SUMMARY
Abscission is a developmental process that enables plants to shed unwanted organs. In Arabidopsis, the floral organ abscission is regulated by a signaling pathway consisting of the peptide ligand IDA, the receptor-like kinases (RLKs) HAE and HSL2, and a downstream MAP kinase (MAPK) cascade. However, little is known about the molecular link between ligand-receptor pairs and intracellular signaling. Here, we report that the SERK family RLKs function redundantly in regulating floral organ abscission downstream of IDA and upstream of the MAPK cascade. IDA induces heterodimerization of HAE/HSL2 and SERKs, which transphosphorylate each other. The SERK3 residues mediating its interaction with the immune receptor FLS2 and the brassinosteroid receptor BRI1 are also required for IDA-induced HAE/HSL2-SERK3 interaction, suggesting SERKs serve as co-receptors of HAE/HSL2 in perceiving IDA. Thus, our study reveals the signaling activation mechanism in floral organ abscission by IDA-induced HAE/HSL2-SERK complex formation accompanied by transphosphorylation.
INTRODUCTION
Abscission is a fundamental physiological process by which multicellular organisms shed various body parts, such as a plant dropping a leaf, flower or seed, or an animal shedding a claw or husk. The floral organ abscission sheds the flower outer organs including sepals, petals and stamens after fertilization of the internal gynoecium. At the cellular level, abscission occurs specifically in the abscission zone, a predetermined cellular layer consisting of small and cytoplasmic dense cells at the boundary between the organ to be abscised and the plant body (Liljegren, 2012). In response to abscission signals, cells in the abscission zone secrete cell wall-modifying and hydrolyzing enzymes to degrade the middle lamella between two adjacent cell layers, which finally leads to organ shedding (Liljegren, 2012; Niederhuth et al., 2013).
A signaling pathway has been defined to regulate the initiation of floral organ separation in Arabidopsis (Liljegren, 2012; Niederhuth et al., 2013). HAESA (HAE) and HAESA-LIKE2 (HSL2), a pair of closely related leucine-rich repeat receptor-like kinases (LRR-RLKs), function redundantly to regulate the floral organ abscission process (Cho et al., 2008; Jinn et al., 2000; Stenvik et al., 2008). The small secreted peptide INFLORESCENCE DEFICIENT IN ABSCISSION (IDA) acts as the ligand of HAE/HSL2 (Butenko et al., 2014; Cho et al., 2008; Stenvik et al., 2008). The signaling pathway downstream of IDA-HAE/HSL2 recognition includes the mitogen-activated protein kinase (MAPK) cascade consisting of the MAPK kinase 4 (MKK4)/MKK5 and the MAPK 3 (MPK3)/MPK6 (Cho et al., 2008), and the transcription factors BREVIPEDICELLUS (BP) and AGAMOUS-like 15 (AGL15) (Patharkar and Walker, 2015; Shi et al., 2011). In addition, the ADP-ribosylation factor GTPase-activating protein NEVERSHED (NEV) is required for floral organ abscission, likely via facilitating the intracellular movement of components involved in abscission (Liljegren et al., 2009).
Ligand-induced receptor heterodimerization has been emerging as one of the common mechanisms to activate downstream intracellular modules in RLK signaling. Perception of plant growth hormone brassinosteroids (BRs) by the LRR-RLK BR-INSENSITIVE 1 (BRI1) receptor triggers heterodimerization with the LRR-RLK BRI1-ASSOCIATED RECEPTOR-LIKE KINASE 1 (BAK1) (also known as SOMATIC EMBRYOGENESIS RECEPTOR KINASE 3, SERK3) and its closest family members SERK1 and SERK4 (Gou et al., 2012; Li et al., 2002; Nam and Li, 2002). BAK1 and SERK4 also heterodimerize with Arabidopsis FLAGELLIN-SENSING 2 (FLS2), the bacterial flagellin receptor, and other immune sensors upon the cognate ligand perception (Chinchilla et al., 2007; Heese et al., 2007; Roux et al., 2011). In addition, BAK1, together with SERK1, SERK2 and SERK4, is involved in male gametophyte development, root growth, stomatal patterning and cell death control (Aan den Toorn et al., 2015; Ladwig et al., 2015; Liebrand et al., 2014; Meng et al., 2015; Wang et al., 2015).
In this study, we report that the SERK family RLKs regulate floral organ abscission through direct heterodimerization with HAE and HSL2 receptors upon the IDA ligand perception. Genetic analyses using a series of serk single and higher-order mutants revealed that all four functional SERKs, SERK1, SERK2, SERK3/BAK1 and SERK4, redundantly regulate floral organ abscission. HAE and BAK1 transphosphorylate each other in vitro. Epistasis analysis indicated that SERKs function genetically downstream of IDA and upstream of the MKK4/MKK5-MPK3/MPK6 cascade in regulating floral organ abscission. Together with our recent finding that SERKs regulate stomatal patterning via peptide ligand-induced association with the ERECTA family RLKs (Meng et al., 2015), our studies have established a parallel pathway in floral organ abscission that recruits SERKs as co-receptors for the IDA-HAE/HSL2 ligand-receptor complex, and revealed a conserved activation and signaling mechanism in plant RLK-mediated diverse biological processes.
RESULTS
Redundant function of SERKs in floral organ abscission
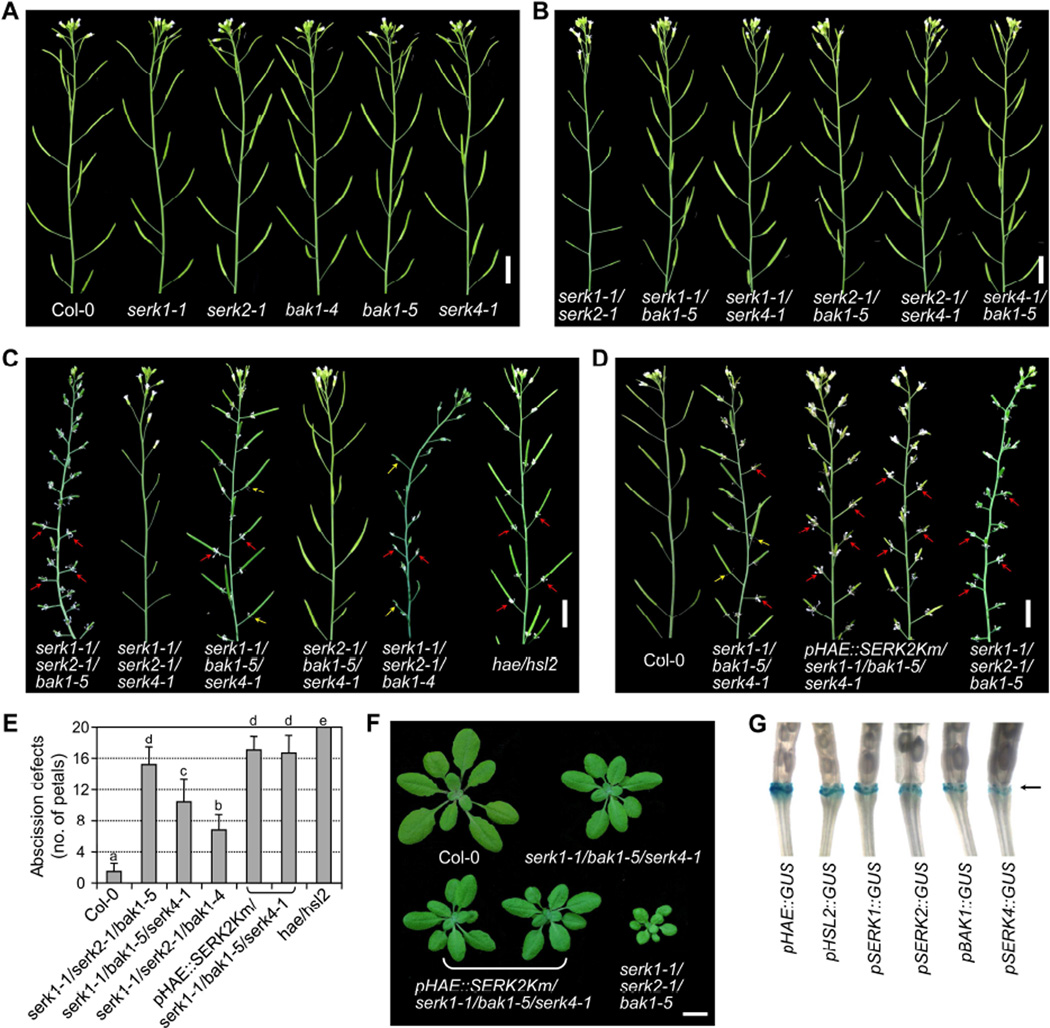
The broad involvement of SERKs in BRI1, ERECTA and FLS2-mediated plant growth and defense prompted us to systematically investigate the potential roles of different SERKs in other LRR-RLK-mediated processes including floral organ abscission. The floral organs including the sepals, petals and stamens abscise shortly after fertilization leaving behind the maturing fruits (siliques) in wild-type (WT) Arabidopsis plants (Figure S1A). The siliques on the main stem of eight-week-old plants were scored for floral organ abscission phenotypes. We did not observe the abnormal floral organ abscission in the serk1-1, serk2-1, bak1-4 and serk4-1 single knockout mutants compared with WT plants (Figures 1A and S1B). The bak1-5 mutant with a missense mutation in the kinase domain also displayed normal floral organ abscission (Figures 1A and S1B). To reveal the potential functional redundancy, we further investigated the abscission phenotypes in the higher-order mutants with different combinations of serk mutations. The bak1-4 null mutant displays seedling lethality when combined with serk4 null mutations (He et al., 2007), whereas the bak1-5 mutant is not impaired in cell death control or BR signaling (Schwessinger et al., 2011), and thus allowed us to generate the higher-order mutants for observing the floral organ abscission phenotype. The double mutants, including serk1-1/serk2-1, serk1-1/bak1-5, serk1-1/serk4-1, serk2-1/bak1-5, serk2-1/serk4-1 and serk4-1/bak1-5, also displayed a similar floral organ abscission phenotype as WT plants (Figures 1B and S1B). Notably, although the serk1-1/serk2-1 mutant is sterile with aborted siliques due to defects in anther development (Albrecht et al., 2005; Colcombet et al., 2005), it showed normal floral organ abscission (Figures 1B and S1B).
Figure 1. The SERK family RLKs redundantly regulate floral organ abscission.
(A–C) The floral organ abscission phenotypes of different single (A), double (B) and triple (C) serk mutants. Eight-week-old inflorescence stems with budding flowers and siliques are shown. Red arrows indicate unabscised floral organs, and yellow arrows indicate the weak abscission defects with only part of petals attached to siliques. (D) Expression of SERK2Km driven by the HAE promoter enhances floral abscission defects in serk1-1/bak1-5/serk4-1. Scale bars = 1 cm (A–D). (E) The number of abscission-defective petals attached to the five most mature siliques from eight-week-old plants with the indicated genotypes. The data are shown as mean + SD (n=50). Different letters above the columns indicate significant differences (p<0.05), as determined by one-way ANOVA with Tukey’s post-test. (F) Expression of SERK2Km driven by the HAE promoter does not exert detectable effects on the vegetative growth of serk1-1/bak1-5/serk4-1. Growth phenotypes of four-week-old mutant or transgenic plants with the indicated genotypes are shown. Scale bar = 1 cm. (G) SERKs are expressed in the floral abscission zones similar as HAE and HSL2. GUS reporter gene expression was analyzed using T1 transgenic plants harboring the GUS gene driven by different promoters. Arrow indicates the floral abscission zones. (see also Figure S1).
Interestingly, the serk triple mutants, serk1-1/serk2-1/bak1-5 and serk1-1/bak1-5/serk4-1 displayed floral organ abscission defects compared to WT plants (Figures 1C–1E and S1B). The floral organs remained attached to the siliques after fertilization in these mutants that resembled the hae/hsl2 double mutant (Figure 1C). The defective floral organ abscission was also observed in serk1-1/serk2-1/bak1-4, although its phenotype was weaker than that of serk1-1/serk2-1/bak1-5 (Figures 1C and 1E). However, the triple mutants serk1-1/serk2-1/serk4-1 and serk2-1/bak1-5/serk4-1, which carry a functional BAK1 or SERK1 respectively, showed normal floral organ abscission (Figure 1C), suggesting that BAK1 and SERK1 might be the two most critical SERKs involved in regulating floral organ abscission. It appears that serk1-1/serk2-1/bak1-5 exhibited more severe abscission defects than serk1-1/bak1-5/serk4-1, suggesting a more pronounced contribution of SERK2 than SERK4 to floral organ abscission (Figures 1C and 1E). Notably, although both serk1-1/serk2-1/bak1-5 and serk1-1/serk2-1/serk4-1 mutants had aborted siliques, serk1-1/serk2-1/bak1-5, but not serk1-1/serk2-1/serk4-1, had defects in floral organ abscission (Figures 1C and S1B), indicating that the involvement of SERKs in floral organ abscission is uncoupled from their function in male gametophyte development. Taken together, the data indicate that SERK1, SERK2, BAK1 and SERK4 function redundantly but contribute unequally in regulating floral organ abscission.
To further confirm whether the four functional SERKs are all involved in floral organ abscission, we introduced a kinase-inactive mutant version of SERK2 (SERK2Km) under the control of the HAE promoter into the serk1-1/bak1-5/serk4-1 mutant. SERK2Km has been suggested to possess a dominant-negative effect and likely suppresses the functions of endogenous SERK2 (Gou et al., 2012). The HAE promoter activity is mainly restricted in the abscission zones (Patharkar and Walker, 2015). Thus, we expected that the HAE promoter would drive SERK2Km expression primarily in the abscission zones and this spatial expression of SERK2Km could overcome pleiotropic effects caused by ectopic expression of SERK2km. Consistent with our expectations, the plant size of multiple lines of pHAE::SERK2Km/serk1-1/bak1-5/serk4-1 transgenic plants at the vegetative growth stage is similar to that of serk1-1/bak1-5/serk4-1 and much larger than that of serk1-1/serk2-1/bak1-5 (Figure 1F). Remarkably, multiple pHAE::SERK2Km/serk1-1/bak1-5/serk4-1 transgenic lines showed more severe floral organ abscission defects than serk1-1/bak1-5/serk4-1 (Figures 1D and 1E). The floral organ abscission defects in pHAE::SERK2Km/serk1-1/bak1-5/serk4-1 are comparable to those in hae/hsl2. The data reinforce that all four SERKs are involved in floral organ abscission.
SERKs are expressed in the floral abscission zones
Using the GUS reporter gene driven by the HAE promoter, it has been shown that HAE is primarily expressed in the floral organ abscission zones (Cho et al., 2008; Jinn et al., 2000). To further confirm the function of SERKs in floral organ abscission, we determined whether they are expressed in the abscission zones using transgenic plants harboring the GUS reporter gene driven by the SERK1, SERK2, BAK1 or SERK4 promoter (pSERK::GUS). As previously reported, HAE and HSL2 promoter activities were observed in the abscission zones (Figure 1G). The promoter activities of all four SERK genes were also clearly detected in the abscission zones, similar with that of HAE and HSL2, supporting their function in floral organ abscission (Figure 1G).
Ligand-induced HAE/HSL2-SERKs interaction
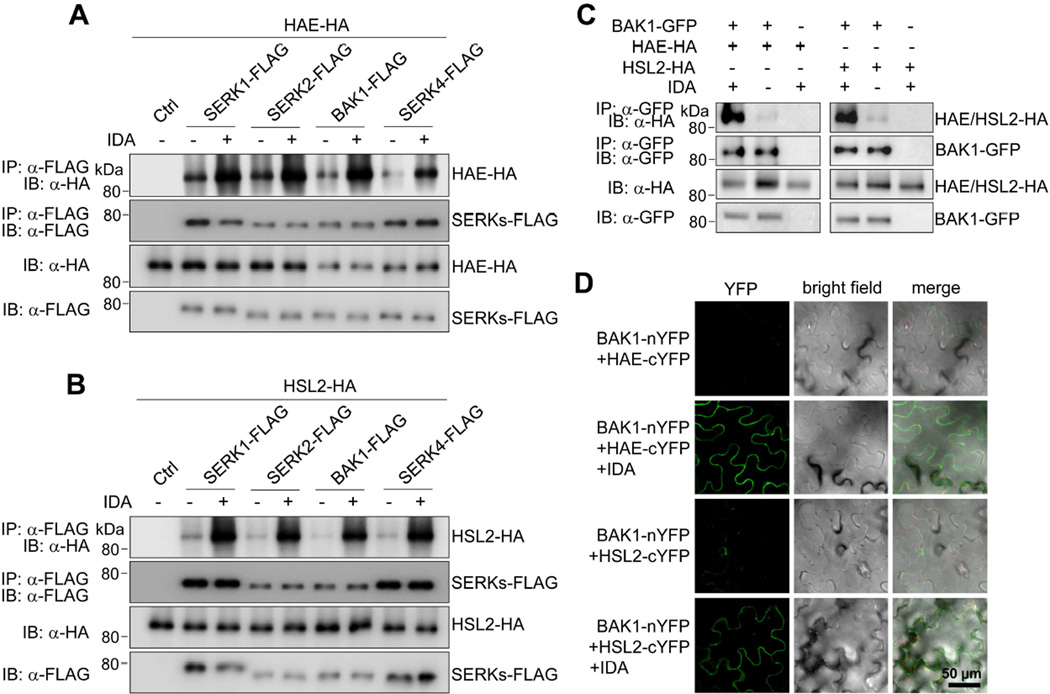
Given that SERKs serve as co-receptors for different LRR-RLKs in plant immunity and development, we tested whether SERKs regulate floral organ abscission through heterodimerization with HAE/HSL2 receptors that recognize the peptide ligand IDA. A co-immunoprecipitation (Co-IP) assay with co-expressing FLAG epitope-tagged SERKs (SERK-FLAG) and HA epitope-tagged HAE or HSL2 (HAE/HSL2-HA) in Arabidopsis protoplasts indicated that SERK1, SERK2, BAK1 and SERK4 were all able to co-immunoprecipitate HAE (Figure 2A) and HSL2 (Figure 2B). Remarkably, co-expression of IDA significantly enhanced the association of SERKs with HAE (Figure 2A) and HSL2 (Figure 2B). In addition, when transiently expressed in Nicotiana benthamiana, BAK1-GFP could immunoprecipitate HAE-HA or HSL2-HA, which was also largely enhanced by co-expression of IDA (Figure 2C). The IDA-induced complex formation between BAK1 and HAE/HSL2 was further confirmed by a split yellow fluorescence protein (YFP)-based bimolecular fluorescence complementation (BiFC) assay. BAK1 and HAE/HSL2 fused with the N- or C-terminal half of YFP respectively (BAK1-nYFP and HAE/HSL2-cYFP) were co-expressed with or without IDA in N. benthamiana. The strong YFP signals were observed when co-expressing BAK1-nYFP and HAE/HSL2-cYFP in the presence of IDA (Figure 2D). Taken together, these data demonstrate that IDA induces complex formation between HAE/HSL2 and SERKs.
Figure 2. Ligand-induced interactions between SERKs and HAE/HSL2.
(A, B) IDA-induced association of SERKs with HAE (A) and HSL2 (B) in Arabidopsis protoplasts. SERK-FLAG and HAE/HSL2-HA proteins were co-expressed with or without IDA expression in protoplasts, immunoprecipitated with α-FLAG antibody (IP: α-FLAG), and immunoblotted with α-HA (IB: α-HA) or α-FLAG antibody (IB: α-FLAG) (top two panels). The protein inputs are shown with immunoblotting before immunoprecipitation (bottom two panels). (C) IDA induces the association between BAK1 and HAE/HSL2 in Nicotiana benthamiana. (D) IDA-induced interaction of BAK1 and HAE/HSL2 in a BiFC assay. BAK1-nYFP and HAE/HSL2-cYFP were transiently co-expressed with or without IDA expression in the leaves of N. benthamiana. YFP signals were observed in the epidermal cells using a confocal microscopy.
Ectodomains of SERKs and HAE/HSL2 are sufficient to form an IDA-induced complex
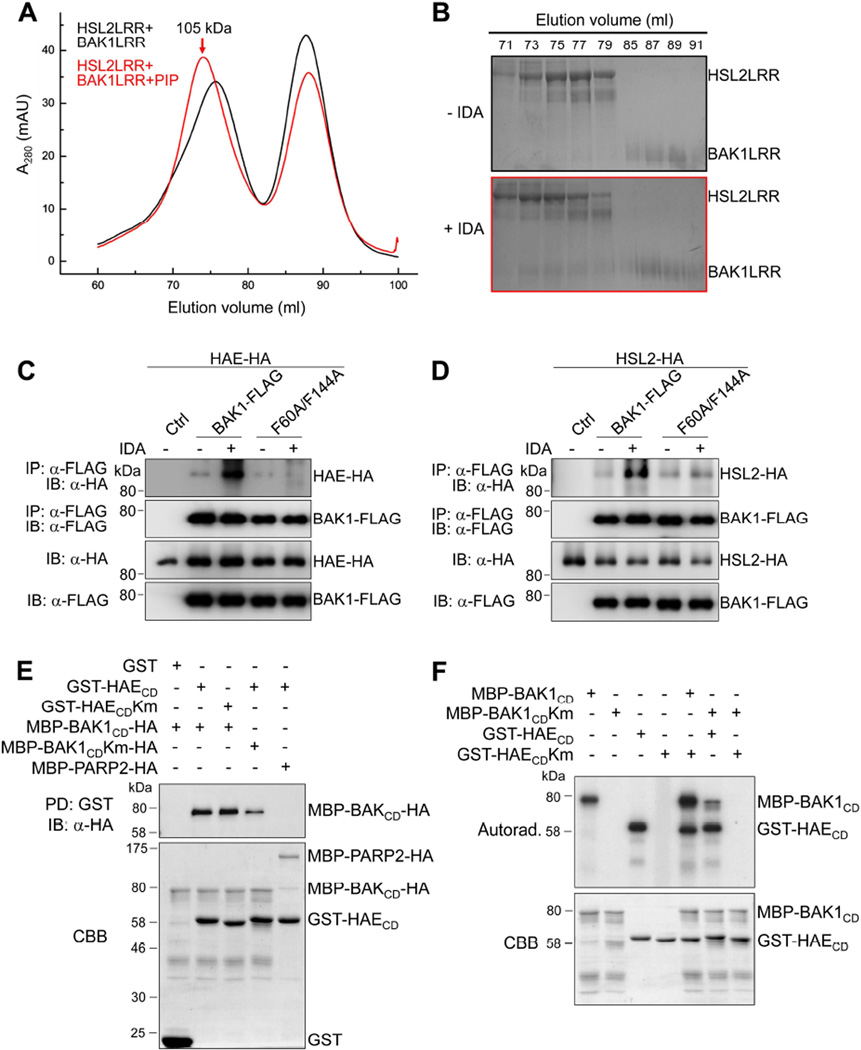
It has been shown that the extracellular LRR domain of BAK1/SERKs forms ligand-induced complexes with the LRR domain of FLS2 or BRI1 and is involved in ligand sensing through contacting the BR-BRI1- or flg22-FLS2-binding interface (Santiago et al., 2013; Sun et al., 2013a; Sun et al., 2013b). To test whether the extracellular domains of SERKs and HAE/HSL2 are sufficient to form an IDA-induced complex, we expressed the LRR domains of BAK1 (residues 1–220, BAK1LRR) and HSL2 (residues 25–632, HSL2LRR) in insect cells, purified the proteins to homogeneity, and performed a gel filtration assay to examine their interaction in the absence or presence of the bioactive IDA peptide. As shown in Figures 3A and 3B, the HSL2LRR and BAK1LRR proteins co-migrated in the assay only in the presence of IDA peptide, indicating that IDA peptide induced the formation of the HSL2LRR-BAK1LRR complex. The complex was eluted at the position corresponding to a monomeric HSL2LRR-BAK1LRR (~105 kD), suggesting that IDA binding did not induce homodimerization of the HSL2LRR-BAK1LRR complex (Figures 3A and 3B). Similarly, the IDA peptide-induced dimerization of HSL2 and SERK1 LRR domains was also observed with the gel filtration assay (Figures S2A and S2B).
Figure 3. Interaction and transphosphorylation between HAE/HSL2 and SERKs.
(A, B) The bioactive PIP peptide of IDA induces HSL2LRR-BAK1LRR heterodimerization in solution. (A) Gel filtration profiles of HSL2LRR and BAK1LRR in the presence (red) and absence (black) of the IDA peptide. The vertical and horizontal axes represent ultraviolet absorbance (λ = 280 nm) and elution volume (ml), respectively. The red arrow indicates the elution position of the PIP-HSL2LRR-BAK1LRR complex (~105 kD). (B) Coomassie Brilliant Blue (CBB) staining of the peak fractions shown on (A). Frame colors indicate their corresponding gel filtration shown on (A). The numbers shown on top of the gels indicate elution volume (ml). (C, D) The F60 and F144 residues of BAK1are required for IDA-induced association of BAK1 with HAE (C) and HSL2 (D) in Arabidopsis protoplasts. (E) BAK1CD interacts with HAECD in an in vitro pull-down (PD) assay. GST, GST-HAECD or GST-HAECDKm immobilized on glutathione sepharose beads was incubated with MBP-BAK1CD-HA, MBP-BAK1CDKm-HA or MBP-PARP2-HA proteins. The beads were washed and pelleted for immunoblotting analysis with α-HA antibody (PD: GST; IB: α-HA) (top panel). The MBP-PARP2-HA protein was used as a negative control. CBB staining of input proteins is shown on the bottom. (F) Transphosphorylation between BAK1CD and HAECD. The in vitro kinase assay was performed using MBP-BAK1CD and GST-HAECD as kinases and MBP-BAK1CDKm and GST-HAECDKm as substrates in the presence of [γ-32P] ATP. Proteins were separated with SDS-PAGE and analyzed by autoradiography (top panel), and the input proteins are shown by CBB staining (bottom panel). (see also Figures S2 and S3).
Crystal structural analysis of the flg22-FLS2-BAK1 and BR-BRI1-BAK1 complexes revealed that several BAK1 residues, including Phe60 and Phe144, directly interact with the FLS2 or BRI1 LRR domain and are critical for flg22-induced FLS2-BAK1 and BR-induced BRI1-BAK1 heterodimerization (Santiago et al., 2013; Sun et al., 2013a; Sun et al., 2013b). We determined whether these residues are also important for BAK1 interaction with HAE and HSL2 upon IDA stimulation by mutating BAK1 Phe60 and Phe144 to Ala (BAK1F60A/F144A). As reported, BAK1F60A/F144A no longer interacted with FLS2 or BRI1 upon flg22 or BR treatment respectively in a protoplast-based Co-IP assay (Figures S3A and S3B). Interestingly, the BAK1F60A/F144A mutant also disrupted the IDA peptide-induced HAE-BAK1 (Figure 3C) and HSL2-BAK1 (Figure 3D) heterodimerizations in the Co-IP assays. The data suggest that BAK1 deploys a similar structural basis to form ligand-induced complexes with different RLK receptors.
Transphosphorylation between BAK1 and HAE
We next investigated whether BAK1 could directly interact with HAE through their cytosolic kinase domains (CD). An in vitro pull-down assay was performed using HAECD fused with the glutathione S-transferase (GST-HAECD) and immobilized on glutathione-sepharose beads as the bait and BAK1CD fused with the maltose-binding protein (MBP) at its N-terminus and an HA epitope tag at its C-terminus as the inputs. As shown in Figure 3E, MBP-BAK1CD-HA could be pulled down by GST-HAECD, but not by GST alone. The MBP-fused poly (ADP-ribose) polymerases 2 (MBP-PARP2)-HA protein was used as a negative control for MBP-BAK1CD-HA. Interestingly, the kinase-inactive mutant of MBP-BAK1CD-HA (MBP-BAK1CDKm-HA) could also be pulled down by GST-HAECD (Figure 3E). Similarly, GST-HAECDKm could pull down MBP-BAK1CD-HA, suggesting that the kinase activities of BAK1 and HAE may not be required for their direct interactions.
Transphosphorylation of RLK complexes often constitutes an important step in the initiation of receptor signaling activation. We examined whether BAK1CD and HAECD could transphosphorylate each other by in vitro kinase assays. The autophosphorylation activity of HAECD is comparable to that of BAK1CD, whereas HAECDKm and BAK1CDKm did not show any detectable kinase activities (Figure 3F). Clearly, BAK1CD phosphorylated HAECDKm, and HAECD also phosphorylated BAK1CDKm, indicating a transphosphorylation event within the HAE-BAK1 receptor complex. Overall, the data suggest that SERK family RLKs regulate floral organ abscission through IDA-induced interaction and transphosphorylation with HAE and HSL2 receptors.
SERKs function genetically downstream of IDA and upstream of the MAPK cascade
To genetically examine whether SERKs function in the IDA signaling pathway, we performed an epistasis analysis through overexpression of IDA under the control of the 35S promoter in the serk1-1/serk2-1/bak1-5 mutant. Consistent with previous reports (Cho et al., 2008; Stenvik et al., 2008), overexpression of IDA in WT plants led to a premature floral organ abscission and the production of a white substance in the abscission zones (Figures 4A and S4). However, this premature abscission caused by IDA overexpression was not observed in the serk1-1/serk2-1/bak1-5 mutant. Instead, these transgenic plants showed severe abscission defects as observed in serk1-1/serk2-1/bak1-5 (Figure 4A), indicating that SERKs function genetically downstream of IDA in floral organ abscission signaling.
Figure 4. SERKs function genetically downstream of IDA and upstream of MKK4/MKK5 in regulating floral organ abscission.
(A) SERKs are required for IDA-triggered floral organ abscission. Floral organ abscission phenotypes of indicated genotypes are shown in floral position order. Overexpression of IDA in the Col-0 background leads to premature abscission (middle panel) and the production of a white substance in receptacle (indicated by the arrow), whereas the IDA-induced premature abscission was blocked in serk1-1/serk2-1/bak1-5 (bottom panel). (B) MKK5 is epistatic to SERKs in regulating floral organ abscission. Eight-week-old inflorescence stems with budding flowers and siliques are shown. Arrows indicate unabscised floral organs of serk1-1/serk2-1/bak1-5. The floral organ abscission defective phenotype of serk1-1/serk2-1/bak1-5 mutant is rescued by the pHSL2::MKK5DD transgene. (C) A model of the IDA signaling pathway. IDA binding to HAE/HSL2 receptors triggers the heterodimerization of HAE/HSL2 and SERKs, followed by transphosphorylation and activation of the receptor complex. The signal from the receptor complex is further transduced via activation of a MAPK cascade consisting of MKK4/MKK5 and MPK3/MPK6, which then phosphorylate downstream transcription factors (TFs), leading to enhanced hydrolyzing enzymatic activity in the abscission zones (AZ) and finally the floral organ shedding. (see also Figure S4).
To determine the genetic relationship between the SERK family members and the MKK4/MKK5-MPK3/MPK6 cascade, a constitutively active variant of MKK5 (MKK5DD) under the control of HSL2 promoter (pHSL2::MKK5DD) was transformed into serk1-1/serk2-1/bak1-5. As shown in Figure 4B, the pHSL2::MKK5DD transgene restored floral organ abscission in serk1-1/serk2-1/bak1-5. The data provide genetic evidence that SERKs function in the same pathway with HAE/HSL2 RLKs upstream of the MKK4/MKK5-MPK3/MPK6 cascade to transduce the IDA signaling.
DISCUSSION
In Arabidopsis, the secreted IDA peptide serves as a signal which is perceived by cell surface-resident RLKs HAE/HSL2 to regulate the floral organ abscission, a highly programmed physiological process that leads to shedding unwanted floral organs during fruit development (Liljegren, 2012; Niederhuth et al., 2013). Here, we show that perception of the IDA ligand by the HAE/HSL2 receptors leads to recruitment of the SERK family RLKs accompanied with transphosphorylation of the HAE/HSL2-SERKs RLK complex, which further relays the signaling to a MAPK cascade consisting of MKK4/MKK5-MPK3/MPK6, to regulate floral organ abscission (Figure 4C). Despite triggering distinct physiological responses, this pathway bears striking similarity with those in flagellin-mediated plant immunity and EPF peptide-mediated stomatal patterning (Chinchilla et al., 2007; Heese et al., 2007; Meng et al., 2015). In addition to the SERK family RLKs, MKK4/MKK5-MPK3/MPK6 cascade is also conserved among these peptide signaling pathways (Meng et al., 2015). Thus, the paradigm in RLK-mediated signal transduction pathways includes ligand-induced heterodimerization of receptors and co-receptors, subsequent transphosphorylation, and relay of the signaling into downstream modules such as MAPK cascades that further amplify the information to cognate effectors for distinct physiological responses.
The SERK family RLKs have been implicated to act as co-receptors of multiple RLK receptors perceiving different extrinsic and intrinsic cues in diverse signaling pathways (Aan den Toorn et al., 2015). It appears that individual SERK family members still exert distinct functions in different physiological responses. For example, SERK1 and SERK2 are dispensable for plant immunity (Roux et al., 2011), whereas SERK2 is nonessential in BR signaling (Gou et al., 2012). An outstanding question is how functional specificity of individual SERKs is controlled. Studies of chimeric proteins with different SERK members indicate that both extracellular and cytoplasmic domains confer functional specificity (Aan den Toorn et al., 2015). The in vivo dynamic binding affinity of individual SERKs with the cognate receptors may partially explain the functional specificity. For instance, SERK2, which is not involved in BR signaling, did not associate with BRI1 in vivo in a Co-IP assay (Aan den Toorn et al., 2015). However, although SERK1 and SERK2 do not have a detectable function in plant immunity, they both associate with the immune receptor FLS2 and EFR in N. benthamiana and Arabidopsis, similar to BAK1 and SERK4 (Roux et al., 2011). In addition, the residues required for interactions with different ligands and receptors are highly conserved in different SERK family members, suggesting they all possess the capacity to complex with different receptors (Aan den Toorn et al., 2015). However, other residues/motifs may also contribute to the interaction strength between receptors and SERK co-receptors. Deployment of more quantitative and sensitive assays of protein-protein interactions, such as microscale thermophoresis and surface plasmon resonance, may further identify different binding kinetics or dissociation constants of different SERK members with receptors.
The ADP-ribosylation factor GTPase-activating protein, NEV, is required for floral organ abscission (Liljegren et al., 2009). Genetic screens for suppressors of the nev mutant, in which the floral organs do not abscise, have identified several components, including EVERSHED (EVE) (Leslie et al., 2010), CAST AWAY (CST) (Burr et al., 2011) and SERK1 (Lewis et al., 2010), that regulate floral organ abscission. NEV is likely involved in protein trafficking between the plasma membrane and recycling endosomes (Liljegren et al., 2009). EVE, also named as SOBIR1, encodes a RLK with a short extracellular LRR domain, structurally similar with SERK family RLKs. Interestingly, EVE/SOBIR1 is involved in plant immunity via association with multiple receptor-like proteins (Liebrand et al., 2014). CST encodes a receptor-like cytoplasmic kinase (RLCK) (Burr et al., 2011). The emerging picture is that RLCKs transduce RLK signaling via interaction and transphosphorylation by RLKs (Lin et al., 2013). It is interesting that SERK1, but not SERK2, could function as a spatial inhibitor of floral organ abscission on the nev mutant (Lewis et al., 2010). Similar with our finding, the serk1 mutant itself did not display any floral organ abscission defects. It has been proposed that SERK1 negatively regulates the nev floral organ abscission in a parallel pathway or upstream of the HAE/HSL2 receptors since the serk1 mutant did not suppress abscission defects of the hae/hsl2 mutant (Lewis et al., 2010). We show here that SERK1, together with other SERKs, also positively regulates floral organ abscission by forming IDA-induced complex with HAE/HSL2. It is possible that SERK1 has distinct functions in different pathways regulating floral organ abscission, or SERK1 is involved in an inhibitory feedback loop in the abscission process.
EXPERIMENTAL PROCEDURES
Plant materials and growth conditions
Arabidopsis thaliana Col-0 accession was used as the wild-type (WT). The T-DNA insertion mutants (hae, SALK_021905 and hsl2, SALK_030520) were obtained from the Arabidopsis Biological Resource Center (ABRC). The serk single, double and triple mutants were reported previously (Meng et al., 2015), and the hae/hsl2 double mutant was generated by the genetic cross. A. thaliana and Nicotiana benthamiana seeds were grown on soil (Metro Mix 360) in a growth room at 23°C, 45% humidity and 75 µE m−2s−1 light with a 12 hr light/12 hr dark photoperiod. Arabidopsis floral organ abscission phenotypes were scored using the number of abscission-defective petals attached to the first five most mature siliques from eight-week-old plants (n=50 siliques from 10 plants). Significant differences (p<0.05) between the values for different Arabidopsis genotypes were assessed by one-way ANOVA with Tukey’s post-test.
Plasmid construction, transient assay and generation of transgenic plants
The promoters of SERK1, SERK2, BAK1, SERK4, HAE and HSL2 were cloned by PCR from Col-0 genomic DNA for GUS reporter constructs. The SERK1, SERK2, BAK1 and SERK4 genes and MKK5DD mutant were reported previously (Meng et al., 2015). The HAE, HSL2 and IDA genes were amplified by PCR from Col-0 cDNA. The construction of plasmids is described in supplemental experimental procedures.
The protoplast isolation and transfection used for transient assays were carried out as reported previously (Meng et al., 2015). Agrobacterium-mediated transformation of Arabidopsis plants was performed using the floral-dip method. For each construct, >20 T1 plants were screened for transgene expression using RT-PCR or immunoblotting. Two to three T2 lines with a single T-DNA insertion and similar transgene expression levels were subjected to phenotypic characterization. The sequences of all primers used for PCR and RT-PCR are listed in Table S1.
Co-immunoprecipitation, GST pull-down, in vitro phosphorylation and gel filtration assay
Co-IP, pull-down and in vitro phosphorylation assays were carried out as reported previously (Meng et al., 2015). Expression and purification of the BAK1LRR protein were reported previously (Sun et al., 2013b). The HSL2LRR and SERK1 LRR gene fragments were cloned into the pFastBac-1 vector (Invitrogen) and expressed in High Five insect cells, and the corresponding proteins were purified using similar procedure as the BAK1LRR purification. The procedure of gel filtration assay is described in the Supplemental Experimental Procedures.
Supplementary Material
Highlights.
The SERK family receptor-like kinases redundantly regulate floral organ abscission
SERKs act downstream of the IDA ligand and upstream of a MAPK cascade
SERKs associate with the HAE and HSL2 receptors in a ligand-induced manner
SERK3 and HAE transphosphorylate each other in their cytosolic domains
Acknowledgments
We thank Dr. Cyril Zipfel and ABRC for Arabidopsis mutant seeds, and Kevin Cox for reading of the manuscript. The work was supported by National Institutes of Health (NIH) (R01GM092893) and National Science Foundation (IOS-1252539) to P.H., NIH (R01GM097247) and the Robert A. Welch foundation (A-1795) to L.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information includes supplemental experimental procedures, 4 Figures and 1 Table.
AUTHOR CONTRIBUTIONS
X.M., J.C., P.H., and L.S. designed experiments, X.M., J.Z., J.T., B.L. and M.O. performed experiments and analyzed data, X.M., P.H., and L.S. wrote the manuscript with input from all co-authors.
The authors have declared no conflict of interests.
REFERENCES
- Aan den Toorn M, Albrecht C, de Vries S. On the Origin of SERKs: Bioinformatics Analysis of the Somatic Embryogenesis Receptor Kinases. Mol Plant. 2015;8:762–782. doi: 10.1016/j.molp.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Albrecht C, Russinova E, Hecht V, Baaijens E, de Vries S. The Arabidopsis thaliana SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASES1 and 2 control male sporogenesis. Plant Cell. 2005;17:3337–3349. doi: 10.1105/tpc.105.036814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr CA, Leslie ME, Orlowski SK, Chen I, Wright CE, Daniels MJ, Liljegren SJ. CAST AWAY, a membrane-associated receptor-like kinase, inhibits organ abscission in Arabidopsis. Plant Physiol. 2011;156:1837–1850. doi: 10.1104/pp.111.175224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenko MA, Wildhagen M, Albert M, Jehle A, Kalbacher H, Aalen RB, Felix G. Tools and Strategies to Match Peptide-Ligand Receptor Pairs. Plant Cell. 2014;26:1838–1847. doi: 10.1105/tpc.113.120071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nurnberger T, Jones JD, Felix G, Boller T. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature. 2007;448:497–500. doi: 10.1038/nature05999. [DOI] [PubMed] [Google Scholar]
- Cho SK, Larue CT, Chevalier D, Wang H, Jinn TL, Zhang S, Walker JC. Regulation of floral organ abscission in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 2008;105:15629–15634. doi: 10.1073/pnas.0805539105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombet J, Boisson-Dernier A, Ros-Palau R, Vera CE, Schroeder JI. Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASES1 and 2 are essential for tapetum development and microspore maturation. Plant Cell. 2005;17:3350–3361. doi: 10.1105/tpc.105.036731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou X, Yin H, He K, Du J, Yi J, Xu S, Lin H, Clouse SD, Li J. Genetic evidence for an indispensable role of somatic embryogenesis receptor kinases in brassinosteroid signaling. PLoS Genet. 2012;8:e1002452. doi: 10.1371/journal.pgen.1002452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Gou X, Yuan T, Lin H, Asami T, Yoshida S, Russell SD, Li J. BAK1 and BKK1 regulate brassinosteroid-dependent growth and brassinosteroid-independent cell-death pathways. Curr Biol. 2007;17:1109–1115. doi: 10.1016/j.cub.2007.05.036. [DOI] [PubMed] [Google Scholar]
- Heese A, Hann DR, Gimenez-Ibanez S, Jones AM, He K, Li J, Schroeder JI, Peck SC, Rathjen JP. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci U S A. 2007;104:12217–12222. doi: 10.1073/pnas.0705306104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinn TL, Stone JM, Walker JC. HAESA, an Arabidopsis leucine-rich repeat receptor kinase, controls floral organ abscission. Genes Dev. 2000;14:108–117. [PMC free article] [PubMed] [Google Scholar]
- Ladwig F, Dahlke RI, Stuhrwohldt N, Hartmann J, Harter K, Sauter M. Phytosulfokine Regulates Growth in Arabidopsis through a Response Module at the Plasma Membrane That Includes CYCLIC NUCLEOTIDE-GATED CHANNEL17, H+-ATPase, and BAK1. Plant Cell. 2015;27:1718–1729. doi: 10.1105/tpc.15.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie ME, Lewis MW, Youn JY, Daniels MJ, Liljegren SJ. The EVERSHED receptor-like kinase modulates floral organ shedding in Arabidopsis. Development. 2010;137:467–476. doi: 10.1242/dev.041335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MW, Leslie ME, Fulcher EH, Darnielle L, Healy PN, Youn JY, Liljegren SJ. The SERK1 receptor-like kinase regulates organ separation in Arabidopsis flowers. Plant J. 2010;62:817–828. doi: 10.1111/j.1365-313X.2010.04194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wen J, Lease KA, Doke JT, Tax FE, Walker JC. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell. 2002;110:213–222. doi: 10.1016/s0092-8674(02)00812-7. [DOI] [PubMed] [Google Scholar]
- Liebrand TWH, van den Burg HA, Joosten MHAJ. Two for all: receptor-associated kinases SOBIR1 and BAK1. Trends in Plant Science. 2014;19:123–132. doi: 10.1016/j.tplants.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Liljegren SJ. Organ abscission: exit strategies require signals and moving traffic. Curr Opin Plant Biol. 2012;15:670–676. doi: 10.1016/j.pbi.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Liljegren SJ, Leslie ME, Darnielle L, Lewis MW, Taylor SM, Luo R, Geldner N, Chory J, Randazzo PA, Yanofsky MF, et al. Regulation of membrane trafficking and organ separation by the NEVERSHED ARF-GAP protein. Development. 2009;136:1909–1918. doi: 10.1242/dev.033605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Ma X, Shan L, He P. Big roles of small kinases: the complex functions of receptor-like cytoplasmic kinases in plant immunity and development. J Integr Plant Biol. 2013;55:1188–1197. doi: 10.1111/jipb.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Chen X, Mang H, Liu C, Yu X, Gao X, Torii KU, He P, Shan L. Differential Function of Arabidopsis SERK Family Receptor-like Kinases in Stomatal Patterning. Curr Biol. 2015;25:2361–2372. doi: 10.1016/j.cub.2015.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam KH, Li J. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell. 2002;110:203–212. doi: 10.1016/s0092-8674(02)00814-0. [DOI] [PubMed] [Google Scholar]
- Niederhuth CE, Cho SK, Seitz K, Walker JC. Letting go is never easy: abscission and receptor-like protein kinases. J Integr Plant Biol. 2013;55:1251–1263. doi: 10.1111/jipb.12116. [DOI] [PubMed] [Google Scholar]
- Patharkar OR, Walker JC. Floral organ abscission is regulated by a positive feedback loop. Proc Natl Acad Sci U S A. 2015;112:2906–2911. doi: 10.1073/pnas.1423595112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux M, Schwessinger B, Albrecht C, Chinchilla D, Jones A, Holton N, Malinovsky FG, Tor M, de Vries S, Zipfel C. The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell. 2011;23:2440–2455. doi: 10.1105/tpc.111.084301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago J, Henzler C, Hothorn M. Molecular mechanism for plant steroid receptor activation by somatic embryogenesis co-receptor kinases. Science. 2013;341:889–892. doi: 10.1126/science.1242468. [DOI] [PubMed] [Google Scholar]
- Schwessinger B, Roux M, Kadota Y, Ntoukakis V, Sklenar J, Jones A, Zipfel C. Phosphorylation-dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor-like kinase BAK1. PLoS Genet. 2011;7:e1002046. doi: 10.1371/journal.pgen.1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi CL, Stenvik GE, Vie AK, Bones AM, Pautot V, Proveniers M, Aalen RB, Butenko MA. Arabidopsis class I KNOTTED-like homeobox proteins act downstream in the IDA-HAE/HSL2 floral abscission signaling pathway. Plant Cell. 2011;23:2553–2567. doi: 10.1105/tpc.111.084608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenvik GE, Tandstad NM, Guo Y, Shi CL, Kristiansen W, Holmgren A, Clark SE, Aalen RB, Butenko MA. The EPIP peptide of INFLORESCENCE DEFICIENT IN ABSCISSION is sufficient to induce abscission in arabidopsis through the receptor-like kinases HAESA and HAESA-LIKE2. Plant Cell. 2008;20:1805–1817. doi: 10.1105/tpc.108.059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Han Z, Tang J, Hu Z, Chai C, Zhou B, Chai J. Structure reveals that BAK1 as a co-receptor recognizes the BRI1-bound brassinolide. Cell Res. 2013a;23:1326–1329. doi: 10.1038/cr.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Li L, Macho AP, Han Z, Hu Z, Zipfel C, Zhou JM, Chai J. Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex. Science. 2013b;342:624–628. doi: 10.1126/science.1243825. [DOI] [PubMed] [Google Scholar]
- Wang J, Li H, Han Z, Zhang H, Wang T, Lin G, Chang J, Yang W, Chai J. Allosteric receptor activation by the plant peptide hormone phytosulfokine. Nature. 2015;525:265–268. doi: 10.1038/nature14858. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.