Abstract
Background and Objectives
Sudden cardiac death (SCD) is a severe burden of modern medicine. Aldosterone antagonist is publicized as effective in reducing mortality in patients with heart failure (HF) or post myocardial infarction (MI). Our study aimed to assess the efficacy of AAs on mortality including SCD, hospitalization admission and several common adverse effects.
Methods
We searched Embase, PubMed, Web of Science, Cochrane library and clinicaltrial.gov for randomized controlled trials (RCTs) assigning AAs in patients with HF or post MI through May 2015. The comparator included standard medication or placebo, or both. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed. Event rates were compared using a random effects model. Prospective RCTs of AAs with durations of at least 8 weeks were selected if they included at least one of the following outcomes: SCD, all-cause/cardiovascular mortality, all-cause/cardiovascular hospitalization and common side effects (hyperkalemia, renal function degradation and gynecomastia).
Results
Data from 19,333 patients enrolled in 25 trials were included. In patients with HF, this treatment significantly reduced the risk of SCD by 19% (RR 0.81; 95% CI, 0.67–0.98; p = 0.03); all-cause mortality by 19% (RR 0.81; 95% CI, 0.74–0.88, p<0.00001) and cardiovascular death by 21% (RR 0.79; 95% CI, 0.70–0.89, p<0.00001). In patients with post-MI, the matching reduced risks were 20% (RR 0.80; 95% CI, 0.66–0.98; p = 0.03), 15% (RR 0.85; 95% CI, 0.76–0.95, p = 0.003) and 17% (RR 0.83; 95% CI, 0.74–0.94, p = 0.003), respectively. Concerning both subgroups, the relative risks respectively decreased by 19% (RR 0.81; 95% CI, 0.71–0.92; p = 0.002) for SCD, 18% (RR 0.82; 95% CI, 0.77–0.88, p < 0.0001) for all-cause mortality and 20% (RR 0.80; 95% CI, 0.74–0.87, p < 0.0001) for cardiovascular mortality in patients treated with AAs. As well, hospitalizations were significantly reduced, while common adverse effects were significantly increased.
Conclusion
Aldosterone antagonists appear to be effective in reducing SCD and other mortality events, compared with placebo or standard medication in patients with HF and/or after a MI.
Introduction
Sudden cardiac death (SCD) is defined as unexpected natural death from a cardiac cause within a short time period, generally within one hour from the onset of symptoms, in a person without any prior condition that would appear fatal [1][2]. Patients with previous myocardial infarctions (MI) or cardiac arrest or congestive heart failure (HF) were much more likely to have inducible arrhythmias, considered as a common cause of SCD [3].
The renin-angiotensin aldosterone hormone system’s (RAAS) main function is to maintain the homeostasis of arterial pressure and of extracellular fluids [4]. Dysregulation of this system leads to cardiovascular (CV) disorders including left ventricular remodeling, vasoconstriction/hypertension, and ventricular hypertrophy which may eventually result in SCD [5]. The hormonal cascade is initially induced by a decrease in blood volume which enhances renin secretion into the blood stream, resulting in the production of angiotensin II that is responsible for blood pressure increase via blood vessel constriction and the stimulation of the aldosterone hormone production. Aldosterone in its turn promotes the reabsorption of sodium and water, also leading to an increase in blood pressure [4].
Aldosterone antagonist (AA) inhibits sodium reabsorption and slightly increases water excretion [6]. This group of drugs, including spironolactone, eplerenone, and canrenone among others, is often used in managing chronic and congestive HF [7][8]. Officially, AA treatment is recommended in clinical practice at a low-dose in all patients with a left ventricular ejection fraction (LVEF) < 35% and severe symptomatic HF, i.e. currently New York Heath Association (NYHA) functional class III or IV, in absence of hyperkalemia and significant renal dysfunction, unless contraindicated or not tolerated. It is also recommended in patients suffering acute myocardial infarction (AMI) with LVEF ≤ 40% and developing HF symptoms or having a history of diabetes mellitus, unless contraindicated [9][10].
The benefits of AA in reducing the negative effects of aldosterone hence decreasing death and hospitalization in HF or AMI patients have been demonstrated in four major trials, including RALES (Randomized Aldactone Evaluation Study) [11], EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure) [12], EPHESUS (Eplerenone Post-AMI Heart Failure Efficacy and Survival Study) [13] and most currently TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist) [14].
Our study aimed to assess the efficacy of AA on SCD, hospitalization admission and several common adverse events in patients with HF or post MI.
Methods
Inclusion and exclusion criteria
We included randomized controlled trials (RCTs) comparing spironolactone or eplerenone or canrenoate potassium to placebo or standard treatment. Studies were included if they recruited patients with left ventricular dysfunction HF (NYHA class I to IV) and/or post AMI with Killip scores between I and IV and indicated at least one assessment criteria. Our meta-analysis classified these patients into two corresponding sub-categories: HF and post-MI. The included studies had to report at least one of the following outcomes: SCD, all-cause/CV mortality, all-cause/CV hospitalization and common side effects (hyperkalemia, renal function degradation and gynecomastia).
We excluded studies with a follow-up period < 8 weeks. Trials with inestimable treatment effect (no event in both arms for all criteria) and small sample size (<40 patients/arm) were excluded. The lack of double-blind and/or intention-to-treat analysis of AA efficacy was not an exclusion criterion but was re-examined by sensibility test afterwards.
Search strategy
The research was conducted systematically from Embase, Medline (Pubmed), Cochrane Library, Web of science and clinicaltrials.gov from 1966 to 31/05/2015 (details of search strategy in S1 App). We searched for studies involving human subjects, clinical trials, RCTs and/or meta-analyses and/or systematic reviews. No language restriction was applied. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [15] were followed (S2 App).
Study screening and analyzing through titles and abstracts was performed independently by several investigators in different periods (HHL, MM, CK, TA, FG), according to the pre-specified selection criteria. Data were extracted independently and compared afterwards. The latest screening and data extraction (through May 2015) were conducted independently by two investigators (HHL & MM) with kappa statistics (S3 App). Cochrane bias criteria [16] were used to evaluate the overall quality of the articles. An included trial was considered as of high quality if all its risks of bias were low. Disagreements were discussed and decisions were made through consensus. A third party (FG) was involved when necessary. The following information was extracted from the studies: the first author or study name, year of publication, baseline patient characteristics, intervention and related outcomes. Besides database searching, reference lists of all included studies, meta-analyses and reviews were manually searched for further potential trials and/or information validation.
Outcomes assessment
The primary endpoints were SCD, total mortality and CV mortality at the end of the follow-up duration. Secondary outcomes were hospitalization (from all causes and CV causes) and adverse reaction events (hyperkalemia, renal function degradation and gynecomastia) by AAs.
Statistical analysis
Kappa statistic was calculated for agreement ratio between two latest reviewers (HHL & MM) (S3 App). We extracted aggregate data, number of events and number of patients in each subgroup from included studies, using fixed-effect and random-effect models to pool the data. Results were reported as relative risk (RR) at 95% confidence intervals (CI) using the Mantel and Haenszel method for the fixed-effect model [17] or the DerSimonian and Laird method for the random-effect model [18]. When similar outcomes were obtained by both methods, we only reported the random-effect results to cover possible heterogeneity as several pharmacologic drugs and different patients were included.
Heterogeneity across studies was estimated using I2 test [18]. I2 values of 25%, 50%, and 75% correspond to low, moderate, and high levels of heterogeneity [19]. Meta-analysis results were considered only if the I2 value was below 75%. Potential existence of publication bias was assessed in both subgroups at each criterion of outcome by funnel plots and verified by the Egger tests [20] using odds ratio (OR) since firm guidance for RR is not yet available [21]. Sensitivity analysis was carried out for each outcome measure to evaluate the contribution of each study to the pooled estimate by excluding important trials/ lack of blinding trials/ lack of intention-to-treat analysis trials at one time and recalculating the combined RR for the remaining studies. Statistical testing was two-tailed, with statistical significance declared at 5%. All analyses were performed using RevMan (version 5.3) and R (version 3.2.2) softwares.
Results
Search results
Our search through Embase, Medline (Pubmed), Cochrane Library, Web of science, clinicaltrials.gov and other sources (www.clinicaltrialsregister.eu & www.trialdetails.com) returned a total of 3653 studies. After elimination of duplicates, 3143 studies were retained for evaluation. Through screening of titles and abstracts, 2644 and 320 irrelevant studies were respectively excluded, respectively. Following full manuscript review of the remaining 80 studies, 54 additional ones were excluded: full-text not available (n = 10) (correspondences to authors were made but we have not received positive responses), study period <8 weeks (n = 8), review, editorial commentary or study design (n = 8), sub-study (n = 3), not RCT (n = 5), and outcomes of interest not available (n = 21). Finally, 25 studies satisfying all selection criteria were included in this meta-analysis (Fig 1). The kappa statistic indicated a subtidal agreement good at 0.75 (IC 95% CI, 0.49–1.02; p = 0.0005) (S3 App).
Fig 1. Study flowchart for the selection process of the final included trials.
The quality of evidence of included studies was relatively high: 100% of low risk for selection, attrition and reporting biases, 70% of low risk for performance bias and >85% of low risk for detection bias (S1 Fig).
Study characteristics
In total, 25 RCTs [11],[13–14],[22–32],[33–43] were selected in this meta-analysis, which enrolled a total of 19333 patients (9750 for AA arm and 9583 for control/placebo arm). The mean follow-up duration was 12.42 months (1.04 year). All trials were placebo controlled except three trials [22][23][24] which applied routine treatment. Nine trials [25][26][27][28][13][29][30][31][24] assessed the effect of AAs in post-AMI patients with left ventricular dysfunction; while the other trials recruited HF patients. Duration of follow-up varied from 3 to 44 months. Spironolactone was the most commonly used AAs (15 studies), followed by eplerenone (7 studies) and canrenone (3 studies) (Table 1). The risk of bias of included trials was presented in S1 Table and S1 Fig.
Table 1. Main characteristics of included studies.
| Studies, (abbreviation name), year of publication | Patients; duration (follow-up); countries | Comparison | Study design, intention to treat analysis (ITTA) | Number of randomized patients (excluded during follow-up) | Mean age (SD) | Male sex (%) | Ischemic etiology (%) | Ejection fraction (%) |
|---|---|---|---|---|---|---|---|---|
| Boccanelli et al. 2009 (AREA-in-HF) [35] | HF; 12 months; Italy | Canrenone 25 mg (titrated to 50 mg/day) vs. Placebo | DB; without ITTA | 231(43)/ 236(42) | 62.3(9.5)/ 62.7(9.5) | 82/85 | 51.1/ 52.1 | 39.9(8.6)/ 39.7(8.6) |
| Chan et al. 2007 [56] | HF; 12 months; China | Spironolactone 25 mg/day + candesartan vs. Placebo + candesartan | DB; with ITTA | 23(0)/25(0) | 61.4(12.3)/ 65(0.6) | 87/80 | 47.8/ 64.0 | 26(2)/28(2) |
| Cicoira et al. 2002 [22] | HF; 12 months; Italy | Spironolactone 25 mg (titrated to 50 mg/day) vs. Routine treatment | Open label, without ITTA | 54(7)/52(6) | 62.5(7.9)/ 61.7(9.8) | 85/88 | 65/63 | 33(7)/34(7) |
| Deswal et al. 2011(RAAM-PEF) [36] | HF; 6 months; USA | Eplerenone 25 mg (titrated to 50 mg/day) vs. Placebo | DB; without ITTA | 23(2)*/23(0)* | 72.2(9.8)*/ 68.7(9.1)* | 95*/91* | NR/NR | 62.1(5)*/ 62.5(7.5)* |
| Di Pasquale et al. 2005 [25] | MI; 6 months; Italy | Canrenoate IV 1 mg/h then 25 mg PO/day + Captopril vs. Placebo + Captopril | DB; without ITTA | 341(33)/ 346(30) | 62.6(6)/ 62.8(5) | 71/71 | 100/100 | NR/NR |
| Edelmann et al. 2013 (Aldo-DHF) [32] | HFPEF; 12 months; Germany &Austria | Spironolactone 25 mg/day vs. Placebo | DB; with ITTA | 213(0)/209(0) | 67(8)/67(8) | 48/47 | NR/NR | 67(8)/68(7) |
| Gao et al. 2007 [57] | HF; 6 months; China | Spironolactone 20 mg/day vs. Placebo | DB, with ITTA | 58(0)/58(0) | 55(13)/54(12) | 64/66 | 50/52 | 42(11)/43(10) |
| Kayrak et al. 2010 [26] | AMI; 6 months; Turkey | Spironolactone 25 mg/day vs. Routine treatment | Open label, without ITTA | 71(16)/71(16) | 55.3(10)*/ 57.2(11)* | 18*/26* | 100/100 | 50.5(8.3)*/ 49.5(8)* |
| Mak et al. 2009 [23] | DHF; 12 months; Ireland | Eplerenone 25 mg (titrated to 50 mg/day) vs. Routine treatment | Open label, without ITTA | 24(1)/20(3) | 80(7.7)/ 79(7.9) | 38/55 | NR/NR | 63(9.0)/64(9.6) |
| Modena et al. 2001 [27] | MI; 12 months; Italy | Potassium canrenoate 50 mg/day vs. Placebo | NR, with ITTA | 24(0)/22(0) | 59(10)/62(13) | 71/77 | 100/100 | 47(6)/46(5) |
| Montalescot et al. 2014 (REMINDER)[28] | MI; 10.5 months; International (11 countries) | Eplerenone 25 mg (titrated to 50 mg/day) vs. Placebo | DB; with ITTA | 506(82)/506(79) | 58.5(10.8)/ 57.8(11.0) | 83/80 | 100/100 | NR/NR |
| Pitt et al. 2014 (TOPCAT) [14] | HF; 3.3 years; International (6 countries) | Spironolactone (15 to 45 mg/day) vs. Placebo | DB; with ITTA | 1722(0)/ 1723(0) | 68.7(median) range 61.0–76.4/ 68.7(median) range 60.7–75.5 | NR/NR | NR/NR | 56(median) range 51–61/ 56(median) range 51–62 |
| Pitt et al. 2003 (EPHESUS) [13] | LVD after MI; 16 months (range 0–33); International (37 countries) | Eplerenone 25 mg (titrated to 50 mg/day) vs. Placebo | DB; with ITTA | 3319(0)/ 3313(0) | 64(11)/64(12) | 72/70 | 100/100 | 33(6)/33(6) |
| Pitt et al. 1999 (RALES) [11] | HF; 24 months; International (15 countries) | Spironolactone 25 mg (titrated to 50 mg/day) vs. Placebo | DB; with ITTA | 822(0)/841(0) | 65(12)/65(12) | 73/73 | 55/54 | 25.6(6.7)/ 25.2(6.8) |
| Taheri et al. 2012 [37] | CHF; 6 months; Iran | Spironolactone 25 mg/day vs. Placebo | DB; without ITTA | 9(2)/9(3) | 50.7(17.4)/ 57.2(13.1) | 55/55 | NR or 0/ NR or 0 | 26.6(8.3)/ 31.1(10.5) |
| Taheri et al. 2009 [38] | HF; 6 months; Iran | Spironolactone 25 mg/day vs. Placebo | DB; without ITTA | 8(3)/8(2) | 59.5(6.5)/ 56.8(9.3) | 63/75 | NR or 0/ NR or 0 | 31.3(8.7)/ 33.8(9.2) |
| The RALES Investigators [58] | HF; 3 months; International | Spironolactone 12.5, 25, 50, 75 mg/day (4 groups) vs. Placebo | DB; with ITTA | 174(0)/40(0) | 63/61(12) | 79/83 | NR/NR | NR/NR |
| Udelson et al. 2010 [39] | HF; 9 months; USA (multicenter) | Eplerenone 25 mg (titrated to 50 mg/day) vs. Placebo | DB; without ITTA | 117(13)/109(20) | 63.3(12.2)/ 62.0(12.9) | 84/84 | 60/61 | 26.2(0.6)/ 27.0(0.6) |
| Uzunhasan et al. 2009 [29] | AMI; 6 months; Turkey | Spironolactone 50 mg/day vs. Placebo | DB; with ITTA | 41(0)/41(0) | 52(10)/52(10) | 79/71 | NR/NR | 47/44 |
| Vatankulu et al. 2013 [30] | AMI; 6 months; Turkey | Spironolactone 12.5 & 25 mg/day (2 groups) vs. Routine treatment | Open label; with ITTA | 104(0)/56(0) | 56/57(11) | 84/80 | 100/100 | NR/NR |
| Vizzardi et al. 2013 [34] | CHF; 44 ± 16 months; Italy | Spironolactone 25 mg (titrated to 100 mg/day) vs. Placebo | SB; without ITTA | 65(5)/65(1) | 61(14.7)/ 65(17.4) | NR/NR | NR/NR | 34.5(6.8)/ 37.7(11) |
| Vizzardi et al. 2010 [59] | HF; 6 months; Italy | Spironolactone 25 mg (titrated to 100 mg/day) vs. Placebo | SB; with ITTA | 79(0)/79(0) | 61(13)/58(13) | 84/82 | NR/NR | 35.2(0.7)/ 35.4(1.0) |
| Weir et al. 2009 [31] | MI; 5.5 months; UK | Eplerenone 25 mg (titrated to 50 mg/day) vs. Placebo | DB; without ITTA | 50(4)/50(3) | 61.0 12.0)*/ 56.8(12.0)* | 74*/80* | 100/100 | 35.2(3.9)*/ 32.3(4.8)* |
| Wu et al. 2013 [24] | AMI; 12 months; China | Spironolactone 20 mg/day vs. Routine treatment | Open label; without ITTA | 308(46)/308(42) | 59.8(11.7)*/ 59.9(10.3)* | 74*/72* | 100/100 | NR/NR |
| Zannad et al. 2011 (EMPHASIS-HF) [33] | HF; 21 months; International | Eplerenone 25 mg (titrated to 50 mg/day) vs. Placebo | DB; with ITTA | 1364(0)/ 1373(0) | 68.7(7.7)/ 68.6(7.6) | 77/78 | 70/68 | 26.2(4.6)/ 26.1(4.7) |
The results are shown according to the mean (SD), except for additional explanation in exceptional cases. BD: double blind; ITTA: intention to treat analysis; HF: Heart failure; DHF: Diastolic heart failure; CHF: congestive heart failure; HFPRE: Heart failure with preserved ejection fraction; MI: Myocardial infarction; LVD: Left Ventricular Dysfunction; IV: Intra-venous; DB: Double blind; SB: Single blind; NR: not reported; AREA-in-HF: Aldosterone Receptor Antagonists improve outcome in severe Heart Failure; RAAM-PEF: Randomized Aldosterone Antagonism in Heart Failure with Preserved Ejection Fraction; Aldo-DHF: Aldosterone Receptor Blockade in Diastolic Heart Failure; TOPCAT: Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist; EPHESUS: Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study; RALES: Randomized Aldactone Evaluation Study; EMPHASIS-HF: Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure.
(*) For only the patients included in final analyses.
Baseline patient characteristics
Most trials included elderly people with mean age ranged from 50–80 years (Table 1). Most of studies consisted dominantly male participants, except two trials [26][23] where more women were recruited and the trial of Edelmann et al. [32] which had a relatively equal sex ratio. All trials were restricted to patients without renal dysfunction (kalemia <5.5 mmol/l and creatinine < 2.5 mg/dL) (Table 2).
Table 2. Main criteria for patients’ eligibility in the included studies.
| Studies | NYHA class | Killip class | Creatinine (mg/dL or other units) | Serum potassium (mmol/L) | Ejection fraction (%) |
|---|---|---|---|---|---|
| Boccanelli et al. 2009 (AREA-in-HF) [35] | II | NR | ≤2.5 | ≤5.0 | ≤45 |
| Chan et al. 2007 [56] | II to III | NR | ≤200 μmol/l | ≤5.0 | <40 |
| Cicoira et al. 2002 [22] | NR | NR | ≤150 μmol/l | ≤5.0 | ≤45 |
| Deswal et al. 2011(RAAM-PEF) [36] | II to III | NR | ≤2.5 | ≤5.0 | ≥50 |
| Di Pasquale et al. 2005 [25] | NR | I to II | <2.0 | <5.0 | NR |
| Edelmann et al. 2013 [32] | II to III | NR | NR | <5.1 | ≥50 |
| Gao et al. 2007 [57] | II to IV | NR | <2.5 | <5.5 | <45 |
| Kayrak et al. 2010 [26] | NR | I to II | ≤2.0 | ≤5.0 | ≥40 |
| Mak et al. 2009 [23] | IV | NR | ≤200 μmol/l | NR | ≥45 |
| Modena et al. 2001 [27] | NR | I to III | ≤2.5 | NR | NR |
| Montalescot et al. 2014 (REMINDER) [28] | NR | NR | ≤2.5 | NR | ≤40 |
| Pitt et al. 2014 (TOPCAT) [14] | I to IV | NR | <2.5 | ≤5.0 | ≥45 |
| Pitt et al. 2003 (EPHESUS) [13] | I to IV | NR | ≤2.5 | ≤5.0 | ≤40 |
| Pitt et al. 1999(RALES) [11] | III to IV | NR | ≤2.5 | ≤5.0 | ≤35 |
| Taheri et al. 2012 [37] | III to IV | NR | NR | <5.5 | ≤45 |
| Taheri et al. 2009 [38] | III to IV | NR | NR | ≤5.5 | ≤45 |
| The RALES Investigators [58] | III to IV | NR | ≤2.0 | <5.5 | ≤35 |
| Udelson et al. 2010 [39] | II to III | NR | NR | ≤5.5 | ≤35 |
| Uzunhasan 2009 [29] | NR | I to II | <2.5 | ≤5.0 | NR |
| Vatankulu et al. 2013 [30] | NR | I to II | ≤2.0 | <5.5 | ≥40 |
| Vizzardi et al. 2013 [34] | I to II | NR | NR | ≤5.0 | <40 |
| Vizzardi et al. 2010 [59] | I to II | NR | ≤2.5 | ≤5.0 | ≤40 |
| Weir et al. 2009 [31] | NR | I to II | ≤2.5 | ≤5.0 | <40 |
| Wu et al. 2013 [24] | NR | I to III | ≤2.5 | ≤5.0 | NR |
| Zannad et al. 2011 (EMPHASIS-HF) [33] | II | NR | NR | ≤5.0 | ≤35 |
NYHA: New York Heath Association; ND: Not Defined; NR: Not Reported; 221 μmol/l ~ 2.5 mg/dL.
Primary outcomes
Sudden cardiac death
In the 25 included articles, six accounting for 8301 subjects (4132 used AAs and 4169 received placebo/control) reported SCD events in patients with HF. In the follow-up duration, the SCD rate in HF patients was 4.89% (n = 202/4132) in those treated with AAs, compared with 6.09% (n = 254/4169) in those treated with placebo/control. In post-MI patients, SCD was reported only in the EPHESUS trial [13] at the rates of 4.88% (n = 162/3319) and of 6.07% (n = 201/3313) in groups receiving AAs and placebo, respectively.
There was a significant reduction of SCD rate with AAs in patients with HF (19% SCD reduction; RR 0.81; 95% CI, 0.67–0.98; p = 0.03) or with post-MI left ventricular dysfunction (20% SCD reduction; RR 0.80; 95% CI, 0.66–0.98; p = 0.03). In total, the SCD rate was 4.88% (n = 364/7451) in those treated with AAs compared with 6.08% (n = 455/7482) in those treated with placebo/control (19% SCD reduction; RR 0.81, 95% CI, 0.71–0.92; p = 0.002) without any evidence of statistical heterogeneity (I2 = 0%) (Fig 2A).
Fig 2. Efficacy of aldosterone antagonist compared with control for the prevention of (A) Sudden death, (B) All-cause mortality, and (C) Cardiovascular death in patients with heart failure or myocardial infarction.
All-cause mortality
All-cause mortality rate in patients with HF were 16.21% (n = 729/4496) in those treated with AAs and 19.96% (n = 903/4523) in those assigned to placebo/control (RR 0.81, 95% CI, 0.74–0.88, p<0.00001) through the follow-up duration. The corresponding numbers in the sub-group of MI were 11.64% (n = 519/4460) and 13.71% (n = 611/4457), respectively, with 15% reduction (RR 0.85; 95% CI, 0.76–0.95, p = 0.003). Altogether, there were 1248/8956 (13.93%) and 1514/8980 (16.86%) deaths from all causes, respectively, observed in treatment and placebo arms with a general reduction rate of 18% (RR 0.82; 95% CI, 0.77–0.88, p<0.00001). Heterogeneity was not found in each sub-group (consisting 10 and 8 trials, respectively) and in the whole population (all I2 = 0%) (Fig 2B).
Cardiovascular mortality
In the follow-up duration, CV mortality rate was 17.03% (n = 541/4205) in those treated with AAs and 22.54% (n = 697/4234) in those received placebo in the HF subgroup, resulting in a reduction rate of 21% (RR 0.79; 95% CI, 0.70–0.89, p<0.00001). In the MI subgroup, the efficacy of AAs was demonstrated by a reduction of 17% (RR 0.83; 95% CI, 0.74–0.94, p = 0.003) of CV mortality in treated patients compared with those receiving placebo (431/4166 vs 517/4165 deaths, respectively). AAs contributed a general reduction of 20% for the two categories of patients (RR 0.80; 95% CI, 0.74–0.87, p<0.00001) (Fig 2C).
Generally, there were likely no heterogeneity found in SCD, all-cause mortality and CV mortality (all I2 = 0%), regarding both categories of patients.
Secondary outcomes
All-cause hospitalization
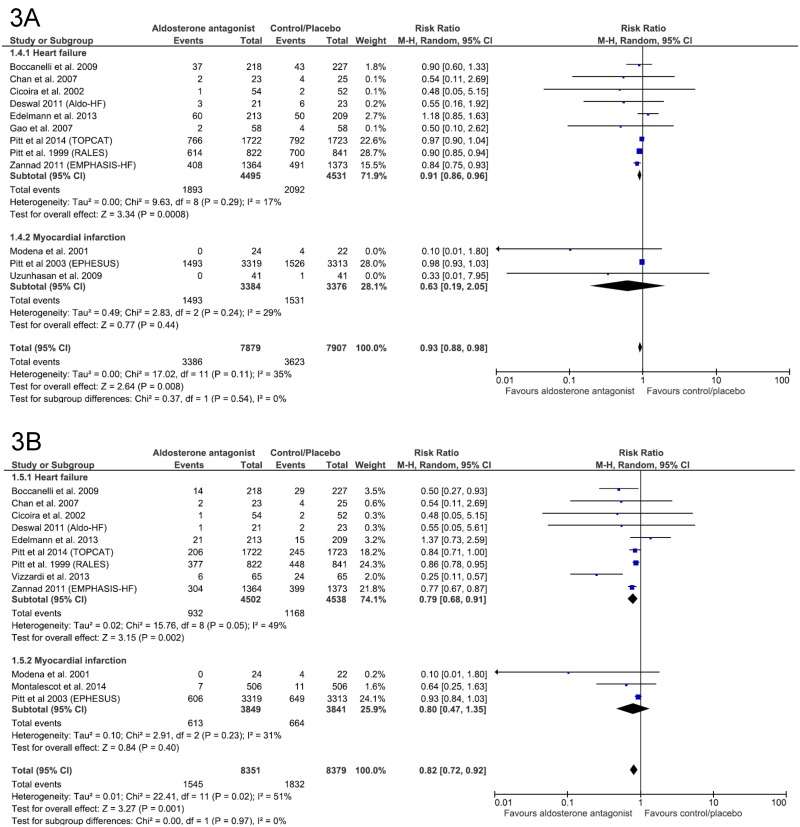
Relative risk reductions in all-cause hospitalization rate by AAs compared with placebo/control were 9% in HF patients (RR 0.91; 95% CI, 0.86–0.96; p = 0.0008) and 37% in post-MI patients (RR 0.63; 95% CI, 0.19–2.05; p = 0.44). In overall analysis, the results showed a significant decrease of 7% of all–cause hospitalization in patients receiving AAs compared with those taking placebo/control (RR 0.93; 95% CI, 0.88–0.98; p = 0.008) (Fig 3A). However, heterogeneity was likely considerable (I2 = 17%, 29% and 35% respectively).
Fig 3. Efficacy of aldosterone antagonist compared to control for the prevention of (A) All-cause hospitalization and (B) Cardiovascular hospitalization in patients with heart failure or myocardial infarction.
Cardiovascular hospitalization
In patients with HF, a significant relative risk reduction of 21% for CV hospitalization was observed in those assigned to AAs, compared with placebo/control (RR 0.79; 95% CI, 0.68–0.91; p = 0.002). In patients with MI, the corresponding value was 20% but not significant (RR 0.80; 95% CI, 0.47–1.35; p = 0.44). An analysis for both subgroups showed a relative risk reduction of 18% (RR 0.82, 95% CI, 0.72–0.92; p = 0.001) (Fig 3B). However, heterogeneity detected was moderate (I2 = 49%, 31% and 51% respectively).
Adverse reactions
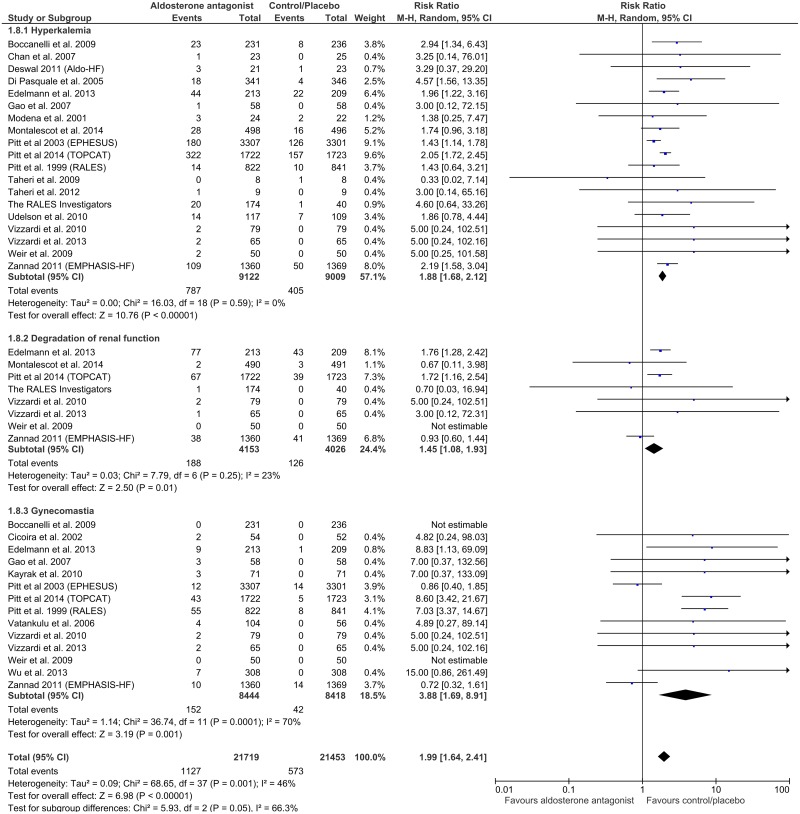
Hyperkalemia, worsening renal function and gynecomastia were the main observed side effects of AAs in the 25 included studies, as compared to placebo or control. In general, the incidence of all considered adverse events significantly doubled in patients treated with AAs, compared to those receiving placebo or reference therapy. Corresponding RRs were 1.88 (Cl 95%, 1.68–2.12, p<0.00001) for hyperkalemia; 1.45 (CI 95%,1.08–1.93, p = 0.01) for degradation of renal function; 3.88 (CI 95%, 1.69–8.91, p = 0.001) for gynecomastia and 1.99 (95% CI, 1.64–2.41; p<0.00001) for all considered side-effects in general, with remarkably various heterogeneities found among the subgroups (0%, 23%, 70% and 46% respectively) (Fig 4). Exceptions appeared for the two big RALES and EMPHASIS-HF trials [11][33], where interestingly enough, patients in the placebo groups had slightly higher rate of gynecomastia (RALES and EMPHASIS-HF) and of renal function degradation (EMPHASIS-HF).
Fig 4. Incidences of adverse effects (hyperkalemia, degradation of renal function and gynecomastia) under aldosterone antagonist treatment, compared with control/placebo group, in patients with heart failure or myocardial infarction.
Publication bias
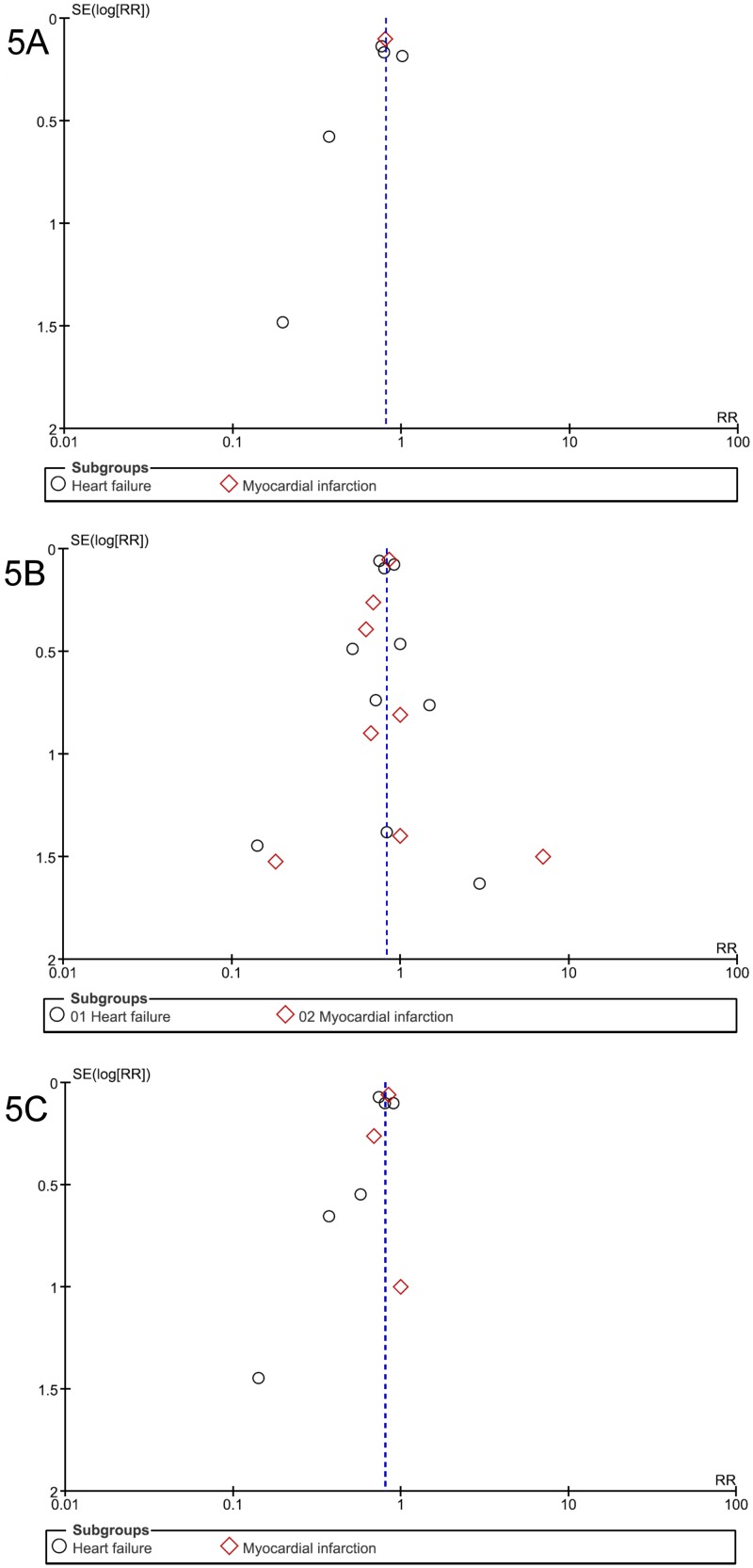
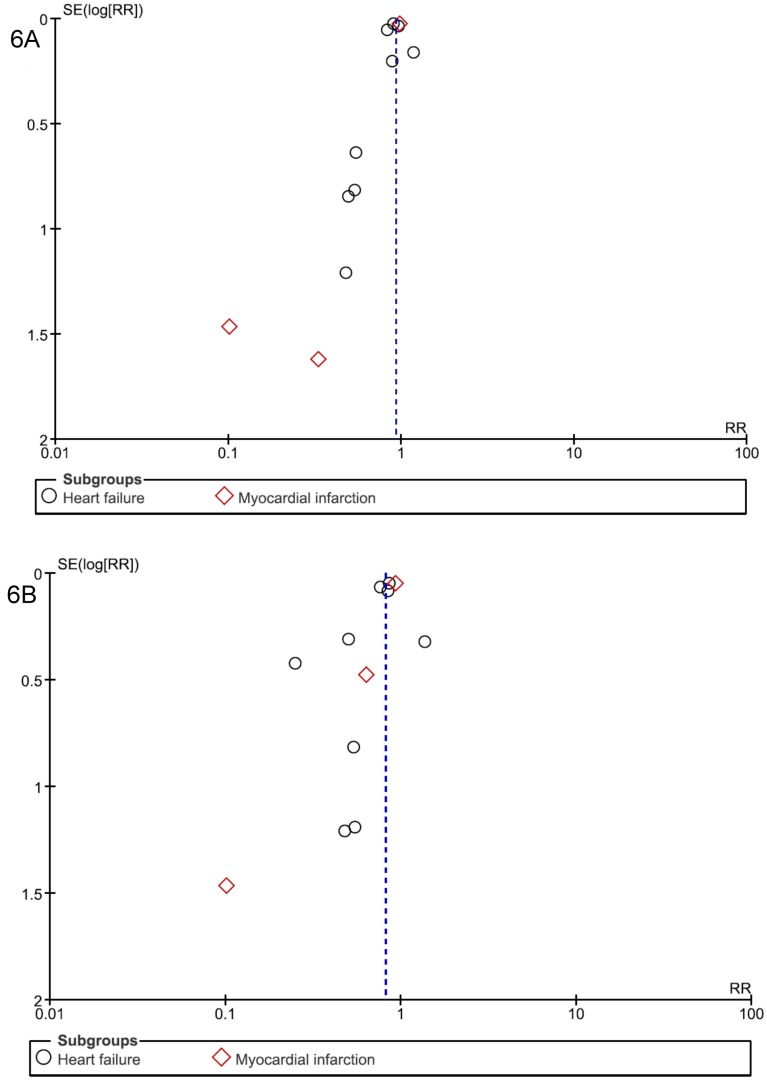
Visual analysis of funnel plots suggested the possibility of publication biases in SCD, CV mortality, total/CV hospitalization analyses, with some asymmetries (Figs 5A, 5C and 6A, 6B); this bias was unlikely in two cases: total mortality (Fig 5B) and side effects (Fig 7).
Fig 5. Funnel plot of standard error (log odds ratio) by odds ratio to evaluate publication bias for effect of aldosterone antagonist treatment in preventing (A) Sudden death, (B) All-cause mortality, and (C) Cardiovascular mortality in patients with heart failure or myocardial infarction.
Fig 6. Funnel plot of standard error (log odds ratio) by odds ratio to evaluate publication bias for effect of aldosterone antagonist treatment in preventing (A) All-cause hospitalization and (B) Cardiovascular hospitalization in patients with heart failure or myocardial infarction.
Fig 7. Funnel plot of standard error (log odds ratio) by odds ratio to evaluate publication bias for effect of aldosterone antagonist treatment in inducing common side effects (hyperkalemia, degradation of renal function, gynecomastia) in comparison with placebo/control, in patients with heart failure or myocardial infarction.
Statistically, potential existence of publication bias was tested by Egger approach, using OR instead of RR for the reason explained in the Method session. For clinical outcome with low incidence (SCD, total/CV mortality, side effects), these two indicators were similar. For example, the SCD prevention effect of AAs estimated by RR was 0.81 (95% CI, 0.71–0.92, p = 0.002) and by OR was 0.80 (95% CI, 0.69–0.92, p = 0.002), both using random effect model. However, the higher the incidence was, the more different these estimators were. For example, for total hospitalization criteria which had the highest incidence (over 40%), intervention effect measured by RR was 0.93 (95% CI, 0.88–0.98, p = 0.008) but by OR was 0.84 (95% CI, 0.72–0.97, p = 0.018), both using random effect model.
Most clinical outcomes in this meta-analysis included at least 10 trials, thus satisfied the recommendations on testing for funnel plot asymmetry, except the primary outcome (SCD). The p-values of Egger tests were 0.21 for SCD, 0.79 for total mortality, 0.17 for CV mortality, 0.13 for total hospitalization, 0.08 for CV hospitalization, 0.23 for hyperkalemia, 0.94 for renal function degradation and 0.29 for gynecomastia, none supporting evidence for publication bias. Of note, regarding both funnel plots & Egger tests, publication biases were not formally assessable for SCD outcome due to the few number of trials included (n = 6).
Sensitivity analysis
Sensitivity analyses were tested for the biggest trial in each subgroup (among the greatest ones REMINDER [28], TOPCAT [14], EPHESUS [13], RALES [11], EMPHASIS-HF [33]) which had the greatest weight percentages, for eight open label/single blind/not reported design trials if applicable (Cicoira et al. [22], Kayrak et al. [26], Mak et al. [23], Modena et al. [27], Vatankulu et al. [30], Vizzardi et al. 2013 [34], Vizzardi et al. 2010 40], Wu et al. [24]) and for 11 trials which had no intention to treat analysis (ITTA) if applicable (Boccanelli et al. [35], Cicoira et al. [22], Deswal et al. [36], Di Pasquale et al. [25], Kayrak et al. [26], Mak et al. [23], Taheri et al. 2012 [37], Taheri et al. 2009 [38], Udelson et al. [39], Vizzardi et al. 2013 [24], Weir et al. [31]) (Table 1). As well, we conducted these analyses only for primary outcome, i.e the preventive effect of AAs on mortality (SCD, total and CV death) in patients with HF or post-MI.
Among all included trials in considering both subgroups, EPHESUS trial [13] contributed the largest weight with relative overall weights of 44.1% for SCD, 34.6% for all-cause mortality and 39.0% for CV mortality analyses. However, when performing a sensitivity test by excluding this trial, no significant differences of RRs were detected for three cases: from (0.81, 95% CI 0.71–0.92, p = 0.002) to (0.81, 95% CI 0.67–0.98, p = 0.03), from (0.82, 95% CI 0.77–0.88, p<0.00001) to (0.80, 95% CI 0.74–0.87, p<0.00001) and from (0.80, 95% CI 0.74–0.87, p<0.00001) to (0.78, 95% CI 0.71–0.86, p<0.00001), respectively.
For patients with HF, the RALES trial [11] had the largest relative weights of 24.6%, 30.8% and 29.4% for these three criteria, respectively. Excluding this trial resulted in no significant difference of estimate effect for SCD analysis: RR (0.81; 95% CI, 0.67–0.98; p = 0.03) changed to (0.82, 95% CI, 0.59–1.14) but the effective estimator turned out non-significant (p = 0.24). The RRs for all-cause and CV mortality changed moderately from (0.81, 95% CI, 0.74–0.88, p <0.00001) to (0.87, 95% CI, 0.77–0.98, p = 0.02) and from (0.79, 95% CI, 0.70–0.89, p = 0.0001) to (0.83, 95% CI, 0.71–0.97, p = 0.02) respectively, with the results remained significant.
In these patients, removing two trials which had no intention-to-treat analysis (ITTA) (Boccanelli et al. [35], Taheri et al. 2012 [37]) gave no remarkable influence on the AAs’ effect in preventing SCD: RR changed from (0.81; 95% CI, 0.67–0.98; p = 0.03) to (0.83; 95% CI, 0.69–0.99; p = 0.04). The same attempt for three trials (Boccanelli et al. [35], Taheri et al. 2012 [37], Taheri et al. 2009 [38]) resulted in slight changes: RR changed from (0.81, 95% CI, 0.74–0.88, p<0.00001) to (0.81, 95% CI, 0.75–0.88, p <0.00001) and from (0.79, 95% CI, 0.70–0.89, p = 0.0001) to (0.79, 95% CI, 0.70–0.90, p = 0.0004) in case of total/CV mortality, respectively.
Open or single blind trials in HF subgroup were also excluded for sensitivity analyses (applicable for total and CV mortality analyses). Removing the three trials Cicoira et al. [22], Mak et al. [23], Vizzardi et al. 2013 [34] for total mortality and removing the trial of Vizzardi et al. 2013 [34] for CV mortality resulted in slight changes: RR changed from (0.81, 95% CI, 0.74–0.88, p<0.00001) to (0.81, 95% CI, 0.73–0.91, p = 0.0004) and from (0.79, 95% CI, 0.70–0.89, p = 0.0001) to (0.83, 95% CI, 0.74–0.94, p = 0.003), respectively.
In those with MI, the EPHESUS trial [13] was the only for SCD prevention analysis. This trial occupied the greatest relative overall weights of 34.6% and 39.0% in case of total and CV mortality, respectively. Removing this trial returned significant changes of RRs from (0.85, 95% CI, 0.76–0.95, p = 0.003) to (0.71, 95% CI 0.48–1.05, p = 0.09) and from (0.83, 95% CI, 0.74–0.94, p = 0.003) to (0.71, 95% CI 0.43–1.18, p = 0.19), respectively.
For total mortality analysis, there was only one trial without ITTA (Weir et al. [31]) presented in the MI subgroup and removing this trial had likely no impact on RR: from (0.85, 95% CI, 0.76–0.95, p = 0.003) to (0.85, 95% CI, 0.76–0.94, p = 0.003). Similarly, when three open design trials (Kayrak et al. [26], Modena et al. [27], Wu et al. [24]) were removed, only slight influences on the final effect were observed: RR changed from (0.85, 95% CI, 0.76–0.95, p = 0.003) to (0.83, 95% CI 0.77–0.88, p = 0.006). No trial without ITTA or with single-blind/open design involved MI patients was included for CV mortality analysis.
For SCD, all the included trials concerned HF patients with reduced LVEF, except TOPCAT trial [14] which recruited HF patients with preserved LVEF. Removing this trial resulted in slight change for treatment effect: RR from (0.81, 95% CI, 0.71–0.92, p = 0.002) to (0.78, 95% CI 0.67–0.90, p = 0.0006 and the heterogeneity remained likely absent (both I2 = 0%).
Discussion
In our meta-analysis, we evaluated the efficacy of AAs in reducing mortality (SCD, overall/CV death) and hospitalization rate, as well as their toxicity via the common side effects in 19,333 patients with HF or post-MI from 25 trials. Our findings demonstrated the effectiveness of AAs in preventing SCD, all-cause mortality and CV mortality, yet a double rate of three studied adverse effects in these patients.
The cardio-protective effect of AAs is quite well proven in literature for CV protection [40]. Some of the proposed mechanisms of action in HF of AAs include (i) inhibition of myocardial and vascular remodeling, (ii) blood pressure reduction, (iii) decreased collagen deposition, (iv) decreased myocardial stiffness, (v) prevention of hypokalemia and arrhythmia, (vi) modulation of nitric oxide synthesis, and (vii) immunomodulation [41]. For instance, the meta-analysis of Li et al. [42] demonstrated beneficial effects of AAs on the reversal of cardiac remodeling and improvement of left ventricular function. Another quantified AAs’ positive effect on ejection fraction (EF) and functional capacity improvement in different HF functional classes [43].
The RALES trial [11], published in 1999 was the first big study concerning AAs’ effect that recommended this treatment which significantly decreased mortality rate (SCD, all cause and CV death) as well as CV hospitalization rate in patients with severe chronic HF (NYHA III to IV). Next, in 2003, the EPHESUS trial [13] re-confirmed the role of AAs for the same outcomes in patients with AMI complicated by left ventricular dysfunction. This therapy was thus limited to patients with severe HF or those with HF following MI until the publication of EMPHASIS-HF trial [12] in 2011, which reported additional beneficial evidence for AAs use in mild-to-moderate HF (NYHA II), regarding the same clinical criteria. However, the current TOPCAT trial [14] finished in 2014 showed only a significant lower incidence of cardiac hospitalization in those treated by spironolactone vs. placebo, but not for total deaths and all-cause hospitalization, in patients with HF and preserved EF. Sensitivity analysis with this trial suggested that the treatment effect of AAs was likely similar in HF patients with reduced or preserved EF for SCD prevention.
The work of Ezekowitz et al. [44] evaluated the effect of aldosterone blockade on left ventricular dysfunction in HF and post-MI participants and reported a significant reduction in overall mortality of 20% (RR 0.80, 95% CI, 0.74–0.87, p<0.00001). That of Hu et al. [45], which showed a 21% (RR 0.79, 95% CI 0.66–0.95, p = 0.65) decrease for overall mortality and a 38% (RR 0.62, 95%, CI 0.52–0.74, p = 0.54) decrease for cardiac re-hospitalization by the use of AAs in patients with mild to moderate chronic HF (NYHA I to II). Another current meta-analysis of Bapoje et al. [46] that included 8 RCTs, concluded a 23% reduction (OR 0.77; 95% CI, 0.66–0.89; p = 0.001) of SCD in patients with a left ventricular systolic dysfunction of ≤ 45%, treated with AAs. On the contrary, the most recent meta-analysis of Chen et al. [47] in 2015 did not observe any all-cause mortality benefit, yet a reduced CV hospitalization rate (RR 0.83; 95% CI; 0.70 to 0.98), in patients with either HF or MI and preserved EF by AA treatment. Our meta-analysis, included MI/ HF patients with both preserved and primarily reduced EF, approved the positive effect of AAs in preventing all considered outcomes: SCD (RR 0.81; 95% CI, 0.71–0.92; p = 0.002), all-cause mortality (RR 0.82; 95% CI, 0.77–0.88, p < 0.0001), CV mortality (RR 0.80; 95% CI, 0.74–0.87, p<0.0001), all–cause hospitalization (RR 0.93; 95% CI, 0.88–0.98; p = 0.008) and CV hospitalization (RR 0.82, 95% CI, 0.72–0.92; p = 0.001) in patients with HF or post MI.
In terms of security, our work demonstrated a doubled rate of common adverse reactions (hyperkalemia, worsening renal function and gynecomastia) in those receiving AAs vs. control or placebo (RR 1.99, 95% CI, 1.64–2.41; p<0.00001). These findings agreed with the results of currently conducted analyses by Clark et al. [48] for renal function insufficiency, or by Rossignol et al. [49] for hyperkalemia and renal function degradation.
In 2013, a systematic study [50] of conventional HF therapies, including angiotensin-converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB), direct renin inhibitor (DRI), and AA compared their effects (on prevention of total death, CV death, non-fatal MI, HF hospitalization and composite of CV death or HF hospitalization) and their safety (on hyperkalemia, hypotension, renal failure). By risk-benefit ratio comparison, this review favored the administration of AA over ARB or DRI, despite its 110% generated increase in hyperkalemia. Likewise, higher proportion of developed hyperkalemia and higher rate of hospitalization for hyperkalemia by AAs in HF patients were recorded in RALES trial, especially in combined use of AAs with either ACEIs or ARBs [51]. Moreover, the benefit of AAs on morbi-mortality prevention seems to overweigh its side-effects, i.e. the reduction in mortality associated with the use of AA was significantly greater than its use complications. Our work estimated numbers of 83, 27 and 18 HF patients need to be treated with AAs to prevent one SCD, one all-cause death and one CV death in one year, respectively. For patients with MI, the corresponding numbers needed to treat (NNT) were 84, 48 and 48, respectively. Considering both patient groups, the estimated NNTs were 83, 34 and 35, respectively. As well, the number needed to harm i.e the number of patients treated on average to have one who suffers at least one of the three common side effects studied, was 77.
Noticeably, focusing on SCD prevention, while AAs help to reduce CV risk factors thus prevent CV accidents including SCD, paradoxically, their side effects of hyperkalemia may induce this accident from cardiac arrhythmia [52]. By this point, a study [53] proved that AAs were independently associated with increased rates of total mortality (hazard ratio HR 1.4; 95% CI 1.1–1.8; P = 0.005), of CV mortality (HR 1.4; 95% CI 1.1–1.9; P = 0.009) and a doubled incidence of SCD (HR 2.0; 95% CI 1.3, 3.0; P = 0.001) in patients with atrial fibrillation and HF. This implied a careful examination of risk/benefit ratio for each individual patient before the prescription of this treatment.
Based on our comprehensive and meticulous search strategy, we believe that we have identified all existing studies that met our inclusion criteria, hence yielding robust results. However, certain limitations should be considered when interpreting these outcomes. For instance, publication bias was not reliably assessed (though seemly negative) for the most important outcome (SCD) when less than 10 studies were included for pooled analyses by funnel plot (Fig 5A) or Egger test.
In summary, to gain the maximum benefit from AAs and reduce possible complications, it is legitimate to individualize and closely monitor their use. For examples, risk-benefit balance should be carefully considered before using AAs in patients with severe renal insufficiency. Also, other factors such as time of treatment initiation [54] and cost difference between AA agents [55] should be taken into account to optimize this therapy.
Conclusion
Our meta-analysis demonstrates that AA treatment may provide beneficial effects on the prevention of SCD, as well as all-cause and CV mortality, for selected patients with HF with altered left ventricular function or after a MI. Nevertheless, careful consideration before prescribing should be given simultaneously to the therapeutic benefit and the overall safety profile of this medication.
Supporting Information
(DOCX)
(DOC)
(DOCX)
(EPS)
(DOCX)
Acknowledgments
We would like to thank Garry Taverny for his bibliographic assistance.
Abbreviations
- AA
Aldosterone Antagonist
- (A)MI
(Acute) Myocardial Infarction
- ACEI
Angiotensin-Converting Enzyme Inhibitor
- ARB
Angiotensin Receptor Blocker
- DRI
Direct Renin Inhibitor
- HF
Heart Failure
- (LV)EF
(Left Ventricular) Ejection Fraction
- LVSD
Left Ventricular Systolic Dysfunction
- NYHA
New York Heath Association
- RAAS
Renin-Angiotensin Aldosterone hormone System
- RCT
Randomized Controlled Trial
- SCD
Sudden Cardiac Death
Data Availability
All relevant data are within the paper.
Funding Statement
The work has been done as a part of master internships of the three co-authors (HHL, CEK and MM). HHL is currently receiving a salary from Claude Bernard Lyon 1 University (scholarship of French Ministry of Higher Education & Research) for her three-year PhD at the UMR 5558 CNRS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Englestein E, Douglas Z. Sudden Cardiac Death, In: Alexander RWS, Schlant RC, Fuster V, The Heart, Arteries and Veins. (New York, NY: McGraw-Hill; ); 1998. [Google Scholar]
- 2.Myerburg R, Castellanos A. Cardiac Arrest and Sudden Death, In: Braunwald E, Ed. Heart Disease: A Textbook of Cardiovascular Medicine., 9th edn Philadelphia: WB Saunders; 1997. [Google Scholar]
- 3.Mehta D, Curwin J, Gomes JA, Fuster V. Sudden Death in Coronary Artery Disease Acute Ischemia Versus Myocardial Substrate. Circulation. 1997;96: 3215–3223. 10.1161/01.CIR.96.9.3215 [DOI] [PubMed] [Google Scholar]
- 4.Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm JMCP. 2007;13: 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nappi JM, Sieg A. Aldosterone and aldosterone receptor antagonists in patients with chronic heart failure. Vasc Health Risk Manag. 2011;7: 353–363. 10.2147/VHRM.S13779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catena C, Colussi G, Marzano L, Sechi LA. Aldosterone and the heart: from basic research to clinical evidence. Horm Metab Res Horm Stoffwechselforschung Horm Métabolisme. 2012;44: 181–187. 10.1055/s-0031-1291318 [DOI] [PubMed] [Google Scholar]
- 7.McMurray J, Swedberg K. Treatment of chronic heart failure: a comparison between the major guidelines. Eur Heart J. 2006;27: 1773–1777. 10.1093/eurheartj/ehl123 [DOI] [PubMed] [Google Scholar]
- 8.Caccamo MA, Eckman PM. Pharmacologic therapy for New York Heart Association class IV heart failure. Congest Heart Fail Greenwich Conn. 2011;17: 213–219. 10.1111/j.1751-7133.2011.00235.x [DOI] [PubMed] [Google Scholar]
- 9.Dickstein K, Authors/Task Force Members, Cohen-Solal A, Filippatos G, McMurray JJV, Ponikowski P, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008‡: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaborati. Eur J Heart Fail. 2008;10: 933–989. 10.1016/j.ejheart.2008.08.005 [DOI] [PubMed] [Google Scholar]
- 10.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128: e240–e327. 10.1161/CIR.0b013e31829e8776 [DOI] [PubMed] [Google Scholar]
- 11.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341: 709–717. 10.1056/NEJM199909023411001 [DOI] [PubMed] [Google Scholar]
- 12.Zannad F, McMurray JJV, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364: 11–21. 10.1056/NEJMoa1009492 [DOI] [PubMed] [Google Scholar]
- 13.Pitt B., Remme W., Zannad F., Neaton J., Martinez F., Roniker B., et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348: 1309–1321. [DOI] [PubMed] [Google Scholar]
- 14.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370: 1383–1392. 10.1056/NEJMoa1313731 [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6: e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cochrane Risk of Bias Criteria (Appendix E) [Internet]. Available: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0049217/
- 17.MANTEL N, HAENSZEL W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22: 719–748. [PubMed] [Google Scholar]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7: 177–188. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327: 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315: 629–634. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sterne JA, Egger M, Moher D. Chapter 10: Addressing reporting biases Cochrane Handbook for Systematic Reviews of Interventions, Version 510 [updated March 2011]. Julian PT Higgins and Sally Green; [Google Scholar]
- 22.Cicoira M, Zanolla L, Rossi A, Golia G, Franceschini L, Brighetti G, et al. Long-term, dose-dependent effects of spironolactone on left ventricular function and exercise tolerance in patients with chronic heart failure. J Am Coll Cardiol. 2002;40: 304–310. [DOI] [PubMed] [Google Scholar]
- 23.Mak GJ, Ledwidge MT, Watson CJ, Phelan DM, Dawkins IR, Murphy NF, et al. Natural history of markers of collagen turnover in patients with early diastolic dysfunction and impact of eplerenone. J Am Coll Cardiol. 2009;54: 1674–1682. 10.1016/j.jacc.2009.08.021 [DOI] [PubMed] [Google Scholar]
- 24.Wu C-T, Wang Z-H, Li Z-Q, Wang L-F. Effect of spironolactone on cardiac remodeling after acute myocardial infarction. World J Emerg Med. 2013;4: 48–53. 10.5847/wjem.j.1920-8642.2013.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Pasquale P, Cannizzaro S, Scalzo S, Parrinello G, Fasullo S, Giambanco F, et al. Effects of canrenoate plus angiotensin-converting enzyme inhibitors versus angiotensin-converting enzyme inhibitors alone on systolic and diastolic function in patients with acute anterior myocardial infarction. Am Heart J. 2005;150: 919.e1–919.e8. 10.1016/j.ahj.2005.03.032 [DOI] [PubMed] [Google Scholar]
- 26.Kayrak M, Bacaksiz A, Vatankulu MA, Ayhan SS, Ari H, Kaya Z, et al. The effects of spironolactone on atrial remodeling in patients with preserved left ventricular function after an acute myocardial infarction: a randomized follow-up study. Coron Artery Dis. 2010;21: 477–85. 10.1097/MCA.0b013e32833fd243 [DOI] [PubMed] [Google Scholar]
- 27.Modena MG, Aveta P, Menozzi A, Rossi R. Aldosterone inhibition limits collagen synthesis and progressive left ventricular enlargement after anterior myocardial infarction. Am Heart J. 2001;141: 41–46. 10.1067/mhj.2001.111258 [DOI] [PubMed] [Google Scholar]
- 28.Montalescot G, Pitt B, Sa de EL, Hamm CW, Flather M, Verheugt F, et al. Early eplerenone treatment in patients with acute ST-elevation myocardial infarction without heart failure: The Randomized Double-Blind Reminder Study. Eur Heart J. 2014; ehu164. 10.1093/eurheartj/ehu164 [DOI] [PubMed] [Google Scholar]
- 29.Uzunhasan I, Yildiz A, Coskun U, Kalyoncuoglu M, Baskurt M, Cakar MA, et al. Effects of aldosterone blockade on left ventricular function and clinical status during acute myocardial infarction. Scand J Clin Lab Invest. 2009;69: 545–549. 10.1080/00365510902802278 [DOI] [PubMed] [Google Scholar]
- 30.Vatankulu M.A., Bacaksiz A., Sonmez O., Alihanoglu Y., Koc F., Demir K., et al. Does spironolactone have a dose-dependent effect on left ventricular remodeling in patients with preserved left ventricular function after an acute myocardial infarction? Cardiovasc Ther. 2013;31: 224–229. 10.1111/1755-5922.12006 [DOI] [PubMed] [Google Scholar]
- 31.Weir RAP, Mark PB, Petrie CJ, Clements S, Steedman T, Ford I, et al. Left ventricular remodeling after acute myocardial infarction: does eplerenone have an effect? Am Heart J. 2009;157: 1088–1096. 10.1016/j.ahj.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 32.Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA J Am Med Assoc. 2013;309: 781–791. 10.1001/jama.2013.905 [DOI] [PubMed] [Google Scholar]
- 33.Zannad F, McMurray JJV, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al. Eplerenone in Patients with Systolic Heart Failure and Mild Symptoms. N Engl J Med. 2011;364: 11–21. 10.1056/NEJMoa1009492 [DOI] [PubMed] [Google Scholar]
- 34.Vizzardi E., Nodari S., Caretta G., D’aloia A., Pezzali N., Faden G., et al. Effects of spironolactone on long-term mortality and morbidity in patients with heart failure and mild or no symptoms. Am J Med Sci. 2014;347: 271–276. 10.1097/MAJ.0b013e31829dd6b1 [DOI] [PubMed] [Google Scholar]
- 35.Boccanelli A, Mureddu GF, Cacciatore G, Clemenza F, Di Lenarda A, Gavazzi A, et al. Anti-remodelling effect of canrenone in patients with mild chronic heart failure (AREA IN-CHF study): final results. Eur J Heart Fail. 2009;11: 68–76. 10.1093/eurjhf/hfn015 [DOI] [PubMed] [Google Scholar]
- 36.Deswal A, Richardson P, Bozkurt B, Mann DL. Results of the Randomized Aldosterone Antagonism in Heart Failure with Preserved Ejection Fraction trial (RAAM-PEF). J Card Fail. 2011;17: 634–642. 10.1016/j.cardfail.2011.04.007 [DOI] [PubMed] [Google Scholar]
- 37.Taheri S, Mortazavi M, Pourmoghadas A, Seyrafian S, Alipour Z, Karimi S. A prospective double-blind randomized placebo-controlled clinical trial to evaluate the safety and efficacy of spironolactone in patients with advanced congestive heart failure on continuous ambulatory peritoneal dialysis. Saudi J Kidney Dis Transplant Off Publ Saudi Cent Organ Transplant Saudi Arab. 2012;23: 507–512. [PubMed] [Google Scholar]
- 38.Taheri S, Mortazavi M, Shahidi S, Pourmoghadas A, Garakyaraghi M, Seirafian S, et al. Spironolactone in chronic hemodialysis patients improves cardiac function. Saudi J Kidney Dis Transplant Off Publ Saudi Cent Organ Transplant Saudi Arab. 2009;20: 392–397. [PubMed] [Google Scholar]
- 39.Udelson JE, Feldman AM, Greenberg B, Pitt B, Mukherjee R, Solomon HA, et al. Randomized, double-blind, multicenter, placebo-controlled study evaluating the effect of aldosterone antagonism with eplerenone on ventricular remodeling in patients with mild-to-moderate heart failure and left ventricular systolic dysfunction. Circ Heart Fail. 2010;3: 347–353. 10.1161/CIRCHEARTFAILURE.109.906909 [DOI] [PubMed] [Google Scholar]
- 40.Munger MA. Use of Angiotensin receptor blockers in cardiovascular protection: current evidence and future directions. P T Peer-Rev J Formul Manag. 2011;36: 22–40. [PMC free article] [PubMed] [Google Scholar]
- 41.Tang WHW, Parameswaran AC, Maroo AP, Francis GS. Aldosterone receptor antagonists in the medical management of chronic heart failure. Mayo Clin Proc. 2005;80: 1623–1630. 10.4065/80.12.1623 [DOI] [PubMed] [Google Scholar]
- 42.Li X, Qi Y, Li Y, Zhang S, Guo S, Chu S, et al. Impact of mineralocorticoid receptor antagonists on changes in cardiac structure and function of left ventricular dysfunction: a meta-analysis of randomized controlled trials. Circ Heart Fail. 2013;6: 156–165. 10.1161/CIRCHEARTFAILURE.112.000074 [DOI] [PubMed] [Google Scholar]
- 43.Phelan D, Thavendiranathan P, Collier P, Marwick TH. Aldosterone antagonists improve ejection fraction and functional capacity independently of functional class: a meta-analysis of randomised controlled trials. Heart Br Card Soc. 2012;98: 1693–1700. 10.1136/heartjnl-2012-302178 [DOI] [PubMed] [Google Scholar]
- 44.Ezekowitz JA, McAlister FA. Aldosterone blockade and left ventricular dysfunction: a systematic review of randomized clinical trials. Eur Heart J. 2009;30: 469–477. 10.1093/eurheartj/ehn543 [DOI] [PubMed] [Google Scholar]
- 45.Hu L, Chen Y, Deng S, Du J, She Q. Additional use of an aldosterone antagonist in patients with mild to moderate chronic heart failure: a systematic review and meta-analysis. Br J Clin Pharmacol. 2013;75: 1202–1212. 10.1111/bcp.12012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bapoje SR, Bahia A, Hokanson JE, Peterson PN, Heidenreich PA, Lindenfeld J, et al. Effects of mineralocorticoid receptor antagonists on the risk of sudden cardiac death in patients with left ventricular systolic dysfunction: a meta-analysis of randomized controlled trials. Circ Heart Fail. 2013;6: 166–173. 10.1161/CIRCHEARTFAILURE.112.000003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y, Wang H, Lu Y, Huang X, Liao Y, Bin J. Effects of mineralocorticoid receptor antagonists in patients with preserved ejection fraction: a meta-analysis of randomized clinical trials. BMC Med. 2015;13: 10 10.1186/s12916-014-0261-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clark H, Krum H, Hopper I. Worsening renal function during renin-angiotensin-aldosterone system inhibitor initiation and long-term outcomes in patients with left ventricular systolic dysfunction. Eur J Heart Fail. 2014;16: 41–48. 10.1002/ejhf.13 [DOI] [PubMed] [Google Scholar]
- 49.Rossignol P, Dobre D, McMurray JJV, Swedberg K, Krum H, van Veldhuisen DJ, et al. Incidence, Determinants, and Prognostic Significance of Hyperkalemia and Worsening Renal Function in Patients With Heart Failure Receiving the Mineralocorticoid Receptor Antagonist Eplerenone or Placebo in Addition to Optimal Medical Therapy: Results From the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circ Heart Fail. 2014;7: 51–58. 10.1161/CIRCHEARTFAILURE.113.000792 [DOI] [PubMed] [Google Scholar]
- 50.Bangalore S, Kumar S, Messerli FH. When conventional heart failure therapy is not enough: angiotensin receptor blocker, direct renin inhibitor, or aldosterone antagonist? Congest Heart Fail Greenwich Conn. 2013;19: 107–115. 10.1111/chf.12011 [DOI] [PubMed] [Google Scholar]
- 51.Anton C, Cox AR, Watson RDS, Ferner RE. The safety of spironolactone treatment in patients with heart failure. J Clin Pharm Ther. 2003;28: 285–287. [DOI] [PubMed] [Google Scholar]
- 52.Sica DA, Gehr TWB, Yancy C. Hyperkalemia, congestive heart failure, and aldosterone receptor antagonism. Congest Heart Fail Greenwich Conn. 2003;9: 224–229. [DOI] [PubMed] [Google Scholar]
- 53.O’Meara E, Khairy P, Blanchet MC, de Denus S, Pedersen OD, Levesque S, et al. Mineralocorticoid receptor antagonists and cardiovascular mortality in patients with atrial fibrillation and left ventricular dysfunction: insights from the Atrial Fibrillation and Congestive Heart Failure Trial. Circ Heart Fail. 2012;5: 586–593. 10.1161/CIRCHEARTFAILURE.111.965160 [DOI] [PubMed] [Google Scholar]
- 54.Rossi R, Crupi N, Coppi F, Monopoli D, Sgura F. Importance of the time of initiation of mineralocorticoid receptor antagonists on risk of mortality in patients with heart failure. J Renin-Angiotensin-Aldosterone Syst JRAAS. 2013; 10.1177/1470320313482603 [DOI] [PubMed] [Google Scholar]
- 55.Chatterjee S, Moeller C, Shah N, Bolorunduro O, Lichstein E, Moskovits N, et al. Eplerenone is not superior to older and less expensive aldosterone antagonists. Am J Med. 2012;125: 817–825. 10.1016/j.amjmed.2011.12.018 [DOI] [PubMed] [Google Scholar]
- 56.Chan AKY, Sanderson JE, Wang T, Lam W, Yip G, Wang M, et al. Aldosterone receptor antagonism induces reverse remodeling when added to angiotensin receptor blockade in chronic heart failure. J Am Coll Cardiol. 2007;50: 591–596. 10.1016/j.jacc.2007.03.062 [DOI] [PubMed] [Google Scholar]
- 57.Gao X, Peng L, Adhikari CM, Lin J, Zuo Z. Spironolactone reduced arrhythmia and maintained magnesium homeostasis in patients with congestive heart failure. J Card Fail. 2007;13: 170–177. 10.1016/j.cardfail.2006.11.015 [DOI] [PubMed] [Google Scholar]
- 58.Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the Randomized Aldactone Evaluation Study [RALES]). Am J Cardiol. 1996;78: 902–907. [DOI] [PubMed] [Google Scholar]
- 59.Vizzardi E., D’Aloia A., Giubbini R., Bordonali T., Bugatti S., Pezzali N., et al. Effect of spironolactone on left ventricular ejection fraction and volumes in patients with class i or II heart failure. Am J Cardiol. 2010;106: 1292–1296. 10.1016/j.amjcard.2010.06.052 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
(DOCX)
(EPS)
(DOCX)
Data Availability Statement
All relevant data are within the paper.