Abstract
Objective
Dihydrofolate reductase (DHFR) is a key protein involved in tetrahydrobiopterin (BH4) regeneration from 7,8-dihydrobiopterin (BH2). Dysfunctional DHFR may induce endothelial nitric oxide synthase (eNOS) uncoupling resulting in enzyme production of superoxide anions instead of nitric oxide (NO). The mechanism by which DHFR is regulated is unknown. Here, we investigate whether eNOS-derived NO maintains DHFR stability.
Approach and Results
DHFR activity, BH4 content, eNOS activity, and S-nitrosylation were assessed in human umbilical vein endothelial cells (HUVECs) and in aortas isolated from wild type and eNOS knockout mice. In HUVECs, depletion of intracellular NO by transfection with eNOS-specific siRNA or by the NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO) --both of which had no effect on DHFR mRNA levels-- markedly reduced DHFR protein levels in parallel with increased DHFR poly-ubiquitination. Supplementation of S-Nitroso-L-glutathione (GSNO), a NO donor, or MG132, a potent inhibitor of the 26S proteasome, prevented eNOS silencing and PTIO-induced DHFR reduction in HUVECs. PTIO suppressed S-nitrosylation of DHFR while GSNO promoted DHFR S-nitrosylation. Mutational analysis confirmed that cysteine 7 of DHFR was S-nitrosylated. Cysteine 7 S-nitrosylation stabilized DHFR from ubiquitination and degradation. Experiments performed in aortas confirmed that PTIO or eNOS deficiency reduces endothelial DHFR, which can be abolished by MG132 supplementation.
Conclusions
We conclude that S-nitrosylation of DHFR at cysteine 7 by eNOS-derived NO is crucial for DHFR stability. We also conclude that NO-induced stabilization of DHFR prevents eNOS uncoupling via regeneration of BH4, an essential eNOS cofactor.
Keywords: Nitric oxide, Tetrahydrobiopterin, Dihydrofolate reductase, eNOS, eNOS uncoupling
Introduction
Nitric oxide (NO), an essential molecule in maintaining cardiovascular health, is derived from L-arginine in an oxidizing reaction catalyzed by NO synthase (NOS). Three distinct isoforms of NOS have been identified in mammalian cells: neuronal NOS (nNOS), inducible NOS (iNOS), and endothelial NOS (eNOS). The eNOS isoform is a major source of NO produced by endothelial cells1, and an overwhelming amount of evidence indicates that dysfunctional eNOS is associated with the pathogenesis of various vascular diseases, including atherosclerosis and hypertension2, 3. In such conditions, endothelial NO bioactivity is diminished and oxidative stress increases, resulting in endothelial dysfunction4, 5.
The enzymatic function of eNOS is tightly regulated by various factors. One cofactor, tetrahydrobiopterin (BH4) helps to maintain enzyme coupling1. When oxidized or reacted with peroxynitrite, BH4 forms 7,8-dihydrobiopterin (BH2) and trihydrobiopterin radical (BH3.) accordingly, which inactivates NOS function by competing with BH4 for eNOS binding, this ultimately causes eNOS uncoupling6–8. Under such condition, the enzyme is converted from an NO-producing enzyme to a molecule that generates superoxide anions9
Two key enzymes regulate the concentration of BH4. GTP cyclohydrolase I (GTPCH) controls de novo biosynthesis from GTP10, and dihydrofolate reductase (DHFR) is the key enzyme responsible for salvation of BH4 from BH2 -at the expense of NAD(P)H11. Recent studies indicate that angiotensin II down-regulates DHFR expression, decreases BH4 levels, and increases eNOS uncoupling in endothelial cells through oxidative stress12. Additionally, DHFR inhibition or knockdown diminishes the BH4:BH2 ratio and exacerbates eNOS uncoupling11, 13. In fact, BH4-deficient (hph-1) mice treated with methotrexate (MTX) to inhibit BH4 recycling by DHFR, displayed strikingly elevated BH2 levels and decreased BH4:BH2 ratio in the aortas7. Despite results from previous studies suggesting that GTPCH and DHFR mediate eNOS function, it is unknown whether eNOS feedback regulates these two enzymes.
In addition to vasodilation, NO is also involved in various signaling pathways. In these pathways, NO targets specific cysteine residues, resulting in covalent incorporation of NO into thiol groups to form S-nitrosothiol, termed S-nitrosylation14. We previously reported that NO can activate GTPCH activity through S-nitrosylation in endothelial cells15. However, it is unknown whether eNOS-derived NO has the potential to S-nitrosylate DHFR to regulate its function.
DHFR expression is regulated by various mechanisms, including transcription and degradation, which are mediated via the ubiquitin-proteasomal pathway16. Previous reports suggest that certain post-translational modifications may regulate protein ubiquitination and function14, 17. S-nitrosylation in particular may inhibit degradation of certain proteins such as Bcl-2 and TRIM72 while promoting degradation of others such as Parkin18–20. In this study, we hypothesized that NO generated by eNOS stabilizes DHFR via S-nitrosylation, which regenerates BH4 in order to maintain the eNOS coupling status.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
eNOS silencing reduces DHFR protein expression in HUVECs
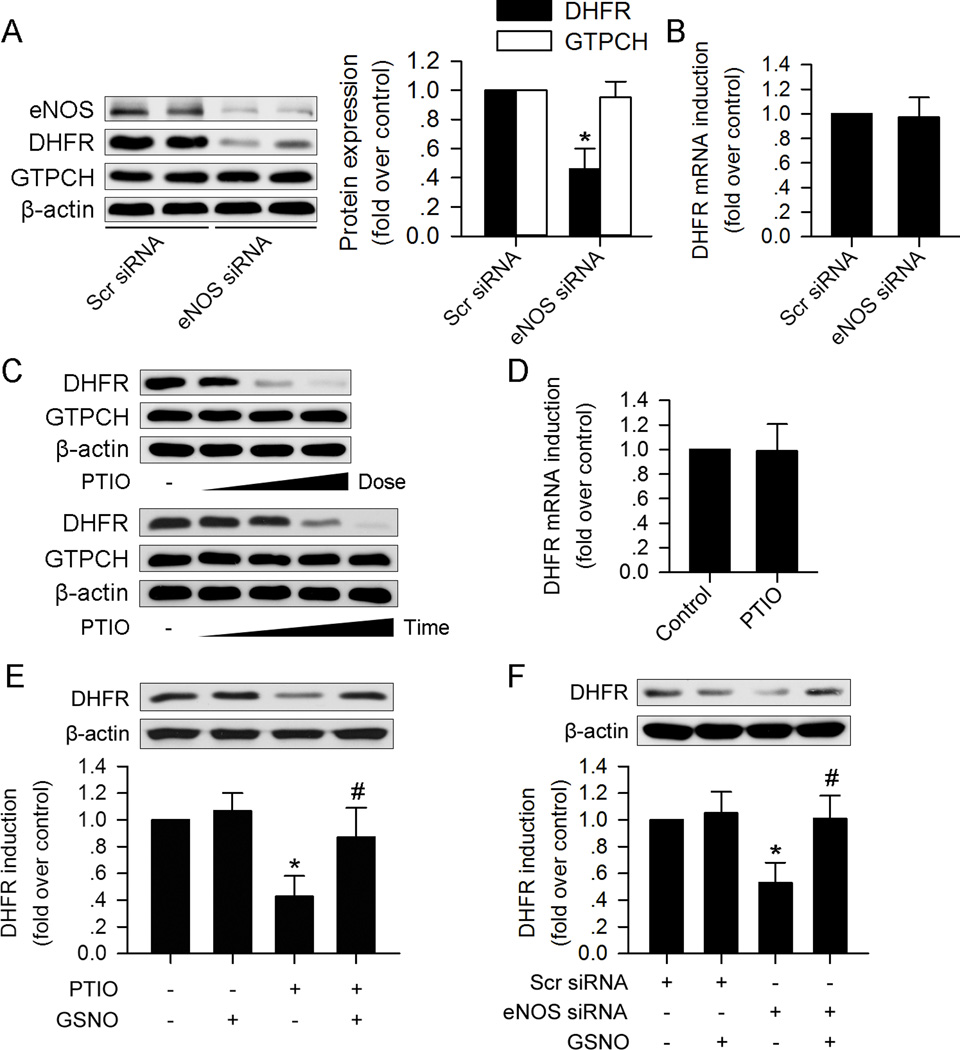
Confluent HUVECs express high levels of eNOS, GTPCH, and DHFR (Figure 1A). To determine whether eNOS expression alters GTPCH and DHFR levels, confluent HUVECs were transfected with scramble siRNA or eNOS-specific siRNA. eNOS-specific siRNA markedly reduced eNOS levels compared to those transfected with control siRNA, 48hr after transfection (Figure 1A), indicating that eNOS-specific siRNA targets only eNOS. Neither control siRNA nor eNOS-specific siRNA affected the levels of GTPCH (Figure 1A). Interestingly, DHFR protein expression in eNOS-silenced HUVECs was markedly lower than that of HUVECs transfected with control siRNA (Figure 1A); however, eNOS silencing did not significantly alter DHFR mRNA levels (Figure 1B).
Figure 1. eNOS derived NO prevents DHFR protein reduction in HUVEC.
(A) eNOS silencing reduced DHFR but not GTPCH protein expression. (B) eNOS silencing did not alter DHFR mRNA expression. (C) NO scavenger PTIO treatment reduced DHFR expression in a dose- (0µM, 75µM, 150µM, 300µM) and time- (0h, 6h, 12h, 24h, 48h) dependent manner, but had no effect on GTPCH expression. (D) PTIO (150µM) had no significant effect on DHFR mRNA expression. (E) NO donor GSNO (100µM) reversed PTIO (150µM) induced DHFR reduction. (F) GSNO (100µM) prevents DHFR reduction induced by eNOS silencing. (n=3 for each group; *p<0.05 vs. Scr siRNA in A and F, or p<0.05 vs. control in E; #p<0.05 vs. PTIO in E, or p<0.05 vs. eNOS siRNA in F)
We also tested the effect of eNOS inhibition by L-NAME on DHFR and GTPCH expression. Similar with the results of eNOS siRNA silencing, L-NAME reduced DHFR expression from the 1mM to 2mM, but had no effect on GTPCH expression (Supplemental Figure I).
eNOS-derived NO prevents DHFR reduction in HUVECs
The major function of eNOS is to generate NO. Next, we investigated whether reduced NO release in eNOS-silenced HUVECs results in reduced expression of DHFR. To this end, HUVECs were exposed to 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO), a known NO scavenger21. As shown in Figure 1C, treatment of HUVECs with PTIO for 24h dose-dependently reduced DHFR protein levels from 150 µM to 300 µM. Exposure of HUVECs to PTIO (150 µM) for 6h and 12h had marginal effects on DHFR expression. Prolonged exposure of HUVECs to PTIO for 24 to 48h significantly reduced DHFR (Figure 1C). PTIO did not alter DHFR mRNA (Figure 1D) levels. In addition, PTIO incubation did not alter GTPCH expression at a maximum dose of 300 µM for 48h (Figure 1C).
Since both eNOS silencing and NO scavenging via PTIO lowered DHFR levels in HUVECs, we reasoned that exposure of HUVECs to a NO donor would ablate DHFR reduction in eNOS-silenced HUVECs. As depicted in Figure1E and 1F, exposure of HUVECs to S-Nitroso-L-glutathione (GSNO), a NO donor, reversed DHFR reduction induced by eNOS siRNA transfection or PTIO incubation. These results indicate that eNOS-derived NO is essential for maintaining DHFR expression without affecting mRNA levels.
ONOO- does not affect DHFR expression in HUVECs
Superoxide anions could deplete NO to generate ONOO-, which is crucial in the pathogenesis in hypertension and endothelial dysfunction. We further tested whether ONOO- may affect DHFR in HUVECs. As shown in Supplemental Figure II, addition of ONOO- range from 50uM to 500uM induced protein tyrosine nitration in a dose-dependent manner. GTPCH expression decreased as the increased dose of ONOO-, which is in consistent with our previous report10. However, DHFR expression remained unchanged, indicating that ONOO- has no effect on DHFR expression.
NO stabilizes the DHFR protein in HUVECs
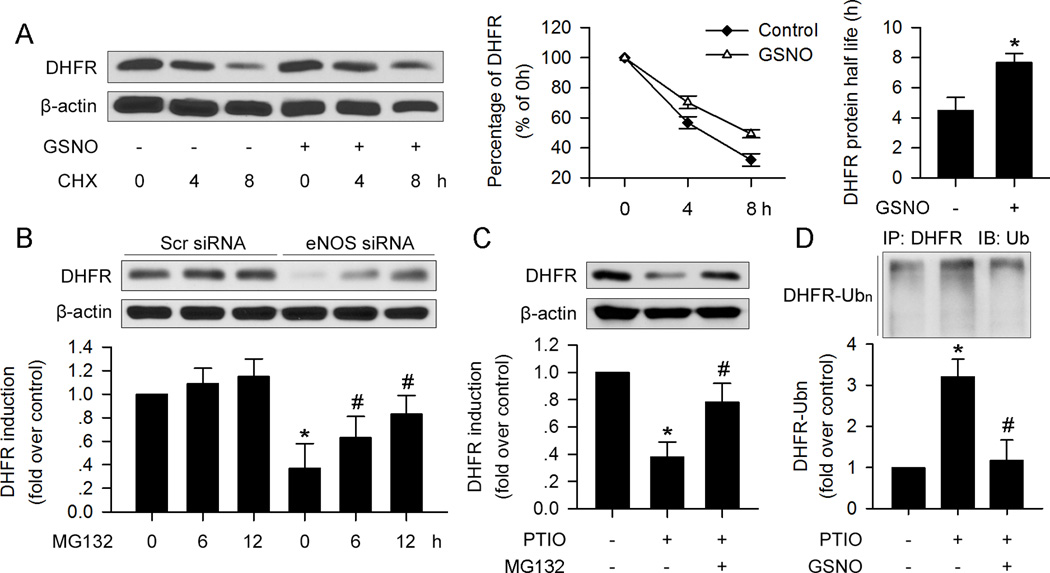
Because NO depletion in HUVECs only suppressed DHFR protein expression without altering mRNA levels, we investigated whether NO affects the stability of the DHFR protein. To this end, protein stability (half-life) was measured in the presence of cycloheximide (CHX), an inhibitor of de novo protein synthesis. As depicted in Figure 2A, in the absence of CHX, the DHFR protein decreased by about 50% after 4h and continued to decline after 8h. As depicted in Figure 2A, GSNO treatment significantly inhibited DHFR reduction in the presence of CHX, and the DHFR half-life was increased to 7.86h.
Figure 2. NO depletion promotes DHFR degradation via ubiquitin-proteasome degradation in HUVEC.
(A) GSNO (100µM) supplementation prevented DHFR reduction induced by CHX (30µg/ml) treatment. DHFR degradation induced by eNOS silencing (B) or PTIO (150µM) (C) could be inhibited by proteasome inhibitor, MG132 (1µM). (D) PTIO- (150µM) induced DHFR poly-ubiquitination, which could be suppressed by GSNO (100µM). (n=3; *p<0.05 vs. control in A, C and D, or p<0.05 vs. Scr siRNA in B; #p<0.05 vs. eNOS siRNA in B, or p<0.05 vs. PTIO in C and D)
NO depletion promotes DHFR degradation via the ubiquitin-proteasome system in HUVECs
The ubiquitin-proteasome system is important for intracellular protein degradation22. It has been reported that DHFR can be ubiquitinated and degraded by the proteasome23. To examine whether NO depletion caused by eNOS silencing or PTIO leads to DHFR reduction via ubiquitin-proteasome degradation, eNOS siRNA-treated or PTIO-treated HUVECs were co-incubated with MG132, a potent 26S proteasome inhibitor. As depicted in Figure2B and 2C, MG132 ablated DHFR reduction caused by eNOS silencing or PTIO. In parallel, PTIO increased the detection of ubiquitinated DHFR (Figure 2D). GSNO pre-treatment abolished PTIO-enhanced DHFR ubiquitination. These data suggest that NO via the ubiquitin-proteasome system promotes DHFR ubiquitination and 26S proteasome-mediated degradation.
NO depletion reduces DHFR activity and BH4 levels in HUVECs
DHFR is the key enzyme responsible for salvation formation of BH411. We therefore determined whether NO depletion altered the function of DHFR in HUVECs. In parallel with decreased protein levels, DHFR activity and intracellular BH4 were markedly suppressed by eNOS silencing. These effects were restored by MG132 or GSNO treatment (Supplemental Figure IIIA–D). Like the effects of eNOS silencing, PTIO also led to a reduction in DHFR activity and BH4 levels, all of which were prevented by addition of MG132 or GSNO (Supplemental Figure IIIE–H).
NO S-nitrosylates DHFR at Cysteine 7 in HUVECs
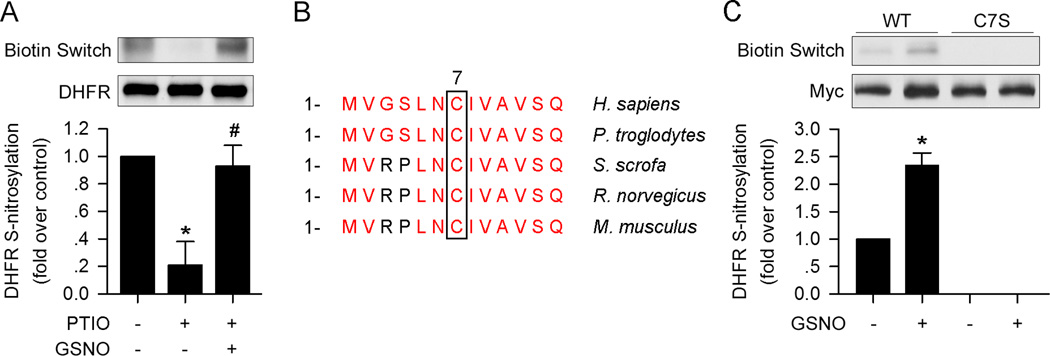
Next, we sought to explore how NO regulates DHFR. Since NO leads to S-nitrosylation of certain proteins18, 20. We determined whether NO could also S-nitrosylate DHFR. As shown in Figure 3A, GSNO markedly increased DHFR S-nitrosylation, while PTIO significantly reduced S-nitrosylation.
Figure 3. Cystein 7 is the site of S-nitrosylation of DHFR.
(A) GSNO (100µM) restored DHFR S-nitrosylation in the presence of PTIO (150µM) as determined by biotin switch assay. (B) Amino acid sequences comparison of DHFR among species. (C) C7S mutation blocked GSNO- (100µM) induced DHFR S-nitrosylation. (n=3; *p<0.05 vs. control in A, or p<0.05 vs. WT in C; #p<0.05 vs. PTIO in A)
There is only one cysteine residue (C7) in human DHFR, which is conserved among species (Figure 3B), suggesting that it is the residue to be S-nitrosylated. To test if cysteine 7 is the target for s-nitrosylation, we generated a DHFR mutant, in which cysteine 7 of DHFR was replaced with serine (C7S). Both wild type (WT) DHFR and the C7S mutant were transfected into HUVECs. After transfection, the cells were treated with GSNO for 6h. As expected, the C7S mutation in DHFR blocked basal- and GSNO-induced S-nitrosylation of DHFR (Figure 3C).
C7 S-nitrosylation stabilizes DHFR in HUVECs
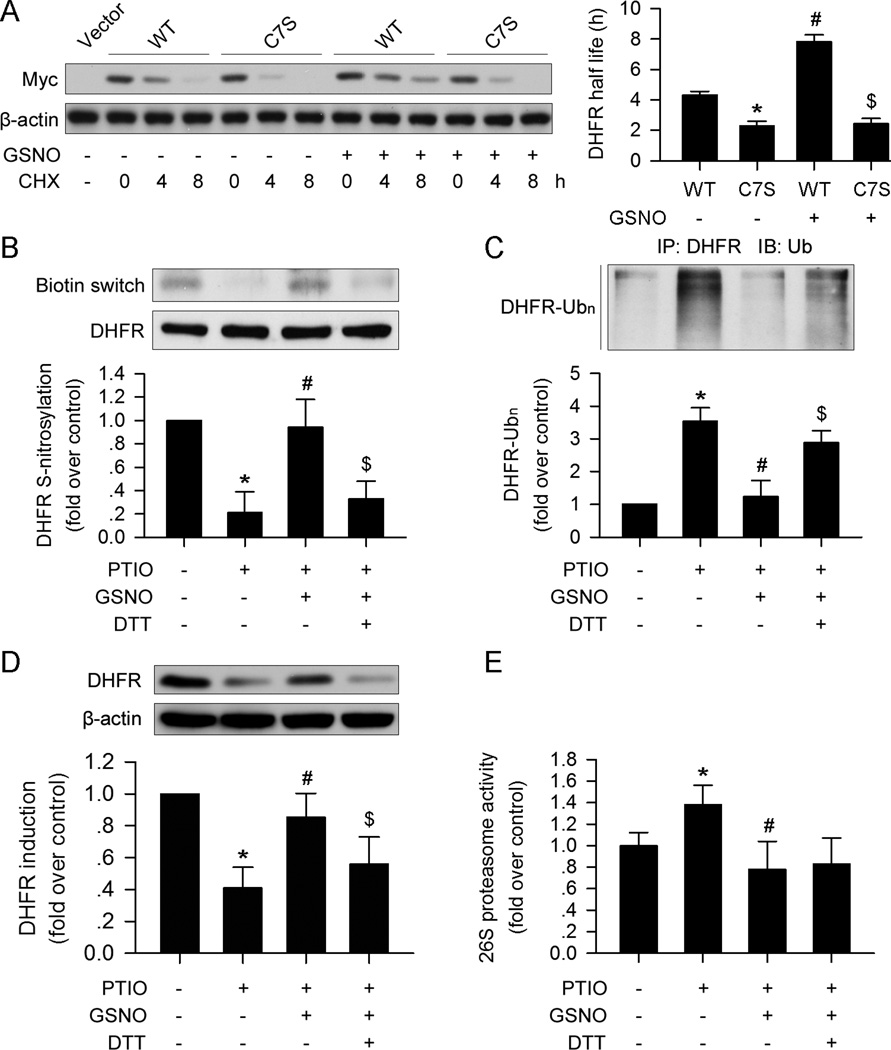
S-nitrosylation of certain proteins may influence their stability19, 24. We explored whether C7 S-nitrosylation of DHFR affects its stability. WT or C7S mutant DHFR plasmids were transfected into HUVECs. The C7S mutation of DHFR shortened the half-life of the protein compared to the WT (Figure 4A). GSNO significantly increased the half-life of WT DHFR, but the C7S mutation blocked this effect in HUVECs (Figure 4A). These data suggest that S-nitrosylation of DHFR at C7 stabilizes the protein.
Figure 4. DHFR S-nitrosylation prevents ubiquitination and degradation.
(A) The C7S mutation in DHFR destabilized the protein compared with the wild type DHFR treated with CHX (30µg/ml). GSNO (100µM) stabilized DHFR, while the C7S mutation abolished the effect. (B) GSNO (100µM) restored DHFR S-nitrosylation, which was suppressed by PTIO (150µM). DTT (10mM) inhibited the effect of GSNO (100µM). (C and D) GSNO (100µM) prevented DHFR ubiquitination and degradation induced by PTIO (150µM); this effect could be blocked by DTT (10mM) supplementation. (E) PTIO (150µM) promoted 26S proteasome activity, which could be reversed by GSNO (100µM). The addition of DTT (10mM) did not affect 26S proteasome activity compared with the PTIO+GSNO group (n=3; *p<0.05 vs. WT in A, or p<0.05 vs. control in B–E; #p<0.05 vs. C7S in A, or p<0.05 vs. PTIO in B–E; $p<0.05 vs. PTIO+GSNO in B–D).
S-nitrosylation of DHFR prevents it from ubiquitination and degradation
Since the ubiquitin-proteasome system is involved in NO depletion-induced DHFR degradation, we next determined whether S-nitrosylation of DHFR initiates ubiquitination and degradation of DHFR. As expected, in parallel with increased S-nitrosylation (Figure 4B), GSNO supplementation markedly lowered PTIO-enhanced DHFR ubiquitination in HUVECs (Figure 4C).
NO directly regulates proteasome activity25. To exclude the possibility that NO-suppressed DHFR degradation occurs via suppressed proteasome activity, HUVECs were treated with dithiothreitol (DTT), a known inhibitor of S-nitrosylation26, in addition to GSNO and PTIO. As shown in Figure 4E, PTIO increased proteasome activity, which was reversed by addition of GSNO. Although supplementation with DTT blocked the effects of GSNO on DHFR ubiquitination and degradation (Figure4C and 4D), it had no effect on proteasome activity (Figure 4E).
S-nitrosylation of DHFR does not affect its activity in vitro
Next, we tested whether S-nitrosylation of DHFR at C7 affects its activity. Recombinant His-tagged WT and C7S DHFR proteins were successfully generated and incubated with GSNO to induce S-nitrosylation. As expected, GSNO increased S-nitrosylation of WT DHFR but had no effect on S-nitrosylation in C7S DHFR mutation in vitro (Supplemental Figure IVA). DHFR activity was further assessed. As shown in Supplemental Figure IVB, the in vitro assay showed no difference between the WT and C7S DHFR activities, and GSNO incubation did not affect their activities.
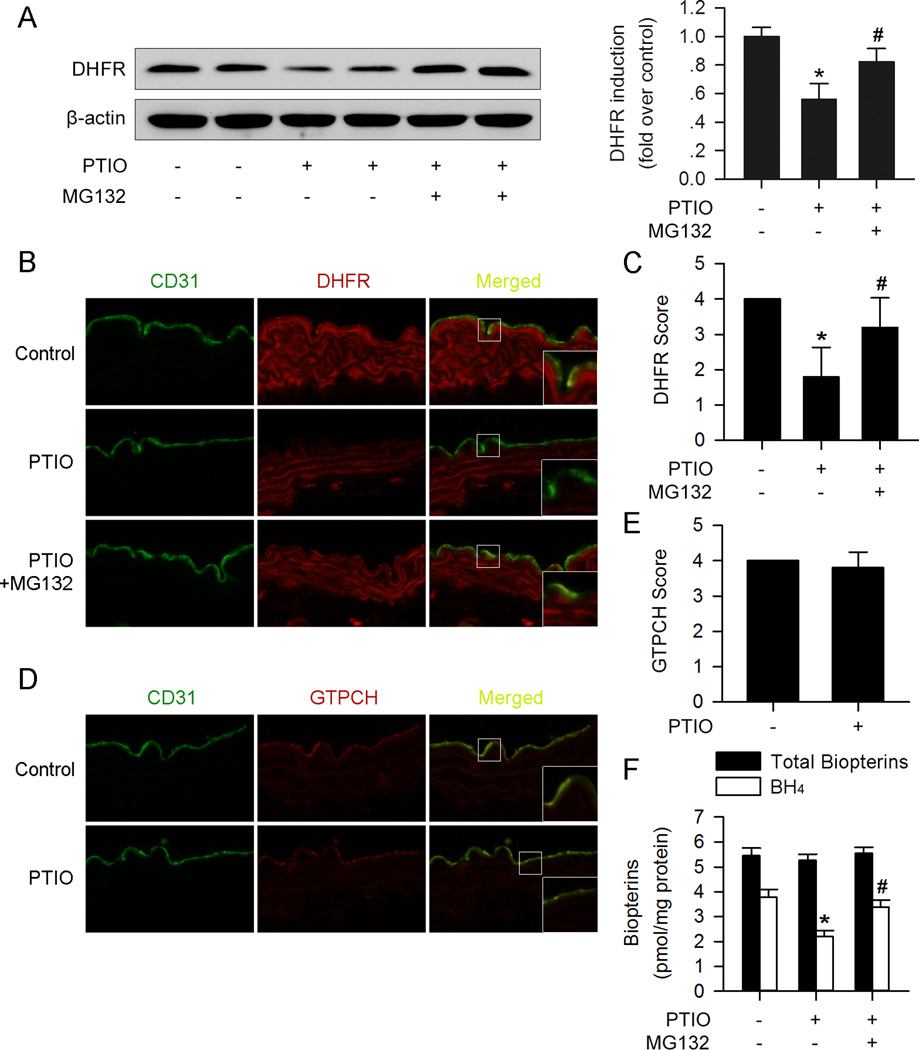
NO depletion suppresses aortic endothelial DHFR expression and BH4 content via proteasomal degradation ex vivo
The effects of NO and proteasome degradation on endothelial DHFR in aortas were further tested in ex vivo system. Aortic segments isolated from 8-week old mice were incubated with PTIO for 24 hours. As shown in Figure 5A–C, PTIO markedly reduced DHFR expression in endothelium, as determined by immunofluorescence staining and western blots. MG132 treatment prevented the effects of PTIO on DHFR (Figure 5A–C). As expected, the endothelial GTPCH expression was not altered by PTIO incubation (Figure5D and 5E).
Figure 5. PTIO reduces aortic endothelial DHFR expression and BH4 content via proteasomal degradation ex vivo.
(A) Western blot analysis showed reduced DHFR expression in PTIO- (150µM) treated aortas, which could be blocked by MG132 (1µM, 6h) supplementation. (B) Representative immunofluorescence staining of DHFR (red) and endothelium marker CD31 (green) of ex vivo cultured aortas. (C) PTIO (150µM) reduced endothelial DHFR expression, while MG132 (1µM, 6h) reversed the effect. (D) Representative immunofluorescence staining of GTPCH (red) and endothelium marker CD31 (green) of ex vivo cultured aortas. (E) PTIO (150µM) had no significant effect on endothelial GTPCH expression. (F) PTIO (150µM) reduced BH4 content, which could be reversed by addition of MG132 (1µM, 6h) in aortas ex vivo. (n=4 for each group; *p<0.05 vs. control; #p<0.05 vs. PTIO)
We next assayed the BH4 and total biopterin levels in ex vivo-cultured aortas. Similar to the effect on DHFR protein expression, PTIO significantly reduced BH4 levels, which was restored by MG132 (Figure 5F).
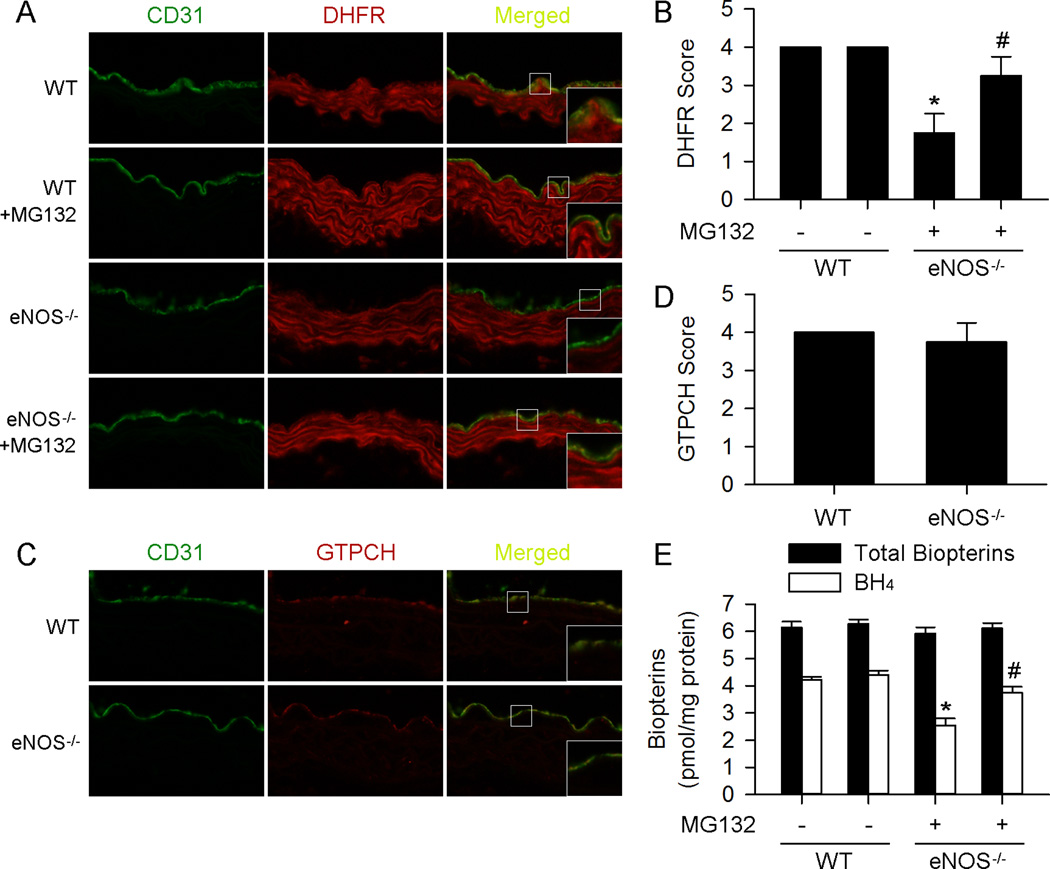
eNOS deficiency reduces endothelial DHFR expression and BH4 content via proteasomal degradation in vivo
Because the above data show that NO prevents endothelial DHFR degradation both in HUVECs and ex vivo aortas, we reasoned that eNOS deficiency may lead to reduced endothelial DHFR expression and BH4 content. Indeed, immunofluorescence staining revealed reduced endothelial DHFR expression in aorta from eNOS−/− mice compared with WT mice (Figure6A and 6B). In contrast, the expression of GTPCH was comparable between WT and eNOS−/− mice (Figure6C and 6D).
Figure 6. MG132 prevents aortic endothelial DHFR expression and BH4 content in eNOS−/− mice.
(A) Representative immunofluorescence staining of DHFR (red) and endothelium marker CD31 (green) of aortas from mice received indicated treatment. (B) MG132 (5mg/kg/d, 3d) suppressed endothelial DHFR reduction in eNOS−/− mice. (C) Representative immunofluorescence staining of GTPCH (red) and endothelium marker CD31 (green) of aortas from indicated treated mice. (D) eNOS deficiency had no significant effect on endothelial GTPCH expression. (E) Supplementation of MG132 (5mg/kg/d, 3d) suppressed aortic BH4 content reduction in eNOS−/− mice. (n=5 for each group; *p<0.05 vs. WT; #p<0.05 vs. eNOS−/−)
Next, we determined if proteasome inhibition increased the levels of DHFR in the aortas of eNOS−/−. To test this, both WT and eNOS−/− mice received i.p. injection of MG132. As depicted in Figure6A and 6B, MG132 increased the levels of DHFR in endothelial cells.
Finally, we detected BH4 and total biopterin levels in aortas. As expected, aortas from eNOS−/− mice showed reduced levels of BH4 compared with WT mice, which was reversed by MG132 i.p. injection (Figure 6E). These data further support that NO prevents endothelial DHFR degradation through proteasome system both in vitro and in vivo.
Discussion
In this study, we demonstrate for the first time that NO derived from eNOS regulates DHFR expression and function. The major finding in the present study is that NO generated by eNOS promotes DHFR S-nitrosylation at C7. Furthermore, we found that S-nitrosylation of DHFR inhibits DHFR ubiquitination and proteasome degradation. Stimuli that deplete intracellular NO lead to the reduction of DHFR S-nitrosylation, and result in its degradation through the ubiquitin-proteasome system. The NO donor GSNO efficiently reversed the effects. Consistently, DHFR is markedly lower in the endothelium from eNOS−/− mice when compared to those in WT. Finally, proteasome inhibition with MG132 increased DHFR expression in the endothelium of eNOS−/− mice. Taken together, we conclude that NO derived from eNOS is a key factor in maintaining DHFR stability, BH4 contents, and eNOS uncoupling.
It has been well documented that DHFR and GTPCH regulate eNOS coupling via maintaining BH4 content9. We show here that eNOS deficiency reduce DHFR but not GTPCH expression both in vivo and in vitro. The major function of eNOS is to generate NO, and studies reveal that NO participates in various signaling pathways and exerts multi-biological functions27. Our data show that NO also regulates DHFR expression. We found that depletion of NO by eNOS deficiency or PTIO reduced DHFR protein levels, paralleled by reduced DHFR activity and intracellular BH4 content, but not by a reduction in mRNA levels. This suggests post-transcriptional regulation of DHFR is responsible for the reduction. Indeed, we found PTIO increased DHFR ubiquitination. The ability of the 26S proteasome inhibitor MG132 to suppress DHFR reduction caused by NO depletion strongly supports the role of the proteasome pathway in DHFR degradation.
NO reacts with cysteine residues to form S-nitrosothiol, and the S-nitrosylated proteins have been increasingly recognized as important determinants of many biochemical processes28. Our lab previously reported that NO is able to S-nitrosylate GTPCH, the key enzyme in de novo BH4 synthesis pathway15. However, whether NO can also S-nitrosylate DHFR and how it affects the protein remains unknown. Here, we found that NO donor GSNO increased DHFR S-nitrosylation, while NO scavenger PTIO decreased its S-nitrosylation. We further identified that C7 of DHFR is the site of S-nitrosylation, which leads to increased stability. Addition of the NO donor GSNO increased DHFR C7 S-nitrosylation and suppressed DHFR reduction in the presence of CHX. However, mutation of this cysteine residue blocked the effect. These data support the role of C7 S-nitrosylation on DHFR degradation process.
S-nitrosylation of proteins is reported to regulate their ubiquitination and stability26, 29. Our results show that S-nitrosylation of DHFR prevents it from ubiquitination, thereby reducing degradation. The mechanism by which S-nitrosylation prevents DHFR ubiquitination is unclear, but may attribute to the conformational change of the protein, which prevents recognition and subsequent attachment of ubiquitin by the enzyme ubiquitin ligases.
The present study demonstrates that NO could stabilize DHFR via S-nitrosylation, and therefore help to maintain eNOS coupling status. However, it is also reported that over-production of NO could also S-nitrosylate eNOS and suppress its function30. These data suggest that two mechanisms of NO S-nitrosylation may work together to maintain eNOS in normal function.
The present study utilized PTIO as an NO scavenger. However, it is reported that PTIO reacts with NO and generates NO2 radicals31, which could then lead to tyrosine nitration of proteins. Despite this side effect of PTIO, our results already showed that tyrosine nitration caused by ONOO- had no effect on DHFR, which indicates that the effect of PTIO on DHFR was not due to tyrosine nitration.
BH4 plays an important role in eNOS coupling, which is crucial in maintaining endothelial function32. In fact, supplementation of BH4 would appear to be a promising strategy for improving the vascular status in such diseases. Unfortunately, application of this strategy to improve endothelial function in vascular disease has resulted in less than satisfactory results33, 34. Studies show that treatment of human endothelial cells with BH4 may only transiently increased intracellular BH4, which is quickly oxidized, causing the accumulation of BH2. This form is inactive as a NOS cofactor and may compete with BH4 for NOS binding and increase eNOS uncoupling13, 34. This may limit the benefits of BH4 therapies, but suggest that DHFR controlling the salvage pathway of BH4 synthesis might be more crucial in balancing intracellular BH4:BH2 ratio than we originally thought.
In summary, the present study provides evidence to suggest that eNOS-derived NO is critical for maintaining DHFR expression in endothelial cells. Stimuli causing NO depletion induces DHFR down-regulation through ubiquitin-proteasome dependent degradation. This is mediated by reduced S-nitrosylation DHFR at C7, which leads to its instability. This signaling cascade may represent a common mechanism that eNOS regulates its homeostasis by maintaining DHFR S-nitrosylation.
Supplementary Material
Significance.
DHFR is a key protein involved in BH4 regeneration from BH2. BH4 is a co-factor that maintains proper function of eNOS and endothelial function. Dysfunctional DHFR is reported to uncouple eNOS resulting in enzyme production of superoxide anions rather than NO. Here, we demonstrate that eNOS may also regulate DHFR, by generating NO leading to DHFR S-nitrosylation. S-nitrosylation stabilizes DHFR and thus maintains BH4 levels in endothelial cells. We also show that stimuli, which deplete NO, induce endothelial DHFR both in vitro and in vivo via ubiquitin-proteasome degradation. These findings highlight the role of NO in maintaining DHFR stability and activity. They also suggest that eNOS has the ability to maintain its coupling status through NO/DHFR S-nitrosylation feedback.
Acknowledgements
None.
Sources of Funding
This study was supported by grants to M.H. Zou (HL079584, HL080499, HL089920, HL105157, HL110488, HL128014, and AG047776) from the National Institutes of Health.
Abbreviations
- DHFR
Dihydrofolate reductase
- GTPCH
GTP cyclohydrolase I
- eNOS
Endothelial nitric oxide synthase
- BH4
Tetrahydrobiopterin
- BH2
7,8-dihydrobiopterin
- HUVEC
Human umbilical vein endothelial cells
- NO
Nitric oxide
- PTIO
2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- GSNO
S-Nitroso-L-glutathione
- DTT
Dithiothreitol
- CHX
Cycloheximide
Footnotes
Disclosures
The authors declare no conflicts of interests.
References
- 1.Bendall JK, Douglas G, McNeill E, Channon KM, Crabtree MJ. Tetrahydrobiopterin in cardiovascular health and disease. Antioxid Redox Signal. 2014;20:3040–3077. doi: 10.1089/ars.2013.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munzel T, Daiber A, Ullrich V, Mulsch A. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Arterioscler Thromb Vasc Biol. 2005;25:1551–1557. doi: 10.1161/01.ATV.0000168896.64927.bb. [DOI] [PubMed] [Google Scholar]
- 3.Li H, Forstermann U. Uncoupling of endothelial NO synthase in atherosclerosis and vascular disease. Curr Opin Pharmacol. 2013;13:161–167. doi: 10.1016/j.coph.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Schulz E, Jansen T, Wenzel P, Daiber A, Munzel T. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal. 2008;10:1115–1126. doi: 10.1089/ars.2007.1989. [DOI] [PubMed] [Google Scholar]
- 5.Abudukadier A, Fujita Y, Obara A, Ohashi A, Fukushima T, Sato Y, Ogura M, Nakamura Y, Fujimoto S, Hosokawa M, Hasegawa H, Inagaki N. Tetrahydrobiopterin has a glucose-lowering effect by suppressing hepatic gluconeogenesis in an endothelial nitric oxide synthase-dependent manner in diabetic mice. Diabetes. 2013;62:3033–3043. doi: 10.2337/db12-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crabtree MJ, Hale AB, Channon KM. Dihydrofolate reductase protects endothelial nitric oxide synthase from uncoupling in tetrahydrobiopterin deficiency. Free Radic Biol Med. 2011;50:1639–1646. doi: 10.1016/j.freeradbiomed.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem. 2003;278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 9.Crabtree MJ and Channon KM. Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide. 2011;25:81–88. doi: 10.1016/j.niox.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J, Wu Y, Song P, Zhang M, Wang S, Zou MH. Proteasome-dependent degradation of guanosine 5'-triphosphate cyclohydrolase I causes tetrahydrobiopterin deficiency in diabetes mellitus. Circulation. 2007;116:944–953. doi: 10.1161/CIRCULATIONAHA.106.684795. [DOI] [PubMed] [Google Scholar]
- 11.Crabtree MJ, Tatham AL, Hale AB, Alp NJ, Channon KM. Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling: relative importance of the de novo biopterin synthesis versus salvage pathways. J Biol Chem. 2009;284:28128–28136. doi: 10.1074/jbc.M109.041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2005;102:9056–9061. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitsett J, Rangel Filho A, Sethumadhavan S, Celinska J, Widlansky M, Vasquez-Vivar J. Human endothelial dihydrofolate reductase low activity limits vascular tetrahydrobiopterin recycling. Free Radic Biol Med. 2013;63:143–150. doi: 10.1016/j.freeradbiomed.2013.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hess DT, Stamler JS. Regulation by S-nitrosylation of protein post-translational modification. J Biol Chem. 2012;287:4411–4418. doi: 10.1074/jbc.R111.285742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Y, Wu J, Zhu H, Song P, Zou MH. Peroxynitrite-dependent zinc release and inactivation of guanosine 5'-triphosphate cyclohydrolase 1 instigate its ubiquitination in diabetes. Diabetes. 2013;62:4247–4256. doi: 10.2337/db13-0751. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Amici M, Sagratini D, Pettinari A, Pucciarelli S, Angeletti M, Eleuteri AM. 20S proteasome mediated degradation of DHFR: implications in neurodegenerative disorders. Arch Biochem Biophys. 2004;422:168–174. doi: 10.1016/j.abb.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Shahani N, Sawa A. Nitric oxide signaling and nitrosative stress in neurons: role for S-nitrosylation. Antioxid Redox Signal. 2011;14:1493–1504. doi: 10.1089/ars.2010.3580. [DOI] [PubMed] [Google Scholar]
- 18.Azad N, Vallyathan V, Wang L, Tantishaiyakul V, Stehlik C, Leonard SS, Rojanasakul Y. S-nitrosylation of Bcl-2 inhibits its ubiquitin-proteasomal degradation. A novel antiapoptotic mechanism that suppresses apoptosis. J Biol Chem. 2006;281:34124–34134. doi: 10.1074/jbc.M602551200. [DOI] [PubMed] [Google Scholar]
- 19.Kohr MJ, Evangelista AM, Ferlito M, Steenbergen C, Murphy E. S-nitrosylation of TRIM72 at cysteine 144 is critical for protection against oxidation-induced protein degradation and cell death. J Mol Cell Cardiol. 2014;69:67–74. doi: 10.1016/j.yjmcc.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung KK, Thomas B, Li X, Pletnikova O, Troncoso JC, Marsh L, Dawson VL, Dawson TM. S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science. 2004;304:1328–1331. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- 21.Amaral JH, Montenegro MF, Pinheiro LC, Ferreira GC, Barroso RP, Costa-Filho AJ, Tanus-Santos JE. TEMPOL enhances the antihypertensive effects of sodium nitrite by mechanisms facilitating nitrite-derived gastric nitric oxide formation. Free Radic Biol Med. 2013;65:446–455. doi: 10.1016/j.freeradbiomed.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 22.Amm I, Sommer T, Wolf DH. Protein quality control and elimination of protein waste: the role of the ubiquitin-proteasome system. Biochim Biophys Acta. 2014;1843:182–196. doi: 10.1016/j.bbamcr.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 23.Peth A, Nathan JA, Goldberg AL. The ATP costs and time required to degrade ubiquitinated proteins by the 26 S proteasome. J Biol Chem. 2013;288:29215–29222. doi: 10.1074/jbc.M113.482570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gould N, Doulias PT, Tenopoulou M, Raju K, Ischiropoulos H. Regulation of protein function and signaling by reversible cysteine S-nitrosylation. J Biol Chem. 2013;288:26473–26479. doi: 10.1074/jbc.R113.460261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu H, Yu S, Zhang H, Xu J. Identification of nitric oxide as an endogenous inhibitor of 26S proteasomes in vascular endothelial cells. PLoS ONE. 2014;9:e98486. doi: 10.1371/journal.pone.0098486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chanvorachote P, Nimmannit U, Stehlik C, Wang L, Jiang BH, Ongpipatanakul B, Rojanasakul Y. Nitric oxide regulates cell sensitivity to cisplatin-induced apoptosis through S-nitrosylation and inhibition of Bcl-2 ubiquitination. Cancer Res. 2006;66:6353–6360. doi: 10.1158/0008-5472.CAN-05-4533. [DOI] [PubMed] [Google Scholar]
- 27.Simon JN, Duglan D, Casadei B, Carnicer R. Nitric oxide synthase regulation of cardiac excitation-contraction coupling in health and disease. J Mol Cell Cardiol. 2014;73C:80–91. doi: 10.1016/j.yjmcc.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Maron BA, Tang SS, Loscalzo J. S-nitrosothiols and the S-nitrosoproteome of the cardiovascular system. Antioxid Redox Signal. 2013;18:270–287. doi: 10.1089/ars.2012.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunne KA, Allam A, McIntosh A, Houston SA, Cerovic V, Goodyear CS, Roe AJ, Beatson SA, Milling SW, Walker D, Wall DM. Increased S-nitrosylation and proteasomal degradation of caspase-3 during infection contribute to the persistence of adherent invasive Escherichia coli (AIEC) in immune cells. PLoS ONE. 2013;8:e68386. doi: 10.1371/journal.pone.0068386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qian J, Fulton D. Post-translational regulation of endothelial nitric oxide synthase in vascular endothelium. Frontiers in physiology. 2013;4:347. doi: 10.3389/fphys.2013.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldstein S, Russo A, Samuni A. Reactions of PTIO and carboxy-PTIO with *NO, *NO2, and O2-*. J Biol Chem. 2003;278:50949–50955. doi: 10.1074/jbc.M308317200. [DOI] [PubMed] [Google Scholar]
- 32.Channon KM. Tetrahydrobiopterin: a vascular redox target to improve endothelial function. Curr Vasc Pharmacol. 2012;10:705–708. doi: 10.2174/157016112803520819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cunnington C, Channon KM. Tetrahydrobiopterin: pleiotropic roles in cardiovascular pathophysiology. Heart. 2010;96:1872–1877. doi: 10.1136/hrt.2009.180430. [DOI] [PubMed] [Google Scholar]
- 34.Cunnington C, Van Assche T, Shirodaria C, et al. Systemic and vascular oxidation limits the efficacy of oral tetrahydrobiopterin treatment in patients with coronary artery disease. Circulation. 2012;125:1356–1366. doi: 10.1161/CIRCULATIONAHA.111.038919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.