Abstract
Individuals who undergo multiplex direct-to-consumer (DTC) genomic testing receive genetic risk results for multiple conditions. To date, research has not investigated the influence of individual differences in disease perceptions among consumers on testing outcomes. A total of 2037 participants received DTC genomic testing and completed baseline and follow-up surveys assessing disease perceptions and health behaviors. Participants were asked to indicate their most feared disease of those tested. Perceived seriousness and controllability of the disease via lifestyle or medical intervention were assessed. Participants most frequently reported heart attack (19.1%) and Alzheimer’s disease (18.6%) as their most feared disease. Perceived seriousness and control over the feared disease both influenced response to DTC genomic testing. Greater perceived seriousness and diminished perceived control were associated with higher, but not clinically significant levels of anxiety and distress. In some cases these associations were modified by genetic risk. No significant associations were observed for diet, exercise and screening behaviors. Individual differences in disease perceptions influence psychological outcomes following DTC genomic testing. Higher perceived seriousness may make a consumer more psychologically sensitive to test results and greater perceived control may protect against adverse psychological outcomes. Findings may inform development of educational and counseling services.
Keywords: direct-to-consumer, direct-to-consumer outcomes, genetic testing, genomic risk assessment, Health Belief Model, personalized medicine, risk perceptions
Direct-to-consumer (DTC) genomic testing utilizes high-throughput genotyping technologies to provide genetic testing and disease risk estimates directly to individuals. Consumers receive risk estimates for a wide range and large number of diseases. However, individuals undergoing testing very likely respond differently to the information depending on what conditions they are most fearful of and their beliefs about those conditions. Taken together with genetic risk results, these factors may impact psychological and behavioral outcomes for DTC genomic testing consumers.
Provision of genetic testing without the involvement of a health care intermediary, i.e. in a ‘DTC’ fashion, has led to controversy among researchers and health care providers (1–3). One primary reason for this is concern regarding the impact of such testing on consumers, including the behavioral and psychological effects. Recently, the US Food and Drug Administration (FDA) weighed in on the debate and issued a warning letter to 23andMe, arguably the leading company specializing in DTC genomic testing (4, 5). The letter questioned the safety of delivering genetic risk information directly to consumers and directed the company to stop marketing their health-related tests. As a result, now 23andMe only provides raw data and ancestry information to new customers and has suspended the return of all health-related genetic risk estimates until the FDA’s concerns are resolved.
Studies carried out to date have suggested that DTC genomic testing does not lead to adverse psychological problems, either at short-term follow-up intervals (2, 6–9) or more than 1 year out from testing (10). Two studies have even suggested that DTC genomic testing may result in healthy behavioral changes (7, 8). Recent current events with respect to DTC genomic testing emphasize the need for further research to generate additional empirical evidence speaking to these issues. This is particularly needed as genomic technologies continue to advance and testing has the potential to become even cheaper and more routine (11, 12). One area in need of study is the extent to which individual differences among consumers play a role in psychological and behavioral outcomes following testing. Specifically, examining an individual’s most feared diseases following DTC genomic testing, and their beliefs about those diseases, can help further clarify the impact of testing.
The Health Belief Model provides a framework for understanding and predicting health-related behavior (13, 14). A specific construct of the model, perceived seriousness, measures an individual’s beliefs regarding the severity and seriousness of a disease. Perceived seriousness of a disease has been shown to impact psychological and health-related behaviors pertaining to that disease (14). Therefore, the perceived seriousness of a feared disease deserves particular consideration. Likewise, in addition to seriousness, perceived level of control over the development of a disease, both via lifestyle or medical intervention, is also thought to impact health-related behaviors, and is thus worthy of study (9).
This study examines consumers’ most feared disease after receiving genetic results, their beliefs about seriousness and control with respect to that disease, and how those beliefs may interact with genetic risk and associate with DTC genomic testing behavioral outcomes. In short, we aimed to examine individual differences in participant health perceptions and beliefs, and whether variations in these beliefs influence levels of psychological distress, lifestyle changes (e.g. exercise and diet), engagement in health screenings, and propensity to share genetic results with a physician. While a few studies have assessed overall response to DTC genomic testing, there are no reports in the literature that have investigated the influence of individual differences in disease perceptions on consumer response to DTC genomic testing.
Materials and methods
Participants
Data were collected as part of the Scripps Genomic Health Initiative (SGHI) which is described in detail elsewhere (15). Briefly, SGHI is a longitudinal cohort study that examines the psychological and behavioral impact of DTC testing for common diseases. Participants purchased the Navigenics Health Compass, a commercially available test at the time of study initiation, at a subsidized rate. Web-based health assessments were completed prior to receiving genetic results and also 3 and 12 months after receiving the results. This study utilizes data from the baseline and 3-month follow-up surveys. Participants who did not complete the original 3-month assessment by a set deadline were given the option of completing a shorter version of the survey. Additional details regarding the follow-up assessments have been previously published (2).
Assessments
As part of the 3-month follow-up assessment, participants were asked to indicate their most feared condition out of the 23 conditions included in the initially deployed version of the Navigenics test. The item read as follows: ‘Since receiving your genetic test results, what are the “top” three medical conditions, of those assessed, you are MOST concerned about’? Participants were then prompted to answer several questions regarding their most concerning disease. For example, participants completed the seriousness subscale of the Health Belief Model scale in relation to their most feared disease.
The Health Belief Model was developed to predict health-related behavior in order to inform the development of effective interventions (13, 14). The model consists of several constructs, which include perceived seriousness, susceptibility, benefits, barriers, cues to actions and self-efficacy. The perceived seriousness sub-scale is a measure of an individual’s beliefs regarding the severity and seriousness of a disease. The scale consists of 12 items and scores can range from 12 to 60, with 25 suggested to represent an average score (16). Seriousness gauges both medical and social consequences of a disease or illness (14).
Perceived control, the belief that an individual can determine his or her own internal state and behavior, was also measured at the 3-month follow-up in reference to the individual’s most feared disease (17). This was measured on a 7-point scale (i.e. ranging from no confidence = 1 to full confidence = 7) in terms of confidence in ability to control disease development via lifestyle changes (e.g. diet, exercise, etc.) or via medical follow-up (e.g. physician appointment, increased frequency of medical check-ups, etc.). The item for control via lifestyle read as follows: ‘This condition is “actionable” in the sense that changes in my lifestyle, diet, exercise routine, etc. could decrease my degree of risk and/or improve my health outcome’. The item for control via medical follow-up read as follows: ‘The condition is “actionable” in the sense that seeing a physician more frequently, having more frequent medical check-ups, tests, etc. could decrease my degree of risk and/or improve my health outcome’.
Genomic test
For the current analyses the risk estimates delivered to the consumer by Navigenics for their disease of most concern were leveraged. Specifically, we focused our analyses on two risk information formats provided to subjects: color-coded, dichotomized high vs low genetic risk and estimated lifetime risk, which was expressed as a percentage.
Outcome measures
Outcome measures included participant changes in anxiety symptoms, genomic-test specific distress, dietary fat intake, and exercise level (18). The anxiety measure used was the Spielberger State-Trait Anxiety Inventory (STAI)(19). Scores on the STAI can range from 20 to 80, and a score greater than 39 is thought to indicate an elevated anxiety state. The Impact of Events Scale-Revised (IES-R), a measure used to gauge traumatic events, was used to measure subjective level of distress after receiving DTC genomic risk results (20, 21). The current analyses relied on the avoidance and intrusion subscales of the IES-R. On these subscales, a score of more than 8 indicates ‘some impact’ whereas 23 or higher is thought to indicate clinically significant distress. Dietary fat was measured with the Block Fat Screener (22). The Godin Leisure-Time Exercise Questionnaire was used to measure exercise activity (23). We also asked whether participants had completed any of 13 health-screening tests since receiving their results, and whether they had shared their results with their physician.
Data analysis
Analyses were conducted using the software package spss 22.0. In addition to descriptive statistics, linear and logistic regressions were used to examine the influence of perceived seriousness and perceived control of the most feared disease on the outcome measures of interest (e.g. anxiety, test-related distress, exercise, diet, physician sharing, and screening test completion). Both main effects and interaction models (perceptions × genetic risk) were tested. The eight covariates included in the analyses were age, sex, education, ancestry (White or non-White), income, health-related occupation (Scripps employee), follow-up interval in days, and completion of the original vs the short 3-month follow-up. The analyses were also adjusted for baseline measures of anxiety, dietary fat intake, and exercise level. All reported p values are corrected for multiple testing using a Bonferroni correction derived by dividing 0.05 by the number of outcome domains (psychological, lifestyle, provider sharing, and screening). As such, a p value of less than 0.0125 was considered statistically significant.
Results
Participants
A total of 2037 participants completed the 3-month follow-up assessment with data passing quality control standards. Participants completed the follow-up survey an average of 5.6 months after receiving genetic risk results. Demographic information is presented in Table 1 and descriptive statistics for the outcome variables of interest are presented in Table S1, Supporting information.
Table 1.
Descriptive statistics for demographic variables and covariates across the full cohort
| Variable | |
|---|---|
| Number of subjects | 2037 |
| Demographics | |
| Sex (% female) | 55 |
| Age in years | |
| Mean, SD | 46.7 ± 12 |
| Range | 19–85 |
| Income (median category) | 100,000–149,000 |
| Education (median category) | Some post-college education |
| Ethnicity (% Caucasian) | 84 |
| Scripps Health employee (%) | 24 |
| Short 3-month assessment (%) | 16 |
| Follow-up interval in days (mean, SD) | 168.6 ± 72.4 (5.6 months) |
Most feared diseases
Table 2 presents the five conditions most commonly selected by participants as being most feared (percentages and a ranking of all 23 diseases can be found in Fig. S1). Heart attack and Alzheimer’s disease were most frequently rated as most feared amongst participants. Frequencies differed slightly for males and females. For females, the most feared disease was Alzheimer’s disease, whereas, for males, heart attack was most feared. Percentages are listed by gender in Table S2. For the top five feared diseases among the full cohort, 69.7% of the individuals represented received a high personal genetic risk estimate.
Table 2.
Conditions most commonly selected by participants as being of most concern (top five)
| Disease | Percentage of individuals who selected (N = 2037) | Seriousnessa | Control lifestyleb | Control medical attentionc |
|---|---|---|---|---|
| Heart attack (N = 390) | 19.1 | 17.6 ± 8.5 | 5.5 ± 1.1 | 4.7 ± 1.4 |
| Alzheimer’s disease (N = 376) | 18.6 | 28.7 ± 9.4 | 3.7 ± 1.6 | 3.0 ± 1.5 |
| Type 2 diabetes (N = 195) | 9.6 | 14.7 ± 9.3 | 5.8 ± 1.1 | 5.0 ± 1.6 |
| Obesity (N = 178) | 8.7 | 17.7 ± 10.1 | 7.0 ± 1.6 | 7.0 ± 1.8 |
| Colon cancer (N = 114) | 5.6 | 18.4 ± 9.5 | 4.8 ± 1.4 | 5.1 ± 1.4 |
Higher scores equal greater level of perceived seriousness.
Higher scores equal greater perceived control via lifestyle changes.
Higher scores equal greater perceived control via seeking medical attention.
Disease perceptions
Disease perceptions, including seriousness and perceived control are presented in Tables 2 and 3. For the cohort as a whole (Table 3), mean seriousness scores were in the low average range suggesting lower levels of perceived seriousness in comparison to a control group assessed for perceived seriousness of cervical cancer (16). Mean perceived control scores for lifestyle and medical were both above neutral suggesting slightly higher than average levels of perceived control. Table 2 shows perceptions within the most frequently selected feared diseases. Alzheimer’s disease showed higher perceived seriousness and lower perceived control (both lifestyle and medical) relative to the other conditions (seriousness p < 0.001; control lifestyle p < 0.001; control medical, p < 0.001). Graphical comparisons of perceptions across the five most commonly selected feared diseases are presented in Fig. 1.
Table 3.
Descriptive statistics for disease perception scales
| Scale/variable | Clinical interpretation | |
|---|---|---|
| Perceived seriousnessa | Sample mean in low average range (z = −0.76)16 | |
| Mean, SD | 19.1 ± 10.9 | Higher levels equal greater level of perceived seriousness |
| Range | 0–48 | |
| Perceived control-lifestyleb | Sample mean above neutral (some confidence) | |
| Mean, SD | 4.7 ± 1.7 | Higher levels equal greater perceived control via lifestyle changes |
| Range | 1–7 | |
| Perceived control-medicalc | Sample mean above neutral (some confidence) | |
| Mean, SD | 4.2 ± 1.6 | Higher levels equal greater perceived control via seeking medical attention |
| Range | 1–7 |
Participant agreement on a 5-point scale on the 12 item Health Belief Model Seriousness Subscale.
Participant confidence rating on a 7-point Likert scale for the following statement: ‘This condition is ‘actionable’ in the sense that changes in my lifestyle, diet, exercise routine, etc. could decrease my degree of risk and/or improve my health outcome’.
Participant confidence rating on a 7-point Likert scale for the following statement: ‘This condition is ‘actionable’ in the sense that seeing a physician more frequently, having more frequent medical check-ups, tests, etc. could decrease my degree of risk and/or improve my health outcome’.
Fig. 1.
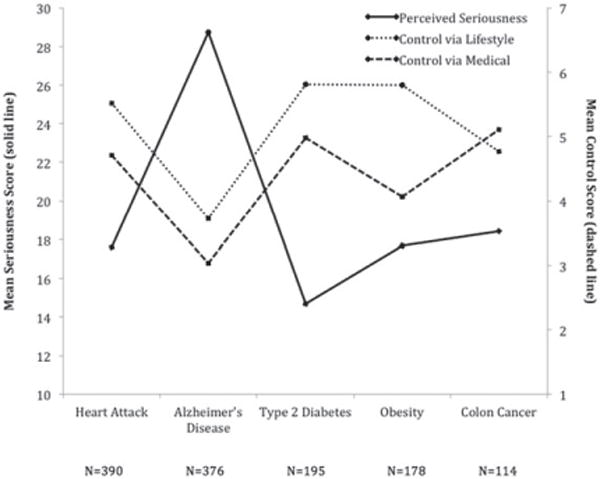
Graph comparing five most commonly selected feared diseases and differences among participant perception of each disease. Participant perception of seriousness or control of a disease varied significantly depending on disease.
Genomic risk for feared disease
A greater proportion of individuals received a high personal genetic risk result for their most feared disease relative to risk distributions for that disease in the remaining sample, suggesting that the risk result itself influenced participants’ selection of their most feared disease (Table S3). However, it is still noteworthy that a large fraction of those individuals, who said they feared a certain disease, were in fact found to be at low genetic risk for that disease suggesting that they felt fearful and concerned about the condition despite any reassurances they may have gleaned from receipt of a low genetic risk result. Consistent with this, there were no significant differences in levels of perceived seriousness or control as a function of genetic risk within those groups of individuals who reported fearing one of the top five conditions (Table S4).
Perceived seriousness, genetic risk, and response to testing
A summary of results from analyses evaluating the influence of disease perceptions and genetic risk on the outcomes of interest is presented in Table 4. There were no significant relationships between either perceived seriousness or genetic risk and follow-up fat intake or exercise behavior. Alternatively, there was a significant main effect of perceived seriousness on follow-up anxiety with higher seriousness being associated with higher anxiety. Furthermore, with respect to genomic test-related distress, there was a significant interaction between perceived seriousness and genetic risk. Specifically, participants who perceived their most feared disease as more serious and who received a high-risk result experienced higher levels of subjective test-related distress, while those at high genetic risk but with low levels of seriousness experienced the same level of distress as low risk individuals (Fig. 2). Importantly, however, the level of subjective test-related distress experienced by all groups was well below clinical threshold levels. Additional results for perceived seriousness are presented in Tables S5 and S6.
Table 4.
Summary of statistically significant results for genetic risk, disease perception and interaction modelsa
| Disease perception | Outcome | Model interpreted | Genetic risk term
|
Disease perception term
|
Interaction term
|
|||
|---|---|---|---|---|---|---|---|---|
| β | p-Value | β | p-Value | β | p-Value | |||
| Seriousness | STAIb | Main effects | −0.014 | 0.302 | 0.060 | <0.001 | – | – |
| IESb | Interaction | −0.072 | 0.081 | 0.155 | <0.001 | 0.249 | <0.001 | |
| Fat intakeb | NS | – | – | – | – | – | – | |
| Exerciseb | NS | – | – | – | – | – | – | |
| Physician sharec | Main effects | 1.5 | 0.002 | 0.993 | 0.135 | – | – | |
| Control via lifestyle changes | STAIb | Interaction | 0.078 | 0.046 | 0.051 | 0.100 | −0.127 | 0.011 |
| IESb | Main effects | 0.092 | <0.001 | −0.112 | <0.001 | – | – | |
| Fat intakeb | NS | – | – | – | – | – | – | |
| Exerciseb | NS | – | – | – | – | – | – | |
| Physician sharec | Main effects | 1.49 | 0.003 | 1.03 | 0.227 | – | – | |
| Control via medical attention | STAIb | Main effects | 0.081 | <0.001 | −0.093 | <0.001 | – | – |
| IESb | NS | – | – | – | – | – | – | |
| Fat intakeb | NS | – | – | – | – | – | – | |
| Exerciseb | NS | – | – | – | – | – | – | |
| Physician Sharec | Main effects | 1.5 | 0.002 | 1.13 | <0.001 | – | – | |
IES, Impact of Events Scale; NS, no significant models, STAI, State-Trait Anxiety Inventory.
Bold entries indicate significant results.
Linear regression models controlled for sex, ethnicity, age, income, education, health-related occupation, follow-up interval, original vs short follow-up, and baseline measures of fat intake, exercise, and STAI.
Logistic regression calculated and odds ratio reported.
Fig. 2.
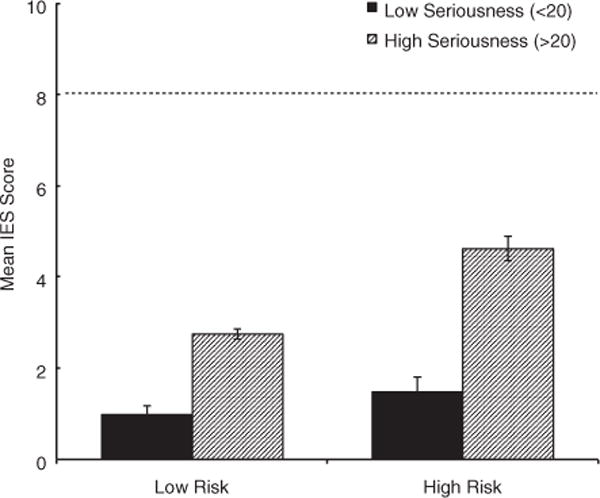
Graph of interaction between genetic risk and perceived seriousness (median split of seriousness) of feared disease on distress levels. Participants who perceived their most feared disease as more serious and received a high-risk result experienced higher levels of subjective test-related distress. Individuals at high genetic risk but with low levels of seriousness experienced the same level of distress as low risk individuals. Dashed line cut off for Impact of Events Scale (IES) indicating ‘some impact’.
Perceived control, genetic risk, and response to testing
There were no significant relationships between either perceived control (lifestyle or medical) or genetic risk and follow-up fat intake or exercise behavior. Alternatively, for both lifestyle and medical, there were significant main effects of both perceived control and genetic risk on follow-up test-related distress, with high genetic risk and low perceived control being associated with higher distress. Furthermore, with respect to anxiety, there was a significant interaction between perceived control via lifestyle and genetic risk. Specifically, participants who not only received a high genetic risk result but also perceived their most feared disease as controllable via lifestyle changes, showed the lowest levels of follow-up anxiety of all groups tested (Fig. 3). Again, however, the levels of subjective test-related distress and anxiety experienced by all groups were well below clinical threshold levels. Additional results for perceived control are presented in Tables S7 and S8.
Fig. 3.
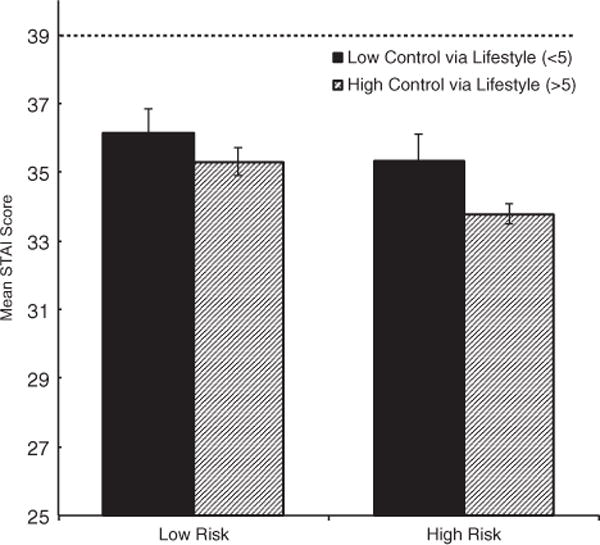
Graph of interaction between genetic risk and perceived control (median split of control) of feared disease on anxiety. Participants who not only received a high genetic risk result but also perceived their most feared disease as controllable via lifestyle changes, showed the lowest levels of follow-up anxiety. Dashed line represents clinically significant cut off for State-Trait Anxiety Inventory (STAI).
Perceived seriousness and control, genetic risk, and physician sharing
Genetic risk, but not perceived seriousness or control via lifestyle changes was associated with physician sharing (Table 4). Specifically, higher genetic risk predicted a greater likelihood of sharing with one’s physician. Alternatively, however, with respect to perceived control via medical changes, there were significant main effects of both genetic risk and perceived control, characterized by greater genetic risk and greater perceived control via medical attention being associated with greater likelihood of sharing with one’s physician (Table 4). Additional analyses can be found in Tables S5–S8.
Perceived seriousness and control, genetic risk, and health screenings
Additional analyses were conducted to determine if a participant’s perceived seriousness or control of a feared disease influenced the completion of health screening tests. Overall, the number of participants who completed health screens for one of the top five feared diseases ranged from 8% to 77% (see Table S9 and S2). In these analyses, only one significant result emerged, which pertained to individuals who endorsed breast cancer as their most feared disease (see Tables S10–S12). In this group, there were significant main effects of both perceived control via lifestyle changes and genetic risk on completion of breast self-checks with higher perceived control and high genetic risk being associated with an increased likelihood of engaging in self-check (see Table S11). None of the interaction models tested was significant (data not shown).
Discussion
This study aimed to assess the impact of DTC genomic testing as a function of individual differences in disease perceptions, beliefs and personal genetic risk for a consumer’s most feared disease. Perceived seriousness and perceived control over a feared disease significantly influenced participants’ psychological, but not behavioral outcomes. While a few studies have assessed overall response to testing, this is the first report of which we are aware that has investigated individual differences in disease perceptions and how those perceptions interact with genetic risk to influence response to DTC genomic testing.
This study found that the combination of high-perceived seriousness and a high-risk genetic result for a feared disease was associated with an increase in genomic test-related distress. Importantly, however, the level of subjective distress associated with DTC testing, even when a feared disease is perceived as serious, is not in the clinically significant range. Similarly, higher perceived control over the development of a feared disease was associated with lower distress, particularly in the face of receiving a high-risk genetic result for that disease. These findings are consistent with prior research on the perceptual and cognitive factors underlying illness representations (24). The Common-Sense Model has demonstrated that the perception of control or curability of an illness is positively associated with better psychological and functional outcomes (25). In short, it appears that higher levels of perceived control over a disease may serve as a protective factor, whereas, higher perceived seriousness may make a participant more sensitive to genetic risk results.
In contrast, however, disease perceptions appeared to have little impact on consulting with a physician or the completion of health screeners, and no measurable impact on lifestyle behaviors (e.g. fat intake or exercise). Given that some recent studies of behavioral response to DTC genomic testing do in fact suggest the presence of behavior changes among consumers in some settings (7, 8), continued study in this domain may be warranted.
Heart attack and Alzheimer’s disease were most commonly selected by participants as being their most feared disease. Similarly, in another study, patients at a preventative care clinic who received genomic risk information from DTC testing also reported high levels of worry for heart attack (Alzheimer’s disease was not included in the study) (26), further underscoring the importance of individual differences with respect to specific diseases and outcomes following DTC genomic testing. In addition, in a study that surveyed participants about potential genetic susceptibility, both risk perception and perceptions of severity for eight common diseases varied among subjects, even for single diseases (27).
Individual differences in disease perceptions may be able to provide guidance for the optimal delivery of educational and counseling services in the context of DTC genomic testing. Increased understanding of disease beliefs and perceptions can help clarify diseases for which testing may be more likely to have a psychological or behavioral impact. Thorough understanding of the views and characteristics of individuals that utilize genetic testing will help guide areas of focus for general education of patients, physicians, genetic counselors and other providers. In addition, prior research has suggested that providers should consider offering patients the option to select which risk information or results they would prefer to receive in a report, again emphasizing the notion and importance of individual differences (28).
Limitations
There are several limitations to this study. First, participants were asked to report their most feared disease after receiving the DTC genetic testing results. Future studies should assess feared diseases prior to and after receiving DTC genetic results. Second, the longitudinal cohort design of the study did not include the use of a control group. In addition, individuals who self-selected into the study probably have an interest in learning more about their health and represent early adopters of genetic testing. Consistent with this, the demographics of the current population was relatively similar to the Navigenics customer base (2). We also studied a selected sample that is primarily Caucasian, college educated, of above average socioeconomic status, and generally in good health. Thus, findings may not be generalizable to the larger public. The study followed participants over a short period of time and does not measure individual differences in the context of long-term follow-up and impact of DTC testing. Finally, participants completed self-report surveys through a web-based approach, which can be less reliable than in-person assessment.
Conclusions
Perceptions of disease seriousness and control impact psychological response to DTC genomic testing over and above the personal genetic risk results disclosed. Higher perceived seriousness of a disease associates with higher levels of anxiety and distress, whereas, higher perceived control associates with lower levels of distress. However, although consumers may experience distress, this level of distress is fairly low and probably comparable to the typical mild distress levels commonly associated with routine management of health and well-being in contexts that do not involve genetics. Information regarding individual differences in disease perceptions can be used to enhance genomic counseling, education, and possibly disease prevention strategies that leverage genetic testing.
Supplementary Material
Table S1. Descriptive statistics for outcome variables (N = 2037)
Table S2. Conditions most commonly selected by participants as being of most concern (top five by gender)
Table S3. Genetic risk distributions as a function of disease being reported as feared vs not being reported as feared
Table S4. Perceptions as a function of genetic risk among individuals who reported a disease as most feared
Table S5. Main effect model results. Impact of genetic risk, and perceived seriousness of most feared disease on behavioral or psychological outcomes
Table S6. Interaction model results. Impact of genetic risk, perceived seriousness of most feared disease, and interaction between risk and seriousness on behavioral or psychological outcomes
Table S7. Main effect model results. Impact of genetic risk and perceived control of the most feared disease on behavioral or psychological outcomes
Table S8. Interaction model results. Impact of genetic risk, perceived control of most feared disease, and interaction between risk and control on behavioral/psychological outcomes
Table S9. Descriptive statistics for screening tests (%)
Table S10. Impact of genetic risk and perceived seriousness of most feared disease on completion of health screenings (main effect model only)
Table S11. Impact of genetic risk and control of lifestyle changes of most feared disease on completion of health screenings (main effect models only)
Table S12. Impact of participant’s most feared disease, genetic risk and control of seeking medical attention of a disease severity on completing health screenings.
Fig. S1. Percentage of participants denoting the disease as their most feared disease.
Fig. S2. Percentage of individuals who completed health screeners for the five most commonly selected feared diseases. Health screeners were not available for Alzheimer’s disease and obesity.
Acknowledgments
This work was supported in part by a NIH/NHGRI R21 grant (1R21HG005747; PI: C. S. B.), a NIH flagship Clinical and Translational Science Award grant (5UL1RR025774, 8UL1 TR000109 and 8UL1 TR001114; PI: E. J. T.), The Scripps Dickinson Fellowship Fund, and Scripps Genomic Medicine Division of Scripps Health.
Footnotes
Supporting Information
The following Supporting information is available for this article:
Additional Supporting information may be found in the online version of this article.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Hall TO, Renz AD, Snapinn KW, Bowen DJ, Edwards KL. Awareness and uptake of direct-to-consumer genetic testing among cancer cases, their relatives, and controls: the Northwest Cancer Genetics Network. Genet Test Mol Biomarkers. 2012;16:744–748. doi: 10.1089/gtmb.2011.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloss CS, Schork NJ, Topol EJ. Effect of direct-to-consumer genome wide profiling to assess disease risk. N Engl J Med. 2011;364:524–534. doi: 10.1056/NEJMoa1011893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samuel GN, Jordens CF, Kerridge I. Direct-to-consumer personal genome testing: ethical and regulatory issues that arise from wanting to ’know’ your DNA. Intern Med J. 2010;40:220–224. doi: 10.1111/j.1445-5994.2010.02190.x. [DOI] [PubMed] [Google Scholar]
- 4.Coghlan A. 23andMe ordered to stop selling $99 genetic test. Genetics: New Scientist. 2013;2945:4. [Google Scholar]
- 5.U.S. Food and Drug Administration. Inspections, Compliance, Enforcement, and Criminal Investigations: 23andMe, Inc. 11/22/13. Warning letter. Accessed at www.fda.gove/iceci/enforcementactions/warningletters/2013/ucm376296.htm Accessed on 28 November, 2013.
- 6.Roberts JS, Ostergren J. Direct-to-consumer genetic testing and personal genomics services: a review of recent empirical studies. Curr Genet Med Rep. 2013;1:182–200. doi: 10.1007/s40142-013-0018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaufman DJ, Bollinger JM, Dvoskin RL, Scott JA. Risky business: risk perception and the use of medical services among customers of DTC personal genetic testing. J Genet Couns. 2012;21:413–422. doi: 10.1007/s10897-012-9483-0. [DOI] [PubMed] [Google Scholar]
- 8.Egglestone C, Morris A, O’Brien A. Effect of direct-to-consumer genetic tests on health behaviour and anxiety: a survey of consumers and potential consumers. J Genet Couns. 2013;22:565–575. doi: 10.1007/s10897-013-9582-6. [DOI] [PubMed] [Google Scholar]
- 9.Collins RE, Wright AJ, Marteau TM. Impact of communicating personalized genetic risk information on perceived control over the risk: a systematic review. Genet Med. 2011;13:273–277. doi: 10.1097/GIM.0b013e3181f710ca. [DOI] [PubMed] [Google Scholar]
- 10.Bloss CS, Wineinger NE, Darst BF, Schork NJ, Topol EJ. Impact of direct-to-consumer genomic testing at long term follow-up. J Med Genet. 2013;50:393–400. doi: 10.1136/jmedgenet-2012-101207. [DOI] [PubMed] [Google Scholar]
- 11.McGuire AL. 1000 genomes on the road to personalized medicine. Per Med. 2008;5:195–197. doi: 10.2217/17410541.5.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caulfield T, McGuire AL. Direct-to-consumer genetic testing: perceptions, problems, and policy responses. Annu Rev Med. 2012;63:23–33. doi: 10.1146/annurev-med-062110-123753. [DOI] [PubMed] [Google Scholar]
- 13.Champion VL. Instrument development for health belief model constructs. ANS Adv Nurs Sci. 1984;6:73–85. doi: 10.1097/00012272-198404000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Champion VL, Skinner CS. The health belief model. In: Glanz K, Rimer BK, Viswanath K, editors. Health behavior and health education. San Francisco, CA: Jossey-Bass; 2008. pp. 45–62. [Google Scholar]
- 15.Bloss CS, Ornowski L, Silver E, et al. Consumer perceptions of direct-to-consumer personalized genomic risk assessments. Genet Med. 2010;12:556–566. doi: 10.1097/GIM.0b013e3181eb51c6. [DOI] [PubMed] [Google Scholar]
- 16.Demirtas B, Acikgoz I. Promoting attendance at cervical cancer screening: understanding the relationship with Turkish womens’ health beliefs. Asian Pac J Cancer Prev. 2013;14:333–340. doi: 10.7314/apjcp.2013.14.1.333. [DOI] [PubMed] [Google Scholar]
- 17.Wallston KA, Strudler Wallston B, Smith S, Dobbins CJ. Perceived control and health. Curr Psychol. 1987;6:5–25. [Google Scholar]
- 18.CHIS. California health interview survey. Vol. 2007. Los Angeles CA: UCLA Center for Health Policy Research; 2007. [Google Scholar]
- 19.Spielberger CD, Gorsuch RL, Lushene PR, Vagg PR, Jacobs AG. Manual for the state-trait anxiety inventory (form Y) Palo Alto, CA: Consulting Psychologists Press; 1983. Incorporated. [Google Scholar]
- 20.Weiss DSM, Marmar CR. The impact of event scale - revised. New York, NY: Kluwer Academic/Plenum; 1996. [Google Scholar]
- 21.Creamer M, Bell R, Failla S. Psychometric properties of the impact of event scale - revised. Behav Res Ther. 2003;41:1489–1496. doi: 10.1016/j.brat.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Block G, Gillespie C, Rosenbaum EH, Jenson C. A rapid food screener to assess fat and fruit and vegetable intake. Am J Prev Med. 2000;18:284–288. doi: 10.1016/s0749-3797(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 23.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–146. [PubMed] [Google Scholar]
- 24.Breland JY, Fox AM, Horowitz CR, Leventhal H. Applying a common-sense approach to fighting obesity. J Obes. 2012;2012:710427. doi: 10.1155/2012/710427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagger MS, Orbell S. A meta-analytic review of the common-sense model of illness representations. Psychol Health. 2003;18:141–184. [Google Scholar]
- 26.James KM, Cowl CT, Tilburt JC, et al. Impact of direct-to-consumer predictive genomic testing on risk perception and worry among patients receiving routine care in a preventive health clinic. Mayo Clin Proc. 2011;86:933–940. doi: 10.4065/mcp.2011.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiloh S, Wade CH, Roberts JS, Alford SH, Biesecker BB. Associations between risk perceptions and worry about common diseases: a between-and within-subjects examination. Psychol Health. 2013;28:434–449. doi: 10.1080/08870446.2012.737464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wade CH, Shiloh S, Roberts JS, Hensley Alford S, Marteau TM, Biesecker BB. Preferences among diseases on a genetic susceptibility test for common health conditions: an ancillary study of the multiplex initiative. Public Health Genomics. 2012;15:322–326. doi: 10.1159/000338114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Descriptive statistics for outcome variables (N = 2037)
Table S2. Conditions most commonly selected by participants as being of most concern (top five by gender)
Table S3. Genetic risk distributions as a function of disease being reported as feared vs not being reported as feared
Table S4. Perceptions as a function of genetic risk among individuals who reported a disease as most feared
Table S5. Main effect model results. Impact of genetic risk, and perceived seriousness of most feared disease on behavioral or psychological outcomes
Table S6. Interaction model results. Impact of genetic risk, perceived seriousness of most feared disease, and interaction between risk and seriousness on behavioral or psychological outcomes
Table S7. Main effect model results. Impact of genetic risk and perceived control of the most feared disease on behavioral or psychological outcomes
Table S8. Interaction model results. Impact of genetic risk, perceived control of most feared disease, and interaction between risk and control on behavioral/psychological outcomes
Table S9. Descriptive statistics for screening tests (%)
Table S10. Impact of genetic risk and perceived seriousness of most feared disease on completion of health screenings (main effect model only)
Table S11. Impact of genetic risk and control of lifestyle changes of most feared disease on completion of health screenings (main effect models only)
Table S12. Impact of participant’s most feared disease, genetic risk and control of seeking medical attention of a disease severity on completing health screenings.
Fig. S1. Percentage of participants denoting the disease as their most feared disease.
Fig. S2. Percentage of individuals who completed health screeners for the five most commonly selected feared diseases. Health screeners were not available for Alzheimer’s disease and obesity.


