Abstract
Objective
High concentrations of leukocytes in blood have been associated with diabetes mellitus. This prospective study aimed to explore whether total and differential leukocyte counts are associated with incidence of diabetes. A missense variant R262W in the SH2B3 (SH2B adaptor protein 3) gene, coding for a protein that negatively regulates hematopoietic cell proliferation, was also studied in relation to incidence of diabetes.
Methods and Results
Leukocyte count and its subtypes (neutrophils, lymphocytes and mixed cells) were analyzed in 26,667 men and women, 45–73 years old, from the population-based Malmö Diet and Cancer study. Information about the R262W polymorphism (rs3184504) in SH2B3 was genotyped in 24,489 subjects. Incidence of diabetes was studied during a mean follow-up of 14 years. Cox proportional hazards regression was used to examine incidence of diabetes by total and differential leukocyte counts. Mendelian randomization analysis using R262W as an instrumental variable was performed with two-stage least squares regression. A total of 2,946 subjects developed diabetes during the follow-up period. After taking several possible confounders into account, concentrations of total leukocyte count, neutrophils and lymphocytes were all significantly associated with incidence of diabetes. The adjusted hazard ratios (95% confidence interval; quartile 4 vs quartile 1) were 1.37 (1.22–1.53) for total leukocytes, 1.33 (1.19–1.49) for neutrophils and 1.29 (1.15–1.44) for lymphocytes. The R262W polymorphism was strongly associated with leukocytes (0.11x109 cells/l per T allele, p = 1.14 x10-12), lymphocytes (p = 4.3 x10-16), neutrophils (p = 8.0 x10-6) and mixed cells (p = 3.0 x10-6). However, there was no significant association between R262W and fasting glucose, HbA1c or incidence of diabetes.
Conclusions
Concentrations of total leukocytes, neutrophils and lymphocytes are associated with incidence of diabetes. However, the lack of association with the R262W polymorphism suggests that the associations may not be causal, although limitations in statistical power and balancing pleiotropic effects cannot be excluded.
Introduction
Inflammation has been repeatedly associated with diabetes mellitus, both in cross-sectional and prospective studies [1–5]. A meta-analysis of 20 prospective cohorts and cross-sectional studies found that raised leukocyte concentrations are associated with higher risk of type 2 diabetes [3]. However, results were inconsistent across studies [2–4, 6, 7], and the authors pointed out that results from the meta-analysis likely represent an overestimate due to publication bias and inability to control for potential confounders in all studies [3]. Most studies are cross-sectional and few prospective studies have examined the role of leukocytes in the development of diabetes. The Atherosclerosis Risk in Communities (ARIC) study from the USA found elevated leukocyte count to be associated with development of diabetes among 12,330 non-diabetic individuals aged 45–64 years after a mean follow-up of 7 years [2]. The National Health and Nutrition Examination Survey Epidemiologic Follow-up Study (NHEFS) found leukocyte count to be significantly and positively related to diabetes incidence with a dose-response relationship [6]. A high leukocyte count was found to predict impaired glucose tolerance and type 2 diabetes in Pima Indians [4]. In the Cardiovascular Health Study (CHS), only C-reactive protein (CRP) and not leukocyte count was associated with the development of diabetes [7].
Since gene variants are inherited and usually not subject to confounding [8], it is possible to use genetic polymorphisms as instrumental variables (IV) to explore the relationship between a trait and a certain outcome, without confounding bias from measured and unmeasured risk factors. We used a missense polymorphism (R262W, SNP rs3184504) in the SH2B adaptor protein 3 (SH2B3) gene to examine the effect of leukocyte count on diabetes. The R262W polymorphism is known to be associated with increased concentrations of leukocytes and its sub-populations [9, 10]. The SH2B3 gene codes for a protein that negatively regulates the hematopoiesis in knock-out models [11]. Loss of function of SH2B3 has been identified as a risk factor for leukemia [12].
The aim of this prospective study was to explore the relationship between total and differential leukocyte count and incidence of diabetes in an urban population.
Subjects and Methods
Study population
The population-based cohort Malmö Diet and Cancer study (MDC), from the city of Malmö in southern Sweden, was used in this study [13, 14]. In brief, all women born between 1923 and 1950 and men born between 1923 and 1945 living in Malmö city were invited to the MDC study during the period March 1991 to September 1996. A total of 30 447 individuals participated in clinical examinations at the screening center and filled in a self-administered questionnaire out of an eligible population of ≈74 000 individuals http://atvb.ahajournals.org/content/32/2/533.long-ref-17#ref-17. DNA was available for 28 767 subjects.
Subjects with history of diabetes (n = 1 311) at the baseline examination were excluded. In order to exclude individuals with severe inflammation, analyses were restricted to participants with information on total leukocyte counts less than 20.0 × 109 cells/L [15]. In addition, 888 participants without complete information on covariates were excluded. Thus, the final study population in the project consisted of 26 667 subjects (10 364 men (38.9%) and 16 303 women (61.1%), aged 45–73 years. A random subsample from the MDC cohort, the MDC cardiovascular cohort (MDC-CV, n = 6,103), was invited to take part in a study of the epidemiology of carotid artery disease between October 1991 and February 1994 [16]. The additional examinations in this sub-cohort included measurements of fasting whole blood glucose, hemoglobin A1c (HbA1c) and CRP.
The ethics committee at Lund University Lund, Sweden, approved the study (LU 51/90) and all participants provided informed written consent.
Baseline examinations
The examinations were performed by trained nurses at the screening center. Blood pressure (mmHg) was measured using a mercury-column sphygmomanometer after 10 minutes of rest in the supine position. Standing height (m) was measured with a fixed stadiometer calibrated in centimeters. Weight was measured to the nearest 0.1 kg using balance-beam scale with subjects wearing light clothing and no shoes. Body mass index (BMI) was calculated as weight (kg) divided by the square of the height (m2). Waist was measured as the circumference (cm) between the lowest rib margin and iliac crest.
Information on family history of diabetes, current use of lipid-lowering, blood pressure-lowering or anti-diabetic medications, smoking habits, leisure-time physical activity, education level and marital status were obtained from a self-administered questionnaire [13]. Family history of diabetes was defined as known diabetes in at least one first-degree relative [17]. History of myocardial infarction or stroke at the baseline examination was retrieved from the Swedish Hospital Discharge Register and the Stroke register in Malmö [18, 19]. Subjects were categorized into current smokers (i.e. those who smoked regularly or occasionally) or non-smokers (i.e. former smokers and never smokers). Low level of leisure-time physical activity was defined as the lowest quartile of a score revealed through 18 questions covering a range of activities in the 4 seasons. The evaluation of the questionnaire has been previously reported [20]. Educational level was divided into three groups: school year <9, 9–12 and > 12, respectively [21]. Marital status was categorized into married or not.
Laboratory measurements
Total and differential leukocyte counts (neutrophils, lymphocytes, and a group of mixed cell types including monocytes, eosinophils and basophils) were counted in heparinized blood samples using a SYSMEX K1000 automatic counter (Sysmex Europe, Norderstedt, Germany). The analyses were performed consecutively at the time of the screening examination, at the central laboratory of Malmö University Hospital.
HbA1c and whole blood glucose was measured according to standard procedures at the Department of Clinical Chemistry. HbA1c was measured by ion exchange chromatography, with reference values of 3.9–5.3% in non-diabetic individuals. Insulin was measured by a radioimmunoassay in mIU/ L and the HOMA index was calculated as fasting insulin*glucose/22.5 [16]. CRP was analyzed using a high-sensitive assay, Tina-quant® CRP latex assay (Roche Diagnostics, Basel, Switzerland).
Incidence of diabetes
All subjects were followed from the baseline examination until first diagnosis of diabetes, death, emigration from Sweden or December 31st, 2009, whichever came first. Cases of new-onset diabetes in the MDC cohort were identified from several sources [22, 23]. In short, incident diabetes was identified from the Malmö HbA1c register (MHR) (56% of all cases), the Swedish National Diabetes Register (NDR) (14%), the Swedish inpatient register (40%), the Swedish outpatient register (38%), the nationwide Swedish drug prescription register (65%) and the regional Diabetes 2000 register of the Skåne region (22%) [22, 23]. In addition, 44% of cases were identified at re-examinations of the cohort [24]. At least two independent sources confirmed the diagnosis for 71.6% of the cases, and 53% of cases were identified in three independent data sources. NDR and the Diabetes 2000 register required a physician´s diagnosis according to established diagnostic criteria (fasting plasma glucose concentration of > = 7.0 mmol/L, which corresponds to a fasting whole blood glucose of > = 6.1 mmol/L, measured on 2 different occasions). The MHR at the Department of Clinical Chemistry, Malmö University Hospital, analyzed and recorded all HbA1c samples taken in institutional and non-institutional care in the greater Malmö area from 1988 onwards. Individuals who had at least two HbA1c recordings > = 6.0% in the MHR with the Swedish Mono-S standardization system (corresponding to 7.0% according to the US National Glycohemoglobin Standardization Program) after the baseline examination were defined as incident diabetes cases.
Polymorphism in the SH2B3 gene
The missense polymorphism R262W (rs3184504), which previously has been associated with concentrations of leukocytes, neutrophils and lymphocytes [9, 10] was genotyped in 24,489 subjects. Based on results from previous GWAS studies of leukocyte count in subjects with European ancestry [25–27], we also tested whether polymorphisms in the 17q21 locus (rs4065321, rs3859192, rs9916158, rs4794822 and rs17609240) were useful as instrumental variables in this study. These genotypes were studied in relation to leukocyte count in a subsample of 4600 individuals, but none of the SNPs from the 17q21 locus reached p<0.5 x10-8 for the association with leukocyte count. Since we also found a significant inverse relationship between insulin resistance and the 17q21 locus, both in the literature [28] and in our data, we decided to use the R262W polymorphism only.
DNA was extracted from peripheral blood cells and assigned to batches without regard to disease status or personal identity. Batches were genotyped using real-time polymerase chain reaction (PCR) with 2.5 ng DNA as PCR template for allelic discrimination on an ABI7900HT (Life Technologies, Carlsbad, CA, USA). Genotype calls were obtained using SDS 2.3 software (Life Technologies, Carlsbad, CA, USA) with manual inspection and curation of fluorescence intensity plots. The call rate was >90%. The Hardy-Weinberg equilibrium (HWE) was calculated using an online calculator (chi-2 = 3.42, df = 2, p = 0.06).
Statistical analysis
Subjects were categorized into sex-specific quartiles of leukocyte concentrations, i.e., four groups with the same proportion of men and women in each group. One-way analysis of variance (ANOVA) and logistic regression was used to assess cross-sectional relationships of leukocyte count to diabetes risk factors. A general linear model was used to adjust glucose and HbA1c for potential confounding factors, in categories of the R262W polymorphism. Cox proportional hazards regression was used to examine hazard ratios (HR) with 95% confidence interval (CI) for incidence of diabetes by total and differential leukocyte counts (neutrophils, lymphocytes, and mixed cells) using the lowest quartile as the reference category. Time axis was follow-up time until death, emigration, incident diabetes or end of follow-up. The results were adjusted for age, sex, BMI and family history of diabetes in the basic model [17, 29]. Secondly, we also adjusted for waist circumference, systolic blood pressure, using of blood pressure- or lipid-lowering medication, history of cardiovascular disease, smoking habits, leisure physical activity, educational level and marital status. CRP was added to the covariates in an additional analysis in the MDC-CV sub-cohort. Possible interaction between leukocyte count and risk factors for diabetes was explored by introducing interaction terms in the fully adjusted multivariate model. The Kaplan-Meier curve was used to demonstrate incidence of diabetes in relation to total leukocyte count during the follow-up. The association between leukocyte count, fasting blood glucose and HbA1c, respectively, was analyzed using general linear model adjusted for age, sex and BMI. Model discrimination was estimated with Harrell’s C-statistics [30].
Sensitivity analyses were performed after excluding individuals having a cold or lung infection within two weeks before the baseline examination. We also explored the effect of non-steroid anti-inflammatory drug (NSAID) medication on the relationships between leukocytes and diabetes.
A Mendelian randomization analysis path diagram was presented in Fig 1. R262W was used as an instrumental variable for leukocyte count to study incidence of diabetes. Mendelian randomization analysis was performed with two-stage least squares regression (2SLS) using the ivreg2 command in STATA [31]. The genetic instrument was validated for association with total and differential leukocyte counts using linear or logistic regression models. A power analysis was performed for the association between R262W and incidence of diabetes. With α = 0.05, there was 64% power to detect a significant relationship, assuming a HR of 1.25 per standard deviation increment of leukocytes, i.e., the age and sex-adjusted HR in the present cohort.
Fig 1. The Mendelian randomization path diagram.
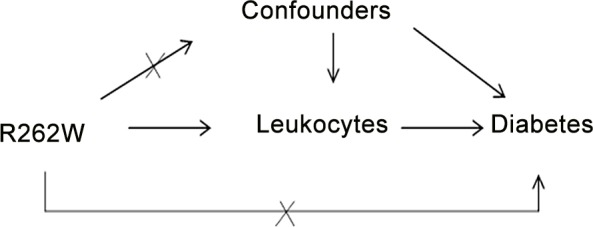
The relationship between the instrumental variable R262W, leukocytes (exposure variable), confounding factors and the outcome diabetes.
All analyses were performed using IBM SPSS statistics (version 20; IBM Svenska AB, Stockholm, Sweden) and STATA12 (Stata Corp, College Station, TX, USA).
Results
Baseline Characteristics
The mean total leukocyte count was 6.37±1.68 x109 /L, and the proportion of neutrophils, lymphocytes, and mixed cells were 61%, 31% and 8%, respectively. The relationships between sex-specific quartiles of total leukocyte count and risk factors for diabetes are presented for all subjects in Table 1, and separately for men and women in S1 Table and S2 Table. Increased leukocyte count was associated with age, BMI, waist circumference, systolic blood pressure, antihypertensive- or lipid-lowering medication, prevalent cardiovascular disease, smoking habits, low physical activity, education level and being married. In addition, HbA1c, glucose and insulin were positively and significantly associated with leukocyte count (p<0.001) in the subgroup, Table 1.
Table 1. Quartiles of total leukocytes and diabetes mellitus risk factors of participants in MDC (n = 26 667) and MDC-CV (n = 5 473).
| MDC (N = 26 667) | |||||
| Sex-specific quartiles | Q1 | Q2 | Q3 | Q4 | p |
| N (men/women) | 2575/4246 | 2548/4097 | 2712/3790 | 2529/4170 | |
| Leukocyte count, Men median (P25—P75), (109/L) | 4.70(4.20–5.20) | 5.60(5.40–5.90) | 6.60(6.40–6.90) | 8.30(7.60–9.10) | |
| Leukocyte count, Women median (P25—P75) (109/L) | 4.70(4.0–5.00) | 5.70(5.40–5.90) | 6.70(6.50–7.00) | 8.30(7.70–9.10) | |
| Sociodemographic variables | |||||
| Age (years) | 57.6±7.0 | 58.4±7.7 | 58.4±7.7 | 57.8±8.0 | <0.001 |
| Married (%) | 68.9 | 66.3 | 66.1 | 59.8 | <0.001 |
| Low education (%) | 37.8 | 41.0 | 42.3 | 45.6 | <0.001 |
| Anthropometric measurements | |||||
| Waist circumference (cm) | 81.6±15.3 | 83.5±12.6 | 85.0±16.0 | 85.0±13.2 | <0.001 |
| BMI (kg/m2) | 25.0±3.5 | 25.7±3.8 | 25.9±4.0 | 26.0±4.2 | <0.001 |
| Medical history variables | |||||
| Family history of diabetes, (%) | 2.0 | 2.0 | 1.8 | 1.7 | 0.226 |
| Prevalent cardiovascular disease (%) | 1.7 | 2.4 | 3.2 | 3.6 | <0.001 |
| Systolic blood pressure (mmHg) | 138±19 | 141±20 | 142±20 | 143±20 | <0.001 |
| Antihypertensive medication (%) | 12.2 | 15.8 | 18.4 | 21.3 | <0.001 |
| Lipid-lowing medication (%) | 2.1 | 2.9 | 3.1 | 3.2 | <0.001 |
| Lifestyle variables | |||||
| Current smoker (%) | 13.1 | 19.6 | 30.0 | 51.2 | <0.001 |
| Low physical activity (%) | 20.8 | 22.5 | 24.3 | 28.5 | <0.001 |
| MDC-CV Subcohort (n = 5 473) | |||||
| N (men/women) | 508/768 | 643/883 | 508/762 | 586/815 | |
| Leukocyte count, median, (men/women) (109/L) | 4.30/4.30 | 5.40/5.40 | 6.40/6.40 | 7.90/7.80 | |
| Glucose (mmol/L) (n = 5082) | 4.87±0.55 | 5.01±0.85 | 5.02±0.71 | 5.07±0.80 | <0.001 |
| HbA1c (%) (n = 5082) | 4.70±0.40 | 4.79±0.49 | 4.84±0.51 | 4.97±0.55 | <0.001 |
| Insulin* (mIU/l) (n = 4912) | 6.00(4.00–8.00) | 7.00(4.00–9.00) | 7.00(5.00–10.00) | 7.00(4.75–10.00) | <0.001 |
| CRP* (mg/L) (n = 4904) | 0.90(0.50–1.80) | 1.30(0.70–2.50) | 1.50(0.80–3.10) | 2.20(1.00–4.30) | <0.001 |
All values are mean±SD, unless otherwise stated.
*insulin, CRP is presented as median and interquartile limits, due to skewed distribution. P value for log-transform value.
MDC, Malmö Diet and Cancer.
Incidence of diabetes in relation to total and differential leukocyte counts
During a mean follow-up of 14 years, 2 946 subjects (1 521 men and 1 425 women, 7.87 per 1000 person-years) developed diabetes. Kaplan-Meier curves of diabetes free survival in relation to sex-specific quartiles of total leukocyte count is shown in Figs 2 and 3. Incidence of diabetes was significantly associated with total and differential leukocyte counts in the basic model 1, Table 2. After adjustment for potential confounding factors, the association remained significant for total leukocyte (4th vs 1st quartiles HR: 95% CI; 1.37; 1.22–1.53), neutrophil (1.33; 95% CI: 1.19–1.49) and lymphocyte (1.29; 95% CI: 1.15–1.44) counts, but not for mixed cells (1.04; 95% CI: 0.94–1.15, not shown in table). Male sex, family history of diabetes, high BMI and waist circumference, high systolic blood pressure, use of antihypertensive and lipid-lowering medications, current smoking and low education level were all significantly associated with incidence of diabetes in the final multivariate model. No significant interaction was observed between total leukocyte count and other risk factors for diabetes. Use of non-steroid anti-inflammatory drugs (NSAID) (n = 822) at baseline was added to the multivariable adjusted model in a sensitivity analysis, but the results were essentially unchanged. NSAID was not a significant risk factor for diabetes in the model.
Fig 2. Diabetes mellitus free survival in relation to quartiles of total leukocytes in men.
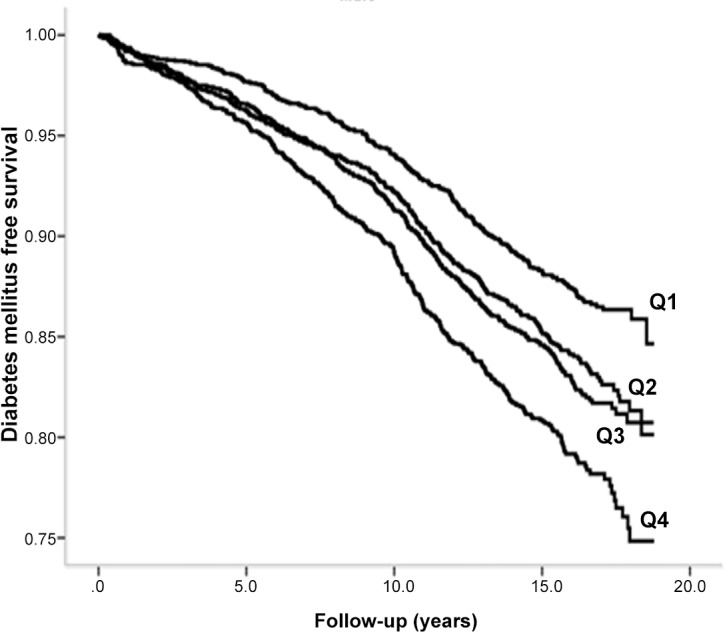
Fig 3. Diabetes mellitus free survival in relation to quartiles of total leukocytes in women.
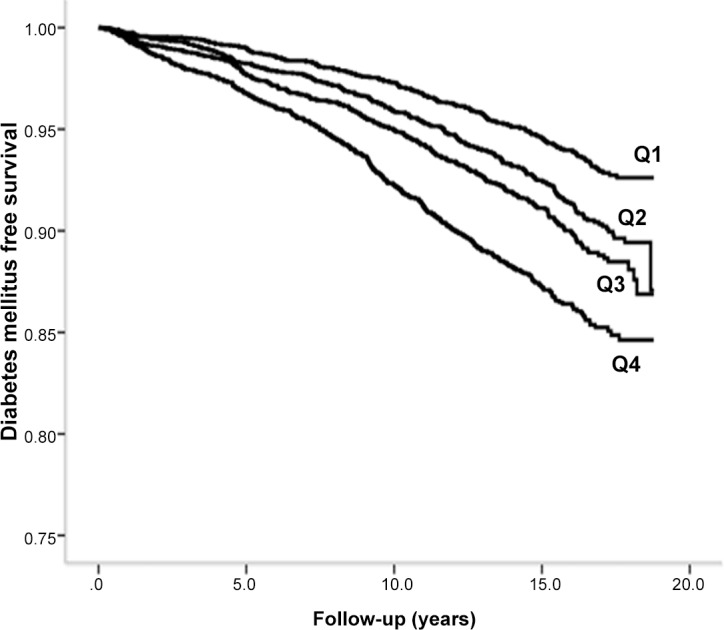
Table 2. Incidence of diabetes mellitus in relation to sex-specific quartiles of total and differential leukocyte counts in MDC cohort (N = 26 667).
| MDC (N = 26 667) | |||||
|---|---|---|---|---|---|
| Sex-specific quartiles | Q1 | Q2 | Q3 | Q4 | p for trend |
| Leukocyte count | |||||
| Incident diabetes, (men/women) (n/n) | 305/249 | 369/324 | 405/346 | 442/506 | |
| All1 | 1.00 | 1.17(1.05–1.31) | 1.26(1.13–1.41) | 1.72(1.54–1.91) | <0.001 |
| All 2 | 1.00 | 1.08(0.97–1.21) | 1.12(0.99–1.25) | 1.37(1.22–1.53) | <0.001 |
| Men 1 | 1.00 | 1.11(0.95–1.29) | 1.17(1.01–1.36) | 1.54(1.33–1.79) | <0.001 |
| Men2 | 1.00 | 1.03(0.88–1.20) | 1.05(0.90–1.23) | 1.26(1.08–1.47) | 0.003 |
| Women1 | 1.00 | 1.24(1.05–1.46) | 1.39(1.18–1.64) | 1.97(1.69–2.30) | <0.001 |
| Women2 | 1.00 | 1.14(0.97–1.35) | 1.21(1.02–1.42) | 1.53(1.30–1.79) | <0.001 |
| Neutrophils | |||||
| Incident diabetes, (men/women) (n/n) | 303/233 | 398/345 | 370/373 | 450/474 | |
| All 1 | 1.00 | 1.22(1.09–1.36) | 1.24(1.11–1.38) | 1.65(1.48–1.83) | <0.001 |
| All 2 | 1.00 | 1.15(1.02–1.28) | 1.10(0.98–1.23) | 1.33(1.19–1.49) | <0.001 |
| Men1 | 1.00 | 1.17(1.01–1.36) | 1.13(0.97–1.32) | 1.54(1.33–1.78) | <0.001 |
| Men2 | 1.00 | 1.09(0.93–1.26) | 1.01(0.87–1.18) | 1.26(1.09–1.47) | 0.008 |
| Women1 | 1.00 | 1.29(1.09–1.52) | 1.40(1.19–1.65) | 1.85(1.58–2.16) | <0.001 |
| Women2 | 1.00 | 1.22(1.03–1.44) | 1.23(1.04–1.45) | 1.46(1.24–1.73) | <0.001 |
| Lymphocytes | |||||
| Incident diabetes, (men/women) (n/n) | 284/233 | 319/370 | 442/353 | 476/469 | |
| All 1 | 1.00 | 1.10(0.98–1.24) | 1.22(1.09–1.37) | 1.46(1.31–1.63) | <0.001 |
| All 2 | 1.00 | 1.06(0.95–1.19) | 1.13(1.01–1.26) | 1.29(1.15–1.44) | <0.001 |
| Men1 | 1.00 | 1.02(0.87–1.19) | 1.12(0.96–1.30) | 1.28(1.10–1.48) | <0.001 |
| Men2 | 1.00 | 0.99(0.85–1.17) | 1.06(0.91–1.23) | 1.16(1.00–1.35) | 0.026 |
| Women1 | 1.00 | 1.20(1.02–1.41) | 1.32(1.12–1.56) | 1.70(1.45–1.99) | <0.001 |
| Women2 | 1.00 | 1.13(0.96–1.34) | 1.20(1.02–1.42) | 1.43(1.22–1.69) | <0.001 |
All values are Hazard ratio (HR) (95%CI), unless otherwise stated.
1HR adjusted for age, sex, BMI and family history of diabetes.
2HR (1) plus adjusted for waist, systolic blood pressure, blood pressure-lowering medication, lipid-lowering medication, prevalent cardiovascular disease, smoking habits, physical activities, marital status and education level. Cl, confidence interval. MDC, Malmö Diet and Cancer.
We also performed a sensitivity analysis after excluding individuals who reported having a cold or lung infection within two weeks before the baseline examination (n = 20 906). After adjustment for potential confounding factors, the association remained significant for total leukocyte (4th vs 1st quartiles HR: 1.39; 95% CI; 1.22–1.58), neutrophil (1.37; 95% CI: 1.21–1.56) and lymphocyte (1.32; 95% CI: 1.16–1.50) counts, while mixed cells remained non-significant (1.03; 95% CI: 0.92–1.16).
In the MDC-CV subcohort, 736 subjects developed diabetes during the follow-up. The HR (95% CI) for total leukocyte count (4th vs 1st quartile) was 1.95 (1.55–2.46, P for trend <0.001) in the basic model, S3 Table. The HR was reduced to 1.53 (1.20–1.95; P for trend 0.004) taking possible confounders into account and remained significant also after adding CRP into the model (HR: 1.37; 1.05–1.77; P for trend 0.044). Among the leukocyte subpopulations, only neutrophils remained significantly associated with incidence of diabetes after adjustment for CRP (HR: 1.39; 1.08–1.78, P for trend 0.022), S3 Table.
The C-statistics value for a model with age, sex and BMI was 0.717 (0.708–0.726) and increased to 0.722 (0.713–0.731) when leukocyte count was added to the model. Leukocyte count significantly improved the discriminatory value (C-statistic) for incidence of diabetes with 0.005 (0.002–0.008) (p<0.001).
Polymorphism in the SH2B3 gene
The association between the R262W polymorphism and leukocyte count is shown in Table 3. The frequency of the minor allele (T) of R262W was 48%. The T allele was strongly associated with increased leukocytes (0.11x109 cells/l per T allele, p = 1.14 x10-12, F statistics = 50.6), lymphocytes (p = 4.3 x10-16), neutrophils (p = 8.0 x10-6) and mixed cells (p = 3.0 x10-6). There was no association between the R262W polymorphism and BMI (p = 0.154), waist (p = 0.495), systolic blood pressure (p = 0.231) and use of lipid-lowering medication (p = 0.723).
Table 3. Baseline characteristics of the study population divided by alleles (CC, CT, TT) of R262W (N = 24 489).
| CC | CT | TT | P for ANOVA | |
|---|---|---|---|---|
| N (%) | 6772 (27.7) | 12074(49.3) | 5643(23.0) | |
| Leukocyte count, (109/L) | 6.3±1.7 | 6.4±2.3 | 6.5±1.9 | <0.001 |
| Sex, men, % | 38.3 | 38.6 | 39.1 | 0.680 |
| Age (years) | 58.3±7.7 | 58.0±7.7 | 58.1±7.6 | 0.049 |
| BMI (kg/m2) | 25.7±3.9 | 25.6±3.9 | 25.7±4.0 | 0.154 |
| Waist (cm) | 83.7±12.8 | 83.6±12.7 | 83.8±12.9 | 0.495 |
| Incident diabetes n (per 1000 person-year) | 762 (8.0) | 1309 (7.7) | 644 (8.2) | 0.465 |
| Glucose (mmol L−1) (n = 4964) | 5.02±0.81 | 4.99±0.71 | 4.99±0.74 | 0.562 |
| HbA1c (%) (n = 4966) | 4.84±0.54 | 4.81±0.48 | 4.81±0.47 | 0.219 |
| Insulin* (mIU/l) (n = 4798) | 6.0(4.0–9.0) | 6.0(4.0–9.0) | 6.0(4.0–9.0) | 0.067 |
Values are as means ± standard deviations or %. BMI: body mass index.
*insulin is presented as median and interquartile limits due to skewed distribution.
We found no statistically significant association between the R262W polymorphism and incidence of diabetes, fasting blood glucose or HbA1c, Table 3. These relationships remained non-significant after full adjustment for potential confounding factors in multivariable Cox regression (diabetes) or general linear models (glucose, HbA1c) (all p>0.288). For glucose, the IV estimator was -0.210 (95% CI: -0.723–0.302) (p = 0.421); for HbA1c, the IV estimator was -0.201 (-0.558–0.157) (p = 0.272). For diabetes, the IV estimator was -0.005 (-0.047–0.057)(p = 0.837).
Discussion
The present study showed a graded association between concentrations of leukocytes, neutrophils and lymphocytes and risk of developing diabetes among middle-aged subjects, taking many potential confounding factors into account. The results confirm that leukocyte count is a risk factor for incidence of diabetes. However, a missense polymorphism in the SH2B3 gene, strongly associated with leukocyte count, was not related to glucose, HbA1c or incidence of diabetes. This suggests that the relationship between leukocytes and diabetes might not be causal.
Previous studies have reported that various inflammation markers, e.g., interleukin-6, tumor necrosis factor α (TNFα) and CRP are associated with diabetes [1, 2, 32] It is believed that TNFα contributes to diabetes through its interaction with insulin signaling pathways and beta-cell function [33]. Since human granulocytes secrete TNFα, this could be a possible link between leukocyte count and diabetes [34]. A polymorphism in the IL-6 gene has been associated with total and differential white blood cell counts [35]. Since IL-6 is produced by human mononuclear cells, this suggests that IL-6 might be a common link between leukocyte count and diabetes [36]. Hence, the relationship between leukocytes and diabetes could be related to the actions of various pro-inflammatory cytokines.
Observational studies could be limited by unmeasured confounding. However, as the alleles are randomly assigned at meiosis and fixed through the lifetime, genetic association studies are usually not subject to confounding. The R262W polymorphism, which is a non-synonymous SNP located in exon 3 of SH2B3, leads to an amino acid change in the pleckstrin homology domain. SH2B3 regulates cytokine receptor-mediated signaling implicated in leukocyte activation [37, 38]. The R262W polymorphism was strongly associated with leukocyte count (F statistics value = 50.6), but we did not find any association between R262W and diabetes, glucose or HbA1c. The results indicate that the relationship between leukocytes and diabetes might not be causal. However, it should be acknowledged that the leukocyte population is highly complex with many different subpopulations [10]. It remains possible that specific populations of leukocytes could be causally associated with diabetes. In addition, even though the number of participants was high and the SNP can be considered a fairly strong instrument, it is still possible that the statistical power was too small in this study. The minor allele of the R262W polymorphism has been previously associated with increased risk of several autoimmune diseases including type 1 diabetes [39], multiple sclerosis [40], blood pressure [41] and MI [9]. Some of these disorders could increase the probability that diabetes is detected and that antihypertensive treatments are prescribed that could increase blood glucose levels. It is not possible to exclude the possibility of pleiotropic effects. However, a potential relationship between the minor allele and increased risk of type 1 diabetes, MI and hypertension should increase the risk of diabetes and cannot explain the negative results in this study.
Strength and limitations
The strength of the study was the large numbers of subjects and events during a long follow-up period. A limitation of the present study is lack of information on type of diabetes. Participants were 45–73 years old and non-diabetic at the baseline examination. It can be assumed that almost all incident cases developed type 2 diabetes, since type 1 diabetes usually has an early onset, and were excluded from analyses as prevalent cases [42]. New cases of diabetes were identified from several independent sources. The registers of out- and in-patients cover all hospital visits in the country and the pharmaceutical register covers all filled prescriptions from all pharmacies in Sweden since 2005. The HbA1c register covers the population in the city of Malmö. The relationship between leukocytes and diabetes was largely the same for each of the data sources. Diabetes can go undetected for several years and individuals that do not seek medical care will be missed. However, the coverage of the registers is very good and we have no reason to question the case validity. Lack of information about change of exposure during the follow-up (e.g. weight change, quitting smoking, new medication, etc.) was another possible limitation. A further limitation is the potential pleiotropy for the SH2B3 missense variant, which is associated with many traits. Although this would be more relevant in the context of a positive finding, it could be hypothesized that a pleiotropic effect could attenuate any association with diabetes. So-called canalization, i.e., compensatory mechanisms that counterbalance the effects of the genetic instrument, is another possibility that hypothetically could explain the negative results for the R262W polymorphism.
Although the leukocyte count significantly increased the model discrimination in terms of C-statistics, it is uncertain whether measurements of leukocytes could improve prediction of future diabetes in clinical practice. However, further studies are needed to confirm this. In conclusion, increased leukocyte counts are associated with incidence of diabetes. However, the negative findings for the R262W polymorphism suggest that the associations may not be causal, although limitations in statistical power and balancing pleiotropic effects cannot be excluded. Further studies are needed for replication of this finding in other cohorts.
Supporting Information
(DOC)
(DOC)
(DOC)
Acknowledgments
The Swedish National Diabetes Register (NDR), the Malmö HbA1c register (MHR), the Diabetes 2000 registers and the National Board of Health and Welfare are acknowledged for valuable assistance in retrieval of diabetes end-points. The study was supported by grants from the Swedish Heart and Lung foundation and the Swedish Research council.
Data Availability
The authors do not own the data underlying this study. The data have been obtained from the steering committee of the Malmö Diet and Cancer study, which coordinates the research from this database. Data are only available upon request because of confidentiality of human research subjects (legal restrictions). A file of data underlying this study is available after application to the Malmö Diet and Cancer study steering committee for the purpose of transparency of the present results (http://snd.gu.se/en/catalogue/study/610). Data manager: Anders Dahlin (e-mail: Anders.dahlin@med.lu.se). Chair of the steering committee: Olle Melander (e-mail: olle.melander@med.lu.se).
Funding Statement
The study was supported by grants from the Swedish Research Council (Dnr 2011-3891; 2014-2265), the Swedish Heart and Lung Foundation (Grant Nos. 20100244; 20130249), the Lundström Foundation and by funds from the Skåne University Hospital. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA: the journal of the American Medical Association. 2001;286(3):327–34. Epub 2001/07/24. . [DOI] [PubMed] [Google Scholar]
- 2.Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, et al. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet. 1999;353(9165):1649–52. Epub 1999/05/21. . [DOI] [PubMed] [Google Scholar]
- 3.Gkrania-Klotsas E, Ye Z, Cooper AJ, Sharp SJ, Luben R, Biggs ML, et al. Differential white blood cell count and type 2 diabetes: systematic review and meta-analysis of cross-sectional and prospective studies. PloS one. 2010;5(10):e13405 Epub 2010/10/27. 10.1371/journal.pone.0013405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vozarova B, Weyer C, Lindsay RS, Pratley RE, Bogardus C, Tataranni PA. High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51(2):455–61. Epub 2002/01/29. . [DOI] [PubMed] [Google Scholar]
- 5.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nature reviews Immunology. 2011;11(2):98–107. 10.1038/nri2925 . [DOI] [PubMed] [Google Scholar]
- 6.Ford ES. Leukocyte count, erythrocyte sedimentation rate, and diabetes incidence in a national sample of US adults. American journal of epidemiology. 2002;155(1):57–64. Epub 2002/01/05. . [DOI] [PubMed] [Google Scholar]
- 7.Barzilay JI, Abraham L, Heckbert SR, Cushman M, Kuller LH, Resnick HE, et al. The relation of markers of inflammation to the development of glucose disorders in the elderly: the Cardiovascular Health Study. Diabetes. 2001;50(10):2384–9. Epub 2001/09/28. . [DOI] [PubMed] [Google Scholar]
- 8.Ebrahim S, Davey Smith G. Mendelian randomization: can genetic epidemiology help redress the failures of observational epidemiology? Human genetics. 2008;123(1):15–33. Epub 2007/11/27. 10.1007/s00439-007-0448-6 . [DOI] [PubMed] [Google Scholar]
- 9.Gudbjartsson DF, Bjornsdottir US, Halapi E, Helgadottir A, Sulem P, Jonsdottir GM, et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nature genetics. 2009;41(3):342–7. Epub 2009/02/10. 10.1038/ng.323 . [DOI] [PubMed] [Google Scholar]
- 10.Orru V, Steri M, Sole G, Sidore C, Virdis F, Dei M, et al. Genetic variants regulating immune cell levels in health and disease. Cell. 2013;155(1):242–56. Epub 2013/10/01. 10.1016/j.cell.2013.08.041 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takaki S, Morita H, Tezuka Y, Takatsu K. Enhanced hematopoiesis by hematopoietic progenitor cells lacking intracellular adaptor protein, Lnk. The Journal of experimental medicine. 2002;195(2):151–60. Epub 2002/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez-Garcia A, Ambesi-Impiombato A, Hadler M, Rigo I, LeDuc CA, Kelly K, et al. Genetic loss of SH2B3 in acute lymphoblastic leukemia. Blood. 2013;122(14):2425–32. Epub 2013/08/03. 10.1182/blood-2013-05-500850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manjer J, Carlsson S, Elmstahl S, Gullberg B, Janzon L, Lindstrom M, et al. The Malmo Diet and Cancer Study: representativity, cancer incidence and mortality in participants and non-participants. Eur J Cancer Prev. 2001;10(6):489–99. Epub 2002/03/28. . [DOI] [PubMed] [Google Scholar]
- 14.Berglund G, Elmstahl S, Janzon L, Larsson SA. The Malmo Diet and Cancer Study. Design and feasibility. J Intern Med. 1993;233(1):45–51. Epub 1993/01/01. . [DOI] [PubMed] [Google Scholar]
- 15.Zia E, Melander O, Bjorkbacka H, Hedblad B, Engstrom G. Total and differential leucocyte counts in relation to incidence of stroke subtypes and mortality: a prospective cohort study. J Intern Med. 2012;272(3):298–304. Epub 2012/02/07. 10.1111/j.1365-2796.2012.02526.x . [DOI] [PubMed] [Google Scholar]
- 16.Hedblad B, Nilsson P, Janzon L, Berglund G. Relation between insulin resistance and carotid intima-media thickness and stenosis in non-diabetic subjects. Results from a cross-sectional study in Malmo, Sweden. Diabet Med. 2000;17(4):299–307. Epub 2000/05/23. . [DOI] [PubMed] [Google Scholar]
- 17.Enhorning S, Wang TJ, Nilsson PM, Almgren P, Hedblad B, Berglund G, et al. Plasma copeptin and the risk of diabetes mellitus. Circulation. 121(19):2102–8. Epub 2010/05/05. doi: CIRCULATIONAHA.109.909663 [pii] 10.1161/CIRCULATIONAHA.109.909663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC public health. 2011;11:450 Epub 2011/06/11. 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zia E, Hedblad B, Pessah-Rasmussen H, Berglund G, Janzon L, Engstrom G. Blood pressure in relation to the incidence of cerebral infarction and intracerebral hemorrhage. Hypertensive hemorrhage: debated nomenclature is still relevant. Stroke; a journal of cerebral circulation. 2007;38(10):2681–5. Epub 2007/09/01. 10.1161/strokeaha.106.479725 . [DOI] [PubMed] [Google Scholar]
- 20.Li C, Aronsson CA, Hedblad B, Gullberg B, Wirfalt E, Berglund G. Ability of physical activity measurements to assess health-related risks. Eur J Clin Nutr. 2009;63(12):1448–51. Epub 2009/07/30. doi: ejcn200969 [pii] 10.1038/ejcn.2009.69 . [DOI] [PubMed] [Google Scholar]
- 21.Rosvall M, Ostergren PO, Hedblad B, Isacsson SO, Janzon L, Berglund G. Occupational status, educational level, and the prevalence of carotid atherosclerosis in a general population sample of middle-aged Swedish men and women: results from the Malmo Diet and Cancer Study. American journal of epidemiology. 2000;152(4):334–46. Epub 2000/09/01. . [DOI] [PubMed] [Google Scholar]
- 22.Enhorning S, Wang TJ, Nilsson PM, Almgren P, Hedblad B, Berglund G, et al. Plasma copeptin and the risk of diabetes mellitus. Circulation. 2010;121(19):2102–8. 10.1161/CIRCULATIONAHA.109.909663 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melander O, Maisel AS, Almgren P, Manjer J, Belting M, Hedblad B, et al. Plasma proneurotensin and incidence of diabetes, cardiovascular disease, breast cancer, and mortality. JAMA: the journal of the American Medical Association. 2012;308(14):1469–75. 10.1001/jama.2012.12998 . [DOI] [PubMed] [Google Scholar]
- 24.Rosvall M, Persson M, Ostling G, Nilsson PM, Melander O, Hedblad B, et al. Risk factors for the progression of carotid intima-media thickness over a 16-year follow-up period: The Malmo Diet and Cancer Study. Atherosclerosis. 2015;239(2):615–21. Epub 2015/03/10. 10.1016/j.atherosclerosis.2015.01.030 . [DOI] [PubMed] [Google Scholar]
- 25.Crosslin DR, McDavid A, Weston N, Nelson SC, Zheng X, Hart E, et al. Genetic variants associated with the white blood cell count in 13,923 subjects in the eMERGE Network. Human genetics. 2012;131(4):639–52. Epub 2011/11/01. 10.1007/s00439-011-1103-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nalls MA, Couper DJ, Tanaka T, van Rooij FJ, Chen MH, Smith AV, et al. Multiple loci are associated with white blood cell phenotypes. PLoS genetics. 2011;7(6):e1002113 Epub 2011/07/09. 10.1371/journal.pgen.1002113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soranzo N, Spector TD, Mangino M, Kuhnel B, Rendon A, Teumer A, et al. A genome-wide meta-analysis identifies 22 loci associated with eight hematological parameters in the HaemGen consortium. Nature genetics. 2009;41(11):1182–90. Epub 2009/10/13. 10.1038/ng.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng JS, Arnett DK, Parnell LD, Lee YC, Ma Y, Smith CE, et al. Genetic variants at PSMD3 interact with dietary fat and carbohydrate to modulate insulin resistance. The Journal of nutrition. 2013;143(3):354–61. Epub 2013/01/11. 10.3945/jn.112.168401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, D'Agostino RB Sr. Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Archives of internal medicine. 2007;167(10):1068–74. Epub 2007/05/30. 10.1001/archinte.167.10.1068 . [DOI] [PubMed] [Google Scholar]
- 30.Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statistics in medicine. 1996;15(4):361–87. Epub 1996/02/28. . [DOI] [PubMed] [Google Scholar]
- 31.Baum CF, Schaffer ME, Stillman S. ivreg2: Stata module for extended instrumental variables/2SLS, GMM and AC/HAC, LIML and k-class regression. http://ideas.repec.org/c/boc/bocode/s425401.html2007. Available: http://ideas.repec.org/c/boc/bocode/s425401.html.
- 32.Nilsson J, Jovinge S, Niemann A, Reneland R, Lithell H. Relation between plasma tumor necrosis factor-alpha and insulin sensitivity in elderly men with non-insulin-dependent diabetes mellitus. Arteriosclerosis, thrombosis, and vascular biology. 1998;18(8):1199–202. Epub 1998/08/26. . [DOI] [PubMed] [Google Scholar]
- 33.Senn JJ, Klover PJ, Nowak IA, Mooney RA. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes. 2002;51(12):3391–9. Epub 2002/11/28. . [DOI] [PubMed] [Google Scholar]
- 34.Smedman C, Gardlund B, Nihlmark K, Gille-Johnson P, Andersson J, Paulie S. ELISpot analysis of LPS-stimulated leukocytes: human granulocytes selectively secrete IL-8, MIP-1beta and TNF-alpha. Journal of immunological methods. 2009;346(1–2):1–8. Epub 2009/04/11. 10.1016/j.jim.2009.04.001 . [DOI] [PubMed] [Google Scholar]
- 35.Fernandez-Real JM, Broch M, Vendrell J, Gutierrez C, Casamitjana R, Pugeat M, et al. Interleukin-6 gene polymorphism and insulin sensitivity. Diabetes. 2000;49(3):517–20. Epub 2000/06/27. . [DOI] [PubMed] [Google Scholar]
- 36.Muller-Steinhardt M, Ebel B, Hartel C. The impact of interleukin-6 promoter -597/-572/-174genotype on interleukin-6 production after lipopolysaccharide stimulation. Clinical and experimental immunology. 2007;147(2):339–45. Epub 2007/01/17. 10.1111/j.1365-2249.2006.03273.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, He X, Schembri-King J, Jakes S, Hayashi J. Cloning and characterization of human Lnk, an adaptor protein with pleckstrin homology and Src homology 2 domains that can inhibit T cell activation. Journal of immunology (Baltimore, Md: 1950). 2000;164(10):5199–206. Epub 2000/05/09. . [DOI] [PubMed] [Google Scholar]
- 38.Fitau J, Boulday G, Coulon F, Quillard T, Charreau B. The adaptor molecule Lnk negatively regulates tumor necrosis factor-alpha-dependent VCAM-1 expression in endothelial cells through inhibition of the ERK1 and -2 pathways. The Journal of biological chemistry. 2006;281(29):20148–59. Epub 2006/04/29. 10.1074/jbc.M510997200 . [DOI] [PubMed] [Google Scholar]
- 39.Reddy MV, Wang H, Liu S, Bode B, Reed JC, Steed RD, et al. Association between type 1 diabetes and GWAS SNPs in the southeast US Caucasian population. Genes and immunity. 2011;12(3):208–12. Epub 2011/01/29. 10.1038/gene.2010.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alcina A, Vandenbroeck K, Otaegui D, Saiz A, Gonzalez JR, Fernandez O, et al. The autoimmune disease-associated KIF5A, CD226 and SH2B3 gene variants confer susceptibility for multiple sclerosis. Genes and immunity. 2010;11(5):439–45. Epub 2010/05/29. 10.1038/gene.2010.30 . [DOI] [PubMed] [Google Scholar]
- 41.Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A, et al. Genome-wide association study of blood pressure and hypertension. Nature genetics. 2009;41(6):677–87. Epub 2009/05/12. 10.1038/ng.384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinology and metabolism clinics of North America. 2010;39(3):481–97. 10.1016/j.ecl.2010.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOC)
Data Availability Statement
The authors do not own the data underlying this study. The data have been obtained from the steering committee of the Malmö Diet and Cancer study, which coordinates the research from this database. Data are only available upon request because of confidentiality of human research subjects (legal restrictions). A file of data underlying this study is available after application to the Malmö Diet and Cancer study steering committee for the purpose of transparency of the present results (http://snd.gu.se/en/catalogue/study/610). Data manager: Anders Dahlin (e-mail: Anders.dahlin@med.lu.se). Chair of the steering committee: Olle Melander (e-mail: olle.melander@med.lu.se).


