Abstract
Cardiorespiratory plasticity induced by acute intermittent hypoxia (AIH) may contribute to recovery following spinal cord injury (SCI). We hypothesized that patients with cervical SCI would demonstrate higher minute ventilation (V̇e) following AIH compared with subjects with thoracic SCI and able-bodied subjects who served as controls. Twenty-four volunteers (8 with cervical SCI, 8 with thoracic SCI, and 8 able-bodied) underwent an AIH protocol during wakefulness. Each subject experienced 15 episodes of isocapnic hypoxia using mixed gases of 100% nitrogen (N2), 8% O2, and 40% CO2 to achieve oxygen saturation ≤90% followed by room air (RA). Measurements were obtained before, during, and 40 min after AIH to obtain ventilation and heart rate variability data [R-R interval (RRI) and low-frequency/ high-frequency power (LF/HF)]. AIH results were compared with those of sham studies conducted in RA during the same time period. Individuals with cervical SCI had higher V̇e after AIH compared with able-bodied controls (117.9 ± 23.2% vs. 97.9 ± 11.2%, P < 0.05). RRI decreased during hypoxia in all individuals (those with cervical SCI, from 1,009.3 ± 65.0 ms to 750.2 ± 65.0 ms; those with thoracic SCI, from 945.2 ± 65.0 ms to 674.9 ± 65.0 ms; and those who were able-bodied, from 949 ± 75.0 to 682.2 ± 69.5 ms; P < 0.05). LH/HF increased during recovery in individuals with thoracic SCI and those who were able-bodied (0.54 ± 0.22 vs. 1.34 ± 0.22 and 0.67 ± 0.23 vs. 1.82 ± 0.23, respectively; P < 0.05) but remained unchanged in the group with cervical SCI. Our conclusion is that patients with cervical SCI demonstrate ventilatory long-term facilitation following AIH compared with able-bodied controls. Heart rate responses to hypoxia are acutely present in patients with cervical SCI but are absent during posthypoxic recovery.
Keywords: hypoxia, long-term facilitation, acute intermittent hypoxia, plasticity, cardiac autonomic response, spinal cord injury
Spinal cord injury (SCI) is the second most common cause of paralysis, affecting more than one million individuals in the United States alone (44). Unfortunately, life expectancy in patients with SCI remains less than the general population, despite improved critical care, targeted rehabilitation, and longer short-term survival (53). Cardiac and respiratory disorders are the two leading causes of morbidity and mortality in patients with SCI (18). In fact, patients with SCI experience repetitive episodes of hypoxia during sleep due to impaired cough, decreased lung volume, impaired chest wall mechanics (16, 49), and higher prevalence of sleep-disordered breathing (SDB) than the general population (47, 48, 52). The ensuing, intermittent hypoxia can induce sensory long-term facilitation (LTF), which manifests by increased peripheral chemo-responsiveness, enhanced LTF following acute intermittent hypoxia (AIH) (41), and increased propensity to central apnea in a similar fashion to that of patients with obstructive sleep apnea (11).
Intermittent hypoxia-induced neural plasticity may play an important role in motor and sensory recovery following SCI (39). Likewise, cardiac and respiratory plasticity induced by AIH may contribute to respiratory recovery following SCI. Tester et al. (56) demonstrated ventilatory LTF in patients with SCI under hypercapnic conditions. However, the presence of hypercapnia during the recovery period may have amplified the ventilatory response. Furthermore, the study did not assess the effect of SCI level on the magnitude of ventilatory LTF, nor did it assess the associated cardiac responses to hypoxia. The purpose of this study was to determine whether LTF evoked by AIH is dependent on the level of SCI and is coupled by cardiac autonomic modulations. Patients with tetraplegia are at risk for developing hypocapnic apneic threshold, indicating increased breathing instability, compared with individuals with thoracic SCI and those who are able-bodied (48). We hypothesized that patients with cervical SCI would demonstrate higher V̇e following AIH compared with individuals with thoracic SCI and subjects who served as able-bodied controls. Results of this study have been previously reported in the form of abstracts (8).
METHODS
The Human Investigation Committee of the Wayne State University and the John D. Dingell VA Medical Center approved the experimental protocol. Informed written consent was obtained from all participating subjects. We studied three groups of adults: those who were able-bodied, and those with cervical (C4–C7) SCI and thoracic (T1–T6) SCI (more than 1 yr after injury) with American Spinal Injury Association (ASIA) scores A, B, C, or D.
Each subject was connected to a breathing circuit with a nasal mask attached to a pneumotachometer (model 3700A; Hans Rudolph, Shawnee, KS) to measure ventilation and timing. End-tidal carbon dioxide (PetCO2) and O2 (PetO2) were measured with CO2 and O2 gas analyzers (models 17515 and 17518, respectively; VacuMed, Ventura, CA) and acquired by a PowerLab data acquisition system (model 16SP, AD Instruments, Colorado Springs, CO). Standard electroencephalography and electrocardiography readings were recorded using a Comet PSG System (Grass Technologies, Warwick, RI). For baseline overnight sleep tests, attended full-polysomnography (PSG) were obtained from all subjects with cervical and thoracic SCI using Comet or Grass Heritage II (Grass Technologies) systems. Four able-bodied control subjects underwent unattended full PSG using a Compumedics Somte PSG System (Compumedics, Charlotte, NC). Arterial O2 saturation (SaO2) was measured by a pulse oximeter using an ear probe (Biox 3740; Datex-Ohmeda, Madison, WI).
Acute Intermittent Hypoxia Protocol
All participants underwent AIH and sham protocols during wakefulness in the supine position on two different occasions during the daytime. Each subject underwent 15 episodes of hypoxia (1 min duration) followed by exposure (2-3 min) to room air (RA) as depicted in Figures 1A and 2A. Three cylinders containing 100% nitrogen (N2), 8% oxygen (O2), and 40% CO2 were connected to the inspiratory circuit through a gas blender (model PMR4, Orangeburg, NY). Isocapnia was maintained throughout the study by bleeding CO2 (40% gas tank, balanced with N2) into the circuit and manually adjusting the flow at the blender as hypoxia was induced to prevent hypocapnia. During this procedure, PetCO2 was measured continuously, and the CO2 flow was adjusted to maintain PetCO2 near its baseline level. In the sham protocols, subjects breathed RA for the same duration of time that elapsed as during the AIH protocols. If breathing was unstable before beginning an AIH protocol, CO2 gas was added to the inspiratory circuit and flow was titrated at the blender until breathing stabilized. This titrated flow of CO2 was then maintained for the entire study in both AIH and sham protocols.
Fig. 1.

A representative polygraph recording of intermittent hypoxia protocol from an able-bodied control subject that illustrates respiratory changes (A) and heart rate changes (B) using R-R interval before, during, and 40 min after acute intermittent hypoxia (AIH).
Fig. 2.

A representative polygraph recording of intermittent hypoxia protocol in a subject with cervical spinal cord injury (SCI) that illustrates respiratory changes (A) and heart rate changes (B) using R-R interval before, during, and 40 min after AIH during recovery while breathing room air.
Peripheral Chemoreceptor Activity Protocol
To determine the relative contribution of the peripheral chemore-ceptors to eupnic ventilation, all participants underwent brief exposure to hyperoxia during wakefulness in the supine position before the AIH protocol. Each subject underwent three episodes of hyperoxia (1 min duration) followed by exposure to RA (2 to 3 min) as depicted in Figure 3. High-flow 100% O2 was connected to the inspiratory circuit through a gas blender (model PMR4).
Fig. 3.

A representative polygraph recording of the hyperoxia protocol from a subject with cervical SCI that illustrates ventilatory changes in room air and during hyperoxia (1 min).
Data Analysis
Ventilatory measurements
Measurements were obtained on a breath-by-breath basis during baseline (1-min segment before first hypoxia) and recovery (measured at 20 [R20], 25 [R25], 30 [R30], and 40 min [R40], respectively) for tidal volume (VT) and V̇e. AIH results were compared with those of sham studies that were conducted in RA over the same period without AIH. The hypoxic ventilatory response (HVR) was measured from the AIH periods and was defined as the change in V̇e for a corresponding change in SaO2 (ΔV̇e/ΔSaO2) during the AIH trials. To assess progressive augmentation in response to AIH, the first and last three hypoxia episodes were compared for each subject. LTF was defined as the percent change in V̇e during the recovery period compared with baseline. Breath-to-breath variability was measured using coefficients of variation for V̇e (CV-V̇e) at baseline and recovery periods in all subjects during AIH and sham protocols. For hyperoxia experiments, the ventilatory response to hyperoxia was defined as the V̇e (nadir breath) following the acute hyperoxia exposure compared with the preceding baseline (10 breaths).
Heart rate variability measurements
The heart rate (HR) and ECG measurements were obtained throughout the study for both AIH and sham protocols. Measurements were obtained on a beat-by-beat basis during baseline and recovery for RRI and HR variability (HRV) using spectral power analysis. Specifically, HRV spectral analysis was performed using a fast Fourier transform size of 1,024, and a Welch window. HRV spectral analysis was employed in 2-min stable wake segments during baseline and recovery for each subject. Normalized low-frequency power (nLF), normalized high-frequency power (nHF), and the low-frequency power to high-frequency power ratio (LF/HF) were determined. To assess immediate cardiac responses to AIH, HR and RRI were also measured during each AIH episode at baseline, at the nadir SaO2 and following AIH termination as depicted in Figs. 1B and 2B.
Statistical Analysis
One-way ANOVA tests were used to compare all demographic variables, baseline characteristics, ventilatory and HR data, and mean HVR. Two-way repeated-measures ANOVA was used to compare V̇e normalized to baseline between R20 and R40 in the three groups (cervical SCI vs. thoracic SCI vs. able-bodied control subjects). A one-way ANOVA was used to compare VT and breathing frequency (FB) in the three groups (cervical SCI vs. thoracic SCI vs. able-bodied control subjects) in each AIH and sham protocol. Two-way repeated-measures ANOVA was used to compare CV-V̇e between baseline and recovery for AIH and sham protocols. Analysis included n = 8/group for AIH studies, and for sham studies data from three subjects were removed (two subjects with cervical SCI and one able-bodied control individual) due to technical problems. Two-way repeated-measures ANOVA was used to compare the mean HRV (LF, HF, and LH/HF) at baseline and recovery periods between the three groups and between AIH and sham protocols. The Student-Newman-Keuls test was used for all post hoc pairwise multiple comparisons. To ascertain potential determinants of the LTF in all three groups, a Spearman’s correlation test was used to assess the relationship between V̇e (% of baseline) and the following variables: age, body mass index (BMI), and apnea hypopnea index (AHI). A multiple linear regression analysis was used to ascertain potential determinants of ventilatory LTF using the correlating variables described above and level of SCI (level 1, able-bodied; level 2, thoracic SCI; level 3, cervical SCI). All analyses were performed using SigmaStat software (v.12.5; Systat Software, Richmond, CA). A value of P < 0.05 was considered significant.
RESULTS
We studied 24 human subjects (8 in each group) during wakefulness in the supine position. All subjects completed both AIH and sham protocols (although the sham protocol was excluded in two subjects with cervical SCI and one able-bodied subject due to technical problems). The three groups had similar demographics; the main difference was a higher AHI and oxygen desaturation index in individuals with cervical SCI compared with those with thoracic SCI (Table 1). The majority of subjects with spinal injury (6 of 8 with cervical SCI and all subjects with thoracic SCI) were classified as A on the ASIA impairment scale. The remaining subjects were classified C or D (both with cervical SCI). There was no difference in ventilatory baseline parameters among the three groups (Table 2). RA ventilation occurred for 2–3 min between hypoxia episodes to achieve a complete return to normoxia in all subjects, especially those who had unstable breathing. The average time between hypoxia trials and RA ventilation was similar among the three groups (cervical SCI, 2.2 ± 0.5 min; thoracic SCI, 2.6 ± 0.9 min; able-bodied controls, 2.3 ± 0.3 min; P = 0.63). The majority of the AIH and sham studies (71%) were performed during a similar time of the day (within 1 h).
Table 1.
Subjects characteristics
| Cervical SCI | Thoracic SCI | Able-bodied | |
|---|---|---|---|
| Number | 8 | 8 | 8 |
| Age, yr | 44.3 ± 15.1 | 37.0 ± 14.9 | 35.5 ± 18.1 |
| BMI, kg/m2 | 25.6 ± 4.9 | 26.9 ± 5.4 | 26.9 ± 4.1 |
| Gender, M/F | 7/1 | 4/4 | 5/3 |
| NC, cm | 39.1 ± 2.9 | 37.2 ± 3.1 | 35.5 ± 4.0 |
| Time since SCI, yr | 11.8 ± 6.5 | 13.9 ± 6.1 | — |
| AHI, event/h | 30.7 ± 18.6* | 10.4 ± 10.5 | 5.5 ± 3.7 |
| ODI, event/h | 20.4 ± 19.2* | 5.5 ± 12.1 | 1.2 ± 1.0 |
AHI, apnea-hypopnea index; BMI, body mass index; NC, neck circumference; ODI, Oxygen desaturation index; SCI, spinal cord injury. Data are means ± SD.
Cervical SCI vs. able-bodied controls, one-way ANOVA P < 0.05.
Table 2.
Ventilatory parameters at baseline and acute hypoxia
| Cervical SCI
|
Thoracic SCI
|
Able-bodied
|
||||
|---|---|---|---|---|---|---|
| Baseline | Hypoxia | Baseline | Hypoxia | Baseline | Hypoxia | |
| V̇e, liter/min | 7.4 ± 2.3 | 16.0 ± 7.4* | 6.7 ± 1.9 | 16.6 ± 4.1* | 8.7 ± 1.9 | 13.6 ± 1.9* |
| VT, liter | 0.5 ± 0.2 | 1.0 ± 0.3* | 0.4 ± 0.1 | 0.9 ± 0.2* | 0.5 ± 0.1 | 0.9 ± 0.3* |
| FB, breaths/min | 14.5 ± 2.3 | 15.2 ± 3.1 | 15.5 ± 2.9 | 18.6 ± 3.5 | 19.2 ± 4.9 | 17.5 ± 4.9 |
| TI, s | 1.9 ± 0.3 | 2.0 ± 0.4 | 1.8 ± 0.4 | 1.5 ± 0.3 | 1.5 ± 0.4 | 1.8 ± 0.8 |
| TE, s | 2.4 ± 0.6 | 2.2 ± 0.6 | 2.2 ± 0.4 | 1.9 ± 0.3 | 1.9 ± 0.4 | 2.1 ± 0.7 |
| PetCO2, mmHg | 40.3 ± 4.5 | 42.5 ± 4.1* | 39.6 ± 2.3 | 41.0 ± 3.0* | 36.6 ± 3.3 | 37.8 ± 3.8 |
| SaO2, % | 96.8 ± 1.3 | 84.9 ± 2.2* | 96.7 ± 2.4 | 86.1 ± 1.3* | 97.3 ± 1.5 | 85.9 ± 1.1* |
V̇e, minute ventilation; VT, tidal volume; FB, breathing frequency; TI, inspiratory time; TE, expiratory time; PetCO2, end-tidal CO2; SaO2, oxygen saturation. Data are means ± SD.
Baseline vs. hypoxia, two-way repeated-measures ANOVA and Student-Newman-Keuls post hoc significance, P < 0.05.
Ventilatory Responses to Acute Hyperoxia and Hypoxia
Compared with able-bodied subjects and those with thoracic SCI, subjects with cervical SCI experienced a significant decrease in V̇e (compared with baseline values) within the first 20 s of hyperoxia (mean FiO270%) as depicted in the representative polygraph (Fig. 3). Figure 4, A and B, shows the summary data for the ventilatory responses to acute hyperoxia and hypoxia, respectively, among the three groups. Note that after hyperoxia, V̇e decreased significantly in individuals with cervical SCI compared with able-bodied control subjects and those with thoracic SCI (P < 0.05).
Fig. 4.

A: summary data to illustrate ventilatory response to hyperoxia (HO-V̇e) presented as (%) a change in minute ventilation (V̇e) from baseline in able-bodied subjects (n = 8) and those with cervical SCI (n = 8) or thoracic SCI (n = 8). B: hypoxic ventilatory response (HVR) during hypoxia episodes in groups of able-bodied individuals (n = 8) and those with cervical SCI (n = 7) or thoracic SCI (n = 8). Note that HO-V̇e decreased significantly in the group of individuals with cervical SCI compared with baseline. *Cervical SCI vs. able-bodied controls (one-way, repeated-measures ANOVA and Student-Newman-Keuls post hoc significance P < 0.05).
Although there was no significant difference in HVR among three groups, HVR in subjects with cervical SCI and thoracic SCI was higher than it was in able-bodied control subjects (P = 0.08). When data were combined from both groups of individuals with cervical and thoracic SCI, the HVR was significantly higher than it was in able-bodied individuals (P = 0.02). Furthermore, there was no significant difference in HVR between the initial (first three) and final (last three) hypoxia episodes in any group as depicted in Figure 5 (P = 0.48).
Fig. 5.

Summary data to illustrate hypoxic ventilatory response (HVR) during early (black bars) and late (white bars) AIH episodes in groups of able-bodied individuals (n = 8) and those with cervical SCI (n = 8) or thoracic SCI (n = 8). Two-way repeated-measures ANOVA, Student-Newman-Keuls post hoc test.
Effect of AIH on ventilation
Exposure to hypoxia was associated with similar degrees of oxyhemoglobin desaturation and a similar increase in V̇e (P < 0.05) (Table 2). The addition of supplemental CO2 maintained isocapnia or mild hypercapnia during the hypoxic periods (P < 0.05). Termination of hypoxia was associated with persistent hyperpnea in subjects with cervical SCI, but not in able-bodied individuals or those with thoracic SCI. Figure 6 depicts the changes in V̇e during the posthypoxic recovery period. V̇e during the recovery period (between R20 and R40) was higher than baseline control levels in individuals with cervical SCI (P < 0.05), but not in individuals with thoracic SCI (P = 0.09) or able-bodied participants (P = 0.16). Increased V̇e in participants with cervical SCI was due to increased VT to 126.3 ± 22.3% of baseline (P < 0.05) without corresponding changes in respiratory frequency (P = 0.30; Fig. 7, A and B). However, increased VT in the recovery period was noted in both groups of individuals with SCI (P < 0.05), but it was higher in individuals with cervical SCI compared with those with thoracic SCI (P < 0.05). There was no increase in VT in the able-bodied group. There was no hyperpnea in the corresponding recovery period during the sham study (Fig. 7, C and D). Specifically, V̇e in the sham protocol during the recovery period was 91.5 ± 8.2% of baseline in individuals with cervical SCI compared with 98.0 ± 9.6% in individuals with thoracic SCI (P = 0.55) and 93.5 ± 9.6% in able-bodied individuals (P = 0.72). The ventilatory variability for V̇e (CV-V̇e) during AIH studies was significantly higher in individuals with cervical SCI than in able-bodied control individuals and those with thoracic SCI, both at baseline and recovery (P < 0.05). Following AIH, individuals with thoracic SCI exhibited an increase in CV-V̇e during recovery periods compared with baseline values (P < 0.05). CV-V̇e, however, did not change either in individuals with cervical SCI (P = 0.14) nor those who were able-bodied (P = 0.20) (Fig. 8A). On the other hand, there was a significant increase in CV-V̇e in individuals with cervical SCI during the sham protocol compared with able-bodied individuals (P < 0.05), but there was not a significant difference in CV-V̇e between those with thoracic SCI and cervical SCI (P = 0.05) or between those with thoracic SCI and able-bodied individuals (P = 0.12) (Fig. 8B). There also was no significant increase in CV-V̇e during the recovery following the sham protocol compared with baseline values (P = 0.17).
Fig. 6.

Minute ventilation (V̇e) changes during recovery from acute intermittent hypoxia at 20 min (R20) through 40 min (R40) in groups of able-bodied individuals (n = 8) and those with cervical SCI (n = 8) or thoracic SCI (n = 8). *Cervical SCI vs. able-bodied controls (two-way repeated-measures ANOVA and Student-Newman-Keuls post hoc significance P < 0.05).
Fig. 7.

Ventilatory changes presented as the average (%) change from baseline in tidal volume (VT) (A) and frequency (FB) (B) during the period between 20 and 40 min of recovery after acute intermittent hypoxia (AIH) in three groups of individuals: able-bodied, and those with cervical SCI or thoracic SCI. Ventilatory changes from baseline are presented as means ± SE for VT (C) and FB (D) during the sham protocol. For AIH studies, n = 8/group except for sham studies where n = 6 for the cervical SCI group and n = 7 for the able-bodied group. +Vs. able-bodied controls, *vs. thoracic SCI group (one-way ANOVA and Student-Newman-Keuls post hoc significance P < 0.05).
Fig. 8.
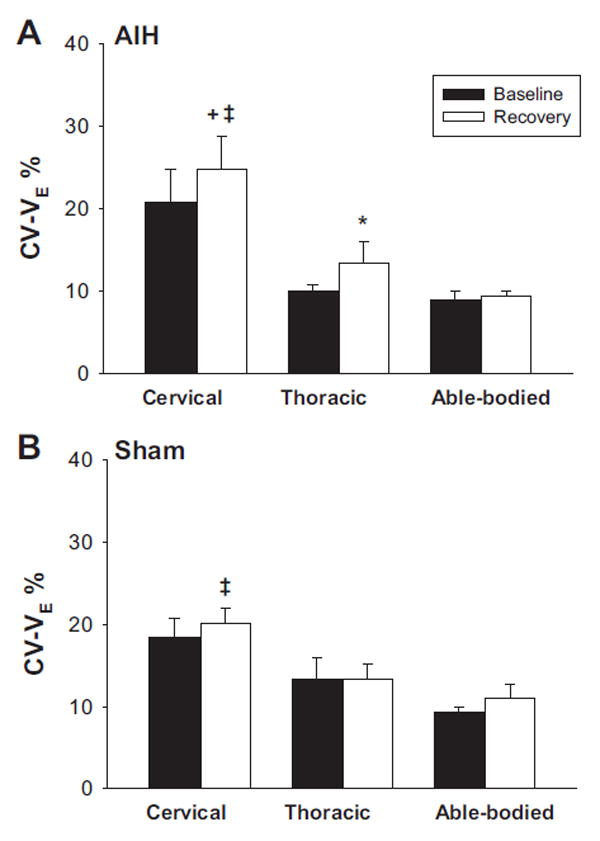
Ventilatory coefficient of variation (CV-V̇e) presented as % change in variation for V̇e during baseline control (black bars) and recovery (white bars) following acute intermittent hypoxia (A) and sham (B) protocols in three groups of able-bodied control subjects and those with cervical SCI or thoracic SCI. Data are means ± SE, n = 8/group for AIH studies except for sham studies for which n = 6 for the cervical SCI group and n = 7 for the able-bodied group. *Vs. baseline, 3vs. thoracic SCI, ‡vs. able-bodied (two-way repeated measures ANOVA and Student-Newman-Keuls post hoc significance P < 0.05).
Determinants of posthypoxic ventilation
To ascertain potential determinants of V̇e in the recovery period, we used a Spearman’s correlation analysis (n = 24). This analysis revealed that V̇e in the recovery period correlated with BMI (R =−0.54, P = 0.006), level of injury (R = 0.62, P = 0.001), and age (R = 0.52, P = 0.009) but did not correlate with AHI (P = 0.18). Using multiple linear regression models, the linear combination of SCI level (P = 0.007) and BMI (P = 0.006) predicted ventilatory LTF. For each level increase (able-bodied control > thoracic SCI > cervical SCI) in the group, posthypoxia recovery V̇e increased 10% from baseline. Moreover, in a subset of subjects matched for AHI and other demographic characteristics (Table 3), V̇e and VT remained higher in individuals with cervical SCI than in the other two groups during recovery.
Table 3.
Results of ventilation change during recovery in a subset of matched subjects for AHI and respective characteristics
| Subject | SCI status | VT, % | VE, % | Age, yr | BMI, kg/m2 | Gender | NC, cm | AHI, event/h |
|---|---|---|---|---|---|---|---|---|
| 1 | SCI-C | 156.7 | 123.5 | 56 | 25.6 | F | 34.5 | 9.3 |
| 2 | SCI-C | 153.8 | 134.5 | 33 | 19.3 | M | 39.0 | 11.7 |
| 3 | SCI-T | 118.5 | 96.8 | 28 | 30.2 | M | 43.5 | 10.3 |
| 4 | SCI-T | 107.3 | 103.5 | 71 | 35.0 | F | 38.0 | 9.9 |
| 5 | Able | 83.5 | 84.9 | 70 | 30.3 | M | 39.0 | 9.7 |
| 6 | Able | 103.2 | 89.4 | 23 | 30.1 | M | 41.0 | 4.1 |
AHI, apnea-hypopnea index; BMI, body mass index; NC, neck circumference; V̇e, minute ventilation; VT, tidal volume (% of baseline).
Effect of AIH on HRV
Figure 9A illustrates the group changes in HR before, during, and after each hypoxia episode. Note that baseline RRIs were similar between able-bodied individuals and those with cervical SCI (P = 0.77). Hypoxia was associated with decreased RRI (74.3 ± 5.7%, 71.4 ± 12.1% and 71.8 ± 13.2% baseline in cervical SCI, thoracic SCI, and able-bodied groups, respectively; P <0.05). Compared with baseline values, nLF and LH/HF during the recovery period did not increase in individuals with cervical SCI, contrary to that in the groups of able-bodied individuals and those with thoracic SCI as shown in Fig. 9B (P <0.05).
Fig. 9.

Cardiac changes in R-R interval (RRI) and heart rate variability. A: RRI at baseline (black bars), during hypoxia (white bars), and during posthypoxia (gray bars) in able-bodied individuals and those with cervical SCI or thoracic SCI. B: heart rate variability changes using power frequency analysis presented as the low-frequency-power to high-frequency-power ratio (LF/HF) for baseline while breathing room air (black bars) and during recovery while breathing room air (gray bars) in the three groups. *Vs. baseline, +vs. baseline, ‡vs. cervical SCI (two-way repeated measures ANOVA and Student-Newman-Keuls post hoc significance P < 0.05).
DISCUSSION
Our study demonstrated the following novel and significant findings regarding the effects of AIH on the respiratory and cardiac systems both in able-bodied subjects and those with chronic SCI: 1) subjects with chronic SCI experience a significant increase in V̇e and VT (compared with prehypoxia base-line levels) during the recovery phase after AIH. The increased VT was most prominent in participants with cervical SCI, less prominent in those with thoracic SCI, and absent in able-bodied subjects. 2) Hyperoxia was associated with decreased V̇e in individuals with cervical SCI compared with those with thoracic SCI and those who were able-bodied. 3) HVR was similar among the three groups. 4) Subjects with cervical SCI demonstrated a cardiac response to acute hypoxia that was manifested by a decreased RRI similar to that in subjects with thoracic SCI and those who were able-bodied. 5) Heart rate variability, specifically nLH and the LH/HF ratio, did not change significantly in subjects with cervical SCI during the recovery period after AIH in contrast to able-bodied subjects and those with thoracic SCI.
Methodological Considerations
Several considerations may influence the interpretation of the findings in this study. First, ventilatory changes in the recovery period could not be due to time-related ventilatory changes, given the lack of change in V̇e and VT during the sham studies. Second, differences in the magnitude of LTF could not be explained by differences in gender distribution or the different prevalence of SDB among the three groups. Previous studies in a rat model showed that there are sex differences in phrenic LTF following C2 hemisection (15). Studies in humans, however, have demonstrated no gender difference in LTF (1, 2, 54). Likewise, LTF following AIH is less pronounced in patients with sleep apnea relative to non-apneic patients (1). Moreover, when we compared a subset of subjects from each group, matching for AHI, those with cervical SCI had higher V̇e than able-bodied subjects and those with thoracic SCI. Therefore, robust LTF in the group with cervical SCI was due to the cervical spine injury per se and not to the underlying SDB. Third, ventilatory variability measured by CV-V̇e increased in subjects with cervical SCI from baseline to recovery in AIH protocols but not the sham protocols, indicating that the increased CV-V̇e is likely due to intermittent hypoxia. Fourth, we were unable to obtain power frequency (nLH and nHF) during brief hypoxia periods because this type of analysis requires longer duration than our brief hypoxic periods. To mitigate this limitation, we measured R-R intervals during hypoxia and the frequency component of the HRV during baseline and recovery periods in all subjects. Finally, our experimental paradigm was limited to wakefulness, and we are unable to draw firm conclusions regarding LTF during sleep in patients with SCI.
Long-Term Facilitation in Chronic SCI
The major finding of our study was that AIH resulted in hyperpnea during the recovery period in individuals with SCI, but not in able-bodied individuals despite similar respiratory parameters at baseline (Table 2). Ventilatory LTF manifested by increased VT and V̇e, without a change in respiratory frequency. This finding corroborates previous studies in awake patients with SCI (56) and in sleeping, able-bodied individuals (2, 41), demonstrating evidence of LTF in the aftermath of repetitive hypoxia. In contrast, the pattern of LTF in humans differs from that in rodent models, which manifests as a significant increase in both frequency and VT after AIH (5), the ventilatory LTF in humans is mainly due to increased VT. Increased VT and V̇e in participants with SCI in our study are suggestive of phrenic LTF.
The lack of ventilatory LTF in able-bodied individuals in this study is in agreement with data from previous studies demonstrating the absence of LTF during wakefulness in healthy humans or during sleep in patients with obstructive sleep apnea (1, 26). LTF during wakefulness in able-bodied humans may require significant hypercapnia (4–6 mmHg greater than baseline levels) that is sustained throughout the hypoxic exposure and the recovery period (24). In contrast, patients with SCI demonstrated ventilatory LTF even under conditions of isocapnia or mild hypercapnia (1–2 mmHg above baseline) during hypoxia trials followed by returning to base-line end-tidal CO2 levels between hypoxic episodes and during the recovery phase. It is of note that the addition of supplemental CO2 alters the relationship between arterial and end-tidal PCO2, resulting in a slightly negative PaCO2-PetCO2 difference of 1.0 to 1.5 mmHg and an underestimation of PaCO2 during the hypoxic episodes (4). Thus our hypoxic episodes were either isocapnic or slightly hypercapnia. Therefore, the propensity to develop LTF following AIH was enhanced in patients with SCI and did not require hypercapnia. Another important observation in this study was the increased VT during recovery after AIH in the group of individuals with cervical SCI compared with able-bodied subjects and those with thoracic SCI (Fig. 7A). This increase in tidal volume during recovery in individuals with cervical SCI compared with those with thoracic SCI suggests that the activation of LTF in humans with SCI could be dependent on the level of injury.
Our study corroborates the findings of Tester et al. (56) who demonstrated, using an AIH protocol, that ventilatory LTF is acutely increased in patients with SCI during wakefulness when end-tidal CO2 remained elevated during recovery. Our study showed, however, that LTF was elicited during RA recovery even in the absence of sustained hypercapnia during the posthypoxic (RA) recovery period, and that the degree of ventilatory LTF was related to the level of SCI (more in cervical SCI than in thoracic SCI).
Several factors may influence the presence of ventilatory plasticity, including arousal state, age, gender, and the presence of SDB. Although it has been shown in animal models that age and gender may affect phrenic LTF (14, 59), human studies did not show gender-related differences in ventilatory LTF (52). SDB may influence the magnitude of LTF in humans during wakefulness and sleep, as has been shown previously in non-injured subjects (1, 2, 51), but not in SCI as we observed in our study. When a subset of subjects matched for AHI and other demographics were compared, ventilatory LTF remained higher in patients with cervical SCI than in able-bodied individuals or those with thoracic SCI (Table 3).
The precise mechanisms responsible for ventilatory LTF in individuals with chronic SCI are unknown. We considered several possible mechanisms on the basis of available literature, mainly performed in animal models of SCI, including central (supraspinal), spinal, or peripheral mechanisms. First, activation of LTF is a serotonin-dependent central nervous system (CNS) phenomenon most likely acting via the serotonin 2A receptor on phrenic motor neurons (27, 35, 37). The neuroplastic changes in respiratory motor output after SCI in animal models coincides with the spontaneous recovery of phrenic function, some of which is modulated by serotonin-containing neurons (19). Chemo-afferent neuron activation during hypoxia results in serotonin receptor activation, which is necessary to initiate phrenic LTF but not to maintain it (17). Second, SCI may be associated with enhanced LTF due to mechanisms that are similar to those triggered by cervical dorsal rhizotomy in the absence of a direct contusion of the spinal cord. Evidence from animal studies has indicated that SCI may cause interruption of the raphe-spinal pathway and lead to a reduction in spinal serotonin content (35). AIH can induce the release of serotonin in rodent models following cervical SCI (3, 20). Moreover, cervical spinal deafferentation enhances phrenic LTF after intermittent hypoxia by increasing brain-derived neurotrophic factor and neurotrophin-3 protein concentrations within neurons of the ventral spinal cord, augmenting the descending serotonergic pathways from the raphe nuclei to the spinal dorsal horn and increasing the serotonin terminal density near phrenic motor neurons (27). Furthermore, it has been reported that exposure to intermittent hypoxia evokes short-term potentiation of phrenic motor neuron discharge after chronic cervical SCI in animal models (14, 29). Accordingly, enhanced LTF in patients with SCI, especially cervical SCI, could be explained by injury-related spinal respiratory motor plasticity. Finally, LTF following AIH may also be explained by increased afferent chemo-transmission from the carotid bodies to the to the raphe nuclei resulting in an increased density of serotonergic neurons and hence the intensity of LTF (42). The increased peripheral chemoreceptor contribution to eupnic ventilation, as evidenced by the augmented response to hyperoxia in patients with SCI is consistent with the hypothesis that augmented LTF in patients with SCI, especially in those with cervical SCI, was due to increased afferent activity from the peripheral chemoreceptors. The dynamic interaction between peripheral and central chemoreceptors in humans may contribute to ventilatory changes and unstable breathing under conditions of hypoxia (13). The development of LTF may reflect an augmentation of the magnitude of activation of sensory LTF by chronic intermittent hypoxia. However, the lack of difference in HVR among individuals in the three groups is inconsistent with sensory LTF playing a significant role.
In summary, LTF is a central neural mechanism elicited by intermittent but not continuous hypoxia (5). The occurrence of LTF in laboratory models requires spinal serotonin receptor activation and spinal protein synthesis (6). LTF in patients with SCI could be due to cervical spinal deafferentation or to prior conditioning with chronic intermittent hypoxia (17, 21). Our data do not permit us to distinguish between these broad possibilities in a given individual. However, it is clear that the net effect of level of injury, CNS adaptive changes, and chronic intermittent hypoxia produce a robust LTF in cervical SCI, which could manifest even during wakefulness.
Effect of Acute Intermittent Hypoxia on the Cardiac Autonomic System
We found that HR increased significantly in response to acute hypoxia in all three groups (those with cervical SCI or thoracic SCI, and able-bodied individuals), indicating preserved sympathetic excitatory input to the heart. Our study demonstrated that acute cardiac response to hypoxic stimulation was preserved in patients with cervical SCI and was similar to that of able-bodied individuals (25% increase in HR in response to 10% drop in SaO2). It has been reported previously that the sympathetic cardiac activity is reduced or absent in humans with SCI (22, 28, 56) and in rats after spinal cord transection (29, 57). Other investigators reported some activity related to sympathetic spinal interneurons in the absence of supraspinal brainstem control (10). The majority of our subjects were classified A on the ASIA impairment scale and had similar reflexes to those of able-bodied controls in their HR response to acute hypoxia. Our finding is consistent with the observation that in animal models, there is significant plasticity after SCI that could explain the observed cardiac reflexes (32, 33, 34).
Our study demonstrated marked differences among the three groups in the response to nLF and LF/HF ratio, surrogate markers of sympathetic and sympathovagal balance, respectively. We observed a significant increase in nLF HRV and LF/HF ratio during RA recovery in able-bodied individuals and those with thoracic SCI but not in those with cervical SCI. Increased sympathetic cardiac response to hypoxia during recovery in able-bodied individuals and those with thoracic SCI are indicative of persistent long-term sympathetic activation following chemical stimulation. Our finding corroborates a previous study in humans showing that brief hypoxia resulted in “long-lasting sympathetic activation” during recovery (40). The lack of persistent elevation in sympathetic outflow during RA recovery in cervical SCI suggests that the sympathovagal balance is impaired. SCI can lead to an autonomic imbalance between the sympathetic and parasympathetic outflow to the heart predominantly due to loss of supraspinal sympathetic control after cervical SCI (25). Therefore, the cardiac autonomic alterations depend on the level of injury, and the severity is dependent on the relative loss of supraspinal control of the afferent inputs (23, 56).
Effect of Acute Intermittent Hypoxia on the Cardiac and Respiratory Systems
The major finding of our study was that AIH resulted in increased ventilation coupled with increased HR in individuals with SCI, even after termination of hypoxia. Our study also demonstrated that cervical SCI is associated with loss of LH, HF, or LH/HF response following AIH. Control of cardiore-spiratory responses occurs on the ventrolateral surface of the medulla, which integrates afferent inputs from central and peripheral receptors, controls the excitatory inputs to preganglionic sympathetic neurons in the spinal cord, and coordinates the primary interactions between neurons responsible for generating the respiratory rhythm and determining HRV (38). Different disturbances such as hypoxia and arousals that commonly occur in patients with SDB affect the afferent rather than the efferent control of respiratory and cardiac systems via sympathetic nerve activity (SNA) (23). Furthermore, there is evidence that SNA modulates the respiratory responses even after vagotomy and decerebration, indicating a role for central coupling between respiratory and sympathetic networks (7). This coupling seems to be an important mechanism of immediately increasing ventilation and cardiac output reflexes to allow for better tissue oxygenation and appropriate cardiovascular and respiratory responses to maintain homeostasis. The dissociation between cardiac and ventilatory responses following intermittent hypoxia in patients with cervical SCI suggests that there are different or redundant pathways for cardiac and ventilatory plasticity.
Clinical Implications
The occurrence of ventilatory LTF in patients with SCI highlights the role of neuroplasticity in ventilatory recovery following SCI and the possible additive contribution of the level of injury and underlying SDB. Accordingly, respiratory plasticity following repetitive intermittent hypoxia may be more pronounced in patients with cervical SCI than thoracic SCI or in able-bodied individuals. Second, the enhanced respiratory output in cervical SCI in response to AIH indicates a potential therapeutic target for patients who experience hypoventilation, particularly during sleep. Conceptually, using a serotonin agonist may facilitate ventilatory recovery without the deleterious effects associated with chronic intermittent hypoxia. Finally, the cardiorespiratory modulations in cervical SCI could help identify the neural pathway and allow differentiation between peripheral vs. central mechanisms of intermittent hypoxia. Thus cervical SCI could represent a potential clinical model representing a mechanism of plasticity common to other neural systems in humans.
In summary, we have shown the occurrence of ventilatory LTF induced by intermittent isocapnic hypoxia in chronic SCI. Ventilatory LTF is primarily dependent on the level of SCI. The cardiac responses to acute hypoxia stimuli are preserved in cervical SCI without significant long-term alterations in HRV during recovery.
Acknowledgments
We thank the subjects who participated in the study. We also thank Scott Maresh, who helped with RRI analysis and figure preparation.
GRANTS
Research work was supported by the United States Department of Veterans Affairs Career Development Award #IK2CX000547 and Merit Review Award #1I01CX001040 from the Clinical Science Research & Development Service of the VA Office of Research and Development.
The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Footnotes
AUTHOR CONTRIBUTIONS
A.S. and M.S.B. conception and design of research; A.T.B. performed experiments; A.T.B. and A.R. analyzed data; A.S. and M.S.B. interpreted results of experiments; A.S. prepared figures; A.S. drafted manuscript; A.S. and M.S.B. edited and revised manuscript; A.S. and M.S.B. approved final version of manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- 1.Aboubakr SE, Taylor A, Ford R, Siddiqi S, Badr MS. Long-term facilitation in obstructive sleep apnea patients during NREM sleep. J Appl Physiol. 2001;91:2751–2757. doi: 10.1152/jappl.2001.91.6.2751. [DOI] [PubMed] [Google Scholar]
- 2.Babcock M, Shkoukani M, Aboubakr SE, Badr MS. Determinants of long-term facilitation in humans during NREM sleep. J Appl Physiol. 2003;94:53–59. doi: 10.1152/japplphysiol.00476.2002. [DOI] [PubMed] [Google Scholar]
- 3.Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol. 1996;104:251–260. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- 4.Badr MS, Skatrud JB, Dempsey JA. Determinants of poststimulus potentiation in humans during NREM sleep. J Appl Physiol. 1992;73:1958–1971. doi: 10.1152/jappl.1992.73.5.1958. [DOI] [PubMed] [Google Scholar]
- 5.Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol. 2000;529:215–219. doi: 10.1111/j.1469-7793.2000.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barman SM, Gebber GL. Sympathetic nerve rhythm of brain stem origin. Am J Physiol Regul Integr Comp Physiol. 1980;239:R42–R47. doi: 10.1152/ajpregu.1980.239.1.R42. [DOI] [PubMed] [Google Scholar]
- 8.Bascom AT, Badr MS, Sankari A. Effect of episodic hypoxia on ventilation and chemosensitivity in patients with chronic spinal cord injury. Am J Respir Crit Care Med. 2014;189:A2220. Abstract. [Google Scholar]
- 9.Berlowitz DJ, Brown DJ, Campbell DA, Pierce RJ. A longitudinal evaluation of sleep and breathing in the first year after cervical spinal cord injury. Arch Phys Med Rehabil. 2005;86:1193–1199. doi: 10.1016/j.apmr.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 10.Cabot JB, Alessi V, Carroll J, Ligorio M. Spinal cord lamina V and lamina VII interneuronal projections to sympathetic preganglionic neurons. J Comp Neurol. 1994;347:515–530. doi: 10.1002/cne.903470404. [DOI] [PubMed] [Google Scholar]
- 11.Chowdhuri S, Shanidze I, Pierchala L, Belen D, Mateika JH, Badr MS. Effect of episodic hypoxia on the susceptibility to hypocapnic central apnea during NREM sleep. J Appl Physiol. 2010;108:369–377. doi: 10.1152/japplphysiol.00308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crosfill ML, Widdicombe JG. Physical characteristics of the chest and lungs and the work of breathing in different mammalian species. J Physiol. 1961;158:1–14. doi: 10.1113/jphysiol.1961.sp006750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dempsey JA, Smith CA, Blain GM, Xie A, Gong Y, Teodorescu M. Role of central/peripheral chemoreceptors and their interdependence in the pathophysiology of sleep apnea. Adv Exp Med Biol. 2012;758:343–349. doi: 10.1007/978-94-007-4584-1_46. [DOI] [PubMed] [Google Scholar]
- 14.Doperalski NJ, Fuller DD. Long-term facilitation of ipsilateral but not contralateral phrenic output after cervical spinal cord hemisection. Exp Neurol. 2006;200:74–81. doi: 10.1016/j.expneurol.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 15.Doperalski NJ, Sandhu MS, Bavis RW, Reier PJ, Fuller DD. Ventilation and phrenic output following high cervical spinal hemisection in male vs. female rats. Respir Physiol Neurobiol. 2008;162:160–167. doi: 10.1016/j.resp.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flavell H, Marshall R, Thornton AT, Clements PL, Antic R, McEvoy RD. Hypoxia episodes during sleep in high tetraplegia. Arch Phys Med Rehabil. 1992;73:623–627. [PubMed] [Google Scholar]
- 17.Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol. 2001;90:2001–2006. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- 18.Garshick E, Kelley A, Cohen SA, Garrison A, Tun CG, Gagnon D, Brown R. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord. 2005;43:408–416. doi: 10.1038/sj.sc.3101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golder FJ, Reier PJ, Bolser DC. Altered respiratory motor drive after spinal cord injury: supraspinal and bilateral effects of a unilateral lesion. J Neurosci. 2001;21:8680–8689. doi: 10.1523/JNEUROSCI.21-21-08680.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci. 2005;25:2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golder FJ. Spinal NMDA receptor activation is necessary for de novo, but not the maintenance of, A2a receptor-mediated phrenic motor facilitation. J Appl Physiol. 2009;107:217–223. doi: 10.1152/japplphysiol.00183.2009. [DOI] [PubMed] [Google Scholar]
- 22.Grigorean VT, Sandu AM, Popescu M, Iacobini MA, Stoian R, Neascu C, Popa F. Cardiac dysfunctions following spinal cord injury. J Med Life. 2009;2:133–145. [PMC free article] [PubMed] [Google Scholar]
- 23.Guzzetti S, Cogliati C, Broggi C, Carozzi C, Caldiroli D, Lombardi F, Malliani A. Influences of neural mechanisms on heart period and arterial pressure variabilities in quadriplegic patients. Am J Physiol Heart Circ Physiol. 1994;266:H1112–H1120. doi: 10.1152/ajpheart.1994.266.3.H1112. [DOI] [PubMed] [Google Scholar]
- 24.Harris DP, Balasubramaniam A, Badr MS, Mateika JH. Long-term facilitation of ventilation and genioglossus muscle activity is evident in the presence of elevated levels of carbon dioxide in awake humans. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1111–R1119. doi: 10.1152/ajpregu.00896.2005. [DOI] [PubMed] [Google Scholar]
- 25.Inskip JA, Ramer LM, Ramer MS, Krassioukov AV. Autonomic assessment of animals with spinal cord injury: tools, techniques and translation. Spinal Cord. 2009;47:2–35. doi: 10.1038/sc.2008.61. [DOI] [PubMed] [Google Scholar]
- 26.Jordan AS, Catcheside PG, O’Donoghue FJ, McEvoy RD. Long-term facilitation of ventilation is not present during wakefulness in healthy men or women. J Appl Physiol. 2002;93:2129–2136. doi: 10.1152/japplphysiol.00135.2002. [DOI] [PubMed] [Google Scholar]
- 27.Kinkead R, Zhan WZ, Prakash YS, Bach KB, Sieck GC, Mitchell GS. Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. J Neurosci. 1998;18:8436–8443. doi: 10.1523/JNEUROSCI.18-20-08436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krassioukov A, Alexander MS, Karlsson AK, Donovan W, Mathias CJ, Biering-Sorensen F. International spinal cord injury cardiovascular function basic data set. Spinal Cord. 2010;48:586–590. doi: 10.1038/sc.2009.190. [DOI] [PubMed] [Google Scholar]
- 29.Krassioukov A, Weaver LC. Episodic hypertension due to autonomic dysreflexia in acute and chronic spinal cord-injured rats. Am J Physiol Heart Circ Physiol. 1995;268:H2077–H2083. doi: 10.1152/ajpheart.1995.268.5.H2077. [DOI] [PubMed] [Google Scholar]
- 30.Lee KZ, Sandhu MS, Dougherty BJ, Reier PJ, Fuller DD. Hypoxia triggers short term potentiation of phrenic motoneuron discharge after chronic cervical spinal cord injury. Exp Neurol. 2015;263:314–324. doi: 10.1016/j.expneurol.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Jr, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci. 2001;21:5381–5388. doi: 10.1523/JNEUROSCI.21-14-05381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lujan HL, Palani G, DiCarlo SE. Structural neuroplasticity following T5 spinal cord transection: increased cardiac sympathetic innervation density and SPN arborization. Am J Physiol Regul Integr Comp Physiol. 2010;299:R985–R995. doi: 10.1152/ajpregu.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lujan HL, Janbaih H, DiCarlo SE. Dynamic interaction between the heart and its sympathetic innervation following T5 spinal cord transection. J Appl Physiol. 2012;113:1332–1341. doi: 10.1152/japplphysiol.00522.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lujan HL, Janbaih H, DiCarlo SE. Structural remodeling of the heart and its premotor cardioinhibitory vagal neurons following T5 spinal cord transection. J Appl Physiol. 2014;116:1148–1155. doi: 10.1152/japplphysiol.01285.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manaker S, Tischler LJ, Morrison AR. Raphe spinal and reticulospinal axon collaterals to the hypoglossal nucleus in the rat. J Comp Neurol. 1992;322:68–78. doi: 10.1002/cne.903220106. [DOI] [PubMed] [Google Scholar]
- 36.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 37.Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol. 1980;42:171–188. doi: 10.1016/0034-5687(80)90113-9. [DOI] [PubMed] [Google Scholar]
- 38.Millhorn DE, Eldridge FL. Role of ventrolateral medulla in regulation of respiratory and cardiovascular systems. J Appl Physiol. 1986;61:1249–1263. doi: 10.1152/jappl.1986.61.4.1249. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited Review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- 40.Morgan BJ, Crabtree DC, Palta M, Skatrud JB. Combined hypoxia and hypercapnia evokes long-lasting sympathetic activation in humans. J Appl Physiol. 1995;79:205–213. doi: 10.1152/jappl.1995.79.1.205. [DOI] [PubMed] [Google Scholar]
- 41.Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci USA. 2003;100:10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng YJ, Prabhakar NR. Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J Appl Physiol. 2004;96:1236–1242. doi: 10.1152/japplphysiol.00820.2003. [DOI] [PubMed] [Google Scholar]
- 43.Pierchala LA, Mohammed AS, Grullon K, Mateika JH, Badr MS. Ventilatory long-term facilitation in non-snoring subjects during NREM sleep. Respir Physiol Neurobiol. 2008;160:259–266. doi: 10.1016/j.resp.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Christopher & Dana Reeve Foundation. Paralysis Resource Center (Online) 2015 Jan 6; http://www.christopherreeve.org/site/c.mtKZKgMWKwG/b.5184189/k.5587/Paralysis_Facts__Figures.htm.
- 45.Quan SF, Gersh BJ. Cardiovascular consequences of sleep-disordered breathing: past, present and future: report of a workshop from the National Center on Sleep Disorders Research and the National Heart, Lung, and Blood Institute. Circulation. 2004;109:951–957. doi: 10.1161/01.CIR.0000118216.84358.22. [DOI] [PubMed] [Google Scholar]
- 46.Salloum A, Rowley JA, Mateika JH, Chowdhuri S, Omran Q, Badr MS. Increased propensity for central apnea in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2010;181:189–193. doi: 10.1164/rccm.200810-1658OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sankari A, Bascom A, Oomman S, Badr MS. Sleep disordered breathing in chronic spinal cord injury. J Clin Sleep Med. 2014;10:65–72. doi: 10.5664/jcsm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sankari A, Bascom AT, Chowdhuri S, Badr MS. Tetraplegia is a risk factor for central sleep apnea. J Appl Physiol. 2014;116:345–353. doi: 10.1152/japplphysiol.00731.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scanlon PD, Loring SH, Pichurko BM, McCool FD, Slutsky AS, Sarkarati M, Brown R. Respiratory mechanics in acute quadriplegia. Lung and chest wall compliance and dimensional changes during respiratory maneuvers. Am Rev Respir Dis. 1989;139:615–620. doi: 10.1164/ajrccm/139.3.615. [DOI] [PubMed] [Google Scholar]
- 50.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, O’Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 51.Shkoukani M, Babcock MA, Badr MS. Effect of episodic hypoxia on upper airway mechanics in humans during NREM sleep. J Appl Physiol. 2002;92:2565–2570. doi: 10.1152/japplphysiol.00938.2001. [DOI] [PubMed] [Google Scholar]
- 52.Short C, Stradling J, Williams S. Prevalence of sleep apnoea in participants over 40 years of age with spinal cord lesions. J Neurol Neurosurg Psychiatry. 1992;55:1032–1036. doi: 10.1136/jnnp.55.11.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strauss CJ, DeVivo MJ, Paculdo DR, Shavelle RM. Trends in life expectancy after spinal cord injury. Arch Phys Med Rehabil. 2006;87:1079–1085. doi: 10.1016/j.apmr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 54.Syed Z, Lin HS, Mateika JH. The impact of arousal state, sex, and sleep apnea on the magnitude of progressive augmentation and ventilatory long-term facilitation. J Appl Physiol. 2013;114:52–65. doi: 10.1152/japplphysiol.00985.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil. 2000;81:506–516. doi: 10.1053/mr.2000.3848. [DOI] [PubMed] [Google Scholar]
- 56.Tester NJ, Fuller DD, Fromm JS, Spiess MR, Behrman AL, Mateika JH. Long-term facilitation of ventilation in humans with chronic spinal cord injury. Am J Respir Crit Care Med. 2014;189:57–65. doi: 10.1164/rccm.201305-0848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wallin G. Abnormalities of sympathetic regulation after cervical cord lesions. Acta Neurochir Suppl (Wien) 1986;36:123–124. doi: 10.1007/978-3-7091-8859-0_33. [DOI] [PubMed] [Google Scholar]
- 58.Weaver EM, Collins EG, Kurichi J, Miskevics S, Smith B, Rajan S, Gater D. Prevalence of obesity and high blood pressure in veterans with spinal cord injuries and disorders: a retrospective review. Am J Phys Med Rehabil. 2007;86:22–29. doi: 10.1097/phm.0b013e31802b8937. [DOI] [PubMed] [Google Scholar]
- 59.Zabka AG, Behan M, Mitchell GS. Long term facilitation of respiratory motor output decreases with age in male rats. J Physiol. 2001;531(Pt 2):509–514. doi: 10.1111/j.1469-7793.2001.0509i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


