Abstract
Studying activity dependent protein expression, subcellular translocation, or phosphorylation is essential to understand the underlying cellular mechanisms of synaptic plasticity. Long-term potentiation (LTP) and long-term depression (LTD) induced in acute hippocampal slices are widely accepted as cellular models of learning and memory. There are numerous studies that use live cell imaging or immunohistochemistry approaches to visualize activity dependent protein dynamics. However these methods rely on the suitability of antibodies for immunocytochemistry or overexpression of fluorescence-tagged proteins in single neurons. Immunoblotting of proteins is an alternative method providing independent confirmation of the findings. The first limiting factor in preparation of subcellular fractions from individual tetanized hippocampal slices is the low amount of material. Second, the handling procedure is crucial because even very short and minor manipulations of living slices might induce activation of certain signaling cascades. Here we describe an optimized workflow in order to obtain sufficient quantity of nuclear enriched fraction of sufficient purity from the CA1 region of acute hippocampal slices from rat brain. As a representative example we show that the ERK1/2 phosphorylated form of the synapto-nuclear protein messenger Jacob actively translocates to the nucleus upon induction of LTP and can be detected in a nuclear enriched fraction from CA1 neurons.
Keywords: Neuroscience, Issue 90, Hippocampal slices, long-term potentiation LTP, nucleus, NMDA receptors, NLS, immunoblotting, Jacob, nuclear enriched protein preparations
Introduction
Synaptic N-methyl-D-aspartate-receptors (NMDARs) play a crucial role in synaptic plasticity and cell survival signaling whereas activation of extrasynaptic NMDARs can trigger neurodegeneration and cell death. These changes depend on tightly controlled/regulated activity dependent gene expression and thus require constant communication between activated synapses or dendrites and the nucleus7. The MAP kinases ERK1/2 are downstream effectors of synaptic NMDARs signaling and are involved in NMDAR-activation-induced gene expression, whereas signaling via extrasynaptic NMDAR has no or an inhibitory effect on ERK1/2 activity8,11.
There are number of proteins that have been shown to shuttle between distal dendrites and the nucleus. Many of these proteins contain a nuclear localization signal and are actively transported along microtubuli in a dynein and importin-dependent manner to the nucleus6,9. Interestingly, some of these messengers only transit to the nucleus in response to specific synaptic stimuli. For example, retrograde transport of cyclic AMP response element binding protein 2 (CREB2) is induced by chemical LTD but not LTP12. Localized NMDAR-dependent synaptic stimulation drives CREB-regulated transcriptional coactivator (CRTC1) into the nucleus, a translocation process, which is involved in long-term hippocampal plasticity4. It was recently shown that the protein messenger Jacob translocates to the nucleus after both, synaptic and extrasynaptic NMDAR activation and regulates CREB dependent gene transcription5. The synaptic or extrasynaptic origin of the signal is encoded in a posttranslational modification of Jacob. Synaptic activity induces ERK1/2 dependent phosphorylation of Jacob at a crucial serine at position 180 (pJacobS 180) which is a requisite for the subsequent translocation to the nucleus in primary hippocampal culture. Moreover, in CA1 neurons of acute hippocampal slices pJacobS 180 translocates to the nucleus after Schaffer collateral LTP but not LTD1,10. pS180 Jacob leads to an increased expression of plasticity related genes and this gene expression feeds back to synaptic function. In sharp contrast, Jacob that translocates to the nucleus after extrasynaptic NMDARs activation is not phosphorylated at Ser180 and might be associated with different protein complex in the nucleus causing ‘CREB shut off’ and a retraction of synaptic contacts10.
Most published studies on the nuclear import of synapto-nuclear protein messenger have been done in dissociated neuronal primary cultures. Therefore it would be interesting to see if such findings can be reproduced in physiologically more relevant conditions using hippocampal slices where neuronal connectivity and function are much better preserved. Here we present an optimized protocol for assessing LTP-dependent nuclear translocation of protein messengers by immunoblotting. This method is also suitable for analyzing activity dependent phosphorylation of proteins in a crude nuclear fraction. Specifically, the current protocol involves preparation of acute CA1 hippocampal slices, induction, and recording of LTP. Next, CA1 region is microscopically dissected to isolate the stimulated region. We combined and modified the protocol for nuclear isolation provided by CellLytic NuCLEAR Extraction Kit with changes introduced by Zhao and colleagues17. The optimized procedure includes the lysis of dissected CA1 regions in hypotonic buffer allowing cell swelling and release of nuclei. Cell lysis and nuclei morphology can be determined by microscopic examination. Nuclear enrichment is achieved by a short centrifugation step. Immunoblotting analysis with antibodies against NeuN and NSE2, specific markers of nuclear or cytosolic fractions, indicates that this approach can be used as a fast and reproducible protocol to isolate these subcellular fractions and to study very labile posttranslational modifications like protein phosphorylation. Additionally, this method is advantageous for small tissue samples deriving from dissected CA1 regions of hippocampal slices and can be used in combination to immunohistochemistry of hippocampal slices.
Protocol
1. Preparation of Acute Hippocampal Slices from Adult Rat Brain
Anesthetize rats with isoflurane. CAUTION: Perform the procedure using a closed exicator, do not inhale isoflurane. Make sure that the animal is completely anesthetized.
Decapitate the rat, quickly isolate the brain, and immerse it in precarbonated (95% O2 / 5% CO2 gas mixture) ice-cold Gey’s solution (composition in mM: 130 NaCl, 4.9 KCl, 1.5 CaCl2·2H2O, 0.3 MgSO4·2H2O, 11 MgCl2·6H2O, 0.23 KH2PO4, 0.8 Na2HPO4·7H2O, 5 Glucose·H2O, 25 HEPES, 22 NaHCO3, pH 7.32) for 30 min1,2,10,16.
Remove the cerebellum and part of the entorhinal cortex. Separate the cortical hemispheres with a mid-sagittal cut, then place each hemisphere down on its medial surface. Thereafter make a 50-70° cut (50-70° transverse) along the dorsal edge of each hemisphere13,14.
Glue each hemisphere with the freshly cut surface on the slicing platform of the sectioning system. The platform should be covered by precarbogenated ice-cold Gey’s solution.
Cut 350 μm 50-70° transverse slices from anterior to posterior side using a vibratome adjusted to minimize z-axis oscillation. The hippocampal formation, subicular and entorhinal cortices, as well as the cortices that are located dorsolateral to the hippocampus will be part of the slices used for experiments13,14.
Transfer hippocampal slices to a U-shape and submerged type incubator and incubate for at least 2 hr at 32 °C with carbogenated artificial cerebrospinal fluid (ACSF containing in mM: 110 NaCl, 2.5 KCl, 2.5 CaCl2·2H2O, 1.5 MgSO4·2H2O, 1.24 KH2PO4, 10 Glucose·H2O, 27.4 NaHCO3, pH 7.3)1,2,10,16.
2. Positioning of Electrodes, Baseline Recording, and Induction of LTP
Transfer the hippocampal slice to a submerged type recording chamber mounted under a microscope. Perfuse (6-7 ml/min) with carbogenated ACSF for at least 30 min at 32 °C.
Prepare glass capillary microelectrodes filled with ACSF (tip resistance is 3-5 MΩ).
Place a glass microelectrodes filled with ACSF in the CA1 Schaffer-collateral fibers for stimulation and in the CA1 stratum radiatum for fEPSP recording1,2,10 (Figure 2). The distance between the electrodes should be about 300 μm.
Evoke field Excitatory Postsynaptic Potentials (fEPSPs) by stimulation of Schaffer-collateral fibers with biphasic rectangular current pulses (200 msec/polarity) in a range of 3-4 V.
Perform the maximum stimulation test by measuring the input-output relationship and define the stimulation strength as 40% of maximum fEPSP-slope values obtained and keep it constant throughout the experiment.
Begin the baseline recording for at least 15 min after the maximum stimulation test. Measure the responses to test stimuli every minute throughout the experiment. Perform baseline recordings with low-frequency stimulation.
Record the baseline for at least 30 min.
For Late-LTP induction apply high-frequency 100 Hz tetanization consisting of three 1 sec stimulus trains at 100 Hz with a 5 min intertrain interval. To increase the field of stimulation within CA1 region 5 μM bicuculline can be washed in 2 min before tetanization and should be washed out immediately after last tetanization.
3. Collection of Slices after Induction of LTP and Snap Freezing
Stop LTP recording 2 min or 30 min after three trains of tetanic stimulation inducing Late-LTP.
Remove the electrodes and quickly transfer the slice onto a precold metal platform placed on dry ice. Repeat the procedure for a control slice kept in U-shaped incubator. CAUTION: Use gloves while working with dry ice.
Collect each slice in a 1.5 ml tube and store at -80 °C.
4. Isolation of Nuclear Enriched Fractions from the CA1 Region of the Hippocampus
Take 1.5 ml Eppendorf tubes with the frozen slices from -80 °C and keep them on ice.
Add 0.5 ml of fresh cold TBS buffer containing protease (PI) and phosphatase (PS) inhibitors, into the tube. Incubate for 2-3 min and transfer to a stereomicroscope. CAUTION: Use protective gloves and a lab coat while working with PI and PS.
Dissect the CA1 stratum pyramidale region of the hippocampus using two needles. Use one needle for holding the slice under the stereomicroscope and the second needle for cutting the CA1 area.
Collect the dissected CA1 regions from 5 slices (for each group) into a new 1.5 ml tube containing 50 μl lysis buffer (1x hypotonic lysis buffer containing in mM: 10 HEPES, 1.5 MgCl2, 10 KCl, pH 7.9, with PI and PS). Note that this amount of material will be sufficient to run 2-3 immunoblots.
Homogenize collected tissue by careful pipetting up and down. Use a 200 μl pipette. Incubate the lysate on ice for 5-7 min to allow the cells to swell.
Take 2 μl of the lysate, drop on a microscopic slide, and visualize the swelling of the cells under a bright field microscope. With proper swelling of the cells the nuclei appear as round intact structures.
Collect 20 μl of sample into a fresh 1.5 ml tube as ‘homogenate fraction’. Add 8 μl of denaturing 4x SDS sample buffer.
Centrifuge the remaining lysates at 11,000 rpm for 1 min.
Carefully collect the supernatant from the top (‘cytosolic fraction’), transfer into a new tube and add 20 μl of 4x sample buffer.
Resuspend the pellet in 60 μl of hypotonic buffer and add 20 μl of 4x sample buffer. This fraction is referred to as a ‘nuclear enriched fraction’.
Homogenate, cytosolic, and nuclear enriched fractions can be stored at -20 °C or -80 °C for later immunoblotting.
5. Semiquantitative Immunoblotting for Synapto-nuclear Proteins
Defreeze the samples and boil them for 5 min at 95 °C.
Take 5 µl from each fraction and determine the protein concentration by amido black test or BCA test.
Load equal amount of protein samples from homogenate, cytoplasmic and nuclear enriched fractions on SDS-PAGE. Samples from control and LTP slices should be placed on the same gel for direct comparison.
Perform a standard western-blotting procedure (wet transfer is recommended).
Probe the membranes with the antibody of choice. Subsequently the same blots (or same samples run in parallel) can be probed with a cytoplasmic marker - neuron specific enolase2 (NSE2) and nuclear marker - NeuN and a -actin antibody as a loading control.
6. Data Analysis
Efficiency of LTP induction can be analyzed by Clampfit software. The average slope of baseline recordings was compared with the slopes after tetanization using two-way ANOVA, p <0.05 was considered significantly different. The fEPSP slope values were depicted in diagrams as the mean ± S.E.M.
For quantification of immunoblots, scan either the autoradiographic film and analyze the integrated density of protein bands by ImageJ or fluorescence bands with a Licor system. Values of immunoreactive bands should be normalized for the loading and blotting control. For comparison of the control and LTP groups a nonparametrical Mann-Whitney U-Test might be used.
| Name of the buffer | Reagent | Concentration (mM) | Amount | Comments/Description |
| Gey’s solution (pH: 7.3~7.4) | NaCl | 130 | 7.6 g | sterile filtration |
| 1,000 ml | KCl | 4.9 | 0.37 g | |
| CaCl2·2H2O | 1.5 | 0.22 g | ||
| MgSO4·2H2O | 0.3 | 0.0739 g | ||
| MgCl2·6H2O | 11 | 2.24 g | ||
| KH2PO4 | 0.23 | 0.0313 g | ||
| Na2HPO4·7H2O | 0.8 | 0.2145 g | ||
| Glucose·H2O | 5 | 0.9909 g | ||
| HEPES | 25 | 5.96 g | ||
| NaHCO3 | 22 | 1.85 g | ||
| ACSF (pH: 7.3~7.4) | NaCl | 110 | 6.428 g | sterile filtration |
| 1,000 ml | KCl | 2.5 | 0.1865 g | |
| CaCl2·2H2O | 2.5 | 0.368 g | ||
| MgSO4·2H2O | 1.5 | 0.370 g | ||
| KH2PO4 | 1.24 | 0.169 g | ||
| Glucose·H2O | 10 | 1.9817 g | ||
| NaHCO3 | 27.4 | 2.3 g | ||
| 1x Hypotonic buffer (pH: 7.9) | HEPES | 10 | 0.23 g | |
| 100 ml | MgCl2·6H2O | 1.5 | 0.0304 g | |
| KCl | 10 | 0.07455 g |
Table 1. Buffers.
Representative Results
We have previously shown that the synapto-nuclear protein messenger Jacob accumulates in the nucleus following the induction of LTP but not LTD1. Moreover, translocation of Jacob after synaptic stimulation requires activation of MAPK ERK1/2 and phosphorylation of Jacob at Ser180 (Figure 1). Phosphorylated Jacob translocates to the nucleus in an importin-dependent manner and the phosphorylated state can be preserved over extended periods of time by association with the intermediate filament αinternexin15 (Figure 1). The phospho-state of Jacob is important for the expression of activity-dependent genes and cell survival. Interestingly, activation of extrasynaptic NMDARs also drives Jacob into the nucleus but in this case Jacob is not phosphorylated at Ser180 and its nuclear accumulation induces CREB ‘shut off’5,10. The results described above were obtained by protocols established in our recent study (see Karpova et al.10 for detailed description of the model).
To prove that Jacob translocates into the nucleus after induction of LTP, we induced and measured the induction of Schaffer collateral fEPSP potentiation in acute hippocampal slices and subsequently isolated neuronal nuclei from CA1 neurons (Figures 2 and 3). We compared two different time points after application of high-frequency stimulation (2 min and 30 min) and recorded the induction of LTP for each slice that was subsequently processed for nuclear isolation and western blotting (Figures 2 and 3). Enrichment of the neuronal nuclear marker NeuN can be seen in the nuclear enriched fraction prepared from the dissected CA1 region, whereas cytosolic marker neuron specific enolase2 (NSE) is mostly present in the supernatant fraction obtained after centrifugation of lysed cells (Figure 4A). The specificity of pS180 Jacob antibody has been characterized previously (for details see Karpova et al.10). pS180 Jacob levels remained unaltered in total CA1 protein homogenates and in the nuclear enriched fraction 2 min after tetanization (Figure 4B), but we found a significant increase in pJacobS 180 immunoreactivity 30 min after the induction of LTP (Figure 4C), confirming that Jacob translocating to the nucleus in this cellular plasticity model is phosphorylated at Ser180.
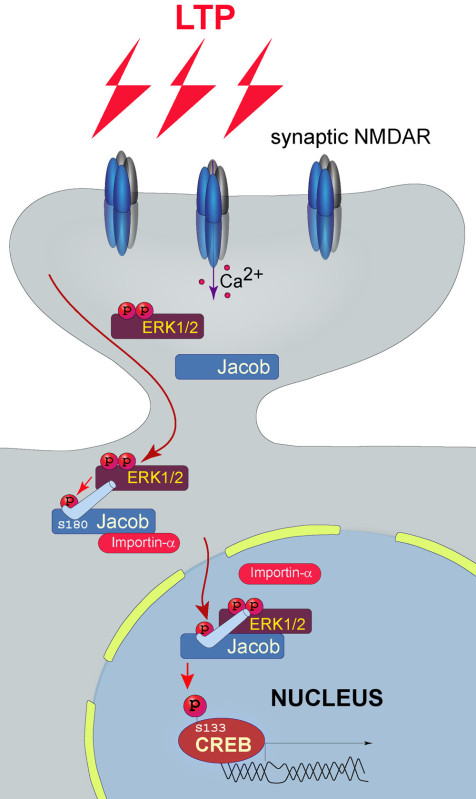 Figure 1. Jacob phosphorylated at S180 encodes the synaptic origin of NMDARs signals. Tetanic stimulation of hippocampal Schaffer collateral fibers results in activation of synaptic NMDARs and subsequent activation of MAPK ERK1/2 signaling cascade. Activated ERK1/2 binds to and phosphorylates Jacob at serine 180 and Jacob-ERK1/2 complex translocates to the nucleus and this correlates with enhanced CREB activity and activation of plasticity related gene expression.
Figure 1. Jacob phosphorylated at S180 encodes the synaptic origin of NMDARs signals. Tetanic stimulation of hippocampal Schaffer collateral fibers results in activation of synaptic NMDARs and subsequent activation of MAPK ERK1/2 signaling cascade. Activated ERK1/2 binds to and phosphorylates Jacob at serine 180 and Jacob-ERK1/2 complex translocates to the nucleus and this correlates with enhanced CREB activity and activation of plasticity related gene expression.
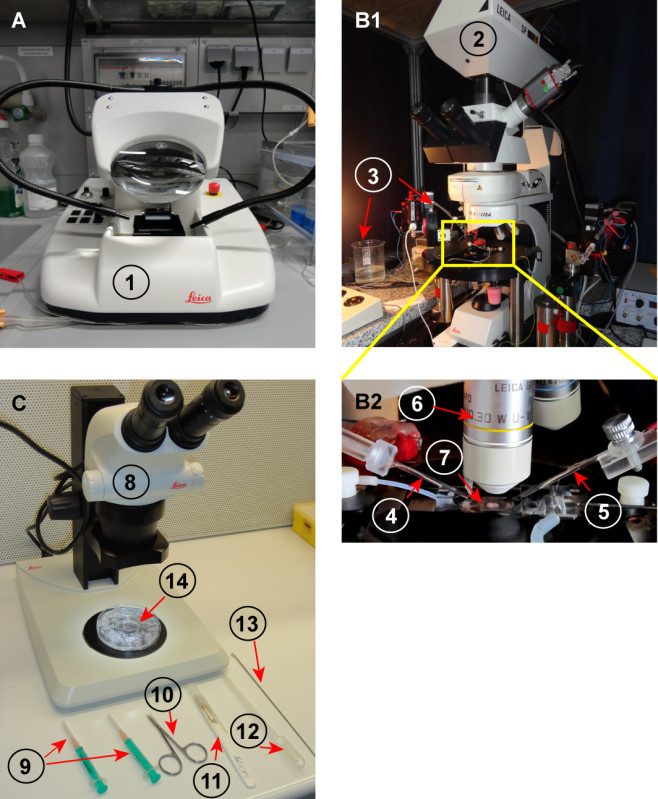 Figure 2.Equipment and tools used for LTP induction and dissecting the CA1 region from hippocampal slices. A) Vibratome used for preparation of acute hippocampal slices (1 - Vibratome) B1-2) Electrophysiology and imaging setup (2 - Microscope; 3 - Micromanipulator and perfusion system; 4 - Recoding electrode; 5 - Stimulation electrode; 6 - Water objective lens; 7 - Slice holder with hippocampal slice) C) Equipment used for dissection of CA1 region from hippocampal slices (8. Stereomicroscope; 9 - Insulin syringe with needles; 10 - Small surgical scissors; 11 - Scalpel; 12 - Plastic Pasteur pipette; 13 - Thin spatula; 14-100 mm plastic culture dish filled with ice-water, and 40 mm plastic culture dish used for dissection of slices.
Figure 2.Equipment and tools used for LTP induction and dissecting the CA1 region from hippocampal slices. A) Vibratome used for preparation of acute hippocampal slices (1 - Vibratome) B1-2) Electrophysiology and imaging setup (2 - Microscope; 3 - Micromanipulator and perfusion system; 4 - Recoding electrode; 5 - Stimulation electrode; 6 - Water objective lens; 7 - Slice holder with hippocampal slice) C) Equipment used for dissection of CA1 region from hippocampal slices (8. Stereomicroscope; 9 - Insulin syringe with needles; 10 - Small surgical scissors; 11 - Scalpel; 12 - Plastic Pasteur pipette; 13 - Thin spatula; 14-100 mm plastic culture dish filled with ice-water, and 40 mm plastic culture dish used for dissection of slices.
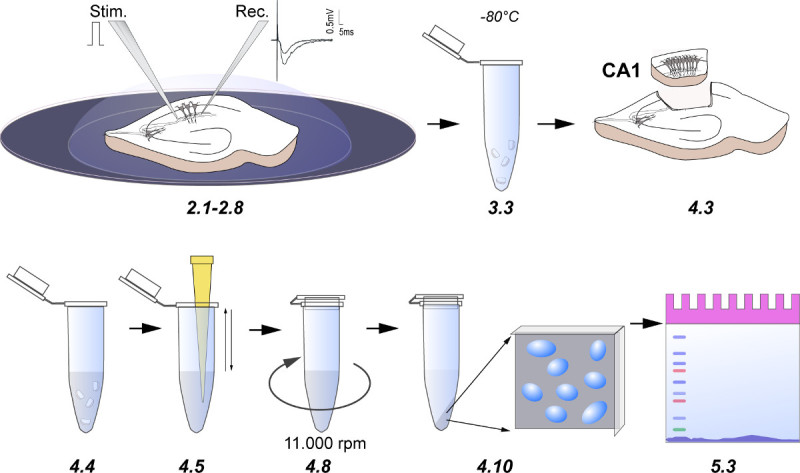 Figure 3. Cartoon demonstrating the experimental procedure. Numbers indicate steps in the protocol. 2.1-2.8) An acute hippocampal slice in the submerged chamber with glass electrodes placed in the stratum radiatum for potentiation of Schaffer collateral-CA1 Synapses. The inset represents the sample fEPSP analog traces, the horizontal bar indicates 5 msec and the vertical bar indicates 0.5 mV. 3.3) The slices were collected into 1.5 ml tubes after LTP recording and snap frozen. 4.3) Dissection of the CA1 region from adult rat hippocampal slices. 4.4-4.5) CA1 regions homogenized in hypotonic lysis buffer, which causes osmotic swelling of cells and release of nuclei. 4.8) Centrifugation of lysates at 11,000 rpm for 1 min. 4.10) Collection of the cytoplasmic and nuclear enriched fractions for immunoblotting. 5.3) Loading of cytoplasmic and nuclear enriched fractions on SDS-PAGE and subsequent standard western-blotting.
Figure 3. Cartoon demonstrating the experimental procedure. Numbers indicate steps in the protocol. 2.1-2.8) An acute hippocampal slice in the submerged chamber with glass electrodes placed in the stratum radiatum for potentiation of Schaffer collateral-CA1 Synapses. The inset represents the sample fEPSP analog traces, the horizontal bar indicates 5 msec and the vertical bar indicates 0.5 mV. 3.3) The slices were collected into 1.5 ml tubes after LTP recording and snap frozen. 4.3) Dissection of the CA1 region from adult rat hippocampal slices. 4.4-4.5) CA1 regions homogenized in hypotonic lysis buffer, which causes osmotic swelling of cells and release of nuclei. 4.8) Centrifugation of lysates at 11,000 rpm for 1 min. 4.10) Collection of the cytoplasmic and nuclear enriched fractions for immunoblotting. 5.3) Loading of cytoplasmic and nuclear enriched fractions on SDS-PAGE and subsequent standard western-blotting.
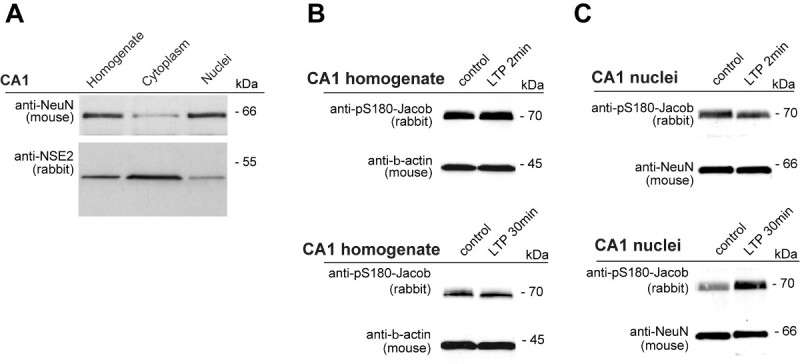 Figure 4. Induction of CA1 LTP leads to the accumulation of pJacS180 in the nucleus. A) Immunoblot for homogenate, cytosolic and nuclear enriched fractions of CA1 lysate probed with cytosolic and nuclear markers. B) Immunoblot analysis of pJac-S180 level in homogenate 2 min and 30 min after induction of tetanization. C) Immunoblot analysis of pJacS180 level in nuclear enriched fraction 2 min and min after tetanization. These results are part of quantification graph published in Karpova et al10. These representative immunoblots are not included in to the original publication.
Figure 4. Induction of CA1 LTP leads to the accumulation of pJacS180 in the nucleus. A) Immunoblot for homogenate, cytosolic and nuclear enriched fractions of CA1 lysate probed with cytosolic and nuclear markers. B) Immunoblot analysis of pJac-S180 level in homogenate 2 min and 30 min after induction of tetanization. C) Immunoblot analysis of pJacS180 level in nuclear enriched fraction 2 min and min after tetanization. These results are part of quantification graph published in Karpova et al10. These representative immunoblots are not included in to the original publication.
Discussion
The steps described in the protocol above provide guidance how to prepare hippocampal acute sliced from young or adult rats, induce and record LTP, rapidly dissect stimulated area of slice, and prepare nuclear enriched fraction for studying activity dependent protein dynamics. This approach derives from combination of several different methods used independently from each other. We optimized a workflow and provide sufficient detail for the beginner to set up their own experiments to study the subcellular redistribution of proteins upon induction of LTP. As an example we demonstrate that the late form of LTP induces the nuclear accumulation of S180 phosphorylated Jacob. To distinguish between the phosphorylation of a pre-existing nuclear pool of a given protein and the translocation of its phosphorylated form after synaptic activity, this approach can be combined with control for total level of protein of interest in the nuclear enriched fraction. Moreover, testing samples over different time points after LTP induction might be very insightful for studying time course of protein activation/inactivation or nuclear turnover.
During the preparation of nuclear enriched fractions from stimulated slices there are several methodological issues that need to be addressed. First, to increase the number of stimulated neurons in the CA1 region and the amount of material for immunoblotting, we apply 5 µM of GABAA receptor blocker biccuculine during tetanization. Biccuculine has been shown to enhance excitatory postsynaptic potentials3.
Second, preservation of slices after induction of LTP by rapid freezing is a critical step for successful experiments because mechanical handling of slices might induce different types of activities directly correlating with changes in protein’s modifications. To shorten the procedure as much as possible, we use a metal bar placed on dry ice. Slices can be transferred in a drop of ACSF using plastic a Pasteur pipette and be frozen within a very few second when placed on prechilled metal.
The third critical step is the enrichment of nuclei in CA1 lysates. We recommend to monitor cell swelling under the microscope and to find the most optimal time point. Sometimes when homogenization of tissue was not done properly, cell debris still remains in the samples. Then the nuclear pellet can be resuspended in lysis buffer again, washed for a few minutes, and then centrifuged to get a purer nuclear fraction.
Overall this protocol can help to further explorer the role of different synapto-nuclear messenger proteins in synaptic plasticity. The same procedure can be applied not only to a high frequency stimulation induced LTP but all other forms of synaptic plasticity like LTD, theta-burst LTP, short term plasticity models, and others.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This work was funded by the DFG (SFB 779 TPB8, Kr1879/3-1 MRK), DIP grant (MRK), EU FP7 MC-ITN NPlast (MRK), Center for Behavioral Brain Sciences (CBBS, Sahsen-Anhalt), (AK and SB), MM is a recipient of European Molecular Biology Organization (EMBO) Long-Term Fellowship (EMBO ALTF 884-2011) and Marie-Curie IEF.
References
- Behnisch T, et al. Nuclear translocation of Jacob in hippocampal neurons after stimuli inducing long-term potentiation but not long-term depression. PLoS One. 2011;6(2):e17276. doi: 10.1371/journal.pone.0017276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai F, Frey JU, Sanna PP, Behnisch T. Protein degradation by the proteasome is required for synaptic tagging and the heterosynaptic stabilization of hippocampal late-phase long-term potentiation. Neuroscience. 2010;169(4):1520–1526. doi: 10.1016/j.neuroscience.2010.06.032. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Perez Y, Lacaille JC. Effects of GABA(A) inhibition on the expression of long-term potentiation in CA1 pyramidal cells are dependent on tetanization parameters. Hippocampus. 1998;8(3):289–298. doi: 10.1002/(SICI)1098-1063(1998)8:3<289::AID-HIPO10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Ch'ng TH, Uzgil B, Lin P, Avliyakulov NK, O'Dell TJ, Martin KC. Activity-dependent transport of the transcriptional coactivator CRTC1 from synapse to nucleus. Cell. 2012;150(1):207–221. doi: 10.1016/j.cell.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich DC, et al. Caldendrin-Jacob: a protein liaison that couples NMDA receptor signalling to the nucleus. PLoS Biol. 2008;6(2):e34. doi: 10.1371/journal.pbio.0060034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fainzilber M, Budnik V, Segal RA, Kreutz MR. From synapse to nucleus and back again--communication over distance within neurons. J. Neurosci. 2011;31(45):16045–16048. doi: 10.1523/JNEUROSCI.4006-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat. Rev. Neurosci. 2010;11(10):682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A, et al. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J. Physiol. 2006;57:789–798. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan BA, Kreutz MR. Nucleocytoplasmic protein shuttling: the direct route in synapse-to-nucleus signaling. Trends Neurosci. 2009;32(7):392–401. doi: 10.1016/j.tins.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Karpova A, et al. Encoding and transducing the synaptic or extrasynaptic origin of NMDA receptor signals to the nucleus. Cell. 2013;152(5):1119–1133. doi: 10.1016/j.cell.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Lai KO, Zhao Y, Ch'ng TH, Martin KC. Importin-mediated retrograde transport of CREB2 from distal processes to the nucleus in neurons. Proc. Natl. Acad. Sci. U.S.A. 2008;105(44):17175–17180. doi: 10.1073/pnas.0803906105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK, Frey JU, Behnisch T. LTP in cultured hippocampal-entorhinal cortex slices from young adult (P25-30) rats. J. Neurosci. Methods. 2003;130(1):19–32. doi: 10.1016/s0165-0270(03)00228-0. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Frey JU, Behnisch T. Single cell analysis of activity-dependent cyclic AMP-responsive element-binding protein phosphorylation during long-lasting long-term potentiation in area CA1 of mature rat hippocampal-organotypic cultures. Neuroscience. 2005;131(3):601–610. doi: 10.1016/j.neuroscience.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Perlson E, Hanz S, Ben-Yaakov K, Segal-Ruder Y, Seger R, Fainzilber M. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron. 2005;45(5):715–726. doi: 10.1016/j.neuron.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Xiang Z, et al. Long-term maintenance of mature hippocampal slices in vitro. J. Neurosci. Methods. 2000;98(2):145–154. doi: 10.1016/s0165-0270(00)00197-7. [DOI] [PubMed] [Google Scholar]
- Zhao M, Adams JP, Dudek SM. Pattern-dependent role of NMDA receptors in actin potential generation: consequences on extracellular signal-regulated kinase activation. J. Neurosci. 2005;25(30):7032–7039. doi: 10.1523/JNEUROSCI.1579-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


