Abstract
Background/Objective
Higher late life body mass index (BMI) is unrelated to or even predicts lower risk of dementia in late-life, a phenomenon that may be explained by reverse causation due to weight loss during pre-clinical phases of dementia. We aim to investigate the association of baseline BMI and changes in BMI with dementia in a large prospective cohort, and to examine whether weight loss predicts cognitive function.
Methods
Using a national cohort of adults average age 58 at baseline in 1994 (n=7,029), we investigated the associations between baseline BMI in 1994 and memory scores from 2000 to 2010. We also examined the association of BMI change from 1994 to 1998 with memory scores from 2000 to 2010. Lastly, to investigate reverse causation, we examined whether memory scores in 1996 predicted BMI trajectories from 2000 to 2010.
Results
Baseline overweight predicted better memory scores 6 to 16 years later (β=0.012, 95%CI=0.001; 0.023). Decline in BMI predicted lower memory scores over the subsequent 12 years (β= −0.026, 95%CI= −0.041; −0.011). Lower memory scores at mean age 60 in 1996 predicted faster annual rate of BMI decline during follow-up (β= −0.158 kg/m2 per year, 95% CI= −0.223;−0.094).
Conclusion
Consistent with reverse causation, greater decline in BMI over the first four years of the study was associated with lower memory scores over the next decade and lower memory scores was associated with a decline in BMI. These findings suggest that pre-clinical dementia predicts weight loss for people as early as their late 50s.
Introduction
Increasing evidence suggests that adiposity increases risk of dementia, particularly Alzheimer’s disease (AD).1 The potential underlying mechanisms linking higher BMI to dementia may include direct effects of adiposity (hyperinsulinemia, advanced glycosylation end products, adipokines and cytokines), as well as indirect effects (increases in the prevalence of other vascular risk factors and related cerebrovascular disease).2 An influential meta-analysis concluded that being underweight, overweight or obese in midlife predicted higher risk of dementia, with much of the evidence resting on a single sample aged 40–45 at body mass index (BMI) assessment.1, 3, 4 On the other hand, results from studies examining late-life BMI and dementia risk are mixed, with many studies reporting no associations of overweight or obesity with dementia or even reporting that individuals with higher BMI had lower risk of dementia.1, 5, 6 These unexpected findings may be explained by reverse causation. Since dementia has a long preclinical phase, weight loss may begin years before the onset of clinical cognitive symptoms.7 Therefore, the null or protective association of adiposity and dementia risk in late-life may be due to weight loss directly related to the developing cognitive impairment or secondary to incipient neuropathological changes that produce both cognitive impairment and weight loss.8
Prior research leaves critical questions unanswered. First, the boundary between “midlife” and “late-life” is unclear. If the weak association between higher BMI and dementia reflects the early consequences of dementia, it is important to know when in the life-course, and how many years prior to detectable cognitive impairments, BMI begins to reflect the influence of incipient dementia. Currently, we have evidence that elevated BMI at ages 40–50 predicts later dementia3, 4, 9–13, but elevated BMI at ages 65+ does not.14–16 There is almost no evidence on whether the association between elevated BMI and later dementia risk persists among individuals in their 50s and early 60s. Second, under the reverse causation hypothesis, decline in BMI should predict future dementia risk. Prior studies support this association among older adults (65 years and older),17–22 but no studies have examined change in BMI and future dementia risk among middle aged individuals. Finally, the reverse causation hypothesis also implies that cognitive assessments indicating higher probability of incipient dementia should predict future BMI decline, but no study to date has examined this association.
To address these gaps, we investigated the association of baseline BMI and change in BMI with future memory scores as well as whether baseline memory scores predict subsequent BMI trajectories in 7,029 Health and Retirement Study (HRS) participants who were average age 58 years at baseline.
Methods
Participants
HRS is a national cohort of Americans born between 1931 and 1941 and their spouses. Participants were enrolled in 1992 and they have been interviewed biennially through 2010. Retention rates were above 80%. Detailed information about HRS can be found elsewhere.23 HRS was approved by the University of Michigan Health Sciences Human Subjects Committee and this analysis was determined exempt by the Harvard School of Public Health Office of Human Research Administration.
For this study, 1994 was considered baseline since most of the information for exposure and covariates were available at this time. We considered 2000 as the first cognitive measure to allow an interval between BMI and cognitive evaluation. We included individuals born 1935 or earlier, alive in 2000, and with complete data for BMI and covariates at baseline. We excluded Hispanics (n=744) because of evidence that the cognitive measures were not valid in this subgroup.24 We also excluded participants with BMI<18.5 kg/m2 at baseline (n=91) to avoid unmeasured confounding associated with baseline severe disease leading to very low BMI.
BMI measurements
The main exposure was BMI in 1994, calculated by dividing self-reported weight in kilograms by the square of the self-reported height in meters. BMI was categorized in 4 groups: 18.5–<20, 20–<25 (reference group), 25–<30, and ≥30 kg/m2.25 We performed additional analyses considering BMI as a continuous variable, categorized in quartiles, quintiles, including additional categories for obesity25, and categorizing BMI at every 2 points.
At up to two interview waves occurring between 2004 and 2010, 10,445 participants had their weight and height measured without shoes and heavy clothing during a home interview. We applied the Bland and Altman method to evaluate agreement between measured and self-reported BMI, considering repeated measures in the same individual.26 Briefly, we plotted the difference between the two measures (y axis) against their mean (x axis). The difference between measured and self-reported BMI was 1.55 kg/m2 (95% CI=1.51 to 1.59). The lower limit of agreement for the difference between measured and self-reported BMI was −2.88 (95% CI=−2.96; −2.80) and upper limit was 5.98 (95% CI=5.90; 6.06) (Supplementary Figure 1A). Using Spearman correlations, we found a high correlation between self-reported and measured BMI (r=0.921; p≤0.0001).
We examined BMI change by subtracting BMI in 1998 from BMI in 1994. To minimize the influence of outliers, BMI change values were truncated at the 1st and 99th percentiles. For our main analyses, we analyzed BMI change as a continuous variable with a quadratic term and also categorized it in quintiles. In addition, we considered the effect of changes in BMI category on cognitive measures, to account for both baseline BMI group and BMI change.27–29 For this analysis, participants were classified into 11 groups, cross-classifying change and baseline category: 4 groups whose BMI category did not change (no change from baseline BMI=18.5–<20, 20–<25, 25–<30, or ≥30 kg/m2), 4 groups whose BMI category increased (increase from baseline BMI=18.5–<20, 20–<25 kg, 25–<30, or ≥30), and 3 groups whose BMI category declined (decrease from baseline BMI=20–<25 kg/m2, 25–<30 kg/m2, or ≥30). For example, if a participant had a BMI of 26 kg/m2 in 1994 and a BMI of 21 kg/m2 in 1998, he or she was included in the group “decrease from baseline BMI=25–<30”.
The agreement between measured and self-reported change in BMI from 2006 to 2010 was investigated in 3,557 participants, using the Bland-Altman plot. The difference between measured and self-reported BMI change was 1.59 (95% CI=1.41 to 1.51), and the lower limit of agreement was −1.72 (95% CI=−1.77; −1.67) and the upper limit was 4.64 (95% CI=4.59; 4.69) (Supplementary Figure 2A). The correlation between self-reported and measured BMI change was moderate (r=0.656; p<0.0001).
In addition, since participants with impaired cognition could report BMI wrongly, we applied the Bland and Altman method to compare measured and self-reported BMI in those in the lowest quantile for the 10-word delayed recall test in 2004. The results were similar to the whole sample for baseline BMI [difference= 1.55 (95% CI=1.51 to 1.59), lower limit of agreement= −2.97 (95% CI=−3.05; −2.89), upper limit= 6.07 (95% CI=5.99; 6.15)] and BMI change [difference=1.37 (95% CI=1.26 to 1.48), lower limit of agreement= −1.80 (95% CI= −1.91; −1.69), upper limit= 4.54 (95% CI=4.43; 4.65)] (Supplementary Figures 1B and 2B).
Cognitive measures
Participants completed cognitive function evaluations biennially from 2000 to 2010. Cognitive function was evaluated using a 10-word immediate and delayed recall and the Telephone Interview for Cognitive Status (TICS).30 When a participant was too impaired to complete the interview (approximately 10% at each wave), a proxy informant, typically a spouse (70%), reported on the participant’s cognitive function using the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE). 31 Composite dementia probabilities and memory scores were calculated by combining direct and proxy cognitive assessments.24 Briefly, the dementia probability score and composite memory score algorithms were developed in a subsample of HRS participants (n=856) who underwent full neuropsychological examinations (CERAD Word Listing learning, delayed recall and recognition and/or Wechsler Memory Scale –Logical Memory) as part of the Aging, Demographics, and Memory Study (ADAMS).32 The dementia probability score algorithm was derived based on a logistic regression model using the cognitive measures available for the full HRS cohort (immediate and delayed recall, TICS or IQCODE) to predict DSM IV-based dementia diagnosis for all ADAMS participants (C-statistic=94.3%). The memory score algorithm was developed using linear regression models to calibrate the cognitive measures available for the full HRS cohort to predict a composite memory Z-score assessed in ADAMS (r2=0.61). These algorithms were applied to the direct or proxy cognitive assessments completed to estimate dementia probability and memory scores for all participants at each wave.24
Covariates
An extensive set of confounders for the association of BMI and cognitive outcomes were evaluated in 1994 and included: age, sex, race, years of education, place of birth (Southern, non-Southern, or outside US), marital status (married vs. non-married), engagement in the labor force (working, retired, or not engaged), annual income per capita, wealth per capita, physical activity (frequency of vigorous activity, such as heavy housework, aerobics, running, swimming, and bicycling, in the past 12 months categorized as 0,<3, or ≥3 times/week), alcohol use (ever consumed any alcohol), current smoking, self-reported health (‘excellent’, ‘very good’, ‘good’ vs. ‘fair’, ‘poor’), basic (ADLs) and instrumental activities of daily living (IADLs), depressive symptoms, and self-report of ever having a physician diagnosis of hypertension, diabetes, stroke, heart disease, and cancer. Income and wealth per household member were highly skewed; therefore natural log transformations were used. ADLs included the ability to bathe, dress, eat, transfer, and walk without help. IADLs included the ability to use the phone, manage money, take medications, shop for groceries, and prepare hot meals. For ADLs and IADLs, participants were considered to have some impairment if they were unable to perform ≥1 of these activities. Depressive symptoms were evaluated with a modified 8-item Centers for Epidemiologic Studies-Depression (CES-D) scale. 33 Depressive symptoms were considered present if the respondent reported ≥3 symptoms in the last week.34
Statistical analysis
Effect of baseline BMI on cognitive measures
Study population characteristics were compared by baseline BMI categories using one-way ANOVA for continuous variables and chi square tests for categorical variables. To estimate the effect of BMI on cognitive function, we used generalized linear models (GLM) with robust variance to account for repeated measures in the same individual. To estimate the effect of BMI category on future dementia probability, we used a logit link in the GLM; therefore, the exponentiated regression coefficients correspond to odds ratios for dementia probability. For memory scores, we used an identity link in the GLM, so regression coefficients correspond to differences in mean memory score at each time point. Though we considered the possibility of non-linear changes in cognitive measures over time, effects appeared approximately linear for both outcomes on graphical inspection, so our main models specify time as linear.
We adjusted for an extensive set of confounders in our primary analyses. In sensitivity analysis, we excluded confounders that may also serve as mediators and adjusted only for age, sex, race, schooling, Southern birth, engagement in the labor force, marital status, income, and wealth. Selection bias due to loss to follow-up and mortality is a concern in longitudinal studies of cognition because cognitive impairment is an important predictor of study participation and survival. To account for possible selection bias, we conducted sensitivity analyses with time-updated inverse probability weighting (IPW).35 We calculated two weights with logistic regression: inverse probability of (1) survival at each wave (conditional on surviving up to and participating in the previous wave) and (2) participation at each wave among those who survived and previously participated.36 To stabilize the weights, models for the numerators of the weights included the following baseline variables: age, sex, race, schooling, BMI, marital status, and dummy variables for each wave of follow-up. The denominators for the weights included the extended set of covariates, BMI category, and dementia probability/memory score in the wave prior to the outcome assessment, and dummy variables for each wave of follow-up. The survival and participation weights were truncated at the 99th percentile to minimize the influence of outliers. We multiplied the weights for survival and participation to create a weight for each observation and applied them in the GLM for dementia probability and memory scores.
Effect of BMI change on cognitive measures
We examined effects of BMI change quintiles and categories from 1994 to 1998 on dementia probability and memory score trajectories from 2000 to 2010. Participants (n=6,690) were on average 58.1 years old (SD=4.3) in 1994 and 74.1 (SD=4.3) in 2010. For these analyses, n=339 participants were excluded because they did not have a BMI measure in 1998. We used a logit link for dementia probability and an identity link for memory scores in the GLMs, adjusted for the extended set of covariates.
Effect of cognitive measures on BMI trajectories
To further investigate the possibility of reverse causation, we examined the effects of dementia probability and memory score quartiles in 1996 on BMI trajectories from 2000 to 2010, using GLM adjusted for the comprehensive set of covariates. As a sensitivity analysis, we used age as the time-dimension and considered models with quadratic terms for time dimensions.
All analyses were performed using SAS 9.2 (SAS Institute, Cary, NC). We present 95% confidence intervals (CIs) with two-sided p-values.
Results
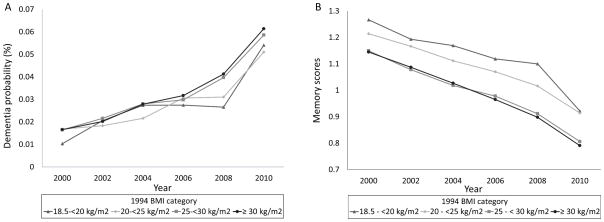
From the 9,285 HRS participants enrolled, 7,029 met the criteria for this study (Supplementary Figure 3). Participants in the analysis sample were older, more likely to be female, and had better overall health compared to those not included (Supplementary Table 1). The 339 participants who were excluded from the BMI change analysis had higher dementia probabilities, higher BMI, lower measures of socioeconomic and health status (Supplementary Table 2). At baseline, the mean age was 58.1 years (SD=4.4) (range: 50–94 years), 44.5% were male, and mean BMI was 27.2 kg/m2 (SD=5.0) (range: 18.5–60.1 kg/m2). Table 1 displays study population characteristics according BMI categories. Overall, the probability of dementia was very low at the first cognitive assessment in 2000 and remained low during most of the follow-up. Memory scores declined during this period (Figure 1). The mean number of cognitive assessments per participant was 4.22 (SD=1.82).
Table 1.
Study population baseline characteristics by BMI categories (Health and Retirement Study, n=7,029)
| Body Mass Index (kg/m2) | 18.5 – < 20 (n=230) | 20 – < 25 (n=2234) | 25 – < 30 (n=2878) | ≥ 30 (n=1687) | p* |
|---|---|---|---|---|---|
| Age (years), mean (SD)* | 57.6 (4.4) | 57.9 (4.3) | 58.4 (4.5) | 57.8 (4.1) | <0.0001 |
| Male, %# | 16.1 | 37.2 | 54.3 | 41.4 | <0.0001 |
| Race, %# | <0.0001 | ||||
| . White | 86.5 | 88.7 | 82.5 | 75.2 | |
| . Black | 9.6 | 8.6 | 15.6 | 23.7 | |
| . Other | 3.9 | 2.6 | 2.0 | 1.1 | |
| Education (years), mean (SD)* | 12.8 (2.7) | 13.0 (2.7) | 12.6 (2.8) | 12.3 (2.7) | <0.0001 |
| Annual income per capita (USD), mean (SD)* | 62,883 (87,268) | 65,661 (104,106) | 58,714 (99,119) | 47,978 (53,102) | <0.0001 |
| Wealth per capita (USD), mean (SD)* | 347,505 (673,101) | 329,695 (543,033) | 278,319 (535,426) | 231,065 (469,052) | <0.0001 |
| Birth place, %# | 0.0002 | ||||
| . US Southern | 35.2 | 34.7 | 38.5 | 40.2 | |
| . US other | 59.1 | 58.4 | 56.0 | 55.6 | |
| . Outside US | 8.7 | 7.0 | 5.5 | 4.2 | |
| Married, %# | 73.0 | 78.5 | 82.1 | 77.4 | <0.0001 |
| Labor force, %# | <0.0001 | ||||
| . Working | 53.0 | 58.6 | 60.3 | 54.8 | |
| . Retired | 27.0 | 27.8 | 29.9 | 31.6 | |
| . Unemployed, disabled, or not in the labor force | 20.0 | 13.5 | 9.8 | 13.6 | |
| Current smoking, %# | 42.6 | 28.2 | 19.7 | 14.9 | <0.0001 |
| Alcohol use, %# | 50.9 | 63.2 | 59.8 | 48.8 | <0.0001 |
| Physical activity, %# | 26.5 | 26.5 | 22.0 | 15.2 | <0.0001 |
| Hypertension, %# | 15.2 | 21.4 | 35.3 | 51.1 | <0.0001 |
| Diabetes, %# | 1.3 | 3.4 | 8.2 | 16.1 | <0.0001 |
| Heart disease, %# | 12.2 | 8.3 | 10.6 | 13.9 | <0.0001 |
| Stroke, %# | 2.2 | 2.2 | 2.1 | 2.8 | 0.49 |
| Cancer, %# | 7.4 | 6.0 | 4.8 | 5.0 | 0.12 |
| Self-reported health, %# | <0.0001 | ||||
| . Excellent, very good, good | 83.0 | 87.5 | 84.4 | 73.9 | |
| . Fair, poor | 17.0 | 12.5 | 15.6 | 26.1 | |
| Basic ADL, %# | <0.0001 | ||||
| . Some impairment | 6.1 | 3.0 | 4.5 | 8.2 | |
| . No impairment | 93.9 | 97.0 | 95.6 | 91.8 | |
| Instrumental ADL, %# | 0.02 | ||||
| . Some impairment | 7.0 | 4.7 | 5.5 | 6.9 | |
| . No impairment | 93.0 | 95.3 | 94.5 | 93.1 | |
| Depression, %# | <0.0001 | ||||
| . CESD < 3 | 19.6 | 85.6 | 85.9 | 79.6 | |
| . CESD ≥ 3 | 80.4 | 14.4 | 14.1 | 20.4 |
SD=standard deviation
ADL: Activities of daily living; CESD: Center for Epidemiologic Studies-Depression Scale
one-way ANOVA,
Chi square test
Figure 1.
Trajectories of dementia probabilities (A) and memory scores (B) from 2000 to 2010 according to BMI category in 1994.
Association of baseline BMI with cognitive measures over follow-up
In models adjusted for the extensive set of covariates, obesity (BMI>30kg/m2) was not significantly associated with memory scores, but overweight (BMI20–<25 kg/m2) predicted higher memory scores (β=0.012, 95% CI=0.001; 0.023, p=0.04) compared to BMI 20–<25 kg/m2. However, this result was not significant when we excluded possible mediators from the covariate list (Table 2).
Table 2.
Coefficients (95% Confidence intervals) for memory scores by BMI categories (Health and Retirement Study, n=7029)
| Body Mass Index (kg/m2) | 18.5 – <20 (n=230) | 20 – < 25 (n=2234) | 25 – < 30 (n=2878) | ≥ 30 (n=1687) |
|---|---|---|---|---|
| Crude | 1.108 (−0.140; 2.355) | 0 | 0.103 (−0.326; 0.531) | 0.178 (−0.320; 0.675) |
| Adjusted for minimal set of confounders, without possible mediators‡ | 0.001 (−0.027; 0.028) | 0 | 0.009 (−0.002; 0.020) | 0.002 (−0.011; 0.015) |
| Adjusted for extensive set of possible confounders* | 0.003 (−0.024; 0.031) | 0 | 0.012 (0.001; 0.023) | 0.012 (−0.002; 0.025) |
| IPCW† | 0.005 (−0.022; 0.032) | 0 | 0.007 (−0.004; 0.018) | 0.008 (−0.006; 0.022) |
Adjusted for age, sex, race, schooling, Southern birth, labor force, marital status, income, wealth
Adjusted for age, sex, race, schooling, Southern birth, labor force, marital status, income, wealth, physical activity, smoking, alcohol use, diabetes, hypertension, stroke, heart disease, cancer, self-reported health, depression, basic and instrumental activities of daily living
IPCW: Inverse probability weighting for censoring.
We investigated factors related to survival and dropout in our study. Higher memory scores (OR=1.81, 95% CI=1.50; 2.19), being married (OR=1.53, 95% CI=1.31; 178), reporting health as ‘excellent, very good, or good’ (OR=1.23, 95%CI=1.05–1.44), alcohol use (OR=1.21, 95%CI=1.05; 1.41), and being physically active (OR=2.07, OR=1.71; 2.49) were related to better survival. On the other hand, older age (OR=0.89, 95%CI=0.88–0.91), underweight (OR=0.54, 95%CI=0.40; 0.72), male sex (OR=0.83, 95%CI=0.71; 0.94), impairment in basic (OR=0.61, 95%CI=0.52;0.73) and instrumental activities of daily life (OR=0.71, 95%CI=0.57; 0.89), heart disease (OR=0.74, 95%CI=0.64–0.86), lung disease (OR=0.61, 95%CI=0.52; 0.73), cancer (OR=0.35, 95%CI=0.30; 0.40), diabetes (OR=0.57, 95%CI=0.49; 0.67), hypertension (OR=0.75, 95% CI=0.65; 0.87), stroke (OR=0.81. 95%CI=0.67; 0.99), and smoking (OR=0.70, 95%CI= 0.59; 0.84) were independently related to shorter survival. Regarding dropout, older age (OR=0.91, 95%CI=0.90; 0.93), higher education (OR=0.97, 95%CI=0.94; 0.99), hypertension (OR=0.75, 95%CI=0.64; 0.87), and reporting health as ‘excellent, very good, or good’ (OR=0.73, 95%CI=0.59; 0.89) predicted higher dropout. Higher memory scores (OR=1.85, 95%CI=1.44; 2.38), male sex (OR=1.78, 95%CI=1.50; 2.10), and being physically active (OR=1.27, 95%CI=1.08; 1.49) predicted lower dropout. Since the outcome, some covariates, and in some situations even the exposure were related to survival and dropout, the use of IPW was justified. When we applied IPW to address selection bias, neither overweight nor obesity predicted memory scores, and the point estimates for the coefficients were slightly positive (Table 2).
In sensitivity analyses, we found a small positive association between continuous BMI with a quadratic term and memory scores. When we categorized BMI into quartiles, quintiles, and additional categories, we found suggestive evidence that BMI in the overweight range was associated with higher memory scores (Supplementary Table 3).
Association of BMI change with cognitive measures
Declines in BMI from 1994 to 1998 predicted lower memory scores from 2000 to 2010 [β for 1st quintile (<−0.8 kg/m2) vs. 3rd quintile (0 to 0.7 kg/m2) = −0.026, 95%CI= −0.041; −0.011, p=0.0008]. In addition, BMI change, analyzed as a continuous variable with a quadratic term, was associated with memory scores (BMI change: β=0.005, 95%CI=0.002; 0.009, p=0.0005; BMI change*BMI change: β= −0.002, 95%CI= −0.003; −0.001, p=0.0007). When we examined change in BMI category stratified by baseline BMI, we found lower memory scores among participants who were overweight (BMI=25–<30 kg/m2) in 1994 and lost weight by 1998 compared to participants who were normal weight (BMI=18.5–<25 kg/m2) in both 1994 and 1998 (β= −0.039, 95%CI= −0.068; −0.010, p=0.008). On the other hand, participants who remained overweight (BMI=25–<30 kg/m2 in both 1994 and 1998) had better memory scores than those who had normal BMI at both time points (β= 0.014, 95% CI=0.001; 0.026, p=0.03) (Table 3).
Table 3.
Coefficients (95% Confidence intervals) for memory scores by BMI change (Health and Retirement Study, n=6690)
| n | β (95% CI) | p* | |
|---|---|---|---|
| BMI change | 6690 | 0.005 (0.002; 0.009) | 0.0005 |
| BMI change*BMI change | −0.002 (−0.003; −0.001) | 0.0007 | |
| BMI change quintiles | 0.0009 | ||
| 1st quintile (< −0.8 kg/m2) | 1434 | −0.026 (−0.041; −0.011) | |
| 2nd quintile (−0.8 to < 0 kg/m2) | 732 | −0.015 (−0.032; 0.002) | |
| 3rd quintile (0 to < 0.7 kg/m2) | 1921 | 0 (reference) | |
| 4th quintile (0.7 to 1.6 kg/m2) | 1215 | 0.003 (−0.009; 0.016) | |
| 5th quintile (≥ 1.6 kg/m2) | 1388 | −0.005 (−0.018; 0.009) | |
| BMI change category | |||
| No change | 0.02 | ||
| . BMI=18.5–<20 kg/m2 in 1994 | 145 | 0.003 (−0.030; 0.036) | |
| . BMI=20–<25 kg/m2 in 1994 | 1682 | 0 (reference) | |
| . BMI=25–<30 kg/m2 in 1994 | 2109 | 0.014 (0.001; 0.026) | |
| . BMI ≥ 30 kg/m2 in 1994 | 1347 | 0.014 (−0.001; 0.029) | |
| BMI category increased | |||
| . BMI=18.5–<20 kg/m2 in 1994 | 74 | 0.016 (−0.029; 0.061) | |
| . BMI=20–<25 kg/m2 in 1994 | 422 | −0.003 (−0.022; 0.016) | |
| . BMI=25–<30 kg/m2 in 1994 | 377 | 0.015 (−0.005; 0.036) | |
| BMI category decreased | |||
| . BMI=20–<25 kg/m2 in 1994 | 58 | −0.037 (−0.111; 0.037) | |
| . BMI=25–<30 kg/m2 in 1994 | 276 | −0.039 (−0.068; −0.010) | |
| . BMI ≥ 30 kg/m2 in 1994 | 200 | 0.001 (−0.032; 0.034) | |
BMI=Body Mass Index
Adjusted for age, sex, race, schooling, Southern birth, labor force, marital status, income, wealth, physical activity, smoking, alcohol use, diabetes, hypertension, stroke, heart disease, cancer, self-reported health, depression, basic and instrumental activities of daily living
Association of cognitive measures with BMI trajectories
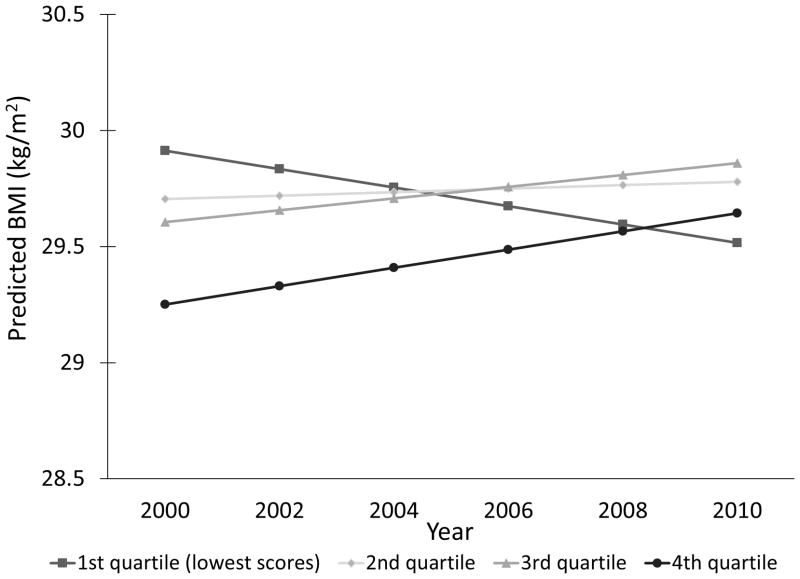
Compared to participants with the higher memory scores in 1996, participants with lowest scores averaged significant declines in BMI from 2000 to 2010 [2nd quartile: β=−0.028 kg/m2/year (95%CI=−0.082; −0.027); 3rd quartile: β= −0.063 kg/m2/year (95%CI=−0.117; −0.010); 4th quartile: β= −0.158 kg/m2/year (95%CI=−0.223; −0.094); p<0.0001] (Figure 2).
Figure 2.
Predicted BMI trajectories according to memory scores quartiles. First quartile was the reference group and included participants with lowest memory scores.
Sensitivity analyses
Similar results were found when we considered dementia probability as the outcome for baseline BMI and BMI change, and the effects of dementia probabilities on BMI trajectories. (Supplementary Tables 4–6)
Discussion
In this large, diverse cohort, we find several lines of evidence that pre-clinical dementia may cause BMI declines in cognitively normal individuals in their late 50s. Overweight at mean age of 58 years predicted higher memory scores through mean age of 74 years. Depending on the categorizations of BMI, higher memory scores prevailed for individuals in the overweight and obese ranges. Declines in BMI over four years predicted lower memory scores over the next decade, especially among individuals who were initially overweight. Finally, participants with lower memory scores at baseline averaged faster declines in BMI during the follow-up. Current research in the dementia field aims to identify individuals who are at the preclinical or early stages of dementia, either for the purposes of early intervention trials or to evaluate etiologic risk factors prior to the influence of dementia. For these purposes, being able to show that even individuals in their 50s were already manifesting physiologic changes that are likely consequences of dementia is important. Another relevant use of this research is to understand the time course from the first pathologic changes to diagnosis, identifying the delay before onset of dementia.
Prior findings on the relationship between late-life BMI and dementia risk are mixed. While retrospectively reported obesity at age 50 was associated with increased risk of dementia in the Cardiovascular Health Study, when BMI was measured at age 65 years or older, underweight participants (BMI <20 kg/m2) had a higher risk of dementia, overweight participants had a similar risk, and obese participants had a lower risk compared to normal weight participants.37 In a male elderly cohort (age range: 65–84 years), being overweight was protective against dementia, and obesity was not associated with risk of dementia.16 In studies treating late-life BMI as a continuous variable, higher BMI is associated with either lower14 or no15 risk of dementia. These findings are in stark contrast to the association between obesity in midlife and increased risk of dementia in late life. The reversal of the association between BMI and dementia in late-life is sometimes referred to as the obesity paradox.38
Although prior studies suggested that elderly individuals who experienced BMI declines are at higher dementia risk,17–21, 39 the average baseline age in our cohort is roughly 10 years younger than prior research. Our results indicate that pre-clinical dementia may influence BMI, via behavioral or physiologic mechanisms, for people in their late 50s, which is surprisingly early considering previous studies and typical age at diagnosis of dementia. Dementia is relatively rare among people in their 60s: the prevalence of dementia is estimated to be <2% among adults age 60–69 year in the United States.40 However, the preclinical phase of dementia is believed to last many years, and our finding may suggests that weight loss caused by incipient dementia begins many years prior to onset of dementia.41, 42
In fact, weight loss may be a prodrome of dementia and is probably related to neuropsychiatric symptoms that are common in very early phases of the disease, like apathy and loss of initiative.43 Moreover, the presence of neuropathological lesions were found in the olfactory bulb in early stages of neurofibrillary tangle deposition (Braak’s stage 0 or 1) in 15 controls and 15 patients with AD.44 As a result, reduced olfaction may contribute to decreased intake of food, as it becomes less tasty. Apathy, reduced initiative and olfaction contribute to reduced food intake mediated by decreased motivation to prepare meals, increased probability of missing meals, and decreased interest in food. Other possible explanations for weight loss in preclinical dementia may include dysfunction in the limbic system and hypothalamus45 and altered automaticity in swallowing and chewing.46
Although survival bias, prior to or during the study period, is another possible explanation for the protective association between obesity and cognition in studies of older adults, we think it is unlikely to account for our findings. Survival bias prior to the start of studies of older adults may occur if individuals who were overweight and obese in midlife die before enrolling in the study, which could result in selection of healthy overweight and obese with better cognition than the normal weight participants in our study. Approximately 23% of the 1930 US birth cohort died between ages 20 and 55, and thus the pattern of selection would have to be exceptionally strong to introduce substantial bias.47 Selection bias during the study may occur if differential loss to follow-up is not considered, since healthier overweight and obese could be preferentially retained. One of the strengths of our study was the use of IPW to account for selective attrition. The results of IPW models were similar to the findings from our main analysis but IPW accounts only for post-enrollment attrition and depends on the assumption that there are no unmeasured common causes of the cognitive measures and survival and dropout.
Other strengths of our study include: (1) the large sample size; (2) sensitivity analyses of alternative functional forms of BMI at baseline (different categories and as a continuous variable); (3) adjustment for confounders, considering the exclusion of possible mediators; (4) the use of GLM with robust variance, and (5) extensive sensitivity analyses that showed the robustness of our results. On the other hand, our study must be interpreted with some limitations in mind. Although the agreement of self-reported and measured baseline BMI and BMI change can be considered satisfactory, some measurement error occurred. This error may have attenuated the true associations. Moreover, we could not investigate the effects of other measures of abdominal obesity, like waist circumference and waist-to-hip ratio, to confirm our conclusions. In addition, some of our findings must be interpreted with caution due to borderline associations in the context of multiple testing (e.g. the association between baseline overweight and memory scores, and the improvement in memory scores in overweight with stable BMI category). Regarding the study outcomes, although memory scores and dementia probabilities were calculated based on the participant’s or proxy’s cognitive evaluation, combining these evaluations was important to keep participants with higher dementia risk in the sample. Finally, the memory scores may have some ambiguity regarding domain specificity, since they had a moderate r2 value with the ADAMS memory composite. This may be due to the fact that multiple aspects of memory were evaluated in the ADAMS subsample, whereas the assessment we used to develop the memory scores included only immediate and delayed word list recall plus the proxy assessment. None of these limitations could explain our key results: BMI in late middle age was not associated with worse subsequent cognition; weight loss in late middle age did not predict better subsequent cognition and for some groups predicted worse cognitive outcomes; and lower cognition in late middle age predicted subsequent weight loss. These results in combination support the reverse causation explanation for the obesity paradox in dementia in late life. Surprisingly, the effects of preclinical dementia on BMI may start decades before average age of dementia diagnosis, since we detected evidence of this process in a sample with a mean age of 58 years.
Supplementary Material
Acknowledgments
Study Funding: The Health and Retirement Study (HRS) is sponsored by the National Institute on Aging (NIA U01AG009740) and is conducted by the University of Michigan. MMG was funded by the American Heart Association (10SDG2640243) and National Institute of Health (R21AG034385). CKS was funded by CAPES (2285/13-4). PG was funded by the National Heart, Lung, and Blood Institute at NIH (NIH 1F31HL112613); the American Heart Association (10SDG2640243), and the Yerby Postdoctoral Fellowship Program.
Footnotes
Potential Conflict of Interest: None
Supplementary information is available at International Journal of Obesity’s website.
Disclosures:
HRS is sponsored by the National Institute on Aging (NIA U01AG009740) and is conducted by the University of Michigan.
Dr. Suemoto was funded by CAPES (2285/13-4).
Dr. Gilsanz was funded by the National Heart, Lung, and Blood Institute at NIH (NIH 1F31HL112613); the American Heart Association (10SDG2640243), and the Yerby Postdoctoral Fellowship Program.
Dr. Mayeda reports no disclosures.
Dr. Glymour was funded by the American Heart Association (10SDG2640243) and National Institute of Health (R21AG034385).
References
- 1.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev. 2011;12(5):e426–37. doi: 10.1111/j.1467-789X.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- 2.Luchsinger JA, Gustafson DR. Adiposity and Alzheimer’s disease. Curr Opin Clin Nutr Metab Care. 2009;12:15–21. doi: 10.1097/MCO.0b013e32831c8c71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330(7504):1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitmer RA, Gunderson EP, Quesenberry CP, Jr, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res. 2007;4(2):103–9. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- 5.Loef M, Walach H. Midlife obesity and dementia: meta-analysis and adjusted forecast of dementia prevalence in the United States and China. Obesity (Silver Spring) 2013;21(1):E51–5. doi: 10.1002/oby.20037. [DOI] [PubMed] [Google Scholar]
- 6.Nourhashemi F, Deschamps V, Larrieu S, Letenneur L, Dartigues JF, Barberger-Gateau P. Body mass index and incidence of dementia: the PAQUID study. Neurology. 2003;60(1):117–9. doi: 10.1212/01.wnl.0000038910.46217.aa. [DOI] [PubMed] [Google Scholar]
- 7.Stewart R, Masaki K, Xue QL, Peila R, Petrovitch H, White LR, et al. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2005;62 (1):55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 8.Sperling R, Mormino E, Johnson K. The evolution of preclinical Alzheimer’s disease: implications for prevention trials. Neuron. 2014;84(3):608–22. doi: 10.1016/j.neuron.2014.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahl AK, Hassing LB, Fransson EI, Gatz M, Reynolds CA, Pedersen NL. Body mass index across midlife and cognitive change in late life. Int J Obes (Lond) 2013;37(2):296–302. doi: 10.1038/ijo.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahl A, Hassing LB, Fransson E, Berg S, Gatz M, Reynolds CA, et al. Being overweight in midlife is associated with lower cognitive ability and steeper cognitive decline in late life. J Gerontol A Biol Sci Med Sci. 2010;65(1):57–62. doi: 10.1093/gerona/glp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu WL, Atti AR, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Midlife overweight and obesity increase late-life dementia risk: a population-based twin study. Neurology. 2011;76(18):1568–74. doi: 10.1212/WNL.0b013e3182190d09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosengren A, Skoog I, Gustafson D, Wilhelmsen L. Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Arch Intern Med. 2005;165(3):321–6. doi: 10.1001/archinte.165.3.321. [DOI] [PubMed] [Google Scholar]
- 13.Hassing LB, Dahl AK, Thorvaldsson V, Berg S, Gatz M, Pedersen NL, et al. Overweight in midlife and risk of dementia: a 40-year follow-up study. International Journal of Obesity. 2009;33(8):893–898. doi: 10.1038/ijo.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahl AK, Lopponen M, Isoaho R, Berg S, Kivela SL. Overweight and obesity in old age are not associated with greater dementia risk. J Am Geriatr Soc. 2008;56(12):2261–6. doi: 10.1111/j.1532-5415.2008.01958.x. [DOI] [PubMed] [Google Scholar]
- 15.Hughes TF, Borenstein AR, Schofield E, Wu Y, Larson EB. Association between late-life body mass index and dementia: The Kame Project. Neurology. 2009;72(20):1741–6. doi: 10.1212/WNL.0b013e3181a60a58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Power BD, Alfonso H, Flicker L, Hankey GJ, Yeap BB, Almeida OP. Body adiposity in later life and the incidence of dementia: the health in men study. PLoS One. 2011;6(3):e17902. doi: 10.1371/journal.pone.0017902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Power BD, Alfonso H, Flicker L, Hankey GJ, Yeap BB, Almeida OP. Changes in body mass in later life and incident dementia. Int Psychogeriatr. 2013;25(3):467–78. doi: 10.1017/S1041610212001834. [DOI] [PubMed] [Google Scholar]
- 18.Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65(6):892. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 19.Driscoll I, Espeland MA, Wassertheil-Smoller S, Gaussoin SA, Ding J, Granek IA, et al. Weight change and cognitive function: findings from the Women’s Health Initiative Study of Cognitive Aging. Obesity. 2011;19(8):1595–600. doi: 10.1038/oby.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson DK, Wilkins CH, Morris JC. Accelerated weight loss may precede diagnosis in Alzheimer disease. Arch Neurol. 2006;63(9):1312–7. doi: 10.1001/archneur.63.9.1312. [DOI] [PubMed] [Google Scholar]
- 21.Knopman DS, Edland SD, Cha RH, Petersen RC, Rocca WA. Incident dementia in women is preceded by weight loss by at least a decade. Neurology. 2007;69(8):739–46. doi: 10.1212/01.wnl.0000267661.65586.33. [DOI] [PubMed] [Google Scholar]
- 22.Barrett-Connor E, Edelstein SL, Corey-Bloom J, Wiederholt WC. Weight loss precedes dementia in community-dwelling older adults. J Am Geriatr Soc. 1996;44(10):1147–52. doi: 10.1111/j.1532-5415.1996.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 23.Juster FT, Suzman R. An overview of the health and retirement study. Journal of Human Resources. 1995;30:S7–S56. [Google Scholar]
- 24.Wu Q, Tchetgen Tchetgen EJ, Osypuk TL, White K, Mujahid M, Maria Glymour M. Combining direct and proxy assessments to reduce attrition bias in a longitudinal study. Alzheimer Dis Assoc Disord. 2013;27(3):207–12. doi: 10.1097/WAD.0b013e31826cfe90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization (WHO) Obesity: Preventing and managing the global epidemic. World Health Organization; Geneva: 1999. [PubMed] [Google Scholar]
- 26.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 27.Droyvold WB, Midthjell K, Nilsen TI, Holmen J. Change in body mass index and its impact on blood pressure: a prospective population study. Int J Obes. 2005;29:650–5. doi: 10.1038/sj.ijo.0802944. [DOI] [PubMed] [Google Scholar]
- 28.Lawlor DA, Benfield L, Logue J, Tilling K, Howe LD, Fraser A, et al. Association between general and central adiposity in childhood, and change in these, with cardiovascular risk factors in adolescence: prospective cohort study. BMJ. 2010;341:c6224. doi: 10.1136/bmj.c6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charakida M, Khan T, Johnson W, Finer N, Woodside J, Whincup PH, et al. Lifelong patterns of BMI and cardiovascular phenotype in individuals aged 60–64 years in the 1946 British birth cohort study: an epidemiological study. Lancet Diabetes Endocrinol. 2014;2(8):648–54. doi: 10.1016/S2213-8587(14)70103-2. [DOI] [PubMed] [Google Scholar]
- 30.Brandt J, Spencer M, Folstein M. The Telephone Interview for Cognitive Status. Neuropsychiatry, Neuropsychology, & Behavioral Neurology. 1988;1(2):111–117. [Google Scholar]
- 31.Jorm AF, Jacomb PA. The Informant Questionnaire On Cognitive Decline In The Elderly (IQCODE) - socio-demographic correlates, reliability, validity and some norms. Psychological Medicine. 1989;19(4):1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 32.Langa KM, Plassman BL, Wallace RB, Herzog AR, Heeringa SG, Ofstedal MB, et al. The aging, demographics, and memory study: Study design and methods. Neuroepidemiology. 2005;25(4):181–191. doi: 10.1159/000087448. [DOI] [PubMed] [Google Scholar]
- 33.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 34.Turvey CL, Wallace RB, Herzog R. A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. Int Psychogeriatr. 1999;11(2):139–48. doi: 10.1017/s1041610299005694. [DOI] [PubMed] [Google Scholar]
- 35.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11(5):561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Weuve J, Tchetgen Tchetgen EJ, Glymour MM, Beck TL, Aggarwal NT, Wilson RS, et al. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology. 2012;23(1):119–28. doi: 10.1097/EDE.0b013e318230e861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O’Meara ES, Longstreth WT, Jr, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66(3):336–42. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oreopoulos A, Kalantar-Zadeh K, Sharma AM, Fonarow GC. The obesity paradox in the elderly: potential mechanisms and clinical implications. Clin Geriatr Med. 2009;25(4):643–59. doi: 10.1016/j.cger.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Dupre ME, Gu D, Warner DF, Yi Z. Frailty and type of death among older adults in China: prospective cohort study. BMJ. 2009;338 doi: 10.1136/bmj.b1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63–75. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Morris JC. Early-stage and preclinical Alzheimer disease. Alzheimer Dis Assoc Disord. 2005;19(3):163–5. doi: 10.1097/01.wad.0000184005.22611.cc. [DOI] [PubMed] [Google Scholar]
- 42.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ready RE, Ott BR, Grace J, Cahn-Weiner DA. Apathy and executive dysfunction in mild cognitive impairment and Alzheimer disease. Am J Geriatr Psychiatry. 2003;11(2):222–8. [PubMed] [Google Scholar]
- 44.Kovács T, Cairns NJ, Lantos PL. Olfactory centres in Alzheimer’s disease: olfactory bulb is involved in early Braak’s stages. NeuroReport. 2001;12(2):285–8. doi: 10.1097/00001756-200102120-00021. [DOI] [PubMed] [Google Scholar]
- 45.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathologica. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 46.Yang EJ, Kim KW, Lim JY, Paik NJ. Relationship between dysphagia and mild cognitive impairment in a community-based elderly cohort: the Korean longitudinal study on health and aging. J Am Geriatr Soc. 2014;62(1):40–6. doi: 10.1111/jgs.12606. [DOI] [PubMed] [Google Scholar]
- 47.Center for Disease Control and Prevention (CDC) [Acessed March 26, 2015];United States Life Tables. http://www.cdc.gov/nchs/products/life_tables.htm#life.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.