Abstract
Although there has been extensive characterization of the Wnt signaling pathway in the osteoblast lineage, the effects of Wnt proteins on the osteoclast lineage are less well studied. We found that osteoclast lineage cells express canonical Wnt receptors. Wnt3a reduced osteoclast formation when applied to early bone-marrow macrophage (BMM) osteoclast differentiation cultures, whereas late addition did not suppress osteoclast formation. Early Wnt3a treatment inactivated the crucial transcription factor NFATc1 in osteoclast progenitors. Wnt3a led to the accumulation of nuclear β-catenin, confirming activation of canonical Wnt signaling. Reducing low-density lipoprotein receptor-related proteins (Lrp) 5 and Lrp6 protein expression prevented Wnt3a-induced inactivation of NFATc1; however, deletion of β-catenin did not block Wnt3a inactivation of NFATc1, suggesting that this effect was mediated by a noncanonical pathway. Wnt3a rapidly activated the cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) pathway and pharmacological stimulation of cAMP/PKA signaling suppressed osteoclast differentiation; Wnt3a-induced NFATc1 phosphorylation was blocked by inhibiting interactions between PKA and A-kinase anchoring proteins (AKAPs). These data indicate that Wnt3a directly suppresses osteoclast differentiation through both canonical (β-catenin) and noncanonical (cAMP/PKA) pathways in osteoclast precursors. In vivo reduction of Lrp5 and Lrp6 expressions in the early osteoclast lineage via Rank promoter Cre recombination reduced trabecular bone mass, whereas disruption of Lrp5/6 expression in late osteoclast precursors via cathepsin K (Ctsk) promoter Cre recombination did not alter the skeletal phenotype. Surprisingly, reduction of Lrp5/6 in the early osteoclast lineage decreased osteoclast numbers, as well as osteoblast numbers. Published studies have previously noted that β-catenin signaling is required for osteoclast progenitor proliferation. Our in vivo data suggest that Rank promoter Cre-mediated deletion of Lrp5/6 may similarly impair osteoclast progenitor proliferation.
Keywords: OSTEOCLAST DIFFERENTIATION, WNT, β-CATENIN, PKA
Introduction
Normal bone metabolism requires a balance of osteoclast-mediated bone resorption and osteoblast-mediated bone formation. Disruption of this balance so that resorption exceeds formation leads to excess bone loss, increasing the risk for pathological fractures. Wnt signaling is crucial to skeletal development and homeostasis, and activation of this pathway in the skeleton has emerged as a promising therapy to increase bone formation and protect against osteoporotic bone fractures.
Canonical Wnt signaling begins with Wnt ligands binding to and activating the low-density lipoprotein receptor-related proteins (Lrp)/frizzled (Fzd) receptors, leading to β-catenin activation. In the absence of Wnts, β-catenin associates with a “destruction complex” that includes glycogen synthase kinase 3 beta (Gsk3β). Gsk3β is a serine-threonine kinase that phosphorylates β-catenin and promotes its degradation through ubiquitin-mediated proteolysis.(1) Wnt binding to canonical Wnt receptors recruits Gsk3β to the membrane,(2–5) releasing β-catenin from the destruction complex. This allows accumulation of cytosolic β-catenin, which subsequently translocates to the nucleus to regulate gene expression.
Much of the research on skeletal Wnt signaling has centered on the influences of canonical Wnt/β-catenin signaling in osteoblasts, where Wnt proteins stimulate commitment and differentiation toward the osteoblast lineage. Canonical Wnt signaling in osteoblasts also promotes expression of osteoprotegerin (OPG) to regulate osteoclast differentiation.(6)
Recent evidence suggests that Wnt signaling has a direct role in regulating osteoclastogenesis. Wei and colleagues(7) examined mice with targeted deletion of β-catenin in very early osteoclast lineage cells and documented that β-catenin deficiency caused osteopetrosis due to lack of precursor proliferation. Deletion of β-catenin in more committed stages of osteoclast differentiation caused osteoporosis because of enhanced osteoclast differentiation. These data support a role for canonical Wnt signaling in promoting osteoclast progenitor proliferation and suppressing osteoclast commitment and/or differentiation. However, β-catenin mediates signaling in other pathways and partners with α-catenin to form adherens junctions. Thus targeting β-catenin will alter Wnt-independent cellular events.
Although the studies of Wei and colleagues(7) may support a direct role for canonical Wnt signaling in suppressing osteoclast differentiation, β-catenin–independent signaling pathways are also activated when Wnts bind to Lrp/Fzd receptors. The effects of these “noncanonical” mechanisms in osteoclasts have remained underexplored. In this study, we examined how Wnts activate both canonical and adenylate cyclase (AC)/cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) signaling to control osteoclast differentiation.
Materials and Methods
Materials
Unless otherwise indicated, all chemicals were purchased from Sigma Chemical Co., St Louis, MO, USA.
Animals
Animals were housed in an accredited facility under a 12-hour light/dark cycle and provided water and food (PicoLab Rodent Diet 20; LabDiet, St. Louis, MO, USA) ad libitum. The facility temperature ranged from 68°F to 70°F and the humidity ranged from 30% to 70%. All animal protocols were approved by the Mayo Clinic Institutional Animal Care and Use Committee (IACUC) before the start of the studies. C57Bl/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA).
In vitro osteoclast cultures
Flushed bone marrow was treated with red blood cell lysis buffer and resuspended in α-Minimal Essential Medium (α-MEM) (Invitrogen, Carlsbad, CA, USA) supplemented with 10% (vol/vol) fetal bovine serum (FBS) (Fisher, Pittsburgh, PA, USA) and 1:40 CMG 14–12 supernatant,(8) which is a source of macrophage colony-stimulating factor (M-CSF). Bone marrow cultures were maintained in a humidified incubator at 37°C, in 5% CO2 for 24 hours. Adherent mesenchymal cells were discarded and the nonadherent cells (bone marrow macrophages [BMMs]) were cultured in the presence of 100 ng/mL receptor activator of NF-κB (RANKL) (generated using a recombinant RANKL expression construct that was the generous gift from Dr. Beth Lee, Ohio State University) and 10 ng/mL M-CSF (R&D Systems, Minneapolis, MN, USA). Osteoclasts were cultured in the presence or absence of Wnt3a (R&D Systems) as described in the figure legends. Cultures were re-fed on day 3 and mature osteoclasts were assessed on day 4.
Osteoclast differentiation quantitation
Mature osteoclasts (day 4) were fixed in 1% paraformaldehyde and stained for Hoechst and tartrate-resistant acid phosphatase (TRAP) activity as described.(9,10) TRAP-positive cells with at least three nuclei were scored as osteoclasts. In some cultures, cells were fixed on day 3 to evaluate whether treatment had accelerated differentiation.
Nuclear isolation and extraction
Bone marrow-derived osteoclast precursors were treated as outlined in the figure legends. Cells were washed, scraped, and pelleted in cold phosphate buffered saline (PBS). The pellet was resuspended in 10 mM Tris-HCL pH 8.0, 10 mM KCl, 0.1 mM EGTA, 0.1 mM EDTA containing 1× Inhibitor cocktail (Complete Mini-EDTA free; Roche Diagnostics, Mannheim, Germany). After incubation on ice for 15 min, 10% (vol/vol) NP-40 was added for a final concentration of 0.7% (vol/vol) and the cell suspension was mixed with a vortexer. Cells were collected by centrifugation at 18,000 g for 15 min at 4°C. The pellet was washed twice with cold PBS and resuspended in 20 mM Tris-HCL pH 8.0, 0.4 M NaCl, 0.1 mM EGTA, 0.1 mM EDTA, and 1× Inhibitor cocktail (Roche Diagnostics). Nuclei were incubated on ice for 15 min and collected by centrifugation at 18,000 g for 15 min at 4°C. Protein was quantitated using the BCA protein assay kit (Pierce, Rockford, IL, USA) and 5 µg/lane analyzed by Western blotting as detailed in the next section.
Western blotting
Total protein concentration was determined using the BCA protein assay kit (Pierce, Rockford, IL, USA). Proteins were separated using 10% SDS-PAGE followed by electroblotting to nitrocellulose (Millipore, Bedford, MA, USA). Membranes were blocked by incubation in 1× PBS containing 5% fat-free dry milk for 1 hour at room temperature. Blots were incubated with the following primary antibodies: Lrp5 (Abcam Inc., Cambridge, MA, USA), Lrp6 (R&D Systems), Fzd4 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), Fzd7 (Abcam Inc.), active β-catenin (Millipore, Temecula, CA, USA), total β-catenin (BD Biosciences, San Jose, CA, USA), PKA targets (Cell Signaling Technology, Danvers, MA, USA), phospho NFATc1 (pNFATc1) (Santa Cruz Biotechnology), total NFATc1 (BD Biosciences), β-actin, or tubulin. Nonspecific protein binding was blocked by 60 min in Tris buffered saline, pH 7.4, containing 0.1% Tween 20 (TBST) in and 5% fat-free dry milk (blocking buffer). Incubation with a 1:1000 dilution of primary antibodies in blocking buffer was carried out for either 60 min at room temperature or overnight at 4°C followed by washing with TBST. Blots were incubated for 1 hour with a 1:5000 dilution of horseradish peroxidase-conjugated secondary antibodies in blocking buffer for 60 min after washing in TBST. Signals were visualized using the ECL Plus detection system (Amersham Biosciences, Buckinghamshire, England) according to the manufacturer’s instructions.
cAMP and PKA activity assay
Bone marrow-derived osteoclast precursors were cultured with vehicle or 100 ng/mL Wnt3a for 15 min and analyzed for cAMP accumulation and PKA activity. cAMP accumulation was measured with the Cyclic AMP XP Assay Kit (Cell Signaling Technology) according to the manufacturer’s protocol. PKA activity was measured with a PKA kinase activity kit (Enzo Life Sciences, Farmingdale, NY, USA) according to the manufacturer’s protocol.
Signaling pathway manipulation
Bone marrow-derived osteoclast precursors were cultured with vehicle or inhibitor as indicated in the figure legends. To inhibit β-catenin signaling, mouse BMMs (obtained from mice carrying β-catenin with required exons 2 to 6 flanked by loxP sequences(11)) was cultured in vitro with Cre adenovirus (Vector Labs, Burlingame, CA, USA; multiplicity of infection [MOI] = 100). To inhibit PKA/A-kinase anchoring protein (AKAP) interactions, cells were serum starved for 60 min and preloaded with 50 µM of control peptide or InCELLect AKAP St-Ht31 Inhibitor Peptide (Promega Corporation, Madison, WI, USA) for 5 min prior to vehicle or Wnt3a treatment as detailed in the figure legends. To inhibit GSK3β, lithium chloride (LiCl) or the control salt NaCl were dissolved in ultrapure H2O and used at a final concentration of 20 nM. Alternatively, GSK inhibitor XI (Calbiochem/EMD4Biosciences, Darmstadt, Germany) was dissolved in DMSO at 25 mM and used at a final concentration of 25 µM. To activate AC, forskolin (Tocris Bioscience, Ellisville, MO, USA) was dissolved in DMSO at 25 µM and used at a final concentration of 25 nM.
Genetically modified mouse strains
Mice with exons 2 of Lrp5 and Lrp6 flanked by loxP sequences(12) were crossed with Rank-promoter(13) or transgenic cathepsin K (Ctsk) promoter–driven Cre recombinase(14) to obtain animals with Lrp5/6 Wnt co-receptors deleted specifically in early (Lrp5/6RankCre) or late (Lrp5/6CtskCre) osteoclast precursors. β-catenin flox mice(11) were used for in vitro studies. Mice were maintained on a mixed C57Bl/6J, 129, and FVB/N background. Ear punches were obtained at weaning and genomic DNA was isolated using the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA, USA). Mice were genotyped for the floxed alleles and Cre recombinase as described.(12–14)
Male animals were assessed in this study and body weight did not differ between knockout and wild-type littermates. For dynamic histomorphometric analyses of bone remodeling, mice were injected subcutaneously with calcein (0.1 mL/animal, 2.5 mg/mL) on days 7 and 3 prior to euthanasia. Mice were sacrificed at 6 weeks of age. Body weights were recorded and serum was collected via cardiac puncture at sacrifice. The right femurs were fixed in 10% neutral buffered formalin and stored in 70% ethanol, to be used for micro–computed tomography (µCT) analysis, histomorphometry, and immunohistochemistry. For in vitro analyses of osteoclasts, left femurs were isolated and dissected free of soft tissue. The epiphyses were removed and the marrow was flushed with PBS.
µCT analysis
The bone structure of 6-week old male mice was evaluated by ex vivo µCT (viva CT40 in vivo µCT; Scanco Medical AG, Basserdorf, Switzerland). The femurs were scanned in the distal femoral metaphysis, 0.5 mm proximal to the growth plate. Bone scans were performed at a resolution of 7 µm per slice, using an energy setting of 70 kVp and an integration time of 300 ms. Isotropic voxel size was 10.5 µm. A region of interest that was 0.7 mm long (100 slices) was analyzed in each mouse. Three-dimensional analyses was conducted to calculate morphometric trabecular parameters including bone volume per tissue volume (BV/TV, %), trabecular number (Tb.N, 1/mm), trabecular thickness (Tb.Th, mm), and trabecular separation (Tb.Sp, mm) using the manufacturer’s software.
Histomorphometric analysis
Femurs were embedded in methyl methacrylate, sectioned, and stained with Goldner’s Trichrome stain to assess bone (total area [Tt.Ar], mm2; bone area [B.Ar], mm2; bone perimeter [B.Pm], µm; osteoid width [O.Wi], µm) and osteoblast parameters (osteoblast surface/bone surface [Ob.S/BS], %; number of osteoblasts [N.Ob], 1/mm2; N.Ob/B.Pm, 1/µm). Sections were TRAP stained to assess osteoclast parameters (osteoclast surface/bone surface [Oc.S/BS], %; number of osteoclasts [N.Oc], 1/mm2; osteoclast perimeter [Oc.Pm], µm; N.Oc/B.Pm, 1/µm). Alternatively, sections were left unstained to quantify mineralizing surfaces (bone formation rate per total volume [BFR/TV], %/year; mineral apposition rate [MAR], µm/day). Cancellous bone histomorphometric indices were measured in an area of tissue (0.700 mm2) beginning 450 µm from the growth plate in the metaphyseal spongiosa. All histomorphometric measurements and calculations were performed with the Osteomeasure Analysis system (Osteometrics, Atlanta, GA, USA).
Statistical analysis
For animal studies, two groups were compared using Student’s t test. Each in vitro experiment contained a minimum of three replicates. Data presented are representative of at least three experiments. The data met the assumptions of homogeneity of variance and normality. Data were analyzed using a one-way analyses of variance (ANOVA) followed by post hoc Student’s t test and are presented as mean ± SE. Significance was determined at p < 0.05 using KaleidaGraph software. The Bonferroni correction was applied to adjust for multiple comparisons in all experiments.
Results
Wnt receptors are expressed by the osteoclast lineage and Wnt3a treatment suppresses osteoclast formation
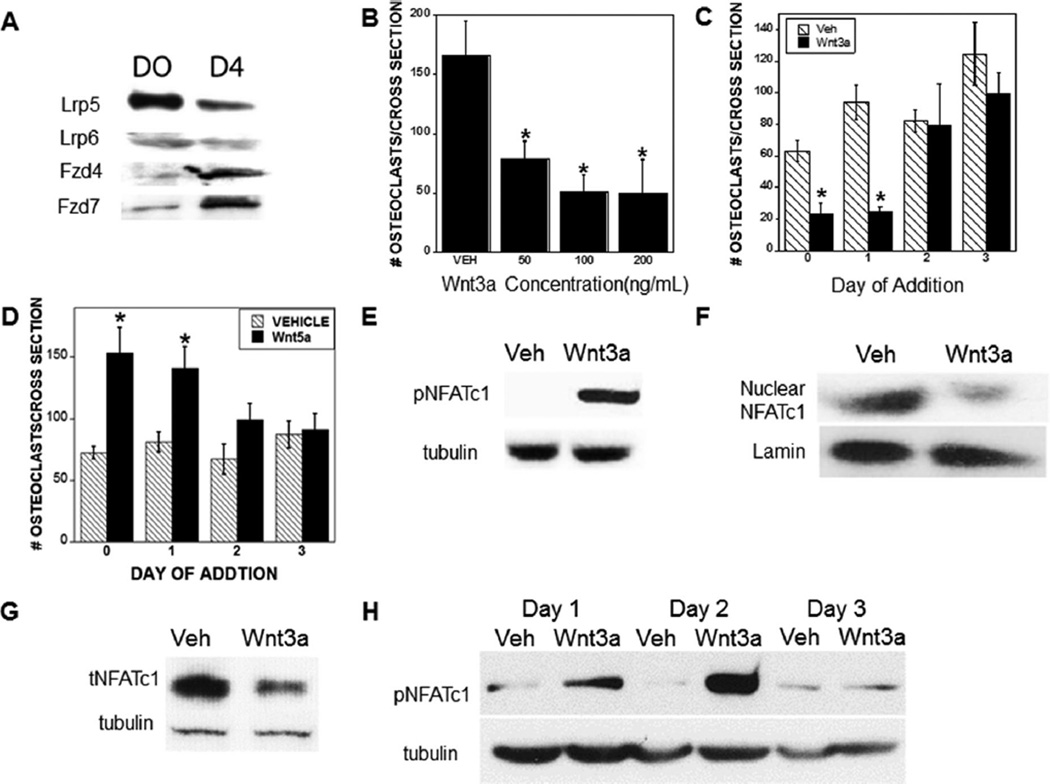
To evaluate the potential influences of Wnt signaling on osteoclast lineage cells, we examined Wnt receptor expression (Fig. 1A). BMM early osteoclast progenitors and mature osteoclasts expressed Lrp5 and Lrp6, as well as Fzd-4 and Fzd-7. Real-time PCR analyses indicated that Fzd1, Fzd2, Fzd3, Fzd5, and Fzd6 message levels were very low (data not shown).
Fig. 1.
Wnt receptors are expressed in the osteoclast lineage and Wnt signaling in early osteoclasts suppresses osteoclast differentiation. (A) Bone marrow–derived osteoclast precursors were harvested (D0) or cultured with RANKL and M-CSF to obtain osteoclasts on day 4 (D4) and protein lysates were examined for Wnt receptor expression. (B,C) Cells were fixed on day 4, TRAP-stained, and quantitated as detailed in the Materials and Methods section. (B) Osteoclast precursors were treated with vehicle or the indicated Wnt3a concentration throughout differentiation culture. (C) Osteoclast precursors were cultured with either vehicle or 100 ng/mL Wnt3a beginning on the indicated day of culture. (D) Osteoclast precursors were cultured with either vehicle or 100 ng/mL Wnt5a beginning on the indicated day of culture. (E) Osteoclast precursors were cultured for 15 min with RANKL and M-CSF and either vehicle or 100 ng/mL Wnt3a. Forty milligrams (40 mg) of cell lysate was analyzed by Western blotting for phosphorylated NFATc1 and tubulin. (F) Osteoclast precursors were cultured for 6 hours with RANKL and M-CSF with either vehicle or 100 ng/mL Wnt3a prior to nuclear isolation and extraction. Five milligrams (5 mg) of nuclear extract was probed by Western blotting for total NFATc1 and lamin. (G) Osteoclast precursors were cultured as in E. Forty milligrams (40 mg) of cell lysate was analyzed by Western blotting for total NFATc1 and tubulin. (H) Osteoclast precursors differentiated for the indicated time were cultured for 15 min with RANKL and M-CSF and either vehicle or 100 ng/mL Wnt3a. Forty milligrams (40 mg) of cell lysate was analyzed by Western blotting for phosphorylated NFATc1 and tubulin. *p < 0.05 compared to vehicle treatment (B) or *p < 0.01 compared to vehicle treatment (C). VEH/Veh = vehicle; NFATc1 = nuclear factor of activated T cells, cytoplasmic 1; M-CSF = macrophage colony-stimulating factor.
Given Wnt receptor expression in osteoclast lineage cells, we evaluated the influences of recombinant Wnt3a on osteoclast differentiation (Fig. 1B, C). Wnt 3a (50, 100, and 200 ng/mL) added at the initiation of osteoclast differentiation, or 100 ng/mL Wnt3a added up to 24 hours after RANKL and M-CSF treatment, significantly suppressed osteoclast formation (Fig. 1B, C). When 100 ng/mL Wnt3a was added at 48 hours, it only partially suppressed osteoclast formation (Fig. 1C). However, addition of 100 ng/mL Wnt3a between 72 and 96 hours after RANKL and M-CSF treatment had no influence on osteoclast formation. These results indicate that early, but not late, Wnt3a addition affects osteoclast differentiation. Of interest, early but not late addition of Wnt5a, a noncanonical Wnt protein, stimulated osteoclast formation (Fig. 1D), suggesting that the effects of Wnt3a are mediated by canonical Wnt receptors.
Wnt3a suppresses RANKL-mediated NFATc1 activation
NFATc1 is required for osteoclast differentiation. NFATc1 phosphorylation blocks nuclear localization by masking the nuclear localization signal.(15) Early in osteoclastogenesis, RANKL stimulates calcineurin-mediated dephosphorylation of NFATc1 in the cytosol, leading to NFATc1 nuclear translocation.(15) However, at late stages of osteoclast differentiation, M-CSF signals downregulate NFATc1 protein levels.(16) Wnt3a (100 ng/mL) treatment rapidly increased NFATc1 phosphorylation (inactivation) in BMM cultures (Fig. 1E), and Wnt3a exposure for 6 hours blocked RANKL-stimulated NFATc1 nuclear localization (Fig. 1F). NFATc1 activation results in autoamplification of NFATc1 expression.(15) We therefore evaluated the influences of Wnt3a on total NFATc1 and observed Wnt3a-mediated suppression of RANKL-driven NFATc1 expression in osteoclast precursors (Fig. 1G). Consistent with the lack of effect of Wnt3a on differentiation of osteoclasts when added late during differentiation, Wnt3a did not affect NFATc1 phosphorylation when administered on day 3 of osteoclast differentiation (Fig. 1H). We did not detect any effects of Wnt3a on activation of NF-κB, MEK, ERK, p38MAPK, or protein kinase B (AKT) (data not shown).
Canonical Wnt signaling suppresses osteoclast differentiation
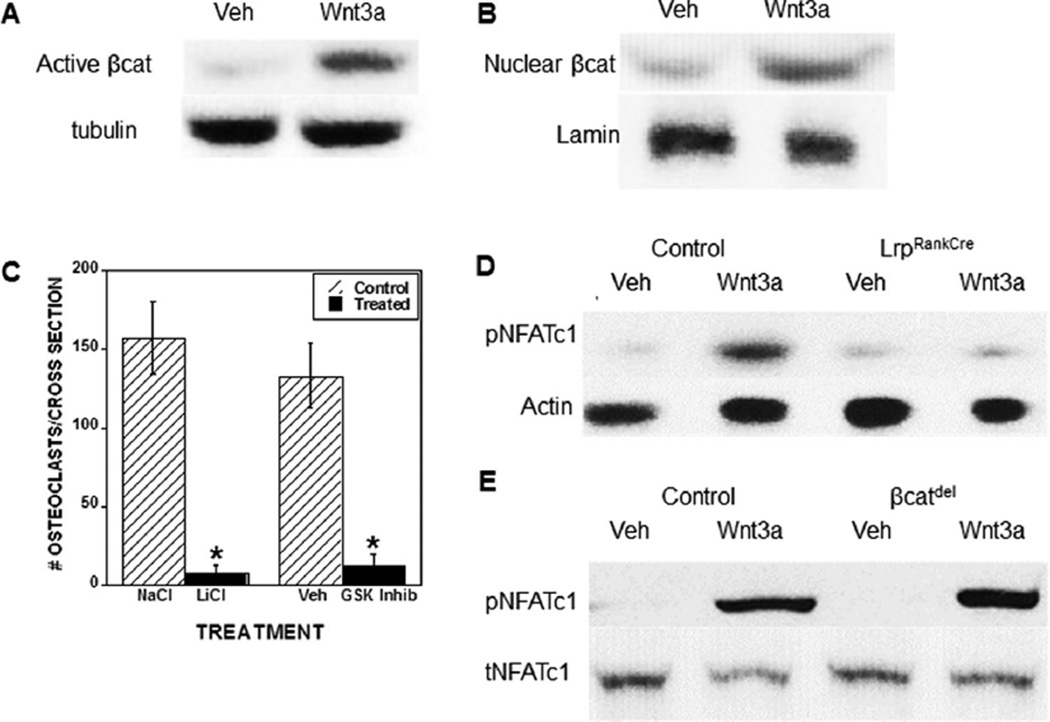
To assess activation of canonical Wnt signaling in the osteoclast lineage, we treated BMM cultures with Wnt3a and assessed β-catenin activation. Wnt3a treatment led to the rapid stabilization (within 15 min) of β-catenin compared to vehicle-treated cultures (Fig. 2A). Six hours after Wnt3a treatment, β-catenin nuclear localization was significantly increased compared to vehicle treatment (Fig. 2B). BMM cultures were treated with GSK3β inhibitors to assess the effect of β-catenin activation on osteoclast differentiation. Both LiCl and GSK3β inhibitor XI significantly suppressed osteoclast formation as compared to their respective controls (Fig. 2C). Lrp5/6 protein reduction in BMM cultures prevented Wnt3a-induced inhibitory phosphorylation of NFATc1 (Fig. 2D). Interestingly, deletion of β-catenin did not prevent Wnt3a-induced inactivation (phosphorylation) of NFATc1 (Fig. 2E). Together these data demonstrate that Wnt3a activates β-catenin signaling to suppress osteoclast differentiation; however, inactivation of NFATc1 occurs through a noncanonical (β-catenin-independent) pathway.
Fig. 2.
Wnt3a activates canonical Wnt signaling in osteoclast precursors to suppress osteoclast differentiation. (A) Osteoclast precursors were serum starved for 60 min, and treated with vehicle or 100 ng/mL Wnt3a for 15 min. Forty milligrams (40 mg) of cell lysate was analyzed by Western blotting for active β-catenin and tubulin. (B) Osteoclast precursors were cultured with vehicle or 100 ng/mL Wnt3a treatment for 6 hours prior to nuclear isolation and extractions. Five milligrams (5 mg) of nuclear extract was probed by Western blotting for total β-catenin and actin. (C) Osteoclast precursors were cultured with either sodium chloride (NaCl) (salt control), lithium chloride (LiCl), vehicle DMSO (Veh, GSK Inhib control), or a GSK3-β inhibitor (GSK Inhib) throughout culture. Cells were fixed on day 4, TRAP-stained, and quantitated as detailed in the Materials and Methods section. Results are the ratio of Wnt3a-treated to vehicle-treated osteoclast numbers. *p < 0.05 compared to vehicle or control treatment. (D, E) Osteoclast precursors from control littermate or LrpRankCre mice (D) or βcat flox osteoclast precursors treated with control or Cre-adenovirus (MOI = 100) (E) were cultured 15 min with RANKL and M-CSF and either vehicle or 100 ng/mL Wnt3a. Forty milligrams (40 mg) of cell lysate was analyzed by Western blotting for phosphorylated and total NFATc1. Veh = vehicle; DMSO = dimethylsulfoxide; GSK Inhib = glycogen synthase kinase 3 beta inhibitor; NFATc1 = nuclear factor of activated T cells, cytoplasmic 1; Lrp = low-density lipoprotein receptor related protein; MOI = multiplicity of infection; TRAP = tartrate-resistant acid phosphatase; pNFTAc1 = phospho NFATc1; tNFTAc1 = total NFATc1; M-CSF = macrophage colony-stimulating factor.
Wnt3a activates the cAMP/PKA to suppress osteoclast differentiation
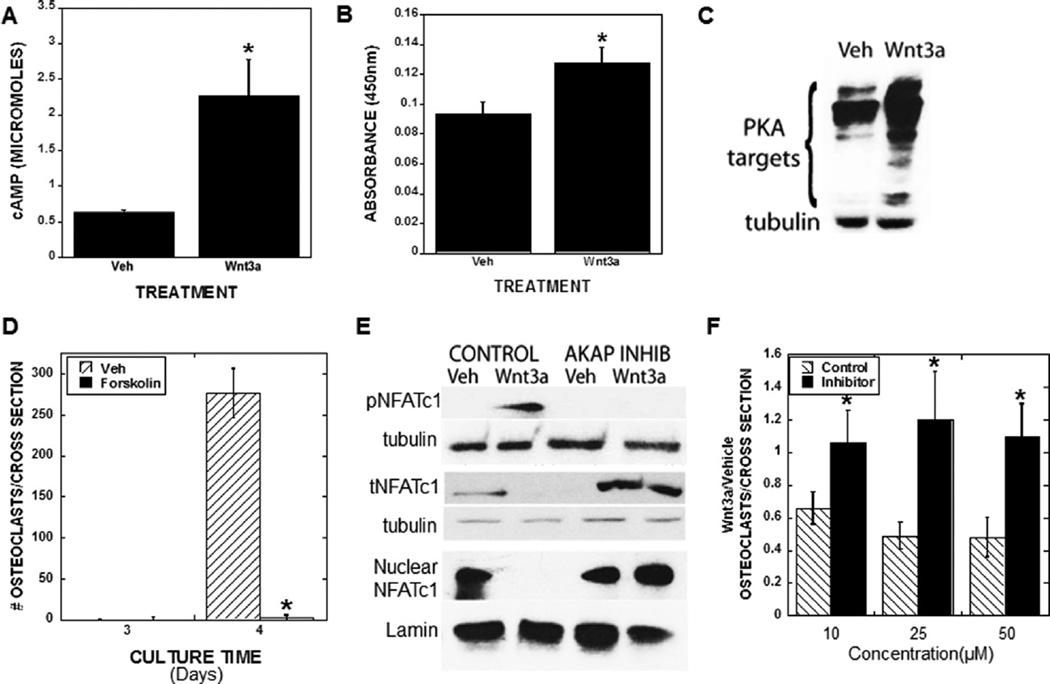
Fzdco-receptors are seven-pass membrane receptors that interact with Lrp5/6 to create a high-affinity Wnt binding complex.(1) Trimeric G proteins are required for some Wnt influences on cells,(17) and because the Fzd receptors are homologous to known G protein coupled receptors, it was proposed that Fzd directly interacts with G proteins (reviewed in Wang and colleagues(18)). More recently, direct interactions between Fzds and G proteins were documented (reviewed in Monroe and colleagues(1)), and Wnt3a was shown to stimulate G protein alpha subunit s (Gαs) and Gαi/o, but not Gαq11 association with Fzd receptors in brain tissue.(19) We therefore asked if Wnt3a altered G protein signaling cascades in the osteoclast lineage. Gαs stimulates the ACs, which in turn catalyze cyclic cAMP formation from ATP and accumulation of cAMP, which activates PKA.(20) Wnt3a treatment rapidly increased cAMP accumulation and PKA activation (Fig. 3A, B). Rapid Wnt3a-mediated phosphorylation of several PKA substrates was observed in osteoclast precursors (Fig. 3C). We also found tha forskolin, a potent PKA activator, significantly suppressed osteoclast differentiation of bone marrow-derived osteoclast precursors, mimicking Wnt3a effects on non-β-catenin signaling (Fig. 3D).
Fig. 3.
Wnt3a activates the cAMP-Protein Kinase A pathway in osteoclast precursors and blocking the PKA/AKAP interaction prevents Wnt3a suppression of NFATc1 activation and osteoclast differentiation. (A, B) Osteoclast precursors were cultured with vehicle or 100 ng/mL Wnt3a for 15 min. Cells were analyzed for cAMP accumulation (A) or PKA activity (B). *p < 0.05 compared to vehicle. (C) Osteoclast precursors cultured for 24 hours with RANKL and M-CSF with either vehicle or 100 ng/mL Wnt3a. Forty milligrams (40 mg) of cell lysate was analyzed by Western blotting for phosphorylated PKA targets and tubulin. (D) Osteoclast precursors were cultured with vehicle or forskolin as detailed in the Materials and Methods section throughout culture. Cells were fixed on day 3 or day 4, TRAP stained, and quantitated as detailed in the Materials and Methods section. *p < 0.05 compared to control treatment. (E, F) Osteoclast precursors were cultured for 24 hours with RANKL and M-CSF. Cells were serum starved and preloaded with 50 mM of control peptide or an AKAP inhibitor peptide for 5 min prior to vehicle or Wnt3a treatment for 15 min (phospho-NFATc1), 6 hours (total and nuclear NFATc1), or throughout differentiation culture (F). (E) Cell lysate was analyzed by Western blotting for phospho-NFATc1, tubulin (15 min), total NFATc1 and tubulin (6 hours), and nuclear NFATc1 and Lamin (6 hours). (F) Cells were fixed on day 4, TRAP stained, and quantitated as detailed in the Materials and Methods section. Results are the ratio of Wnt3a treated to vehicle treated osteoclast numbers. *p < 0.05 compared to control peptide treatment. Veh = vehicle; TRAP = tartrate-resistant acid phosphatase; pNFTAc1 = phospho NFATc1; tNFTAc1 = total NFATc1; M-CSF = macrophage colony-stimulating factor.
PKA association with AKAPs is required for Wnt3a to suppress NFATc1 activation and osteoclast differentiation
The myriad of PKA influences on cell functions is controlled to a great extent by PKA binding to AKAPs. AKAPs are a large and diverse family of proteins that selectively tether PKA to specific intracellular structures to form a signaling complex, therefore regulating PKA subcellular localization and function.(21) We used a competitive binding inhibitor peptide that prevents PKA interactions with AKAPs to determine the role of AKAP binding in Wnt3a signaling. Blocking AKAP interactions with PKA prevented Wnt3a-mediated increases in NFATc1 phosphorylation (inactivation) and decreases in NFATc1 accumulation (Fig. 3E). In addition, blocking AKAP interactions with PKA prevented Wnt3a suppression of osteoclast formation (Fig. 3F).
Lrp5 and Lrp6 deletion in early but not late osteoclast precursors causes osteopenia
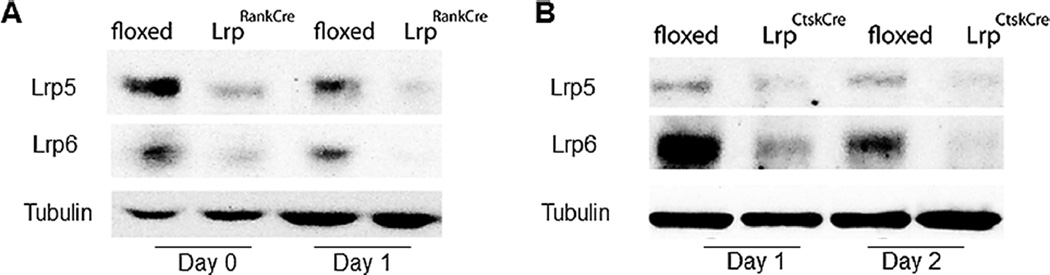
Because of the temporal responses to Wnt3a during osteoclast differentiation, Lrp5 and Lrp6 were deleted selectively in the early or late stages of osteoclast differentiation. Lrp5 fl/fl and Lrp6 fl/fl mice were crossed with mice expressing Rank or Ctsk promoter-driven Cre recombinase to delete Lrp5 and Lrp6 from early or late osteoclast precursors, respectively. We were unable to generate Lrp5 fl/fl Lrp6 fl/fl Rank-Cre animals; therefore, Rank-Cre–positive Lrp5 fl/+ Lrp6 fl/fl and Lrp5 fl/fl Lrp6 fl/+ were analyzed together and referred to collectively as LrpRankCre. Ctsk-Cre–positive Lrp5 fl/fl Lrp6 fl/fl animals were born at normal Mendelian ratios (LrpCtskCre). Rank-Cre and Ctsk-Cre effectively reduced Lrp5 and Lrp6 in osteoclast differentiation cultures in vitro (Fig. 4A, B).
Fig. 4.
Deletion of Lrp5/6 protein from the early osteoclast lineage prevents the suppressive effects of Wnt3a on osteoclastogenesis. (A, B) Validation of receptor deletion. Bone marrow-derived osteoclast precursors were harvested (Day 0) or cultured with RANKL and M-CSF to obtain osteoclast precursors on Day 1 or Day 2. Protein lysates were examined for Lrp5 and 6 and tubulin in the Rank-Cre (A) and Ctsk-Cre (B) mice. Lrp = low-density lipoprotein receptor related protein; Ctsk = cathepsin K; M-CSF = macrophage colony-stimulating factor.
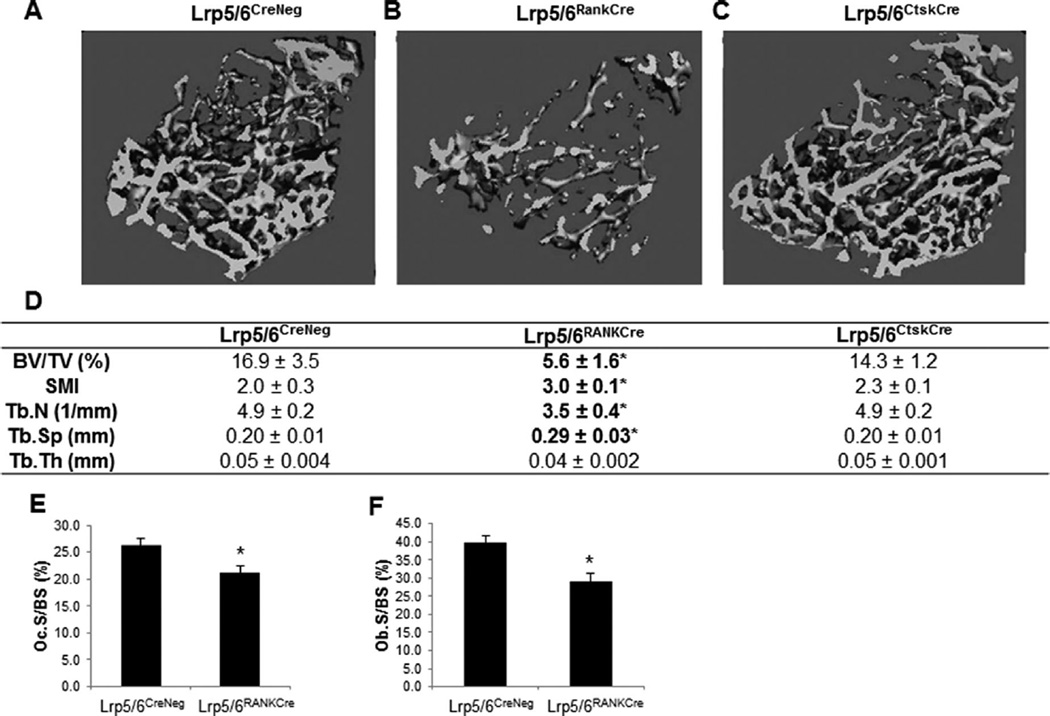
Deletion of Lrp5 and Lrp6 in the early osteoclast precursors (LrpRankCre) resulted in a decreased cancellous bone mass compared to Cre negative littermates (Fig. 5A, B, D), measured by µCT. The phenotypes of Rank-Cre Lrp5 fl/+ Lrp6 fl/fl and Rank-Cre Lrp5 fl/fl Lrp6 fl/+ did not differ significantly (data not shown). BV/TV and Tb.N were significantly decreased, whereas Tb.Sp and SMI, a ratio of weaker rod-like to stronger plate-like bone structures, were significantly increased (Fig. 5B, D). In contrast, deletion of Lrp5 and Lrp6 in late osteoclast precursors (LrpCtskCre) did not alter cancellous parameters (Fig. 5C, D).
Fig. 5.
Deletion of canonical Wnt receptors in early, but not late, osteoclast precursors causes low bone mass phenotype. Micro–computed tomography images of Cre negative (A), LrpRankCre (B), or LrpCtsk-Cre (C) femurs with analyses of trabecular bone parameters (D). n = 5, *p < 0.05 compared to Cre negative littermate mice. (E, F) Histomorphometric analyses of Cre-negative (n = 12) and Lrp5/6RankCre (n = 10) mice. TRAP stain was used to quantify (E) Oc.S/BS (%), and Goldner’s stain was used to quantify (F) Ob.S/BS (%).*p < 0.05 compared to Cre-negative control littermates. Lrp = low-density lipoprotein receptor related protein; Ctsk = cathepsin K; Oc.S/BS = osteoclast surface per bone surface; Ob.S/BS = osteoblast surface per bone surface; TRAP = tartrate-resistant acid phosphatase; SMI = structure model index; BV/TV = bone volume fraction; Tb.N = trabecular number; Tb.Sp = trabecular spacing; Tb.Th = trabecular thickness.
Surprisingly, histomorphometric analyses of LrpRankCre trabeculae showed a significant reduction in Oc.S/BS (Fig. 5E). Ob.S/BS was also significantly reduced in LrpRankCre trabeculae (Fig. 5F).
Discussion
Over the past decade, activation of skeletal Wnt signaling has emerged as a promising therapy to increase bone formation. Neutralizing antibodies to the Wnt antagonist Sclerostin increase bone formation and reverse the effects of estrogen deficiency on bone loss in animals.(22,23) In humans, antisclerostin monoclonal antibodies, romosozumab and blosozumab, increase bone formation markers while suppressing bone resorption markers.(22–25) A monoclonal antibody to the Wnt antagonist DKK1 increases bone formation to prevent bone loss related to tumor-driven osteolysis.(26–28) Although the majority of studies have focused on the effects of activating Wnt signaling in the osteoblast lineage, we show here that these therapies also may affect the osteoclast lineage to contribute to the overall net increase in bone mass.
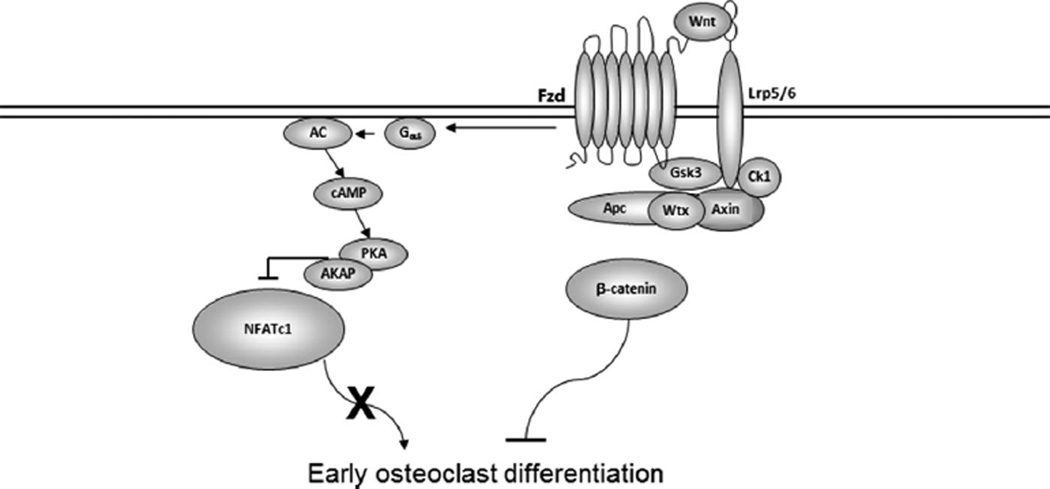
The study by Wei and colleagues(7) indicated that β-catenin activation is required for proliferation of early osteoclast precursors but must be suppressed for transition from the proliferative to the commitment phase of differentiation. Consistent with this, Modarresi and colleagues(29) showed that adenovirus-mediated overexpression of β-catenin in vitro inhibited osteoclast differentiation. In vitro studies of Wnt signaling in human osteoclast precursors isolated from multiple myeloma patients indicated that Wnts may suppress human osteoclast precursor differentiation as well.(30) Because β-catenin has additional cellular functions not directly related to Wnt signaling, we investigated canonical and noncanonical Wnt signaling influences on osteoclast differentiation by targeting Wnt co-receptors instead of β-catenin. Our studies document that Wnt/Lrp signaling suppresses early osteoclast differentiation in vitro through both canonical and noncanonical mechanisms (Fig. 6).
Fig. 6.
Model for the mechanism of Wnt-mediated suppression of osteoclast differentiation. Wnt interactions with Fzd and Lrp5/6 co-receptors leads to activation of both canonical (β-catenin-driven) and cAMP/PKA signaling pathways. The cAMP/PKA pathway blocks osteoclast differentiation of early osteoclast progenitors by PKA-mediated phosphorylation of NFATc1, which masks its nuclear localization signal, preventing nuclear accumulation. Lrp = low-density lipoprotein receptor related protein; Gsk = glycogen synthase kinase; NFATc1 = nuclear factor of activated T cells, cytoplasmic 1; A-KAP = Akinase anchoring protein; Fzd = frizzled.
Canonical Wnt signaling is initiated by the binding of Wnts to the Lrp/Fzd receptor complex. This leads to the recruitment, phosphorylation, and inactivation of Gsk3β and the consequent activation of β-catenin. Our data document that two different Gsk3β inhibitors suppressed osteoclast differentiation, indicating that canonical Wnt signaling inhibits differentiation. In contrast, Jang and colleagues(31) found that Gsk3β suppression was required for osteoclast differentiation. In vitro silencing of Gsk3β with siRNA stimulated osteoclast differentiation and overexpression of constitutively active Gsk3β inhibited RANKL-mediated NFATc1 induction. Additionally, Jang and colleagues(31) observed that mice overexpressing constitutively active Gsk3β driven by the TRAP promoter are osteopetrotic due to reduced osteoclast numbers. Gsk3β is an integral signaling component in many pathways including Wnts, hedgehog, G protein coupled ligands, growth factors, and cytokines,(32,33) and Gsk3β is a key downstream effector of Akt, which is required for osteoclast differentiation.(34) Thus, manipulating Gsk3β likely altered multiple signaling pathways, which may explain these contradictory observations.
Some consideration of the specificity of the promoter is also required to interpret the conflicting data between Jang and colleagues(31) and the studies reported here and by Wei and colleagues.(7) Analysis of TRAP Cre Rosa reporter mice showed that the TRAP promoter is active in many tissues.(14,35) Additionally, overexpression of a cellular protein may produce nonphysiological responses due to the high levels of expression overriding normal cellular controls.
Surprisingly, our in vivo model revealed reduced osteoclast numbers with deletion of Lrp5/6 in the early osteoclast lineage. Wei and colleagues(7) documented the biphasic effect of β-catenin deletion in the osteoclast lineage, with early deletion via the Tie2 promoter Cre or PPARχ-tTA TRECre causing decreased osteoclast numbers because of impaired progenitor proliferation. It is possible that the RANK-promoter Cre-mediated reduction in Lrp5/6 similarly impairs osteoclast progenitor proliferation. Preliminary data from our laboratory show that LrpRankCre bone marrow has reduced osteoclast progenitor colony formation units (data not shown), supporting this hypothesis. However, in contrast to the osteopetrotic phenotype depicted by early osteoclast deletion of β-catenin, LrpRankCre mice exhibited an osteopenic phenotype with reduced osteoblast numbers. The mice examined in this study were 6 weeks of age, whereas the mice examined by Wei and colleagues(7) were 4 months of age, and this may account for the discrepancy in bone phenotype. Alternatively, Wnt signaling may have a role in coupling factor expression by osteoclast progenitors that is differentially affected by β-catenin versus Lrp5/6 deletion. Our preliminary data show that Wnt3a stimulates osteoclast progenitor expression of Ctgf, Cthrc1, Pdgfa, Pdgfb, and Sphk1 (data not shown), which previous studies have demonstrated to be involved in migration and or differentiation of osteoblast progenitors.(36–41) However, it also remains possible that the reduction in osteoblast numbers is due to nonspecific expression of RANK-promoter Cre. RANK is expressed by a variety of different tissues including liver and skeletal muscle.(42,43) In unpublished studies we have found that RANK-promoter Cre induced 22-fold greater Cre reporter (eYFP) expression in mature osteoclasts compared to liver. RANK-promoter Cre-reporter expression was threefold greater in mature osteoclasts as compared to skeletal muscle (data not shown).
In addition to forming a complex with Lrp, Fzds interact with the transmembrane receptors Vangl1, Ror2, and Ryk to activate noncanonical Wnt signaling.(44) Maeda and colleagues(13) showed that deletion of osteoclast Ror2 decreases osteoclast numbers and increases bone mass, suggesting that noncanonical Wnt signaling (through Wnt5a) stimulates osteoclastogenesis. These data are supported by our current results in which early but not late addition of Wnt5a promoted osteoclast differentiation. Similarly, Santiago and colleagues(45) showed that the noncanonical Wnt5b promoted osteoclast differentiation through Ryk. β-catenin–independent Wnt signaling pathways include the planar cell polarity pathway, trimeric G-protein–coupled receptor pathways, Rho GTPase signaling, and Jnk signaling (reviewed in Monroe and colleagues(1)). Noncanonical Wnt/Fzd activation of the cAMP/PKA pathway has been reported(46,47); however, whether the observed Wnt3a stimulation of the cAMP/PKA pathway involved Lrps or the noncanonical receptors remained unresolved.
We document here that Wnt3a stimulated cAMP accumulation and activated PKA in early osteoclast precursors. PKA is a well-established regulator of NFATc1 because it phosphorylates NFATc1 to suppress its nuclear localization.(48) Yoon and colleagues(49) investigated the effects of the AC/cAMP/PKA pathway on osteoclast differentiation. They found that activation of cAMP and PKA suppressed osteoclast differentiation by PKA-mediated phosphorylation and inactivation of NFATc1. Accordingly, we examined the role of the cAMP/PKA pathway on mediating Wnt suppression of osteoclast differentiation. Blocking PKA interactions with AKAPs suppressed Wnt3a influences on osteoclast differentiation, indicating a crucial role for PKA/AKAP interactions in Wnt3a regulation of osteoclastogenesis. In addition to inhibiting NFATc1, it is also possible that PKA/AKAP signaling are inhibiting GSK3β·<p&#-4027;ΦNϒΜΛιΝΚ>(50) This would be consistent with our data that inhibiting GSK3β impairs osteoclast differentiation. Importantly, the effects of Wnts to stimulate PKA-mediated inactivation of NFATc1 or inhibit osteoclast differentiation were suppressed in the absence of canonical Wnt receptors Lrp5 and Lrp6.
PKA has also been shown to negatively regulate NF-κB nuclear localization; however, we did not detect a change in NF-κB activation (data not shown). The activity of PKA is dependent on the targeting of its activities via AKAP proteins. We are currently working to determine the AKAP involved in facilitating Wnt3a-induced phosphorylation of NFATc1.
It is likely that altered Wnt responses in osteoclast lineage cells contribute to the bone preserving effects of anti-sclerostin and anti-DKK antibody therapies. Non-targeted activation of Wnt signaling may have deleterious effects including driving cancer development.(51–61) Thus it is important to understand the mechanisms by which Wnts influence cells outside of the osteoblast lineage.
Our results demonstrate that Wnt signaling inhibits the early stages of osteoclast differentiation through both canonical and noncanonical cAMP/PKA signaling. Deletion of Lrp5/Lrp6 in osteoclasts prevented the negative effect of Wnt3a on NFATc1 activity. Importantly, deletion of β-catenin did not prevent the negative effects of Wnt3a on NFATc1. Resolution of the mechanisms by which Wnt signaling in osteoclast lineage cells prevents bone loss may provide new therapeutic targets to selectively retain bone-preserving functions of Wnts while avoiding the pro-tumor progression components of Wnt signaling.
Acknowledgments
This work was supported by NIH grants F32 AR064679, T32 AR056950, R01 AR053293, P01 AG004875P3, P01 AG004875P4, R01 GM097495, and R01 DE020194.
Authors’ roles: Data collection: LP, MR, MMW, and MJO. Animal breeding strategies: CH, RD, JZ, YK, MMW, BOW, and MJO. Animal maintenance: CH. Study design: AH, BW, JJW, SK, and MJO. Drafting manuscript: MMW and MJO. Revising manuscript content: MMW, RD, JJW, SK, AH, BOW and MJO. Approving final version of manuscript: MMW, LP, MR, CH, AH, YK, RD, JZ, BOW, JJW, SK, and MJO. MJO takes responsibility for the integrity of the data analysis.
Footnotes
Disclosures
BOW is a consultant to Amgen. The other authors have no conflicts of interest.
References
- 1.Monroe DG, McGee-Lawrence ME, Oursler MJ, Westendorf JJ. Update on Wnt signaling in bone cell biology and bone disease. Gene. 2012;492(1):1–18. doi: 10.1016/j.gene.2011.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niehrs C, Shen J. Regulation of Lrp6 phosphorylation. Cell Mol Life Sci. 2010;67(15):2551–2562. doi: 10.1007/s00018-010-0329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilic J, Huang YL, Davidson G, et al. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316(5831):1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- 5.Zheng M, Messerschmidt D, Jungblut B, Sommer RJ. Conservation and diversification of Wnt signaling function during the evolution of nematode vulva development. Nat Genet. 2005;37(3):300–304. doi: 10.1038/ng1512. [DOI] [PubMed] [Google Scholar]
- 6.Fujita K, Janz S. Attenuation of WNT signaling by DKK-1 and -2 regulates BMP2-induced osteoblast differentiation and expression of OPG, RANKL and M-CSF. Mol Cancer. 2007;6:71. doi: 10.1186/1476-4598-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei W, Zeve D, Suh JM, et al. Biphasic and dosage-dependent regulation of osteoclastogenesis by beta-catenin. Mol Cell Biol. 2011;31(23):4706–4719. doi: 10.1128/MCB.05980-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeshita S, Kaji K, Kudo A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J Bone Miner Res. 2000;15(8):1477–1488. doi: 10.1359/jbmr.2000.15.8.1477. [DOI] [PubMed] [Google Scholar]
- 9.Gingery A, Bradley E, Shaw A, Oursler MJ. Phosphatidylinositol 3-kinase coordinately activates the MEK/ERK and AKT/NFkappaB pathways to maintain osteoclast survival. J Cell Biochem. 2003;89(1):165–179. doi: 10.1002/jcb.10503. [DOI] [PubMed] [Google Scholar]
- 10.Gingery A, Bradley EW, Pederson L, Ruan M, Horwood NJ, Oursler MJ. TGF-beta coordinately activates TAK1/MEK/AKT/NFkB and SMAD pathways to promote osteoclast survival. Exp Cell Res. 2008;314(15):2725–2738. doi: 10.1016/j.yexcr.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brault V, Moore R, Kutsch S, et al. Inactivation of the (β)-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128(8):1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 12.Joeng KS, Schumacher CA, Zylstra-Diegel CR, Long F, Williams BO. Lrp5 and Lrp6 redundantly control skeletal development in the mouse embryo. Dev Biol. 2011;359(2):222–229. doi: 10.1016/j.ydbio.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maeda K, Kobayashi Y, Udagawa N, et al. Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat Med. 2012;18(3):405–412. doi: 10.1038/nm.2653. [DOI] [PubMed] [Google Scholar]
- 14.Chiu WS, McManus JF, Notini AJ, Cassady AI, Zajac JD, Davey RA. Transgenic mice that express Cre recombinase in osteoclasts. Genesis. 2004;39(3):178–185. doi: 10.1002/gene.20041. [DOI] [PubMed] [Google Scholar]
- 15.Song I, Kim JH, Kim K, Jin HM, Youn BU, Kim N. Regulatory mechanism of NFATc1 in RANKL-induced osteoclast activation. FEBS Lett. 2009;583(14):2435–2440. doi: 10.1016/j.febslet.2009.06.047. [DOI] [PubMed] [Google Scholar]
- 16.Kim JH, Kim K, Jin HM, et al. Negative feedback control of osteoclast formation through ubiquitin-mediated down-regulation of NFATc1. J Biol Chem. 2010;285(8):5224–5231. doi: 10.1074/jbc.M109.042812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koval A, Purvanov V, Egger-Adam D, Katanaev VL. Yellow submarine of the Wnt/Frizzled signaling: submerging from the G protein harbor to the targets. Biochem Pharmacol. 2011;82(10):1311–1319. doi: 10.1016/j.bcp.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Wang HY, Liu T, Malbon CC. Structure-function analyses of Frizzleds. Cell Signal. 2006;18(7):934–941. doi: 10.1016/j.cellsig.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Koval A, Katanaev VL. Wnt3a stimulation elicits G-protein-coupled receptor properties of mammalian Frizzled proteins. Biochem J. 2011;433(3):435–440. doi: 10.1042/BJ20101878. [DOI] [PubMed] [Google Scholar]
- 20.Taylor SS, Kim C, Cheng CY, Brown SH, Wu J, Kannan N. Signaling through cAMP and cAMP-dependent protein kinase: diverse strategies for drug design. Biochim Biophys Acta. 2008;1784(1):16–26. doi: 10.1016/j.bbapap.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skroblin P, Grossmann S, Schafer G, Rosenthal W, Klussmann E. Mechanisms of protein kinase a anchoring. Int Rev Cell Mol Biol. 2010;283:235–330. doi: 10.1016/S1937-6448(10)83005-9. [DOI] [PubMed] [Google Scholar]
- 22.Ominsky MS, Li C, Li X, et al. Inhibition of sclerostin by monoclonal antibody enhances bone healing and improves bone density and strength of nonfractured bones. J Bone Miner Res. 2011;26(5):1012–1021. doi: 10.1002/jbmr.307. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Ominsky MS, Warmington KS, et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009;24(4):578–588. doi: 10.1359/jbmr.081206. [DOI] [PubMed] [Google Scholar]
- 24.Padhi D, Jang G, Stouch B, Fang L, Posvar E. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res. 2011;26(1):19–26. doi: 10.1002/jbmr.173. [DOI] [PubMed] [Google Scholar]
- 25.McColm J, Hu L, Womack T, Tang CC, Chiang AY. Single- and multiple-dose randomized studies of blosozumab, a monoclonal antibody against Sclerostin, in healthy postmenopausal women. J Bone Miner Res. 2014;29(4):935–943. doi: 10.1002/jbmr.2092. [DOI] [PubMed] [Google Scholar]
- 26.Fulciniti M, Tassone P, Hideshima T, et al. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood. 2009;114(2):371–379. doi: 10.1182/blood-2008-11-191577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diarra D, Stolina M, Polzer K, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13(2):156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 28.Munshi NC, Abonour R, Beck JT, et al. Early evidence of anabolic bone activity of BHQ880, a fully human anti-DKK1 neutralizing antibody: results of a phase 2 study in previously untreated patients with smoldering multiple myeloma at risk for progression. Paper presented at: 54th American Society of Hematology (ASH) Annual Meeting and Exposition; ASH Annual Meeting Abstracts; December 8–11, 2012; Atlanta, GA. 2012. [cited 2015 Aug 1]. p. 331. Available from: https://ash.confex.com/ash/2012/webprogram/Paper48568.html. [Google Scholar]
- 29.Modarresi R, Xiang Z, Yin M, Laurence J. WNT/β-catenin signaling is involved in regulation of osteoclast differentiation by human immunodeficiency virus protease inhibitor ritonavir: relationship to human immunodeficiency virus-linked bone mineral loss. Am J Pathol. 2009;174(1):123–135. doi: 10.2353/ajpath.2009.080484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiang YW, Chen Y, Brown N, et al. Characterization of Wnt/beta-catenin signalling in osteoclasts in multiple myeloma. Br J Haematol. 2010;148(5):726–738. doi: 10.1111/j.1365-2141.2009.08009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jang HD, Shin JH, Park DR, et al. Inactivation of glycogen synthase kinase-3b is required for osteoclast differentiation. J Biol Chem. 2011;286(45):39043–39050. doi: 10.1074/jbc.M111.256768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doble BW, Woodgett JR. Role of glycogen synthase kinase-3 in cell fate and epithelial-mesenchymal transitions. Cells Tissues Organs. 2007;185(1–3):73–84. doi: 10.1159/000101306. [DOI] [PubMed] [Google Scholar]
- 33.Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci. 2010;35(3):161–168. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang J, Slingerland JM. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle. 2003;2(4):339–345. [PubMed] [Google Scholar]
- 35.Yu H, Levesque MA, Khosravi MJ, Papanastasiou-Diamandi A, Clark GM, Diamandis EP. Associations between insulin-like growth factors and their binding proteins and other prognostic indicators in breast cancer. Br J Cancer. 1996;74:1242–1247. doi: 10.1038/bjc.1996.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanchez-Fernandez MA, Gallois A, Riedl T, Jurdic P, Hoflack B. Osteoclasts control osteoblast chemotaxis via PDGF-BB/PDGF receptor beta signaling. PLoS One. 2008;3(10):e3537. doi: 10.1371/journal.pone.0003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lotinun S, Kiviranta R, Matsubara T, et al. Osteoclast-specific cathepsin K deletion stimulates S1P-dependent bone formation. J Clin Invest. 2013;123(2):666–681. doi: 10.1172/JCI64840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryu J, Kim HJ, Chang EJ, Huang H, Banno Y, Kim HH. Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast-osteoblast coupling. EMBO J. 2006;25(24):5840–5851. doi: 10.1038/sj.emboj.7601430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeshita S, Fumoto T, Matsuoka K, et al. Osteoclast-secreted CTHRC1 in the coupling of bone resorption to formation. J Clin Invest. 2013;123(9):3914–3924. doi: 10.1172/JCI69493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quint P, Ruan M, Pederson L, et al. Sphingosine 1-phosphate (S1P) receptors 1 and 2 coordinately induce mesenchymal cell migration through S1P activation of complementary kinase pathways. J Biol Chem. 2013;288(8):5398–5406. doi: 10.1074/jbc.M112.413583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li A, Xia X, Yeh J, et al. PDGF-AA promotes osteogenic differentiation and migration of mesenchymal stem cell by down-regulating PDGFRalpha and derepressing BMP-Smad1/5/8 signaling. PLoS ONE. 2014;9(12):e113785. doi: 10.1371/journal.pone.0113785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kiechl S, Wittmann J, Giaccari A, et al. Blockade of receptor activator of nuclear factor-[kappa]B (RANKL) signaling improves hepatic insulin resistance and prevents development of diabetes mellitus. Nat Med. 2013;19(3):358–363. doi: 10.1038/nm.3084. [DOI] [PubMed] [Google Scholar]
- 43.Anderson DM, Maraskovsky E, Billingsley WL, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390(6656):175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 44.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10(7):468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- 45.Santiago F, Oguma J, Brown AMC, Laurence J. Noncanonical Wnt signaling promotes osteoclast differentiation and is facilitated by the human immunodeficiency virus protease inhibitor ritonavir. Biochem Biophys Res Commun. 2012;417(1):223–230. doi: 10.1016/j.bbrc.2011.11.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Witze ES, Litman ES, Argast GM, Moon RT, Ahn NG. Wnt5a control of cell polarity and directional movement by polarized redistribution of adhesion receptors. Science. 2008;320(5874):365–369. doi: 10.1126/science.1151250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen AE, Ginty DD, Fan C-M. Protein kinase A signalling via CREB controls myogenesis induced by Wnt proteins. Nature. 2005;433(7023):317–322. doi: 10.1038/nature03126. [DOI] [PubMed] [Google Scholar]
- 48.Sheridan CM, Heist EK, Beals CR, Crabtree GR, Gardner P. Protein kinase A negatively modulates the nuclear accumulation of NF-ATc1 by priming for subsequent phosphorylation by glycogen synthase kinase-3. J Biol Chem. 2002;277(50):48664–48676. doi: 10.1074/jbc.M207029200. [DOI] [PubMed] [Google Scholar]
- 49.Yoon SH, Ryu JY, Lee Y, Lee ZH, Kim HH. Adenylate cyclase and calmodulin-dependent kinase have opposite effects on osteoclastogenesis by regulating the PKA-NFATc1 pathway. J Bone Miner Res. 2011;26(6):1217–1229. doi: 10.1002/jbmr.310. [DOI] [PubMed] [Google Scholar]
- 50.Tanji C, Yamamoto H, Yorioka N, Kohno N, Kikuchi K, Kikuchi A. A-kinase anchoring protein AKAP220 binds to glycogen synthase kinase-3β (GSK-3β) and mediates protein kinase A-dependent inhibition of GSK-3β. J Biol Chem. 2002;277(40):36955–36961. doi: 10.1074/jbc.M206210200. [DOI] [PubMed] [Google Scholar]
- 51.Zardawi SJ, O’Toole SA, Sutherland RL, Musgrove EA. Dysregulation of Hedgehog, Wnt and Notch signalling pathways in breast cancer. Histol Histopathol. 2009;24(3):385–398. doi: 10.14670/HH-24.385. [DOI] [PubMed] [Google Scholar]
- 52.Ayyanan A, Civenni G, Ciarloni L, et al. Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc Natl Acad Sci USA. 2006;103(10):3799–3804. doi: 10.1073/pnas.0600065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olson DJ, Papkoff J. Regulated expression of Wnt family members during proliferation of C57mg mammary cells. Cell Growth Differ. 1994;5(2):197–206. [PubMed] [Google Scholar]
- 54.Wong GT, Gavin BJ, McMahon AP. Differential transformation of mammary epithelial cells by Wnt genes. Mol Cell Biol. 1994;14(9):6278–6286. doi: 10.1128/mcb.14.9.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benhaj K, Akcali KC, Ozturk M. Redundant expression of canonical Wnt ligands in human breast cancer cell lines. Oncol Rep. 2006;15(3):701–707. [PubMed] [Google Scholar]
- 56.Bankfalvi A, Terpe HJ, Breukelmann D, et al. Immunophenotypic and prognostic analyses of E-cadherin and beta-catenin expression during breast carcinogenesis and tumour progression: a comparative study with CD44. Histopathology. 1999;34(1):25–34. doi: 10.1046/j.1365-2559.1999.00540.x. [DOI] [PubMed] [Google Scholar]
- 57.Hatsell S, Rowlands T, Hiremath M, Cowin P. Beta-catenin and Tcfs in mammary development and cancer. J Mammary Gland Biol Neoplasia. 2003;8(2):145–158. doi: 10.1023/a:1025944723047. [DOI] [PubMed] [Google Scholar]
- 58.A J, Nakopoulou L, Gakiopoulou H, Keramopoulos A, Davaris PS, Pignatelli M. Expression patterns of beta-catenin in in situ and invasive breast cancer. Eur J Surg Oncol. 2001;27(1):31–36. doi: 10.1053/ejso.1999.1017. [DOI] [PubMed] [Google Scholar]
- 59.Wong SC, Lo SF, Lee KC, Yam JW, Chan JK, Hsiao WWL. Expression of frizzled-related protein and Wnt-signalling molecules in invasive human breast tumours. J Pathol. 2002;196(2):145–153. doi: 10.1002/path.1035. [DOI] [PubMed] [Google Scholar]
- 60.Li Y, Hively WP, Varmus HE. Use of MMTV-Wnt-1 transgenic mice for studying the genetic basis of breast cancer. Oncogene. 2000;19(8):1002–1009. doi: 10.1038/sj.onc.1203273. [DOI] [PubMed] [Google Scholar]
- 61.Jonsson M, Borg A, Nilbert M, A Andersson T. Involvement of adenomatous polyposis coli (APC)/beta-catenin signalling in human breast cancer. Eur J Cancer. 2000;36(2):242–248. doi: 10.1016/s0959-8049(99)00276-2. [DOI] [PubMed] [Google Scholar]