in the process of including patients in the study or in diagnostic procedures. Psychometric tests have been assigned the same independent status as biochemical and imaging markers. This is an advantage as to the evaluation of the importance of various modalities for the prediction of cognitive decline. Thus, in the Gothenburg MCI study we have been able to evaluate not only markers of different modalities but also combinations of markers.81,82 As high tech methods will not be available in every context dealing with mental and cognitive health issues there is a need for reliable and low tech methods to identify cognitive impairment and their relationship with diseases. The comprehensive, relatively time-consuming neuropsychological measurement in the Gothenburg MCI study has been used as a reference for the development of tools for abbreviated measurement of cognitive impairment such as CAB, which has to date been used in several studies besides MCI and dementia, e.g., heart failure83 varicella-zoster virus infection,84 and Parkinson’s disease.85 The neuropsychological battery has also undergone two major revisions, to keep it up to date. The first one was in 2001, when Wechsler’s Logical Memory, Cognitive Estimation Test, Stroop, and Wisconsin Card Sorting Test were added. The second one was in 2006, the WAIS-R versions were replaced with WAIS-III versions of the tests and the Digit letter and Matrices subtests from WAIS-III were added. Dual Task was also excluded.
As there is a clear risk of misidentifying individuals when the previous level of cognitive functioning is not taken into account, measures of premorbid IQ may enable more precise estimates. To this end, the Swedish National Adult Reading Test was developed.55
The imaging data set from the study has been analyzed using both low-tech methods (WMC rating scales) and more advanced methodologies (manual and automated volumetry of hippocampus and WMC). A comparison of different methods for WMC estimation found that only high burden WMC could be reliably detected. While similar results have been reported previously,86 most patients in the study had only mild WMC, which calls for methods with increased reliability to further clarify the clinical significance of WMC. One lesson learned from the Gothenburg MCI study is to plan in advance for technological development and increased availability of MRI scanners. The 0.5-T scanner used from the start became outdated during the course of the study and the replacement from 0.5 to 1.5 T was delayed because the transition had not been planned in advance.
Analysis of CSF biomarkers is a cross-cutting theme in the Gothenburg MCI study. Since the initiation of this study in 1999, CSF biomarkers are increasingly used both for clinical diagnosis and in clinical trials.47 Further, CSF biomarkers may give important clues on pathogenic mechanisms, and several studies from the Gothenburg MCI study have resulted in support for the AD-SVD spectrum and shown that biomarkers could be an important addition in the clinical differential diagnostics as early as at the MCI stage. The utility of biomarkers as clinical tools could be readily translated into the clinical trial setting since the inclusion of well-defined patient groups already early in the course of disease is a prerequisite for targeting the right disease mechanisms and to prevent the spread of the disease. It is well recognized that to reliably use biomarkers for all of these purposes and also to be able to compare research findings across the community a lot of effort is being put into reducing measurement variability between different centers by investigating confounding factors.49,62,63
There are some drawbacks of the study such as potential cohort effects, drop-outs, low number of cases that has developed specific dementia diseases, the presence of lacunes has not been rated in the diagnostic process, and that for practical reasons not all patients eligible for inclusion have been included in the study. Time constraints in the clinic may be a contributing factor to noninclusion. However, no systematic bias regarding age or sex is evident in the comparison between patients in the Memory clinic at large and patients included in the study. Thus, it can be argued that the sample is representative of a population of Memory clinic patients, albeit with fewer comorbid conditions. However, the representativeness is not a major issue as long as specific groups of patients are identified.
The advantages of the study are the longitudinal design, strict diagnostic procedures, and multimodal approach with focus on the AD-SVD spectrum that has been rated as a top priority field in AD research.64
In summary, the main findings from the Gothenburg MCI study so far are that there are different longitudinal features of SCI and MCI and that incipient AD and SVD are possible to separate in a memory clinic setting. This type of study may fill the gap between basic (preclinical) research on one hand and general population- and register-based research on the other hand. In particular, the study has the potential to identify differentiating and overlapping features in AD and SVD when the disease processes first manifest themselves. Our opinion is that this type of knowledge is a prerequisite for successful design of treatment trials. The case record forms, including instructions and definitions of items, may also function as self-instructive material for physicians who undergo education.
Acknowledgement
The authors are grateful to Mona Pedersen for recruitment of patients and Ewa Styrud, Marie C Johansson, Ing-Marie Isgaard, and Eva Bringman for their technical support and Niklas Klasson for comments.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from Sahlgrenska University Hospital, Swedish Research Council, Swedish Brain Power, the Alzheimer’s Association, the Inga-Britt and Arne Lundberg Research Foundation, the Gothenburg Medical Society, the Swedish Medical Society, the Swedish Dementia Foundation, Stiftelsen Gamla Tjänarinnor, Gun och Bertil Stohnes stiftelse, Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse, the Adlerbert Research Foundation, Swedish Alzheimer Foundation, and Stiftelsen Psykiatriska forskningsfonden.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
The following authors contributed substantially to the conception and design of the study: AW, AN, MB, CE, MG, and SR. Data were acquired by AW, AN, MJ, KL, ÅE, ME, JSt, JSv, SR, CE, SK, and AB-H. Analysis of data was performed by MG. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work. Interpretation of data as well as drafting and revising the manuscript critically for intellectual content was jointly performed by all authors.
References
- 1.Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, et al. The Alzheimer's Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement 2013; 9: e111–e194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellis KA, Szoeke C, Bush AI, Darby D, Graham PL, Lautenschlager NT, et al. Rates of diagnostic transition and cognitive change at 18-month follow-up among 1,112 participants in the Australian Imaging, Biomarkers and Lifestyle Flagship Study of Ageing (AIBL). Int Psychogeriatr 2014; 26: 543–554. [DOI] [PubMed] [Google Scholar]
- 3.Rizzi L, Rosset I, Roriz-Cruz M. Global epidemiology of dementia: Alzheimer's and vascular types. BioMed Res Int 2014; 2014: 908915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. New Engl J Med 2014; 370: 322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. New Engl J Med 2014; 370: 311–321. [DOI] [PubMed] [Google Scholar]
- 6.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011; 42: 2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshitake T, Kiyohara Y, Kato I, Ohmura T, Iwamoto H, Nakayama K, et al. Incidence and risk factors of vascular dementia and Alzheimer's disease in a defined elderly Japanese population: the Hisayama Study. Neurology 1995; 45: 1161–1168. [DOI] [PubMed] [Google Scholar]
- 8.Zekry D, Gold G. Management of mixed dementia. Drugs Aging 2010; 27: 715–728. [DOI] [PubMed] [Google Scholar]
- 9.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999; 56: 303–308. [DOI] [PubMed] [Google Scholar]
- 10.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004; 256: 240–246. [DOI] [PubMed] [Google Scholar]
- 11.Reisberg B, Prichep L, Mosconi L, John ER, Glodzik-Sobanska L, Boksay I, et al. The pre-mild cognitive impairment, subjective cognitive impairment stage of Alzheimer's disease. Alzheimer's Dement 2008; 4(Suppl 1): S98–S108. [DOI] [PubMed] [Google Scholar]
- 12.Auer S, Reisberg B. The GDS/FAST staging system. Int Psychogeriatr 1997; 9(Suppl 1): 167–171. [DOI] [PubMed] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 14.Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr 1997; 9(Suppl 1): 173–176. discussion 177–8. [DOI] [PubMed] [Google Scholar]
- 15.Wallin A, Edman A, Blennow K, Gottfries CG, Karlsson I, Regland B, et al. Stepwise comparative status analysis (STEP): a tool for identification of regional brain syndromes in dementia. J Geriatr Psychiatry Neurol 1996; 9: 185–199. [DOI] [PubMed] [Google Scholar]
- 16.Royall DR, Mahurin RK, Gray KF. Bedside assessment of executive cognitive impairment: the executive interview. J Am Geriatr Soc 1992; 40: 1221–1226. [DOI] [PubMed] [Google Scholar]
- 17.Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjogren M, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 2001; 32: 1318–1322. [DOI] [PubMed] [Google Scholar]
- 18.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984; 34: 939–944. [DOI] [PubMed] [Google Scholar]
- 19.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998; 51: 1546–1554. [DOI] [PubMed] [Google Scholar]
- 20.McKeith IG, Perry EK, Perry RH. Report of the second dementia with Lewy body international workshop: diagnosis and treatment. Consortium on Dementia with Lewy Bodies. Neurology 1999; 53: 902–905. [DOI] [PubMed] [Google Scholar]
- 21.Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology 2011; 76: 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erkinjuntti T, Inzitari D, Pantoni L, Wallin A, Scheltens P, Rockwood K, et al. Research criteria for subcortical vascular dementia in clinical trials. J Neural Transm Suppl 2000; 59: 23–30. [DOI] [PubMed] [Google Scholar]
- 23.Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 1993; 43: 250–260. [DOI] [PubMed] [Google Scholar]
- 24.Wechsler D. WAIS-R Manual, New York, NY, USA: The Psychological Corporation, 1981. [Google Scholar]
- 25.Wechsler D. Wechsler Memory Scale-Revised manual, San Antonio, TX, USA: The Psychological Corporation, 1987. [Google Scholar]
- 26.Wechsler D. WAIS-III Manual (Swedish version), New York, NY, USA: Harcourt Assessment, Inc, 2003. [Google Scholar]
- 27.Reitan RM, Wolfson D. The Halstead–Reitan Neuropsycholgical Test Battery: Therapy and clinical interpretation, Tucson, AZ, USA: Neuropsychological Press, 1985. [Google Scholar]
- 28.Geffen GM, Butterworth P, Geffen LB. Test-retest reliability of a new form of the auditory verbal learning test (AVLT). Arch Clin Neuropsychol 1994; 9: 303–316. [PubMed] [Google Scholar]
- 29.Meyers JE, Meyers KR. Rey Complex Figure test and Recognition Trial, Odessa, FL, USA: Psychological Assessment Resources, 1995. [Google Scholar]
- 30.Binetti G, Cappa SF, Magni E, Padovani A, Bianchetti A, Trabucchi M. Disorders of visual and spatial perception in the early stage of Alzheimer's disease. Ann NY Acad Sci 1996; 777: 221–225. [DOI] [PubMed] [Google Scholar]
- 31.Bandera R, Capitani E, Della Sala S, Spinnler H. Discrimination between senile dementia Alzheimer type patients and -education matched normal controls by means of a 6-test set. Italian J Neurol Sci 1985; 6: 339–344. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan E, Weintraub S. The Boston Naming Test, 2nd ed Philadelphia, PA, USA: Lea & Febiger, 1983. [Google Scholar]
- 33.Crossley M, D'Arcy C, Rawson NS. Letter and category fluency in community-dwelling Canadian seniors: a comparison of normal participants to those with dementia of the Alzheimer or vascular type. J Clin Exp Neuropsychol 1997; 19: 52–62. [DOI] [PubMed] [Google Scholar]
- 34.Lezak M, Howieson D, Bigler E, Tranel D. Neuropsychological Assessment, USA: Oxford University Press, 2012. [Google Scholar]
- 35.Della Sala S, Baddeley A, Papagno C, Spinnler H. Dual-task paradigm: a means to examine the central executive. Ann NY Acad Sci 1995; 769: 161–171. [DOI] [PubMed] [Google Scholar]
- 36.Paolo AM, Axelrod BN, Troster AI, Blackwell KT, Koller WC. Utility of a Wisconsin Card Sorting Test short form in persons with Alzheimer's and Parkinson's disease. J Clin Exp Neuropsychol 1996; 18: 892–897. [DOI] [PubMed] [Google Scholar]
- 37.Regard M. Cognitive Rigidity and Flexibility: A Neuropsychological Study, University of Victoria, 1981. [Google Scholar]
- 38.Shallice T, Evans ME. The involvement of the frontal lobes in cognitive estimation. Cortex 1978; 14: 294–303. [DOI] [PubMed] [Google Scholar]
- 39.Eckerstrom C, Olsson E, Borga M, Ekholm S, Ribbelin S, Rolstad S, et al. Small baseline volume of left hippocampus is associated with subsequent conversion of MCI into dementia: the Goteborg MCI study. J Neurol Sci 2008; 272: 48–59. [DOI] [PubMed] [Google Scholar]
- 40.Eckerstrom C, Olsson E, Klasson N, Bjerke M, Gothlin M, Jonsson M, et al. High white matter lesion load is associated with hippocampal atrophy in mild cognitive impairment. Dement Geriatr Cogn Disord 2011; 31: 132–138. [DOI] [PubMed] [Google Scholar]
- 41.Convit A, De Leon MJ, Tarshish C, De Santi S, Tsui W, Rusinek H, et al. Specific hippocampal volume reductions in individuals at risk for Alzheimer's disease. Neurobiol Aging 1997; 18: 131–138. [DOI] [PubMed] [Google Scholar]
- 42.Maller JJ, Reglade-Meslin C, Anstey KJ, Sachdev P. Sex and symmetry differences in hippocampal volumetrics: before and beyond the opening of the crus of the fornix. Hippocampus 2006; 16: 80–90. [DOI] [PubMed] [Google Scholar]
- 43.Fischl B. FreeSurfer. NeuroImage 2012; 62: 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33: 341–355. [DOI] [PubMed] [Google Scholar]
- 45.Holland CM, Smith EE, Csapo I, Gurol ME, Brylka DA, Killiany RJ, et al. Spatial distribution of white-matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging. Stroke 2008; 39: 1127–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edman A, Edenbrandt L, Freden-Lindqvist J, Nilsson M, Wallin A. Asymmetric cerebral blood flow in patients with mild cognitive impairment: possible relationship to further cognitive deterioration. Dement Geriatr Cogn Disord Extra 2011; 1: 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol 2010; 6: 131–144. [DOI] [PubMed] [Google Scholar]
- 48.Blennow K, Ricksten A, Prince JA, Brookes AJ, Emahazion T, Wasslavik C, et al. No association between the alpha2-macroglobulin (A2M) deletion and Alzheimer's disease, and no change in A2M mRNA, protein, or protein expression. J Neural Transm 2000; 107: 1065–1079. [DOI] [PubMed] [Google Scholar]
- 49.Palmqvist S, Zetterberg H, Blennow K, Vestberg S, Andreasson U, Brooks DJ, et al. Accuracy of brain amyloid detection in clinical practice using cerebrospinal fluid beta-amyloid 42: a cross-validation study against amyloid positron emission tomography. JAMA Neurol 2014; 71: 1282–1289. [DOI] [PubMed] [Google Scholar]
- 50.Blennow K, Wallin A, Agren H, Spenger C, Siegfried J, Vanmechelen E. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol 1995; 26: 231–245. [DOI] [PubMed] [Google Scholar]
- 51.Vanmechelen E, Vanderstichele H, Davidsson P, Van Kerschaver E, Van Der Perre B, Sjogren M, et al. Quantification of tau phosphorylated at threonine 181 in human cerebrospinal fluid: a sandwich ELISA with a synthetic phosphopeptide for standardization. Neurosci Lett 2000; 285: 49–52. [DOI] [PubMed] [Google Scholar]
- 52.Andreasen N, Hesse C, Davidsson P, Minthon L, Wallin A, Winblad B, et al. Cerebrospinal fluid beta-amyloid(1-42) in Alzheimer disease: differences between early- and late-onset Alzheimer disease and stability during the course of disease. Arch Neurol 1999; 56: 673–680. [DOI] [PubMed] [Google Scholar]
- 53.Edman A, Mahnfeldt M, Wallin A. Inter-rater reliability of the STEP protocol. J Geriatr Psychiatry Neurol 2001; 14: 140–144. [DOI] [PubMed] [Google Scholar]
- 54.Astrand R, Rolstad S, Wallin A. Cognitive Impairment Questionnaire (CIMP-QUEST): reported topographic symptoms in MCI and dementia. Acta Neurol Scand 2010; 121: 384–391. [DOI] [PubMed] [Google Scholar]
- 55.Rolstad S, Nordlund A, Gustavsson MH, Eckerstrom C, Klang O, Hansen S, et al. The Swedish National Adult Reading Test (NART-SWE): a test of premorbid IQ. Scand J Psychol 2008; 49: 577–582. [DOI] [PubMed] [Google Scholar]
- 56.Nordlund A, Pahlsson L, Holmberg C, Lind K, Wallin A. The Cognitive Assessment Battery (CAB): a rapid test of cognitive domains. Int Psychogeriatr 2011; 23: 1144–1151. [DOI] [PubMed] [Google Scholar]
- 57.Nystrom O, Wallin A, Nordlund A. MCI of different etiologies differ on the Cognitive Assessment Battery. Acta Neurol Scand 2014; 132: 31–36. [DOI] [PubMed] [Google Scholar]
- 58.Eckerstrom M, Skoogh J, Rolstad S, Gothlin M, Steineck G, Johansson B, et al. Sahlgrenska Academy Self-reported Cognitive Impairment Questionnaire (SASCI-Q)—a research tool discriminating between subjectively cognitively impaired patients and healthy controls. Int Psychogeriatr 2013; 25: 420–430. [DOI] [PubMed] [Google Scholar]
- 59.Olsson E, Klasson N, Berge J, Eckerstrom C, Edman A, Malmgren H, et al. White matter lesion assessment in patients with cognitive impairment and healthy controls: reliability comparisons between visual rating, a manual, and an automatic volumetrical MRI method-the Gothenburg MCI study. J Aging Res 2013; 2013: 198471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vanderstichele H, Van Kerschaver E, Hesse C, Davidsson P, Buyse MA, Andreasen N, et al. Standardization of measurement of beta-amyloid(1-42) in cerebrospinal fluid and plasma. Amyloid 2000; 7: 245–258. [DOI] [PubMed] [Google Scholar]
- 61.Olsson A, Vanderstichele H, Andreasen N, De Meyer G, Wallin A, Holmberg B, et al. Simultaneous measurement of beta-amyloid(1-42), total tau, and phosphorylated tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin Chem 2005; 51: 336–345. [DOI] [PubMed] [Google Scholar]
- 62.Bjerke M, Portelius E, Minthon L, Wallin A, Anckarsater H, Anckarsater R, et al. Confounding factors influencing amyloid Beta concentration in cerebrospinal fluid. Int J Alzheimers Dis 2010; 2010: pii: 986310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carrillo MC, Blennow K, Soares H, Lewczuk P, Mattsson N, Oberoi P, et al. Global standardization measurement of cerebral spinal fluid for Alzheimer's disease: an update from the Alzheimer's Association Global Biomarkers Consortium. Alzheimers Dement 2013; 9: 137–140. [DOI] [PubMed] [Google Scholar]
- 64.Montine TJ, Koroshetz WJ, Babcock D, Dickson DW, Galpern WR, Glymour MM, et al. Recommendations of the Alzheimer's disease-related dementias conference. Neurology 2014; 83: 851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer's Coordinating Centre. Brain 2013; 136 (Pt 9): 2697–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poggesi A, Salvadori E, Pantoni L, Pracucci G, Cesari F, Chiti A, et al. Risk and determinants of dementia in patients with mild cognitive impairment and brain subcortical vascular changes: a study of clinical, neuroimaging, and biological markers-the VMCI-Tuscany study: rationale, design, and methodology. Int J Alzheimers Dis 2012; 2012: 608013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jellinger K, Neumayer E. [Binswanger's Progressive Subcortical Vascular Encephalopathy. A Clinico-Neuropathological Study]. Arch Psychiatr Nervenkr 1964; 205: 523–554. [DOI] [PubMed] [Google Scholar]
- 68.Matsui Y, Tanizaki Y, Arima H, Yonemoto K, Doi Y, Ninomiya T, et al. Incidence and survival of dementia in a general population of Japanese elderly: the Hisayama study. J Neurol Neurosurg Psychiatry 2009; 80: 366–370. [DOI] [PubMed] [Google Scholar]
- 69.Brun A. Pathology and pathophysiology of cerebrovascular dementia: pure subgroups of obstructive and hypoperfusive etiology. Dementia 1994; 5: 145–147. [DOI] [PubMed] [Google Scholar]
- 70.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr., Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011; 7: 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol 2002; 1: 426–436. [DOI] [PubMed] [Google Scholar]
- 72.Defrancesco M, Marksteiner J, Deisenhammer E, Kemmler G, Djurdjevic T, Schocke M. Impact of white matter lesions and cognitive deficits on conversion from mild cognitive impairment to Alzheimer's disease. J Alzheimers Dis 2013; 34: 665–672. [DOI] [PubMed] [Google Scholar]
- 73.Radanovic M, Pereira FR, Stella F, Aprahamian I, Ferreira LK, Forlenza OV, et al. White matter abnormalities associated with Alzheimer's disease and mild cognitive impairment: a critical review of MRI studies. Expert Rev Neurother 2013; 13: 483–493. [DOI] [PubMed] [Google Scholar]
- 74.Selnes P, Blennow K, Zetterberg H, Grambaite R, Rosengren L, Johnsen L, et al. Effects of cerebrovascular disease on amyloid precursor protein metabolites in cerebrospinal fluid. Cerebrospinal Fluid Res 2010; 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiong YY, Mok V. Age-related white matter changes. J Aging Res 2011; 2011: 617927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mitchell AJ, Monge-Argiles JA, Sanchez-Paya J, Do CSF. biomarkers help clinicians predict the progression of mild cognitive impairment to dementia? Pract Neurol 2010; 10: 202–207. [DOI] [PubMed] [Google Scholar]
- 77.Nordlund A, Rolstad S, Gothlin M, Edman A, Hansen S, Wallin A. Cognitive profiles of incipient dementia in the Goteborg MCI study. Dement Geriatr Cogn Disord 2010; 30: 403–410. [DOI] [PubMed] [Google Scholar]
- 78.Nordlund A, Rolstad S, Klang O, Edman A, Hansen S, Wallin A. Two-year outcome of MCI subtypes and aetiologies in the Goteborg MCI study. J Neurol Neurosurg Psychiatry 2010; 81: 541–546. [DOI] [PubMed] [Google Scholar]
- 79.Roberts RO, Knopman DS, Mielke MM, Cha RH, Pankratz VS, Christianson TJ, et al. Higher risk of progression to dementia in mild cognitive impairment cases who revert to normal. Neurology 2014; 82: 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.American Psychiatric Association Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: Author 2013.
- 81.Eckerstrom C, Olsson E, Klasson N, Berge J, Nordlund A, Bjerke M, et al. Multimodal prediction of dementia with up to years follow up: the Gothenburg MCI study. J Alzheimers Dis 2014; 44: 205–214. [DOI] [PubMed] [Google Scholar]
- 82.Eckerstrom C, Olsson E, Bjerke M, Malmgren H, Edman A, Wallin A, et al. A combination of neuropsychological, neuroimaging, and cerebrospinal fluid markers predicts conversion from mild cognitive impairment to dementia. J Alzheimers Dis 2013; 36: 421–431. [DOI] [PubMed] [Google Scholar]
- 83.Nordlund A, Berggren J, Holmström A, Fu M, Wallin A. Frequent mild cognitive deficits in several functional domains in elderly patients with heart failure without known cognitive disorders. J Card Fail 2015. advance online publication, 20 April 2015; pii: S1071-9164(15)00108-6; doi: 10.1016/j.cardfail.2015.04.006 (e-pub ahead of print) PMID:25908019. [DOI] [PubMed] [Google Scholar]
- 84.Grahn A, Nilsson S, Nordlund A, Linden T, Studahl M. Cognitive impairment 3 years after neurological Varicella-zoster virus infection: a long-term case control study. J Neurol 2013; 260: 2761–2769. [DOI] [PubMed] [Google Scholar]
- 85.Pohl P, Dizdar N, Hallert E. The Ronnie Gardiner Rhythm and Music Method - a feasibility study in Parkinson's disease. Disabil Rehabil 2013; 35: 2197–2204. [DOI] [PubMed] [Google Scholar]
- 86.Wardlaw JM, Ferguson KJ, Graham C. White matter hyperintensities and rating scales-observer reliability varies with lesion load. J Neurol 2004; 251: 584–590. [DOI] [PubMed] [Google Scholar]
- 87.Petrovitch H, Ross GW, Steinhorn SC, Abbott RD, Markesbery W, Davis D, et al. AD lesions and infarcts in demented and non-demented Japanese-American men. Ann Neurol 2005; 57: 98–103. [DOI] [PubMed] [Google Scholar]


