Abstract
Background
Obesity is a risk factor for heart failure (HF), but is associated with lower N-terminal of pro-Brain Natriuretic Peptide (NT-proBNP) levels. It is unclear whether the prognostic value and implications of NT-proBNP levels for HF risk differ across body mass index (BMI) categories.
Methods and Results
We followed 12,230 ARIC participants free of prior HF at baseline (visit 2, 1990–1992) with BMI ≥18.5 kg/m2. We quantified and compared the relative and absolute risk associations of NT-proBNP with incident HF across BMI categories. There were 1,861 HF events during a median 20.6 years of follow-up. Despite increased HF risk in obesity, a weak inverse association was seen between baseline BMI and NT-proBNP levels (r = −0.10). Nevertheless, higher baseline NT-proBNP was associated with increased HF risk in all BMI categories. NT-proBNP improved HF risk prediction overall and even among those with severe obesity (BMI ≥35 kg/m2; improvement in c-statistic +0.032 [95% CI 0.011–0.053]). However, given higher HF rates among those with obesity, at each NT-proBNP level, higher BMI was associated with greater absolute HF risk. Indeed, among those with NT-proBNP 100 to < 200 pg/ml, the average 10-year HF risk was <5% among normal weight individuals but >10% if severely obese.
Conclusions
Despite its inverse relationship with BMI, NT-proBNP provides significant prognostic information regarding the risk of developing HF even among individuals with obesity. Given the higher baseline HF risk among persons with obesity, even slight elevations in NT-proBNP may have implications for increased absolute HF risk in this population.
Keywords: obesity, natriuretic peptides, heart failure, risk prediction, epidemiology
Brain natriuretic peptide (BNP) is a hormone with natriuretic and vasodilatory properties that is secreted by cardiac ventricular myocytes in response to elevated ventricular filling pressures and increased wall stress1–3. The N-terminal fragment of the prohormone for BNP (NT-proBNP) is a diagnostic marker for heart failure (HF) severity4, 5 and is also associated with subclinical left ventricular (LV) dysfunction among asymptomatic individuals6. In addition to its relationship with structural heart disease, elevated NT-proBNP has been independently linked to incident HF among individuals within the general population7–9. Prospective analyses have demonstrated that the addition of NT-proBNP to traditional risk factors significantly improves HF risk prediction10. Interestingly, NT-proBNP is inversely associated with BMI and known to be lower among obese individuals with and without HF11–15, despite the higher burden of HF associated with obesity16, 17. Multiple studies have demonstrated relatively poor correlation between natriuretic peptide levels and LV filling pressures among individuals with obesity compared to those who are normal weight18, 19. Additionally, among patients undergoing cardiac catheterization, obese subjects have been shown to have lower NT-proBNP levels despite higher average LV end diastolic pressures20. Although the inverse relationship between BMI and NT-proBNP has led to concerns regarding the use of NT-proBNP levels for HF diagnosis among people with obesity21, data on NT-proBNP for HF risk prediction among individuals with obesity are lacking. Therefore, we evaluated the utility of NT-proBNP for HF risk prediction among individuals with different obesity status in the Atherosclerosis Risk in Communities (ARIC) study, a community-based predominately bi-ethnic cohort of middle-aged individuals.
Methods
The ARIC study is an ongoing prospective cohort study of 15,792 individuals enrolled from four U.S. communities: Washington County, Maryland; Jackson, Mississippi; Forsyth County, North Carolina; and the suburbs of Minneapolis, Minnesota. The initial recruitment and baseline evaluation took place between 1987 and 1989. Participants were subsequently examined at three follow-up visits at approximately 3 year intervals; after an extended interval, a fifth study visit was recently completed between 2011 and 2013. The design, recruitment and examination protocols for ARIC has been described in detail previously22. The institutional review boards at each site approved all study protocols and informed consent was provided by all study participants. Visit 2 (1990–1992), at which NT-proBNP was measured for all participants, was the baseline for the present study.
Of the 14,348 participants who attended visit 2 in 1990 to 1992, we excluded individuals with self-reported HF at Visit 1 or with a HF event at or prior to Visit 2 (N=955). We additionally excluded those with a BMI < 18.5 kg/m2 (N=124), the small number of individuals not of black or white race (N=38), those individuals missing data on clinical covariates (N=168 for smoking, systolic blood pressure, diabetes mellitus, heart rate and prevalent coronary heart disease (CHD)) and those individuals missing data on NT-proBNP or BMI (N=833), for a final sample size of 12,230 individuals at baseline.
All covariates were assessed at baseline (visit 2, 1990–1992). BMI was calculated from measured height and weight and categorized as normal weight (18.5 to < 25 kg/m2), overweight (25 to < 30 kg/m2), obese (30 to < 35 kg/m2) or severely obese (≥ 35 kg/m2). We also evaluated variables included in the ARIC HF risk score, a parsimonious algorithm of traditional risk factors that has previously been shown to strongly predict incident HF among men and women in the general community10. The variables included in the ARIC HF risk score are age, race, sex, smoking status, prior CHD, diabetes mellitus (DM), mean systolic blood pressure, use of anti-hypertensive medications, and heart rate. Smoking status was categorized as current, former or never smoker. CHD was defined as self-reported CHD at Visit 1 or a CHD event (validated non-fatal myocardial infarction or coronary revascularization, or silent myocardial infarction by ECG criteria) at or prior to Visit 2. Individuals were classified as having DM if they had a self-reported history of diabetes, used hypoglycemic medications, had a fasting blood glucose of ≥ 126 mg/dl or had a non-fasting blood glucose of ≥ 200 mg/dl. Systolic blood pressure was measured three times during the same examination, and the mean of the last two values was used for analysis. The use of anti-hypertensive medications over the 2 weeks prior to the exam was ascertained by use of a standardized questionnaire.
NT-proBNP was measured in 2012–2013 from thawed Visit 2 serum samples that had previously been stored at −70 degrees Celsius. Measurements were performed using a sandwich immunoassay method on the on the Roche Elecsys 2010 Analyzer (Roche Diagnostics Corporation, Indianapolis, IN). The assay coefficients of variation were 5.4% at an NT-proBNP value of 133 pg/ml and 4.3% at a value of 4,516 pg/ml. The value of 2.5 pg/ml was imputed for those individuals with undetectable NT-proBNP (n=248). For this analysis, NT-proBNP was both assessed continuously and categorized using prespecified cutpoints of 50, 100, 200 and 400 pg/ml.
Participants were contacted annually and active surveillance of hospitals in the ARIC communities was performed to obtain information about interim hospitalizations, and vital records were examined for all deaths. The outcome of interest was incident HF occurring after Visit 2, defined as the first hospitalization or death related to HF. Hospitalizations and deaths related to HF were identified by HF discharge codes (ICD-9 code 428 for hospitalizations and deaths early during follow-up and ICD-10 code I50 for later deaths). After 2004, HF events were additionally adjudicated by an expert panel23. Follow-up for HF events was available through December 31, 2012.
Statistical Analysis
Baseline characteristics across BMI categories were compared using analysis of variance for continuous variables and the chi-squared test for categorical variables. NT-proBNP levels were compared across BMI categories for the overall study population, and within subgroups defined by race and gender.
In prospective analyses, Cox proportional hazards models were used to estimate adjusted incidence rates, adjusted hazard ratios and corresponding 95% confidence intervals for the association of NT-proBNP with incident HF within baseline BMI categories. Follow-up began at ARIC Visit 2 (1990–92) and continued until incident HF or censoring, with Cox models accounting for unequal follow-up time. Censoring occurred if the participant was lost to follow-up or died from a non-HF etiology, or if the participant was administratively censored at December 31, 2012. Cox proportional hazards models were adjusted for: age, race, sex, smoking status, prior CHD, DM, mean systolic blood pressure, use of anti-hypertensive medications, and heart rate (variables included in the ARIC HF risk score). NT-proBNP was modeled as a linear spline with knots at NT-proBNP levels of 50, 100, 200 and 400 pg/ml and with 25 pg/ml as the reference.
To evaluate potential effect modification of obesity on the NT-proBNP-HF risk association, likelihood ratio tests were used to assess for interactions across BMI categories on the multiplicative scale for comparisons of hazard ratios, and on the additive scale for comparisons of adjusted incidence rates. Pointwise interactions were evaluated by comparing the relative and absolute risk differences associated with each 1.08-fold higher NT-proBNP between individuals who were normal weight (reference group) and those in other BMI categories. The statistical approach for evaluating pointwise interactions has been has been described in detail in prior analyses24. Tests for overall interactions across BMI categories were also performed.
We assessed whether NT-proBNP added predictive value beyond traditional risk factors for the outcome of incident HF across BMI categories, by evaluating changes in prediction statistics associated with the addition of NT-proBNP to the variables in the ARIC HF risk score. We evaluated changes in the net reclassification index (NRI), C-statistic and integrated discrimination improvement (IDI) within each BMI category, using methods that account for censoring25, 26. Changes in the NRI were calculated for events and non-events, and assessed using both continuous and categorical NRI (with categories for 10-year HF risk of <5%, 5 to <10%, 10 to <20% and ≥20%). 95% confidence intervals for NRI and IDI changes were calculated using bootstrapping methods, as described previously26. In assessing its contribution to risk prediction, NT-proBNP was modeled both continuously and categorically, using the aforementioned cutpoints.
To further assess the implications of NT-proBNP levels for absolute HF risk among individuals with and without obesity, we used Cox models to calculate the average predicted 10-year HF risk within NT proBNP ranges of <50, 50 to <100, 100 to <200, 200 to <400 and ≥ 400 pg/ml for each BMI category, with risks calculated at mean levels of each of the ARIC HF Risk Score predictors. Additionally, we estimated the NT-proBNP levels associated with pre-specified levels of absolute HF risk (5%, 10% and 20% over 10 years) within each BMI category at mean levels of each of the covariates, and used bootstrapping to calculate 95% confidence intervals.
Additional analyses were performed with stratification by gender, race or age (≥ or < 60 years). Analyses were also performed substituting waist circumference (assessed categorically, by dividing into quartiles) for BMI as the metric for adiposity. In sensitivity analyses, we repeated assessments of the relative and absolute risk associations between NT-proBNP and HF across BMI categories using only adjudicated HF events.
Statistical analyses were performed with Stata version 13.1. All reported p-values are 2-sided.
Results
Individuals with higher BMI were more likely to be African-American and less likely to be current smokers (Table 1). Higher BMI was also associated with a greater burden of HF risk factors, including higher systolic blood pressure, more anti-hypertensive medication use, a slightly higher mean heart rate and a greater prevalence of diabetes. As expected, overall, NT-proBNP was inversely correlated with BMI (Pearson’s correlation coefficient: −0.10; p<0.001). Examining this in more detail, compared to those with normal weight, NT-proBNP levels were lower in the overweight and obese groups, with a slight uptick in NT-proBNP levels among those with severe obesity (Table 1). This pattern was generally maintained across subgroups defined by race and gender (Figure 1), although more of a U-shaped association was seen among white men, with individuals with severe obesity having similar average NT-proBNP levels to those with normal weight.
Table 1.
Characteristics of Study Population, Stratified by BMI Category
| Variable | Normal Weight N=3,802 |
Overweight N=4,953 |
Obese N=2,356 |
Severely Obese N=1,119 |
|---|---|---|---|---|
| Mean Age, in years (SD) | 57 (6) | 58 (6) | 57 (6) | 56 (6) |
| Female – % | 62 | 46 | 54 | 75 |
| African American – % | 15 | 22 | 33 | 44 |
| Current Smokers – % | 28 | 21 | 16 | 13 |
| Mean systolic blood pressure, in mmHg (SD) | 117 (18) | 121 (18) | 125 (19) | 130 (19) |
| Anti-hypertensive medicine – % | 20 | 29 | 41 | 50 |
| Mean heart rate (SD) | 65 (10) | 65 (10) | 66 (10) | 69 (11) |
| Diabetes – % | 5 | 12 | 23 | 33 |
| History of CHD - % | 4 | 6 | 6 | 4 |
| Median NT-proBNP, in pg/ml (25th–75th percentile) | 63 (34–108) | 46 (24–85) | 45 (24–85) | 51 (27–91) |
Figure 1.
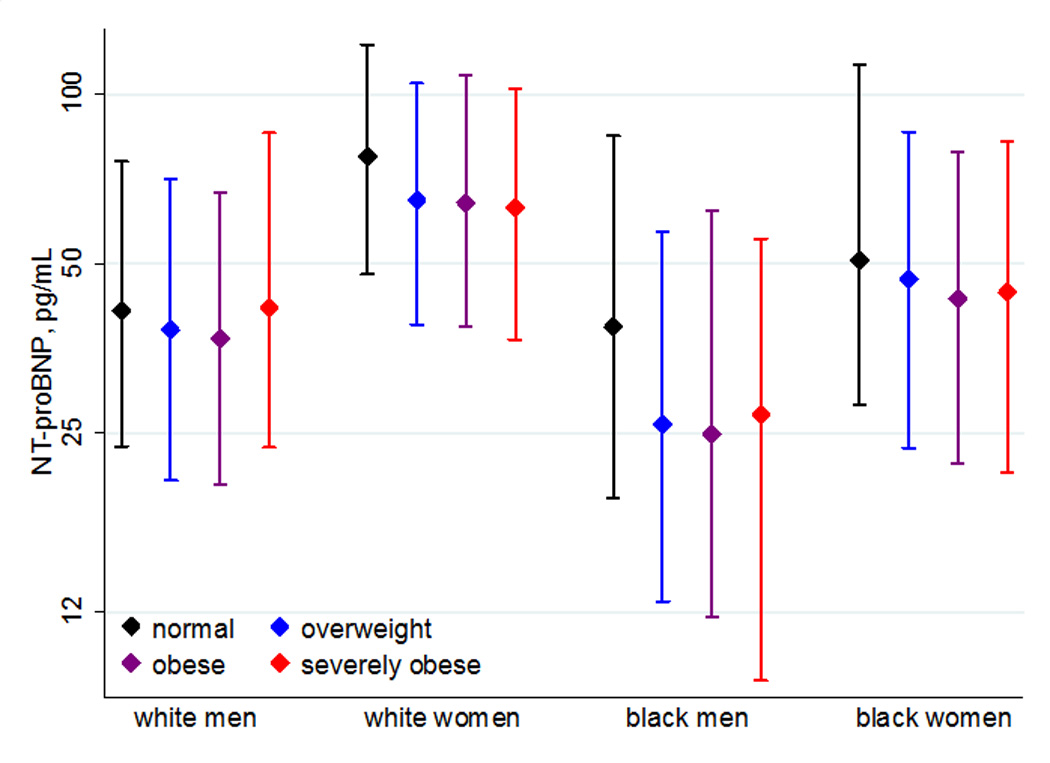
Median NT-proBNP levels within BMI Categories, Stratified by Race/Gender Subgroups. The median NT-proBNP levels and associated interquartile ranges within each BMI category are displayed for white men, white women, black men and black women.
Over a median of 20.6 years of follow-up, there were 1,861 cases of incident heart failure. Higher BMI category at baseline was associated with higher rates of HF, with incidence rates (per 1000 person years) for those with normal weight of 5.6, overweight of 7.5, obesity of 11.6 and severe obesity of 16.8.
Despite the inverse relationship between BMI and NT-proBNP, higher NT-proBNP levels were associated with greater relative risks of HF within each BMI category even after adjusting for ARIC HF risk score predictors (Figure 2a). The relative risk gradient was blunted among those in the highest BMI categories, with significant pointwise interactions seen in some ranges of NT proBNP (denoted as Δ in the bottom of Figure 2a). The significant interactions at these points indicate a difference in the slope of risk from the reference (25 pg/ml of NT-proBNP) to the indicated NT-proBNP value for individuals with obesity or severe obesity relative to those with normal weight. Significant overall interactions for the association between continuous log-transformed NT-proBNP and HF risk were also seen across BMI categories (overall p for interaction <0.01 for severe obesity versus normal weight and <0.05 for obesity versus normal weight).
Figure 2.
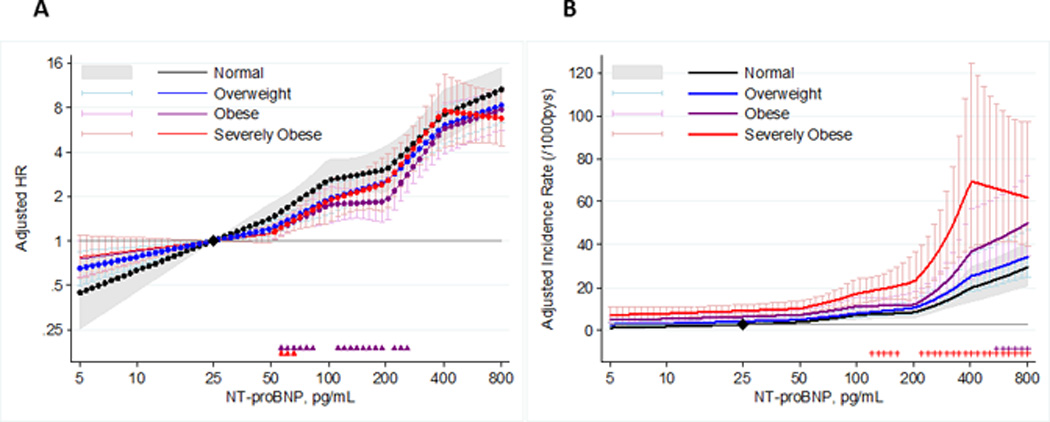
Relative and Absolute Risks of HF Associated with Higher NT-proBNP Within BMI Categories. Linear spline models demonstrating the relative (Figure 2a) and absolute (Figure 2b) risk associations of NT-proBNP with incident HF within each BMI category, using a common reference of 25 pg/ml and knots at NT-proBNP levels of 50, 100, 200 and 400 pg/ml. The color-coded triangles and plus signs at the bottom of each panel indicate significantly negative (smaller effect size) and positive (greater effective size) pointwise interactions, respectively, for a given BMI category compared with normal weight. All regression models are adjusted for the variables in the ARIC HF Risk score: age, race, sex, smoking status, prior CHD, DM, mean systolic blood pressure, use of anti-hypertensive medications, and heart rate.
However, despite the shallower risk gradients for hazard ratios in obesity and severe obesity than normal weight, because of the higher rates of HF associated with higher BMI, the absolute risk differences associated with higher NT-proBNP were actually greater among those with obesity and severe obesity at higher levels of NT-proBNP (significant pointwise interactions indicated as + in the bottom of Figure 2b). Accordingly, the NT-proBNP increment from 25 to 150 pg/ml was associated with an incidence rate difference for HF (per 1000 person years) of 11.0 among individuals with severe obesity and 5.1 among those with normal weight.
These risk associations were similar across demographic subgroups defined by age, gender and race, and when waist circumference was used as an alternative measure for adiposity. Analogous findings were seen when we restricted our analyses to adjudicated HF cases only (N=893). Findings were also similar when we excluded those individuals with HF events in the first 3 years after Visit 2 (n=165), to account for the possibility of undiagnosed early HF at the baseline visit. To account for the possibility of dramatic weight loss after the baseline visit affecting the observed risk associations, we performed a sensitivity analysis excluding 18 individuals who underwent bariatric surgery after the baseline visit and our findings were largely unchanged.
We evaluated the impact of NT-proBNP on HF risk prediction within each BMI category (Table 2). Overall, as reflected by the C-statistic, traditional risk factors were less predictive of HF in higher BMI categories, with an associated C-statistic of 0.827 (95% CI: 0.795–0.858) for normal weight vs. 0.754 (95% CI: 0.715–0.792) for severe obesity. Nonetheless, the addition of NT-proBNP to traditional risk factors within the ARIC HF Risk Score significantly improved HF risk prediction and discrimination in all BMI categories, as reflected by the NRI, IDI and changes in the C-statistic. C-statistic values across the range of NT-proBNP within each BMI category are displayed in the Supplemental Figure. Improvements in risk prediction were similar when NT-proBNP was modeled as a continuous or a categorical variable.
Table 2.
Heart Failure Risk Prediction Statistics Associated with the Addition of NT-proBNP, by BMI category
| C statistic | Difference in C statistic* |
Categorical NRI† |
Categorical NRI, event |
Categorical NRI, nonevent |
Continuous NRI |
Continuous NRI event |
Continuous NRI, nonevent |
IDI | |
|---|---|---|---|---|---|---|---|---|---|
| Normal Weight | |||||||||
| ARIC basic HF risk score | 0.827 (0.795 – 0.858) |
||||||||
| +categorical NT-BNP | 0.849 (0.816 – 0.881) |
0.022 (0.004 – 0.039) |
0.141 (0.032 – 0.253) |
0.104 (−0.005 – 0.218) |
0.038 (0.025 – 0.051) |
0.390 (0.222 – 0.540) |
0.075 (−0.093 – 0.225) |
0.316 (0.283 – 0.345) |
0.063 (0.042 – 0.087) |
| +continuous NT-BNP | 0.854 (0.822 – 0.885) |
0.027 (0.009 – 0.045) |
0.196 (0.088 – 0.298) |
0.166 (0.061 – 0.272) |
0.030 (0.018 – 0.043) |
0.414 (0.246 – 0.566) |
0.171 (0.006 – 0.318) |
0.243 (0.212 – 0.274) |
0.080 (0.053 –0.111) |
| Overweight | |||||||||
| ARIC basic HF risk score | 0.802 (0.775 – 0.828) |
||||||||
| +categorical NT-BNP | 0.829 (0.802 – 0.855) |
0.027 (0.013 – 0.041) |
0.229 (0.146 – 0.306) |
0.170 (0.090 – 0.246) |
0.058 (0.045 – 0.071) |
0.509 (0.392 – 0.633) |
0.124 (0.006 – 0.245) |
0.385 (0.357 – 0.411) |
0.077 (0.059 –0.098) |
| +continuous NT-BNP | 0.826 (0.798 – 0.853) |
0.024 (0.009 – 0.040) |
0.243 (0.169 – 0.311) |
0.206 (0.132 – 0.271) |
0.038 (0.026 – 0.050) |
0.451 (0.339 – 0.572) |
0.250 (0.140 – 0.358) |
0.201 (0.173 – 0.233) |
0.059 (0.044 – 0.079) |
| Obese | |||||||||
| ARIC basic HF risk score | 0.771 (0.739 – 0.803) |
||||||||
| +categorical NT-BNP | 0.809 (0.777 – 0.841) |
0.038 (0.019 – 0.057) |
0.213 (0.117 – 0.309) |
0.124 (0.033 – 0.214) |
0.090 (0.065 – 0.111) |
0.597 (0.445 – 0.755) |
0.223 (0.077 – 0.376) |
0.374 (0.332 – 0.410) |
0.053 (0.035 – 0.069) |
| +continuous NT-BNP | 0.808 (0.776 – 0.839) |
0.037 (0.016 – 0.057) |
0.218 (0.123 – 0.303) |
0.153 (0.062 – 0.236) |
0.065 (0.041 – 0.085) |
0.477 (0.326 – 0.627) |
0.300 (0.152 – 0.449) |
0.177 (0.134 – 0.216) |
0.053 (0.034 – 0.070) |
| Severely Obese | |||||||||
| ARIC basic HF risk score | 0.754 (0.715 – 0.792) |
||||||||
| +categorical NT-BNP | 0.784 (0.747 – 0.821) |
0.030 (0.008 – 0.052) |
0.119 (0.020 – 0.216) |
−0.004 (−0.090 – 0.088) |
0.123 (0.086 – 0.161) |
0.323 (0.133 – 0.517) |
−0.022 (−0.202 – 0.147) |
0.345 (0.283 – 0.405) |
0.053 (0.031 – 0.076) |
| +continuous NT-BNP | 0.786 (0.750 – 0.821) |
0.032 (0.011 – 0.053) |
0.123 (0.027 – 0.215) |
0.059 (−0.029 – 0.146) |
0.064 (0.031 – 0.098) |
0.275 (0.086 – 0.450) |
0.111 (−0.063 – 0.270) |
0.164 (0.099 – 0.224) |
0.043 (0.025 – 0.063) |
Normal weight: BMI 18.5 to <25 kg/m2; Overweight: BMI 25 to <30 kg/m2; Obese: BMI 30 to <35 kg/m2; Severely Obese: BMI ≥ 35 kg/m2
The difference in C-statistic indicates the difference in discrimination between the baseline model (ARIC HF risk score covariates) and the model additionally including NT-proBNP.
Categorical NRI = Net Reclassification Improvement across four risk categories over 10 years: 0–5%, 5–10%, 10–20%, 20%+
We then calculated the average predicted HF risk over 10 years within NT-proBNP ranges of <50, 50 to <100, 100 to <200, 200 to <400 and ≥400 pg/ml for each BMI category, when all other ARIC HF Risk Score predictors were at the average levels of our study population. As demonstrated in Figure 3, within each range of NT-proBNP, those in higher BMI categories had a higher predicted 10-year HF risk. For example, among those in the NT-proBNP range of 100 to <200 pg/ml, the average predicted 10-year HF risk was <5% if weight was normal but >10% if severely obese. Nevertheless, lower levels of NT-proBNP were associated with lower predicted risk within each BMI category, with NT-proBNP levels of 50 to <100 pg/ml associated with 3.0% and 7.1% average 10-year HF risk among those with normal weight and severe obesity, respectively.
Figure 3.

Predicted 10-year HF Risk (and Associated 95% Confidence Intervals) within NT-proBNP Ranges for Each BMI Category. The predicted risk of incident HF events over 10 years was calculated at mean levels of each of the covariates in the ARIC HF Risk Score (age, race, sex, smoking status, prior CHD, DM, mean systolic blood pressure, use of anti-hypertensive medications, and heart rate) using Cox regression. The average predicted 10-year HF risk for the NT-proBNP ranges of <50, 50 to <100, 100 to <200, 200 to <400 and ≥400 pg/ml are displayed for each BMI category. 95% confidence intervals were calculated using bootstrapping techniques.
From another perspective, we assessed the NT-proBNP levels leading to prespecified 10-year HF risks (5%, 10% and 20%) in each BMI category at mean levels of all covariates (Table 3). For example, a 10% risk of HF over 10 years was associated with median NT-proBNP levels of 119 pg/ml (95% CI: 83–177 pg/ml) in the severely obese category, 289 pg/ml (95% CI: 195–500 pg/ml) in the obese category, 473 pg/ml (95% CI: 318–778 pg/ml) in the overweight category, and 489 pg/ml (95% CI: 367–703 pg/ml) in the normal weight category. Generally, relatively modest NT-proBNP elevations among those with severe obesity in the general community were associated with a high absolute risk of developing HF.
Table 3.
NT-proBNP Levels* (pg/ml) with 95% Confidence Intervals Associated with Levels of 10–year HF Risk
| BMI Category | 10–year HF risk | ||
|---|---|---|---|
| 5% | 10% | 20% | |
| Normal Weight (BMI 18.5 to < 25 kg/m2) | 160 (129 – 202) | 489 (367 – 703) | 1563 (1014 – 2781) |
| Overweight (BMI 25 to < 30 kg/m2) | 118 (96 – 154) | 473 (318 – 778) | 2012 (1055 – 4526) |
| Obese (BMI 30 to < 35 kg/m2) | 57 (41 – 73) | 289 (195 – 500) | 1562 (785 – 4460) |
| Severely Obese (BMI ≥ 35 kg/m2) | 23 (14 – 35) | 119 (83 – 177) | 653 (358 – 1631) |
Using log-linear NT-proBNP.
Discussion
In this prospective cohort study of 12,230 individuals in the general population, we found that higher NT-proBNP levels were associated with an increased risk of HF even among individuals with obesity, despite an inverse association between NT-proBNP and BMI. We observed slightly lesser relative risk associations, but greater absolute risk associations among those with obesity and severe obesity compared to those with normal weight. Our findings were largely consistent across different demographic subgroups. Furthermore, NT-proBNP significantly improved HF risk prediction in all BMI categories. Because of the higher rates of HF associated with obesity, at each level of NT-proBNP, higher BMI categories were associated with increased absolute HF risk. For example, among those with severe obesity, the average 10-year HF risk was 4.7% if NT-proBNP was less than 50 but exceeded 10% with NT-proBNP in the 100 to <200 pg/ml range. Corresponding risks in the normal weight group were 1.5% and 4.4%. Similarly, equivalent levels of absolute HF risk were reflected by lower NT-proBNP levels among those with higher BMI, with NT-proBNP levels of 119 pg/ml (95% CI: 83–177 pg/ml) and 489 pg/ml (95% CI: 367–703) associated with a 10% 10-year HF risk among those with severe obesity and normal weight, respectively. These results suggest that even mild elevations in NT-proBNP among those with severe obesity identify individuals with a high absolute risk of developing HF.
Prior studies have demonstrated that higher natriuretic peptide levels among individuals in the general community are independently associated with increased risk for future HF and mortality7, 9, 15. In a community-based study, NT-proBNP levels were shown to improve HF risk prediction beyond traditional risk factors10. However, the utility of NT-proBNP for risk prediction among individuals with obesity has been unclear. Hemodynamic studies comparing obese and non-obese patients have demonstrated lower levels and lesser correlations of natriuretic peptide levels with LV end-diastolic pressures among those with obesity, despite higher LV filling pressures in this group18–20. It has been suggested that among HF patients with obesity, lower BNP thresholds for “abnormal” values may be needed to maximize the accuracy of HF diagnoses11. Our analysis supports this approach of utilizing lower NT-proBNP thresholds for HF prediction among obese individuals in the community.
In our spline models, at the highest levels of NT-proBNP a decrease in the slope of the risk association was seen for those with severe obesity. This most likely represents statistical imprecision due to the small number of individuals (n=31) with both severe obesity and very high NT-proBNP, and in fact the overwhelming majority of this subgroup went on to develop incident HF (n=25). However, further studies should explore the implications of very elevated NT-proBNP for HF risk among those with severe obesity.
The mechanisms and pathophysiologic significance of the inverse relationship between measures of adiposity and natriuretic peptide levels remain unclear. There is an intricate interrelationship between obesity and natriuretic peptides, with adipose tissue having a high concentration of receptors that promote the clearance of BNP, and higher BNP levels being associated with enhanced lipolysis and metabolism27. A recent study demonstrated that mice genetically engineered to lack receptors promoting the clearance of BNP had lesser fat mass and a greater expression of thermogenic genes characteristically found in brown adipose tissue that stimulate energy expenditure28. Treatment with natriuretic peptide infusions similarly induced “browning” of adipocytes, lending further credence to a causal association between BNP and metabolic rates. In our analysis, a J-shaped relationship between BMI and NT-proBNP was seen, with the highest levels among those with normal weight. It is possible that the slight increase in NT-proBNP levels observed in the severe obesity category may reflect the balance between the negative association between BMI and NT-proBNP and the increased risk of incident HF among those with very high BMI.
This study has significant clinical implications. First, it confirms that regardless of obesity status, NT-proBNP measurements among adults in the general community provide prognostic information beyond traditional risk factors regarding HF risk. Additionally, higher BMI was associated with greater absolute HF risk at each level of NT-proBNP, with NT-proBNP levels as low as 100 to <200 pg/ml being associated with greater than 10% predicted HF risk among those with severe obesity (in contrast with a 7.1% 10-year average HF risk if NT-proBNP levels were 50 to <100 pg/ml). The finding that modest NT-proBNP levels are associated with high predicted HF risk among individuals with severe obesity may also have implications for HF prevention. Assessments of global risk are central to prevention strategies for CHD and stroke29, but are not currently widely accepted in clinical practice for approaches to HF prevention. Our results suggest that if such a risk centered approach is applied for HF, even mild elevations of NT-proBNP warrant attention among those with severe obesity. Further analyses incorporating both HF events and cost-benefit assessments will be needed to establish thresholds for “abnormal” NT-proBNP levels for risk prediction within each BMI category.
There are some limitations of this analysis. Because it is an observational study, we cannot rule out the possibility of residual confounding. This study didn’t account for medical therapies during the follow-up period that may have influenced the development of heart failure. It is also possible that differences with respect to lifestyle factors such as diet, physical activity and adherence not captured in this analysis may have additionally influenced the associations of interest. Additionally, the use of hospitalization and discharge codes for the diagnosis of incident HF may have resulted in some misclassification, although sensitivity analyses using only adjudicated HF events demonstrated similar outcomes. Additionally, this analysis doesn’t capture outpatient HF cases; however, the use of inpatient HF cases in cohort studies has been associated with relatively high diagnostic specificity23 and is most relevant to the high clinical, public health and economic burdens associated with HF. Nonetheless, this is a large, prospective analysis of a well-characterized, bi-racial community-based cohort that extends knowledge regarding the utility and implications of NT-proBNP measurements for HF risk prediction among individuals across BMI categories.
In conclusion, this study demonstrates that despite an inverse relationship between BMI and NT-proBNP levels, NT-proBNP is independently associated with incident HF and improves HF risk prediction beyond traditional risk factors even among individuals with obesity. If a risk-centered prevention approach is applied to HF, because of the greater risk of HF associated with obesity, even modest NT-proBNP elevations may warrant clinical attention in obese individuals.
Supplementary Material
Clinical Perspectives.
The N-terminal of pro-Brain Natriuretic Peptide (NT-proBNP) is used clinically for the diagnosis of heart failure (HF). NT-proBNP is also useful for predicting future HF in the general community. However, NT-proBNP levels are known to be lower among obese versus non-obese individuals, despite increased HF risk associated with obesity. Consequently, the utility of NT-proBNP levels for HF prediction among individuals with obesity is unclear. Therefore, in this analysis of 12,230 individuals without baseline HF in the Atherosclerosis Risk in Communities (ARIC) Study, we quantified and compared the risk associations of NT-proBNP with incident HF across body-mass index (BMI) categories. Despite a weak inverse association between NT-proBNP and BMI (r= −0.10), higher NT-proBNP was associated with increased HF risk and improved HF risk prediction in all BMI categories. However, because of higher HF rates associated with obesity, at each level of NT-proBNP, higher BMI categories were associated with greater absolute HF risk. For example, among those with NT-proBNP 100–200 pg/ml, the average ten-year HF risk was <5% among normal weight individuals but >10% if severely obese. Similarly, at mean levels of clinical covariates, ten-year HF risk exceeded 10% at median NT-proBNP levels of 119 pg/ml (95% CI: 83–177 pg/ml) in the severely obese category and 489 pg/ml (95% CI: 367–703 pg/ml) in the normal weight category. These findings demonstrate the prognostic value of NT-proBNP for HF risk even among individuals with obesity, and suggest that even mild NT-proBNP elevations warrant attention among those with severe obesity in terms of future HF risk.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Funding Sources: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). This work was supported by the Robert E. Meyerhoff Professorship, a Robert Wood Johnson Amos Medical Faculty Development Award and an NIH/NHLBI grant (K23HL12247) awarded to Dr. Ndumele and by an NIH/NIDDK grant (R01DK089174) awarded to Dr. Selvin. Dr. Selvin was also supported by NIH/NIDDK grant K24DK106414. Reagents for the NT-proBNP assays were donated by Roche Diagnostics.
Disclosures: Dr. Selvin and Ballantyne have served on an advisory board for Roche Diagnostics. Drs. Ballantyne and Nambi along with Roche and Baylor College of Medicine have filed a provisional patent (patent #61721475) entitled “Biomarkers to Improve Prediction of Heart Failure Risk”.
References
- 1.Kinnunen P, Vuolteenaho O, Ruskoaho H. Mechanisms of atrial and brain natriuretic peptide release from rat ventricular myocardium: effect of stretching. Endocrinology. 1993;132:1961–1970. doi: 10.1210/endo.132.5.8477647. [DOI] [PubMed] [Google Scholar]
- 2.Kim HN, Januzzi JL., Jr Natriuretic peptide testing in heart failure. Circulation. 2011;123:2015–2019. doi: 10.1161/CIRCULATIONAHA.110.979500. [DOI] [PubMed] [Google Scholar]
- 3.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 4.Bettencourt P, Azevedo A, Pimenta J, Frioes F, Ferreira S, Ferreira A. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004;110:2168–2174. doi: 10.1161/01.CIR.0000144310.04433.BE. [DOI] [PubMed] [Google Scholar]
- 5.Hartmann F, Packer M, Coats AJ, Fowler MB, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Anker SD, Amann-Zalan I, Hoersch S, Katus HA. Prognostic impact of plasma N-terminal pro-brain natriuretic peptide in severe chronic congestive heart failure: a substudy of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) trial. Circulation. 2004;110:1780–1786. doi: 10.1161/01.CIR.0000143059.68996.A7. [DOI] [PubMed] [Google Scholar]
- 6.Luers C, Wachter R, Kleta S, Uhlir M, Koschack J, Scherer M, Binder L, Herrmann-Lingen C, Zapf A, Kulle B, Kochen MM, Pieske B. Natriuretic peptides in the detection of preclinical diastolic or systolic dysfunction. Clin Res Cardiol. 2010;99:217–226. doi: 10.1007/s00392-009-0108-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.deFilippi CR, Christenson RH, Gottdiener JS, Kop WJ, Seliger SL. Dynamic cardiovascular risk assessment in elderly people. The role of repeated N-terminal pro-B-type natriuretic peptide testing. J Am Coll Cardiol. 2010;55:441–450. doi: 10.1016/j.jacc.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 9.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, Benjamin EJ, D'Agostino RB, Vasan RS. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal SK, Chambless LE, Ballantyne CM, Astor B, Bertoni AG, Chang PP, Folsom AR, He M, Hoogeveen RC, Ni H, Quibrera PM, Rosamond WD, Russell SD, Shahar E, Heiss G. Prediction of incident heart failure in general practice: the Atherosclerosis Risk in Communities (ARIC) Study. Circ Heart Fail. 2012;5:422–429. doi: 10.1161/CIRCHEARTFAILURE.111.964841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniels LB, Clopton P, Bhalla V, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, McCullough PA, Maisel AS. How obesity affects the cut-points for B-type natriuretic peptide in the diagnosis of acute heart failure. Results from the Breathing Not Properly Multinational Study. Am Heart J. 2006;151:999–1005. doi: 10.1016/j.ahj.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Horwich TB, Hamilton MA, Fonarow GC. B-type natriuretic peptide levels in obese patients with advanced heart failure. J Am Coll Cardiol. 2006;47:85–90. doi: 10.1016/j.jacc.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 13.Krauser DG, Lloyd-Jones DM, Chae CU, Cameron R, Anwaruddin S, Baggish AL, Chen A, Tung R, Januzzi JL., Jr Effect of body mass index on natriuretic peptide levels in patients with acute congestive heart failure: a ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) substudy. Am Heart J. 2005;149:744–750. doi: 10.1016/j.ahj.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Mehra MR, Uber PA, Park MH, Scott RL, Ventura HO, Harris BC, Frohlich ED. Obesity and suppressed B-type natriuretic peptide levels in heart failure. J Am Coll Cardiol. 2004;43:1590–1595. doi: 10.1016/j.jacc.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 15.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, Vasan RS. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 16.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 17.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 18.Dokainish H, Gonzalez R, Hartley WB, Caldera A, Koshy S, Sengupta R, Lakkis NM. Usefulness of B-type natriuretic peptide levels to predict left ventricular filling pressures in patients with body mass index >35, 31 to 35, and < or =30 kg/m2. Am J Cardiol. 2007;100:1166–1171. doi: 10.1016/j.amjcard.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 19.Tosa S, Watanabe H, Iino K, Terui G, Kosaka T, Hasegawa H, Ito H. Usefulness of plasma BNP levels as a marker of left ventricular wall stress in obese individuals. Int Heart J. 2009;50:173–182. doi: 10.1536/ihj.50.173. [DOI] [PubMed] [Google Scholar]
- 20.Taylor JA, Christenson RH, Rao K, Jorge M, Gottlieb SS. B-type natriuretic peptide and N-terminal pro B-type natriuretic peptide are depressed in obesity despite higher left ventricular end diastolic pressures. Am Heart J. 2006;152:1071–1076. doi: 10.1016/j.ahj.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Madamanchi C, Alhosaini H, Sumida A, Runge MS. Obesity and natriuretic peptides, BNP and NT-proBNP: mechanisms and diagnostic implications for heart failure. Int J Cardiol. 2014;176:611–617. doi: 10.1016/j.ijcard.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 23.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hallan SI, Matsushita K, Sang Y, Mahmoodi BK, Black C, Ishani A, Kleefstra N, Naimark D, Roderick P, Tonelli M, Wetzels JF, Astor BC, Gansevoort RT, Levin A, Wen CP, Coresh J. Age and association of kidney measures with mortality and end-stage renal disease. JAMA. 2012;308:2349–2360. doi: 10.1001/jama.2012.16817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 26.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang TJ. The natriuretic peptides and fat metabolism. N Engl J Med. 2012;367:377–378. doi: 10.1056/NEJMcibr1204796. [DOI] [PubMed] [Google Scholar]
- 28.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


