Beginning with the report in 1994 that an adult male with homozygous loss of estrogen receptor α (ERα) had unfused epiphyses and osteopenia,(1) there is now considerable evidence from observational and interventional studies (summarized in Khosla and colleagues(2)) that estrogen is the dominant regulator of bone metabolism in men. Indeed, a legitimate argument can be made that testosterone is largely a prohormone for the skeleton, with its effects on bone mediated principally via aromatization to estrogen, although it remains likely that testosterone contributes to bone size via effects on periosteal apposition and perhaps indirectly to bone mass by increasing muscle mass.
A series of human investigations from our group have certainly contributed to building this argument. Perhaps the most convincing of these was a direct interventional study published almost 15 years ago,(3) which is summarized briefly here, because it is instructive to revisit the data from that human study in light of the article in this issue of JBMR by Ucer and colleagues(4) on the contributions of skeletal androgen receptor (AR) versus ERα signaling toward regulating bone metabolism in mice. Thus, the key questions are: 1) Are the findings from the mouse study translatable to humans? 2) Can the new mouse data help us better understand previous findings in humans?
In our human study, we used an experimental design in which sex steroid production was suppressed in adult men using a GnRH agonist followed by selective replacement of either estrogen or testosterone, or both.(3) An aromatase blocker was administered to all subjects in order to examine effects of testosterone on bone in the absence of conversion to estrogen. For the purposes of this discussion, the bone resorption marker, N-telopeptide of type I collagen (NTx) will be used to reflect sex steroid effects on bone turnover; independent effects of estrogen and testosterone on bone formation markers were also observed, but these were largely concordant with the findings for NTx, so to simplify the discussion, NTx is used here to reflect bone remodeling changes in this study.
As shown in Fig. 1A, compared with the sex steroid replete state (+T, +E), complete sex steroid deficiency (−T, −E) resulted in an increase in NTx of ~35% over 3 weeks. Estrogen replacement alone, in the absence of testosterone (−T, +E), was able to almost completely prevent this increase in bone resorption, which increased only by ~10%; by contrast, testosterone replacement alone (+T, −E) was largely ineffective, with NTx increasing in this group by ~25%. Using a rigorous 2-factor ANOVA model and these percentages, we concluded that even in men, estrogen exerted the dominant effect on bone resorption, with testosterone making a much smaller contribution. Based on the relative magnitude of the changes, our best estimate was that of the total effect of sex steroids on bone resorption in men, estrogen accounted for ~70% and testosterone for at most ~30% of the effect.(3)
Fig. 1.
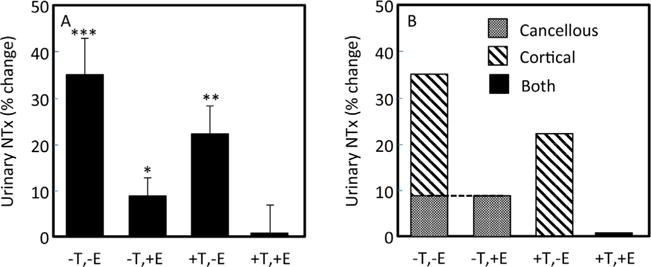
(A) Percent changes in urinary NTx excretion in men made acutely hypogonadal, treated with an aromatase inhibitor, and replaced with estrogen, testosterone, both, or neither.*p< 0.05, **p< 0.01, and ***p< 0.001 for change from baseline. The estrogen and testosterone effects were analyzed using a 2-factor ANOVA model: E effect, p = 0.0002; T effect, p = 0.085. Adapted from Falahati-Niniand colleagues(3) with permission. (B) Data from panel A but now depicting postulated changes in cancellous bone resorption (testosterone effect) and in cortical bone resorption (estrogen effect) based on the mouse genetic studies of Ucerand colleagues.(4) See text for discussion.
The findings of this interventional study were largely confirmed by a similar study by Leder and colleagues(5) using bone turnover markers and a more recent study by Yu and colleagues(6) demonstrating that even in the setting of testosterone sufficiency, induced estrogen deficiency led to significant bone loss in men. In addition, several studies found that selective estrogen receptor modulators exerted a protective effect on bone in men with low endogenous estrogen levels,(7–9) whereas treatment with an aromatase inhibitor(10) or with the nonaromatizable androgen, dihydrotestosterone, resulted in bone loss.(11) Finally, a large body of observational data (summarized in Khosla and colleagues(2)) have shown that in men, serum estrogen levels were better predictors than serum testosterone levels of bone mineral density, rates of bone loss, and fracture risk. Thus, the collective evidence from both interventional and observational human studies is now overwhelming that estrogen plays a much more significant role in regulating bone metabolism in men than testosterone.
To better understand the human data at a mechanistic level, Ucer and colleagues(4) from the Manolagas group report in the current issue of JBMR on the use of a genetic approach to evaluate the role of AR versus ER signaling in bone cells on bone metabolism in mice. Although some data on female mice were also included in the article, this discussion will be limited to the male mice in order to compare the findings to our human male study. Thus, Ucer and colleagues(4) generated mice with targeted deletion of the AR or ERα either in osteoblast lineage (ARf/y;Prx1-Cre or ERαf/f;Osx1-Cre) or myeloid lineage cells (and hence osteoclasts) (ARf/y;LysM-Cre or ERαf/f;LysM-Cre). Although ERβ deletion was not included in any of these models, the available evidence from previous mouse models is that ERβ may play a role in the female, but not male, skeleton,(12,13) so the authors accordingly focused on the AR and ERα.
One of the key findings of this study, consistent with three previous publications in male mice using similar models,(14–16) was that AR deletion in osteoblast lineage cells resulted in decreased cancellous bone volume and trabecular number associated with an increase in osteoclast number. By contrast, ERα deletion in osteoblast lineage cells had no effect on cancellous bone in male mice, nor did AR or ERα deletion in myeloid (osteoclast) lineage cells. In addition, none of these deletions had any discernable effects on cortical bone. Thus, the genetic mouse data are incontrovertible: Androgens act directly via the AR in osteoblast lineage cells to regulate cancellous, but not cortical, bone mass and turnover. Moreover, ERα signaling, at least through osteoblast or osteoclast lineage cells, plays no role in regulating cortical bone in male mice. The issue of ERα signaling in cancellous bone in male mice is more complex (see Vanderschueren and colleagues(17) for review): Although the Manolagas group, in either the present study(4) or in a previous report,(18) found no effect of ERα deletion in osteoblast lineage cells on cancellous bone, two previous studies using either the osteocalcin(19) or DMP1(20) Cre did observe decreased cancellous bone mass in young adult male mice lacking ERα in osteoblast lineage cells.
These discrepancies between laboratories regarding ERα signaling in cancellous bone in male mice notwithstanding, there is no escaping the conclusion that the mouse genetic findings from Ucer and colleagues(4) demonstrating a major role for the AR but little or no role for ERα in bone metabolism in male mice are at odds with the large body of human evidence that estrogen is the dominant regulator of bone metabolism in men. Clearly, one possibility is that as far as sex steroid regulation of bone metabolism is concerned, mice are different from humans. For example, aromatase expression in rodents is predominantly in the brain and gonads, whereas primates and humans have much more widespread expression of aromatase in multiple tissues.(21) Thus, there may be fundamental differences between the rodent and human skeletons in terms of regulation by testosterone versus estrogen.
There are, however, other possible explanations for the apparent discrepancy between the human and mouse findings that may, in fact, shed some light on the underlying biology. Thus, in our human study, we did observe a small increase (~10%) in NTx in the −T, +E group (Fig. 1A), which represented ~30% of the NTx increase observed after complete sex steroid deficiency (−T, −E). If we accept the mouse data demonstrating that testosterone action via the AR (but not estrogen action via the ER) regulates cancellous bone remodeling and extrapolate that this is also true in humans, then the inevitable conclusion is that the increase in bone resorption in the −T, +E group must reflect loss of the direct effects of testosterone (without aromatization to estrogen) on bone resorption in cancellous bone. Under this scenario, estrogen alone was not able to fully suppress cancellous bone resorption in men. If we then transpose the amount of bone resorption found in the −T, +E group to the −T, −E group (dashed line in Fig. 1B), then we have to attribute the remaining 70% or so of the increase in NTx in the −T, −E group to estrogen effects on cortical bone remodeling because the data of Ucer and colleagues(4) indicate that ER signaling plays no role in cancellous bone in males. This parsing of the resorption data to reflect estrogen effects on cortical bone versus testosterone effects on cancellous bone (70:30) is remarkably consistent with the fact that the skeleton is ~80% cortical bone and ~20% cancellous bone.(22) As such, in our human model, it is possible that estrogen emerged as the dominant regulator of NTx production not only because it may be a more potent antiresorptive (or suppressor of bone remodeling) than testosterone, but also because it predominantly regulates cortical bone, which comprises much more of the total skeletal mass. This would also explain why bone resorption in the +T, −E group increased by ~25% compared with the larger increase of ~35% in the −T, −E group: Loss of estrogen led to increased bone resorption in cortical bone but the presence of testosterone in the +T, −E group did, in fact, suppress bone resorption in cancellous bone. Because measurement of bone turnover markers in serum or urine provides no information regarding whether the source is from cortical or cancellous bone, the power of combining our previous human data(3) with the new mouse data of Ucer and colleagues(4) is that it now allows us to reinterpret our earlier findings and to construct a plausible hypothesis that reconciles the mouse and human findings. Specifically, it shows that the mouse findings regarding the key role of the AR in regulating cancellous bone remodeling may also be true in humans.
The hypothesis proposed in Fig. 1B also leads to a number of predictions that can be tested. For example, loss of estrogen with preservation of testosterone levels in men should result principally in changes in cortical, but not in cancellous, bone. Although the findings have thus far only been presented in abstract form, Yu and colleagues(6) recently conducted a study that tested this prediction. In this study, men were made hypogonadal using a GnRH agonist and treated with increasing doses of testosterone (including into the supraphysiological range) but in the presence of an aromatase blocker. Thus, all men had low estrogen levels and varying degrees of testosterone sufficiency. High-resolution peripheral quantitative computed tomography (HRpQCT) imaging at the distal radius and tibia was obtained at baseline and 16 weeks after treatment in order to evaluate changes in cortical and cancellous bone microarchitecture. Consistent with the prediction of the hypothesis in Fig. 1B, maintenance of serum testosterone levels but loss of estrogen production led to significant decreases in cortical area and thickness (and increases in cortical porosity) but no changes in trabecular number, thickness, or spacing, although there were small reductions in trabecular volumetric bone mineral density. Because these men were testosterone replete, cancellous bone was relatively protected, but cortical bone did suffer the consequences of estrogen deficiency. Thus, a reasonable scenario can be constructed whereby the mouse data would be consistent with the human data in terms of protective androgen effects on cancellous bone.
There is, however, still a problem withcortical bone. As shown in Fig. 1A, ~70% of the total effect of sex steroids on bone resorption in men was owing to estrogen. If we assume that the mouse findings regarding AR regulation of cancellous bone (and the lack of effects of ERα deletion on cancellous bone in male mice, at least as observed by Ucer and colleagues(4)) are translatable to humans, then estrogen effects on bone remodeling in human males in our study must have been on cortical bone (Fig. 1B), including endocortical resorption and intracortical, osteonal (Haversian) remodeling. Furthermore, for concordance between humans and mice, deletion of ERα in osteoblast and/or osteoclast lineage cells should have had clear effects on cortical bone in male mice, but this was not observed by Ucer and colleagues.(4) Admittedly, there was a transient decrease in cortical thickness in 6-week-old male ERαf/f;Osx1-Cre mice compared with the Osx1-Cre littermates, but this was no longer present at 10 or 26 weeks. Thus, there is a hint of estrogen regulation of cortical bone, and perhaps of endocortical bone resorption, in male mice through the osteoblastic ERα, but certainly the findings are not as clear as the human data in terms of the estrogen effects on cortical bone resorption depicted in Fig. 1.
Here, we may have little choice but to resort to the “mice are different from humans” argument. Indeed, mouse cortical bone is quite different from human cortical bone, particularly because humans have extensive intracortical, osteonal remodeling that is absent in mice.(23) Thus, estrogen may only regulate endocortical resorption in mice versus endocortical and perhaps the much more extensive intracortical remodeling in humans, making it much easier to detect estrogen effects on cortical bone in humans compared with mice.
It is also important to note that the cancellous/cortical regulation by testosterone versus estrogen depicted in Fig. 1B is probably not all or none—biology rarely is. As such, it is likely that, in males, estrogen (via the ER) has some effects on cancellous bone (as shown by some studies(19,20)) and testosterone (via the AR) has some effects on cortical bone, but these were not evident in the mouse studies because of inherent limitations of the Cre-lox models, as appropriately discussed by Ucer and colleagues.(4) Nonetheless the predominant effects of testosterone versus estrogen on the two bone compartments as depicted in Fig. 1B are most consistent with the available evidence and reconcile at least some of the differences between the mouse and human findings.
Ucer and colleagues(4) do raise another possibility to explain the lack of AR or ERα deletion effects on cortical bone in their models. They argue that effects of sex steroids on cortical bone could be mediated not by osteoblast lineage cells or osteoclasts (ie, the cells in which they deleted AR or ERα) but by some other cell type—for example, B cells. They point to earlier evidence that androgens and estrogens suppress B-cell production(24) and that mice lacking RANKL in B lymphocytes are partially protected from the loss of cancellous bone caused by ovariectomy.(25) However, the problem is with cortical bone, and B-cell RANKL-deficient mice were not protected from bone loss at cortical sites.(25) Moreover, at least in the adult human, there is little hematopoietic marrow, but rather mostly fatty marrow, at appendicular cortical sites that nonetheless undergo substantial bone loss after gonadectomy,(26) making it somewhat problematic to invoke a role for B cells (or other cells in the hematopoietic bone marrow) in influencing bone loss at these distant cortical sites. Nevertheless, not only B cells(25) but also T cells(27) have been implicated in mouse models in mediating ovariectomy-induced bone loss, the latter through increased TNF-α production that may well modulate bone resorption at distant sites. As such, the possibility that sex steroid effects on bone are mediated by one or more of these immune cells certainly warrants further investigation, particularly in humans.
In summary, the article by Ucer and colleagues(4) does provide important new insights into AR versus ERα regulation of the male skeleton. At first glance, the findings from the mouse models of Ucer and colleagues(4) and others(14–16) do not seem to fit with the large body of human data demonstrating a dominant role for estrogen in bone metabolism in men. Although this may be because of fundamental species differences between mice and men, a plausible case can be made that the findings in mice regarding AR regulation of cancellous bone in male mice may also be true in men. Subtracting out the possible effects of testosterone (in the absence of aromatization) on bone resorption in cancellous bone, however, still leaves the remaining effects of estrogen on cortical bone in men as being distinctly different from those observed by Ucer and colleagues(4) in male mice. Finally, this discussion should remind us that whether one studies mice or humans, the challenge is to develop a comprehensive understanding of the underlying biology by combining the mechanistic information that the mouse models are able to provide with the relevance to humans inherent in clinical studies—realizing that both are important and both have limitations.
Footnotes
Disclosures
The author states that he has no conflicts of interest.
References
- 1.Smith EP, Boyd J, Frank GR, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–61. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 2.Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29:441–64. doi: 10.1210/er.2008-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falahati-Nini A, Riggs BL, Atkinson EJ, O’Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest. 2000;106:1553–60. doi: 10.1172/JCI10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ucer S, Iyer S, Bartell SM, et al. The effects of androgens on murine cortical bone do not require AR or ERalpha signaling in osteoblasts and osteoclasts. J Bone Miner Res. 2015;30 doi: 10.1002/jbmr.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leder BZ, LeBlanc KM, Schoenfeld DA, Eastell R, Finkelstein JS. Differential effects of androgens and estrogens on bone turnover in normal men. J Clin Endocrinol Metab. 2003;88:204–10. doi: 10.1210/jc.2002-021036. [DOI] [PubMed] [Google Scholar]
- 6.Yu E, Wulcyzn K, Perros N, Bouxsein M, Finkelstein J. Hypogonadism with estrogen removal (HER): differential effects of androgens and estrogens on bone microarchitecture in adult men. J Bone Miner Res. 2012;27(Suppl 1):S66. [Google Scholar]
- 7.Doran PM, Riggs BL, Atkinson EJ, Khosla S. Effects of raloxifene, a selective estrogen receptor modulator, on bone turnover markers and serum sex steroid and lipid levels in elderly men. J Bone Miner Res. 2001;16:2118–25. doi: 10.1359/jbmr.2001.16.11.2118. [DOI] [PubMed] [Google Scholar]
- 8.Uebelhart B, Herrmann F, Pavo I, Draper MW, Rizzoli R. Raloxifene treatment is associated with increased serum estradiol and decreased bone remodeling in healthy middle-aged men with low sex hormone levels. J Bone Miner Res. 2004;19:1518–24. doi: 10.1359/JBMR.040503. [DOI] [PubMed] [Google Scholar]
- 9.Smith MR, Malkowicz SB, Brawer MK, Hancock ML, Morton RA, Steiner MS. Toremifene decreases vertebral fractures in men younger than 80 years receiving androgen deprivation therapy for prostate cancer. J Urol. 2011;186:2239–44. doi: 10.1016/j.juro.2011.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnett-Bowie S-AM, Roupenian KC, Dere ME, Lee H, Leder BZ. Effects of aromatase inhibition in hypogonadal older men: a randomized, double-blind, placebo-controlled trial. Clin Endocrinol. 2009;70:116–23. doi: 10.1111/j.1365-2265.2008.03327.x. [DOI] [PubMed] [Google Scholar]
- 11.Idan A, Griggiths KA, Harwood T, et al. Long-term effects of dihydrotestosterone treatment on prostate growth in healthy, middle-aged men without prostate disease: a randomized,;1; placebo-controlled trial. Ann Intern Med. 2010;153:621–32. doi: 10.7326/0003-4819-153-10-201011160-00004. [DOI] [PubMed] [Google Scholar]
- 12.Sims NA, Dupont S, Krust A, et al. Deletion of estrogen receptors reveals a regulatory role for estrogen receptors beta in bone remodeling in females but not in males. Bone. 2002;30:18–25. doi: 10.1016/s8756-3282(01)00643-3. [DOI] [PubMed] [Google Scholar]
- 13.Sims NA, Clement-Lacroix P, Minet D, et al. A functional androgen receptor is not sufficient to allow estradiol to protect bone after gonadectomy in estradiol receptor-deficient mice. J Clin Invest. 2003;111:1319–27. doi: 10.1172/JCI17246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiang C, Chiu M, Moore AJ, et al. Mineralization and bone resorption are regulated by the androgen receptor in male mice. J Bone Miner Res. 2009;24:621–31. doi: 10.1359/jbmr.081217. [DOI] [PubMed] [Google Scholar]
- 15.Notini AJ, McManus JF, Moore A, et al. Osteoblast deletion of Exon 3 of the androgen receptor gene results in trabecular bone loss in adult male mice. J Bone Miner Res. 2007;22:347–56. doi: 10.1359/jbmr.061117. [DOI] [PubMed] [Google Scholar]
- 16.Sinnesael M, Claessens F, Laurent M, et al. Androgen receptor (AR) in osteocytes is important for the maintenence of male skeletal integrity: evidence from targeted AR disruption in mouse osteocytes. J Bone Miner Res. 2012;27:2535–43. doi: 10.1002/jbmr.1713. [DOI] [PubMed] [Google Scholar]
- 17.Vanderschueren D, Laurent M, Claessens F, et al. Sex steroid actions in male bone. Endocr Rev. 2014;35:906–60. doi: 10.1210/er.2014-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almeida M, Iyer S, Martin-Millan M, et al. Estrogen receptor-alpha signaling in osteoblast progenitors stimulates coritcal bone accrual. J Clin Invest. 2013;123:394–404. doi: 10.1172/JCI65910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maatta JA, Buki KG, Gu G, et al. Inactivation of estrogen receptor a in bone-forming cells induces bone loss in female mice. FASEB. J. 2013;27:478–88. doi: 10.1096/fj.12-213587. [DOI] [PubMed] [Google Scholar]
- 20.Windahl SH, Borjesson AE, Farman HH, et al. Estrogen receptor-a in osteocytes is important for trabecular bone formation in male mice. Proc Natl Acad Sci USA. 2013;110:2294–9. doi: 10.1073/pnas.1220811110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simpson ER, Zhao Y, Agarwal VR, et al. Aromatase expression in health and disease. Recent Prog Horm Res. 1997;52:185–214. [PubMed] [Google Scholar]
- 22.Bonnick SL. Skeletal anatomy in densitometry. In: Bonnick SL, editor. Bone densitometry in clinical practice. New York: Humana Press; 1998. pp. 35–78. [Google Scholar]
- 23.Jikla RL. The relevance of mouse models for investigating age-related bone loss in humans. J Gerontol A Biol Sci Med Sci. 2013;68:1209–17. doi: 10.1093/gerona/glt046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakiani S, Olsen NJ, Kovacs WJ. Gonadal steroids and humoral immunity. Nat Rev Endocrinol. 2013;9:56–62. doi: 10.1038/nrendo.2012.206. [DOI] [PubMed] [Google Scholar]
- 25.Onal M, Xiong J, Chen X, et al. Receptor activator of nuclear factor kB ligand (RANKL) protein expression by B lymphocytes contributes to ovariectomy-induced bone loss. J Biol Chem. 2012;287:29851–60. doi: 10.1074/jbc.M112.377945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricci C, Cova M, Kang YS, et al. Normal age-related patterns of cellular and fatty bone marrow distribution in the axial skeleton: MR imaging study. Radiology. 1990;177:83–8. doi: 10.1148/radiology.177.1.2399343. [DOI] [PubMed] [Google Scholar]
- 27.Pacifici R. T cells: critical bone regulators in health and disease. Bone. 2010;47:461–71. doi: 10.1016/j.bone.2010.04.611. [DOI] [PMC free article] [PubMed] [Google Scholar]


