Abstract
BACKGROUND
Although virologically confirmed dengue fever has been recognized in Jeddah, Saudi Arabia, since 1994, causing yearly outbreaks, no proper seroepidemiologic studies on dengue virus have been conducted in this region. Such studies can define the extent of infection by this virus and estimate the proportion that may result in disease. The aim of this study was to measure the seroprevalence of past dengue virus infection in healthy Saudi nationals from different areas in the city of Jeddah and to investigate demographic and environmental factors that may increase exposure to infection.
METHODS
Sera were collected from 1984 Saudi subjects attending primary health care centers in six districts of Jeddah. These included general patients of various ages seeking routine vaccinations, antenatal care or treatment of different illnesses excluding fever or suspected dengue. A number of blood donors were also tested. Serum samples were tested by enzyme immunoassay (EIA) for IgG antibodies to dengue viruses 1, 2, 3, 4. A questionnaire was completed for each patient recording various anthropometric data and factors that may indicate possible risk of exposure to mosquito bites and dengue infection. Patients with missing data and those who reported a history of dengue fever were excluded from analysis, resulting in a sample of 1939 patients to be analyzed.
RESULTS
The overall prevalence of dengue virus infection as measured by anti-dengue IgG antibodies from asymptomatic residents in Jeddah was 47.8% (927/1939) and 37% (68/184) in blood donors. Infection mostly did not result in recognizable disease, as only 19 of 1956 subjects with complete information (0.1%) reported having dengue fever in the past. Anti dengue seropositivity increased with age and was higher in males than females and in residents of communal housing and multistory buildings than in villas. One of the six districts showed significant increase in exposure rate as compared to the others. Availability of public sewage was associated with lower infection at a nearly significant level. No other clear risk factors were identifiable. Infection was not related to travel abroad.
CONCLUSIONS
Our results indicate a relatively high exposure of Jeddah residents to infection by dengue viruses, which must be considered endemic to this region. Infection largely remained asymptomatic or was only associated with minor illness for which patients did not seek treatment. These results call for continued vigilance for clinical cases of dengue that may arise from this wide exposure. They also call for more extensive control efforts to reduce exposure to and transmission of dengue viruses.
Keywords: dengue, seroepidemiology, infection
Introduction
Dengue fever is the most common arboviral (arthropod-transmitted) disease of mankind. Around 2.5 billion people in the world are at risk of infection. More than 100 countries are affected by dengue outbreaks.1 An estimated 390 million infections occur annually in the world, of which 96 million have some clinical manifestations.2 This includes about 250,000 cases with the significant and severe manifestations of dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS). The global incidence of DHF/DSS has increased more than 500-fold in recent years.3 While these statistics reflect the major health impact of dengue, they also indicate that the majority of people infected with dengue viruses remain asymptomatic. This aspect has prompted us to estimate the extent of asymptomatic infection by dengue viruses in the Jeddah region of Saudi Arabia. Historical description of dengue-like illness (breakbone fever) suggests that dengue has occurred in this region for several decades. Virologically-confirmed dengue was discovered when type 2 dengue virus was isolated from a fatal case of dengue hemorrhagic fever in Jeddah in 1993.4 Since then, many laboratory confirmed cases have been reported. The Ministry of Health Statistical Records indicate a total of 20,034 reported clinical cases in Saudi Arabia between 2004 and 2013, mostly in the cities of Jeddah and Mecca on the Western coast of the country,5–10 confirming the endemicity and yearly occurrence of dengue.
The aim of this study was to measure the seroprevalence of dengue virus infection in healthy asymptomatic Saudi nationals, male and female, of different ages and professions residing in different areas of the city of Jeddah. Understanding the epidemiology of the disease is essential in guiding effective control measures and in determining vaccination strategy once a dengue vaccine becomes available.
Materials and Methods
Subjects and questionnaire
The total population of the study was 1984 subjects of Saudi nationality attending six primary health care centers in various districts of Jeddah (total population around 3.5 million). These included students from elementary, middle, and secondary schools and university, adult females seeking antenatal care, and general patients of various ages. The mean age was 38.6 years (range 3–80 years, standard deviation ±14.7). The median was 38 years. The subjects were not suspected cases of dengue and did not suffer from fever. The reasons for their visits were for primary care (eg vaccination, antenatal care, etc.) or for illnesses other than dengue fever or similar illnesses. Most patients (1637 out of 1974 with completed information, 83%) had no symptoms. The rest had various conditions (eg diabetes, respiratory or diarrheal disease) but were not suspected of dengue. Forty-five patients were excluded from the statistical analysis. Nineteen of these reported a history of dengue fever while 26 had incomplete questionnaires.
The location of primary health care centers and the number of patients are included in Table 1.
Table 1.
anti-dengue IgG antibody positivity and demographic information of asymptomatic saudi subjects attending primary health care centers.
| VARIABLE | NO. | ANTI-DENGUE IgG | P-VALUE* | ODDS RATIO | (95% CI) | ||
|---|---|---|---|---|---|---|---|
| POSITIVE | % | ||||||
| Total no. studied | 1939 | 927 | 47.8 | 0.001 (Male > female) | 1.374 | (1.139–1.658) | |
| Male** | 656 | 348 | 53.0 | ||||
| Female | 1280 | 577 | 45.1 | ||||
| Age (years) | |||||||
| <11 | 11 | 3 | 27.3 | 0.000 (compared to age < 11 y) | |||
| 11–20 | 237 | 76 | 32.1 | 1.259 | (0.325–4.878) | ||
| 21–30 | 399 | 156 | 39.1 | 1.712 | (0.447–6.552) | ||
| 31–40 | 462 | 197 | 42.6 | 1.982 | (0.519–7.568) | ||
| 41–50 | 429 | 228 | 53.1 | 3.025 | (0.792–11.557) | ||
| >50 | 366 | 244 | 66.7 | 5.333 | (1.390–20.462) | ||
| District of health center | |||||||
| –Kilo 14 | 828 | 391 | 47.2 | 0.004 (Aziziah > other districts) | 1.326 | (1.093–1.607) | |
| –Al-Montazaht | 204 | 101 | 49.5 | ||||
| –Al-Amir abdulmajeed | 52 | 17 | 32.7 | ||||
| –Al-Aziziah | 591 | 312 | 52.8 | ||||
| –Al-Matar algadeem | 148 | 65 | 43.9 | ||||
| –Al-Bawadi | 11 6 | 41 | 35.3 | ||||
| Type of housing | |||||||
| Villa | 61 | 15 | 24.6 | 0.004 (compared to villa) | |||
| Apartment | 1177 | 567 | 48.2 | 2.795 | (1.569–4.978) | ||
| Communal housing | 581 | 292 | 50.3 | 3.052 | (1.694–5.497) | ||
| Collective housing | 49 | 26 | 53.1 | 3.391 | (1.529–7.523) | ||
| Employment | 422 | 208 | 49.3 | 0.227 | NS | ||
| Non-employment | 1214 | 557 | 46.0 | ||||
| Travel outside saudi Arabia | No | 221 | 111 | 50.2 | 0.022 (did not travel > travelled | 1.869 | (1.092–3.198) |
| Yes | 77 | 27 | 35.0 | ||||
Notes:
(Significance: P < 0.05).
Gender information missing for three patients.
Official governmental approval for the collection of samples was secured by writing to the Deputy Director of Health of the Jeddah Municipality and ethical approval was obtained from the Ethical Committee at King Fahd Medical Research Center of the King Abdulaziz University, Jeddah. A patient consent form was completed for each patient. This work complies with the Declaration of Helsinki.
Questionnaires were completed for 1958 patients. The measured variables were sex, age, occupation, residence location, type of housing, water supply, water storage, stagnant water bodies in the house and vicinity, sewage drainage, history of dengue fever or other ailments, exposure to mosquitoes, mosquito protection measures, mosquito control activities, travel abroad, and dengue-specific health education.
In addition, samples were collected from 184 anonymous Saudi blood donors at King Abdulaziz University Hospital, but no questionnaire was conducted.
Specimen collection and testing
About 5 mL of blood was collected in plain Vacutainer tubes. Blood was allowed to clot at room temperature for half an hour and the specimens were centrifuged at 3000 rpm for 20 minutes. Serum was transferred to 5 mL screw cap containers and stored at −20°C until tested. Frozen samples from the primary health care centers were shipped in ice boxes to the main lab at King Fahd Medical Research Center (KFMRC) for further storage and testing.
Serum samples were tested by enzyme immunoassay (EIA) for anti-dengue virus IgG antibodies to dengue viruses 1, 2, 3 and 4, using Dengue IgG Indirect ELISA Test kits manufactured by Panbio (Inverness Medical/Panbio Australia). This kit is for the purpose of qualitative detection of IgG antibodies to dengue virus serotypes 1, 2, 3 and 4 in serum. It is the recommended kit for detection of any past exposure to dengue. It has high sensitivity for detecting low concentration of IgG antibodies. Compared to the reference method of hemagglutination inhibition, the kit is reported by the manufacturer to have a sensitivity of 87% for primary dengue and 97.9% for secondary dengue and a specificity of 100% with negative sera. The indirect ELISA kit is to be distinguished from another kit made by the same company, Dengue IgG Capture ELISA, which detects only higher concentrations of the IgG antibodies (equivalent to hemagglutination inhibition titer of 1:2560) and is mainly used for the diagnosis of secondary dengue virus infection in patients with clinical symptoms of dengue.
Data entry and analysis
Data analysis was by the Statistical Package for Social Studies, Version 22 (IBM-SPSS PC+ version 22, SPSS Inc). Descriptive statistics, χ2 test, odds ratio, and binary logistic regression analysis were used. Odds ratio and 95% confidence intervals (CI) were calculated for variables with significant differences.
Results
Table 1 indicates the result of the anti-dengue IgG test in 1939 subjects attending primary health care centers. These subjects were not suspected of dengue or dengue-like illnesses and did not report any history of dengue. The percentage of dengue IgG positive subjects was 47.8%.
Prevalence of dengue IgG antibody was significantly higher in males than females (P < 0.001, odds ratio 1.374). Prevalence of anti-dengue IgG in different age groups is indicated in Table 1 and Figure 1. The prevalence of anti-dengue IgG was significantly increased with increasing age as compared to children less than 11 years old.
Figure 1.
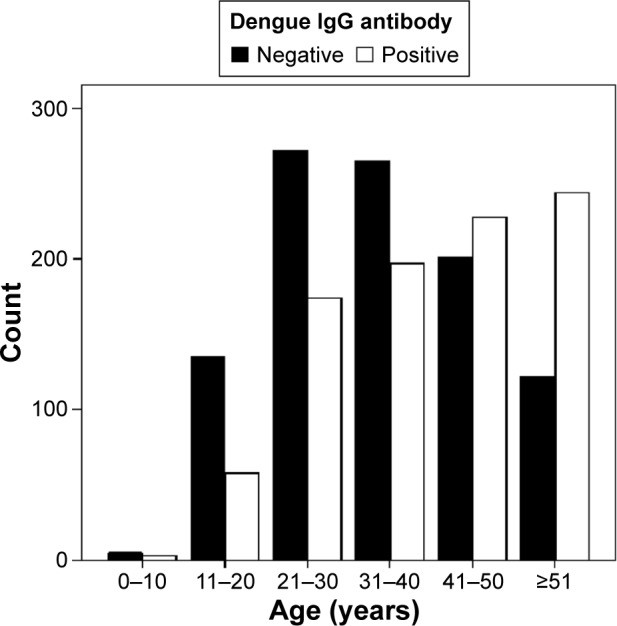
Age-related prevalence of anti-dengue IgG.
Residents of communal and collective housing had significantly higher anti-dengue seroprevalence, followed by residents in apartments as compared with residents in villas, who had the lowest prevalence. However, no significant differences were found according to the number of members or number of rooms in each housing arrangement (χ2 0.225, 0.807 respectively). Among the six locations of primary health centers where samples were collected, only Al-Aziziah district showed a significantly higher seroprevalence as compared to the five other locations.
Out of 298 subjects who were questioned about travel, dengue IgG was positive in 111/221 (50.2%) who had not travelled outside Saudi Arabia compared to 27/77 (35%) who had travelled outside the country, indicating that infection was mostly acquired locally (P = 0.022, OR 1.869, 95% CI 1.092–3.198).
Table 2 indicates the association of different environmental variables with seropositivity to dengue. These variables are grouped under four categories: availability of municipal water and sewage, presence of open water tanks or other sources of stagnant water in the house or surrounding areas, frequency of mosquito biting, and mosquito control efforts. Only the availability of a municipal sewage system was nearly significantly associated (P = 0.052) with a lower dengue infection. On the other hand, mosquito spraying in the house by municipal workers was significantly associated with a higher rate of dengue seropositivity. Our interpretation of this finding is found in the Discussion section below.
Table 2.
Association of different environmental variables with the prevalence of anti-dengue IgG antibodies.
| VARIABLE | NO. | ANTI-DENGUE IgG | P-VALUE* | SIGNIFICANCE | ||
|---|---|---|---|---|---|---|
| POSITIVE | % | |||||
| A) Municipal water and sewage | ||||||
| Availability of municipal water | Yes | 1405 | 680 | 48.4 | 0.124 | NS |
| No | 452 | 200 | 44.2 | |||
| Regular availability of municipal water | Yes | 732 | 355 | 48.5 | 0.525 | NS |
| No | 891 | 418 | 46.9 | |||
| Availability of public sewage | Yes | 586 | 261 | 44.5 | 0.052 No > yes or 1.210 (95% Cl 0.996–1.471) | Sig? |
| No | 1290 | 637 | 49.4 | |||
| Overflow of sewage water | Yes | 654 | 320 | 48.9 | 0.435 | NS |
| No | 1140 | 536 | 47.0 | |||
| B) Open water systems | ||||||
| Water storage in tanks and containers in house | Yes | 1041 | 510 | 49.0 | 0.524 | NS |
| No | 654 | 310 | 47.4 | |||
| Coverage of water containers | Yes | 1006 | 493 | 49.0 | 0.111 | NS |
| No | 103 | 42 | 40.8 | |||
| Any stagnant water in house | Yes | 158 | 77 | 48.7 | 0.085 | NS |
| No | 1159 | 578 | 49.9 | |||
| Presence of uncovered flower vases | Yes | 83 | 42 | 50.6 | 0.405 | NS |
| No | 1708 | 827 | 48.4 | |||
| Presence of stagnant water in fountains or swimming pools | Yes | 73 | 35 | 47.9 | 0.902 | NS |
| No | 1726 | 825 | 47.8 | |||
| Presence of open water tanks in nearby construction sites | Yes | 27 | 10 | 37.0 | 0.517 | NS |
| No | 1148 | 553 | 44.3 | |||
| Water accumulation from air conditioners | Yes | 138 | 64 | 46.4 | 0.436 | NS |
| No | 809 | 379 | 46.8 | 0.474 | ||
| Stagnant water in animal pits | Yes | 27 | 10 | 37.0 | 0.404 | NS |
| No | 1513 | 739 | 48.8 | |||
| Car tire in yard | Yes | 27 | 12 | 44.4 | 0.701 | NS |
| No | 1425 | 694 | 48.7 | |||
| Stagnant water outside house | Yes | 36 | 14 | 38.9 | 0.501 | NS |
| No | 959 | 454 | 47.3 | |||
| C) Mosquito biting | ||||||
| Presence of window screens | Yes | 615 | 300 | 48.8 | 0.16 2 | NS |
| No | 285 | 120 | 42.1 | |||
| Frequent mosquito biting | Yes | 1384 | 667 | 48.2 | 0.739 | NS |
| No | 505 | 239 | 47.3 | |||
| Presence of mosquitoes inside house | Yes | 1214 | 591 | 48.7 | 0.365 | NS |
| No | 673 | 313 | 46.5 | |||
| Presence of mosquitoes outside house | Yes | 1254 | 616 | 4 9.1 | 0.316 | NS |
| No | 592 | 276 | 46.6 | |||
| D) Mosquito control | ||||||
| Insecticide spraying (fogging) in street | Yes | 659 | 309 | 46.9 | 0.823 | NS |
| No | 1206 | 572 | 47. 4 | |||
| Insecticide spraying inside house by municipality workers | Yes | 1121 | 558 | 49.8 | 0.029 Yes > no or 1.216 (95 Cl 1.012–1.462) | Significant |
| No | 768 | 343 | 44.7 | |||
| Frequency of mosquito control awareness or education | Yes | 1163 | 568 | 48.8 | 0.268 | NS |
| No | 695 | 321 | 46.2 | |||
Note:
Significance: P < 0.05.
Abbreviation: NS, not significant.
In addition to asking subjects whether they had previous dengue fever infection, they were asked if they suffered from diabetes, liver disease, heart disease, kidney disease, thyroid disease, or any other disease. No significant association with seropositivity was observed (P = 0.267).
Anti-dengue IgG was also tested in 184 Saudi blood donors, of whom 68 (36.95%) were found positive.
Discussion
The main significant finding of the present study is the high prevalence of infection (47.8%) with dengue viruses in the city of Jeddah, Saudi Arabia. While clinical cases are now frequently diagnosed and reported in this city and neighboring areas (eg Mecca), the extent of infection by dengue viruses in this region has not been reported. The current work indicates widespread infection by these viruses and supports the conclusion that dengue is currently endemic in this region. This calls for more vigilance for the diagnosis of clinical dengue and the study of factors that affect the balance between asymptomatic and clinically significant infections.
While measurement of anti-dengue IgG antibody may reflect infection in the past several months or years, it may not detect ongoing or very recent infection at the time of sample collection, i.e. until IgG increases to a detectable level. This may require testing for anti-dengue IgM antibodies. However, the interval between IgM and IgG appearance is expected to be very short (a few days to a few weeks) compared to the total period during which IgG remains stable in the blood (up to many years). Therefore, the expected effect of this point on the result of the study would be small if not negligible.
Of several possible risk factors, only male sex, older age and communal and multistory housing could be identified as significant. Higher prevalence amongst males may reflect the tendency of males to spend more time outside the house, which may increase their exposure to mosquito biting, or the custom for females in this region to cover their bodies more extensively, reducing exposure to mosquito biting. The increased seroprevalence with older age most likely reflects the increased chance of infection with time rather than higher susceptibility of older people. We also observed a significant association between anti-dengue IgG and mosquito spraying in the house by municipal workers. This may reflect targeted house spraying by the municipality in areas from which clinical dengue cases are reported.
One district out of the six tested had a significantly higher infection rate. This calls for inspection of factors that may have led to this increase. For reasons unknown to us, this study did not show a significant association between several factors related to water storage or mosquito activity and dengue infection. We consider it possible that the high incidence of infection may have masked the identification of some specific factors. Ghaznawi et al reported a significant association in 1995 between dengue fever cases and construction sites near the affected patients’ houses.11 This was attributed to the presence of open water tanks at these sites. Our study indicates that this may no longer be a major risk factor at the present time. Most houses and apartment buildings in Jeddah have two water reservoirs, a lower one underground in the yard and an upper one on the roof. The larger underground tank is usually built mostly from concrete or fiberglass. The smaller one, or several smaller ones, were in the past usually made from concrete, but most are now fiberglass. Municipal water supply is stored in these smaller tanks and the underground tank. During shortages of municipal water supply, water is supplemented by potable water trucks. In addition, most houses hold an underground septic tank. Septic tanks are emptied by sewage trucks in areas of the city where public sewers are not yet present.
Jeddah holds a special importance due to its close proximity to Mecca and surrounding holy sites, so that it is the main entry point for pilgrimage to these sites. Endemic diseases in this area are thus of concern to pilgrims as well as to residents. The population of Jeddah is multiethnic, as pilgrims from many Islamic countries, especially from the Middle East, East Asia, and Africa, have settled in and intermixed with the local inhabitants from the Arabian peninsula and Yemen.
The high level of dengue IgG positivity found in this study is not unique. Similar high rates have been reported from many countries in which dengue is endemic. Burke et al reported in 1988 that 50% of the children in Bangkok had antibodies to dengue by the age of 7 years.12 Yamashiro et al reported in 2004 that 98% of blood donors and 56% of children in the Dominican Republic were positive for dengue IgG.13 Balmaseda et al reported an overall seroprevalence of 91% among children in urban Nicaragua in 2006.14 Yew et al reported that 59% of adults in Singapore had anti-dengue IgG indicating past infection.15 In the Aseer and Jazan regions in Southern Saudi Arabia, Al-Azraqi et al found a seroprevalence of anti-dengue IgG of 31.7% in 2013.16
The presumed high level of dengue infection suggested by the high IgG seropositivity relative to the number of confirmed clinical dengue cases reported in Jeddah strongly suggests that the vast majority of infections are either asymptomatic or cause minor symptoms that do not prompt medical attention or require hospitalization. Still, it cannot be ruled out that some clinically diagnosed dengue cases are not reported. This is not unexpected since inapparent dengue infection has been generally recognized and widely reported. Thus, Endy et al reported that the majority of dengue infections in children were inapparent or undifferentiated febrile illnesses.17,18 Burke et al reported that 87% of students in Bangkok who became infected by dengue viruses were either asymptomatic or minimally symptomatic.12 Yew et al estimated that only 1 out of 23 individuals recently infected with dengue was reported to the health authority as a clinical case.15 Bhatt et al estimate that of the estimaed 390 million dengue infections per year in the world, only 96 million (24.6%) manifest apparently at any level of clinical or subclinical severity.3 In their study of dengue vaccine efficacy in Latin America, Villar et al (2014) reported that 79.4% of children in their study had seropositive status at baseline for one or more dengue sero-types.19 Even in regions with rare incidence of dengue, eg the United States of America, a serosurvey conducted in Florida in 2009 reported that 5.4% of households had evidence of recent dengue virus infection (Centers for Disease Control and Prevention).20
With the indirect anti-dengue IgG kit used in this study, the manufacturers report a 5–10% level of cross reactivity with sera from patients infected by the related flaviviruses West Nile and Japanese Encephalitis. While we cannot exclude the possibility of cross-reactivity, infection or disease by these two viruses has not been reported or recognized in Jeddah, and further study is required.
Inapparent dengue infection is important in understanding the full picture of dengue burden in a population. Inapparent infection provides a source of virus to mosquitoes and constitutes a fundamental link in the chain of transmission of dengue in a community. In addition, a major point of concern related to existing dengue antibody in the population is its effect on enhancement of subsequent (secondary) infections in future epidemics. A secondary dengue infection results when a person previously infected with one serotype is exposed to a different serotype, and is the single most important risk factor for severe dengue disease (DHF/DSS).2,12 The co-circulation of several dengue serotypes in Jeddah has been reported.21,22 In this regard, it may be pointed out that the global incidence of DHF/DSS in recent years has increased more than 500-fold.4
Finally, the prevalence of anti-IgG antibody is important for assessment of vaccine efficiency when a dengue vaccine becomes available. Hopefully, the presence of previous anti-dengue antibodies may not interfere with vaccine efficacy. In fact, Villar et al reported higher efficacy in dengue seropositive children after vaccination with a tetravalent recombinant dengue vaccine in South America.19
Acknowledgments
We would like to express our sincere thanks to Dr. Ezz Abdulfattah, Dr. Amna Jamjoom, and Mr. Mohammed Saadi for help with statistical analysis. Our thanks are extended to Mr. Esam Al-Arabi for supervising sample collection, Nassrin Badroon for technical help and Samah Alahmadi for secretarial help.
Footnotes
ACADEMIC EDITOR: Wen-Zhe Ho, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1144 words, excluding any confidential comments to the academic editor.
FUNDING: This work was supported by grant 451/142/1431 from King Abdulaziz University, Deanship of Scientific Research. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: GAJ, EIA, MAK, RMR. Analyzed the data: GAJ, EIA, MAK. Wrote the first draft of the manuscript: GAJ. Contributed to the writing of the manuscript: EIA, MAK. Agree with manuscript results and conclusions: GAJ, EIA, MAK, RMR. Jointly developed the structure and arguments for the paper: GAJ, EIA, MAK, RMR. Made critical revisions and approved final version: GAJ. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Knipe DM, Howley PM, editors. Fields Virology. 6th ed. Philadelphia: Walters Klewer/Lippincott, Williams, and Wilkins; 2014. [Google Scholar]
- 2.Bhartt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496:504–7. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyle JL, Harris E. Global spread and persistence f dengue. Annu Rev Microbiol. 2008;62:71–92. doi: 10.1146/annurev.micro.62.081307.163005. http://www.ncbi.nlm.nih.gov/pubmed/18429680. [DOI] [PubMed] [Google Scholar]
- 4.Fakeeh M, Zaki AM. Virologic and serologic surveillance for dengue fever in Jeddah, Saudi Arabia, 1994–1999. Am J Trop Med Hyg. 2001;65:764–7. doi: 10.4269/ajtmh.2001.65.764. [DOI] [PubMed] [Google Scholar]
- 5.Ministry of Health . Statistical Year Book 2005. Riyadh: Saudi Ministry of Health; 2005. [Google Scholar]
- 6.Ministry of Health . Ministry of Health Report 2006. Riyadh: Saudi Ministry of Health; 2007. [Google Scholar]
- 7.Ministry of Health . Statistical Year Book 2007. Riyadh: Saudi Ministry of Health; 2007. [Google Scholar]
- 8.Ministry of Health, Department of Statistics . Health Statistical Year Book 2009. Riyadh: Saudi Ministry of Health; 2010. [Google Scholar]
- 9.Ministry of Health . Statistical Year Book 2012. Riyadh: Saudi Ministry of Health; 2012. [Google Scholar]
- 10.Aziz A-T, Al-Shami SA, Mahyoub JA, Hatabbi M, Ahmad AH, Rawi CS. An update on the incidence of dengue gaining strength in Saudi Arabia and current control approaches for its vector mosquito. Parasites & Vectors. 2014;7:258. doi: 10.1186/1756-3305-7-258. http://www.parasitesandvectors.com/content/7/1/258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghaznawi HI, Al-Khateeb TO, Akbar N, et al. Surveillance for dengue fever in Jeddah. East Mediterr Health J. 1997;3:567–70. [Google Scholar]
- 12.Burke DS, Nisalak A, Johnson DE, et al. A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg. 1988;38(1):172–180. doi: 10.4269/ajtmh.1988.38.172. http://www.ncbi.nlm.nih.gov/pubmed/3341519. [DOI] [PubMed] [Google Scholar]
- 13.Yamashiro T, Disla M, Petit A, et al. Seroprevalence of IgG specific for dengue virus among adults and children in Santo Dominigo, Dominican Republic. Am J Trop Med Hyg. 2004;71(2):138–143. [PubMed] [Google Scholar]
- 14.Balmaseda A, Hammond SN, Tellez Y, et al. High seroprevalence of antibodies against dengue virus in a prospective study of schoolchildren in Managua, Nicaragua. Trop Med Int Health. 2006;11(6):935–942. doi: 10.1111/j.1365-3156.2006.01641.x. http://www.ncbi.nlm.nih.gov/pubmed/16772016. [DOI] [PubMed] [Google Scholar]
- 15.Yew YW, Ye T, Wei L, et al. Seroepidemiology of dengue virus infection among adults in Singapore. Ann Acad Med Singapore. 2009;38:667–75. [PubMed] [Google Scholar]
- 16.Al-Azraqi TA, El Mekki AA, Mahfouz AA. Seroprevalence of dengue virus infection in Aseer and Jazan regions, Southwestern Saudi Arabia. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2013;107(6):368–71. doi: 10.1093/trstmh/trt022. [DOI] [PubMed] [Google Scholar]
- 17.Endy TP, Chunsuttiwat S, Nisalak A, et al. Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol. 2002;156(1):40–51. doi: 10.1093/aje/kwf005. http://www.ncbi.nlm.nih.gov/pubmed/12076887. [DOI] [PubMed] [Google Scholar]
- 18.Endy TP, Anderson KB, Nisalak A, et al. Determinants of Inapparent and Symptomatic Dengue Infection in a Prospective Study of Primary School Children in Kamphaeng Phet, Thailand. PLoS. 2011;5(3):e975, 1–10. doi: 10.1371/journal.pntd.0000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villar L, Dayan GH, Arredondo-García JL, et al. Efficacy of a Tetravalent Dengue Vaccine in Children in Latin America. New England J Med. 2015;372:113–123. doi: 10.1056/NEJMoa1411037. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC) Locally acquired Dengue–Key West, Florida, 2009–2010. MMWR Morb Mortal Wkly Rep. 2010;59(19):577–581. [PubMed] [Google Scholar]
- 21.Zaki A, Perera D, Janhan SS, Cardosa MJ. Phylogeny of dengue viruses circulating in Jeddah, Saudi Arabia: 1994 to 2006. Trop Med Int Health. 2008;13:584–92. doi: 10.1111/j.1365-3156.2008.02037.x. [DOI] [PubMed] [Google Scholar]
- 22.Azhar E, Kao M, Niedrig M, et al. Virologic diagnosis of dengue fever in Jeddah, Saudi Arabia: comparison between RT-PCR and virus isolation in cell culture. Journal of Infectious Diseases and Immunity. 2010;2(2):24–29. [Google Scholar]


