Abstract
Hypertension (HTN) doubles the risk of Alzheimer’s disease (AD), but the mechanisms remain unclear. Amyloid-β (Aβ), a key pathogenic factor in AD, induces cerebrovascular dysfunction. We hypothesized that HTN acts in concert with Aβ to amplify its deleterious cerebrovascular effects and to increase Aβ production. Infusion of angiotensin II (ANGII; intravenously) elevated blood pressure and attenuated the cerebral blood flow (CBF) response to whisker stimulation or the endothelium-dependent vasodilator acetylcholine (ACh) (P < 0.05). Neocortical application of Aβ in mice receiving ANGII worsened the responses to ACh (P < 0.05). The cerebrovascular dysfunction observed in Tg2576 mice, in which Aβ is elevated both in blood and in brain due to expression of mutated amyloid precursor protein (APP), was not aggravated by neocortical application of ANGII or by a 2-week administration of ‘slow pressor’ of ANGII (600 ng/kg per minute; subcutaneously). In contrast, ANGII aggravated the dysfunction in TgSwDI mice, in which Aβ is increased only in brain. Slow-pressor ANGII induced microvascular amyloid deposition in Tg2576 mice and enhanced β-secretase APP cleavage. In Chinese hamster ovary (CHO) cells producing Aβ, ANGII increased β-secretase activity, Aβ1–42, and the Aβ42/40 ratio. We conclude that HTN enhances amyloidogenic APP processing, effects that may contribute to the pathogenic interaction between HTN and AD.
Keywords: β-CTF, endothelium-dependent vasodilatation, functional hyperemia, Tg2576, TgSwDI
Introduction
There is increasing evidence that Alzheimer’s disease (AD), the leading cause of dementia in the elderly, results from multiple pathogenic factors including structural and functional alterations of cerebral blood vessels.1,2,3 Thus, impairment of cerebral blood flow (CBF) and alterations in blood–brain barrier function have been observed in AD, even in the presymptomatic stages of the disease.4,5,6 Furthermore, epidemiologic and clinico-pathologic studies have suggested that vascular risk factors increase the risk of AD,7,8 and that improved vascular health may underlie the recently reported decrease in AD prevalence.9 Finally, amyloid-β (Aβ), the main constituent of amyloid plaques and a key pathogenic factor in AD, has potent effects on cerebral blood vessels resulting in disruption of critical regulatory mechanisms of the cerebral circulation, such as the increase in CBF produced by neural activity and the ability of endothelial cells to regulate microvascular flow.10,11,12 These findings have increased the awareness of the role of vascular dysfunction in AD and have raised the possibility that aggressive control of vascular risk factors may delay the onset or slow down the progression of this devastating disease.13,14
Hypertension is one of the vascular risk factors implicated in AD.8,15 Midlife HTN increases the risk for AD later in life, and HTN has been associated with increased amyloid deposition and neurofibrillary tangles, neuropathologic hallmarks of AD.16,17 Furthermore, studies in experimental models have showed that HTN increases Aβ formation and amyloid accumulation.18,19 Hypertension disrupts the structure and function of cerebral blood vessels,15 which could act in concert with the vascular effects of Aβ to aggravate cerebral perfusion and increase the susceptibility of the brain to injury. However, little is known about how HTN interacts with the vascular effects of Aβ and with the molecular mechanisms of amyloidogenesis.
In this study, we sought to examine the impact of HTN on the vascular dysfunction induced by Aβ to gain insight into whether HTN may interact synergistically with Aβ to worsen the ability of cerebral blood vessels to regulate blood flow. Furthermore, we used in vivo and in vitro approaches to examine whether HTN enhances Aβ production and amyloid accumulation. We found that, in some models, HTN aggravates the neurovascular dysfunction induced by Aβ and, at the same time, increases the cleavage of Aβ from the amyloid precursor protein (APP). The findings shed further light on the pathogenic interactions between HTN and Aβ and provide potential mechanistic bases for the increased risk of AD associated with HTN.
Materials and methods
Mice
The methods used in this study have been described in detail in previous publications from this laboratory,20,21 and are briefly summarized. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Weill Cornell Medical College and conducted in accordance with ARRIVE guidelines (http://www.nc3rs.org/ARRIVE) and Weill Cornell Medical College guidelines, which are based on the National Institutes of Health (NIH) guide for care and use of laboratory animals. Experiments were performed in male C57BL/6, Tg2576, and Tg-SwDI male mice at 3 months of age.22 In Tg2576 mice, Aβ is elevated both in plasma and in brain. However, in TgSwDI mice Aβ is increased only in brain, but to a level higher than in Tg2576 mice.22 In studies with transgenic mice, age-matched littermates were used as controls.
Osmotic Minipumps and DOCA Pellets Implantation
In some mice, osmotic minipumps containing vehicle (saline) or ANGII (600 ng/kg per minute) were implanted subcutaneously (n = 5/group) under isoflurane anesthesia. Systolic blood pressure was monitored in awake mice using tail-cuff plethysmography, as previously described.21 In other studies, mice were anesthetized by isoflurane inhalation for subcutaneous implantation of a 50-mg pellet of DOCA (21-day release, Innovative Research of America) or sham implantation.23 After recovery from anesthesia, animals were maintained on standard chow and ad libitum access to 0.9% NaCl in tap water. Control animals were maintained on standard chow and ad libitum access to tap water. After ANGII pumps or DOCA pellets implantation (14 and 21 days, respectively), the mice were anesthetized and instrumented for assessment of cerebrovascular reactivity by laser-Doppler flowmetry as described in Monitoring cerebral blood flow.
General Surgical Procedures for Cerebral Blood Flow Studies
Mice were anesthetized with isoflurane in a mixture of N2 and O2 (Induction, 5%; Maintenance, 2%). The trachea was intubated and mice were artificially ventilated with an oxygen–nitrogen mixture. The O2 concentration in the mixture was adjusted to provide an arterial pO2 (PaO2) of 120 to 130 mmHg. One of the femoral arteries was cannulated for recording mean arterial pressure (MAP) and collecting blood samples. Rectal temperature was maintained at 37°C using a thermostatically controlled rectal probe connected to a heating pad. End tidal CO2, monitored by a CO2 analyzer (Capstar-100, CWE Inc., Ardmore, PA, USA), was maintained at 2.6% to 2.7% to provide a pCO2 of 33 to 36 mmHg. After surgery, isoflurane was discontinued and anesthesia was maintained with urethane (750 mg/kg, intraperitoneally) and chloralose (50 mg/kg, intraperitoneally). Throughout the experiment, the level of anesthesia was monitored by testing corneal reflexes and motor responses to tail pinch.
Monitoring Cerebral Blood Flow
A small craniotomy (2 × 2 mm) was performed to expose the parietal cortex, the dura was removed, and the site was superfused with Ringer’s solution (37°C; pH 7.3 to 7.4; composition in mmol/L: 137 NaCl, 5 KCl, 1 MgCl2, 1.95 Na2HPO3, 15 NaHCO3, 2 CaCl2). Cerebral blood flow was continuously monitored at the site of superfusion with a laser-Doppler probe (Perimed, Ardmore, PA, USA) positioned stereotaxically on the cortical surface. The outputs of the flowmeter and blood pressure transducer were connected to a data acquisition system (PowerLab, Colorado Springs, CO, USA) and saved on a computer for off-line analysis. Ccerebral blood flow values were expressed as percentage increases relative to the resting level. Zero values for CBF were obtained after the heart was stopped by an overdose of isoflurane at the end of the experiment. Although laser Doppler flowmetry is not quantitative, it monitors relative changes in CBF quite accurately.
Experimental Protocol for Cerebral Blood Flow Experiments
After MAP and blood gases were stable (pCO2: 33 to 36 mmHg; pO2: 120 to 130 mmHg; pH: 7.3 to 7.4), the cranial window was superfused with Ringer’s solution (vehicle), and CBF responses were recorded. To minimize the confounding effects of anesthesia on vascular reactivity, the time interval between the administration of urethane–chloralose and the testing of CBF responses was kept consistent among the different groups of mice studied. The whisker-barrel cortex was activated for 60 seconds by stroking the contralateral vibrissae, and the evoked changes in CBF were recorded. The endothelium-dependent vasodilator acetylcholine (ACh; 10 µmol/L; Sigma, St Louis, MO, USA) or the smooth muscle relaxant adenosine (400 µmol/L; Sigma) was superfused on the exposed neocortex for 5 minutes. In some studies, CBF responses were tested before and after 30 to 45 minutes of superfusion with Aβ (5 µmol/L)24 or 30 to 45 minutes of intravenous infusion of ANGII (0.25 µg/kg per minute).20
Detection of Cerebral Amyloid Angiopathy
For the detection of cerebral amyloid angiopathy, mice (n = 3 per group) were perfused transcardially with phosphate-buffered saline followed by 4% paraformaldehyde in phosphate-buffered saline. Brains were removed and postfixed in the same fixative overnight at 4°C. Free-floating coronal brain sections (thickness, 40 µm) were cut through the somatosensory cortex using a vibratome. After mounting on slides and postfixation with 4% paraformaldehyde in phosphate-buffered saline for 10 minutes, brain sections were washed and labeled with 0.05% (wt/vol) thioflavine-S in 50% (vol/vol) ethanol for 10 minutes to identify cerebral amyloid angiopathy. A Leica confocal microscope was used to visualize the FITC signal associated with thioflavine-S.25
Cell Cultures and Western Blot
Chinese hamster ovary (CHO) cells overexpressing the human V717F mutant APP26 were incubated with ANGII (0.5 to 2.5 µmol/L) in serum-free medium overnight. After incubation, the cell culture medium was collected for Aβ assay by ELISA and the cells were lysed. After protein concentration determination, equal amount of proteins was loaded and separated in a 4% to 16% polyacrylamide gradient gel and transferred onto a PVDF membrane. The membrane was incubated with an antibody specific for the β-CTF (RU369).27 This is a widely used model well suited to assess quantitatively changes in APP processing.26
Amyloid-β Measurement
Brain tissues were homogenized in RIPA buffer supplemented with protease inhibitor cocktail followed by centrifugation (20,000 × g) for 15 minutes at 4°C. The supernatants were transferred to new tubes and the lysis buffer was added to the pellets and homogenized again. After centrifugation, the supernatants were combined and used to determine the SDS soluble Aβ contents. To extract SDS insoluble Aβ in the pellets, guanidine solution (5 mol/L guanidine-HCl, 50 mM Tris-Cl, pH 8.0, protease inhibitor cocktail) was added to the pellets and homogenized. After incubation in cold room overnight on a shaking platform, the solutions were centrifuged 20,000 × g for 15 minutes at room temperature. The supernatants were used to assay for SDS insoluble Aβ. Aβ40 and 42 in the culture medium were quantitatively determined by a sandwich ELISA as described27 with commercially available assay plates.
Data Analysis
Sample size was determined according to power analysis based on the previous studies published by our laboratory on CBF regulation. No animal was excluded from the study. Animals were randomly assigned to treatment and control groups and analysis was performed in a blinded manner. Data are expressed as mean ± s.e.m. Intergroup differences were analyzed using the analysis of variance with Tukey’s post-hoc analysis, as appropriate. Differences were considered statistically significant for P < 0.05.
Results
Neocortical Application of Amyloid-β Exacerbates the Neurovascular Dysfunction Induced by Acute Angiotensin II Administration
First, we examined the effect of neocortical application of Aβ in a model of acute HTN induced by intravenous administration of ANGII.28 We have previously established that the cerebrovascular effects of ANGII in this model depend on NADPH oxidase-derived reactive oxygen species (ROS) in cerebral endothelial cells.28 ANGII elevated MAP and attenuated the increase in CBF evoked by whisker stimulation or topical application of the endothelium-dependent vasodilator ACh (Figures 1A to 1C). The CBF increase induced by topical application of the smooth muscle relaxant adenosine was not affected (Figure 1D). In agreement with the previous studies,24 neocortical application of Aβ also attenuated functional hyperemia and the response to ACh, without affecting the response to adenosine. However, Aβ application in mice receiving ANGII exacerbated the attenuation of the CBF response to ACh, but not whisker stimulation (Figures 1B and 1C).
Figure 1.
Effect of angiotensin II (ANGII) intravenous (i.v.) infusion on neurovascular responses, with and without neocortical application of amyloid-β (Aβ) to the cerebral cortex. (a) ANGII infusion increases mean arterial pressure (MAP) equally in mice superfused with Aβ or vehicle. (b) Topical Aβ does not aggravate the attenuation in the cerebral blood flow (CBF) response to whisker stimulation induced by ANGII. (c) Topical Aβ aggravates the attenuation in the CBF response to acetylcholine induced by ANGII. (d) The increase in CBF produced by adenosine is not affected. *P < 0.05 from vehicle; #P < 0.05 from saline; ANOVA and Tukey’s test; n = 5 per group.
Next, we also examined the effect of topical Aβ in a model of HTN in which subpressor doses of ANGII are administered for 2 weeks using osmotic minipumps (slow-pressor HTN).21,29 In this model, the neurovascular dysfunction involves central autonomic pathways and release of vasopressin leading to increases in ROS in brain cells as well as vascular and perivascular cells.21,29 When tested separately, ANGII or Aβ attenuated the responses to whisker stimulation and ACh, but when Aβ was tested in mice treated with ANGII the cerebrovascular dysfunction was not enhanced (Figures 2A to 2D).
Figure 2.
Effect of slow-pressor angiotensin II (ANGII) hypertension on cerebral blood flow (CBF) responses, with and without topical application of amyloid-β (Aβ) to the cerebral cortex. (a) ANGII administration increases mean arterial pressure (MAP). (b and c) The increase in CBF induced by whisker stimulation (b) or acetylcholine (c) is attenuated by ANGII hypertension, but Aβ does not increase the attenuation. (d) The CBF response to adenosine is not affected. *P < 0.05 from saline vehicle; ANOVA and Tukey’s test; n = 5 per group.
Neocortical Application of Amyloid-β Exacerbates the Neurovascular Dysfunction Induced by DOCA-Salt Hypertension
Next, we sought to establish whether Aβ could enhance neurovascular dysfunction in a model of HTN independent of circulating ANGII. To this end, we used a model of DOCA-salt HTN characterized by salt and water retention, and reduced circulating levels of ANGII.23 Mice treated with the DOCA-salt protocol developed an increase in blood pressure after 7 to 14 days (Figures 3A and 3B). DOCA-salt HTN attenuated the increase in CBF induced by whisker stimulation or ACh, but did not affect the response to adenosine (Figures 3C to 3E). Neocortical application of Aβ in DOCA-salt treated mice attenuated further the response to ACh, but not whisker stimulation (Figures 3C and 3D). These data indicate that Aβ exacerbates the cerebrovascular dysfunction also in a model of HTN independent of circulating ANGII.
Figure 3.
Effect of topical amyloid-β (Aβ) on cerebrovascular regulation in DOCA-salt hypertensive mice. (a) DOCA-salt hypertension develops over 3 weeks as assessed in awake mice by tail cuff plethysmography. (b) The MAP increases measured by a femoral catheter at the time of cerebral blood flow (CBF) measurement do not differ with vehicle or topical Aβ. (c) Functional hyperemia is attenuated in DOCA-salt hypertension. Topical Aβ attenuates the response further, but the effect does not reach statistical significance. (d) The response to acetylcholine is also attenuated by DOCA salt and the effect is aggravated by topical Aβ. (e) The increase in CBF produced by adenosine was not affected. *P < 0.05 from sham; #P < 0.05 from DOCA vehicle; ANOVA and Tukey’s test; n = 5 per group. SAP, systolic arterial pressure.
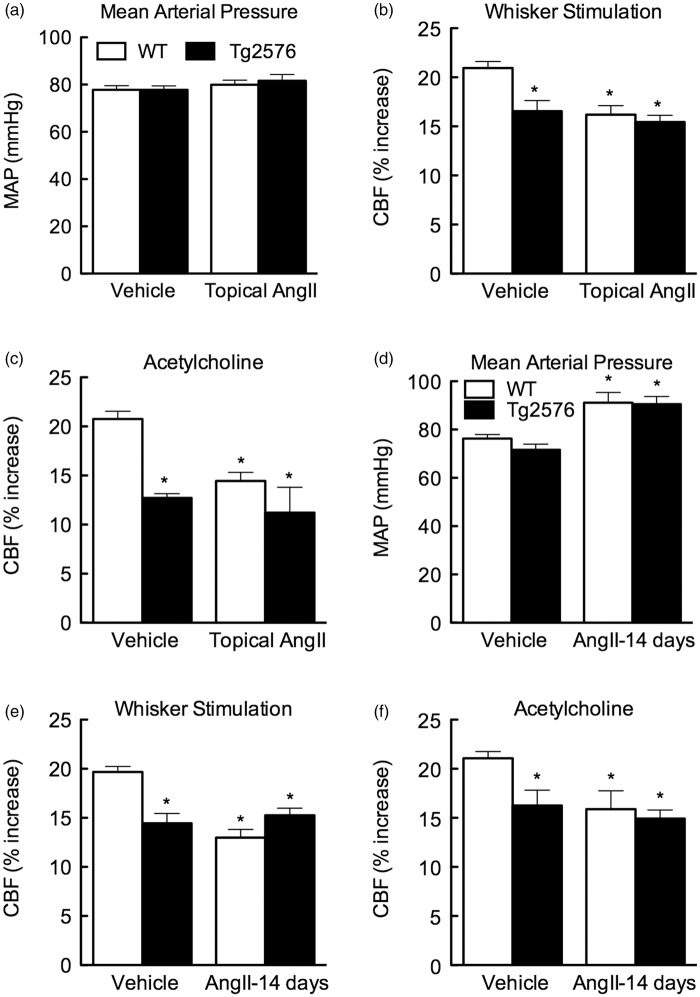
Slow-Pressor Angiotensin II Hypertension Does Not Exacerbate Cerebrovascular Dysfunction in Tg2576 Mice
In these studies, we tested whether ANGII exacerbates cerebrovascular dysfunction in Tg2576 mice, which have elevated levels of Aβ in blood and brain.22 In agreement with the previous studies, Tg2576 mice exhibited impaired responses to whisker stimulation and ACh (Figures 4A to 4F). Administration of slow-pressor doses of ANGII did not cause additive dysfunction in Tg2576 mice (Figures 4E and 4F). Similarly, neocortical application of ANGII did not aggravate the neurovascular alterations observed in these mice (Figures 4B and 4C). Therefore, ANGII did not worsen cerebrovascular dysfunction in Tg2576 mice.
Figure 4.
Effect of angiotensin II (ANGII) hypertension on neurovascular responses in Tg2576 mice. (a) Topical ANGII application to the neocortex does not alter mean arterial pressure (MAP). (b and c) Cerebral blood flow (CBF) responses to whisker stimulation (b) or acetylcholine (c) are attenuated in Tg2676 mice, but ANGII does not attenuate the responses further. (d) Slow-pressor ANGII administration increases MAP equally in wild-type (WT) and Tg2576 mice. (e and f) Responses to whisker stimulation (e) or acetylcholine (f) are not attenuated further by ANGII slow-pressor hypertension. *P < 0.05 from WT vehicle; ANOVA and Tukey’s test; n = 5 per group.
Angiotensin II Administration Worsens Cerebrovascular Dysfunction in TgSwDI Mice
Next, we examined the effects of ANGII HTN in TgSwDi mice, a model in which Aβ is elevated only in brain and not in blood.22 Cerebrovascular responses to whisker stimulation and ACh were attenuated in these mice, as previously reported (Figure 5).22 Acute administration of ANGII increased blood pressure equally in WT and TgSwDI mice, and exacerbated the increase in CBF induced by whisker stimulation, but not ACh (Figures 5E and 5F). In contrast, slow-pressor ANGII HTN did not worsen the cerebrovascular dysfunction in TgSwDI mice (Figures 5B and 5C).
Figure 5.
Effect of angiotensin II (ANGII) hypertension on cerebral blood flow (CBF) regulation in TgSwDI mice. (a) Slow-pressor hypertension increases systolic blood pressure (SBP) in TgSwDI mice. (b and c) Slow-pressor ANGII does not exacerbate the attenuation in the CBF response to whisker stimulation (b) or acetylcholine (c) in TgSwDI mice. (d) Acute ANGII intravenous (i.v.) infusion increases mean arterial pressure (MAP) equally in wild-type (WT) and TgSwDI mice. (E) ANGII aggravates the attenuation in the CBF response to whisker stimulation observed in TgSwDI mice. (f) ANGII does not aggravate the attenuation in the CBF response to acetylcholine observed in TgSwDI mice. *P < 0.05 from vehicle; #P < 0.05 from saline; ANOVA and Tukey’s test; n = 5 per group.
Angiotensin II Increases β-Secretase Cleavage of Amyloid Precursor Protein and Promotes Amyloidogenesis
Angiotensin II hypertension could also contribute to AD by promoting Aβ accumulation,19 as also reported in a model of HTN induced by aortic coartation.18 To address this issue, we examined the effect of slow-pressor ANGII HTN on amyloid pathology in Tg2576 mice at 6 months of age when amyloid deposition is not yet present.30 We found that ANGII increases amyloid deposition in cerebrovascular profiles as revealed by thioflavin-S staining (Figure 6A). To determine whether ANGII promotes amyloidogenic APP cleavage in wild-type (WT) mice, we examined the effect of subpressor ANGII HTN on the β-CTF fragment of APP, reflecting β-secretase cleavage31 and we found that ANGII increases the β-CTF (Figure 6B).
Figure 6.
Effect of angiotensin II (ANGII) hypertension on amyloid accumulation and amyloid precursor protein (APP) processing. (a) ANGII administration for 2 weeks increases vascular amyloid deposition, assessed by thioflavin-S, in 6- to 8-month-old Tg2576 mice. (b) Ang II administration for 2 weeks increases β-CTF in the neocortex of WT mice, suggesting increased APP cleavage at the β-secretase site. (c and d) Overnight ANGII treatment of Chinese hamster ovary (CHO) cells expressing mutated APP increases β-CTF dose dependently. (e) Overnight ANGII treatment of CHO cells increases Aβ 1–42 production and the Aβ 42/40 ratio, a major determinant of Aβ toxicity. *P < 0.05 from sham; ANOVA and Tukey’s test; n = 5 per group. Aβ, amyloid-β.
To provide more direct evidence of an effect of ANGII on β-secretase activity, we used CHO cells expressing a mutated form of APP and producing Aβ oligomers.26 Consistent with the findings in vivo, ANGII increases the β-CTF fragment of APP. Angiotensin II also increases Aβ1–42 and reduced Aβ1–40, resulting in a marked increase of the Aβ42/Aβ40 ratio (Figures 6C to 6E), a major determinant of Aβ toxicity.32 These observations establish that ANGII has potent effects on APP processing that promote amyloidogenesis.
Discussion
New Findings of the Study
This paper reported several novel findings. First, we found that neocortical application of Aβ exacerbates the endothelial dysfunction associated with the elevation in blood pressure caused by acute intravenous administration of ANGII, but neocortical application of Aβ did not exacerbate the vasomotor dysfunction induced by slow-pressor doses of ANGII. We also found that the HTN induced by DOCA salt is associated with neurovascular dysfunction, which is exacerbated by neocortical application of Aβ, suggesting that the deleterious interaction between HTN and Aβ occurs also in a model of HTN independent of circulating ANGII. We then examined the effect of ANGII in mouse models of endogenous Aβ overproduction. In Tg2576 mice, in which Aβ is increased both in blood and in brain, ANGII, either topically applied to the neocortex or in the slow-pressor model, failed to induce additional dysfunction. However, in TgSwDI mice, in which Aβ is increased only in brain, ANGII enhanced the dysfunction when administered acutely, but not chronically in the slow-pressor model. Finally, we explored the possibility that HTN may also promote Aβ accumulation in brain. Consistent with this hypothesis, we found that slow-pressor ANGII HTN increases Aβ deposition in cerebral blood vessels of Tg2576 mice. Furthermore, ANGII increased the β-CTF fragment of APP in WT mice, suggesting enhanced β-secretase APP processing. Supporting an effect on β-secretase activity, ANGII increased the β-CTF in CHO cells expressing mutated APP, and increased Aβ production and the Aβ42/40 ratio. These observations, collectively, provide the first demonstration that Aβ aggravates the cerebrovascular dysfunction induced by acute administration of ANGII and DOCA-salt HTN, and that ANGII is able to promote amyloidogenesis by enhancing β-secretase-dependent APP processing.
Vascular and Parenchymal Components of the Cerebrovascular Dysfunction
In wild-type mice in which both Aβ and ANGII were administered, worsening of the vascular dysfunction was observed depending on whether ANGII was administered acutely or chronically (slow-pressor model). Thus, we found that topical application of Aβ aggravates the vasomotor dysfunction induced by ANGII only in the acute administration model and not in the slow-pressor model. Since the elevation in blood pressure does not have a role in the neurovascular dysfunction induced by ANGII,28,29 it is unlikely that the difference in the effects of Aβ can be attributed to differences in the time course and magnitude of the blood pressure elevation. However, there are important differences in the mechanisms of the neurovascular dysfunction in these models of ANGII HTN. The neurovascular dysfunction induced by acute ANGII administration is mediated by a direct action of ANGII on the endothelium leading to ROS production,28 whereas the dysfunction induced by slow-pressor ANGII involves both brain tissue and endothelial generation of ROS.29 Although ROS was not measured in the present paper, one possibility is that topical application of Aβ is able to aggravate the dysfunction induced by intravenous angiotensin II by triggering ROS generation from the brain tissue. In contrast, in slow-pressor ANGII administration parenchymal ROS production is already increased and topical application of Aβ would be unable to increased ROS further. This hypothesis is supported by experiments in transgenic mice overexpressing mutated APP. In Tg2576 mice, in which Aβ is increased both in blood and in brain, ANGII was unable to worsen the dysfunction, whereas in TgSwDi mice, in which Aβ is increased only in brain, ANGII is able to aggravate the vasomotor deficit. A similar result was obtained in a study on the vascular effects of brain versus intravascular Aβ, in which intravascular administration of Aβ aggravated the cerebrovascular dysfunction in TgSwDI mice, but not in Tg2576 mice.22 These observations, collectively, support to the hypothesis that the vascular dysfunction depends on distinct endothelial and parenchymal/perivascular factors. Several lines of evidence suggest that the cross-talk between the ANGII and the AGE-RAGE system may have a role in the deleterious effects of HTN.33 Furthermore, HTN induced by aortic coartation increases Aβ deposition through oxidative stress RAGE18 highlighting the importance of the AGE-RAGE and HTN cross talk in the interaction between HTN and AD pathology.
Effects of Angiotensin II on Amyloid Precursor Protein Cleavage and Amyloid-β Production
We also found that ANGII treatment increases the cleavage of APP both in vivo and in vitro and enhances Aβ deposition in vivo. Thus, in wild-type mice ANGII increases β-CTF in brain and promotes microvascular deposition of Aβ in Tg2576 mice. In vitro, exposure to ANGII of CHO cells genetically engineered to secrete Aβ oligomers26 leads to increased β-secretase activity, as reflected by increased β-CTF. The effect was associated with elevated levels of Aβ 1–42 and an increase in the Aβ 42/40, which is a key determinant of Aβ aggregation and neurotoxicity.32,34 Therefore, ANGII is also able to enhance APP cleavage and to increase the toxic potential of Aβ by altering the Aβ 42/40 ratio. Although a link between HTN, vascular oxidative stress and amyloid was previously established,18,19 the present results show a previously unrecognized effect of ANGII on β-secretase processing of APP, which may provide the molecular bases for the effect of HTN on amyloid pathology and AD risk.8,16 However, the specific molecular mechanisms of the effects of ANGII on β-secretase cleavage remain to be determined. Possibilities, to be examined in future studies, include ANGII-induced activation of AT1 receptors and NADPH oxidase-2-dependent ROS production, which may enhance APP cleavage and Aβ production.35 Alternatively, ANGII HTN could upregulate β-secretase expression, as it has been reported to occur in hypoxia.36 However, since ANGII HTN does not increase β-secretase mRNA expression,37 this possibility seems unlikely. Furthermore, it is unclear whether the vascular dysfunction induced by Aβ and ANGII could leads to a level of brain hypoxia sufficient to promote APP β-secretase cleavage.36
Implications for Hypertension as a Risk Factor for Alzheimer's Disease
Inasmuch as ANGII has a role in human HTN38,39 and not withstanding the limitations of the short-term HTN models used in the present study, our findings suggest that HTN can influence AD pathology through different mechanisms. On the one hand, HTN could promote vascular Aβ accumulation by enhancing the vascular dysfunction induced by Aβ and reducing Aβ vascular and perivascular clearance,15 with the caveat that additive vascular effects are observed only in selected models. However, as shown here, HTN can promote Aβ production by enhancing APP cleavage at the β-secretase site. These experimental observations are in agreement with data in patients demonstrating that HTN increases the amyloid deposition assessed by amyloid imaging, an effect attenuated by antihypertensive treatment.16 Consistent with an effect of ANGII on brain Aβ homeostasis, inhibitors of the renin–angiotensin system have been shown to reduce amyloid accumulation in mouse models.40 However, randomized clinical trials have reported conflicting results on the efficacy of renin–angiotensin system inhibitors on AD progression,14 and larger clinical trials in well-characterized patients with proper cognitive assessment and longer follow-up are needed.
Conclusions
We investigated the interaction between HTN and Aβ in cerebrovascular function and the effect of ANGII HTN on APP processing. We found that acute administration of ANGII or DOCA-salt HTN aggravates the cerebrovascular effects of Aβ in WT mice or in TgSwDI mice with elevated brain levels of Aβ. Angiotensin II enhanced APP cleavage at the β-secretase site and promoted vascular Aβ accumulation in vivo. While reduced Aβ clearance secondary neurovascular dysfunction may have a role, increased β-secretase APP cleavage resulting in increased Aβ production could also be factor. Taking together, these novel data provide insight into potential mechanisms by which HTN may promote AD, and provide the rational bases for further clinical studies assessing the impact of blood pressure control on the progression of AD.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants P01-HL96571, R01-NS37853, R37-NS89323 and by a Zenith Fellow Award from the Alzheimer’s Association (ZEN202707) (CI), and by a SDG award from the American Heart Association (15SDG22760007) (GF).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
GF and CI were responsible for the study design and for drafting and revising the manuscript. GF and LP were responsible for CBF studies and related analyses. WL, JA, and PZ were responsible for the biochemical and molecular studies. WL, SP, and JA provided critically input in the final version of the manuscript.
References
- 1.Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell 2012; 148: 1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iadecola C. The pathobiology of vascular dementia. Neuron 2013; 80: 844–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zlokovic BV. Cerebrovascular effects of apolipoprotein E: implications for Alzheimer disease. JAMA Neurol 2013; 70: 440–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wierenga CE, Hays CC, Zlatar ZZ. Cerebral blood flow measured by arterial spin labeling MRI as a preclinical marker of Alzheimer's disease. J Alzheimers Dis 2014; 42: S411–S419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDade E, Kim A, James J, Sheu LK, Kuan DC, Minhas D, et al. Cerebral perfusion alterations and cerebral amyloid in autosomal dominant Alzheimer disease. Neurology 2014; 83: 710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 2015; 85: 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaffe K, Vittinghoff E, Pletcher MJ, Hoang TD, Launer LJ, Whitmer R, et al. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation 2014; 129: 1560–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorelick PB, Scuteri A, Black SE, DeCarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011; 42: 2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larson EB, Yaffe K, Langa KM. New insights into the dementia epidemic. N Engl J Med 2013; 369: 2275–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci 2004; 5: 347–360. [DOI] [PubMed] [Google Scholar]
- 11.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci 2011; 12: 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamel E. The cerebral circulation: function and dysfunction in Alzheimer's disease. J Cardiovasc Pharmacol 2015; 65: 317–324. [DOI] [PubMed] [Google Scholar]
- 13.Deschaintre Y, Richard F, Leys D, Pasquier F. Treatment of vascular risk factors is associated with slower decline in Alzheimer disease. Neurology 2009; 73: 674–680. [DOI] [PubMed] [Google Scholar]
- 14.Valenti R, Pantoni L, Markus HS. Treatment of vascular risk factors in patients with a diagnosis of Alzheimer's disease: a systematic review. BMC Med 2014; 12: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faraco G, Iadecola C. Hypertension: a harbinger of stroke and dementia. Hypertension 2013; 62: 810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodrigue KM, Rieck JR, Kennedy KM, Devous MD, Diaz-Arrastia R, Park DC. Risk factors for beta-amyloid deposition in healthy aging: vascular and genetic effects. JAMA Neurol 2013; 70: 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman LB, Schmeidler J, Lesser GT, Beeri MS, Purohit DP, Grossman HT, et al. Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons. Neurology 2009; 72: 1720–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carnevale D, Mascio G, D'Andrea I, Fardella V, Bell RD, Branchi I, et al. Hypertension induces brain beta-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension 2012; 60: 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cifuentes D, Poittevin M, Dere E, Broqueres-You D, Bonnin P, Benessiano J, et al. Hypertension accelerates the progression of Alzheimer-like pathology in a mouse model of the disease. Hypertension 2015; 65: 218–224. [DOI] [PubMed] [Google Scholar]
- 20.Faraco G, Moraga A, Moore J, Anrather J, Pickel VM, Iadecola C. Circulating endothelin-1 alters critical mechanisms regulating cerebral microcirculation. Hypertension 2013; 62: 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Capone C, Faraco G, Peterson JR, Coleman C, Anrather J, Milner TA, et al. Central cardiovascular circuits contribute to the neurovascular dysfunction in angiotensin II hypertension. J Neurosci 2012; 32: 4878–4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park L, Zhou P, Koizumi K, El JS, Previti ML, Van Nostrand WE, et al. Brain and circulating levels of Abeta1-40 differentially contribute to vasomotor dysfunction in the mouse brain. Stroke 2013; 44: 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grobe JL, Buehrer BA, Hilzendeger AM, Liu X, Davis DR, Xu D, et al. Angiotensinergic signaling in the brain mediates metabolic effects of deoxycorticosterone (DOCA)-salt in C57 mice. Hypertension 2011; 57: 600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park L, Wang G, Zhou P, Zhou J, Pitstick R, Previti ML, et al. Scavenger receptor CD36 is essential for the cerebrovascular oxidative stress and neurovascular dysfunction induced by amyloid-beta. Proc Natl Acad Sci USA 2011; 108: 5063–5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park L, Zhou J, Zhou P, Pistick R, El JS, Younkin L, et al. Innate immunity receptor CD36 promotes cerebral amyloid angiopathy. Proc Natl Acad Sci USA 2013; 110: 3089–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 2002; 416: 535–539. [DOI] [PubMed] [Google Scholar]
- 27.Bettayeb K, Oumata N, Zhang Y, Luo W, Bustos V, Galons H, et al. Small-molecule inducers of Abeta-42 peptide production share a common mechanism of action. FASEB J 2012; 26: 5115–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kazama K, Anrather J, Zhou P, Girouard H, Frys K, Milner TA, et al. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ Res 2004; 95: 1019–1026. [DOI] [PubMed] [Google Scholar]
- 29.Capone C, Faraco G, Park L, Cao X, Davisson RL, Iadecola C. The cerebrovascular dysfunction induced by slow pressor doses of angiotensin II precedes the development of hypertension. Am J Physiol Heart Circ Physiol 2011; 300: H397–H407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J Neurosci 2001; 21: 372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De SB, Vassar R, Golde T. The secretases: enzymes with therapeutic potential in Alzheimer disease. Nat Rev Neurol 2010; 6: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuperstein I, Broersen K, Benilova I, Rozenski J, Jonckheere W, Debulpaep M, et al. Neurotoxicity of Alzheimer's disease Abeta peptides is induced by small changes in the Abeta42 to Abeta40 ratio. EMBO J 2010; 29: 3408–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura K, Yamagishi S, Nakamura Y, Takenaka K, Matsui T, Jinnouchi Y, et al. Telmisartan inhibits expression of a receptor for advanced glycation end products (RAGE) in angiotensin-II-exposed endothelial cells and decreases serum levels of soluble RAGE in patients with essential hypertension. Microvasc Res 2005; 70: 137–141. [DOI] [PubMed] [Google Scholar]
- 34.Hasegawa K, Yamaguchi I, Omata S, Gejyo F, Naiki H. Interaction between A beta(1-42) and A beta(1-40) in Alzheimer's beta-amyloid fibril formation in vitro. Biochemistry 1999; 38: 15514–15521. [DOI] [PubMed] [Google Scholar]
- 35.Iadecola C, Zhang F, Niwa K, Eckman C, Turner SK, Fischer E, et al. SOD1 rescues cerebral endothelial dysfunction in mice overexpressing amyloid precursor protein. Nat Neurosci 1999; 2: 157–161. [DOI] [PubMed] [Google Scholar]
- 36.Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, et al. Hypoxia facilitates Alzheimer's disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci USA 2006; 103: 18727–18732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Csiszar A, Tucsek Z, Toth P, Sosnowska D, Gautam T, Koller A, et al. Synergistic effects of hypertension and aging on cognitive function and hippocampal expression of genes involved in beta-amyloid generation and Alzheimer's disease. Am J Physiol Heart Circ Physiol 2013; 305: H1120–H1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reudelhuber TL. Where hypertension happens. J Clin Invest 2013; 123: 1934–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coffman TM. Under pressure: the search for the essential mechanisms of hypertension. Nat Med 2011; 17: 1402–1409. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Ho L, Chen L, Zhao Z, Zhao W, Qian X, et al. Valsartan lowers brain beta-amyloid protein levels and improves spatial learning in a mouse model of Alzheimer disease. J Clin Invest 2007; 117: 3393–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]