SUMMARY
Ambient temperature affects energy intake and expenditure to maintain homeostasis in a continuously fluctuating environment. Here, mice with an adipose-specific defect in fatty acid oxidation (Cpt2A−/−) were subjected to varying temperatures to determine the role of adipose bioenergetics in environmental adaptation and body weight regulation. Microarray analysis of mice acclimatized to thermoneutrality revealed that Cpt2A−/− interscapular brown adipose tissue (BAT) failed to induce the expression of thermogenic genes such as Ucp1 and Pgc1α in response to adrenergic stimulation, and increasing ambient temperature exacerbated these defects. Furthermore, thermoneutral housing induced mitochondrial DNA stress in Cpt2A−/− BAT and ultimately resulted in a loss of interscapular BAT. Although the loss of adipose fatty acid oxidation resulted in clear molecular, cellular and physiologic deficits in BAT, body weight gain and glucose tolerance were similar in control and Cpt2A−/− mice in response to a high fat diet, even when mice were housed at thermoneutrality.
INTRODUCTION
Obesity is driven by energy imbalance. Overconsumption of a calorie dense diet increases energy storage mainly as triglyceride in white adipose tissue. Concomitantly, the inability to utilize this energy potentiates adiposity and body weight gain. Although great strides are being made in understanding the regulation of food intake, much less is known about the regulation and contribution of energy expenditure to obesity. Determining the tissue-specific contribution of macronutrient metabolism to energy expenditure is critical for understanding the balance of energy intake and expenditure.
White adipose tissue (WAT) plays an important role in energy storage, but contains few mitochondria and contributes minimally to organismal bioenergetics in an autonomous manner. Alternatively, brown adipose tissue (BAT) is densely packed with mitochondria and can rapidly and robustly affect whole animal energy expenditure when deployed during a cold challenge (Harms and Seale, 2013). The physiological role of BAT is to produce heat to maintain body temperature during a cold challenge. This is accomplished via uncoupling cellular respiration from ATP generation via the mitochondrial transporter Uncoupling Protein-1 (Ucp1). Fatty acid oxidation is critical for this process (Guerra et al., 1998; Schuler et al., 2005; Tolwani et al., 2005) and provides the biophysical activator of uncoupling in BAT (Fedorenko et al., 2012). Mice with an adipose-specific loss of fatty acid oxidation are severely cold intolerant, demonstrating an autonomous requirement for adipose fatty acid oxidation in cold-induced thermogenesis (Ellis et al., 2010; Lee et al., 2015).
Due to the large potential to alter energy expenditure, it is tempting to suggest that defects in brown or beige adipocytes can lead to an obesogenic phenotype. Therefore, it was somewhat surprising that Ucp1KO mice were resistant to rather than prone to diet-induced obesity (Enerback et al., 1997; Liu et al., 2003). One caveat is that standard laboratory animal housing is below the thermal preference for mice, generating a mild cold stress. Mice have a large surface to volume ratio and need to expend large amounts of energy defending their body temperature against the environment. Removing this cold stress by rearing mice at thermoneutrality (30°C) acutely reversed this phenotype and generated obese prone Ucp1KO mice, revealing a strong environmental impact on body weight and energy expenditure (Feldmann et al., 2009).
Recently, we generated mice with a loss of adipose fatty acid oxidation by knocking out Carnitine Palmitoyltransferase 2 (Cpt2), an obligate step in mitochondrial long chain fatty acid β-oxidation (Lee et al., 2015). Similar to Ucp1KO mice, Cpt2A−/− mice were severely cold intolerant, but mildly resistant to high fat diet-induced adiposity. To understand the effect of ambient temperature on BAT deficient in fatty acid oxidation, we acclimatized Cpt2A−/− mice to thermoneutrality (30°C) and demonstrated a severe loss of agonist-induced thermogenic gene induction, and loss of interscapular BAT after long term housing of Cpt2A−/− mice at thermoneutrality. Surprisingly, given the severity of cold intolerance and suppression of molecular and cellular thermogenic programing in Cpt2A−/− BAT, thermoneutral housing did not affect diet-induced obesity in Cpt2A−/− mice. These data show that bioenergetically and transcriptionally incompetent BAT does not potentiate obesity, even at thermoneutrality.
RESULTS
The loss of adipose fatty acid oxidation results in broad transcriptional dysregulation at Thermoneutrality
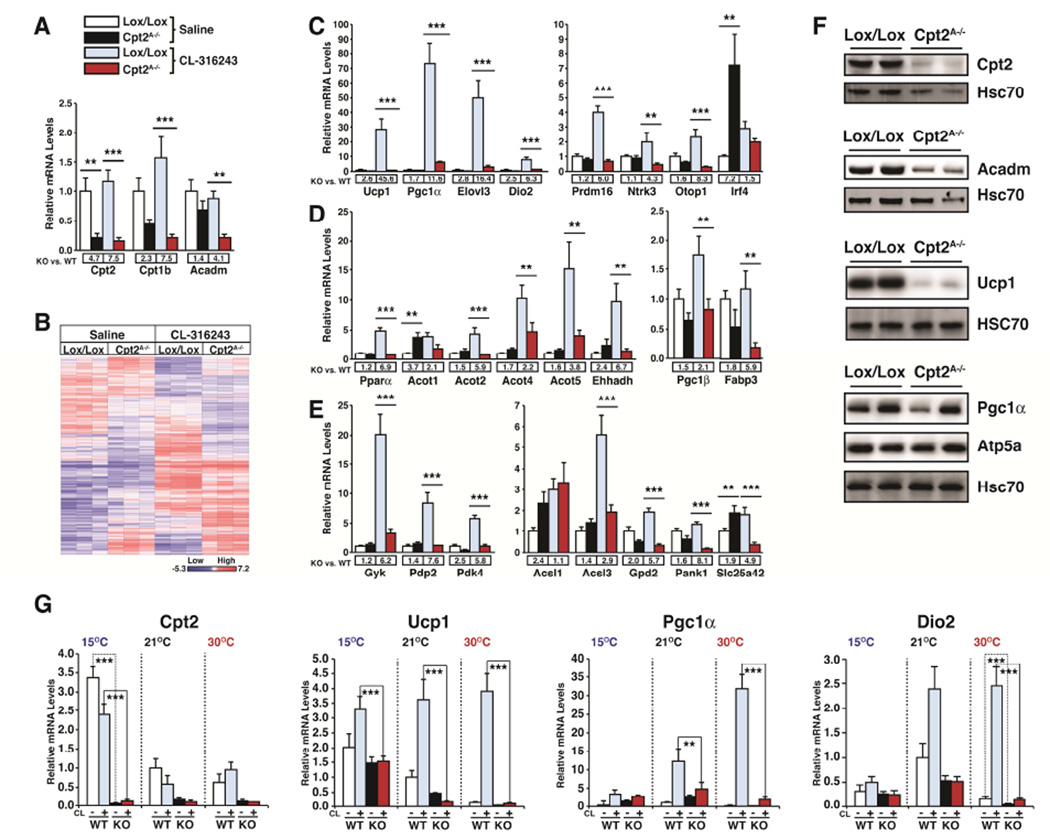
Previously, we showed that the loss of an obligate step in mitochondrial long chain fatty acid β-oxidation specifically in adipocytes (Cpt2A−/−) resulted in severe cold intolerance (Lee et al., 2015). Cold intolerance was expected given the critical role of fatty acid oxidation for providing the mitochondrial bioenergetics as well as biophysical activator of uncoupling in BAT (Guerra et al., 1998; Schuler et al., 2005; Tolwani et al., 2005). Unexpectedly, Cpt2A−/− BAT failed to up-regulate thermogenic programming in response to cold, β3-adrenergic agonists or forskolin (Lee et al., 2015). To better understand the role of fatty acid oxidation on the transcriptional control of BAT, we acclimatized Cpt2A−/− and littermate control mice to thermoneutrality (30°C) for two weeks. Thermoneutrality relieves facultative thermogenesis and reduces the requirement for basal tonic adrenergic signaling in BAT. We then injected the mice with the selective β3-adrenergic agonist CL-316243 (10mg/kg) or saline and collected interscapular BAT 3hrs later and preformed genome wide gene expression profiling on BAT mRNA via DNA microarrays (Fig. 1A,B). Microarray analysis revealed broad transcriptional dysregulation upon the loss of fatty acid oxidation, particularly in response to the β3-adrenergic agonist.
Figure 1. Transcriptional response of Cpt2A−/− BAT to adrenergic stimulation at thermoneutrality.
(A) Gene expression profiling via microarray of Cpt2, Cpt1b and Acadm at thermoneutrality 3 hrs after injection with vehicle or CL-316243 injected control and Cpt2A−/− BAT (n=5).
(B) Heat map of genes exhibiting statistically significant (p<0.05) changes from microarray analyses are shown for vehicle or CL-316243 injected control and Cpt2A−/− BAT (n=3).
(C) mRNA expression in BAT of thermogenic and brown fat enriched genes (n=5).
(D) mRNA expression in BAT of Pparα and Pparα target genes (n=5).
(E) mRNA expression in BAT of metabolic genes (n=5).
(F) Western blots of BAT from control and Cpt2A−/− mice at thermoneutrality.
(G) mRNA expression of Cpt2, Ucp1, Pgc1α and Dio2 in BAT of control and Cpt2A−/− mice after 2wks at 15°C, 22°C and 30°C injected with vehicle or CL-316243 (n=5).
Data are expressed as mean ± SEM. *p<0.05; **p<0.01; ***p<0.001.
In order to validate the microarray results in a larger cohort of mice, we analyzed a subset of genes identified in the microarray analysis by qRT-PCR. In agreement with our previous studies (Lee et al., 2015), thermogenic genes Ucp1, Pgc1α, Elovl3 and Dio2 failed to be induced upon β3-adrenergic stimulation in vivo (Fig 1C). Alternatively, inguinal white adipose tissue (iWAT) of Cpt2A−/− mice exhibited a significant suppression of Ucp1 but was still responsive to β3-adrenergic stimulation (Fig. S1). Other models of BAT dysfunction have shown a switch in identity from BAT to WAT (Cohen et al., 2014; Harms et al., 2014); however, Cpt2A−/− BAT largely maintained its transcriptional identity but again lost β3-adrenergic induction (Fig. 1C). Loss of triglyceride hydrolysis results in defects in β-oxidation and a loss of Pparα signaling (Ahmadian et al., 2011; Haemmerle et al., 2011; Mottillo et al., 2012; Schoiswohl et al., 2015). Cpt2A−/− BAT did not exhibit a defect in Pparα or the canonical Pparα target Acot1 was increased at baseline; however, these genes were again not induced by the β3-adrenergic agonist (Fig. 1D). Consistent with the metabolic defects induced upon the loss of β-oxidation, Cpt2A−/− BAT exhibited defects in β3-adrenergic induction of genes in several metabolic pathways such as Gyk (glycerolipid synthesis), Pdp2 and Pdk4 (pyruvate/TCA cycle flux) (Fig. 1E). Alternatively, iWAT remained sensitive to β3-adrenergic stimulation and Pdp2 and Pdk4 showed increased expression following CL-316243 injection (Fig. S1). The transcriptional deficits in BAT were mirrored by alterations in the protein abundance for Ucp1, Pgc1α, and Acadm (Fig. 1F). These data show that the loss of fatty acid β-oxidation via genetic perturbation of Cpt2 results in a defective transcriptional response to β3-adrenergic stimulation specifically in BAT.
The difference between agonist-induced thermogenic gene induction between control and Cpt2A−/− BAT at 30°C was much larger than observed at lower ambient temperatures (Lee et al., 2015). To understand if this was due to a change in basal expression or inducibility, control and Cpt2A−/− mice were acclimatized for two weeks to 15°C, 21°C, or 30°C and then injected with saline or CL-316243 (10mg/kg) and interscapular BAT was collected 3 hrs later. We then analyzed the expression of Ucp1, Pgc1α, and Dio2 (Fig. 1G). Basal Ucp1 expression in WT BAT increased with decreasing temperature but was induced to the same degree. Ucp1 expression in Cpt2A−/− BAT also increased with decreasing temperature but was not induced at any temperature. Basal Pgc1α expression was minimally altered by temperature acclimatization or genotype. However, Pgc1α was induced by adrenergic stimulation in WT BAT to a greater degree upon increasing temperature, while Cpt2A−/− BAT remained non-induced under all conditions (Fig. 1G). These data show that the primary transcriptional defect in Cpt2A−/− BAT is a failure in β3-adrenergic-induced rather than basal thermogenic gene expression.
Role of adipose fatty acid oxidation on thermogenic plasticity
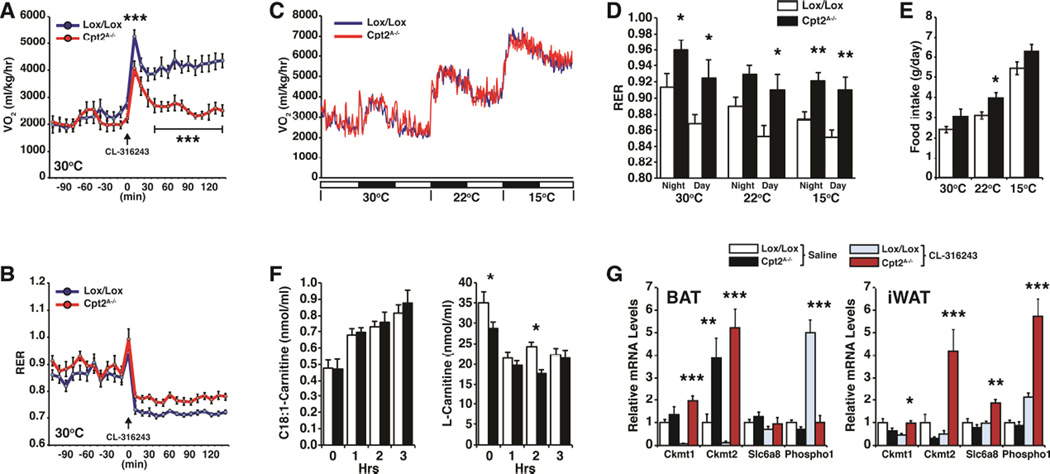
Adipose fatty acid oxidation is required for acute cold-induced thermogenesis (Ellis et al., 2010; Lee et al., 2015). Pre-acclimatization to different ambient temperatures has dramatic effects on the response to cold challenge. To understand the role of adipose fatty acid oxidation on the physiology of thermogenic plasticity, Cpt2A−/− mice were acclimatized at thermoneutrality (30°C) for two weeks and then placed in individual metabolic cages also maintained at 30°C. After several days to further acclimatize the mice to the metabolic cages, control and Cpt2A−/− mice were injected with CL-316243 (10mg/kg) and their energy expenditure was monitored. As expected, Cpt2A−/− mice failed to increase their energy expenditure in response to β3-adrenergic stimulation and also did not drop their RER, showing that they exhibited less fatty acid oxidation in response to the adrenergic challenge (Fig. 2A,B). We then measured the energy expenditure of control and Cpt2A−/− mice at 30°C, and directly before the dark cycle, the temperature was reduced (~1°C/4min) to 22°C (standard housing temperature) for 24hrs and then to 15°C for an additional 24hrs. The energy expenditure during this paradigm of temperature shifting was unchanged between control and Cpt2A−/− mice (Fig. 2C). However, consistent with the lack of adipose fatty acid oxidation in Cpt2A−/− mice, there was a strong shift towards carbohydrate oxidation as revealed by the RER (Fig. 2D). Additionally, all mice showed an increase in food intake, and there was a modest increase in food consumption of Cpt2A−/− mice during this experimental paradigm (Fig. 2E). These data show that although the Cpt2A−/− mice could not increase energy expenditure via fatty acid oxidation in adipose tissue, a systemic compensation enables homeostatic regulation of energy expenditure at different temperatures.
Figure 2. Contribution of adipose fatty acid oxidation to energy expenditure at different temperatures.
(A) O2 consumption of control and Cpt2A−/− mice injected with vehicle or CL-316243 (10 mg/kg) at thermoneutrality (n=7).
(B) Respiratory exchange ratio of control and Cpt2A−/− mice injected with either vehicle or CL-316243 (10 mg/kg) at thermoneutrality (n=7).
(C) O2 consumption of control and Cpt2A−/− mice at 30°C, 22°C and 15°C (n=7).
(D) Respiratory exchange ratio of control and Cpt2A−/− mice at 30°C, 22°C and 15°C under dark and light cycles (n=7).
(E) Food intake of control and Cpt2A−/− mice at 30°C, 22°C and 15°C (n=7).
(F) Hourly blood C18:1-carntine and L-carnitine concentrations of thermoneutral acclimatized control and Cpt2A−/− mice injected with CL-316243 (10 mg/kg) over 3 hr (n=5).
(G) mRNA expression in BAT and iWAT of genes in creatine metabolism (n=5).
Data are expressed as mean ± SEM. *p<0.05; **p<0.01; ***p<0.001.
Next we wanted to better understand how mice that could not oxidize fatty acids in adipose tissue could maintain systemic energy homeostasis. First, we measured blood acylcarnitines of thermoneutral acclimatized control and Cpt2A−/− mice injected with CL-316243 (10mg/kg) over 3 hours. Cpt2A−/− mice were slightly carnitine deficient, but exhibited a rise in blood long chain acylcarnitines and a suppression of short chain acylcarnitines similar to controls (Fig. 2F, Table S1). These data support a systemic redistribution of fatty acids (Bartelt et al., 2011; Lee et al., 2015). To understand possible compensatory adaptations in Cpt2A−/− adipose tissue, we mined our microarray data for genes in creatine metabolism (Kazak et al., 2015). Consistent with Ucp1KO mice, Cpt2A−/− BAT exhibited an increase in Ckmt2; however, Cpt2A−/− BAT exhibited a loss of β3-adrenergic induced Phospho1 expression, consistent with other thermogenic genes (Fig 2G). Additionally, Cpt2A−/− iWAT exhibited increased β3-adrenergic induction of Ckmt1, Ckmt2, Slc6a8 and Phospho1 (Fig 2G). These data support the notion that when BAT thermogenesis is lost, temperature acclimatization results in systemic compensation to maintain energy homeostasis during gradual changes in temperature.
Thermoneutrality induces mitochondrial DNA stress that is potentiated by the loss of fatty acid oxidation
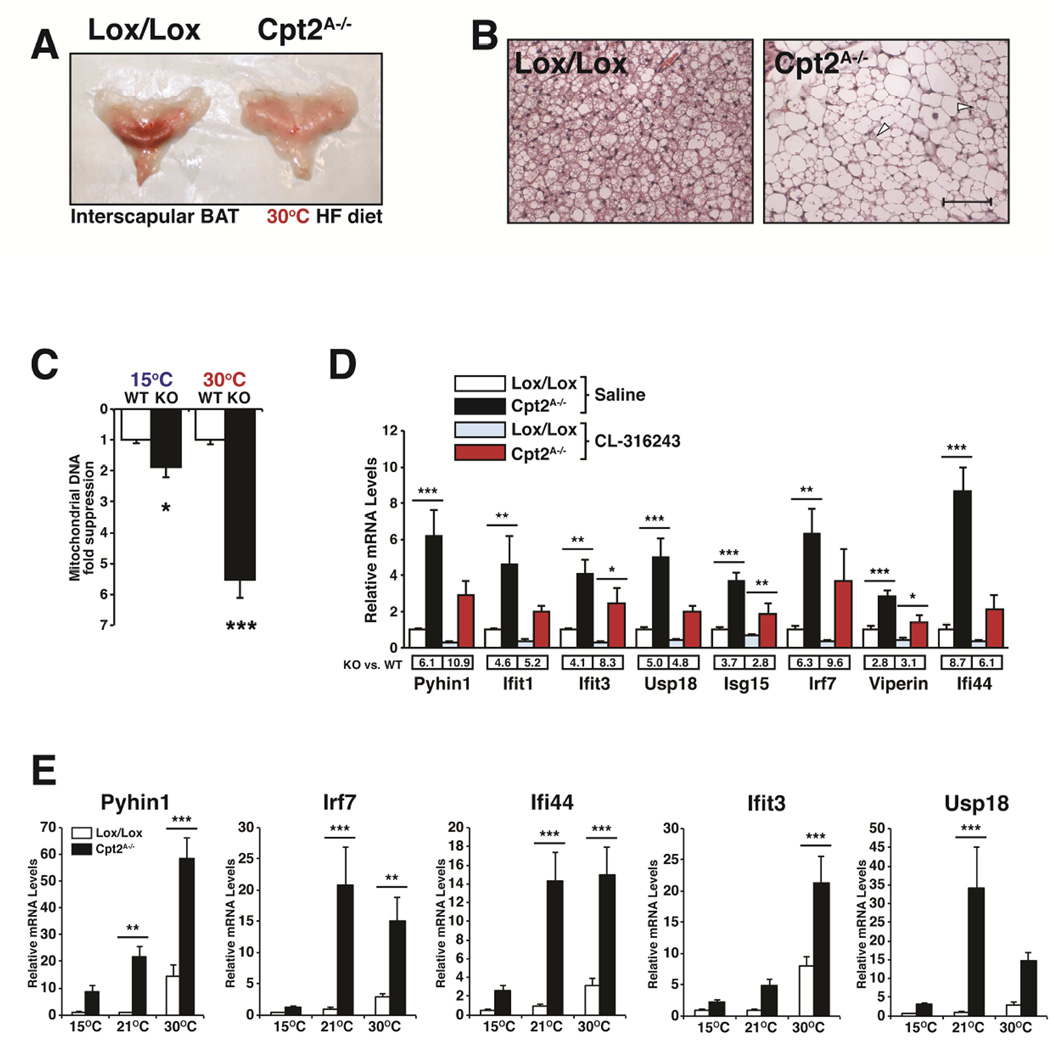
To determine the role of adipose fatty acid oxidation during long-term housing at thermoneutrality, we placed control and Cpt2A−/− male mice on a high fat diet for 12 weeks housed in a temperature-and light-controlled animal cabinet at 30°C. Upon necropsy, control mice had normal interscapular BAT. However, we could not identify morphologically distinct BAT in Cpt2A−/− mice (Fig. 3A). Upon histological examination of interscapular adipose tissue, almost all of the classical BAT morphology was absent (Fig. 3B). The loss of adipose tissue triglyceride lipase results in a suppression of Pparα, and therefore a switch of identity from BAT to a more WAT-like identity (Ahmadian et al., 2011; Schoiswohl et al., 2015). We did not observe defects in Pparα or Pparα target genes at baseline (Fig. 1D). Additionally Cpt2A−/− BAT did not exhibit alterations in BAT-specific identity (Fig. 1C). However, increasing ambient temperature potentiated the loss of mitochondrial DNA in Cpt2A−/− BAT (Fig. 3C). These data show that adipose fatty acid oxidation is required to maintain BAT at thermoneutrality.
Figure 3. Loss of fatty acid oxidation potentiates mitochondrial DNA stress.
(A) Gross interscapular BAT morphology of control and Cpt2A−/− mice after 12 wks of HFDat thermoneutrality.
(B) Hematoxylin and Eosin (H&E) stained BAT from control and Cpt2A−/− mice after 12 wksof HFD at thermoneutrality. Arrows indicate BAT morphology. Scale bar is 100 μM.
(C) Mitochondrial DNA fold suppression of control and Cpt2A−/− BAT at 15°C and 30°C (n=5).
(D) mRNA expression in BAT of interferon stimulated genes in BAT of control and Cpt2A−/− mice injected with vehicle or CL-316243 at thermoneutrality (n=5).
(E) mRNA expression in BAT of interferon stimulated genes in control and Cpt2A−/− mice after 2wks at 15°C, 22°C and 30°C (n=5).
Data are expressed as mean ± SEM. *p<0.05; **p<0.01; ***p<0.001.
The loss of mitochondrial DNA via genetic deletion of the mitochondrial transcriptional regulator Tfam was shown to induce Interferon Stimulated Genes (ISG) and initiate an innate antiviral response (West et al., 2015). Microarray analysis of thermoneutral acclimatized Cpt2A−/− BAT identified a similar gene expression profile. A broad range of ISGs, such as Oasl2, Ifih1,Sp110 and p200 family proteins Ifi203 and Ifi204, were induced in Cpt2A−/− BAT. We went on to verify a subset of these genes (Pyhin1, Ifit1, Ifit3, Usp18, Isg15, Irf7, Viperin and Ifi44) by qRT-PCR (Fig. 3D). Interestingly, these genes were induced regardless of adrenergic stimulation. In fact, adrenergic stimulation suppressed these genes in both control and Cpt2A−/− BAT (Fig. 3D). To determine the role of ambient temperature on the regulation of these ISGs, we profiled a subset of genes (Pyhin1, Irf7, Ifi44, Ifit3, Usp18 and Viperin) in control and Cpt2A−/− BAT from mice acclimatized to 15°C, 21°C, and 30°C. All of the ISGs increased in BAT as ambient temperature increased, and the loss of fatty acid oxidation greatly potentiated this response (Fig. 3E). These data show that temperature imparts a mitochondrial DNA stress in BAT and this is greatly potentiated by a loss in mitochondrial fatty acid β-oxidation.
The absence of adipose fatty acid oxidation does not affect obesity at thermoneutrality
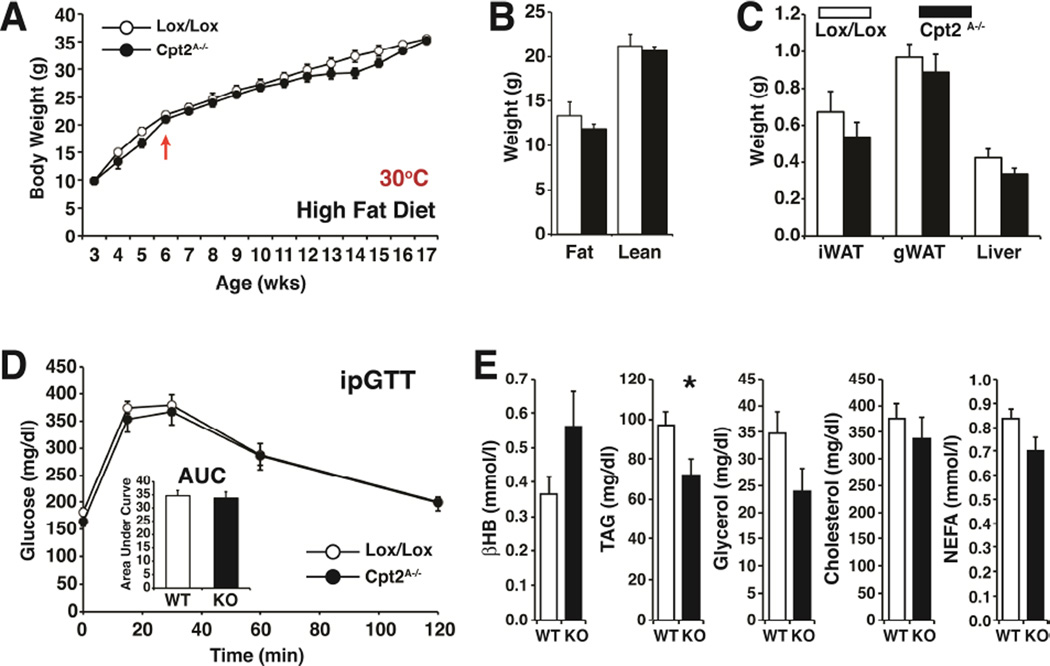
Thermoneutrality has been shown to dramatically and acutely increase weight gain of Ucp1KO mice, particularly when they are fed a high fat diet (Feldmann et al., 2009). We predicted that thermoneutrality would impart an obesogenic phenotype on Cpt2A−/− mice since Ucp1KO and Cpt2A−/− mice have a similar degree of cold intolerance. Cpt2A−/− BAT is bioenergetically and transcriptionally incompetent for thermogenesis, and long-term housing at 30°C facilitates an almost complete loss of BAT. Therefore, we measured the body weight weekly of control and Cpt2A−/− mice for 12 weeks fed a high fat diet and housed at 30°C. Surprisingly, thermoneutral housing did not affect weight gain in Cpt2A−/− mice (Fig. 4A). Adiposity was also not affected and even trended lower (Fig. 4B,C), similar to high fat feeding at room temperature (Lee et al., 2015). Additionally, there was no change in glucose tolerance (Fig. 4D), little change in serum chemistry (Fig. 4E), and no significant beiging of iWAT (Fig. S2) upon deletion of mitochondrial fatty acid β-oxidation in adipose tissue. These data suggest that defects in adipose tissue bioenergetics alone cannot account for the etiology of obesity or diabetes.
Figure 4. The loss of adipose fatty acid oxidation does not potentiate diet-induced obesity at thermoneutrality.
(A) Body weights of control and Cpt2A−/− male mice fed a high fat diet from 6 wks of age at thermoneutrality (n=11–13). Arrow indicates beginning of high fat diet.
(B) Fat and lean mass of control and Cpt2A−/− mice at 18 weeks of age after 12 weeks on high fat diet at thermoneutrality (n=11–13).
(C) Wet weights of iWAT, gWAT (unilateral depots) and liver (left lobe) of control and Cpt2A−/− mice (n=11–13).
(D) Intraperitoneal glucose tolerance test (ipGTT) including area under the curve for control and Cpt2A−/− mice at 10 weeks on HFD at thermoneutrality (n=11–13).
(E) Serum metabolite levels of control and Cpt2A−/− mice fed a high fat diet at thermoneutrality (n=9).
Data are expressed as mean ± SEM. *p<0.05; **p<0.01; ***p<0.001.
DISCUSSION
BAT gene expression and thermogenic potential are highly correlated with the regulation of body weight and susceptibility to high fat diet-induced obesity across many mouse models (Harms and Seale, 2013). The loss of Ucp1 (Enerback et al., 1997; Liu et al., 2003) or adipose fatty acid oxidation (Ellis et al., 2010; Lee et al., 2015) generates severe cold intolerance but does not result in susceptibility to diet-induced obesity under standard laboratory housing conditions. Therefore, it seems unlikely that mice with an obesogenic phenotype at room temperature can be explained via a defect in BAT bioenergetics alone. Thermoneutrality revealed an obesogenic phenotype of Ucp1KO mice (Feldmann et al., 2009); however, we were not able to recapitulate this effect in Cpt2A−/− mice although they had molecular, cellular and biochemical defects that prevented canonical BAT or beige cell function.
It seems logical that losing the ability to burn lipid stores within adipocytes would result in increased triglyceride accumulation and potentiate obesity. Therefore, it is somewhat counterintuitive that Cpt2A−/− mice did not show increased high fat diet-induced weight gain at any temperature. In fact, long term housing of Cpt2A−/− mice at thermoneutrality resulted in an almost complete loss of classical BAT without increasing adiposity or glucose intolerance. It is not clear how aging might affect body weight gain in Cpt2A−/− mice (Kontani et al., 2005). These data suggest the current model for BAT function/dysfunction in obesity is much more complex than originally envisioned. Our results suggest that, although BAT dysfunction is tightly correlated with obesity, BAT function itself is likely correlating with the main driver of whole body energy expenditure that has yet to be defined mechanistically. In support of this notion, Fgf21 has a strong effect on BAT but can increase energy expenditure and induce weight loss equally well in wild-type or Ucp1KO mice even at thermoneutrality (Fisher et al., 2012; Samms et al., 2015; Veniant et al., 2015). Additionally, the transplantation of large quantities of BAT in mice produced only mild benefits to body weight gain that were dependent on Il-6, suggesting an endocrine rather than bioenergetic role of BAT (Stanford et al., 2013).
Triglyceride lipolysis and fatty acid oxidation are largely interdependent. Consequently, the loss of the rate-setting step in triglyceride hydrolysis, Adipocyte Triglyceride Lipase (Atgl), results in cold intolerance (Ahmadian et al., 2011). Interestingly, the knockout of Atgl with ap2/Fabp4-Cre generates mice that are obese with increased adiposity (Ahmadian et al., 2011). However, the knockout of Atgl with the more specific adiponectin-Cre generates mice that are not more prone to obesity even after high fat feeding (Schoiswohl et al., 2015). This suggests that the increase in body weight and adiposity induced by a loss in adipocyte triglyceride hydrolysis requires simultaneous deletion in cells other than adipocytes (such as macrophages).
The loss of adipose fatty acid catabolism at either β-oxidation or triglyceride hydrolysis has little effect on the progression of obesity. These models may be mitigated by suppressing lipid uptake or de novo synthesis or by shifting bioenergetic demands to other tissues. Alternatively, adipose bioenergetics per say may be dispensable in body weight regulation in relation to whole animal physiology. The loss of Atgl results in the loss of Pparα directed transcription and the loss of BAT identity presumably from the loss of an endogenous Pparα ligand produced upon hydrolysis (Ahmadian et al., 2011; Haemmerle et al., 2011; Mottillo et al., 2012; Schoiswohl et al., 2015). Cpt2 deficient BAT maintains a BAT-like transcriptional program and is not deficient in Pparα mediated transcription. In fact, there is more Pparα target gene expression in Cpt2A−/− BAT likely due in part to the increase in basal adrenergic activity and PKA signaling (Lee et al., 2015). The main defect in Cpt2A−/− BAT is due to the loss in adrenergic-induced gene expression with limited changes to basal gene expression. Although lipolysis and β-oxidation are intimately linked within a continuous pathway, these data show that fatty acid catabolism affects transcription at multiple independent steps, suggesting a complex integration of metabolites and transcriptional programming.
One of the most surprising results from the current study was the increase in ISGs in Cpt2A−/− BAT. The loss of Cpt2 and the loss of Tfam have a high degree of concordance between the ISGs induced (West et al., 2015). However, the suppression in mitochondrial DNA was specific to BAT in Cpt2A−/− mice as mtDNA was unchanged in gWAT, iWAT or MEFs deleted in Cpt2 (Lee et al., 2015). This suggests that cells that undergo high fatty acid oxidation may be more susceptible to mtDNA stress, while loss of Tfam affects mtDNA stress more globally. It will be interesting to see if other tissue specific KOs of Cpt2 enhance innate immune priming, particularly macrophages. The mtDNA stress and ISG expression was increased in wild-type mice with increasing temperature, and this was greatly potentiated by the loss of fatty acid oxidation. Thermoneutrality exhibits a broad array of beneficial physiologic adaptations not overtly associated with energy homeostasis, including significant improvements in tumor therapy (Eng et al., 2015; Kokolus et al., 2013). Additionally, thermoneutrality increases macrophage recruitment to adipose tissue (Tian et al., 2015). It is tempting to speculate that thermoneutrality initiates a priming of the innate immune system via mitochondrial metabolism that improves tumor clearance and antiviral activity.
Small mammals with large surface to volume ratios can expend a large amount of energy defending their body temperatures against the environment and have a large capacity for thermogenesis. Altering the ambient temperature results in an amazing array of molecular, cellular and physiological adaptations to maintain body temperature and energy homeostasis. Adipocyte fatty acid oxidation plays a major role in both mediating and regulating these processes. Mice with an adipose-specific loss of fatty acid oxidation are unable to maintain their body temperature during an acute cold challenge (Lee et al., 2015). However, in response to moderate environmental challenges, they are able to maintain energy homeostasis via alternative mechanisms. The control of energy expenditure represents a critical evolutionarily conserved survival mechanism that is likely regulated by multiple overlapping and compensatory systems, which may present both opportunities and challenges for developing treatments aimed at reversing human obesity.
EXPERIMENTAL PROCEDURES
Animals
Cpt2lox/lox and Cpt2A−/− mice and diets were previously described (Lee et al., 2015). Control mice are defined as sex matched Cpt2lox/lox littermates. For the diet study, Cpt2lox/lox and Cpt2A−/− mice were fed a 60% high-fat diet (D12492, Research Diets) starting at 6 weeks of age and continuing for 12 weeks. For temperature acclimation studies, Cpt2lox/lox and Cpt2A−/− mice were housed at the indicated temperatures for 2 weeks in an animal incubator (Key Scientific, Inc.) on a 12 hr light/12 hr dark cycle. Serum was collected from all mice to measure free glycerol and TAG (sigma), β-hydroxybutyrate (StanBio), total cholesterol (Wako), and NEFA (Wako). Blood acylcarnitines were quantified from dried blood spots (DBS) with modifications (Chace et al., 1997; Sandlers et al., 2012). Punched 1/8″ DBS samples were submerged in 100 μl of methanol solution containing internal acyl carnitine standards (NSK B, Cambridge Isotopes). Samples were incubated at 4°C for 20min, dried under the nitrogen and then 60 μl 3N HCl in n-butanol was added. The samples were incubated for 15 min at 65 °C then dried under LN2 , and butylated acyl carnitines were reconstituted in 100 μl of mobile phase acetonitrile/water/formic acid (H2O:CH3CN:HCOOH; 80:19.9:0.1 v/v%). Samples were vortexed, transferred to a centrifuge filter, spun and transferred to injection vial. Acyl carnitines were analyzed on an API 3200 (AB SCIEX, Foster City, CA) operated in positive ion mode employing precursor ion scan for m/z 85 which is generated as a characteristic product ion of butyl ester of acyl carnitine species. Quantitation of acyl carnitines was achieved by Chemoview (AB SCIEX) application. Body composition was measured using quantitative-NMR (Echo-MRI-100) in order to determine fat and lean mass. Energy expenditure and RER were measured using Oxymax indirect calorimetry cages (Columbus Instruments). All procedures were performed in accordance with the NIH’s Guide for the Care and Use of Laboratory Animals and under the approval of the Johns Hopkins Medical School Animal Care and Use Committee.
Analysis of gene expression and mitochondrial DNA
Trizol followed by the RNeasy Mini Kit (QIAGEN) was used to obtain total RNA. Microarray analysis was done using affymetrix mouse exon arrays (GSE72210). For qRT-PCR, 1-2 μg of RNA was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosciences). cDNA was diluted to 2 ng/μL and was amplified by specific primers in a 20 μL reaction using SsoAdvanced SYBR Green Supermix (Bio-Rad). Analysis of gene expression was carried out in a CFX Connect Real-Time System (Bio-Rad). For each gene, mRNA expression was calculated as 2^deltaCT relative to cyclophilin A expression. For mitochondrial DNA analysis, total DNA was prepared using the QIAmp DNA mini Kit (QIAGEN). Primer and gene information are provided (Table S2).
Western Blots
For western blots, protein was obtained by homogenizing tissues in 1x RIPA buffer (50 mM Tris-Cl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.25% deoxycholate). The concentration of the protein was obtained using the BCA Protein Assay Reagent (Pierce, Rockford, IL). 30 μg of protein was used in an SDS-PAGE and transferred to either a nitrocellulose (Protran BA 83, Whatman) or a polyvinylidene difluoride (PVDF) membrane and blocked with 3% BSA-TBST (tris buffer saline with tween 20). The blots were probed with the following antibodies: CPT2 (Thermoscientific), ACADM (GeneTex), UCP1 (Abcam), Pgc1α (Abcam), ATP5A (Abcam), and Hsc70 (Santa Cruz Biotechnology). Cy3 fluorescent antibody was used for Hsc70 and the corresponding secondary antibodies conjugated to horseradish peroxidase were used for other primary antibodies.
Statistic Analyses
Multiple comparisons were calculated using ANOVA. Acylcarnitines were analyzed using repeated measures ANOVA with a with Bonferroni post hoc correction. Pairwise comparisons were calculated using a two-tailed Student’s T-test. Significance was determined as: *p<0.05; **p<0.01;***p<0.001.
Supplementary Material
HIGHLIGHTS.
Fatty acid oxidation (FAO) is required for the induction of Ucp1 and Pgc1α in BAT.
Increasing ambient temperature potentiates defects in FAO-deficient BAT.
Loss of adipose FAO induces mitochondrial DNA stress in BAT.
Loss of adipose FAO does not alter body weight or adiposity at thermoneutrality.
Acknowledgments
This work was supported in part by American Diabetes Association grant #1-16-IBS-313 and National Institutes of Health grants R01NS072241 to M.J.W., K08NS069815 to S.S. and J.L was supported in part by T32GM007445.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession Numbers
The Gene Expression Omnibus (GEO) accession number for the mRNA expression data reported in this paper is GSE72210.
Conflict of Interest
The authors have no competing financial interests.
Author Contributions
J.L., J.C., S.A., S.S. and M.J.W. performed experiments and J.L., J.C. and M.J.W. wrote the manuscript.
REFERENCES
- Ahmadian M, Abbott MJ, Tang T, Hudak CS, Kim Y, Bruss M, Hellerstein MK, Lee HY, Samuel VT, Shulman GI, et al. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell metabolism. 2011;13:739–748. doi: 10.1016/j.cmet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17:200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- Chace DH, Hillman SL, Van Hove JL, Naylor EW. Rapid diagnosis of MCAD deficiency: quantitative analysis of octanoylcarnitine and other acylcarnitines in newborn blood spots by tandem mass spectrometry. Clinical chemistry. 1997;43:2106–2113. [PubMed] [Google Scholar]
- Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell. 2014;156:304–316. doi: 10.1016/j.cell.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis JM, Li LO, Wu PC, Koves TR, Ilkayeva O, Stevens RD, Watkins SM, Muoio DM, Coleman RA. Adipose acyl-CoA synthetase-1 directs fatty acids toward beta-oxidation and is required for cold thermogenesis. Cell metabolism. 2010;12:53–64. doi: 10.1016/j.cmet.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- Eng JW, Reed CB, Kokolus KM, Pitoniak R, Utley A, Bucsek MJ, Ma WW, Repasky EA, Hylander BL. Housing temperature-induced stress drives therapeutic resistance in murine tumour models through beta2-adrenergic receptor activation. Nature communications. 2015;6:6426. doi: 10.1038/ncomms7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell. 2012;151:400–413. doi: 10.1016/j.cell.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell metabolism. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes & development. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra C, Koza RA, Walsh K, Kurtz DM, Wood PA, Kozak LP. Abnormal nonshivering thermogenesis in mice with inherited defects of fatty acid oxidation. The Journal of clinical investigation. 1998;102:1724–1731. doi: 10.1172/JCI4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haemmerle G, Moustafa T, Woelkart G, Buttner S, Schmidt A, van de Weijer T, Hesselink M, Jaeger D, Kienesberger PC, Zierler K, et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat Med. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nature medicine. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- Harms MJ, Ishibashi J, Wang W, Lim HW, Goyama S, Sato T, Kurokawa M, Won KJ, Seale P. Prdm16 is required for the maintenance of brown adipocyte identity and function in adult mice. Cell Metab. 2014;19:593–604. doi: 10.1016/j.cmet.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P, Vetrivelan R, Lu GZ, Laznik-Bogoslavski D, Hasenfuss SC, et al. A Creatine- Driven Substrate Cycle Enhances Energy Expenditure and Thermogenesis in Beige Fat. Cell. 2015;163:643–655. doi: 10.1016/j.cell.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokolus KM, Capitano ML, Lee CT, Eng JW, Waight JD, Hylander BL, Sexton S, Hong CC, Gordon CJ, Abrams SI, et al. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:20176–20181. doi: 10.1073/pnas.1304291110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontani Y, Wang Y, Kimura K, Inokuma KI, Saito M, Suzuki-Miura T, Wang Z, Sato Y, Mori N, Yamashita H. UCP1 deficiency increases susceptibility to diet-induced obesity with age. Aging Cell. 2005;4:147–155. doi: 10.1111/j.1474-9726.2005.00157.x. [DOI] [PubMed] [Google Scholar]
- Lee J, Ellis JM, Wolfgang MJ. Adipose fatty acid oxidation is required for thermogenesis and potentiates oxidative stress-induced inflammation. Cell Rep. 2015;10:266–279. doi: 10.1016/j.celrep.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Rossmeisl M, McClaine J, Riachi M, Harper ME, Kozak LP. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. The Journal of clinical investigation. 2003;111:399–407. doi: 10.1172/JCI15737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottillo EP, Bloch AE, Leff T, Granneman JG. Lipolytic products activate peroxisome proliferator-activated receptor (PPAR) alpha and delta in brown adipocytes to match fatty acid oxidation with supply. The Journal of biological chemistry. 2012;287:25038–25048. doi: 10.1074/jbc.M112.374041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samms RJ, Smith DP, Cheng CC, Antonellis PP, Perfield JW, 2nd, Kharitonenkov A, Gimeno RE, Adams AC. Discrete Aspects of FGF21 In Vivo Pharmacology Do Not Require UCP1. Cell Rep. 2015;11:991–999. doi: 10.1016/j.celrep.2015.04.046. [DOI] [PubMed] [Google Scholar]
- Sandlers Y, Moser AB, Hubbard WC, Kratz LE, Jones RO, Raymond GV. Combined extraction of acyl carnitines and 26:0 lysophosphatidylcholine from dried blood spots: prospective newborn screening for X-linked adrenoleukodystrophy. Mol Genet Metab. 2012;105:416–420. doi: 10.1016/j.ymgme.2011.11.195. [DOI] [PubMed] [Google Scholar]
- Schoiswohl G, Stefanovic-Racic M, Menke MN, Wills RC, Surlow BA, Basantani MK, Sitnick MT, Cai L, Yazbeck CF, Stolz DB, et al. Impact of reduced ATGL-mediated adipocyte lipolysis on obesity-associated insulin resistance and inflammation in male mice. Endocrinology. 2015;156:3610–3624. doi: 10.1210/en.2015-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler AM, Gower BA, Matern D, Rinaldo P, Vockley J, Wood PA. Synergistic heterozygosity in mice with inherited enzyme deficiencies of mitochondrial fatty acid beta-oxidation. Molecular genetics and metabolism. 2005;85:7–11. doi: 10.1016/j.ymgme.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. The Journal of clinical investigation. 2013;123:215–223. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian XY, Ganeshan K, Hong C, Nguyen KD, Qiu Y, Kim J, Tangirala RK, Tonotonoz P, Chawla A. Thermoneutral Housing Accelerates Metabolic Inflammation to Potentiate Atherosclerosis but Not Insulin Resistance. Cell Metab. 2016 doi: 10.1016/j.cmet.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolwani RJ, Hamm DA, Tian L, Sharer JD, Vockley J, Rinaldo P, Matern D, Schoeb TR, Wood PA. Medium-chain acyl-CoA dehydrogenase deficiency in genetargeted mice. PLoS genetics. 2005;1:e23. doi: 10.1371/journal.pgen.0010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veniant MM, Sivits G, Helmering J, Komorowski R, Lee J, Fan W, Moyer C, Lloyd DJ. Pharmacologic Effects of FGF21 Are Independent of the "Browning" of White Adipose Tissue. Cell Metab. 2015;21:731–738. doi: 10.1016/j.cmet.2015.04.019. [DOI] [PubMed] [Google Scholar]
- West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, Bestwick M, Duguay BA, Raimundo N, MacDuff DA, et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520:553–557. doi: 10.1038/nature14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.