Abstract
Epidemiologic studies on acute effects of air pollution have generally been limited to larger cities, leaving questions about rural populations behind.
Recently, we had developed a spatiotemporal model to predict daily PM2.5 level at a 1 km2 using satellite aerosol optical depth data. Based on the results from the model, we applied a case-crossover study to evaluate the acute effect of PM2.5 on mortality in North Carolina, South Carolina, and Georgia between 2007 and 2011.
Mortality data were acquired from the departments of public health in the states and modeled PM2.5 exposures were assigned to the zip code of residence of each decedent. We performed various stratified analyses by age, sex, race, education, cause of death, residence, and EPA standards. We also compared results of analyses using our modeled PM2.5 levels and those imputed daily from the nearest monitoring station.
848,270 non-accidental death records were analyzed and we found each 10 μg/m3 increase in PM2.5 (mean lag0 and lag1) was associated with a 1.56% (1.19, 1.94) increase in daily deaths. Cardiovascular disease (2.32%, 1.57–3.07) showed the highest effect estimate. Blacks (2.19%, 1.43–2.96) and persons with education ≤8 yr (3.13%, 2.08–4.19) were the most vulnerable populations. The effect of PM2.5 on mortality still exists in zip code areas that meet the PM2.5 EPA annual standard (2.06%, 1.97–2.15). The effect of PM2.5 below both EPA daily and annual standards was 2.08% (95% CI = 1.99 to 2.17). Our results showed more power and suggested that the PM2.5 effects on rural populations have been underestimated due to selection bias and information bias.
We have demonstrated that our AOD-based exposure models can be successfully applied to epidemiologic studies. This will add new study populations in rural areas, and will confer more generalizability to conclusions from such studies.
INTRODUCTION
Numerous epidemiologic studies have reported a positive relationship between acute exposures to fine particles (particles less than 2.5 micrometers in diameter, PM2.5) and mortality ranging from all-cause deaths to respiratory or cardiovascular death 1–7. PM2.5 is believed to increase mortality by producing inflammation and oxidative stress 8, in part because its small size allows it to penetrate into the alveoli and be retained in the lung parenchyma 9. Toxicological research has shown that the PM2.5 components such as metals, sulfate, nitrate, or organic compounds also elicit reactive oxygen species (ROS), inflammatory injury, oxidative DNA damage, and other biological effects 10.
Traditionally, PM2.5 measurements from ground-level monitoring stations centrally located in the study domain have been used as surrogates for individual-level exposures to PM2.5. Ecological exposure assignment is more subject to biases than individual-level exposure measurement, and the increased measurement error generally makes the risk estimates attenuated 11 and results in less statistical power 12. The use of the existing monitoring stations imposes temporal limitations as well as spatial. Many monitors in the U.S. operate only every third or even sixth day.
As a result, a majority of studies are done in cities or urban areas close to the location of those monitors. Restriction in study population imposes a problem of generalizability of those epidemiologic studies.
However, the characteristics of rural populations may be different from urban populations with regard to such factors as education, housing, and the accessibility to health care facilities; this may lead to a different response to environmental stimuli. Also urban and rural air pollution mixtures are different in composition. Therefore, there have been a lot of uncertainties about acute PM2.5 effects outside of large cities.
Recently, we have developed a spatiotemporal model to predict daily PM2.5 level at a 1 km×1km resolution for the Southeastern United States between 2003 through 2011. It allowed us to estimate spatially resolved PM2.5 on a daily basis throughout the 7 states located in the Southeastern U.S: North Carolina, South Carolina, Georgia, Tennessee, Alabama, Mississippi, and Florida. We have also obtained zip-code level mortality data in three of those states: North Carolina, South Carolina, and Georgia between 2007 and 2011. Therefore, we use our model generated predictions to study the acute effect of PM2.5 on mortality in the entire population of North Carolina, South Carolina, and Georgia between 2007 and 2011.
DATA AND METHODS
Outcome
We acquired mortality data from the departments of public health in Georgia, North Carolina, and South Carolina. Data was available between 2007 through 2011. The data variables include the date of death, age, sex, race, education, primary cause of death in ICD-code 10th version, and residential zip code. We restricted our analyses to deaths from internal causes by excluding ICD codes V01 through Y98. Specific causes were derived from the ICD code for the underlying cause of death: respiratory disease (ICD codes J00 through J99), cardiovascular disease (ICD codes I01 through I52), and stroke (ICD codes I60 through J69).
As a result, we used 848,270 non-accidental deaths occurring in the study area from 2007 through 2011. Mortality data were unidentifiable, therefore, our research was exempted by the Harvard School of Public Health’s Human Subjects Committee.
Exposure
PM2.5 exposure estimates were generated by our prediction model, which covers Southeastern United States consists of North Carolina, South Carolina, Georgia, Tennessee, Alabama, Mississippi, and Florida for the years 2007–2011 at a 1×1 km resolution. To begin with, daily PM2.5 measurements were associated with Aerosol Optical Depth (AOD) data from the Moderate Resolution Imaging Spectroradiometer (MODIS) on the Aqua satellite and other meteorological and land use predictors. We used ground-level PM2.5 measurements from 277 monitoring sites from the Environmental Protection Agency (EPA) and Interagency Monitoring of Protected Visual Environments (IMPROVE) monitoring networks. Our predictors were AOD data at a 1 km resolution, land use terms (elevation, distance to major roads, percent of open space, point and area emissions), and meteorological variables (temperature, wind speed, relative humidity and visibility). We applied inverse probability weights (IPW) to the models to adjust for the non-random missingness of AOD. Since AOD measurement are missing mainly due to weather conditions such as cloud and snow cover, covariates that affects them were used to model the probability of missingness in the AOD data: temperature, wind speed, sea level pressure, elevation and the season. Once the probability is inversed and normalized by its mean, the weight was placed on AOD values present.
To accommodate the fact that the PM-AOD calibration factors can vary spatially between large regions, we divided the Southeastern area into 3 regions and their 31 sub-regions. The intercept, AOD, and temperature random effects in the model are nested within regions of the study. In the next stage of modeling, we simply predicted PM2.5 concentrations to grid cells without monitors using the model fit. For the grid cells that were missing AOD observations, we fitted a model by each year to spatially interpolate predictions from the second stage. Specifically, for each region in the Southeast, we used the PM2.5 predictions for the days when AOD was not missing, and estimated a smooth function of latitude and longitude with a random intercept for each cell and a slope for the mean of PM2.5 monitors within 100 km of the grid cell on that day. The results of this model were used to estimate the PM2.5 for grid cell-days where AOD was missing. To validate our model, we performed 10-fold out-of-sample cross-validation. Specifically, data for each cross-validation set were reserved from the 90% training set and repeated in turn for 10 times. As a result, we obtained reasonable and reliable PM2.5 predictions for the study area (mean cross-validated R2 of 0.77, 81, 0.70 for region 1, 2, 3, respectively) with modest daily predictions errors (RMSPE -Root of the mean squared prediction errors = 2.89, 2.51, 2.82 μg/m3 for region 1, 2, 3, respectively). Among the regions, Region 2 displayed the best fit, and includes North Carolina, South Carolina, and part of Georgia. The slopes for the observations and predictions were almost 1, indicating no bias. Further details are described in elsewhere13.
For this study, the daily predictions of PM2.5 at a 1 km2 resolution were aggregated into the zip code level. We matched 1 km grid cells with zip code area by assigning the centroid of each 1 km grid cell to the centroid of the closest zip code. And then, PM2.5 predictions at a 1 km2 level were averaged for zip codes and dates. In that way, the zip code areas that contained one or more 1 km grid cells were given the averaged PM2.5 predictions, which is the most case. Zip code areas smaller than 1 km2 are given the predictions from the closest grid cell. Finally, PM2.5 by zip code was assigned to the zip code of each decedent on each day.
Ground-level particulate matter measurements
To compare the results from modeled PM2.5 exposure and the one from the nearest monitor, we collected PM2.5 mass concentration data from the EPA and IMPROVE monitors described previously. The resident zip code of the deceased was assigned the nearest monitor without a distance limit. 24-hr PM2.5 level for the corresponding lag day (lag 0 for the day of death and lag 1 for the previous day of death). Our analysis included 62,467 observations from 96 PM2.5 monitoring stations operating in the three states between 2007 and 2011.
Covariates
We downloaded daily temperature data from the Climate Data Online website hosted by NOAA’s National Data Centers (NNDC) at The National Oceanic and Atmospheric Administration (NOAA) National Climate Data Center (NCDC, 2010). 26 stations were used and grid cells were matched to the closest weather station.
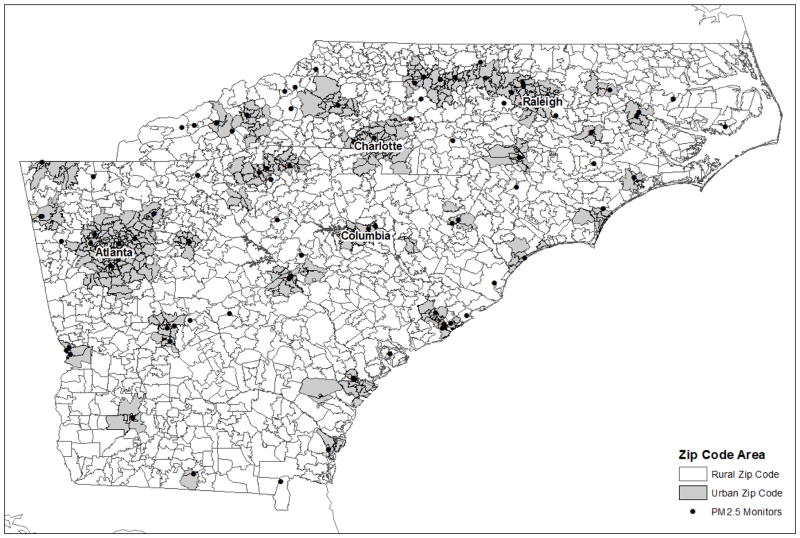
To classify zip codes into urban versus rural areas, we downloaded the 2004 ZIP Rural-Urban Commuting Area Codes (RUCAs) from the website of the Washington, Wyoming, Alaska, Montana, and Idaho Rural Health Research Center (WWAMI RHRC). RUCA is a census tract-based classification scheme to characterize all of the nation's Census tracts regarding their rural and urban status. It was based on the 2000 Census work commuting information, and Census Bureau defined Urbanized Areas (cities of 50,000 and greater population) and Urban Clusters (cities/towns with populations from 2,500 through 49,999) and a ZIP Code RUCA approximation has been developed by Health Resources and Service Administration's (HRSA's ) Office of Rural Health Policy (ORHP), the Department of Agriculture's Economic Research Service (ERS), and the WWAMI RHRC. We utilized that data to classify zip codes in the study area as being urban or rural. RUCA version 2 was used. When the RUCA code is 1.0 or 1.1 which stands for the metropolitan area core, the corresponding zip code area was defined as an urban area. The spatial distribution of urbaneness by zip code is given by Figure 1.
Figure 1.
Map of study area
Statistical analyses
Zip code-specific deaths were matched with our exposure estimates for each grid cell. For monitoring data, we assigned the closest monitoring stations on a specific day. Since many monitoring stations operate on every third or sixth day, we found the next closest monitoring station, if the closest monitoring station didn’t run on a specific day.
We used a case-crossover design14 with time-stratified control selection since our research question lies in the acute effect of PM2.5 on mortality. This harmonizes with the assumptions of case-crossover design which is acute exposures and acute outcome without carry-over effect. To avoid seasonal confounding, only the corresponding month to death was served as control time period. Within that month, control days were chosen every third day from the case day bidrectionally15.
We defined the relevant exposure time window as the mean exposure on the day of and day before the decedent’s death controlling for temperature and day of the week. Specifically, a conditional logistic regression was fitted as follows,
where pi is the probability of death for the ith person, PredPM2.5jk is the predicted two day mean PM2.5 level in zip code j on day k, Tempjk is the temperature for zip code j on day k, and the remaining are the indicator variables for day of the week. To deliver results in a more meaningful way, the effect estimate was converted to percent change (%) in mortality by 10 μg/m3 increase in PM2.5. Specifically, the following formula was used,
where β1 is the logarithm of odds ratio of death when the PM2.5 level increases per unit from the previous model.
For stratified analysis, we performed separate analyses to generate the effect estimate but used the interaction term with the exposure and the effect modifier to test for significant modification. For those sub-region analyses, we used the same exposure window as in the main analysis. Various stratified analyses were conducted by age, sex, race, education, the primary cause of death.
To evaluate whether the effect of PM2.5 still exists under the EPA standards, we performed two separate analyses for both EPA annual and daily standard. First, we restricted zip code areas to where the annual average of predicted PM2.5 is less than 12 ug/m3 (EPA annual standard for PM2.5) and perform the analysis. In terms of daily standard, we conducted analysis on the days less than 35 ug/m3 in the zip code areas below the annual EPA standard.
We also conducted analyses to compare the results that used modeled PM2.5 with those using nearest monitor values PM2.5 measurement data.
In sensitivity analysis, we evaluated the potential non-linear relationship between temperature and mortality to assess how the main effect is robust to the control of residual confounding. We applied natural spline to the temperature term with the degree of freedom of 3.
The data analysis for this paper was generated using the PROC PHREG procedure in Base SAS software, version 9.3 of the SAS system for windows (Copyright © 2014 SAS Institute Inc., Cary, NC, USA).
RESULTS
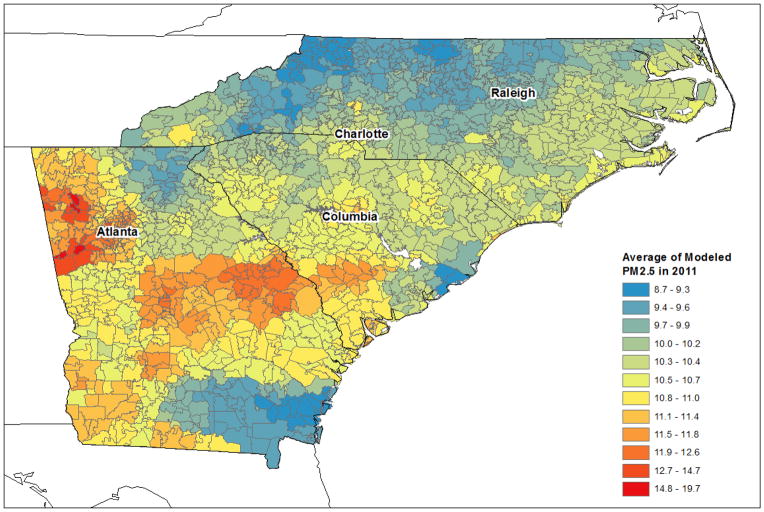
Figure 1 shows the study area. Zip codes are divided by being urban or rural. Main cities in the study areas included: Atlanta (GA), Charlotte (NC), Raleigh (NC), and Columbia (SC). Most of the PM2.5 monitors operated in urban areas. Figure 2 presents one of the examples of the annual mean of PM2.5 concentrations at the zip code level in 2011. PM2.5 concentrations at 1 km grid cells were summarized into a zip code level for the year of 2011. It shows the spatial distribution of the average of modeled PM2.5 in the study area in 2011 by zip code. The spatial distribution of PM2.5 was relatively high in Georgia among three states and urban areas showed higher PM2.5 level compared to the surrounding rural areas.
Figure 2.
Average of predicted PM2.5 by zip code in 3 States in 2011
We used 848,270 non-accidental death records in three states of North Carolina, South Carolina, and Georgia between 2007 and 2011 (Table 1). By state, 42% of the total record was collected from North Carolina, 37% of data from Georgia, and 21% was from South Carolina. The average age of the decedents was 73 years old with a standard deviation of 17 years. Most of the decedents were aged over 65 (71%) and about the half of the deceased died at 75 or over. The sex ratio was close to 1:1 with a slight excess of females (52%). By race, whites accounted for 75% of deaths, blacks for 24%, and other races for 1%. The percentage of whites was highest in North Carolina (78%). For education level, less than high school education (years less than 12 years) occurred with the highest frequency (27%). However 24% of the education records were missing and most of that originated from data collected from Georgia (63% of Georgia records). 53% of the population resided in urban areas and the proportion was highest in Georgia (59%). It is worth noting that the 4,339 (0.5%) missing data on being urban or not includes true missing data as well as those with a residential zip code outside the study area.
Table 1.
Characteristics of mortality and modeled PM2.5, 2007–2011
| Total | GA | NC | SC | |
|---|---|---|---|---|
| Deaths (%) | 848,270 | 311,831 (37) | 357,915 (42) | 178,524 (21) |
| Age, mean (SD), yr | 73 (17) | 72 (18) | 73 (17) | 73 (17) |
| > 65, No. (%) | 603,600 (71) | 216,416 (69) | 260,544 (73) | 126,640 (71) |
| > 75, No. (%) | 438,728 (52) | 156,805 (50) | 190,724 (53) | 91,199 (51) |
| Missing | 43 (≤1) | 0 (0) | 0 (0) | 43 (≤1) |
| Sex, No. (%) | ||||
| Male | 409,408 (48) | 149,790 (48) | 172,171 (48) | 87,447 (49) |
| Female | 438,847 (52) | 162,041 (52) | 185,739 (52) | 91,067 (51) |
| Missing | 15 (≤1) | 0 (0) | 5 (≤1) | 10 (≤1) |
| Race, No. (%) | ||||
| White | 631,701 (75) | 223,727 (72) | 279,375 (78) | 128,599 (73) |
| Black | 206,226 (24) | 85,020 (27) | 73,812 (21) | 47,394 (27) |
| Other | 9,202 (1) | 3,084 (1) | 4,728 (1) | 1,390 (1) |
| Missing | 1141 (≤1) | 0 (0) | 0 (0) | 1,141 (1) |
| Education, No. (%) | ||||
| ≤8 yr | 118,283 (14) | 18,915 (6) | 66,198 (19) | 33,170 (19) |
| ≤ 11 | 112,699 (13) | 17,580 (6) | 67,463 (19) | 27,656 (16) |
| ≤12 | 227,791 (27) | 44,535 (14) | 118,816 (33) | 64,440 (37) |
| ≤15 | 109,018 (13) | 27,698 (9) | 53,576 (15) | 27,744 (16) |
| ≥16 | 77,187 (9) | 8,201 (3) | 46,645 (13) | 22,341 (13) |
| Missing | 203,292 (24) | 194,902 (63) | 5217 (1) | 3,173 (2) |
| Residence, No. (%) | ||||
| Urban | 445,393 (53) | 182,461 (59) | 174,830 (49) | 88,102 (49) |
| Rural | 398,538 (47) | 129,095 (41) | 179,381 (51) | 90,062 (51) |
| Missing | 4,339 (0.5) | 275 (≤1) | 3,704 (1) | 360 (≤1) |
| Cause of Death, No. (%) | ||||
| CVD | 194,180 (55) | 71,643 (55) | 81,604 (55) | 40,933 (55) |
| Stroke | 46,535 (13) | 16,252 (12) | 20,116 (13) | 10,167 (14) |
| CHF | 23,119 (7) | 10,364 (8) | 8,180 (5) | 4,575 (6) |
| MI | 42,371 (5) | 13,686 (4) | 18,105 (5) | 10,580 (6) |
| Respiratory | 89,280 (25) | 32,475 (25) | 38,791 (26) | 18,014 (24) |
| Missing | 38 (≤1) | 38 (≤1) | 0 (0) | 0 (0) |
| PM2.5 mean (SD), μg/m3 | 11.1 (4.4) | 11.6 (4.5) | 10.7 (4.3) | 10.9 (4.2) |
| Range (min, max) | 0.02, 86.2 | 0.8, 80.2 | 0.02, 86.2 | 0.5, 70.99 |
Table 1 also shows a summary of the modeled PM2.5 level for rural/urban areas and temperature. The average PM2.5 level from 2007 through 2011 was 11.1 μg/m3 with the standard deviation of 4.4 μg/m3. The average PM2.5 in Georgia was slightly higher than the other states.
Table 2 presents the estimated percent increase in mortality for a 10 μg/m3 increase in PM2.5 by lag period and its comparison with the results from nearest monitor data. For all non-accidental deaths, we found a 1.56% increase in mortality (95% CI = 1.19 to 1.94). Compared to the result from our prediction model, the estimate from the existing monitoring stations showed a lower effect estimate, which was a 1.21% increase. The mean distance to the assigned monitors was around 55 km. It is worth noting the distances were different between lag 0 and lag 1, because many monitoring stations don’t operate on a daily basis.
Table 2.
Comparison of effect estimates between modeled and measured PM2.5
| LAG | Modeled PM2.5 | Measured PM2.5 | Mean Distance |
|---|---|---|---|
| 0 | 1.12 (0.80, 1.43) | 0.85 (0.63, 1.07) | 54.96 km |
| 1 | 1.19 (0.86, 1.52) | 0.90 (0.68, 1.13) | 54.93 km |
| 0–1 | 1.56 (1.19, 1.94) | 1.21 (0.94, 1.47) | N/A |
Increase in mortality in percentage and (95% confidence intervals)
Mean distance is the average distance between the centroid of the zip code and the closest monitoring station.
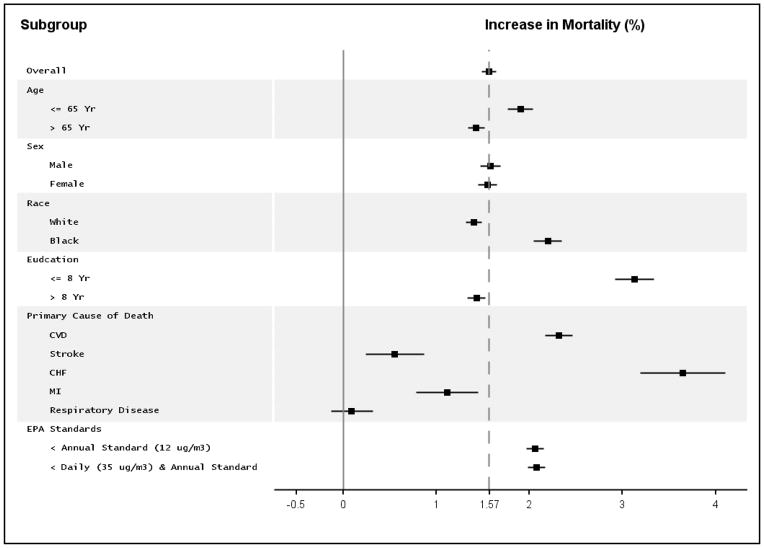
We conducted various subgroup analyses (Figure 3). The dashed gray line is a reference line from the overall effect estimate to make comparison easier. There was no significant effect modification by sex. We found that black population showed substantially higher risk (2.19%, 95% CI=1.43–2.96) than white population (1.40%). While not significant, a 50% difference in effect size is noteworthy, and the significance may have been affected by the small percentage of deaths that were blacks. For education level, the region less than 8 years of education showed more than double the risk (3.13%) compared to the more educated regions (1.43%), and that difference was marginally significant.
Figure 3.
Stratified analyses on the association between PM2.5 and mortality
The impact of PM2.5 on CVD and CHF death rates was larger than for all natural causes; we found a 2.32% (95% CI = 1.57 to 3.07) and a 3.64% (1.35, 5.99) respectively. In contrast, the impact on deaths from myocardial infarction (MI) (1.12%) and stroke (0.55%) was lower. We did not observe a significant association with pulmonary deaths (0.09 %, 95% CI = −0.13 to 0.32).
There was statistically significant effect of PM2.5, even below the EPA standards. The effect of PM2.5 on mortality in zip code areas that meet EPA annual standard for PM2.5 (12 μg/m3), was 2.06% (95% CI = 1.97 to 2.15), which was higher than the overall effect, 1.57%. When further restricted the analysis to the days below the EPA daily standard among the zip code areas that meet EPA annual standard, the effect estimate has slightly increased to 2.08% (95% CI = 1.99 to 2.17).
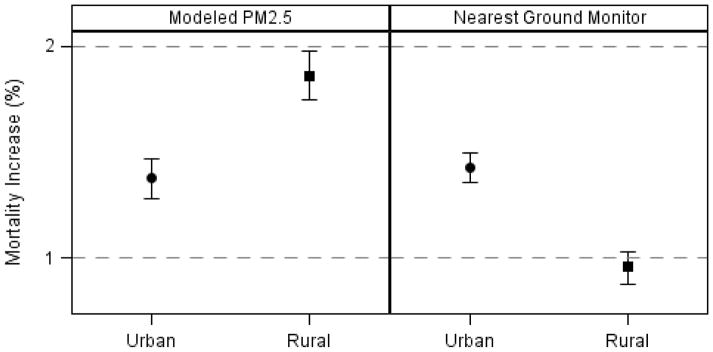
We also observed differences in the PM2.5 associations with mortality between people living in urban areas and those in rural areas and those results were reversed between modeled PM2.5 and measured PM2.5 (Figure 4). Rural areas showed the higher risk for mortality (1.86%; 1.75, 1.98) than the urban areas (1.38%; 1.28, 1.47) in our analyses. Conversely, results based on the existing monitoring stations, showed a higher increase in mortality in urban areas (1.43%; 1.36, 1.50) than rural areas (0.96%; 0.88, 1.03).
Figure 4.
Difference in the effect of PM2.5 by residence and exposure metric
Sensitivity analysis revealed the model was robust to the shape of association between temperature and mortality (Table 3). The PM2.5 effect hardly changed when the temperature function was defined as natural spline term (1.57 %, 1.59%, 1.63%, for d.f.=2, 3, 4, respectively)
Table 3.
Sensitivity analysis on the control for the shape of temperature-mortality relationship
| D.F. | Effect Estimate (%)* |
|---|---|
| 2 | 1.57 (0.82, 2.32) |
| 3 | 1.59 (0.84, 2.34) |
| 4 | 1.63 (0.88, 2.39) |
Increase in the odds of mortality in percentage and (95% confidence intervals)
DISCUSSION
In this paper, we examine associations between model-predicted PM2.5 exposures and increased-mortality in the three states of Georgia, North Carolina, and South Carolina between 2007 through 2011.
Compared to the effect estimate from our modeled PM2.5, monitor-based estimates tended to be attenuated, and have wider confidence intervals. The use of the modeled PM2.5 levels enabled us to investigate the entire region, with more power to conduct stratified analyses. We found differences in effect size by race and education level, with blacks and less educated people having higher risk. While some studies using central monitoring sites have identified such effect modification before, the nature of the study design made it impossible to determine whether these effects were due to differential exposure or differential response. Our results, based on 1x1 km spatially resolved exposure estimates, strongly suggest that in addition to any differential exposure, there is differential response. Whether these differences relate to psychosocial stress, housing, or other factors needs further investigation.
In the stratified analyses for the primary cause of death, CVD and CHF exhibited the highest increase in mortality in response to PM2.5. Compared to them, stroke and MI showed the less amount of increase in mortality. Meanwhile, death by respiratory cause showed the least increase and was not statistically significant. This finding is contradictory to the existing studies16–18, where showed about 1 ~ 2% increase in respiratory mortality. This might be because any specific speciation which is most associated with respiratory death lacks in PM2.5 in the southeastern area. Further study by PM2.5 specification in this area will be helpful to evaluate this hypothesis.
The effect of PM2.5 still existed under both EPA annual and daily standards. This result is consistent with those reported by other studies19, 20. Although those studies used the previous EPA annual standard of 15 μg/m3, their results showed a linear relationship with mortality without any threshold under the current standard.
We also found differences in the PM2.5 associations between people living in rural areas and urban areas. Interestingly, the effects of acute PM2.5 exposure appeared stronger in rural areas. Kloog et al.21 reported results consistent with this in his article where he used a similar PM2.5 exposure model in the mid-Atlantic region of U.S. This finding was not revealed when the nearest monitor was used for an exposure metric which showed a contrary result. This likely reflects the higher measurement error in exposure in rural areas, because most residents live further from a monitor.
A possible explanation for the higher risk in rural areas can be sought in the socioeconomic characteristics of rural populations who tend to be in low income and education level compared to the population in urban areas, or in a difference in the prevalence of smoking. Alternatively, these differences may be related to prolonged outdoor time by farmers or accessibility to hospitals21.
In the perspective of exposure and confounding, this may also due to the difference in the composition of air pollution between urban areas and rural areas. For instance, more ozone can be found in rural areas where there is not much nitrogen monoxide (NO) to deplete ozone. NO, abundant in an urban area from combustion reacts with ozone (O3) to transform into nitrogen dioxide (NO2) and oxygen (O2). Therefore, the effect of PM2.5 may be confounded by the effect of ozone on mortality.
Regardless, this finding implies that the acute effects of particulate matter may have been underestimated because an important part of the population was excluded from most previous epidemiologic studies due to the lack of monitoring sites. The majority of epidemiologic studies have not studied an important fraction of the population due to the low density of monitors in the rural areas. Now, we found that this subpopulation which was not studied happens to be in a higher risk.
There are still limitations in our study. The resident location of the deceased was in an aggregated form of zip codes, not individual addresses which were not available due to privacy and confidentiality issues. This ecological attribute in exposure measurement still lends more measurement error than individual-level studies. With 1 km resolution exposure day, once we obtain the street-level address from study subjects, our research question will be better served. Yet, considering the area of zip code is much smaller than a city or a county, the magnitude of such error seems much smaller than that of many previous studies relying on sparse network of monitoring stations.
In terms of the exposure history, there might be some level of discrepancy between the location of assumed exposed place and actual place. We used zip code for residence, which implies that we assumed that the exposure to PM2.5 in their resident zip code areas between the day of death and the previous day is associated with their death. Considering the exposure time window in our study, it is very unlikely that subjects change their residence on the day of death or the previous day. Therefore, a discrepancy between the record and the actual ambient environment that is related to the subject is low.
We also expect further improvements of the prediction. With an advent of finer satellite remote sensing and processing algorithms, we will be able to produce more reliable and accurate predictions of PM2.5.
The same issue also exists for the covariate, temperature. Temperature was controlled as a possible confounder and its measurement was not based on the individual-level as well. The residual confounding in temperature is expected.
To the best of our knowledge, this is the first study that included the entire population in the three states in the Southeastern U.S. to assess the relationship between short-term PM2.5 exposures and mortality.
In conclusion, our findings indicate that increased mortality especially for CVD and CHF were associated with PM2.5 exposures. In addition, we have demonstrated that our AOD-based exposure models can be successfully applied to epidemiologic studies investigating the acute effects of PM2.5. This has been possible because these models estimate spatially resolved PM2.5 exposures for specific zip codes on a daily basis. This will add a new study population in rural areas, and in doing so, the result from those analyses will be more generalizable to other populations and areas.
Acknowledgments
This publication was made possible by USEPA grant RD 83479801. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the USEPA. Further, USEPA does not endorse the purchase of any commercial products or services mentioned in the publication.
References
- 1.Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N Engl J Med. 2000;343(24):1742–1749. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- 2.Wichmann HE, Spix C, Tuch T, et al. Daily mortality and fine and ultrafine particles in Erfurt, Germany part I: role of particle number and particle mass. Res Rep Health Eff Inst. 2000;(98):5–86. discussion 87–94. [PubMed] [Google Scholar]
- 3.Mar TF, Ito K, Koenig JQ, et al. PM source apportionment and health effects. 3. Investigation of inter-method variations in associations between estimated source contributions of PM2.5 and daily mortality in Phoenix, AZ. J Expos Sci Environ Epidemiol. 2005;16(4):311–320. doi: 10.1038/sj.jea.7500465. [DOI] [PubMed] [Google Scholar]
- 4.Boldo E, Medina S, Le Tertre A, et al. Apheis: Health Impact Assessment of Long-term Exposure to PM2.5 in 23 European Cities. Eur J Epidemiol. 2006;21(6):449–458. doi: 10.1007/s10654-006-9014-0. [DOI] [PubMed] [Google Scholar]
- 5.Ostro BD, Broadwin R, Lipsett MJ. Coarse and fine particles and daily mortality in the Coachella Valley, California: a follow-up study. J Expo Anal Environ Epidemiol. 2000;10(5):412–419. doi: 10.1038/sj.jea.7500094. [DOI] [PubMed] [Google Scholar]
- 6.Franklin M, Zeka A, Schwartz J. Association between PM2.5 and all-cause and specific-cause mortality in 27 US communities. J Expos Sci Environ Epidemiol. 2006;17(3):279–287. doi: 10.1038/sj.jes.7500530. [DOI] [PubMed] [Google Scholar]
- 7.Ruchirawat M, Settachan D, Navasumrit P, Tuntawiroon J, Autrup H. Assessment of potential cancer risk in children exposed to urban air pollution in Bangkok, Thailand. Toxicol Lett. 2007;168(3):200–209. doi: 10.1016/j.toxlet.2006.09.013. S0378-4274(06)01323-3 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Risom L, Moller P, Loft S. Oxidative stress-induced DNA damage by particulate air pollution. Mutat Res. 2005;592(1–2):119–137. doi: 10.1016/j.mrfmmm.2005.06.012. S0027-5107(05)00246-0 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Dockery DW. Health effects of particulate air pollution. Ann Epidemiol. 2009;19(4):257–263. doi: 10.1016/j.annepidem.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valavanidis A, Fiotakis K, Vlachogianni T. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2008;26(4):339–362. doi: 10.1080/10590500802494538. [DOI] [PubMed] [Google Scholar]
- 11.Brenner H, Savitz DA, Jockel KH, Greenland S. Effects of nondifferential exposure misclassification in ecologic studies. Am J Epidemiol. 1992;135(1):85–95. doi: 10.1093/oxfordjournals.aje.a116205. [DOI] [PubMed] [Google Scholar]
- 12.Greenland S. Divergent biases in ecologic and individual-level studies. Stat Med. 1992;11(9):1209–1223. doi: 10.1002/sim.4780110907. [DOI] [PubMed] [Google Scholar]
- 13.Lee M, Kloog I, Chudnovsky A, et al. Spatiotemporal prediction of fine particulate matter using high-resolution satellite images in the Southeastern US 2003–2011. J Expos Sci Environ Epidemiol. 2015 doi: 10.1038/jes.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133(2):144–153. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- 15.Medina-Ramon M, Schwartz J. Temperature, temperature extremes, and mortality: a study of acclimatisation and effect modification in 50 US cities. Occup Environ Med. 2007;64(12):827–833. doi: 10.1136/oem.2007.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atkinson RW, Kang S, Anderson HR, Mills IC, Walton HA. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: a systematic review and meta-analysis. Thorax. 2014;69(7):660–665. doi: 10.1136/thoraxjnl-2013-204492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franklin M, Zeka A, Schwartz J. Association between PM2.5 and all-cause and specific-cause mortality in 27 US communities. J Expo Sci Environ Epidemiol. 2007;17(3):279–287. doi: 10.1038/sj.jes.7500530. 7500530 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Ostro B, Broadwin R, Green S, Feng WY, Lipsett M. Fine particulate air pollution and mortality in nine California counties: results from CALFINE. Environ Health Perspect. 2006;114(1):29–33. doi: 10.1289/ehp.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz J, Coull B, Laden F, Ryan L. The effect of dose and timing of dose on the association between airborne particles and survival. Environ Health Perspect. 2008;116(1):64–69. doi: 10.1289/ehp.9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lepeule J, Laden F, Dockery D, Schwartz J. Chronic exposure to fine particles and mortality: an extended follow-up of the Harvard Six Cities study from 1974 to 2009. Environ Health Perspect. 2012;120(7):965–970. doi: 10.1289/ehp.1104660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kloog I, Nordio F, Zanobetti A, Coull BA, Koutrakis P, Schwartz JD. Short term effects of particle exposure on hospital admissions in the Mid-Atlantic states: a population estimate. PLoS One. 2014;9(2):e88578. doi: 10.1371/journal.pone.0088578. [DOI] [PMC free article] [PubMed] [Google Scholar]