Abstract
Purpose
Idiopathic juvenile osteoporosis (IJO) is a rare condition in children, characterized by bone pain, long bone and vertebral fractures. Previously, IJO bone was solely characterized by histomorphometry and quantitative computed tomography. The goal of this study is to describe IJO bone composition.
Materials and Methods
Fourier transform infrared imaging (FTIRI), a vibrational spectroscopic technique providing spatially resolved images of chemical composition, was used to determine whether iliac crest biopsies from children with IJO differed in composition from and age- and sex-matched controls, and, as a secondary analysis, whether IJO-bone showed the same disease dependent change in composition as do iliac crest bone biopsies from women with postmenopausal osteoporosis (PMO). Wilcoxon rank-tests and linear regressions were used to analyze FTIRI variables (mineral-to-matrix ratio, carbonate-to-phosphate ratio, crystallinity, acid-phosphate substitution, collagen maturity) and their individual pixel distributions (Heterogeneity).
Results
Mineral-to-matrix ratio was comparable in IJO and age-matched controls. Contrastingly, collagen maturity (also known as collagen cross-link ratio) was higher in cortical and cancellous IJO bone compared to juvenile controls. Acid-phosphate substitution was greater in IJO cancellous bone than in age-matched controls, suggesting IJO bone mineral is formed more recently, reflecting a slower mineralization process. This agrees with findings of increased heterogeneity for mineral-to-matrix and collagen maturity ratios in IJO cancellous bone. There were negative correlations between cancellous collagen maturity and previously reported histomorphometric bone formation markers. There were no correlations with indices of remodeling.
Conclusions
IJO bone, similar to PMO bone, had elevated collagen maturity relative to its age-matched controls. This emphasizes the importance of the collagen matrix for bone health. IJO bone differed from PMO bone as IJO bone contains more recently formed mineral than age-matched controls but has a more mature matrix, whereas in PMO-bone both mineral and matrix have older characteristics.
Keywords: FTIRI, Osteoporosis, Idiopathic juvenile osteoporosis, Collagen Maturity, material properties
Introduction
Osteoporosis is a disease characterized by a loss of bone mass and architecture [1] associated with an increased risk of fragility fracture and significant morbidity and mortality [2]. Recent studies suggest that in addition to loss of bone mineral density, the composition of bone in osteoporosis, and in particular in patients with fractures, is altered compared to sex- and age-matched populations [3–8]. Increases in enzymatic-collagen cross-links and crystal size were found in iliac crest biopsies from post-menopausal women with fragility fractures using vibrational spectroscopy [3]. Using a similar technique, Malluche reported an increase in collagen maturity in women with low-T scores and fragility fractures [4]. Tamminen, using similar methods, also found elevated collagen cross-links in children with vertebral fractures [5]. These findings suggest that increased collagen cross-links may be a feature of fragile bones rather than an indication of older bones. Generally, osteoporosis results from an imbalance of osteoclast-mediated resorption and osteoblast-mediated bone formation [1, 9], with fracture incidence increasing with age [10]. To address the question of whether spectroscopic changes in collagen cross-links are directly related to chronologic age or incidental to the aging process, we examined bone biopsies from a rare form of osteoporosis occurring in children and compared their composition to that in age- and sex-matched controls. We compared this information to published compositional analysis of adults with and without fragility fractures (osteoporosis) analyzed using the same methodology [3].
Idiopathic juvenile osteoporosis (IJO) is a rare disease in children [11]. IJO is characterized by pre-pubertal onset and spontaneous remission with progression of puberty [11,12]. Two large IJO clinical cohorts have been reported [13,14] and smaller populations of patients have been characterized by histomorphometry [15,16,17] and quantitative computed tomography [17], providing insight into mineral but not matrix changes. According to recommendations of the International Society for Clinical Densitometry on DXA in Children, osteoporosis in juveniles is defined as patients having one or more fractures and a bone density significantly less than age- and sex-matched individuals (z-score < −0.2) [18]. There are three types of osteoporosis in children, each of which is rare. Primary osteoporosis is associated with a genetic abnormality or enzyme defects [19]. Secondary osteoporosis occurs in response to a distinct cause, either an underlying medical condition or medication intake, e.g., corticosteroid use. Idiopathic juvenile osteoporosis (IJO), rarer still, is generally found in healthy children and adolescents with symptoms developing just prior to puberty [11,18–20]. The reported average age of onset of IJO is 7 years; however this condition has been observed in children between the age of 1 and 13 years of age. IJO is distinguished from primary and secondary juvenile osteoporosis, by the absence of underlying causes or metabolic abnormalities and by its ability to resolve spontaneously [16]. There are few reports on the composition of IJO bone, the original report by Rausch [15,16] and a more recent report by Bacchetta et al [17]. In the current investigation, all biopsies from the first published report of patients with IJO and the age-matched “healthy” controls from the same study [15,16], were used to determine if changes in bone composition in IJO compared to age-matched controls, are distinct from previously reported changes in matrix and mineral composition of post-menopausal osteoporotic (PMO) bones, compared to their age-matched controls.
Fracture risk in osteoporosis is predicated on the amount of bone present (bone quantity) and a series of properties including architecture, collagen cross-links, and mineralization that are defined as bone quality [21]. Fourier transform infrared imaging (FTIRI) provides chemical images of the composition of thin sections of bone and is a technique previously used to study bone quality in terms of composition and heterogeneity, by age [22] and disease [3,4,5,22,23]. In the present study, FTIRI was used to characterize and compare previously described transiliac bone biopsies from patients with IJO and juvenile controls [15,16] according to five validated variables. These variables, as detailed elsewhere [24], are indicative of the mineral content (ash weight of bone), collagen maturity or collagen cross-link distribution [25], size and perfection of hydroxyapatite (HA) crystals [26], and carbonate [24] and acid phosphate substitution into newly formed HA [27].
Materials and Methods
Materials
Transiliac bone biopsies from the 9 patients with IJO (age 10 – 12) and 12 age-matched controls that had been previously described in terms of histomorphometry [15,16] were provided by Dr. Rauch (McGill University, CA), and analyzed by FTIRI. All the biopsies from the original study [15] were used for this investigation. The IJO patients had been identified based on the presence of vertebral and long bone fractures occurring in otherwise healthy children with no evidence of secondary osteoporosis due to drugs or metabolic abnormalities. There had been a deceptive onset of diffuse pain and difficulty in ambulation. Radiographically, the new bone formed in metaphyseal areas appeared radiolucent. Biopsies were from 2 males (one male had two biopsies, both of which were analyzed and averaged together) and 7 females with IJO and 7 males and 5 females as juvenile controls (Juvenile). The mean ages were 10.8 ± 0.8 for IJO and 11.6 ± 0.7 for juvenile controls. FTIRI analysis of these iliac crest biopsies was approved by the Internal Review Board of the Hospital for Special Surgery (HSS #93019). Each bone biopsy was previously embedded in polymethyl methacrylate (PMMA) [28]. For each bone sample, three sequential 1–3μm sections were collected using a Leica microtome model SM2500 (Leica Microsystems, Buffalo Grove, IL, USA) and placed on 25 mm diameter, 2 mm thick barium fluoride windows (Spectral Systems, Hopewell, NY, USA).
FTIRI Data Acquisition
Fourier transform infrared imaging is a spectroscopic method applied to thin (1–3um) sections of tissue providing spatially resolved maps of the molecular environments of tissue components. Visible survey images were taken of each section of each biopsy to select regions of interest containing either intact cancellous or cortical bone. Infrared images were collected from these regions using a Spectrum Spotlight 300 FTIR imaging microscope (PerkinElmer, Waltham, MA, USA) in transmission, using the imaging mode. Spectral resolution was 4 cm−1, and 16 scans were obtained and co-added for each 6.25 × 6.25 μm2 pixel. For every biopsy, three adjacent sections were examined, and FTIRI was used to image three areas of cancellous bone and three areas of cortical bone (each extending from the periosteal to the endosteal side of the cortex), resulting in a total of 18 images for each individual biopsy. All images were approximately 500 μm × 500 μm in area.
FTIRI Data Processing
Base-line subtraction using a second-order polynomial, followed by subtraction of the PMMA contribution were performed for each image using ISYS 5.0 Spectral software (ISYS Image Analysis Software, Malvern Instruments, Columbia, MD). The following five variables were calculated for each image, averaged, and expressed as mean ± SD for cortical and cancellous regions of each biopsy, separately. (1) Mineral-to-matrix ratio, the peak area ratio of the phosphate vibration at 900–1159 cm−1 divided by the peak area of the amide I band (1585–1700 cm−1), corresponding to mineral content or ash weight [26]. (2) Carbonate-to-phosphate ratio, the peak area ratio of the carbonate band at 860–890 cm−1 divided by the area of the mineral band at 900-1159 cm−1, indicating the extent of carbonate substitution into the mineral lattice [24]. Carbonate substitutes for phosphate or hydroxide ions [29]. (3) Crystallinity, the intensity ratio of sub-bands at 1030cm−1/1020cm−1, related to the crystal size and lattice perfections as measured by x-ray diffraction wide-angle line-broadening [26]. (4) Acid phosphate substitution, the intensity ratio of subbands at 1128cm−1/1096cm−1, related to the extent of acid phosphate substitution in the apatite lattice and inversely related to the crystallinity parameter [27]. Collagen maturity (also referred to as collagen cross-link ratio), the peak height intensity of subbands at 1660cm−1/1690 cm−1, has been shown to decrease as the trivalent cross-links are depleted or the divalent cross-links are increased [25]. The spectroscopic method provides a ratio that correlates with enzymatic trivalent/divalent cross-links, as confirmed by HPLC [25]; there is no correlation with non-enzymatic cross-links.
Following processing, means and pixel distribution for each image were calculated. Line widths at half-maximum (FWHM) of each pixel distribution, used as a measure of tissue heterogeneity for the parameter in question, were calculated from each ISYS-generated pixel histogram using ISYS 5 software, as detailed elsewhere [23].
Statistics
Wilcoxon rank tests were used to examine differences between disease and control in cancellous and cortical bone. Linear regression analyses were conducted to compare IJO and juvenile controls while adjusting for age, gender, and whether the bone was trabecular or cortical. Significance level was set at 0.05. All data were analyzed in SAS version 9.3 (SAS Institute, Cary, NC, USA).
FTIRI collagen maturity values for each individual subject were analyzed vs. previously reported histomorphometric data [15], provided for each individual biopsy by Dr Rausch, to determine whether values were related to bone formation (bone formation rate (BFR/BS) and mineralizing surface/bone surface (MS/BS)), remodeling (osteoclast surface/bone surface (OcS/BS) or eroded surface/bone surface (ES/BS)) or bone size (trabecular thickness (TbTh) or cortical width (CtW)). All comparisons except cortical width were based on cancellous bone collagen maturity values. Analyses were done using GraphPad Prism version 3.0 (Carlsbad, CA, USA). All data, independent of disease or sex were correlated with the histomorphometric parameters for cortical and cancellous bones, as appropriate. Only significant (p<0.05) correlations are reported.
Results
The spectral regions used for analysis, along with typical images of the collagen cross-link ratio (also known as collagen maturity) variable and the carbonate-to-phosphate ratio for cortical bones are seen in Figure 1A,B. The IJO cortical bone, in agreement with histomorphometry [15,16], appeared thinner than that in the juvenile controls. The mean values for IJO and juvenile control iliac crest bone biopsies (Table 1) and the distribution of the data from each case (Figure 2A) indicated the IJO biopsies had significantly higher collagen maturity levels in both cortical and cancellous bones than juvenile controls based on Wilcoxon rank tests. There was also a statistically significant difference between juvenile controls and IJO cancellous bones for acid phosphate variables (Figure 2A). No other statistically significant differences were seen in the compositional parameters between IJO and juvenile controls in either cortical or cancellous bones.
Figure 1.
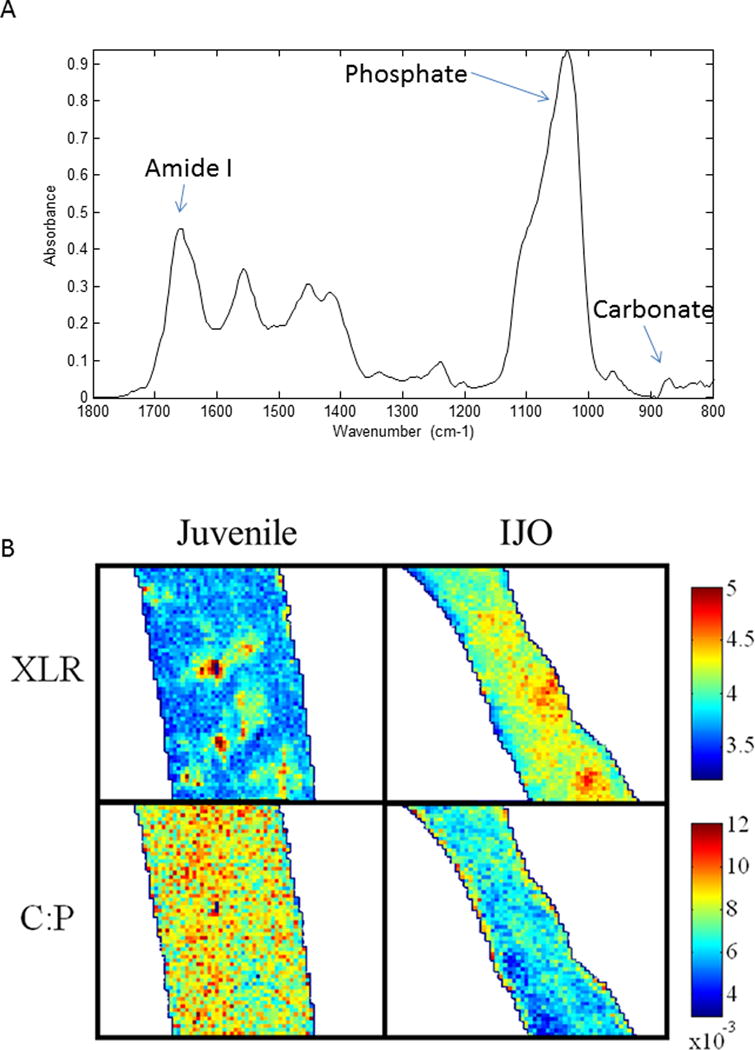
A) Typical spectrum of newly forming bone in a juvenile control. The bands used for calculations of variables are indicated. B) Representative infrared images of the collagen cross-link maturity parameter (XLR) and the carbonate-to-phosphate ratio (C:P) in cortical bone in IJO and juvenile controls (Juvenile). Color bar units are arbitrary (AU). The structures are outlined to separate the image from the background. XLR = collagen cross-link ratio; C:P = carbonate-to-phosphate ratio.
Table 1.
Mean values of FTIRI outcome variables and their standard deviations and p-Values
| Parameter | Parameter Mean ± SD | p-values* | ||||
|---|---|---|---|---|---|---|
| IJO | Juvenile | |||||
| Cortical | Cancellous | Cortical | Cancellous | Cortical | Cancellous | |
| Min/Mat | 4.32±0.38 | 4.08±0.4 | 4.19±0.38 | 4.14±0.43 | 0.6 | 0.8 |
| XLR | 4.45±0.27 | 4.47±0.39 | 3.90±0.26 | 3.85±0.23 | 0.004* | 0.0009* |
| XST | 1.22±0.02 | 1.18±0.01 | 1.21±0.03 | 1.19±0.02 | 1 | 0.2 |
| C/P | 0.0071±0.0006 | 0.0069±0.0008 | 0.0077±0.0006 | 0.0076±0.0006 | 0.2 | 0.06 |
| HPO4 | 0.44±0.04 | 0.48±0.04 | 0.40±0.04 | 0.42±0.02 | 0.2 | 0.002* |
Statistical data result from Wilcoxon rank tests. p-values compare IJO to Juvenile Control for each bone type.
p<0.05
Min/Mat = mineral/matrix ratio; XLR = collagen cross link ratio; XST = crystallinity; C/P = carbonate/phosphate ratio; HPO4 = acid phosphate substitution.
Figure 2.


Distribution of FTIRI variables for IJO (closed symbols) and juvenile controls (open symbols) illustrated as box-whisker plots, where the box indicates the upper and lower quartiles, the line within the box indicates the median value and the whiskers indicates the extreme values. Cortical bone is shown as circles and cancellous bone as triangles. * p<0.05 based on a linear regression model comparing IJO with juvenile control while adjusting for age, gender, and cortical or cancellous bone. (A) Variable means (B) Heterogeneity means.
Based on the statistical model (data not shown), the mean collagen cross-link ratio showed a positive trend with age (p = 0.0037), as did the mean carbonate-to-phosphate ratio (p = 0.0063). Mean mineral-to-matrix differed by gender, with males greater than females (p = 0.0007). The crystallinity ratio (Figure 2A) was greater in cortical bone than cancellous bone (p < 0.0001).
There was a significant (p<0.05) negative correlation between collagen maturity and bone formation markers based on Spearman’s correlation (Table 2), but not with markers of resorption. Collagen maturity was highest where bone formation was slowest. There was no correlation between cortical collagen maturity and any cortical histomorphometric parameter. Trabecular collagen maturity was negatively correlated with bone volume fraction (BV/TV), trabecular thickness (TbTh) and trabecular number (TbN). There was also a negative correlation with mineral apposition rate (MAR) and bone formation rate (BFR/Bs).
Table 2.
| Histomorphometric Parameter | Spearman r | 95% CI*** | p-value |
|---|---|---|---|
| Bone volume fraction (BV/TV) | −0.7649 | −0.9070 to −0.4647 | 0.0001 |
| Trabecular thickness (TbTh) | −0.7018 | −0.8790 to −0.3506 | 0.0008 |
| Trabecular number (TbN) | −0.8018 | −0.9229 to −0.5363 | <0.0001 |
| Bone formation rate (BFr/BS) | −0.6382 | −0.8655 to −0.1929 | 0.0078 |
| Mineral apposition rate (MAR) | −0.6018 | −0.8046 to 0.00843 | 0.0476 |
Statistically significant differences (Wilcoxon rank tests) were found between IJO and juvenile control cancellous and cortical bone FTIRI heterogeneities for mineral-to-matrix (p=0.019) and collagen cross-links (p=0.0002) (Table 3, Figure 2B). In both cortical and cancellous bones, FTIRI heterogeneities were greater for IJO than juvenile controls indicating that the IJO bones were more heterogeneous.
Table 3.
Average FWHMs* for FTIRI outcome variables and their corresponding standard deviations and p-values comparing IJO and Juvenile Controls of same Bone Type
| Parameter | FWHM± SD | p-values** | ||||
|---|---|---|---|---|---|---|
| IJO | Juvenile | |||||
| Cortical | Cancellous | Cortical | Cancellous | Cortical | Cancellous | |
| Min/Mat | 0.73±0.2 | 1.01±0.18 | 0.64±0.07 | 0.85±0.16 | 0.3 | 0.05*** |
| XLR | 0.39±0.13 | 0.45±0.14 | 0.3±0.09 | 0.31±0.07 | 0.2 | 0.01*** |
| XST | 0.06±0.008 | 0.059±0.01 | 0.053±0.01 | 0.053±0.012 | 0.07 | 0.3 |
| C/P | 0.002±0.0002 | 0.002±0.0002 | 0.002±0.0003 | 0.002±0.0003 | 0.6 | 0.2 |
| HPO4 | 0.045±0.02 | 0.061±0.015 | 0.042±0.008 | 0.054±0.006 | 0.8 | 0.2 |
FWHM = full width at half maximum of the pixel distribution defined here as heterogeneity;
Statistical data results from Wilcoxon; comparisons are IJO vs. Juvenile for each bone type;
Statistically Significant, p<0.05; Min/Mat = mineral/matrix ratio; XLR = collagen cross link ratio; XST = crystallinity; C/P = carbonate/phosphate ratio; HPO4 = acid phosphate substitution.
The percent change in FTIRI parameters, calculated as = 100*(xOP-xc)/xc), where xop is the mean parameter value for the osteoporotic patient (IJO or PMO) and xc the mean parameter value for the control, showed reproducibility in published and unpublished PMO values, an agreement of crystallinity and mineral-to-matrix changes in IJO and PMO and elevated collagen maturity values in cortical and cancellous IJO data compared to PMO (Table 4).
Table 4.
Percent Change* of Average FTIRI Variables relative to Age-Matched Controls
| Variable | IJO** | Osteoporosis (n=52)*** | Osteoporosis(n=120)**** | |||
|---|---|---|---|---|---|---|
| Cortical | Cancellous | Cortical | Cancellous | Cortical | Cancellous | |
| Mineral-to-Matrix | −3.0 | 1.5 | −1.3 | 2.1 | −1.6 | −0.47 |
| Carbonate-to-Mineral | 8.2 | 8.3 | 23 | 29 | 1.9 | 4.7 |
| Crystallinity | 0.3 | 0.8 | 0.35 | 2.2 | 1.9 | −0.9 |
| Collagen Maturity | −15.4 | −16.2 | −9.67 | −1.77 | −4.0 | −6.0 |
| Acid Phosphate | −11.4 | −14.2 | – | – | −.49 | −15.8 |
% Change = 100×(control variable-patient variable)/control variable
males and females combined
calculated from raw data files from ref 3
calculated from larger data set from presented at Orthopaedic Research Society: Boskey A, Spevak L, Watson P, Bare S, Recker R. (2015) Tissue Heterogeneity Determined by FTIR Imaging Decreases with Increasing Fracture Count. ORS Transactions. PS1-040. (Full MS Boskey AL, Donnelly E, Boskey E, Spevak L, Ma Y, Zhang W, Lappe J, Watson P, Recker, RR. Bone Tissue Composition, Compositional Heterogeneity and Fragility Fracture Risk: A Matched Case Controlled FTIRI Study, under revision for JBMR – when accepted will modify reference).
Discussion
This study found that iliac crest biopsies from patients with IJO presented with elevated levels of collagen maturity (also known as collagen cross-link ratio) compared to age-matched controls. The finding of an altered matrix in the IJO biopsies is an important observation, and agrees with similarly observed matrix alterations in children with vertebral fractures [5], young women with fractures [4,30] and patients with post-menopausal osteoporosis [3]. The average mineral content of IJO bones was comparable to the controls. The averaged compositional properties of the IJO bones were more characteristic of younger, newly formed bone similar to juvenile controls. Evidence of impaired new bone formation and decreased resorption in IJO patients, relative to controls, reported by histomorphometry [15], was confirmed on the basis of compositional and heterogeneity data using FTIRI. Histomorphometric data for these very bones [15,16], indicated defective bone formation, with markedly impaired mineralization rates, as well as impaired osteoblastic, and to a lesser extent, osteoclastic activities. Based on a larger study of patients with various types of juvenile osteoporosis, IJO patients were reported to have a wide range of resorption and formation rates [31]. This was also reflected in the smaller population of our study. Comparing patient-specific histomorphometric data to FTIRI data from that patient’s biopsy allowed correlation of bone composition with bone formation markers.
Mean changes in collagen cross-link ratios (collagen maturity) in iliac crest biopsies from IJO compared to age-matched healthy controls, were relatively higher than those in biopsies from post-menopausal osteoporotic women, using the same experimental procedure and the analytical device [3]. The relative increase in collagen maturity in IJO was not as large as that found in line scans across trabeculae of female patients < 40 years of age who suffered unexplained spontaneous fragility fractures [30]. In that study, values as high as 4x normal were found in the center of the trabeculae using FTIR microscopy. Mean values for a single trabeculum or patient were not reported, barring comparison of FTIRI data to the referenced study [30]. Generally, IJO, older post-menopausal osteoporotic women [10], younger women with low bone mineral density [4] and young women with unexplained fragility fractures [30], all presented with elevated collagen cross-link ratios (collagen maturity) relative to average values in age-matched controls, as measured by FTIRI. This increase in collagen cross-link ratios (collagen maturity) could simply reflect persistence of older bone in a highly resorbed matrix, suggested by the weak negative correlation between bone thickness and cortical collagen maturity (p<0.15), or, more likely, may be indicative of differences in the rate or nature of matrix collagen enzymatic cross-linking, in other words we suggest the matrix has time to form additional enzymatic crosslinks due to the delay in mineralization. The latter mechanism (matrix collagen enzymatic cross-linking) is suggested by the observation that collagen crosslink ratio correlated with bone formation, not resorption markers. In this respect, we might suggest that as the maximum accumulation of stable enzymatic collagen crosslinks occurs during adolescence [32,33], the delay in mineralization may allow the cross-linking process to continue. It will be important, when more material is available, to analyze the relative proportion of enzymatic and non-enzymatic collagen crosslinks in patients with IJO to see if the relative proportion of enzymatic cross-links is increased. The FTIRI data suggest that the amount of trivalent cross-links should be elevated and that the delay in mineralizing the matrix causes an accumulation of additional cross-links.
Supporting the latter mechanism, osteoclast changes reported in IJO patients were not significant [16]. Osteoblast changes were, however, large (54% reduction in bone formation/osteoblast surface) [15]. This also suggests that excessive osteoclastic activity was not a factor. There is one report of reduced collagen production by fibroblasts cultured from an IJO patient’s skin, but no information was provided on cross-linking in these cultures [34]. This data suggests that if the mineralization process is delayed or slowed, additional enzymatic cross-linking may be formed; the more highly cross-linked collagen may not be as receptive to mineral deposition [35]. This too is supported by the high collagen maturity on the edges of trabeculae where new mineralization should commence. In contrast, biopsies from post-menopausal osteoporotic women, show elevated levels of collagen maturity throughout the sample; the highest values in the center, suggesting that the matrix throughout the tissue is more mature.
The increase in collagen maturity was found in the presence of an invariant mean mineral content, increased acid phosphate substitution in cancellous IJO bone relative to controls and a trend towards decreased carbonate-to-phosphate ratio in the IJO cancellous bone. In general, increased carbonate-to-phosphate ratio reflects increased remodeling and is usually found in “older” bone [36], again suggesting a relatively “newer” mineral in the IJO cancellous bone and a delayed mineralization process. This is in agreement with an increased acid phosphate substitution, which is characteristic of a more recently formed bone [27]. There was also greater heterogeneity of mineral-to-matrix ratio and of collagen maturity in IJO, compared to controls. Increases in heterogeneity were unexpected, as iliac crest biopsies from post-menopausal osteoporotic patients with fractured bones, have decreased levels of heterogeneity [37], suggesting in turn, that IJO bone is distinct in some ways from PMO bone. The increase in heterogeneity and small compositional changes in the mineral, along with previously reported histomorphometric findings on these very same IJO patients, supports the notion that the formation of the apatite mineral in IJO bone is normal, but the rate of bone is formation is slow.
The lower bone turnover, suggested by the relatively lower carbonate-to-phosphate ratio for IJO compared to juvenile controls, is in contrast to what one would expect from a study of post-menopausal osteoporotic bone, where the turnover is higher [36]. The reduced carbonate-to-phosphate ratio suggesting a lower bone turnover in patients with IJO, is consistent with previous histomorphometric studies postulating an imbalance between osteoclast mediated erosion and osteoblast mediated refilling, resulting in decreased trabecular thickness in IJO patients [1].
HA crystallinity was not significantly different when IJO and juvenile controls were compared; yet acid phosphate substitution was elevated in cancellous bones and carbonate-to- phosphate ratio decreased. This may appear counterintuitive, but the sites for carbonate and acid phosphate substitutions in the bone mineral hydroxyapatite lattice are different [29,38]; thus, an increase of acid phosphate increasing the disorder (crystallinity) of the lattice and a decrease in carbonate content decreasing [29,39] this disorder, may result in a net zero change in the overall crystallinity parameter. It is equally plausible that the crystallinity parameter is less sensitive to variations than is the carbonate-to-phosphate ratio or the acid phosphate substitution.
This study had several limitations. The number of biopsies from patients with IJO that were available is small. Our sample size, similar to that of children with vertebral fractures in the Tamminen study [5], limits the power of our conclusions. None-the-less, we developed new information on the collagen quality in this condition, and believe that this information will be helpful in designing short term therapies for these children. For example, rather than using anti-resorptive agents, pharmaceuticals that stimulate mineralization (parathyroid hormone, vitamin D, sclerostin antibodies, or calcium supplementation) might accelerate the mineralization process, preventing the accumulation of a matrix in which the collagen maturity is elevated. There are case reports where vitamin D and calcitonin [40] or calcium, vitamin D, bisphosphonates and activity restriction [41], normalized BMD and eliminated pain in children with IJO treated for 2 years or until puberty. Since children with IJO recover without treatment after puberty, one cannot tell whether the correction in these case reports would have occurred overtime without any therapy. None-the-less, our data confirmed and correlated with the histomorphometric information on these children. The spread in the data is likely due to the differences in age and other characteristics among children with IJO [31]. Despite these limitations, IJO bone, like post-menopausal osteoporotic bone, had elevated collagen maturity. The fractional increase in collagen maturity exceeded that of post-menopausal osteoporosis. Unlike post-menopausal osteoporotic bone, the IJO bone lacked mature mineral crystals and had an increased amount of acid phosphate substitution.
In conclusion, taken together, since osteoporosis can and does occur in children, chronologic age cannot be the sole determinant of the osteoporosis phenotype. The bone composition in IJO and PMO has some similarities, especially the presence of elevated collagen cross-links. The mineral changes in PMO and IJO, however, are different and occur in opposite directions; increased acid phosphate substitution in IJO vs. a decrease in PMO, increases in heterogeneity in IJO vs. decreased heterogeneity in PMO, and decreased carbonate-to-phosphate ratios in IJO vs. increased ratios in PMO. These changes point to the importance of the matrix (collagen) structure in contributing to bone fragility, and suggest that osteoporosis is not only caused by increased chronologic age.
Acknowledgments
This study was supported by NIH grant AR041325. Dr. Ma’s research was partially supported by grant number R01HS021734 from the Agency for Healthcare Research and Quality. The authors gratefully appreciate the contribution of biopsies for use in this study from Dr. Frank Rauch and Dr. Francis Glorieux, Shriner’s Hospital, Montreal Canada.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Statement of Human Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Hospital for Special Surgery and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Raisz LG, Rodan GA. Pathogenesis of osteoporosis. Endocrinol Metab Clin North Am. 2003;32:15–24. doi: 10.1016/s0889-8529(02)00055-5. [DOI] [PubMed] [Google Scholar]
- 2.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. JAMA. 2001;285:785–795. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 3.Gourion-Arsiquaud S, Faibish D, Myers E, Spevak L, Compston J, Hodsman A, Shane E, Recker RR, Boskey ER, Boskey AL. Use of FTIR spectroscopic imaging to identify parameters associated with fragility fracture. J Bone Miner Res. 2009;24:1565–1571. doi: 10.1359/JBMR.090414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malluche HH, Porter DS, Mawad H, Monier-Faugere MC, Pienkowski D. Low-energy fractures without low T-scores characteristic of osteoporosis: a possible bone matrix disorder. J Bone Joint Surg Am. 2013;95:e1391–e1396. doi: 10.2106/JBJS.L.01281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tamminen IS, Mäyränpää MK, Turunen MJ, Isaksson H, Mäkitie O, Jurvelin JS, Kröger H. Altered bone composition in children with vertebral fracture. J Bone Miner Res. 2011;26:2226–2234. doi: 10.1002/jbmr.409. [DOI] [PubMed] [Google Scholar]
- 6.Wallace RJ, Pankaj P, Simpson AH. The effect of strain rate on the failure stress and toughness of bone of different mineral densities. J Biomech. 2013;46:2283–2287. doi: 10.1016/j.jbiomech.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Misof BM, Gamsjaeger S, Cohen A, Hofstetter B, Roschger P, Stein E, Nickolas TL, Rogers HF, Dempster D, Zhou H, Recker R, Lappe J, McMahon D, Paschalis EP, Fratzl P, Shane E, Klaushofer K. Bone material properties in premenopausal women with idiopathic osteoporosis. J Bone Miner Res. 2012;27:2551–2561. doi: 10.1002/jbmr.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paschalis EP, Mendelsohn R, Boskey AL. Infrared assessment of bone quality: a review. Clin Orthop Relat Res. 2011;469:2170–2178. doi: 10.1007/s11999-010-1751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: Now and in the Future. Lancet. 2009;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ensrud KE. Epidemiology of fracture risk with advancing age. J Gerontol A Biol Sci Med Sci. 2013;68:1236–1242. doi: 10.1093/gerona/glt092. [DOI] [PubMed] [Google Scholar]
- 11.Ward L, Glorieux F. The spectrum of pediatric osteoporosis. In: Glorieux F, editor. Pediatric bone, biology and diseases. Elsevier Science; San Diego Ca, USA: 2003. pp. 401–431. [Google Scholar]
- 12.Kulkarni ML, Keshavamurthy KS. Juvenile Idiopathic Osteoporosis. Indian Pediatrics. 2004;41:737–740. [PubMed] [Google Scholar]
- 13.Smith R. Idiopathic juvenile osteoporosis: experience of twenty-one patients. Br J Rheumatol. 1995;34:68–77. doi: 10.1093/rheumatology/34.1.68. [DOI] [PubMed] [Google Scholar]
- 14.Lorenc RS. Idiopathic juvenile osteoporosis. Calcif Tissue Int. 2002;70:395–397. doi: 10.1007/s00223-001-0045-y. [DOI] [PubMed] [Google Scholar]
- 15.Rauch F, Travers R, Norman ME, Taylor A, Parfitt AM, Glorieux FH. Deficient Bone Formation in Idiopathic Juvenile Osteoporosis: A Histomorphometric Study of Cancellous Iliac Bone. J Bone Miner Res. 2000;15:957–963. doi: 10.1359/jbmr.2000.15.5.957. [DOI] [PubMed] [Google Scholar]
- 16.Rauch F, Travers R, Norman ME, Taylor A, Parfitt AM, Glorieux FH. The Bone Formation defect in Idiopathic Juvenile Osteoporosis is Surface-Specific. Bone. 2002;31:85–89. doi: 10.1016/s8756-3282(02)00814-1. [DOI] [PubMed] [Google Scholar]
- 17.Bacchetta J, Wesseling-Perry K, Gilsanz V, Gales B, Pereira RC, Salusky IB. Idiopathic Juvenile Osteoporosis: A cross-sectional Single-Centre Experience with Bone Histomorphometry and Quantitative Computed Tomography. Pediatr Rheumatol Online J. 2013;11:6. doi: 10.1186/1546-0096-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bianchi ML. Osteoporosis in Children and Adolescents. Bone. 2007;41:486–495. doi: 10.1016/j.bone.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Uziel Y, Zifman E, Hashkes PJ. Osteoporosis in Children: Pediatric and Pediatric Rheumatology Perspective: A Review. Pediatr Rheumatol Online J. 2009;7:16. doi: 10.1186/1546-0096-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pludowsky P, Lebiedowski M, Olszaniecka M, Marowska J, Matusik H, Lorenc RS. Idiopathic Juvenile Osteoporosis – an Analysis of the Muscle-Bone Relationship. Osteoporos Int. 2006;17:1681–1690. doi: 10.1007/s00198-006-0183-1. [DOI] [PubMed] [Google Scholar]
- 21.Fonseca H, Moreira-Gonçalves D, Coriolano HJ, Duarte JA. Bone quality: the determinants of bone strength and fragility. Sports Med. 2014;44:37–53. doi: 10.1007/s40279-013-0100-7. [DOI] [PubMed] [Google Scholar]
- 22.Gourion-Arsiquaud S, Burket JC, Havill LM, DiCarlo E, Doty SB, Mendelsohn R, van der Meulen MC, Boskey AL. Spatial Variation in osteonal Bone Properties Relative to Tissue and Animal Age. J Bone Miner Res. 2009;24:1271–1281. doi: 10.1359/JBMR.090201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donnelly E, Meredith DS, Nguyen JT, Gladnick BP, Rebolledo BJ, Shaffer AD, Lorich DG, Lane JM, Boskey AL. Reduced Cortical Bone Compositional Heterogeneity with Bisphosphonate Treatment in Postmenopausal Women with Intertrochanteric and Subtrochanteric Fractures. J Bone Miner Res. 2012;27:672–678. doi: 10.1002/jbmr.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gourion-Arsiquaud S, West PA, Boskey AL. Fourier Transform-Infrared Microspectroscopy and Microscopic Imaging. In: Westendorf JJ, editor. Methods in Molecular Biology, Vol 455: Osteoporosis: Methods and Protocols. 1st. Totowa, NJ: Humana Press; 2008. pp. 293–303. [DOI] [PubMed] [Google Scholar]
- 25.Paschalis EP, Tatakis DN, Robins S, Fratzl P, Manjubala I, Zoehrer R, Gamsjaeger S, Buchinger B, Roschger A, Phipps R, Boskey AL, Dall’Ara E, Varga P, Zysset P, Klaushofer K, Roschger P. Lathyrism-Induced Alterations in Collagen Cross-Links Influence the Mechanical Properties of Bone Material Without Affecting the Mineral. Bone. 2011;49:1232–1241. doi: 10.1016/j.bone.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faibish D, Gomes A, Boivin G, Binderman I, Boskey A. Infrared imaging of calcified tissue in bone biopsies from adults with osteomalacia. Bone. 2005;36:6–12. doi: 10.1016/j.bone.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 27.Spevak L, Flach CR, Hunter T, Mendelsohn R, Boskey A. Fourier-Transform Infrared Spectroscopic Imaging Parameters Describing Acid Phosphate Substitution in Biologic Hydroxyapatite. Calcif Tissue Int. 2013;92:418–428. doi: 10.1007/s00223-013-9695-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erben RG. Embedding of Bone Samples in Methylmethacrylate: An Improved Method Suitable for Bone Histomorphometry, Histochemistry, and Immunohistochemistry. J Histochem Cytochem. 1997;45:307–313. doi: 10.1177/002215549704500215. [DOI] [PubMed] [Google Scholar]
- 29.Fleet ME. The carbonate ion in hydroxyapatite: recent X-ray and infrared results. Front Biosci (Elite Ed) 2013;5:643–52. doi: 10.2741/e645. [DOI] [PubMed] [Google Scholar]
- 30.Paschalis EP, Shane E, Lyritis G, Skarantavos G. Bone Fragility and Collagen Cross-Links. J Bone Miner Res. 2004;19:2000–2004. doi: 10.1359/JBMR.040820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jobke B, Delling G. Juvenile osteoporosis: A retrospective histomorphological study on 628 iliac crest biopsies. Bone. 2007;40:S53–S54. [Google Scholar]
- 32.Eyre DR, Dickson IR, Van Ness K. Collagen crosslinking in human bone and articular cartilage. Age-related changes in the content of mature hydroxypyridinium residues. Biochem J. 1988;252:495–500. doi: 10.1042/bj2520495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saito M, Marumo K, Fujii K, Ishioka N. Single column high – performance liquid chromatographic – fluorescence detection of immature, mature and senescent cross-links of collagen. Anal Biochem. 1997;253:26–32. doi: 10.1006/abio.1997.2350. [DOI] [PubMed] [Google Scholar]
- 34.Pocock AE, Francis MJ, Smith R. Type I collagen biosynthesis by skin fibroblasts from patients with idiopathic juvenile osteoporosis. Clin Sci (Lond) 1995;89:69–73. doi: 10.1042/cs0890069. [DOI] [PubMed] [Google Scholar]
- 35.Knott L, Bailey AJ. Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance. Bone. 1998;22:181–187. doi: 10.1016/s8756-3282(97)00279-2. [DOI] [PubMed] [Google Scholar]
- 36.McCreadie BR, Morris MD, Chen TC, Sudhaker Rao D, Finney WF, Widjaja E, Goldstein SA. Bone tissue compositional differences in women with and without osteoporotic fracture. Bone. 2006;39:1190–1195. doi: 10.1016/j.bone.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Gourion-Arsiquaud S, Lukashova L, Power J, Loveridge N, Reeve J, Boskey AL. Fourier transform infrared imaging of femoral neck bone: reduced heterogeneity of mineral-to-matrix and carbonate-to-phosphate and more variable crystallinity in treatment-naive fracture cases compared with fracture-free controls. J Bone Miner Res. 2013;28:150–161. doi: 10.1002/jbmr.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peroos S, Du Z, de Leeuw NH. A computer modelling study of the uptake, structure and distribution of carbonate defects in hydroxy-apatite. Biomaterials. 2006;27:2150–2161. doi: 10.1016/j.biomaterials.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 39.Young RA, Holcolmb DW. The role of acid phosphate in hydroxyapatite lattice expansion. Calcif Tiss Int. 1984;36:60–63. doi: 10.1007/BF02405294. [DOI] [PubMed] [Google Scholar]
- 40.Imerci A, Canbek U, Haghari S, Sürer L, Kocak M. Idiopathic juvenile osteoporosis: A case report and review of the literature. International Journal of Surgery Case Reports. 2015;9:127–129. doi: 10.1016/j.ijscr.2015.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kauffman RP, Overton TH, Shiflett M, Jennings JC. Osteoporosis in children and adolescent girls: case report of idiopathic juvenile osteoporosis and review of the literature. Obstet Gynecol Surv. 2001;56:492–504. doi: 10.1097/00006254-200108000-00023. [DOI] [PubMed] [Google Scholar]


