Abstract
Plasmacytoid dendritic cells (pDCs) exhibit both innate and adaptive functions. In particular they are the main source of type I IFNs and directly impact T cell responses through antigen presentation. We have previously demonstrated that during experimental autoimmune encephalomyelitis (EAE) initiation, myelin-antigen presentation by pDCs is associated with suppressive Treg development and results in attenuated EAE. Here, we show that pDCs transferred during acute disease phase confer recovery from EAE. Clinical improvement is associated with migration of injected pDCs into inflamed CNS and is dependent on the subsequent and selective chemerin-mediated recruitment of endogenous pDCs to the CNS. The protective effect requires pDC pre-loading with myelin antigen, and is associated with the modulation of CNS-infiltrating pDC phenotype and inhibition of CNS encephalitogenic T cells. This study may pave the way for novel pDC-based cell therapies in autoimmune diseases, aiming at specifically modulating pathogenic cells that induce and sustain autoimmune inflammation.
Keywords: Central nervous system, Plasmacytoid dendritic cells, Experimental autoimmune encephalomyelitis, Cell therapy
Highlights
-
•
pDC therapy ameliorates established EAE.
-
•
CNS inflammation is locally modulated after pDC transfer.
-
•
Upon pDC transfer, resting endogenous pDCs are selectively recruited to the CNS via chemerin/CMKLR1 axis.
-
•
Therapeutic pDC injection promotes a tolerogenic environment and inhibits encephalitogenic T cells in the CNS.
1. Introduction
Plasmacytoid dendritic cells (pDCs) are the main producers of type I interferons (IFN-I) in response to foreign nucleic acids, thereby indirectly influencing immunity. They can also differentiate into antigen presenting cells (APCs) to directly stimulate and modulate T cell responses [1]. pDCs have been implicated in the pathogenesis of many human inflammatory diseases and their corresponding mouse models. For example, chronic pDC activation and subsequent IFN-I production can promote autoimmune diseases, such as lupus erythematosus (SLE) [2] and psoriasis [3].
Multiple Sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS). In MS patients, pDCs are present in the cerebrospinal fluid (CSF), leptomeninges and demyelinating lesions [4], [5]. The exact role of pDCs in the pathogenesis of MS is controversial, as they might have both pathogenic and protective functions. In one hand, pDC-derived cytokines, including type-I IFN and IL-6 induces pro-pathogenic Th1 and Th17 cells, which are implicated in MS pathogenesis [6], [7]. On the other hand, pDCs can exert a protective role in MS through the production of type-I IFN [8]. For instance, relapsing patients treated with IFN-β exhibit reduced disease severity [9]. In experimental autoimmune encephalomyelitis (EAE), the rodent model of MS, IFN-β and IFNAR-deficiency exacerbates disease severity [10], [11]. Furthermore, one study suggests that pDCs inhibit pro-pathogenic conventional DCs (cDCs) functions in the CNS and, consequently locally inhibit encephalitogenic Th17 cells [12]. In addition to a controversial local role in the CNS, pDCs have also been implicated in the modulation of autoimmune Th1 and Th17 priming in secondary lymphoid organs (SLOs) during early phases of EAE development. It was suggested that pDCs promote Th17 priming [13], whereas we have demonstrated that the selective abrogation of MHCII expression by pDCs leads to increased MOG-specific Th1 and Th17 and impaired Treg proliferation. As a result, lack of MHCII on pDCs correlates with exacerbated EAE [14].
Thus, although many questions remain to be elucidated, modulation of pDC functions as an approach to treat MS is currently an axis of intense investigation [15]. We have previously demonstrated that the adoptive transfer of MHCII-sufficient pDCs prior to EAE induction significantly dampened disease severity, whereas MCHII-deficient pDC had no effect [14]. Here we investigated whether and how pDCs may modulate the course of EAE after disease onset. We found that when transferred during acute EAE phase, pDCs led to dramatic disease remission. Protection was pDC-dependent, correlated with reduced CNS inflammation, and decreased encephalitogenic Th1 and Th17 cells. Our results demonstrated that pDC transfer induced the recruitment of resting endogenous pDCs to the CNS via a chemerin dependent mechanism, and confer a tolerogenic environment, which, together with local myelin peptide delivery, inhibits encephalitogenic T cells and results in disease improvement. Therefore, modulation of CNS inflammation by pDCs in EAE mice is an interesting axis of investigation to ameliorate disease clinical outcome. Up to date, almost all current therapies in autoimmune diseases are based on the systemic suppression of immune functions and are not curative. Our work reinforces and validates the relevance of emerging therapeutic concepts regarding the use of cell therapies for autoimmune diseases.
2. Materials and methods
2.1. Mice
H2-Aa–/– (MHCII–/–) [16], Ubiquitin-GFP [17], IFN-β–/– [10], IFNAR–/– [18], BDCA2-DTR [19], CD45.1 (Charles River, France) and 2D2 [20] mice were in a C57BL/6 background. WT C57BL/6 mice were purchased from Harlan laboratories. Mice were bred and maintained under SPF conditions at Geneva medical school animal facility and under EOPS conditions at Charles River, France. Bone marrow (BM) chimeric mice were generated as described [14]. All procedures were approved by and performed in accordance with the guidelines of the animal research committee of Geneva.
2.2. EAE induction
Active EAE was induced as described by immunizing mice with 100 μg of MOG35-55 peptide (Biotrend) emulsified in incomplete Freund's adjuvant (BD Diagnosis) supplemented with 500 μg/ml Mycobacterium tuberculosis H37Ra (BD Diagnosis). At the time of immunization and 48 h later, mice also received 300 ng of pertussis toxin (Sigma–Aldrich) into the tail vein. For passive EAE induction, encephalitogenic CD4+ T cells were generated in vitro from LN and spleen cells of 2D2 mice as described [21]. 1–2 × 106 total cells were injected i.p. into recipient mice. Mice received 67 ng of pertussis toxin at the day of cell injection and 48 h later. Mice were monitored daily for disease clinical symptoms, and blindly scored as follows. 1, flaccid tail; 2, impaired righting reflex and hind limb weakness; 3, complete hind limb paralysis; 4, complete hind limb paralysis with partial fore limb paralysis; 5,moribund. In some experiments, EAE mice were treated at indicated time points with α-NETA (Abcam) (10 mg/kg/daily), DT (100 ng/mouse), or 10 μg/mL of MOG35-55 peptide in PBS.
2.3. BM-pDC and cDC generation
cDCs and pDCs were generated as described [22] from BM of WT for cDCs and WT, MHCII–/–, Ubi-GFP, CD45.1, and IFN-β–/– mice for pDCs. For some experiments, pDCs were treated with 1 μg/mL CpG-B (Invivogen) for the last 24 h of culture.
2.4. pDC and cDC adoptive transfer
pDCs and cDCs were generated and purified from BM cell cultures. pDCs were enriched from BM cell cultures after 7 days or purified ex-vivo from BM using a pDC isolation kit (Mylteniy biotec) according to manufacturer's instructions. Purity generally exceeded 95%. cDCs were purified by cell sorting from BM-cell cultures as CD11chiPDCA-1–, using a MoFlowAstrios (Beckman Coulter). cDCs and pDCs were loaded or not with 10 μg/mL MOG35-55 or OVA323-339 peptide and 10 × 106 cells were injected i.v. into EAE recipient mice, 10–12 days after MOG35-55 + CFA immunization.
2.5. Ex vivo DC isolation
DCs were isolated from Lymph nodes (LN), spleen, liver and Spinal cord (SC). by digesting organ fragments with an enzymatic mix containing collagenase D (1 mg/mL) and DNAse I (10 μg/mL) (Roche) in HBSS [14]. For liver and SC, single cell suspensions were further centrifuged through a discontinuous 30:70% percoll (Invitrogen) gradient.
2.6. Antibodies and flow cytometry
Monoclonal antibodies used for flow cytometry were from: Biolegend; anti-CD11c (N418), anti–I-Ab (AF6-120.1), anti-CD11b (M1/70), anti-CD86 (P03), anti-PD-1 (10F.9G2) (PMP1-30); anti-CD8 (53–6.7); anti-VLA-4 (HMB1-1), anti-Ly6C (HK1.4), anti-ICOS-L (HK5.3); from eBioscience: anti-CD4 (GK1.5), anti-CD69 (H1.2F3), VEGFR2 (FLK1), anti-TER119 (TER-119); anti-CMKLR1 (BZ194), Anti-Foxp3 (FJK-16s), anti–IL-17 (ebio17B7), anti-FLT3 (CD135) (A2F10), anti-SIGLEC H (ebio440c); from BD: anti–PDCA-1 (BST-2, CD317), anti-CD45 (30F11), anti-CD16/32 FcyRIII (clone 2.4G2), anti-CD45R/B220 (RA3-6B2), anti-CD19 (1D3), anti-CD3 (145-2C11), anti-c-kit (CD117) (2B8), anti-Sca-1 (D7), Ki67 (B56) and anti–IFN-γ (XMG1.2). Anti-Ly49q (clone 2E6) was from MBL.
For flow cytometry analysis of DCs, single cell suspensions were incubated with FcBlock (anti-CD16/32 FcγRIII) for 10 min, at 4 °C and stained using antibodies against CD11c and PDCA-1 or Siglec-H. cDCs were defined as CD11chiPDCA-1− and pDCs as CD11cintPDCA-1+ or CD11cintSiglec-H+. For hematopoietic progenitor analysis, red cells from BM were lysed with NH4ClNaHCO3 buffer. BM cells were stained with the following biotinylated lineage markers: anti-CD3, anti-B220, anti-CD19, anti-CD11b, anti-TER119, anti- Ly6C followed by streptavidin FITC conjugated staining. LSK BM progenitors were defined as Lin−Sca-1+c-kit+FLT3−. Microglial cells were defined as CD11c−CD11b+ CD45int. Data were acquired in a Cyan™ ADP (Beckman Coulter) and analysed using FlowJo software (Tree Star).
Intracellular cytokine stainings were done with the Cytofix/Cytoperm kit (BD) for IFN-γ and IL-17 staining. Foxp3 staining was performed with the eBioscience kit, according to manufacturer's instructions. Cell proliferation was assessed by flow cytometry using anti–human Ki67 and respective isotype control. For IFN-γ and IL-17 staining, SC, LN and spleen cells were cultured in RPMI containing 10% heat-inactivated fetal bovine serum, 50 mM 2-mercaptoethanol, 100 mM sodium pyruvate, and 100 μM penicillin/streptomycin at 37 °C, 5% CO2. Cells were stimulated for 18 h with PMA/ionomycin and Golgi stop solution (BD) was added to the last 4 h of culture.
2.7. Immunofluorescence microscopy
Mice were transcardiacally perfused with PBS followed by 4% paraformaldehyde (PFA). Cryopreserved SC, post-fixed with 4% PFA and embedded in OCT (Sakura Finetek), were cut into 10 μm-thick sections, and stained using primary antibodies against CD45 and PDCA-1 appropriate species-specific Alexa555-labelled, Cy3-, or Cy5-conjugated secondary antibodies (all from Jackson ImmunoResearch Laboratories, Inc.) with DAPI (Sigma–Aldrich) counterstaining. Sections were mounted with Mowiol fluorescent mounting medium (EMD). Images were acquired with a confocal microscope (LSM 700; Carl Zeiss, Inc.)
2.8. Histological analysis
Mice were transcardiacally perfused with PBS followed by 4% PFA and post-fixed overnight. Brain and SC, dehydrated and embedded in paraffin, were cut into 2 μm thick sections and stained with Mayer's hemalum, differentiated in acidic-alcohol and co-stained with eosin and coverslipped with DePeX mounting medium (Serva Electrophoretics GmbH, Heidelberg, Germany) using Tissue Tek Prisma slide stainer (Sakura Seiki C.o., Nagano, Japan).
2.9. Statistical analysis
Statistical significance was assessed by the two-tailed unpaired Student's t test or 1-way ANOVA with Bonferroni post Hoc test. EAE incidence was analysed using the 2-way ANOVA with Bonferroni post Hoc test, using Prism 5.0 software (GraphPad Software).
3. Results
3.1. Adoptive transfer of pDCs during EAE acute phase inhibits CNS inflammation and induces disease remission
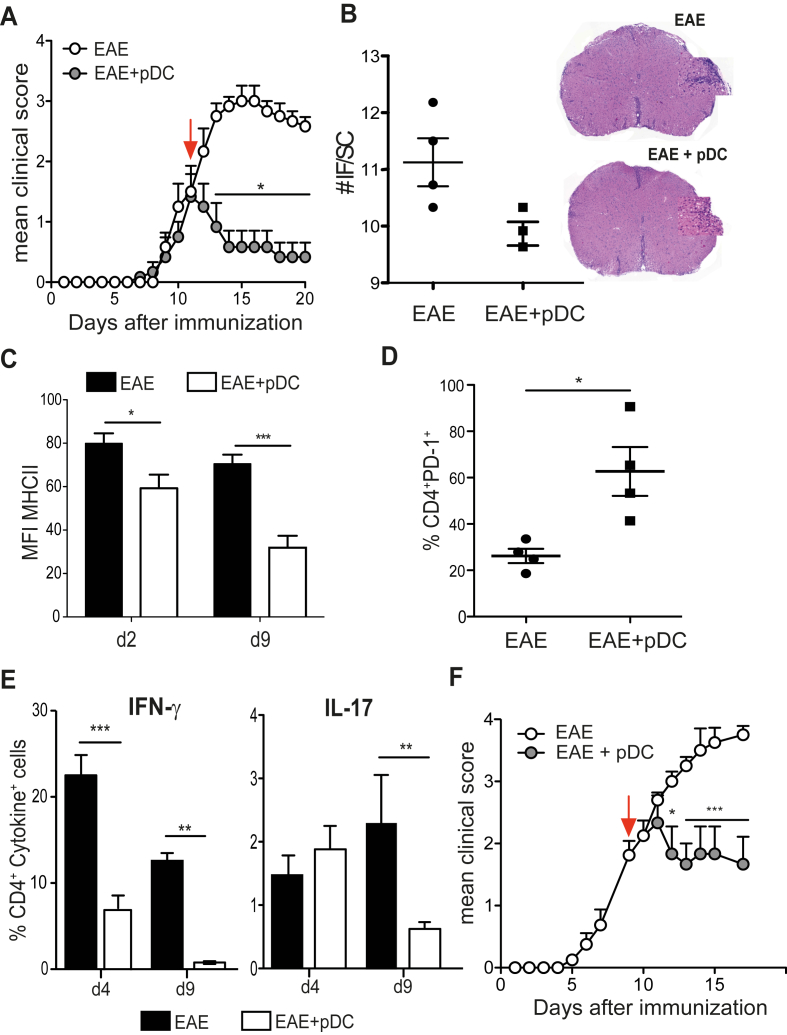
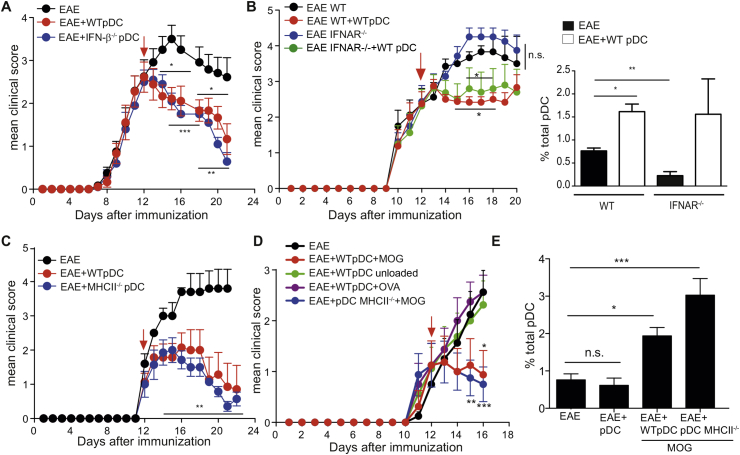
In order to explore a potential therapeutic role for pDCs in EAE, we adoptively transferred in vitro bone marrow (BM) derived WT MOG35-55 loaded pDCs into C57BL/6 mice after disease onset, 12 days after immunization with MOG35-55 + CFA. Strikingly, whereas clinical scores of control non pDC-injected mice continued to rise, those of pDC-injected mice stayed stable for few days post-pDC transfer, and then rapidly decreased, resulting in a significant inhibition of EAE (Fig. 1A). Clinical symptoms almost completely disappeared 10 days after pDC injection (Fig. 1A). Moreover, mice presenting a clinical score of 3 at the time of pDC transfer either significantly recovered or exhibited stabilized disease development, whereas scores of control animals maintained their progression (Supplementary Fig. 1A, B). Consistent with an amelioration of clinical scores, as early as four days post-pDC transfer, inflammatory foci were reduced in spinal cord (SC) of pDC-transferred mice, compared to control EAE mice (Fig. 1B). In agreement with reduced CNS inflammation, microglial cells downmodulated the expression of MHCII molecules (Fig. 1C). In spite of this, four days after pDC transfer, we did not observe any differences regarding the frequency of CD4+ and CD8+ T cells infiltrating the SC (Supplementary Fig. 2A), nor in the expression of VLA-4 by T cells (Supplementary Fig. 2B) between pDC transferred and control EAE mice. Consequently, T cell migration from SLOs to SC was not altered upon pDC transfer. However, the expression of PD-1, a molecule that delivers negative signals to T cells [23], was increased on SC-infiltrating CD4+ T cells one day after pDC transfer (Fig. 1D). Remarkably, encephalitogenic Th1 (at day 4 and 9 after pDC transfer) and Th17 frequencies (at day 9 after pDC transfer) were substantially decreased in SC of pDC-transferred mice (Fig. 1E). These data demonstrate that adoptive transfer of BM-derived pDCs after EAE onset induces a strong and rapid improvement of disease clinical symptoms and CNS inflammation. This effect appeared to be pDC-specific, since transfer of MOG35-55-loaded cDCs did not significantly impact EAE development (Supplementary Fig. 3A). In addition, although most of our experiments were done using 5–10 × 106 of transferred pDCs, a similarly efficient therapeutical effect was observed by using only 1 × 106 cells (Supplementary Fig. 3B). Importantly, adoptively transferred MOG35-55 loaded ex vivo BM resident pDCs efficiently induced EAE amelioration (Fig. 1F), demonstrating that in vivo, terminally differentiated pDCs efficiently inhibit the disease.
Fig. 1.
pDC adoptive transfer during disease acute phase inhibits EAE. EAE was induced in WT mice by immunization with MOG35-55. (A–E) EAE mice were injected i.v. (EAE + pDC) or not (EAE) with MOG35-55-loaded WT BM-pDCs during disease acute phase (arrow). (A) Clinical scores were followed daily. (B) SCs of indicated mice were harvested four days after transfer, processed and stained for H&E. Graph represents the mean number of inflammatory foci (IF) per SC from 3 to 4 individual mice per group (left). Representative SC images (right). (C) MHCII MFI on microglial cells in SC of EAE mice at day 2 and 9 after pDC transfer. Frequencies of (D) PD-1 expressing CD4+ T cells at day 1, and (E) IFN-γ (left panel) and IL-17 producing (right panel) CD4+ T cells in SC at day 4, and day 9 after pDC transfer. (F) EAE clinical scores of WT mice transferred (EAE + pDC) or not (EAE) during EAE acute phase (arrow) with 5 × 106 ex-vivo purified from the BM, MOG35-55-loaded, WT pDCs. (A–F) Data are representative of at least 2 experiments with minimum 5 mice per group. Data represent mean ± SEM. (A and F) 2-way ANOVA with Bonferroni post hoc test; (B–E) Standart two-tailed Student's t test; *P < 0.05; **P < 0.01; ***P < 0.001.
3.2. pDC transfer induces the selective recruitment of endogenous pDCs to the CNS and inhibits EAE effector phase
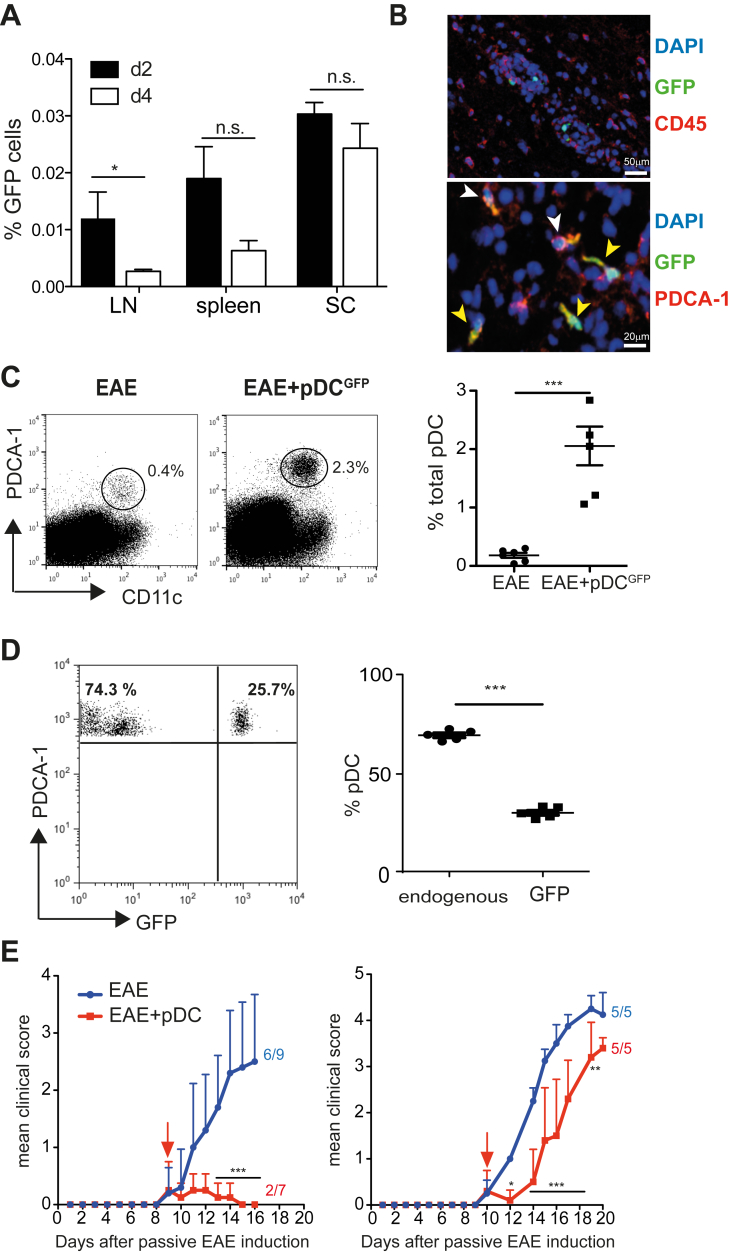
To determine the mechanisms accounting for pDC-mediated EAE amelioration, we analysed the body distribution of injected BM-pDCs derived from Ubiquitin-GFP mice. Two days post-injection, GFP+ pDCs were detectable in LN, spleen and inflamed SC (Fig. 2A). In addition, a very low frequency of GFP+ pDCs was observed in the liver and the BM (data not shown). In situ immunofluorescence staining of SC revealed that, GFP+ pDCs localized within EAE lesions (Fig. 2B, top), expressed the pDC-specific marker PDCA-1 (yellow arrows) and were surrounded by endogenous infiltrating pDCs (white arrows) (Fig. 2B, bottom). While the frequencies of cDCs, macrophages and microglial cells were similar in SC of pDC-transferred and control EAE mice (Supplementary Fig. 4), we observed a massive (six-fold) increase of total pDC frequencies in SC of pDC-transferred EAE mice (Fig. 2C). Injected pDCs (GFP+) represented only 25% of total SC pDCs (Fig. 2D), suggesting that endogenous pDCs were recruited to the SC following exogenous pDC transfer. In agreement, increased endogenous pDC frequencies in SC of pDC-transferred animals did not result from local pDC proliferation (data not shown). Therefore, we hypothesized that they would be recruited from the BM, where pDCs originate from. Accordingly, four days post-pDC transfer into EAE mice, we observed in the BM a substantial increase of total pDC frequencies, with only a minor proportion consisting of transferred GFP+ pDCs (Supplementary Fig. 5A, B). These results suggest that the increase in endogenous pDC frequency could be due to active proliferation or enhanced de novo generation. Ki67 staining revealed a negligible proliferation rate of pDCs in the BM, in both control and pDC-transferred EAE mice (Supplementary Fig. 5C). In contrast, we observed a significant increase in LSK (lineage-negative, SCA1+, c-Kit+) progenitor's frequencies in the BM, one-day post-pDC transfer (Supplementary Fig. 5D), suggesting a general enhancement of hematopoietic cell generation. In line with this notion, both cDC and pDC frequencies were significantly increased in LN, 4 days after pDC-transfer (Supplementary Fig. 5E). Furthermore, at the same time point, BM-resident pDCs, from pDC-transferred mice, exhibited an upregulation of Ly49q, a marker of fully differentiated pDCs [24] compared to control mice (Supplementary Fig. 5F). Altogether, these data show that pDC transfer in EAE mice increases hematopoietic cell generation in the BM, leading to enhanced cDC and pDC frequencies in SLOs, and resulting in the selective recruitment of pDCs to the CNS.
Fig. 2.
pDC transfer induces the selective recruitment of pDCs to SC and inhibits EAE effector phase. (A–D) EAE was induced in WT mice and BM-derived, MOG35-55-loaded, pDCs from Ubi-GFP mice were transferred into mice during EAE acute phase. SC cells from control EAE (EAE) or GFP+pDC transferred EAE (EAE + pDCGFP) mice were analysed after pDC transfer. (A) Graph shows the frequency of CD11cint PDCA-1+ GFP+ transferred pDCs infiltrating the LNs, spleen and SC at indicated time after pDC transfer. (B) Representative confocal images of SC from GFP+pDC transferred mice (day 4), show GFP+ cells among CD45+ cells (top) and PDCA-1 co-localization with GFP+ cells (bottom). Arrows depict PDCA-1+GFP− cells (white) and PDCA-1+GFP+ cells (yellow). (C) Representative FACS profile (left) and quantification (right) of total pDCs in SC from control (EAE) or pDC transferred (EAE + pDCGFP) EAE mice (day 4). (D) Representative FACS profile (left) and quantification (right) of GFP+ (exogenous) and GFP− (endogenous) pDC frequencies among total pDCs in SC (day 4). (A–D) Data are representative of 4 independent experiments with 6–8 mice/group. (E) EAE was induced in WT mice by adoptive transfer of 1 × 106 (left) or 2 × 106 (right) effector MOG35-55-specific 2D2 transgenic CD4+ T cells. BM-derived WT pDCs were transferred i.v. into EAE mice at the appearance of clinical symptoms (arrows). Clinical scores were followed daily. Incidence is indicated for each group. Data are representative of 2 experiments with at least 5 mice/group. Data represent mean ± SEM. (A, C and D) Standard two-tailed Student's t test; (E) 2-way ANOVA with Bonferroni post hoc test * P < 0.05; **P < 0.01; ***P < 0.001.
Increased pDC frequencies in SC of EAE mice following pDC transfer suggests that these cells might play a protective role locally in the CNS. To test this hypothesis, we induced passive EAE by injecting in vitro differentiated MOG35-55-specific 2D2 CD4+ T cell effectors [14]. Upon both mild (1 × 106 2D2 effectors, Fig. 2E, left) and strong (2 × 106 2D2 effectors, Fig. 2E, right) EAE induction, pDCs injected at early disease clinical symptoms onset were able to inhibit disease development, with significantly reduced EAE incidence and clinical scores. These results demonstrated that pDC transfer inhibits CNS-local effector phase of EAE.
3.3. Recruitment of endogenous pDCs to the inflamed CNS mediates EAE protection
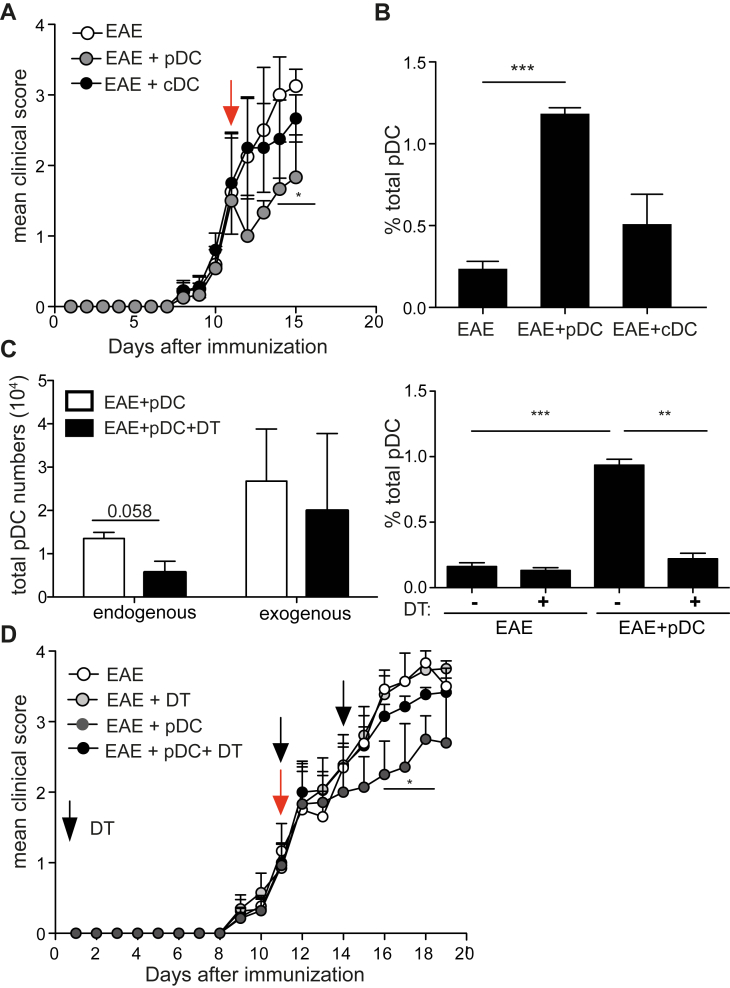
Our data strongly suggests that endogenous pDCs recruited to the CNS following pDC transfer are implicated in EAE amelioration. Indeed, exogenous MOG35-55 loaded cDCs, which did not significantly ameliorate EAE upon transfer (Supplementary Fig. 3A; Fig. 3A), also did not induce endogenous pDC recruitment to SC (Fig. 3B). To firmly demonstrate that endogenous pDC recruitment to SC was necessary for disease amelioration following exogenous pDC transfer, we used a genetic mouse model to deplete endogenous pDCs. For this purpose, CD45.1 WT pDCs were transferred into BDCA-2 DTR EAE mice [19], and mice were injected or not with DT at the same time pDCs were transferred. Endogenous pDCs were efficiently depleted in the spleens of DT-treated mice (not shown) [25]. However, exogenous (CD45.1+) pDC numbers reaching the SC were not affected (Fig. 3C, left), but as a consequence of BDCA-2 DTR pDC depletion, endogenous pDC recruitment to the SC was substantially impaired in DT treated, compared to untreated animals (Fig. 3C, right). Importantly, EAE amelioration following MOG35-55 loaded pDC transfer was totally abrogated in DT treated mice (Fig. 3D). Therefore, endogenous pDC recruitment to SC after pDC transfer is mandatory to confer EAE protection.
Fig. 3.
Endogenous pDC recruitment to the SC following pDC transfer is mandatory for EAE protection. (A, B) EAE was induced in WT mice and BM-derived, MOG35-55-loaded, WT pDCs (EAE + pDC) or cDCs (EAE + cDC) were transferred or not (EAE) into mice during disease acute phase (arrow). (A) Clinical scores were followed daily. (B) Total pDC frequencies were analysed in SC of EAE mice four days after cell transfer. (C, D) EAE was induced in BDCA2-DTR:WT BM chimeric mice and BM-derived, MOG35-55-loaded, CD45.1 WT pDCs were transferred into EAE mice during EAE acute phase (D, red arrow, day 11). When indicated, mice were also injected with DT (D, black arrows, day 11 and day 14 after EAE induction). (C) Endogenous (gated on CD45.2 cells) and exogenous (gated on CD45.1 cells) pDC numbers (left) and total pDC frequencies (right) were analysed in SC of EAE mice four days after pDC transfer. (D) Clinical scores were followed daily. (A–D) Data are representative of at least 2 independent experiments with 8 mice per group. Data represent mean ± SEM. (A and D) 2-way ANOVA with Bonferroni post hoc test; (B, C right panel) 1-way ANOVA with Bonferroni post hoc test *P < 0.05; **P < 0.01; ***P < 0.001.
3.4. Chemerin-CMKLR1 axis mediates endogenous pDC recruitment to the CNS following pDC transfer
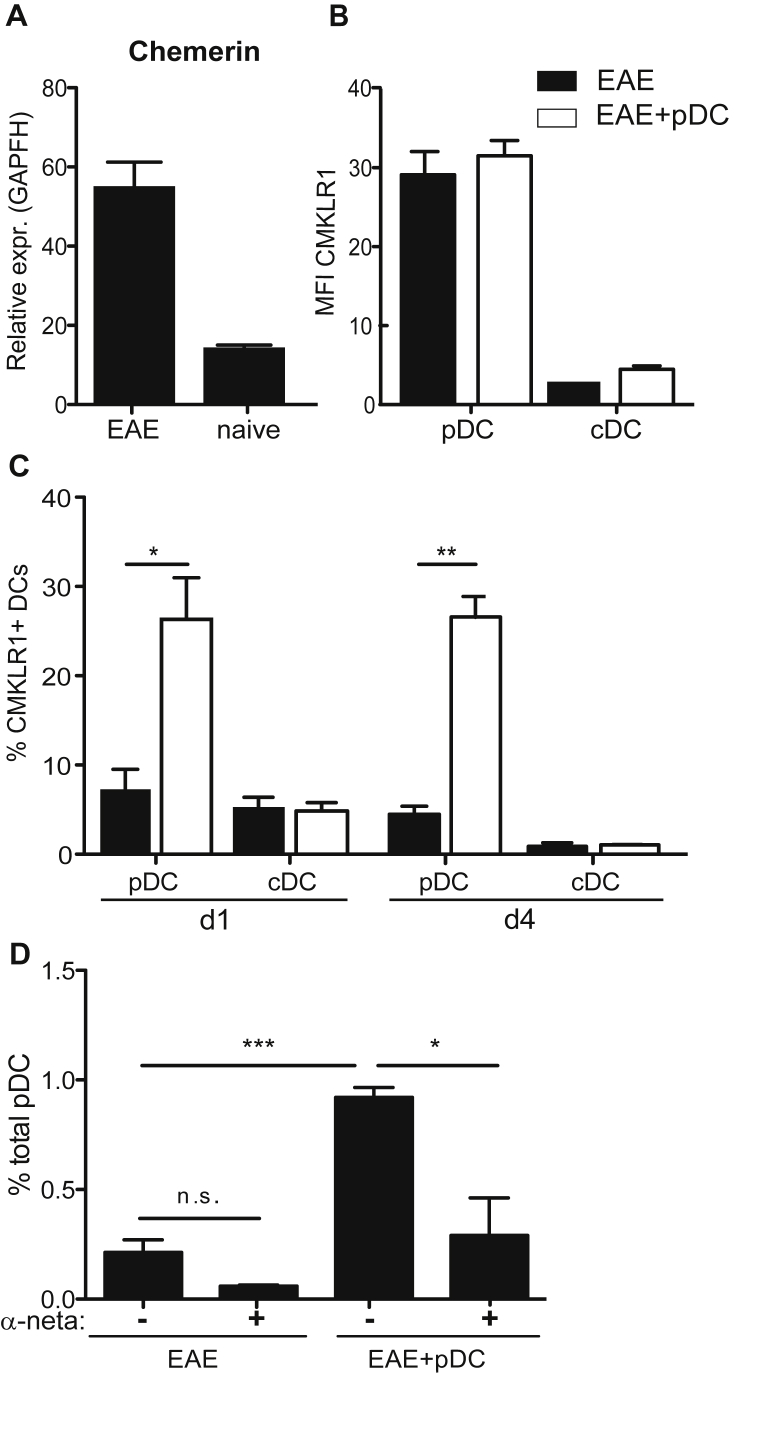
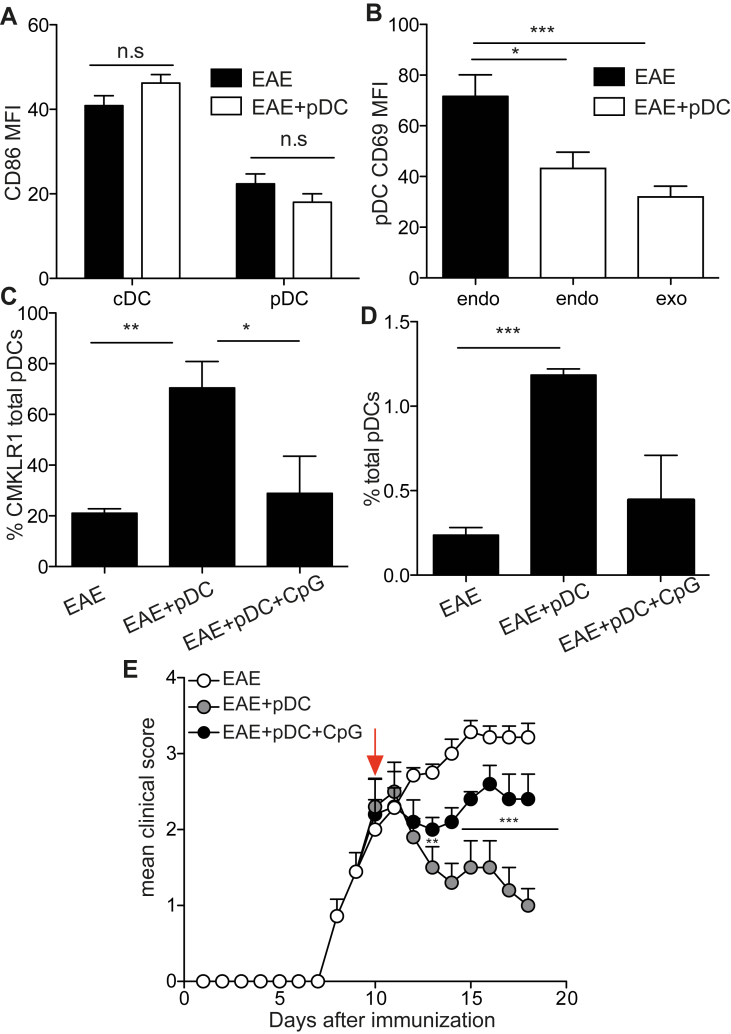
Although a specific receptor mediating pDC migration to inflamed tissues has not yet been identified, different chemokines and chemotactic factors, as well as its receptors have been related to pDC homing to peripheral tissues under pathological conditions [26]. In order to identify possible mechanism(s) responsible for pDC recruitment to CNS, we analysed the implication of Chemerin/CMKLR1 (chemerin receptor), an axis linked to pDC migration in humans [27]. First, and as previously observed [28], we found that SC of EAE mice contained increased chemerin mRNA levels, compared to naive SC (Fig. 4A). In addition, in spleens of EAE mice, pDCs expressed high levels of CMKLR1, compared to cDCs, irrespective of pDC transfer (Fig. 4B). Interestingly, the frequency of pDCs expressing CMKLR1 was substantially increased in SC as early as one day after pDC transfer, and was sustained four days after transfer (Fig. 4C). In contrast, CMKLR1 expression remained low and unaltered on SC infiltrating cDCs from both pDC-transferred and control EAE mice (Fig. 4C). In order to test a possible involvement of the Chemerin/CMKLR1 axis in the recruitment of endogenous pDCs to the SC following pDC transfer, we treated pDC transferred or control EAE mice with a CMKLR1 small molecule antagonist, α-naphthoyl ethyltrimethylammonium iodide (α-NETA), known to block CMKLR1+ cell migration [29]. α-NETA treatment, for 3 consecutive days following pDC transfer, significantly blocked pDC recruitment to SC of EAE mice (Fig. 4D). These results demonstrate that pDC recruitment to SC following pDC transfer is mediated by the chemerin-CMKLR1 axis. However, given that this molecule not only interferes with pDC homing but also affects migration of additional key effector cells in EAE, such as macrophages and microglial cells (Supplementary Fig. 6) [29], we could not use this experimental approach to evaluate the impact of blocking endogenous pDC recruitment to the CNS on disease evolution.
Fig. 4.
Chemerin-CMKLR1 axis mediates pDC recruitment to SC. EAE was induced or not in WT mice and BM-derived, MOG35-55-loaded, pDCs were transferred (EAE + pDC) or not (EAE) into mice during EAE acute phase. (A) Chemerin mRNA levels were analysed in SC of naïve and EAE mice day 12 after immunization. (B, C) CMKLR1 expression levels by cDCs and pDCs (B) in spleens one day after pDC transfer and (C) in SC one and four days after pDC transfer. (B) Graphs show the MFI and (C) the frequency of expressing cells. (D) EAE was induced in WT mice and WT BM-pDCs were transferred or not into mice during EAE acute phase. α-NETA was injected i.p. or not one day following pDC transfer for 3 consecutive days. Frequency of total pDCs was analysed in SC 4 days after pDC transfer. (A–D) Data are representative of 2 independent experiments with 4 mice per group each. Data represent mean ± SEM.(A–C) Standard two tailed Student's t test; (D) 1-way ANOVA with Bonferroni post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001.
3.5. Transferred pDCs provide myelin antigen and modulate pDC activation state in the CNS
pDCs are important producers of type-I IFN upon viral infections. In addition, IFN-β is widely used as a treatment for MS. However, the precise mechanisms involved in IFN-β mediated disease amelioration are still incompletely understood. In order to check whether IFN-β was involved in pDC-mediated protection in EAE, we transferred IFN-βko or WT MOG35-55 loaded pDCs into EAE recipient mice. Both WT and IFN-βko pDCs mediated the same level of disease inhibition, thus excluding a role for IFN-β production by exogenous pDCs in disease amelioration (Fig. 5A). Similarly, WT pDCs induced the same level of disease amelioration (Fig. 5B, left), as well as a similar increase in SC pDC frequencies (Fig. 5B, right), when transferred into either WT or IFN-I receptor deficient (IFNAR–/–) EAE recipient mice. Altogether, these results firmly rule out a role for IFN-I in disease amelioration induced by pDC transfer. We have previously showed that when transferred prior to EAE induction, pDC-mediated protection was MHCII dependent [14]. However, in post-EAE settings, disease inhibition was as efficient using either MHCII sufficient or deficient pDCs (Fig. 5C), suggesting a MHCII independent role for injected MOG35-55 loaded pDCs in regulating CNS-inflammation. A requirement for MHCII expression by endogenous pDCs, that are recruited to the SC after pDC injection, was also ruled out, since EAE protection after WT pDC transfer was not altered in mice selectively lacking MHCII expression by pDCs (μMTxpIII + IV–/–:WT BM chimeric mice, as described before) [14] (Supplementary Fig. 7A). Surprisingly, EAE protection was abolished when transferred pDCs were either unloaded or loaded with an irrelevant peptide (OVA323-339), prior to their injection (Fig. 5D). Importantly, in contrast to high peptide concentrations (100–200 μg/mL) that have been described to confer EAE amelioration [30], intravenously injection of 10 μg/mL of MOG35-55 peptide, the concentration used to load pDCs, did not confer any disease improvement (Supplementary Fig. 7B), emphasizing the requirement of pDCs for therapeutic effect. Notably, disease amelioration in EAE mice injected with MOG35-55-loaded WT or MHCII–/– pDCs correlated with pDC recruitment to SC (Fig. 5E). In contrast, pDC frequencies were not increased in SC of EAE mice injected with unloaded pDCs, in which no disease protection was observed (Fig. 5D, E), again supporting the need of endogenous pDC recruitment to SC for pDC therapeutic effect.
Fig. 5.
Transferred pDCs deliver myelin antigen to SC. (A–C) EAE was induced in indicated mice and BM-derived, WT or IFN-β–/– (A), WT (B) WT or MHCII–/– (C) MOG35-55-loaded pDCs were transferred into mice during EAE acute phase (red arrows). Clinical scores were followed daily. (A) EAE clinical scores in WT mice transferred with WT or IFN-β–/– pDCs. (B) EAE clinical scores from WT or IFNAR–/– mice transferred or not with WT pDCs (left). Total pDC frequencies were analysed in SC of mice four days after pDC transfer (right). (C) EAE clinical scores from WT mice transferred or not with WT or MHCII–/– pDCs. Data are representative of 5 independent experiments with 8 mice/group each. (D, E) EAE was induced in WT mice and BM-derived WT or MHCII–/– pDCs, loaded or not with MOG35-55 or OVA323-339 as indicated, were transferred into mice during EAE acute phase. (D) Clinical scores were followed daily. (E) Total pDC frequencies were analysed four days after pDC transfer in SC of mice from. (A–E) Data are representative of 3 independent experiments with 8 mice/group each. Data represent mean ± SEM. (A, B left panel, C, D) 2-way ANOVA with Bonferroni post hoc test; (B right panel) Standart two-tailed Student's t test; (E) 1-way ANOVA with Bonferroni post hoc test * P < 0.05; **P < 0.01; ***P < 0.001.
In order to investigate the features related to pDC tolerogenic effect, we analysed pDC phenotype in SC of EAE mice. Irrespective of pDC transfer, CNS pDCs expressed lower levels of CD86, compared to cDCs (Fig. 6A). Conversely, the expression of CD69 by endogenous pDCs was substantially downregulated upon pDC transfer (Fig. 6B). These findings suggest that the inflammatory status of pDCs in SC from EAE mice was markedly altered following pDC transfer. To determine whether the lack of pDC activation is involved in the pDC transfer-therapeutic effect, we treated pDCs with CpG-B prior to their injection. CpG-treated pDCs did not induce in situ upregulation of CMKLR1 by pDCs (Fig. 6C), neither the recruitment of endogenous pDCs to SC of EAE mice (Fig. 6D). Importantly, we further observed that injection of CpG-B activated MOG35-55 loaded pDCs did not confer any significant disease amelioration, compared to untreated MOG35-55 loaded pDCs (Fig. 6E), supporting a role for steady-state pDCs in disease amelioration. Altogether, these results demonstrate that steady-state pDC-transfer promotes a pDC-dependent tolerogenic environment in SC of EAE mice, resulting in the general suppression of EAE pathogenicity and clinical symptoms.
Fig. 6.
EAE amelioration is mediated by resting pDCs. (A, B) EAE was induced in WT mice and BM-derived, MOG35-55-loaded, CD45.1 WT pDCs were transferred into mice during EAE acute phase. DC phenotype was analysed in SC of EAE mice four days after pDC transfer. (A) CD86 MFI on cDCs and pDCs. (B) CD69 MFI on endogenous pDCs (EAE), and on endogenous and exogenous pDCs (EAE + pDC). (C–E) EAE was induced in WT mice and BM-derived, MOG35-55-loaded, WT pDCs, previously treated (EAE + pDC + CpG) or not (EAE + pDC) with CpG-B, were transferred or not (EAE) into mice during EAE acute phase (arrow). Frequencies of (C) CMKLR1 expressing pDCs and (D) total pDCs were analysed in SC of EAE mice 4 days after pDC transfer. (E) Clinical scores were followed daily. (A–E) Data are representative of 2 independent experiments with 6–8 mice per group each. Data represent mean ± SEM. (A) Standart two-tailed Student's t test; (B–D) 1-way ANOVA with Bonferroni post hoc test; (E) 2-way ANOVA with Bonferroni post hoc test. *P < 0.05; **P < 0.01; ***P < 0.001.
4. Discussion
In EAE and MS, whether pDCs have a prominent pathogenic or tolerogenic role is still controversial. Activated pDCs, through the production of pro-inflammatory cytokines, such as TNF and IL-6, have been claimed to be pathogenic and promote disease progression, both in mice and humans. In agreement, pDCs were shown to be pathogenic in EAE by inducing Th17 responses [13]. On the other hand, constitutive or antibody mediated pDC depletion during peak disease correlates with EAE exacerbation [12], [31]. We have previously demonstrated that pDCs can present myelin-derived autoantigens via MHCII to CD4+ T cells in an EAE context and promote the expansion of suppressive Treg cells [14]. In the present work, in order to explore pDC tolerogenic potential in a therapeutic EAE setting, we performed adoptive transfer of syngenic pDCs after disease onset. Strikingly, pDCs induced a rapid and substantial amelioration of both CNS inflammation and EAE clinical scores upon transfer into sick mice. Transferred pDCs rapidly reached the SC, localized in lesion areas in the CNS, and inhibited already primed encephalitogenic MOG35-55-specific 2D2 CD4+ T effector cells, pointing out to a local immunoregulatory role of these cells during EAE effector phase. However, we cannot rule out a possible systemic impact on immune cells in other organs following pDC transfer, and future studies will determine whether this might be beneficial in other inflammatory diseases.
The idea that few exogenous pDCs reaching the SC could confer such strong disease amelioration seemed rather puzzling. When investigating the cellular compartments in the SC after pDC transfer, we observed a tremendous increase in total pDC frequency, but not of other cell types such as cDCs, macrophages or microglial cells, suggesting a specific recruitment of endogenous pDCs to the SC. Importantly, selective depletion of endogenous pDCs completely abrogated disease amelioration following pDC adoptive transfer. Thus, the accumulation of endogenous pDCs in the CNS of EAE mice following pDC transfer accounts for EAE protection. Interestingly, we observed a significant increase in de novo HSC progenitors' generation in the BM of pDC transferred compared to control EAE mice. One possibility is that upon transfer, few exogenous pDCs reach the BM and locally produce a factor promoting HSC generation. In agreement, it has been recently demonstrated that angiopoietin-like 7, which is produced by pDCs, regulates the expansion and repopulation of human hematopoietic stem and progenitor cells [32]. Alternatively, LSK progenitor increase in BM might reflect a physiologic demand for pDC generation, as an indirect consequence of pDC-recruitment to the CNS following pDC transfer. Newly generated BM-pDCs would then be specifically recruited to the inflamed CNS through chemerin/CMKLR1 axis, an inflammatory chemotactic factor that is involved in pDC migration [33]. In vivo, chemerin expression correlated with pDC infiltration into peripheral tissues during autoimmune diseases, such as SLE [26]. Most importantly, chemerin was detected in intralesional cerebrovascular endothelial cells of MS patients, and its receptor is expressed by pDCs [34]. In agreement, we observed an increase in chemerin expression in the SC of EAE mice compared to naïve mice. Furthermore, we found a significant and specific increase in the expression of CMKLR1 (or Chem23R) by pDCs in the SC of pDC-transferred mice. Blockade of CMKLR1 induced cell migration abrogated pDC recruitment to the SC, demonstrating that following pDC transfer, endogenous pDC homing to inflamed CNS is mediated by the chemerin/CMKLR1 axis. However, microglia cells and CNS-infiltrating myeloid dendritic cells also express CMKLR1, and EAE clinical and histological disease was found less severe in CMKLR1 deficient mice when compared to WT counterparts [28]. One possibility is that under inflammatory EAE condition, activated CNS pDCs indeed promote disease pathogenesis, and that in CMKLR-1 deficient mice, pDC recruitment to the CNS is impaired and mice consequently develop attenuated EAE. In our settings of pDC transfer in EAE mice, endogenous pDCs would be deflected from pro-pathogenic to pro-tolerogenic functions after downmodulation of their activation state.
In our system, pDC protective role was not related to type I IFN production. Rather, a “non-activated” state for pDCs seemed to be required. Accordingly, CpG-B activated pDCs did not induce endogenous pDC recruitment to the CNS of EAE mice, neither did they conferred any significant level of disease amelioration, when compared to steady-state pDCs. Strikingly, resting pDCs transferred in EAE mice downmodulated the activation of local pDCs. Notably, expression of CD69, a marker for activated pDCs [35], was substantially reduced on endogenous pDCs recruited to SC after pDC transfer. Therefore, the combination of several pDC-specific features in the CNS: i) increased pDC frequencies; ii) modulation of pDC activation status; iii) ability to provide myelin antigen; iv) production of a state permissive for tolerance, altogether likely contribute to pDC protective role in EAE. MHCII deficient pDCs, were able to induce the same degree of disease inhibition compared to WT pDCs, ruling out a role for MHCII mediated antigen presentation by exogenous pDCs in this protective setting. Our data support a local inhibition of encephalitogenic effector T cells in the CNS of EAE mice after pDC transfer. First, as early as 1 day post-pDC injection, T cells up-regulated the inhibitory molecule PD-1, and second, after 4 days, we observed reduced frequencies of encephalitogenic Th1 and Th17 cells in the SC.
Current available antibody based therapies target various immune cell populations mediating the autoimmune chronic reaction for the treatment of MS, rheumatoid arthritis and other autoimmune diseases. Autologous stem cell transplantations are currently tested as immunotherapy for autoimmune diseases, including MS [36]. Although visionary, therapies using terminally differentiated cells for the treatment of autoimmune diseases are emerging and are up to date mainly focused on the adoptive transfer of Tregs. Both preclinical and clinical data in support for Treg infusional therapy suggest that ex vivo expanded, autologous Tregs might have a beneficial effect in patients for T1D [37] and SLE [38], [39]. In MS, the rationale for Treg therapy is less clear. Indeed, while Tregs infused prior EAE induction confer protection, their therapeutic value is considerably diminished when infused after disease initiation [40], [41]. Accordingly, Tregs immersed in inflamed CNS of EAE mice lost their suppressive activity [42]. Whether Tregs acquire suppressive functions and are directly linked to EAE protection after pDC-transfer will require further investigation.
Adoptive transfer of tolerogenic APCs for MS therapies has also been considered. If these approaches efficiently suppress EAE when injections were performed prior disease induction [43], tolerogenic APCs however impact mainly the priming of encephalitogenic T cells, and no efficacy was consequently proven when effector T cell responses and disease symptoms are already established. These observations make our results even more attractive in the sense that pDC suppressive effect on established EAE is not APC dependent, and directly impact disease effector phase in the CNS. Together, our results highlight pDCs as important mediators in MS immunotherapy. Manipulation of pDC numbers as well as enhancement of pDC potential immunoregulatory properties could be exploited in order to treat MS patients.
Competing interests
The authors have no competing financial interests.
Acknowledgements
The authors thank J.P Aubry-Lachainaye and Cécile Gameiro for excellent assistance in flow-cytometry, and Walter Reith and Julien Bertrand for helpful discussions. S.H. and D.M. are supported by the Swiss National Science Foundation (PP00P3_152951 to S.H and PP00P3_152928 to D.M.) and by the Swiss Multiple Sclerosis Society. S.H. is supported by the European Research Council (281365).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jaut.2015.08.014.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Soumelis V., Liu Y.J. From plasmacytoid to dendritic cell: morphological and functional switches during plasmacytoid pre-dendritic cell differentiation. Eur. J. Immunol. 2006;36:2286–2292. doi: 10.1002/eji.200636026. [DOI] [PubMed] [Google Scholar]
- 2.Chan V.S., Nie Y.J., Shen N., Yan S., Mok M.Y., Lau C.S. Distinct roles of myeloid and plasmacytoid dendritic cells in systemic lupus erythematosus. Autoimmun. Rev. 2012;11:890–897. doi: 10.1016/j.autrev.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Nestle F.O., Conrad C., Tun-Kyi A., Homey B., Gombert M., Boyman O., Burg G., Liu Y.J., Gilliet M. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J. Exp. Med. 2005;202:135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pashenkov M., Huang Y.M., Kostulas V., Haglund M., Soderstrom M., Link H. Two subsets of dendritic cells are present in human cerebrospinal fluid. Brain. 2001;124:480–492. doi: 10.1093/brain/124.3.480. [DOI] [PubMed] [Google Scholar]
- 5.Longhini A.L., von Glehn F., Brandao C.O., de Paula R.F., Pradella F., Moraes A.S., Farias A.S., Oliveira E.C., Quispe-Cabanillas J.G., Abreu C.H. Plasmacytoid dendritic cells are increased in cerebrospinal fluid of untreated patients during multiple sclerosis relapse. J. Neuroinflammation. 2011;8:2. doi: 10.1186/1742-2094-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 7.Serada S., Fujimoto M., Mihara M., Koike N., Ohsugi Y., Nomura S., Yoshida H., Nishikawa T., Terabe F., Ohkawara T. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U. S. A. 2008;105:9041–9046. doi: 10.1073/pnas.0802218105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reder A.T., Feng X. Aberrant Type I interferon regulation in autoimmunity: opposite directions in MS and SLE, shaped by evolution and body ecology. Front. Immunol. 2013;4:281. doi: 10.3389/fimmu.2013.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodkin D.E. Interferon beta treatment for multiple sclerosis: persisting questions. Mult. Scler. 1996;1:321–324. doi: 10.1177/135245859600100605. [DOI] [PubMed] [Google Scholar]
- 10.Teige I., Treschow A., Teige A., Mattsson R., Navikas V., Leanderson T., Holmdahl R., Issazadeh-Navikas S. IFN-beta gene deletion leads to augmented and chronic demyelinating experimental autoimmune encephalomyelitis. J. Immunol. 2003;170:4776–4784. doi: 10.4049/jimmunol.170.9.4776. [DOI] [PubMed] [Google Scholar]
- 11.Prinz M., Schmidt H., Mildner A., Knobeloch K.P., Hanisch U.K., Raasch J., Merkler D., Detje C., Gutcher I., Mages J. Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity. 2008;28:675–686. doi: 10.1016/j.immuni.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Bailey-Bucktrout S.L., Caulkins S.C., Goings G., Fischer J.A., Dzionek A., Miller S.D. Cutting edge: central nervous system plasmacytoid dendritic cells regulate the severity of relapsing experimental autoimmune encephalomyelitis. J. Immunol. 2008;180:6457–6461. doi: 10.4049/jimmunol.180.10.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isaksson M., Ardesjo B., Ronnblom L., Kampe O., Lassmann H., Eloranta M.L., Lobell A. Plasmacytoid DC promote priming of autoimmune Th17 cells and EAE. Eur. J. Immunol. 2009;39(10):2925–2935. doi: 10.1002/eji.200839179. [DOI] [PubMed] [Google Scholar]
- 14.Irla M., Kupfer N., Suter T., Lissilaa R., Benkhoucha M., Skupsky J., Lalive P.H., Fontana A., Reith W., Hugues S. MHC class II-restricted antigen presentation by plasmacytoid dendritic cells inhibits T cell-mediated autoimmunity. J. Exp. Med. 2010;207:1891–1905. doi: 10.1084/jem.20092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Glehn F., Santos L.M., Balashov K.E. Plasmacytoid dendritic cells and immunotherapy in multiple sclerosis. Immunotherapy. 2012;4:1053–1061. doi: 10.2217/imt.12.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kontgen F., Suss G., Stewart C., Steinmetz M., Bluethmann H. Targeted disruption of the MHC class II Aa gene in C57BL/6 mice. Int. Immunol. 1993;5:957–964. doi: 10.1093/intimm/5.8.957. [DOI] [PubMed] [Google Scholar]
- 17.Schaefer B.C., Schaefer M.L., Kappler J.W., Marrack P., Kedl R.M. Observation of antigen-dependent CD8+ T-cell/dendritic cell interactions in vivo. Cell Immunol. 2001;214:110–122. doi: 10.1006/cimm.2001.1895. [DOI] [PubMed] [Google Scholar]
- 18.Muller U., Steinhoff U., Reis L.F., Hemmi S., Pavlovic J., Zinkernagel R.M., Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 19.Swiecki M., Gilfillan S., Vermi W., Wang Y., Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity. 2010;33:955–966. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bettelli E., Pagany M., Weiner H.L., Linington C., Sobel R.A., Kuchroo V.K. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J. Exp. Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domingues H.S., Mues M., Lassmann H., Wekerle H., Krishnamoorthy G. Functional and pathogenic differences of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. PLoS One. 2010;5:e15531. doi: 10.1371/journal.pone.0015531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guery L., Dubrot J., Lippens C., Brighouse D., Malinge P., Irla M., Pot C., Reith W., Waldburger J.M., Hugues S. Ag-presenting CpG-activated pDCs prime Th17 cells that induce tumor regression. Cancer Res. 2014;74:6430–6440. doi: 10.1158/0008-5472.CAN-14-1149. [DOI] [PubMed] [Google Scholar]
- 23.Yogev N., Frommer F., Lukas D., Kautz-Neu K., Karram K., Ielo D., von Stebut E., Probst H.C., van den Broek M., Riethmacher D. Dendritic cells ameliorate autoimmunity in the CNS by controlling the homeostasis of PD-1 receptor(+) regulatory T cells. Immunity. 2012;37:264–275. doi: 10.1016/j.immuni.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 24.Sawai C.M., Sisirak V., Ghosh H.S., Hou E.Z., Ceribelli M., Staudt L.M., Reizis B. Transcription factor Runx2 controls the development and migration of plasmacytoid dendritic cells. J. Exp. Med. 2013;210:2151–2159. doi: 10.1084/jem.20130443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swiecki M., Wang Y., Vermi W., Gilfillan S., Schreiber R.D., Colonna M. Type I interferon negatively controls plasmacytoid dendritic cell numbers in vivo. J. Exp. Med. 2011;208:2367–2374. doi: 10.1084/jem.20110654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sozzani S., Vermi W., Del Prete A., Facchetti F. Trafficking properties of plasmacytoid dendritic cells in health and disease. Trends Immunol. 2010;31:270–277. doi: 10.1016/j.it.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Zabel B.A., Silverio A.M., Butcher E.C. Chemokine-like receptor 1 expression and chemerin-directed chemotaxis distinguish plasmacytoid from myeloid dendritic cells in human blood. J. Immunol. 2005;174:244–251. doi: 10.4049/jimmunol.174.1.244. [DOI] [PubMed] [Google Scholar]
- 28.Graham K.L., Zabel B.A., Loghavi S., Zuniga L.A., Ho P.P., Sobel R.A., Butcher E.C. Chemokine-like receptor-1 expression by central nervous system-infiltrating leukocytes and involvement in a model of autoimmune demyelinating disease. J. Immunol. 2009;183:6717–6723. doi: 10.4049/jimmunol.0803435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham K.L., Zhang J.V., Lewen S., Burke T.M., Dang T., Zoudilova M., Sobel R.A., Butcher E.C., Zabel B.A. A novel CMKLR1 small molecule antagonist suppresses CNS autoimmune inflammatory disease. PLoS One. 2014;9:e112925. doi: 10.1371/journal.pone.0112925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H., Zhang G.X., Chen Y., Xu H., Fitzgerald D.C., Zhao Z., Rostami A. CD11c+CD11b+ dendritic cells play an important role in intravenous tolerance and the suppression of experimental autoimmune encephalomyelitis. J. Immunol. 2008;181:2483–2493. doi: 10.4049/jimmunol.181.4.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reizis B., Bunin A., Ghosh H.S., Lewis K.L., Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annu. Rev. Immunol. 2011;29:163–183. doi: 10.1146/annurev-immunol-031210-101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao Y., Jiang Z., Li Y., Ye W., Jia B., Zhang M., Xu Y., Wu D., Lai L., Chen Y. ANGPTL7 regulates the expansion and repopulation of human hematopoietic stem and progenitor cells. Haematologica. 2015;100:585–594. doi: 10.3324/haematol.2014.118612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vermi W., Riboldi E., Wittamer V., Gentili F., Luini W., Marrelli S., Vecchi A., Franssen J.D., Communi D., Massardi L. Role of ChemR23 in directing the migration of myeloid and plasmacytoid dendritic cells to lymphoid organs and inflamed skin. J. Exp. Med. 2005;201:509–515. doi: 10.1084/jem.20041310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lande R., Gafa V., Serafini B., Giacomini E., Visconti A., Remoli M.E., Severa M., Parmentier M., Ristori G., Salvetti M. Plasmacytoid dendritic cells in multiple sclerosis: intracerebral recruitment and impaired maturation in response to interferon-beta. J. Neuropathol. Exp. Neurol. 2008;67:388–401. doi: 10.1097/NEN.0b013e31816fc975. [DOI] [PubMed] [Google Scholar]
- 35.Gao Y., Majchrzak-Kita B., Fish E.N., Gommerman J.L. Dynamic accumulation of plasmacytoid dendritic cells in lymph nodes is regulated by interferon-beta. Blood. 2009;114:2623–2631. doi: 10.1182/blood-2008-10-183301. [DOI] [PubMed] [Google Scholar]
- 36.Muraro P.A., Robins H., Malhotra S., Howell M., Phippard D., Desmarais C., de Paula Alves Sousa A., Griffith L.M., Lim N., Nash R.A. T cell repertoire following autologous stem cell transplantation for multiple sclerosis. J. Clin. Invest. 2014;124:1168–1172. doi: 10.1172/JCI71691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang Q., Henriksen K.J., Bi M., Finger E.B., Szot G., Ye J., Masteller E.L., McDevitt H., Bonyhadi M., Bluestone J.A. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J. Exp. Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyara M., Amoura Z., Parizot C., Badoual C., Dorgham K., Trad S., Nochy D., Debre P., Piette J.C., Gorochov G. Global natural regulatory T cell depletion in active systemic lupus erythematosus. J. Immunol. 2005;175:8392–8400. doi: 10.4049/jimmunol.175.12.8392. [DOI] [PubMed] [Google Scholar]
- 39.Valencia X., Yarboro C., Illei G., Lipsky P.E. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J. Immunol. 2007;178:2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 40.Van de Keere F., Tonegawa S. CD4(+) T cells prevent spontaneous experimental autoimmune encephalomyelitis in anti-myelin basic protein T cell receptor transgenic mice. J. Exp. Med. 1998;188:1875–1882. doi: 10.1084/jem.188.10.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olivares-Villagomez D., Wang Y., Lafaille J.J. Regulatory CD4(+) T cells expressing endogenous T cell receptor chains protect myelin basic protein-specific transgenic mice from spontaneous autoimmune encephalomyelitis. J. Exp. Med. 1998;188:1883–1894. doi: 10.1084/jem.188.10.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bailey S.L., Schreiner B., McMahon E.J., Miller S.D. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ T(H)-17 cells in relapsing EAE. Nat. Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- 43.Faunce D.E., Terajewicz A., Stein-Streilein J. Cutting edge: in vitro-generated tolerogenic APC induce CD8+ T regulatory cells that can suppress ongoing experimental autoimmune encephalomyelitis. J. Immunol. 2004;172:1991–1995. doi: 10.4049/jimmunol.172.4.1991. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.