Summary
Treatment of Wilson’s disease (WD) with anti-copper agents is effective in most compliant patients. During long-term treatment with chelating agents, a two-day interruption of the treatment should result in normal urinary copper concentrations (<50 μg/dl). The aim of this study was to establish the usefulness of this method as a compliance assessment in these patients.
We examined consecutive patients treated with d-penicillamine (DPA) undergoing routine follow-up studies at our center. We performed 24-h urinary copper excretion analysis 48 h after interruption of chelating therapy.
Thirty-two patients were enrolled. After DPA cessation, normalization of copper excretion was observed in 91% of reportedly compliant patients. The specificity and sensitivity values of this test were 87% and 77%, respectively.
Measurement of 24-h urinary copper excretion after a 48-h interruption of DPA therapy in patients with WD is a reliable method for confirming patients’ compliance.
Keywords: compliance assessments, d-penicillamine, urinary copper excretion, Wilson’s disease
Introduction
Wilson’s disease (WD, OMIM: 277900) is an inherited autosomal recessive disorder of copper metabolism caused by mutations in the ATP7B gene that lead to copper accumulation in the body. Patients present with hepatic, neurological and psychiatric symptoms (Ala et al., 2007; Ferenci et al., 2003).
Diagnostic biochemical tests include measurement of serum ceruloplasmin concentration or activity (typically decreased), total serum copper concentration (typically decreased), and 24-h urinary copper excretion (increased). Approximately 95% of the serum copper in healthy individuals is incorporated into ceruloplasmin. The remaining 5% is loosely bound to albumin (Desai and Kaler, 2008; EASL, 2012). In patients with WD, non-ceruloplasmin-bound copper (NCC) is elevated and has toxic effects. In untreated and ineffectively treated patients, NCC is usually above 25 μg/dl, whereas the normal range is suggested in most publications to be 10–15 μg/dl (Roberts and Schilsky, 2008; EASL, 2012; Walshe, 2012).
The aims of treatment in WD are to remove excess copper from the blood and tissues, prevent its re-accumulation, and maintain a negative copper balance (Roberts and Schilsky, 2008; EASL, 2012). There are two groups of drugs used to treat WD: chelators (d-penicillamine [DPA]; trientine), which promote urinary copper excretion, and zinc salts, which interfere with intestinal copper absorption (Roberts and Schilsky, 2008; EASL, 2012).
Compliance is essential for a good therapeutic effect and non-compliance is the major problem with lifelong therapy. Our previous studies showed that 25–45% of patients with WD are non-compliant (Masełbas et al., 2010; Dzieżyc et al., 2014). To date, there are no reliable tests that can be used to monitor copper metabolism and proper treatment and compliance. Normalization of NCC in blood reflects the effectiveness of anti-copper therapy (Desai and Kaler, 2008; EASL, 2012). Although direct measurement of free copper is possible, it is not currently routinely available, and most measurements of free copper are not standardized (Twomey et al., 2005, 2007; El Balkhi et al., 2011).
Twenty-four-hour urinary copper excretion is a very important parameter for the diagnosis of WD as well as for treatment monitoring, as it reflects the fraction of free copper in serum (EASL, 2012). Normal urinary copper excretion is below 50 μg/24 h. In patients with WD, it is often above 100 μg/24 h (EASL, 2012). Wide variations in urinary copper excretion levels are observed in patients with WD. The first urinary copper excretion values were reported by Cumings (1959), who found levels of 48 μg/24 h (standard deviation [SD], 16.3 μg/24 h) in healthy individuals and 703 μg/24 h (SD, 308 μg) in patients with WD. In recent years it has been shown that patients with hepatic features tend to have higher 24-h urinary copper excretion than those with neurological features (Walshe, 2011).
Some previous studies suggested that following one year of chelation treatment, 24-h urinary copper excretion values fell nearly to normal after chelator cessation. However, no compliance or clinical state change assessment was performed in these studies (Walshe, 2012).
In the 2012 European Association for the Study of the Liver guidelines on WD (EASL, 2012), urinary copper excretion evaluation 48 hours after DPA cessation is advised as a class C recommendation for monitoring treatment and assessing compliance. However, this recommendation is not supported by any large studies, and to our knowledge the sensitivity and specificity of this test have never been investigated.
The aim of this study was to establish the diagnostic value of 24-h urinary copper excretion two days after interruption of DPA therapy as a compliance assessment in a cohort of patients with WD.
Materials and methods
We examined consecutive patients who were undergoing routine follow-up studies at our center between March 2013 and April 2014 and had been treated only with DPA for at least two years. The diagnosis of WD was based on a combination of abnormal copper metabolism tests, the presence of a Kayser-Fleisher ring, and the results of genetic testing, performed as described previously (Członkowska et al., 1996, 2014).
For the present analysis, all the enrolled patients underwent 24-h urinary copper excretion measurement during and two days after interruption of DPA treatment. Serum ceruloplasmin and serum copper concentrations were measured, and basic hepatic tests were also performed.
The patients were divided into groups according to their dominant symptoms: neurological or hepatic. To assess the severity of hepatic involvement, we divided the patients into five groups on the basis of a previously established staging system used in our department (Członkowska et al., 2014). Neurological examinations were performed according to the Unified Wilson’s Disease Rating Scale (UWDRS) (Członkowska et al., 2007). Compliance during the course of treatment was assessed by asking questions during a structured interview, and findings were supported by basic liver function tests and neurological assessments. Non-compliance was defined as discontinuation of therapy lasting at least three months or irregular treatment. Worsening of hepatic symptoms was assessed according to our hepatic staging system and neurological worsening was defined according to the UWDRS as described previously (≤ 4 points scored in part III and any point scored in part II of the UWDRS) (Członkowska et al., 2007).
Serum ceruloplasmin was measured using the improved colorimetric enzymatic assay developed by Ravin (1961). Total serum copper concentration and 24-h urinary copper excretion were measured by flame atomic absorption spectrometry. In cases not showing negative values, free serum copper was calculated as previously described using the following formula: (total serum copper concentration in μg/dl)–(serum ceruloplasmin in mg/dl×3)=(free serum copper concentration in μg/dl) (Desai and Kaler, 2008; EASL, 2012).
The study protocol was approved by the Institutional Review Board of the Institute of Psychiatry and Neurology in Warsaw.
Statistical analysis
Categorical variables are presented as ratios of the number of valid observations. Continuous variables are presented as means and standard deviation (SD). For basic comparisons, the two-tailed Fisher’s exact test and Mann-Whitney U-test were used. Sensitivity, specificity and positive and negative predictive values were calculated in the traditional manner. The relationship between urinary copper excretion and duration of treatment was analyzed using a Spearman’s rank correlation coefficient. All calculations were performed with STATISTICA 10.0 (2011; StatSoft, Tulsa, OK, USA). A p-value of <0.05 was considered significant. Receiver operating characteristic (ROC) curve analysis was performed in PQStat version 1.4.4.126 (PQStat Software, Poznań, Poland).
Results
Thirty-two patients (12 females, 37%) were enrolled. Their age on entering the study ranged from 22 to 65 years (mean, 40.37±13.65 years). Their mean age at diagnosis was 28.42±11.66 years and their mean treatment duration was 12.12±11.77 years (range: 2–45 years, median of 8 years, interquartile range: 4–17).
Hepatic features were dominant in 11 (34%) patients and neurological symptoms in 21(65%). The clinical details and study results are shown in table I.
Table I.
Copper metabolism tests at the time of diagnosis and at the time of study in 32 Wilson’s disease patients.
| All patients (n=32) |
Hepatic presentation (n=11) |
Neurological presentation (n=21) |
p | |
|---|---|---|---|---|
| Duration of treatment (years) | 12.1±11.7 | 11.4±11.1 | 12.0±11.6 | 0.44 |
| Baseline ceruloplasmin serum concentration (mg/dl)** | 6.4±4.9 | 5.4±5.0 | 5.5±4.8 | 0.72 |
| Baseline total serum copper (μg/l)** | 34.2±22.6 | 38.7±23.2 | 38.2±20.4 | 0.52 |
| Baseline NCC (μg/l)***† | 18.2±14.2 | 18.1±15.1 | 17.1±13.4 | 0.76 |
| NCC at the time of study (μg/l) ***† | 8.0±7.7 | 6.0±7.8 | 7.9±6.7 | 0.42 |
| 24-h urinary copper excretion at diagnosis (μg/24 h)** | 657.8±582.6 | 695.0±613.6 | 644.9±600.5 | 0.72 |
| 24-h urinary copper excretion at the time of study (μg/24 h)** | 653.9±354.1 | 683.3±391.1 | 679.2±386.1 | 0.74 |
| 24-h urinary copper excretion after DPA interruption (μg/24h)** | 48.4±44.6 | 48.7±48.42 | 44.68±40.51 | 0.65 |
| Non-compliant patients* | 10 (31.2%) | 3(27.2%) | 7(33.3%) | 0.29 |
Data are presented as mean±SD or
number of observations (%)
Normal ranges of copper metabolism tests: serum ceruloplasmin concentration 25.0–45.0 mg/dl, serum copper concentration 70.0–140.0 μg/dl, and urinary copper excretion <50 μg/24 h.
according to European and American recommendations normal values for free copper concentration are in the range 10–15 μg/dl. Negative values of NCC: in 5 patients at the time of diagnosis (2 with hepatic features, 3 with neurological features) and in 12 patients at the time of study (5 with hepatic features, 7 with neurological features).
Abbrevations: NCC=non-ceruloplasmin-bound copper; DPA=d-penicillamine.
All the recruited patients had increased urinary copper excretion at the time of diagnosis (657.87±582.60; range: 57–2308 μg/24 h). No statistically significant differences in urinary copper excretion were found between the patients with predominantly neurological or hepatic features at diagnosis or at the time of the study (Table I).
Twenty-two (68%) patients were classified as compliant with therapy. Most patients [19(86%)] had clinical improvements. The clinical status had not markedly changed since diagnosis in three (13%) patients. Normalization (<50 μg/24 h) of copper excretion after interruption of therapy was observed in 20 (91%) of the 22 compliant patients. In two patients who declared compliance, urinary copper excretion was higher than 50 μg/24 h (values of 62, 72), but no clinical or biochemical worsening was observed in these patients. They had been treated for five and 10 years, respectively. One patient with neurological symptoms had improved, and the other with mild hepatic features had remained in a stable state since diagnosis.
Ten patients were classified as non-compliant. In seven (70%) of these patients, copper excretion was above 50 μg/24 h after cessation of DPA (Table II). In five (50%) of the 10 non-compliant patients, clinical worsening was observed (neurological deterioration, n=3; hepatic symptom worsening, n=2).
Table II.
Comparison of copper metabolism parameters at the time of diagnosis and at the time of study in compliant and non-compliant Wilson’s disease patients.
| Compliant (n=22) | Non-compliant (n=10) | p | |
|---|---|---|---|
| Duration of treatment (years) | 12.0±11.9 | 11.1±11.3 | 0.46 |
| Neurological presentation* | 13 (59.0%) | 7 (70.0%) | 0.28 |
| Hepatic presentation* | 8 (41.0%) | 3 (30.0%) | 0.28 |
| Serum ceruloplasmin (mg/dl) | |||
| - baseline | 6.2±4.6 | 6.7±5.2 | 0.41 |
| - at the time of study | 8.06±8.1 | 7.8±7.9 | 0.54 |
| Total serum copper (μg/dl) | |||
| - baseline | 34.9±20.9 | 34.7±22.3 | 0.75 |
| - at the time of study | 24.8±21.2 | 22.3±19.2 | 0.88 |
| NCC (μg/dl)** | |||
| - baseline *** | 21.2±14.7 | 16.0±15.6 | 0.32 |
| - at the time of study † | 5.9±6.7 | 6.5±4.2 | 0.62 |
| Urinary copper excretion (μg/24 h) | |||
| - baseline | 657.87±582.60 | 715.1±604.7 | 0.45 |
| - at the time of study | 653.95±354.19 | 690.5±422.1 | 0.38 |
| - after DPA cessation | 48.08±46.10 | 90.0±64.7 | <0.001 |
| Clinical deterioration | 1(4.54%) | 5 (50.0%) | 0.005 |
Data are expressed as mean±SD or
number of observations (%)
according to European and American recommendations normal values for free copper concentration are in the range 10–15 μg/dl.
calculated for patients who did not obtain negative values (compliant=19, non-compliant n=9)
calculated for patients who did not obtain negative values (compliant n=13, non-compliant n=8).
Abbreviations: NCC=non-ceruloplasmin-bound copper; DPA=d-penicillamine.
Three non-compliant patients showed normal urinary copper excretion after the interruption of DPA therapy (values of 39, 39.5, and 40 μg/24 h, respectively) and no clinical deterioration. All had been treated for between six and 17 years. Two patients reported interruptions in therapy lasting six and 18 months, respectively, prior to the present evaluation. The third patient had taken half of the prescribed dose for 10 years, although he had previously been compliant for seven years.
We did not find statistically significant differences in free copper concentration between compliant and non-compliant patients either at baseline or at the time of the study. However, negative values of NCC were obtained in five (15%) patients at the time of diagnosis and in 12 (37%) patients at the time of the study.
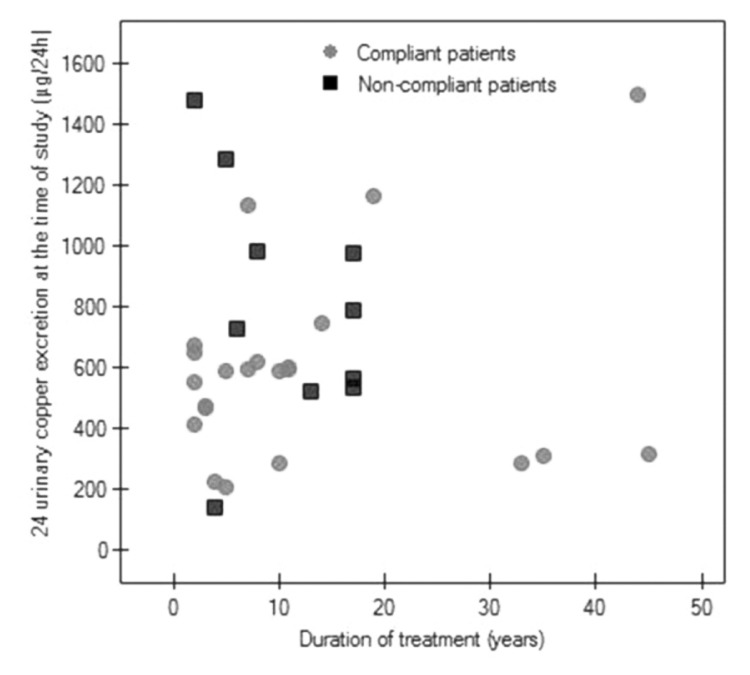
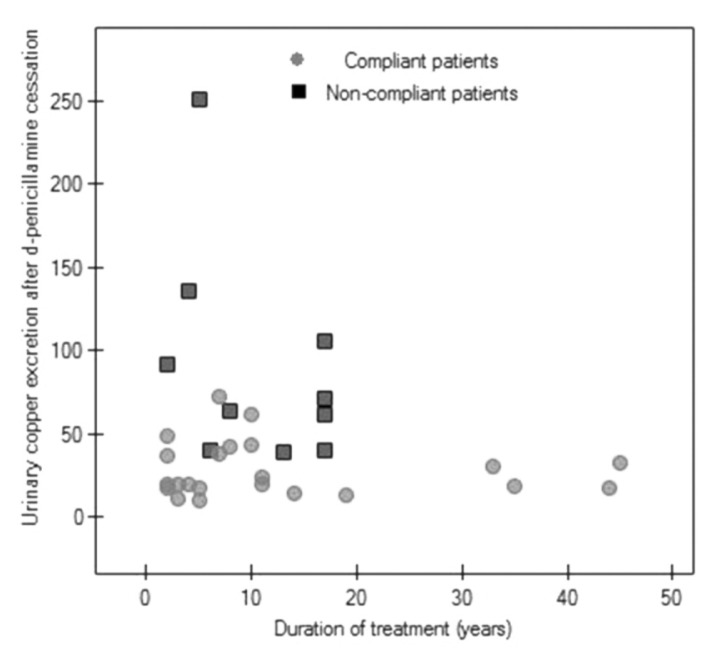
We did not find any correlation between duration of treatment and urinary copper excretion in all the analyzed WD patients or between duration of treatment and urinary copper excretion after DPA cessation in compliant and non-compliant patients (Figs 1, 2).
Figure 1.
A scatter diagram of 24-h urinary copper excretion in all 32 enrolled patients versus duration of treatment (Spearman correlation, r=0.07, p=0.68).
Figure 2.
A scatter diagram of urinary copper excretion (μg/24h) after 48h of d-penicillamine interruption versus duration of treatment in 22 compliant patients (Spearman correlation, r=0.11, p=0.603) and 10 non-compliant patients (Spearman correlation, r=−0.37, p=0.28).
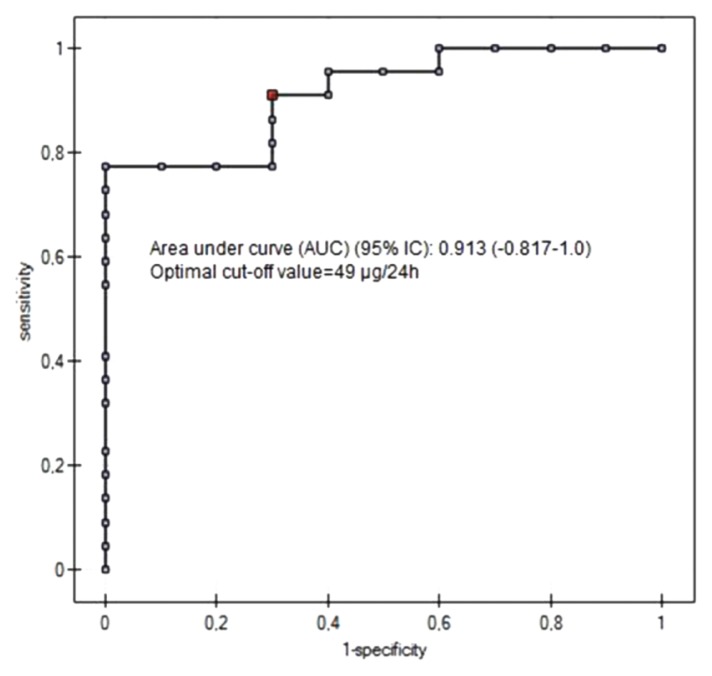
The specificity and sensitivity values of this test were 87% and 77%, respectively (Table III). The results of the ROC curve analysis showed that the optimal cutoff of 24-h urinary copper excretion after DPA cessation in compliant patients was 49 μg/24 h (Fig. 3). The area under the ROC curve was equal to 0.913 (95% CI; −0.817 to 1).
Table III.
Sensitivity, specificity and positive and negative predictive values for 24-h urinary copper excretion after cessation of d-penicillamine treatment.
| Sensitivity (95% CI) | Specificity (95% CI) | Positive predictive value (95% CI) | Negative predictive value (95% CI) | |
|---|---|---|---|---|
| Urinary copper excretion <50 μg/24 h | 77.78% (40.06–96.53) | 86.96% (66.38–97.07) | 90.91% (70.80–98.62) | 70.00% (34.84–92.97) |
Figure 3.
ROC curve for the cut-off level of 24-h urinary copper excretion after 48h of d-penicillamine interruption in a compliance assessment of patients with Wilson’s disease.
Discussion
Our findings show a high specificity (87%) for 24-h urinary copper excretion after a 48-h cessation of DPA as a compliance assessment in patients with WD. According to the ROC curve analysis, a value of 49 μg/24 h was established as the cut-off point; however, the sensitivity of this test was 77%. This test cannot completely exclude that patients are non-compliant. However, 80% of clinically deteriorated noncompliant patients had increased 24-h urinary copper excretion after a 48-h interruption of DPA treatment.
Our results showed that some compliant patients showed increased urinary copper excretion after interrupting treatment while some non-compliant patients showed normal urinary copper excretion. Information about compliance cannot always be reliable, but 50% of the patients in the non-compliant group deteriorated. On the other hand, some of the non-compliant patients had taken their drugs regularly for many years before they became non-compliant, and their copper metabolism had normalized and did not return to abnormal values at the time of the present study.
One patient without clinical deterioration treated for 45 years, in whom we suspect low copper excretion, was found to have very high urinary copper excretion (1500 μg/24 h) prior to interruption of DPA. Although he reported compliance, it seems that he was irregularly treated and had taken DPA just before the follow-up visit, and that this was the cause of his very high urinary copper excretion value. After interrupting DPA for two days, he showed normal urinary copper excretion (17 μg/24 h) (Figs 1, 2).
The duration of therapy and the duration of non-compliance seem to play an important role in 24-h urinary copper excretion assessments. However, clinical symptoms may develop later than abnormal copper metabolic parameters. Our previous study on pre-symptomatic patients showed that the time to symptom development in non-compliant patients may be as long as 14 years (Dzieżyc et al., 2014).
According to the present recommendations, treatment monitoring includes copper metabolism parameters and should be performed at least twice yearly and more frequently at the beginning of therapy (Desai and Kaler, 2008; EASL, 2012). In patients treated with DPA, urinary copper excretion should be in the range 200–500 μg/24 h (EASL, 2012). However, this value is not supported by larger studies. Our results also showed that even in compliant patients values are often higher. Copper urinary excretion at diagnosis is highly variable, and response to therapy may depend on the patient’s baseline copper level (Walshe et al., 2011). Non-compliant patients who start taking drugs just before their assessment may show high urinary copper excretion. On the other hand, low values may indicate non-compliance as well as excessive anti-copper treatment. To differentiate between these situations, we can assess NCC. In cases of non-compliance, NCC will be high (>25 μg/dl), whereas it will be very low (<5 μg/dl) in cases of overtreatment (EASL, 2012). Unfortunately, the calculated NCC in our group of patients showed false negative values in five (15%) patients at the time of diagnosis and in 12 (37%) at the time of the study. These results suggest that NCC calculation is not reliable and further studies on methods for measuring free copper are needed. Moreover, comparing our compliant and non-compliant patients, we did not find statistically significant differences in the calculated NCC. These results indicate that urinary copper excretion after interruption of DPA is more helpful as a compliance assessment.
Clinical and basic laboratory tests remain the most important tests for patient management. Some compliant patients may not respond to treatment well or may deteriorate on therapy, and will need to be switched to another drug. Therefore, when making decisions on therapy in patients with WD in everyday practice, we must consider current as well as previous copper metabolism tests, liver function tests, and clinical status.
Our study has some limitations. Even though we did not assess compliance only on the basis of interviews, but also though biochemical assessments, we cannot exclude the possibility that some patients in whom we did not detect any clinical or biochemical disturbances were in fact non-compliant (especially for short periods of time). Furthermore, we calculated NCC using an indirect method, therefore our results for this variable are not reliable. However, no method of free copper measurement in routine practice is standardized.
In conclusion, when assessing compliance in patients with WD, we should consider many factors like the duration of treatment, biochemical tests and worsening of symptoms. Twenty-four-hour urinary copper excretion after interruption of DPA therapy may lend additional support to a suspicion of non-compliance, but it cannot exclude non-compliance. However, normal 24-h excretion of copper provides an indication of normalization of copper storage.
Acknowledgments
KD is supported by the “START” stipend awarded by the Foundation for Polish Science.
References
- Ala A, Walker AP, Askhan K, et al. Wilson’s disease. Lancet. 2007;369:397–408. doi: 10.1016/S0140-6736(07)60196-2. [DOI] [PubMed] [Google Scholar]
- Cumings JN. Heavy Metals and The Brain. Oxford: Blackwell Scientific Publications; 1959. [Google Scholar]
- Członkowska A, Litwin T, Karliński M, et al. D-penicillamine versus zinc sulfate as first-line therapy for Wilson’s disease. Eur J Neurol. 2014;21:599–606. doi: 10.1111/ene.12348. [DOI] [PubMed] [Google Scholar]
- Członkowska A, Tarnacka B, Moller JC, et al. Unified Wilson’s Disease Rating Scale - proposal for the neurological scoring of Wilson’s disease patients. Neurol Neurochir Pol. 2007;41:1–12. [PubMed] [Google Scholar]
- Członkowska A, Gajda J, Rodo M. Effects of long-term treatment in Wilson’s disease with D-penicillamine and zinc sulphate. J Neurol. 1996;243:269–273. doi: 10.1007/BF00868525. [DOI] [PubMed] [Google Scholar]
- Desai V, Kaler SG. Role of copper in human neurological disorders. Am J Clin Nutr. 2008;88:855S–858S. doi: 10.1093/ajcn/88.3.855S. [DOI] [PubMed] [Google Scholar]
- Dzieżyc K, Karliński M, Litwin T, et al. Compliant treatment with anti-copper agents prevents clinically overt Wilson’s disease in pre-symptomatic patients. Eur J Neurol. 2014;21:332–337. doi: 10.1111/ene.12320. [DOI] [PubMed] [Google Scholar]
- El Balkhi S, Trocello JM, Poupon J, et al. Relative exchangeable copper: a new highly sensitive and highly specific biomarker for Wilson’s disease diagnosis. Clin Chim Acta. 2011;412:2254–2260. doi: 10.1016/j.cca.2011.08.019. [DOI] [PubMed] [Google Scholar]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Wilson’s disease. J Hepatol. 2012;56:671–685. doi: 10.1016/j.jhep.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Ferenci P, Caca K, Loudianos G, et al. Diagnosis and phenotypic classification of Wilson disease. Liver Int. 2003;23:139–142. doi: 10.1034/j.1600-0676.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- Masełbas W, Chabik G, Członkowska A. Persistence with treatment in patients with Wilson disease. Neurol Neurochir Pol. 2010;44:260–263. doi: 10.1016/s0028-3843(14)60040-2. [DOI] [PubMed] [Google Scholar]
- Ravin HA. An improved colometric enzymatic assay of ceruloplasmin. J Lab Clin Med. 1961;58:161–168. [PubMed] [Google Scholar]
- Roberts EA, Schilsky ML. Diagnosis and treatment of Wilson’s disease: an update. Hepatology. 2008;47:2089–2111. doi: 10.1002/hep.22261. [DOI] [PubMed] [Google Scholar]
- Twomey PJ, Viljoen A, House IM, et al. Copper:caeruloplasmin ratio. J Clin Pathol. 2007;60:441–442. doi: 10.1136/jcp.2006.041756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twomey PJ, Viljoen A, House IM, et al. Relationship between serum copper, ceruloplasmin, and non-ceruloplasmin-bound copper in routine clinical practice. Clin Chem. 2005;51:1558–1559. doi: 10.1373/clinchem.2005.052688. [DOI] [PubMed] [Google Scholar]
- Walshe JM. Serum ‘free’ copper in Wilson disease. QJM. 2012;105:419–423. doi: 10.1093/qjmed/hcr229. [DOI] [PubMed] [Google Scholar]
- Walshe JM. The pattern of urinary copper excretion and its response to treatment in patients with Wilson’s disease. QJM. 2011;104:775–778. doi: 10.1093/qjmed/hcr073. [DOI] [PubMed] [Google Scholar]