Abstract
Stroke is the leading cause of seizures and epilepsy in the aged population, with post-stroke seizures being a poor prognostic factor. The pathological processes underlying post-stroke seizures are not well understood and studies of these seizures in aging/aged animals remain scarce. Therefore, our primary objective was to model post-stroke seizures in aging mice (C57 black strain, 16–20 month-old), with a focus on early-onset, convulsive seizures that occur within 24-hours of brain ischemia. We utilized a middle cerebral artery occlusion model and examined seizure activity and brain injury using combined behavioral and electroencephalographic monitoring and histological assessments. Aging mice exhibited vigorous convulsive seizures within hours of the middle cerebral artery occlusion. These seizures manifested with jumping, rapid running, barrel-rolling and/or falling all in the absence of hippocampal-cortical electrographic discharges. Seizure development was closely associated with severe brain injury and acute mortality. Anticonvulsive treatments after seizure occurrence offered temporary seizure control but failed to improve animal survival. A separate cohort of adult mice (6–8 months-old) exhibited analogous early-onset convulsive seizures following the middle cerebral artery occlusion but had better survival outcomes following anticonvulsive treatment. Collectively, our data suggest that early-onset convulsive seizures are a result of severe brain ischemia in aging animals.
Keywords: Aging, Animal model, Anticonvulsant, Convulsion, EEG, Epilepsy, Ischemia, Mice, Seizures, Stroke
Introduction
Stroke is the most common cause of seizures in the elderly population, with reported incidences ranging from 3–10% of all stroke cases (Bladin and Bornstein, 2009; Brodie et al., 2009; Menon and Shorvon et al., 2009; Chen et al., 2010; Balami et al., 2011; Gilad 2012; Guekht and Bornstein 2012; Procaccianti et al., 2012; Guth et al., 2014). Post-stroke seizures are associated with higher mortality rates, prolonged hospitalization, and increased incidences of long-term disability (Waterhouse et al., 1998; Vespa et al., 2003; Szaflarski et al., 2008; Burneo et al., 2010; Huang et al., 2014). These consequences contribute significantly to the socioeconomic burden of stroke, already the greatest amongst all disease in North America, in addition to the massive personal burden felt by patients and their families (Smurawska et al., 1994; Zorowitz et al., 2009; Publication Health Agency of Canada. 2011; Mozaffarian et al., 2015). Specific guidelines for the definitive treatment of post-stroke seizures have yet to be established but may require a deeper understanding of seizure pathogenesis (Gilad, 2012; Guekht and Bornstein 2012; Procaccianti et al., 2012; Kulhari et al., 2014; Sykes et al., 2014).
Early-onset seizures are largely observed within 24 hours and are a medical emergency as life-threatening status epilepticus may follow (Waterhouse et al., 1998; Waterhouse and DeLorenzo 2001). Early-onset seizures can manifest as generalized tonic-clonic convulsions, or non-convulsive seizures which require EEG monitoring for diagnosis (Silverman et al., 2002; Jordan, 2004; Claassen et al., 2007; Chung, 2014). Stroke severity and the degree of cortical involvement have been recognized as risk factors for early-onset seizure development following ischemic stroke (Bladin and Bornstein, 2009; Brodie et al., 2009; Menon and Shorvon et al., 2009; Balami et al., 2011; Chen et al., 2010; Gilad 2012; Guekht and Bornstein 2012; Procaccianti et al., 2012; Chung, 2014). However, the regional initiation and progression of early-onset seizures are often difficult to assess clinically in patients with severe brain ischemia. Therefore, it may be of more practical value to study these phenomena in a viable animal model.
Previous studies have characterized early-onset, non-convulsive seizures (NCS) in adult rats following a middle cerebral artery occlusion (MCAO; Hartings et al., 2003; Williams et al., 2004; 2006; Karhunen et al., 2006; Lu et al., 2009; Cuomo et al., 2013). These NCS were generally observed a few hours following the MCAO with matching cortical EEG discharges. A link between development of these NCS and brain injury was established as anticonvulsant treatment reduced both ischemic injury and acute mortality (Williams et al., 2004; 2006; Cuomo et al., 2013). Other studies investigated early-onset, convulsive seizures (CS) in adult animals following brain ischemia (Reglodi et al., 2000; Wang et al., 2001; Shabanzadeh et al., 2005; El-Hayek et al., 2011a). However, inconsistencies in the EEG discharges corresponding to these CS demonstrate a need for further examination. These prior studies also utilized adult animals exclusively, overlooking aging as the most important non-modifiable risk factor for stroke and its link to greater brain damage and poor outcomes (Mozaffarian et al., 2015). While late-onset seizures have been documented in aged rats following MCAO and photothrombotic ischemia (Kelly, 2006; Karhunen et al., 2007; Kelly et al., 2001; 2011), very little information remains available on the behavioral and EEG characteristics of early-onset, post-ischemic seizures in aging/aged animals. Therefore, for our study, we aimed to develop a viable model of early-onset, post-MCAO seizures (CS, NCS) in aging mice in order to characterize the prognostic role of these seizures and the pathophysiological processes underlying their development.
Material and Methods
Animals
Male C57 black mice (C57BL/6; Charles River, Senneville St-Constant, Quebec, Canada) were used. C57 mice that are ≥24-months-old may correspond to a human age of approximately ≥70 years (Flurkey et al., 2007). However, aged C57 mice often encounter many health-related complications such as skin lesions, ear infections and tumors (Flurkey et al., 2007). Therefore we chose to conduct our MCAO experiments in 16–20 month-old C57 mice in order to minimize confounding health complications while effectively modeling brain ischemia in aging animals. Overall, 89 aging mice were used: 67 animals underwent a MCAO, 15 underwent a sham surgery or occlusion of the common carotid artery alone (n=7 or 8), and 7 died during the course of surgery/anesthesia due to either respiratory suppression or bleeding. A separate cohort of adult C57 black mice (male, 6–8 months-old, n=38) were used to compare the effects of age on early-onset seizures.
The animals were housed in a vivarium that was maintained at 22–23°C with a 12-hour light on/off cycle. Food and water were available ad libitum. All experiments detailed were reviewed and approved by the animal care committee of the University Health Network in accordance with the Canadian Guidelines for Animal Care. In line with the guidelines, animals with severe CS were treated with clinically appropriate anticonvulsants. Mandatory euthanization was conducted if animals exhibited severe, recurrent CS inadequately suppressed by anticonvulsant treatments and/or presented in poor physical condition such as with persistent immobility, lack of eating and drinking, irresponsiveness to touch, loss of the righting reflex, and/or a substantial reduction in body weight (≥20% of baseline level). In our study, acute mortality was defined as spontaneous death or mandatory euthanization within 48 hours post-ischemia.
Intracranial EEG recordings
Electrode construction, implantation and EEG recordings were conducted as previously described (Wu et al., 2008; Wais et al., 2009; El-Hayek et al., 2011a, b; Jeffrey et al., 2014). All electrodes were constructed using polyamide-insulated stainless steel wires (outside diameter of 200 μm for monopolar electrodes and 125 μm for twisted bipolar electrodes; Plastics One, Ranoake, VA, USA). Monopolar electrodes were pre-assembled in an array before implantation (Wu et al., 2008). The tips of the twisted bipolar wires were separated by approximately 100 μm (Jeffrey et al., 2014). EEG recordings were made using a dual-channel AC microelectrode amplifier with extended headstages (model 1800, AM Systems, Carlsborg, WA, USA). Signals were collected in a frequency bandwidth of 0.1–1000 Hz, amplified 1000 times and then digitized at ≥5 KHz (Digidata 1300, Molecular Devices; Sunnyvale, CA, USA). Data acquisition, storage, and analysis were conducted using pClamp software (version 9 or 10; Molecular Devices).
Monopolar recordings were conducted in the majority of animals since electrode implantation involved a shorter surgery with minimal perioperative complications, a particularly important consideration in more susceptible aging animals (Wu et al., 2008). EEG activity recorded with monopolar electrodes represented the signal difference between the recording and reference electrodes. Therefore, this method was more sensitive to movement artifacts and other remote signals. Twisted bipolar electrodes were used for local differential recordings in some animals but the relatively long surgery for implantation was associated with greater perioperative complications including mortality. EEG activity in these local differential recordings represented the signal difference between the tips of the two twisted electrodes. Therefore, this method was less susceptible to other artifacts or remote signals, and was preferable for sampling the local circuitry activity.
For monopolar recordings, electrodes were implanted bilaterally into the hippocampal CA1 (bregma −2.3 mm, lateral 2.0 mm and depth 2.0 mm) and parietal cortex (bregma −0.6 mm, lateral 1.5 mm and depth 1 mm; Franklin and Paxinos, 1997) or unilaterally into the hippocampal CA3 (bregma: −2.6 mm, lateral 2.5 mm and depth 3.0 mm) and parietal cortex. For local differential recordings, twisted bipolar electrodes were implanted bilaterally into the hippocampal CA3 or into CA3 and the parietal cortex. The locations of the implanted electrodes were verified histologically (Fig 3C, Supplemental Fig 1).
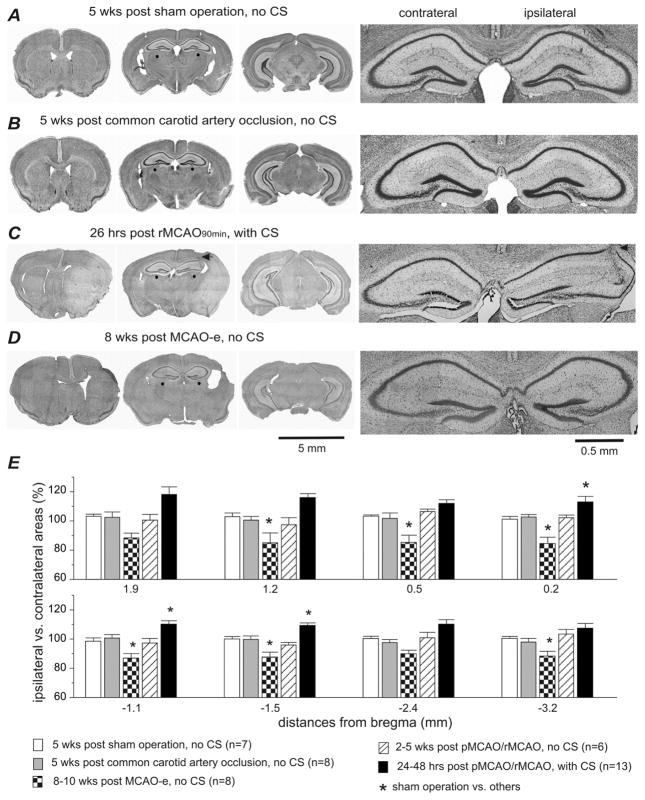
Fig. 3. Extensive ipsilateral brain injury in aging mice with CS.
Data were collected from aging mice. A–D, images of cresyl violet-stained brain sections obtained from 4 representative animals. Time of histological examination, experimental protocol, and the presence/absence of CS are indicated above each row of images. Left, low power views at three coronal levels. Right, magnified views of dorsal hippocampal areas (denoted by red dots on low power view). ‘Ipsilateral’ and ‘contralateral’ are relative to unilateral MCAO. Note the weakly stained ipsilateral regions and the enlarged ipsilateral to contralateral hemispheric area in the animal with CS (C). The track of an implanted hippocampal electrode is denoted by black arrows in C. E, the ratio of the ipsilateral to contralateral hemispheric areas were calculated from brain sections at 8 coronal levels (from bregma −3.2 mm to 1.9 mm). Data were grouped according to the specific experimental protocol and the presence/absence of CS. *, sham controls vs. other groups, p<0.05, one way ANOVA.
Most EEG recordings were performed in free-moving animals. During some EEG recordings, animals were placed in a mouse restrainer (Type C or D, Canadawide Scientific, Ottawa, Canada) to limit the amount of CS-related movement artifacts. Although the restrainers inhibited vigorous convulsive behavior such as jumping and rapid running, other characteristic seizure activity such as barrel-rolling, rapid limb movements, and tail erections remained observable and analogous to those seen in their unrestrained counterparts.
To quantify EEG changes over time, we used the root mean square (RMS) of the EEG signals as this has been shown to be a sensitive measure of ischemic EEG suppression in adult C57 black mice following hypoxia-ischemia (El-Hayek et al., 2011b). Others have used EEG RMS to assess brain activities in a rat model of post hemorrhagic seizures (Klahr et al., 2015). RMS calculations were made from 30-sec EEG segments collected while animals were immobile as these data segments were minimally contaminated by movement-related artifacts. Power spectra were then generated with 50% window overlap at a spectral resolution of 0.3 Hz. The RMS of the EEG power spectrum was automatically calculated in pClamp.
Middle cerebral artery occlusion (MCAO)
Animals were anesthetized with 2% isoflurane. During surgery, animal rectal temperatures were maintained between 35.5–36.5°C via an automatic heating device (DC temperature controller, FHC Inc., Bowdoin, ME, USA). Permanent and reversible MCAO, referred to as pMCAO and rMCAO in the following text, were conducted via intra-luminal suture insertion (Durukan and Tatlisumak 2007; Hossmann, 2008; Howells et al., 2010; Liu and McCullough, 2011). Each surgery lasted 20–40 minutes. The protocol for electrocoagulation of the MCA (MCAO-e) was modified from previous studies in adult mice (Liu et al., 2005; Cipriani et al., 2011; Moyanova et al., 2011; Wang et al., 2011).
For the pMCAO, a silicon-coated fine suture (#6, Doccol Corporation, Redlands, CA, USA) was inserted into the common carotid artery and advanced through the internal carotid artery with its tip 7–8 mm distal to the carotid bifurcation (the Koizumi’s method; see Durukan and Tatlisumak 2007). The common carotid artery and the inserted suture were then permanently ligated.
For the rMCAO, the suture was inserted through the external carotid artery and advanced through the internal carotid artery (the Longa’s method; see Durukan and Tatlisumak 2007). After suture placement, the external carotid artery was temporarily ligated and the skin wound was loosely sutured. Animals were split into two groups and were allowed to recover from anesthesia with the suture in for either 45 or 90 minutes (referred to as the rMAO45min and rMCAO90min group respectively in the following text). Different durations of occlusion were utilized to explore the relationship between the duration of ischemia, reperfusion, and seizure occurrence. The suture was then withdrawn under isoflurane anesthesia and the external carotid artery was permanently ligated.
For the MCAO-e, animals received a skin incision to expose the skull between the right ear and eye. A small hole was drilled through the skull, and the main ascending branch of the MCA was cauterized using a clinical-grade cautery (Bovie, Clearwater, FL, USA).
Aging mice that underwent either an irreversible ligation of the common carotid artery or a sham surgery were used as controls. The sham surgery consisted of surgical exposure of the common carotid artery in the anesthetized animal, followed by subsequent skin closure.
Detection of early-onset seizures
Baseline monitoring was performed 1 week after electrode implantation with continuous behavioral and EEG monitoring for up to 6 hours. To detect early-onset seizures following MCAO, animals were placed under continuous visual surveillance by experimenters with concurrent EEG recordings for the first 4–6 hours post-surgery, followed by overnight video monitoring for an additional 10–14 hours. Behavioral and EEG monitoring was resumed the next day and at later serial time points when permitted by animal survival.
The appearance of vigorous CS were exemplified by rapid running, jumping, barrel rolling (≥3 turns), falling (loss of righting reflex) with tonic limb flexion, and repetitive tail erection (Supplementary video 1 and 2). These CS were in keeping with generalized tonic-clonic seizures described for rodent models in the past (Velíšková 2006). Previous studies have reported similar CS in other mouse models (Ivanov et al., 2004; Muramatsu et al., 2008; Silva-Fernandes et al., 2010; El-Hayek et al., 2011a). Subtle or ambiguous convulsive behaviors, such as head nodding, jerking, and/or shaking were not classified as CS.
Ictal-like EEG discharges were defined as repetitive single-spike or poly-spike waveforms lasting ≥5 seconds in duration with amplitudes ≥2 times that of the background signals (Wais et al., 2009; He et al., 2009; Jeffrey et al., 2014).
Anticonvulsive drug treatments
Lorazepam (Ativan) and fosphenytoin (Cerebyx) were obtained in clinically available injectable forms (Sandoz Canada Inc. and Erfa Canada Inc., Quebec, Canada). These drugs were diluted in saline and administered via intra-peritoneal (IP) injections at dosages of 1.5mg/kg for lorazepam and 30mg/kg for fosphenytoin (equivalent to phenytoin 20mg/kg).
Brain histology
Brain histological assessments were conducted as previously described (Wais et al., 2009; He et al., 2009; El-Hayek et al., 2011a). Animals were anesthetized using an IP injection of sodium pentobarbital (70 mg/kg) and then transcardially perfused with 10% neutral buffered formalin solution. The brain was removed and further fixed in 10% formalin with 20% sucrose. Cryostat coronal sections 30 μm thick were obtained of the entire brain and stained with cresyl-violet to evaluate gross brain injury at different post-ischemic time points (Liu and McCullough, 2011) and verify implanted electrode tracks in their appropriate anatomical locations. The timing of histological processing was dependent on animal survival. All aging mice with CS were processed at 24–48 hours post-ischemia due to early mortality (or mandatory euthanization) while CS-free aging mice survived for up to 10 weeks prior to processing. Images were obtained using a Leica (DMRN) upright microscope and analyzed using Image J software (National Institute of Health, USA).
To quantify the regions with weak cresyl-violet staining, the brightness and contrast of the Leica microscope captured images were adjusted to clearly demarcate boundaries. Only regions that were clearly stained lighter compared to adjacent areas were included for analysis. To assess ipsilateral edema or atrophy, the area of the ipsilateral hemisphere was normalized as a percentage of the corresponding contralateral hemisphere at 8 coronal levels (from bregma −3.2 to 1.9 mm; El-Hayek et al., 2011a). Ipsilateral brain injury, defined as weakly stained areas, or cavities and scar tissue with visible margins, was also quantified as a percentage of the total ipsilateral area at the same 8 coronal levels.
Statistical analysis
Statistical tests were done using the SigmaStat software (Systat Software Inc, San Jose, California, USA). A Student’s t-test or Mann-Whitney Rank Sum Test was used for two group comparisons. For multiple group comparisons, a one-way ANOVA was used, followed by a multiple comparison Dunn’s test versus baseline control or a Holm-Sidak multiple comparison pairwise test. A Chi-square or Fischer exact test was used for rate comparisons. Data were presented as mean and standard error of the mean (SEM) throughout the text and figures. Statistical significance was set at the level of p≤0.05.
Results
Data collected from aging C57 black mice (C57BL/6, 16–20 months-old) are presented below except where use of adult mice (6–8 months-old) is specified. In the following text, ‘ipsilateral’ and ‘contralateral’ are relative to the unilateral MCAO.
1. Behavioral and EEG features of CS and NCS
Vigorous CS (Supplementary video 1) were observed in 8/13 (62%), 12/19 (63%), and 15/27 (56%) aging mice that underwent the pMCAO, rMCAO90min, and rMCAO45min respectively, but in none of the aging mice (0/8) that underwent the MCAO-e (Fig 1A). Neither CS nor NCS were observed in any control mice following a sham surgery (n=7) or occlusion of the common carotid artery alone (n=8; Fig 1A).
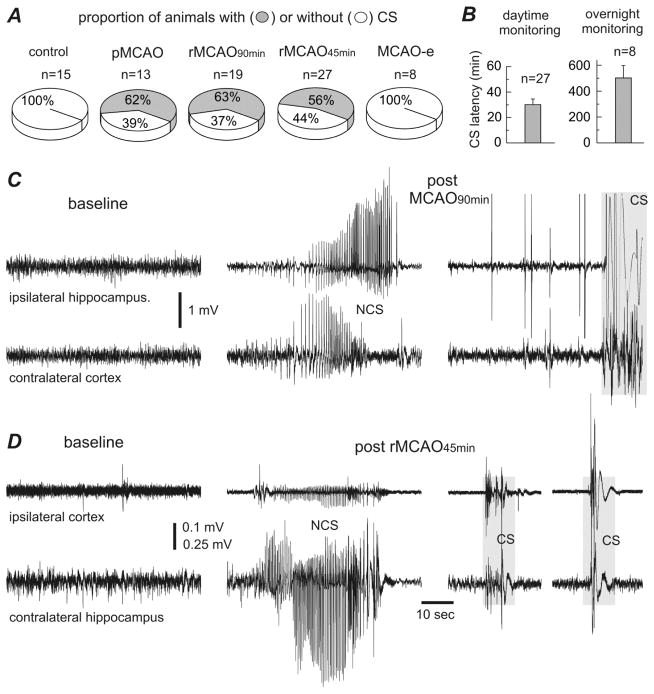
Fig. 1. Incidence and latency of early-onset convulsive seizures observed from aging mice.
Data were collected from aging mice (16–20 months-old). Abbreviations: MCAO - middle cerebral artery occlusion; pMCAO - permanent MCAO; rMCAO90min and rMCAO45min - reversible MCAO for 90 min and 45 min; MCAO-e - MCAO via electrocoagulation. A, the proportion of animals with and without convulsive seizures (CS) in different MCAO procedural groups. B, CS latencies were determined from the termination of suture-insertion surgery (pMCAO/rMCAO) to the onset of the first spontaneous CS. Left, data (mean±SE) from animals with CS onset during daytime monitoring. Right, data from animals with CS onset during overnight video monitoring. C–D, representative EEG traces collected from 2 aging mice before and following rMCAO90min (C) or rMCAO45min (D). Signals were collected via monopolar (C) or local differential (D) recordings from indicated recording sites. Original data were treated with a band-pass filter (0.5–500 Hz) for illustrative purposes. Left, baseline signals. Middle, EEG discharges corresponding to NCS. Right, large movement-related artifacts but no evident discharges during CS (gray boxes).
CS onset was sudden, without any appreciable prodromes. However, animal handling or moderate auditory stimuli (such as cage disturbance and hand clapping) could sometimes trigger these CS. CS latency was measured from termination of surgical suture-insertion to the first spontaneous CS. Of the 35 aging mice with CS post pMCAO/rMCAO, 27 (77%) exhibited their first CS within 7–110 min. The mean CS latency in these animals was 30.1±4.6 min (Fig 1B). The remaining 8 animals (23%) exhibited their first CS during overnight video monitoring (Supplementary video 2). Their mean CS latency was 502.1±96.7 min (Fig 1B).
We recorded EEG signals from bilateral hippocampal-cortical areas or ipsilateral hippocampal-cortical areas in different animal cohorts to examine ischemia- and seizure-related forebrain activity. EEG signals during CS were analyzed in 28 aging mice (2–3 CS per animal) that underwent either mono-polar (n=16) or local differential recordings with twisted bipolar electrodes (n=12). No hippocampal or cortical EEG discharges preceding or coinciding with the CS were seen in any of the 28 animals examined (Fig 1C, D). However, hippocampal and hippocampal-cortical EEG discharges during periods of immobility, indicative of NCS (Hartings et al., 2003; Williams et al., 2004; 2006; Karhunen et al., 2006; Lu et al., 2009; Cuomo et al., 2013), were observed in 14 aging mice following pMCAO, rMCAO90min, rMCAO45min, and MCAO-e (n=2, 3, 7 and 2 respectively; Fig 1C, D). Mean NCS latency, measured from the termination of surgery to the first non-convulsive discharge, was 24.4±3.7 min (n=13; Table 1). One animal was an outlier with a NCS latency of 300 min. The mean duration of the NCS was 35.6±4.5 sec (measured from 19 hippocampal discharge events in 14 animals; Table 1). Interestingly, in aging mice with both NCS and CS following pMCAO or rMCAO, 1–2 NCS always preceded the first CS but did not recur afterwards.
Table 1.
Early-onset seizures and related measures in aging and adult mice
| Aging mice | Adult mice | p value | |
|---|---|---|---|
| Animals with CS following MCAO | |||
| pMCAO | 8/13 (62%) | 11/17 (65%) | 1.0 |
| rMCAO90min | 12/19 (63%) | 11/15 (73%) | 0.715 |
| rMCAO45min | 15/27 (56%) | 3/6 (50%) | 1.0 |
| CS latencies (min) | 30.1±4.6 (n=27) | 45.6±9.9 (n=24) | 0.307 |
| Proportion of animals with CS onset during overnight video monitoring | 8/35 | 1/25 | 0.067 |
| NCS latencies (min) | 24.4±3.7 (n=13) | 17.8±5.1 (n=5) | 0.095 |
| NCS durations (sec) | 35.6±4.5 (n=14) | 42.9±4.3 (n=5) | 0.968 |
| Ipsilateral EEG changes in animals with CS (% of baseline) | |||
| 1 hr post MCAO | 41.4±5.4 (n=27) | 67.4±6.6 (n=11) | 0.010 |
| 24 hrs post MCAO | 57.8±16.5 (n=5) | 80.9±6.0 (n=8) | 0.391 |
| Weakly stained regions in animals with CS (% of ipsilateral hemisphere) | |||
| bregma 1.9 mm | 35.1±14.5 (n=3) | 36.7±7.1% (n=5) | 0.911 |
| bregma 1.2 mm | 18.2±7.4 (n=7) | 44.5±3.8 (n=5) | 0.048 |
| bregma 0.5 mm | 27.2±12.5 (n=6) | 52.9±2.8 (n=5) | 0.106 |
| bregma 0.2 mm | 24.7±12.9 (n=6) | 48.2±9.6 (n=6) | 0.176 |
| bregma −1.1 mm | 20.7±9.8 (n=4) | 45.8±8.1 (n=5) | 0.086 |
| bregma −1.5 mm | 28.9±11.1 (n=3) | 39.1±9.5 (n=5) | 0.525 |
| bregma −2.4 mm | 37.1 (n=1) | 36.2±10.9 (n=4) | |
| bregma −3.2 mm | 14.2 (n=1) | ||
| Area ratios of ipsilateral/contralateral hemisphere in animals with CS (%) | |||
| bregma 1.9 mm | 118.1±5.2 (n=13) | 114.6±3.2 (n=16) | 0.948 |
| bregma 1.2 mm | 116.1±2.1 (n=13) | 117.3±3.6 (n=17) | 0.801 |
| bregma 0.5 mm | 112.0±2.4 (n=11) | 117.1±3.3 (n=16) | 0.271 |
| bregma 0.2 mm | 113.0±3.7 (n=10) | 113.6±2.7 (n=16) | 0.890 |
| bregma −1.1 mm | 110.3±2.3 (n=11) | 111.7±2.3 (n=16) | 0.677 |
| bregma −1.5 mm | 109.3±1.7 (n=12) | 110.0±1.5 (n=15) | 0.791 |
| bregma −2.4 mm | 110.23.0 (n=13) | 109.7±2.7 (n=15) | 0.903 |
| bregma −3.2 mm | 107.5±3.3 (n=10) | 107.5±3.3 (n=12) | 0.167 |
| Acute mortality in animals received post-MCAO anticonvulsive treatment | |||
| pMCAO | 8/8 (100%) | 11/11 (100%) | 1.0 |
| rMCAO90min | 12/12 (100%) | 7/11 (64%) | 0.037 |
| rMCAO45min | 12/15 (80%) | 1/3 (33%) | 0.621 |
Numbers of animals examined are indicated in parentheses. Rate comparison was done using a Chi-square or Fisher exact test. Other comparisons were done using a Student’s t-test or Mann-Whitney Rank Sum Test. Two-tailed p values are presented, and bold font denotes where p≤0.05. For post-MCAO anticonvulsive treatment, intra-peritoneal injections of lorazepam (1.5mg/kg) and fosphenytoin (30mg/kg) were applied to individual animals after two observed CS events.
2. Development of CS is closely associated with severe brain injury
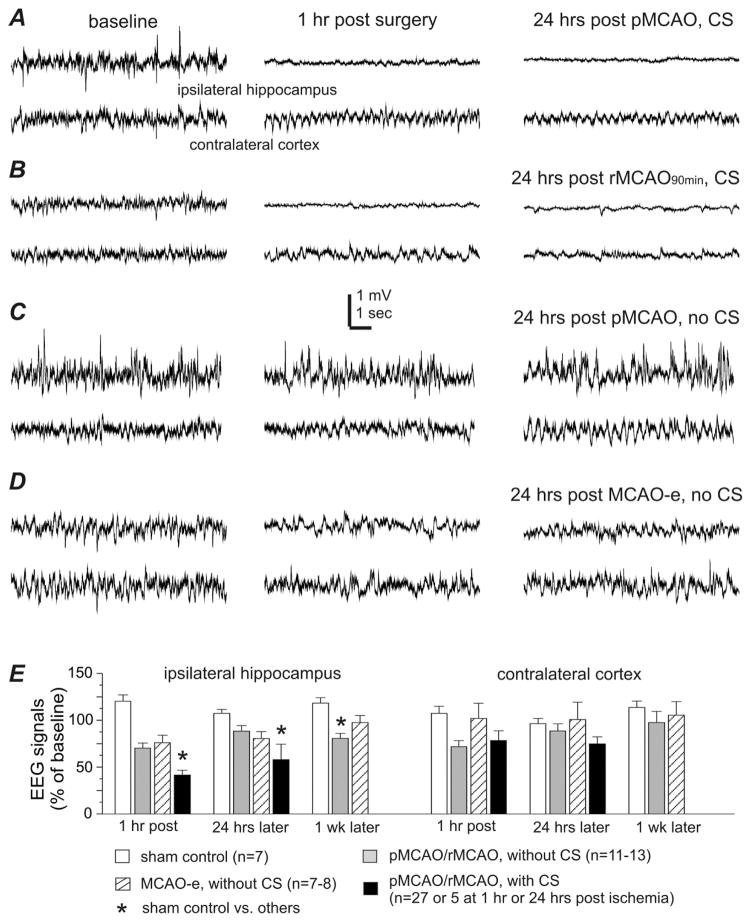
A positive correlation has been found between EEG signal suppression and the degree of brain ischemia in human subjects (Faught 1993; Jordan 2004). Previous work has shown that an early decrease in ipsilateral hippocampal EEG signal also closely correlates with ischemic brain injury and CS development in adult mice following an ischemic insult (El-Hayek et al., 2011b). In aging mice with CS following pMCAO/rMCAO, post-ischemic EEG signals from the ipsilateral hippocampus were decreased to 41% and 58% of baseline at 1 hour (n=27) and 24 hours (n=5) respectively, which significantly differed from EEG changes seen in the sham controls (p<0.05, Fig 2A, B, E). In CS-free aging mice, ipsilateral EEG signals were only moderately decreased following pMCAO/rMCAO to 70% of the baseline at 1-hour post-ischemia with subsequent recovery to 88% at 24 hours (n=13; Fig 2C, E). Similarly moderate decreases were seen in the CS-free aging mice that underwent MCAO-e (75–80% of sham control levels) at 1 hour and 24 hours post electrocoagulation (Fig 2D, E).
Fig. 2. EEG signal changes in aging mice with or without early-onset CS.
Data were collected from aging mice. A–D, representative EEG traces collected from 4 animals with simultaneous recording from the ipsilateral hippocampus and contralateral parietal cortex. Left, baseline signals. Middle, signals collected 1 hour after termination of surgery. Right, signals collected 24 hours post-surgery. Note that ipsilateral hippocampal signals were suppressed in animals with CS and remained suppressed at 24 hours post-surgery (A, B); whereas ipsilateral EEG suppression was not seen in CS-free animals post-surgery (C, D). E, the root mean square (RMS) of EEG signals measured from data segments collected during baseline monitoring and at 1 hour and 24 hours post-surgery or post-MCAO. Data were presented as a percentage of the baseline EEG signals and grouped for animals with or without CS following sham operation or different MCAO procedures. *, sham controls vs. other groups, p<0.05, one way ANOVA.
Brain histological assessments were conducted in all groups at different time points. At 24–48 hours post-pMCAO/rMCAO, brain injury was identifiable by areas of weak cresyl-violet staining in the ipsilateral hemisphere, and by a significantly increased ipsilateral to contralateral hemispheric area ratio (Fig 3C, E). These injury features were observed in all 13 aging mice that exhibited CS and underwent successful histological processing. The weakly stained areas predominated in the rostral to mid coronal planes and affected the striatal, hippocampal, and lateral cortical sites (Fig 3C). These areas corresponded to 18–35% of the total ipsilateral hemisphere across 6 coronal levels (bregma 1.9 mm to −1.5 mm, n=9; Table 1). The ratio of the ipsilateral to contralateral hemispheric area was increased to 108–118% in these animals (n=13 aging mice), significantly different from the sham controls (Fig 3E). This was suggestive of substantial ipsilateral edema seen in early brain injury. CS-free aging mice (n=6) did not have any evidence of cystic infarctions and had ipsilateral to contralateral hemispheric area ratios comparable to the sham controls when examined 2–5 weeks post-surgery (Fig 3E). In the MCAO-e group, focal ipsilateral infarcts were evident in the rostral coronal planes of all 8 aging mice at 8–10 weeks post-surgery (Fig 3D). The ipsilateral to contralateral hemispheric area ratio was decreased in these animals at several coronal levels (Fig 3E), suggesting ipsilateral atrophy. No evident brain injuries and no differences between the ipsilateral and contralateral hemispheric areas were observed in any control mice (n=7, 8; Fig 3A, B, E).
3. Occurrence of CS is tightly linked to acute mortality
All animals with CS during daytime monitoring were treated with anticonvulsive drugs as per the animal care guidelines. However, 8 aging mice with CS onset during overnight video monitoring (see Section 1 of Results) could not be treated in a timely manner. Of these untreated animals, 5 died immediately after the first 1–3 CS (Supplementory video 2) while the other 3 animals exhibited 7–15 CS throughout the entire duration of overnight video monitoring and underwent mandatory euthanization the next morning due to their poor physical condition. This suggests that if left untreated, post-ischemic CS can cause sudden death or lead to status epilepticus-like conditions in aging animals. In the following text, we use the term ‘acute mortality’ to denote spontaneous death or mandatory euthanization within a 48-hour post-ischemic window.
IP injections of lorazepam (1.5 mg/kg) and fosphenytoin (30 mg/kg) were given to individual animals after two observed CS. The intention of treatment was to prevent recurrent seizures to potentially improve CS-induced mortality. The treatment provided temporary seizure control as CS ceased 4–6 hours following treatment but reappeared later during overnight video monitoring. Despite offering temporary seizure control, the treatment failed to improve overall animal survival. Of the 27 aging mice treated after two CS, 24 encountered acute mortality and only 3 survived following rMCAO45min but ultimately either died spontaneously or underwent mandatory euthanization 9–21 days later (Table 1).
4. Comparison with adult mice
We performed pMCAO/rMCAO in adult mice (6–8 months-old) to explore the effect of animal age on ischemia severity, seizure genesis and anticonvulsive treatment outcomes. The protocols for the MCAO, hippocampal-cortical EEG recordings, behavioral monitoring, anticonvulsive treatments, and histological assessments were identical to those applied to aging mice. The motor manifestations of CS were analogous to those seen in aging mice and were observed in 11/17 (65%), 11/15 (73%), and 3/6 (50%) adult mice following pMCAO, rMCAO90min, and rMCAO45min respectively (Fig 4A). The mean CS latency for all adult animals with the exception of one was 45.6±9.9 min (n=24). One adult animal exhibited its first CS approximately 7.5 hours after the pMCAO (Fig 4A). The mean CS incidence and latency did not significantly differ from those documented in aging mice (Table 1). However, relatively fewer adult mice exhibited their first CS during overnight monitoring (1/25 vs. 8/35, p=0.067; Table 1). As with their aging counterparts, no CS or NCS were observed in sham control adult mice (n=5; Fig 4A).
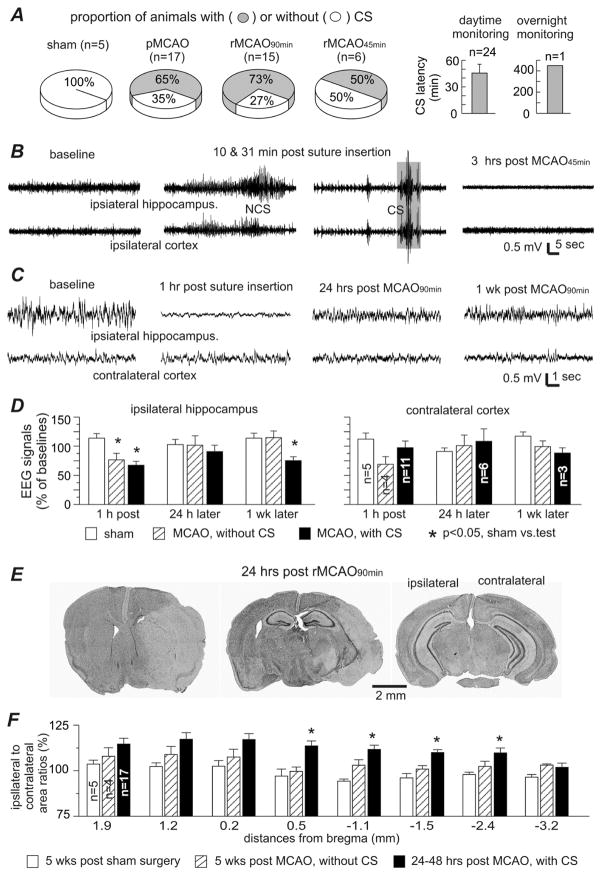
Fig. 4. Early-onset CS and related measures in adult mice.
Data were collected from adult mice (6–8 months-old). A, CS incidences in different procedural groups (left) and CS latencies (right). Only one adult mouse had CS onset during overnight video monitoring. B, representative EEG traces collected from one animal via local differential recordings from the ipsilateral hippocampus and parietal cortex). Left to right, signals collected during baseline monitoring, 10 min post surgery, 31 min post surgery, and 3 hours following rMCAO45min. C, representative EEG traces collected from another animal via monopolar recordings from the ipsilateral hippocampus and contralateral cortex. Left to right, signals collected during baseline monitoring, 1 hour post surgery, 24 hours post rMCAO90min, and 1 week later. D, the root mean square (RMS) of EEG signals measured from data segments collected during baseline monitoring and at 1 hour, 24 hours and 1 week post sham operation or post MCAO. Data were presented as a percentage of the baseline EEG signals and grouped for animals with or without CS following sham operation or pMCAO/rMCAO. E, images of cresyl violet-stained brain sections obtained from one animal 24 hours post rMCAO90min. Note the weakly stained ipsilateral regions and enlarged ipsilateral to contralateral hemispheric areas in left and middle images. F, ipsilateral and contralateral hemispheric areas measured from brain sections at 8 coronal levels (from bregma −3.2 mm to 1.9 mm). Area of the ipsilateral hemisphere was normalized as a percentage of the corresponding contralateral hemisphere, and data were grouped for sham controls and animals with or without CS following either pMCAO or rMCAO. *, sham controls vs. other groups, p<0.05, one way ANOVA.
As with aging animals, no hippocampal-cortical EEG discharges were detected during CS (n=11) but NCS were evident following pMCAO/rMCAO (n=5; Fig 4B). Mean NCS latency (17.8±5.1 min) and duration (42.9±4.3 sec) did not significantly differ from aging mice (Table 1). Like with aging animals, NCS preceded CS in adult mice that developed both, and did not recur after the CS.
In adult mice with CS following pMCAO, rMCAO90min, and rMCAO45min, ipsilateral hippocampal EEG signals decreased to 67% of baseline at 1 hour (n=11) post-surgery and recovered to 91% at 24 hours (n=6) (Fig 4C, D). The early ipsilateral EEG suppression at 1 hour was significantly less than that observed in the aging mice (41%, p=0.01, Table 1). In CS-free adult animals, ipsilateral EEG signals decreased to 77% of baseline at 1 hour with subsequent recovery to 102% at 24 hours (Fig 4D) post-pMCAO/rMCAO.
Ipsilateral brain injury was seen in 16 adult mice with early-onset CS that successfully underwent histological processing 24–48 hours post pMCAO/rMCAO. Weakly stained ipsilateral regions were quantifiable in 6/16 adult animals (Fig 4E), and appeared to be less variable than those observed in aging mice (Table 1). The ipsilateral to contralateral hemispheric area ratio was increased to 108–117% at 8 coronal levels (Fig 4E, F), similar to increases seen in aging mice (Table 1). In addition, no histological evidence of gross brain injury was found in CS-free adult mice following pMCAO/rMCAO (n=4). The ipsilateral to contralateral hemispheric area ratio of these animals also did not differ from their age-matched sham controls (Fig 4F).
Adult mice (n=24) were treated identically to the aging mice, with IP lorazepam and fosphenytoin after two observed CS. Despite temporary seizure suppression following treatment, 18 animals still encountered acute mortality while 6 survived for 10–28 days after rMCAO90min and rMCAO45min (n=4 and 2 respectively). Gross ipsilateral infarctions were evident in 3 of the 5 surviving animals that underwent successful histological processing. The acute mortality rate in treated, adult animals following rMCAO90min was significantly lower than their aging counterparts (7/11 vs. 12/12; p=0.037; Table 1).
Discussion
Four main findings emerged from our study. 1) Aging mice (16–20 months-old) are susceptible to early-onset CS development following pMCAO/rMCAO. 2) Development of CS is closely associated with severe brain injury. 3) CS occurrence is strongly linked to acute mortality, and while anticonvulsive treatment can transiently suppress seizure genesis, it does not decrease overall mortality. 4) Compared to aging mice, adult mice (6–8 months) exhibit morphologically identical early-onset, post MCAO CS but have better survival when treated following rMCAO90min.
Modeling early-onset post-ischemic seizures in aging mice
C57 black mice (C57BL/6) are commonly utilized in aging and neuroscience research (Flurkey et al., 2007). However, the posterior communicating arteries that connect the posterior cerebral artery and internal carotid artery territories are often underdeveloped or hypoplastic in this strain. This inherited cerebrovascular defect may contribute to the extensive brain injuries seen in C57 black mice following periods of global or focal brain ischemia (Fujii et al., 1997; Kitagawa et al., 1998; Ozdemir et al., 1999; Majid 2003; McColl et al., 2004; Adhami et al., 2006; El-Hayek et al., 2011a). Since stroke severity is a risk factor for post-ischemic seizure genesis, this anatomical defect may have contributed to the higher prevalence of experimental post-stroke seizures in our model than what has been documented clinically (Bladin and Bornstein, 2009; Brodie et al., 2009; Menon and Shorvon, 2009; Balami et al., 2011; Chen et al., 2010; Gilad 2012; Guekht and Bornstein, 2012; Procaccianti et al., 2012; Chung, 2014).
We utilized four different MCAO protocols (pMCAO, rMCAO45min, rMCAO90min, MCAO-e) in order to evaluate the effects of different durations of ischemia and reperfusion on CS development. The MCAO-e group was included in order to evaluate the effect of small, focal infarcts on CS development without the inherent variability of a suture-insertion method. Although we may have expected to see a lower incidence of CS in the rMCAO45min group compared to the rMCAO90min group and the pMCAO group (Barber 2005), there were no significant differences in CS incidence between any of the groups in either aging or adult mice (Table 1). Therefore, the complete cohort was used in the analysis for both age groups.
The association we established between early-onset CS and acute mortality is in keeping with post-stroke seizures as a poor prognostic factor clinically including its association with higher mortality (Waterhouse et al., 1998; Vespa et al., 2003; Szaflarski et al., 2008; Burneo et al., 2010; Huang et al., 2014). The greater mortality rates in aging mice compared to adult mice may reflect the relative intolerance of aging/aged animals to ischemic insults and a blunted recovery response (Popa-Wagner et al., 2011). Aging animals are also more susceptible to the perioperative complications of the MCAO, including the use of anesthesia, which may exacerbate ischemic brain injury leading to poorer physical presentation. Aging animals with CS were seemingly dichotomized into those with short CS latencies, and a minority with long CS latencies (Results section 1). This variability may have arisen due to differences in the severity of the induced ischemia amongst animals or to documented differences in the susceptibility of different aging animals to anesthesia and surgical procedures (Liu and McCullough, 2011). Based on our findings and the corroborating clinical evidence, our model encapsulates key clinical features of post-stroke seizures that make it potentially viable for future translational studies.
Development of CS is closely associated with severe brain injury
In our study, severe brain injury was classified as a decrease in the ipsilateral EEG signal to ≤50% of the baseline level and/or histologically evident gross brain injury in the ipsilateral hemisphere (Figs 2–3). CS were found to have occurred exclusively in animals with severe brain injury, as evidenced by widespread ipsilateral edema and loss of cresyl-violet stained neurons in the pMCAO/rMCAO animals. This is further supported by the observation that no CS were seen in any animals from the MCAO-e group where histology showed only small, focal infarctions without the aforementioned features. Furthermore, histological evidence of gross brain injury was conspicuously absent in all CS-free animals. For example, there was no evidence of ipsilateral edema or atrophy (Fig 3E), or regions of evident weak cresyl-violet staining in animals without CS that survived 2–5 weeks following pMCAO/rMCAO. This suggests a potential relationship between both the presence and degree of brain injury to the subsequent development of early-onset CS. Whether the CS themselves exacerbate brain injury or if the eventual development of severe, ischemic brain injury is a requisite feature for early CS development remain to be determined.
Significant suppression of the ipsilateral EEG signals at 1 hour post-ischemia was closely associated with CS onset in the majority of cases. Since EEG suppression has been shown to be a sensitive measure of ischemia, this finding implies a close temporal relationship between brain ischemia and subsequent CS development (Faught 1993; Jordan 2004; El-Hayek et al., 2011a, b). Animals with only moderate ipsilateral EEG suppression (Fig 2C, E) without CS or histological signs of brain injury may have undergone insufficient brain ischemia. These findings together suggest that early-onset CS are a consequence of severe, ischemic brain injury.
Influence of CS on acute mortality and the effects of anticonvulsive drug treatments
Aging mice with CS had a high acute mortality rate despite anticonvulsive treatment. When left untreated, all aging mice with CS died shortly after or developed a status epilepticus-like condition, eventually leading to acute mortality. However, CS-free aging mice remained in acceptable physical condition post-MCAO and survived for several weeks prior to histological processing. These observations suggest that early-onset CS development is a major contributing factor to acute mortality.
Lorazepam and fosphenytoin are recommended for controlling status epilepticus clinically (Meierkord et al., 2010; Shorvon 2011). Lorazepam enhances GABAA receptor-mediated inhibition with lasting effects while fosphenytoin suppresses excessive activity of voltage-gated Na+ channels with less adverse effects than phenytoin. We combined lorazepam and fosphenytoin in an attempt to control recurrent CS in our animals through the synergistic anticonvulsive effects of these drugs. However, treatment after CS development only offered transient seizure control and did not significantly improve survival (Table 1). This phenomenon may be analogous to the refractory nature of recurrent seizures or status epilepticus-like conditions clinically and in other animal models (Reddy and Kuruba, 2013). It is also unlikely that the severe, progressive ischemic brain injury leading to the poor physical condition of these aging mice can be reversed by anticonvulsive treatment alone. However, the effects of repetitive anticonvulsive drug treatments, similar to those used clinically for status epilepticus (Meierkord et al., 2010; Shorvon 2011) remain to be evaluated. This may be accomplished using continuous intravenous infusion or systemic application through a pump. Whereas repeated dosing may be able to rescue additional animals, prophylactic or early anticonvulsive treatments (given prior to the very first CS or NCS) may also offer neuroprotection (Calabresi et al., 2003) in ischemic aging mice and may be a topic worth exploring in the future.
Influence of age on genesis of early-onset post-ischemic CS
We found that the post-MCAO CS observed in aging and adult mice were similar in regards to motor behavior, incidence, latency, the lack of associated hippocampal-cortical EEG discharges, and its association with severe brain injury. These notable similarities suggest that there may be a common mechanism underlying the development of these early-onset CS in aging and adult animals. However, both the early ipsilateral EEG suppression (at 1 hour post-ischemia) and the acute mortality rate following rMCAO90min were greater in aging mice than in adult mice (Table 1). Aging mice were also more likely to demonstrate poorer physical condition (such as with prolonged immobility or lack of eating or drinking) following pMCAO/rMCAO and a greater proportion of aging mice had their first CS later on during overnight monitoring compared to adult mice (Table 1). The decreased tolerance of aging animals to anesthesia and surgery in addition to an age-related increase in vulnerability to brain ischemia (Liu and McCullough, 2011; Popa-Wagner et al., 2011) may have contributed to both poorer outcomes and a longer CS latency in some instances.
Generation of early-onset CS may involve or affect deeper subcortical structures
Current clinical evidence has implicated deeper sub-cortical structures in the pathogenesis of generalized seizures. Seizures and seizure-like behaviors have been observed in patients with brainstem stroke (Saposnik, 2001; Rollins et al., 2013). Furthermore, generalized tonic-clonic seizures in patients with temporal lobe epilepsy do not always have corresponding cortical EEG discharges (Schindler et al., 2007) but tend to correlate with abnormally increased cerebral blood flow in deeper brain structures, including the brainstem (Blumenfeld et al., 2009). The role of subcortical structures in seizure generation and propagation has long been recognized in animal models (Gale 1992; Miller 1992). In particular, CS with rapid running, jumping, and/or barrel-rolling have been observed in rodent models of brainstem electrical kindling (Omori et al., 2001; Lam et al., 2010) and audiogenic seizures during ethanol withdrawal (Yang et al., 2003). In these models, the CS were thought to involve or affect the midbrain reticular formation, periaqueductal gray matter, and/or superior colliculus based on local EEG discharges and spike activity. Brainstem areas have also been implicated in seizure genesis in rats following systemic injections of the GABAA receptor antagonist bicuculline (DeSalvo et al., 2010).
Behaviorally, the CS we observed from both aging and adult mice resemble the generalized tonic-clonic seizures described in previous rodent models (Velíšková 2006; Ivanov et al., 2004; Muramatsu et al., 2008; Silva-Fernandes et al., 2010; El-Hayek et al., 2011a). Hippocampal and parietal cortical discharges were absent during all CS regardless of whether the EEG recordings were made with monopolar or twisted bipolar electrodes (Figs 1, 4). This was unlikely due to the limitations of our EEG recordings since we were able to reliably detect NCS in a large number of animals using the same recording protocols and technology. In light of the similarities between the post-MCAO CS observed in our study and the brainstem CS previously observed from other models (Omori et al., 2001; Yang et al., 2003; Lam et al., 2010), we suggest that generation of early-onset CS following MCAO may involve and/or affect deeper subcortical structures.
The mechanisms underlying the pathogenesis of these CS still remain unclear. Gross brainstem injury may not be a primary factor due to the short latency of the CS (mean latencies of 32.8 min and 45.6 min in aging and adult mice respectively). Therefore, CS onset may precede the development of structural neuronal damage in the majority of ischemic animals. There was also a conspicuous lack of gross, histological brainstem injury when examined 24–48 hours and 2–5 weeks post-ischemia. In light of observations that tactile handling or moderate auditory stimuli could trigger CS in ischemic animals, it is possible that peripheral ascending inputs may facilitate CS generation when cortical descending inhibition is compromised during early ischemia. Further experiments examining EEG signals in multiple subcortical areas simultaneously in individual mice, although technically challenging, may reveal the precise regional initiation and subsequent spread of these early-onset CS.
Other study limitations
In our study, we did not measure cerebral blood flow to confirm the success of the MCAO. This was in part due to the technical constraints of the EEG electrode headset, which physically obstructs the mouse calvarium. Furthermore, an accurate CBF measurement may require animals to be anesthetized, which would suppress early-onset CS. Instead of measuring cerebral blood flow, we utilized EEG suppression as a previously validated surrogate measure of brain ischemia (Faught 1993; Jordan 2004; El-Hayek et al., 2011a, b). The reliability of our EEG recordings was validated by the demonstrated ability to detect NCS and to assess EEG suppression at early post-ischemic time points. However, there were a group of aging mice that did not have EEG suppression or histologic evidence of brain injury. These animals may have undergone a technically unsuccessful MCAO due to inappropriate suture insertion. Further methodological improvements in the MCAO procedure may diminish this underlying variability in the future.
Our study was designed to study the effects of brain ischemia on seizure genesis. Our data were limited in terms of evaluating hemorrhagic stroke-like events and post-hemorrhagic seizures (Vespa et al., 2003; Balami and Buchan, 2012; Klahr et al., 2015). The presence of a hemorrhage could not be histologically determined as all animals underwent cardiac perfusion prior to brain dissection and histological processing. Therefore, even in the presence of visible ventricular blood in certain animals, it could not be conclusively determined if the bleeding originated from the MCAO or as a direct consequence of the transcardiac perfusion. The issue of a potential hemorrhagic component contributing to early-onset seizures in our model remains to be addressed.
In our study, there was a high acute mortality rate in aging mice following “successful” pMCAO/rMCAO. While we established a close relationship between acute mortality and the development of early-onset CS, the high mortality rate still imposed limitations on several experimental measures. In particular, the histological assessment of brain injury was limited by animal survival particularly at later post-ischemic time points. Therefore, a direct comparison of histological parameters between aging and adult mice (Table 1) was inconclusive due to the low sample sizes and resulting high variability within each group. In addition, the acute mortality of many animals in our study precludes an adequate examination of late-onset, epileptic seizures and other chronic behavioral abnormalities in ischemic aging mice. Further strategies to reduce acute mortality will be needed in order to assess post-stroke epilepsy and its associated effects on outcome.
Conclusion
We describe here a model of early-onset post-ischemic seizures in aging mice that replicates some common features of clinical post-stroke seizures. Our data suggest that early-onset CS are a poor prognostic factor and CS generation may involve or affect deeper subcortical structures. Our study may provide useful information to further elucidate the pathophysiology of these post-stroke seizures such that in the future, more timely diagnoses and effective interventions may be developed clinically.
Supplementary Material
Acknowledgments
This work was supported by research grants from the Canadian Institute of Health Research to LZ and RM (Grant # 219171); from Ontario Brain Institute Epilepsy Research Program to JE and LZ; and from Natural Science and Engineering Research Council of Canada to LZ.
Abbreviations
- CS
convulsive seizures
- EEG
electroencephalography
- MCAO
middle cerebral artery occlusion
- MCAO-e
middle cerebral artery occlusion via electrocoagulation
- NCS
non-convulsive seizures
- pMCAO
permanent middle cerebral artery occlusion
- rMCAO
reversible middle cerebral artery occlusion
- RMS
root mean square
Footnotes
Author contribution statement
CPW and JW conducted most of experiments and data analysis. JP, NP, YH, XG and SA conducted experiments and data analysis. JW, JHE, RM and LZ contributed in experimental design and manuscript writing.
Disclosure/Conflict of Interest
No complicit of interest needs to be disclosed for all authors.
References
- Adhami F, Liao GH, Morozov YM, Schloemer A, Schmithorst VJ, Lorenz JM, Dunn RS, Vorhees CV, Wills-Karp M, Degen JL, Davis RJ, Mizushima N, Rakic P, Dardzinski BJ, Holland SK, Sharp FR, Kuan CY. Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am J Pathol. 2006;169:566–583. doi: 10.2353/ajpath.2006.051066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balami JS, Buchan AM. Complications of intracerebral haemorrhage. Lancet Neurol. 2012;11:101–118. doi: 10.1016/S1474-4422(11)70264-2. [DOI] [PubMed] [Google Scholar]
- Balami JS, Chen RL, Grunwald IQ, Buchan AM. Neurological complications of acute ischaemic stroke. Lancet Neurol. 2011;10:357–371. doi: 10.1016/S1474-4422(10)70313-6. [DOI] [PubMed] [Google Scholar]
- Barber PA, Hoyte L, Kirk D, Foniok T, Buchan A, Tuor U. Early T1 and T2-weighted MRI signatures of transient and permanent middle cerebral artery occlusion in a murine stroke model studied at 9.4T. Neurosci Lett. 2005;388:54–59. doi: 10.1016/j.neulet.2005.06.067. [DOI] [PubMed] [Google Scholar]
- Bladin CF, Bornstein N. Post-stroke seizures. In: Fisher M, editor. Stroke Part II: Clinical Manifestations and Pathogenesis, Handbook Clin Neurol. Vol. 93. Elsevier B.V; 2009. pp. 613–621. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Varghese GI, Purcaro MJ, Motelow JE, Enev M, McNally KA, Levin AR, Hirsch LJ, Tikofsky R, Zubal IG, Paige AL, Spencer SS. Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain. 2009;132:999–1012. doi: 10.1093/brain/awp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MJ, Elder AT, Kwan P. Epilepsy in later life. Lancet Neurol. 2009;8:1019–1030. doi: 10.1016/S1474-4422(09)70240-6. [DOI] [PubMed] [Google Scholar]
- Burneo JG, Fang J, Saposnik G Investigators of the Registry of the Canadian Stroke Network. Impact of seizures on morbidity and mortality after stroke: a Canadian multi-centre cohort study. Eur J Neurol. 2010;17:52–58. doi: 10.1111/j.1468-1331.2009.02739.x. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Cupini ML, Centonze D, Pisani F, Bernardi G. Antiepileptic drugs as a possible neuroprotective strategy of brain ischemia. Ann Neurol. 2003;53:693–702. doi: 10.1002/ana.10603. [DOI] [PubMed] [Google Scholar]
- Claassen J, Jetté N, Chum F, Green R, Schmidt M, Choi H, Jirsch J, Frontera JA, Connolly ES, Emerson RG, Mayer SA, Hirsch LJ. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurol. 2007;69:1356–1365. doi: 10.1212/01.wnl.0000281664.02615.6c. [DOI] [PubMed] [Google Scholar]
- Chen RL, Balami JS, Esiri MM, Chen LK, Buchan AM. Ischemic stroke in the elderly: an overview of evidence. Nat Rev Neurol. 2010;6:256–265. doi: 10.1038/nrneurol.2010.36. [DOI] [PubMed] [Google Scholar]
- Chung JM. Seizures in the acute stroke setting. Neurol Res. 2014;36:403–406. doi: 10.1179/1743132814Y.0000000352. [DOI] [PubMed] [Google Scholar]
- Cipriani R, Villa P, Chece G, Lauro C, Paladini A, Micotti E, Perego C, De Simoni MG, Fredholm BB, Eusebi F, Limatola C. CX3CL1 is neuroprotective in permanent focal cerebral ischemia in rodents. J Neurosci. 2011;31:16327–16335. doi: 10.1523/JNEUROSCI.3611-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuomo O, Rispoli V, Leo A, Politi GB, Vinciguerra A, di Renzo G, Cataldi M. The antiepileptic drug levetiracetam suppresses non-convulsive seizure activity and reduces ischemic brain damage in rats subjected to permanent middle cerebral artery occlusion. PLoS One. 2013;8:e80852. doi: 10.1371/journal.pone.0080852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSalvo MN, Schridde U, Mishra AM, Motelow JE, Purcaro MJ, Danielson N, Bai X, Hyder F, Blumenfeld H. Focal BAGING fMRI changes in bicuculline-induced tonic-clonic seizures in the rat. Neuroimage. 2010;50:902–909. doi: 10.1016/j.neuroimage.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durukan A, Tatlisumak T. Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav. 2007;87:179–197. doi: 10.1016/j.pbb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- El-Hayek YH, Wu CP, Chen R, Al-Sharif AR, Huang S, Patel N, Du C, Ruff CA, Fehlings MG, Carlen PL, Zhang L. Acute post-ischemic seizures are associated with increased mortality and brain damage in adult Mice. Cereb, Cortex. 2011a;21:2863–2875. doi: 10.1093/cercor/bhr080. [DOI] [PubMed] [Google Scholar]
- El-Hayek YH, Wu CP, Zhang L. Early suppression of intracranial EEG signals predicts ischemic outcome in adult mice following hypoxia-ischemia. Exp Neurol. 2011b;231:295–303. doi: 10.1016/j.expneurol.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Faught E. Current role of electroencephalography in cerebral ischemia. Stroke. 1993;24:609–613. doi: 10.1161/01.str.24.4.609. [DOI] [PubMed] [Google Scholar]
- Flurkey K, Currer JM, Harrison DE. Mouse Models in Aging Research. In: Fox JG, Davisson MT, Quimby FW, Barthaging SW, Newcomer CE, Smith AL, editors. The Mouse in Biomedical Research. Academic Press; New York: 2007. pp. 637–672. [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- Fujii M, Hara H, Meng W, Vonsattel JP, Huang Z, Moskowitz MA. Strain-related differences in susceptibility to transient forebrain ischemia in SV-129 and C57black/6 mice. Stroke. 1997;28:1805–1811. doi: 10.1161/01.str.28.9.1805. [DOI] [PubMed] [Google Scholar]
- Gale K. Subcortical structures and pathways involved in convulsive seizure generation. J Clin Neurophysiol. 1992;9:264–277. doi: 10.1097/00004691-199204010-00007. [DOI] [PubMed] [Google Scholar]
- Gilad R. Management of Seizures following a Stroke. What are the Options? Drugs Aging. 2012;29:533–538. doi: 10.2165/11631540-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Guekht A, Bornstein NM. Seizures after stroke. In: Stefan H, Theodore WH, editors. Epilepsy, Handbook of Clinical Neurology. Vol. 108. Elsevier B.V; 2012. pp. 570–583. 3rd series. [DOI] [PubMed] [Google Scholar]
- Guth JC, Gerard EE, Nemeth AJ, Liotta EM, Prabhakaran S, Naidech AM, Maas MB. Subarachnoid extension of hemorrhage is associated with early seizures in primary intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2014;23:2809–2813. doi: 10.1016/j.jstrokecerebrovasdis.2014.07.023. [DOI] [PubMed] [Google Scholar]
- Hartings JA, Williams AJ, Tortella FC. Occurrence of nonconvulsive seizures, periodic epileptiform discharges, and intermittent rhythmic delta activity in rat focal ischemia. Exp Neurol. 2003;179:139–149. doi: 10.1016/s0014-4886(02)00013-4. [DOI] [PubMed] [Google Scholar]
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota 1935–1984. Epilepsia. 1993;34:453–458. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- He J, Hsiang HL, Wu C, Hes L, Mylvagnanam S, Carlen PL, Zhang L. Cellular mechanisms of cobalt-induced hippocampal epileptiform discharges. Epilepsia. 2009;50:99–115. doi: 10.1111/j.1528-1167.2008.01767.x. [DOI] [PubMed] [Google Scholar]
- Hossmann KA. Cerebral ischemia: models, methods and outcomes. Neuropharmacol. 2008;55:257–270. doi: 10.1016/j.neuropharm.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Howells DW, Porritt MJ, Rewell SS, O’Collins V, Sena ES, van der Worp HB, Traystman RJ, Macleod MR. Different strokes for different folks: the rich diversity of animal models of focal cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:1412–1431. doi: 10.1038/jcbfm.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CW, Saposnik G, Fang J, Steven DA, Burneo JG. Influence of seizures on stroke outcomes: A large multicenter study. Neurol. 2014;82:768–776. doi: 10.1212/WNL.0000000000000166. [DOI] [PubMed] [Google Scholar]
- Ivanov SV, Ward JM, Tessarollo L, McAreavey D, Sachdev V, Fananapazir L, Banks MK, Morris N, Djurickovic D, Devor-Henneman DE, Wei MH, Alvord GW, Gao B, Richardson JA, Minna JD, Rogawski MA, Lerman MI. Cerebellar ataxia, seizures, premature death, and cardiac abnormalities in mice with targeted disruption of the Cacna2d2 gene. Am J Pathol. 2004;165:1007–1018. doi: 10.1016/S0002-9440(10)63362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey M, Lang M, Gane J, Chow E, Wu CP, Zhang L. Novel anticonvulsive effects of progesterone in a mouse model of hippocampal electrical kindling. Neurosci. 2014;257:65–75. doi: 10.1016/j.neuroscience.2013.10.074. [DOI] [PubMed] [Google Scholar]
- Jetter GM, Cavazos JE. Epilepsy in the elderly. Semi Neurol. 2008;28:336–341. doi: 10.1055/s-2008-1079338. [DOI] [PubMed] [Google Scholar]
- Jordan KG. Emergency EEG and continuous EEG monitoring in acute ischemic stroke. J Clin Neurophysiol. 2004;21:341–352. [PubMed] [Google Scholar]
- Karhunen H, Nissinen J, Sivenius J, Jolkkonen J, Pitkänen A. A long-term video-EEG and behavioral follow-up after endothelin-1 induced middle cerebral artery occlusion in rats. Epilepsy Res. 2006;72:25–38. doi: 10.1016/j.eplepsyres.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Karhunen H, Bezvenyuk Z, Nissinen J, Sivenius J, Jolkkonen J, Pitkänen A. Epileptogenesis after cortical photothrombotic brain lesion in rats. Neurosci. 2007;148:314–324. doi: 10.1016/j.neuroscience.2007.05.047. [DOI] [PubMed] [Google Scholar]
- Kelly KM, Shiau DS, Jukkola PI, Miller ER, Mercadante AL, Quigley MM, Nair SP, Sackellares JC. Effects of age and cortical infarction on EEG dynamic changes associated with spike wave discharges in F344 rats. Exp Neurol. 2011;232:15–21. doi: 10.1016/j.expneurol.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KM. Stroke. In: Pitkänen A, Schwartzkroin PA, Moshe SL, editors. Animal models of seizures and epilepsy. Elsevier Academic Press; San Diego: 2006. pp. 501–519. [Google Scholar]
- Kelly KM, Kharlamov A, Hentosz TM, Kharlamova EA, Williamson JM, Bertram EH, 3rd, Kapur J, Armstrong DM. Photothrombotic brain infarction results in seizure activity in aging Fischer 344 and Sprague Dawley rats. Epilepsy Res. 2001;47:189–203. doi: 10.1016/s0920-1211(01)00294-7. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Yang G, Mabuchi T, Yagita Y, Hori M, Yanagihara T. Cerebral ischemia after bilateral carotid artery occlusion and intraluminal suture occlusion in mice: evaluation of the patency of the posterior communicating artery. J Cereb Blood Flow Metab. 1998;18:570–579. doi: 10.1097/00004647-199805000-00012. [DOI] [PubMed] [Google Scholar]
- Klahr AC, Dickson CT, Colbourne F. Seizure activity occurs in the collagenase but not the blood infusion model of striatal hemorrhagic stroke in rats. Transl Stroke Res. 2015;6:29–38. doi: 10.1007/s12975-014-0361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulhari A, Strbian D, Sundararajan S. Early onset seizures in stroke. Stroke. 2014;45:e249–251. doi: 10.1161/STROKEAHA.114.006974. [DOI] [PubMed] [Google Scholar]
- Lam A, Whelan N, Corcoran ME. Susceptibility of brainstem to kindling and transfer to the forebrain. Epilepsia. 2010;51:1736–1744. doi: 10.1111/j.1528-1167.2010.02551.x. [DOI] [PubMed] [Google Scholar]
- Liu D, Wu L, Breyer R, Mattson MP, Andreasson K. Neuroprotection by the PGE2 EP2 receptor in permanent focal cerebral ischemia. Ann Neurol. 2005;57:758–761. doi: 10.1002/ana.20461. [DOI] [PubMed] [Google Scholar]
- Liu FD, McCullough LD. Middle cerebral artery occlusion model in rodents: methods and potential pitfalls. J Biomed Biotech. 2011;2011:Article ID 464701. doi: 10.1155/2011/464701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XC, Si Y, Williams AJ, Hartings JA, Gryder D, Tortella FC. NNZ-2566, a glypromate analog, attenuates brain ischemia-induced non-convulsive seizures in rats. J Cereb Blood Flow Metab. 2009;29:1924–1932. doi: 10.1038/jcbfm.2009.109. [DOI] [PubMed] [Google Scholar]
- Majid A, He YY, Gidday JM, Kaplan S, Gonzales ER, Park TS, Fenstermacher JD, Wei L, Choi DW, Hsu CY. Differences in vulnerability to permanent focal cerebral ischemia among 3 common mouse strains. Stroke. 2003;31:2707–2714. doi: 10.1161/01.str.31.11.2707. [DOI] [PubMed] [Google Scholar]
- McColl BW, Carswell HV, McCulloch J, Horsburgh K. Extension of cerebral hypoperfusion and ischaemic pathology beyond MCA territory after intraluminal filament occlusion in C57Bl/6J mice. Brain Res. 2004;997:15–23. doi: 10.1016/j.brainres.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Meierkord H, Boon P, Engelsen B, Göcke K, Shorvon S, Tinuper P, Holtkamp M European Federation of Neurological Societies. EFNS guideline on the management of status epilepticus in adults. Eur J Neurol. 2010;17:348–55. doi: 10.1111/j.1468-1331.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- Menon B, Shorvon SD. Ischaemic stroke in adults and epilepsy. Epilepsy Res. 2009;87:1–11. doi: 10.1016/j.eplepsyres.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Miller JW. The role of mesencephalic and thalamic arousal systems in experimental seizures. Prog Neurobiol. 1992;39:155–178. doi: 10.1016/0301-0082(92)90009-4. [DOI] [PubMed] [Google Scholar]
- Moyanova SG, Mastroiacovo F, Kortenska LV, Mitreva RG, Fardone E, Santolini I, Sobrado M, Battaglia G, Bruno V, Nicoletti F, Ngomba RT. Protective role for type 4 metabotropic glutamate receptors against ischemic brain damage. J Cereb Blood Flow Metab. 2011;31:1107–1118. doi: 10.1038/jcbfm.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB American Heart Association Statistics Committee and Stroke, Statistics Subcommittee. Heart disease and stroke statistics- 2015 update: a report from the American Heart Association. Circulation. 131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Muramatsu R, Ikegaya Y, Matsuki N, Koyama R. Early-life status epilepticus induces ectopic granule cells in adult mice dentate gyrus. Exp Neurol. 2008;211:503–510. doi: 10.1016/j.expneurol.2008.02.026. [DOI] [PubMed] [Google Scholar]
- Noebels J. A perfect storm: Converging paths of epilepsy and Alzheimer’s dementia intersect in the hippocampal formation. Epilepsia. 2011;52(suppl 1):39–46. doi: 10.1111/j.1528-1167.2010.02909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouh A, Remke J, Ruland S. Ischemic posterior circulation stroke: a review of anatomy, clinical presentations, diagnosis, and current management. Front Neurol. 2014;5:30. doi: 10.3389/fneur.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori N, Ishimoto T, Mutoh F, Chiba S. Kindling of the midbrain periaqueductal gray in rats. Brain Res. 2001;903:162–167. doi: 10.1016/s0006-8993(01)02436-2. [DOI] [PubMed] [Google Scholar]
- Ozdemir YG, Bolay H, Erdem E, Dalkara T. Occlusion of the MCA by an intraluminal filament may cause disturbances in the hippocampal blood flow due to anomalies of circle of Willis and filament thickness. Brain Res. 1999;822:260–264. doi: 10.1016/s0006-8993(99)01175-0. [DOI] [PubMed] [Google Scholar]
- Popa-Wagner A, Buga AM, Kokaia Z. Perturbed cellular response to brain injury during aging. Ageing Res Rev. 2011;10:71–79. doi: 10.1016/j.arr.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Procaccianti G, Zaniboni A, Rondelli F, Crisci M, Sacquegna T. Seizures in acute stroke: incidence, risk factors and prognosis. Neuroepidemiol. 2012;39:45–50. doi: 10.1159/000338374. [DOI] [PubMed] [Google Scholar]
- Publication Health Agency of Canada. Tracking heart disease and stroke in Canada - Stroke highlights 2011. 2011 http://www.phac-aspc.gc.ca/cd-mc/cvd-mcv/sh-fs-2011/index-eng.php>.
- Reddy DS, Kuruba R. Experimental models of status epilepticus and neuronal injury for evaluation of therapeutic interventions. Int J Mol Sci. 2013;14:18284–18318. doi: 10.3390/ijms140918284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reglodi D, Somogyvari-Vigh A, Vigh S, Maderdrut JL, Arimura A. Neuroprotective effects of PACAP38 in a rat model of transient focal ischemia under various experimental conditions. Ann N Y Acad Sci. 2000;921:119–128. doi: 10.1111/j.1749-6632.2000.tb06958.x. [DOI] [PubMed] [Google Scholar]
- Rollins N, Pride GL, Plumb PA, Dowling MM. Brainstem strokes in children: an 11-year series from a tertiary pediatric center. Pediatr Neurol. 2013;49:458–464. doi: 10.1016/j.pediatrneurol.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Saposnik G, Caplan LR. Convulsive-like movements in brainstem stroke. Arch Neurol. 2001;58:654–657. doi: 10.1001/archneur.58.4.654. [DOI] [PubMed] [Google Scholar]
- Schindler K, Leung H, Lehnertz K, Elger CE. How generalised are secondarily “generalised” tonic clonic seizures? J Neurol Neurosurg Psych. 2007;78:993–9966. doi: 10.1136/jnnp.2006.108753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabanzadeh AP, Shuaib A, Wang CX. Simvastatin reduced ischemic brain injury and perfusion deficits in an embolic model of stroke. Brain Res. 2005;1042:1–5. doi: 10.1016/j.brainres.2005.01.105. [DOI] [PubMed] [Google Scholar]
- Shorvon S. The treatment of status epilepticus. Curr Opin Neurol. 2011;24:165–170. doi: 10.1097/WCO.0b013e3283446f31. [DOI] [PubMed] [Google Scholar]
- Silva-Fernandes A, Oliveiral P, Sousa1 N, Maciel P. Motor and behavioural abnormalities Associated with persistent spontaneous epilepsy in the fvb/n mouse strain. Scand J Lab Anim Sci. 2010;37:213–222. [Google Scholar]
- Silverman IE, Restrepo L, Mathews GC. Post stroke Seizures. Arch Neurol. 2002;59:195–201. doi: 10.1001/archneur.59.2.195. [DOI] [PubMed] [Google Scholar]
- Smurawska LT, Alexandrov AV, Bladin CF, Norris JW. Cost of acute stroke care in Toronto, Canada. Stroke. 1994;25:1628–1631. doi: 10.1161/01.str.25.8.1628. [DOI] [PubMed] [Google Scholar]
- Sykes L, Wood E, Kwan J. Antiepileptic drugs for the primary and secondary prevention of seizures after stroke. Cochrane Database Syst Rev. 2014;1:CD005398. doi: 10.1002/14651858.CD005398.pub3. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Rackley AY, Kleindorfer DO, Khoury J, Woo D, Miller R, Alwell K, Broderick JP, Kissela BM. Incidence of seizures in the acute phase of stroke: A population-based study. Epilepsia. 2008;49:974–981. doi: 10.1111/j.1528-1167.2007.01513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velíšková J. Behavioral characterization of seizures in rats. In: Pitkänen A, Schwartzkroin PA, Moshé SL, editors. Models of seizures and epilepsy. Elsevier Academic Press; San Diego: 2006. pp. 601–610. [Google Scholar]
- Vespa PM, O’Phelan K, Shah M, Mirabelli J, Starkman S, Kidwell C, Saver J, Nuwer MR, Frazee JG, McArthur DA, Martin NA. Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurol. 2003;60:1441–1446. doi: 10.1212/01.wnl.0000063316.47591.b4. [DOI] [PubMed] [Google Scholar]
- Wais M, Wu CP, Zahid T, Del Campo M, Zhang L. Repeated hypoxic episodes induce seizures and alter hippocampal network activities in mice. Neurosci. 2009;161:599–613. doi: 10.1016/j.neuroscience.2009.03.036. [DOI] [PubMed] [Google Scholar]
- Wang B, Cao W, Biswal S, Doré S. Carbon monoxide-activated Nrf2 pathway leads to protection against permanent focal cerebral ischemia. Stroke. 2011;42:2605–2610. doi: 10.1161/STROKEAHA.110.607101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CX, Yang T, Shuaib A. An improved version of embolic model of brain ischemic injury in the rat. J Neurosci Meth. 2001;109:147–151. doi: 10.1016/s0165-0270(01)00408-3. [DOI] [PubMed] [Google Scholar]
- Waterhouse EJ, DeLorenzo RJ. Status epilepticus in aginger patients: epidemiology and treatment options. Drugs Aging. 2001;18:133–142. doi: 10.2165/00002512-200118020-00006. [DOI] [PubMed] [Google Scholar]
- Waterhouse EJ, Vaughan JK, Barnes TY, Boggs JG, Towne AR, Kopec-Garnett L, DeLorenzo RJ. Synergistic effect of status epilepticus and ischemic brain injury on mortality. Epilepsy Res. 1998;29:175–183. doi: 10.1016/s0920-1211(97)00071-5. [DOI] [PubMed] [Google Scholar]
- Williams AJ, Tortella FC, Lu XM, Moreton JE, Hartings JA. Antiepileptic drug treatment of nonconvulsive seizures induced by experimental focal brain ischemia. J Pharmacol Exp Ther. 2004;311:220–227. doi: 10.1124/jpet.104.069146. [DOI] [PubMed] [Google Scholar]
- Williams AJ, Bautista CC, Chen RW, Dave JR, Lu XC, Tortella FC, Hartings JA. Evaluation of gabapentin and ethosuximide for treatment of acute nonconvulsive seizures following ischemic brain injury in rats. J Pharmacol Exp Ther. 2006;318:947–955. doi: 10.1124/jpet.106.105999. [DOI] [PubMed] [Google Scholar]
- Wu CP, Wais M, Sheppy E, del Campo M, Zhang L. A glue-based, screw-free method for implantation of intra-cranial electrodes in young mice. J Neurosci Meth. 2008;171:126–131. doi: 10.1016/j.jneumeth.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Yang L, Long C, Randall ME, Faingaging CL. Neurons in the periaqueductal grey are critically involved in the neuronal network for audiogenic seizures during ethanol withdrawal. Neuropharmacol. 2003;44:275–281. doi: 10.1016/s0028-3908(02)00367-2. [DOI] [PubMed] [Google Scholar]
- Zorowitz RD, Chen E, Tong KB, Laouri M. Costs and rehabilitation use of stroke survivors: a retrospective study of Medicare beneficiaries. Top Stroke Rehabil. 2009;16:309–320. doi: 10.1310/tsr1605-309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.