Abstract
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by extracellular deposits of amyloid β-protein (Aβ) in the brain. The conversion of soluble monomers to amyloid Aβ fibrils is a complicated process and involves several transient oligomeric species, which are widely believed to be highly toxic and play a crucial role in the etiology of AD. The development of inhibitors to prevent formation of small and mid-sized oligomers is a promising strategy for AD treatment. In this work, we employ ion mobility spectrometry (IMS), transmission electron microscopy (TEM) and molecular dynamics (MD) simulations to elucidate the structural modulation promoted by two potential inhibitors of Aβ oligomerization, cucurbit[7]uril (CB[7]) and 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose (PGG), on early oligomer and fibril formation of the Aβ25–35 fragment. One and two CB[7] molecules bind to Aβ25–35 monomers and dimers, respectively, and suppress aggregation by remodeling early oligomer structures and inhibiting the formation of higher-order oligomers. On the other hand, non-selective binding was observed between PGG and Aβ25–35. The interactions between PGG and Aβ25–35, surprisingly, enhanced the formation of Aβ aggregates by promoting extended Aβ25–35 conformations in both homo- and hetero-oligomers. When both ligands were present, the inhibitory effect of CB[7] overrode the stimulatory effect of PGG on Aβ25–35 aggregation, suppressing the formation of large amyloid oligomers and eliminating the structural conversion from isotropic to β-rich topologies induced by PGG. Our results provide mechanistic insights into CB[7] and PGG action on Aβ oligomerization. They also demonstrate the power of the IMS technique to investigate mechanisms of multiple small-molecule agents on the amyloid formation process.
Keywords: amyloids, polyphenols, cucurbiturils, ion-mobility mass spectrometry, computational modeling, amyloid-β
INTRODUCTION
Alzheimer’s disease (AD) is a neurodegenerative disease pathologically characterized by extracellular deposits of amyloid β-protein (Aβ) in the brain, resulting in neuronal cell loss and subsequent dementia.1–6 However, the toxicity of Aβ is not mediated by these large aggregates of Aβ. Rather, neurotoxicity correlates with the rapid self-assembly of soluble, oligomeric forms of Aβ. Interestingly, there are two major forms of Aβ: Aβ1–42 constitutes only ~9 % of the total Aβ in the brain and is the more toxic alloform, whereas the more abundant species Aβ1–40 (~90 %) is less toxic and has a slower aggregation rate.7–11 The conversion of soluble Aβ monomers to amyloid Aβ fibrils is a complicated, dynamic process involving several transient oligomeric species,12–17 several of which appear to be highly toxic and play a crucial role in the development of AD.18,19
Although there are presently no effective treatments for AD, several therapeutic strategies are under development for prevention and treatment.20–25 Approaches based on suppressing Aβ fibril formation appear to be insufficient and are likely to be unsuccessful given that soluble oligomers, rather than fibrils, are the probable toxic agents.14,26,27 In this context, the search for potential inhibitors to shorten the lifetime of small and mid-sized oligomers has attracted great attention.25,28–32 Promising results have been reported employing polyphenol derivatives as inhibitors of amyloid formation.30,31,33–35 In particular, 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose (PGG) (see Scheme 1), a large polyphenol from the traditional medicinal herb Paeonia suffruticosa, was reported to be a potent inhibitor for the aggregation of both Aβ1–40 and Aβ1–42.33 However, it is unclear whether there are specific interaction motifs between polyphenols and amyloid peptides, as these compounds tend to form complexes with a wide range of proteins and peptides including Aβ, islet amyloid polypeptide (IAPP), α-synuclein, and tau.30,34,36,37 It has been suggested that the inhibitory mechanism of polyphenols may be size and shape-dependent. For example, a previous study has shown that epigallocatechin gallate (EGCG; a polyphenol extracted from green tea) is an effective inhibitor of Aβ25–35 aggregation because the peptide can tightly wrap around three faces of the inhibitor, whose shape resembles a propeller.31 Another study has shown that the effects of inositols, a class of small polyphenols, on amyloid aggregation are stereochemistry dependent.38 However, there is not enough evidence to correlate inhibitory effects with the shapes of the polyphenols and the lengths of the peptide targets.
Scheme 1.
Structural formulas of 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose (PGG), cucurbit[7]uril (CB[7]) and Aβ25–35.
Recently, a new supramolecular strategy approach has been employed to target certain types of residues explicitly.32,39–41 For example, cucurbit[7]uril (CB[7]) (see Scheme 1), a synthetic macrocyclic receptor molecule,42–44 has demonstrated inhibitory effects by locking aromatic residues inside its hollow core.40 Each cucurbituril possesses a rigid hydrophobic cavity lying between the two oxygen-atom-rich rims, making it a compatible structure for host-guest chemistry.45 The inhibitory effect of CB[7] on Aβ aggregation was suggested to be associated primarily with its hydrophobic cavity capable of capturing aromatic residues.46 Polar and charged residues can in principle also interact with the hydrophilic surfaces of the molecule, but there is currently lack of experimental support for this effect.
The mechanistic effect of ligands like PGG and CB[7], individually or in combination, on transient early-stage soluble Aβ oligomers is unknown. Here, we investigate the effect of these ligands on oligomer and fibril formation of Aβ25–35 (see Scheme 1), a cytotoxic fragment of Aβ that has been studied extensively by experimental and computational techniques.31,47–54 The transient and polymorphic nature of aggregating systems poses significant challenges for traditional techniques aimed at detecting and characterizing early oligomer formation. Ion mobility spectrometry coupled mass spectrometry (IMS-MS) overcomes many of these challenges, and has been applied successfully to studies of structural changes in the self-assembly of Aβ and similar systems,14,55,56 as well as to the evaluation of small-molecules that interfere with the Aβ assembly process.29,31,32,57–60 We report herein the effects of CB[7] and PGG on oligomer formation and aggregate morphologies of Aβ25–35 using IMS-MS, transmission electron microscopy (TEM), and molecular dynamics (MD) simulations.
RESULTS and DISCUSSION
Aβ25–35 aggregation and the transition of oligomeric states from isotropic to β-sheet
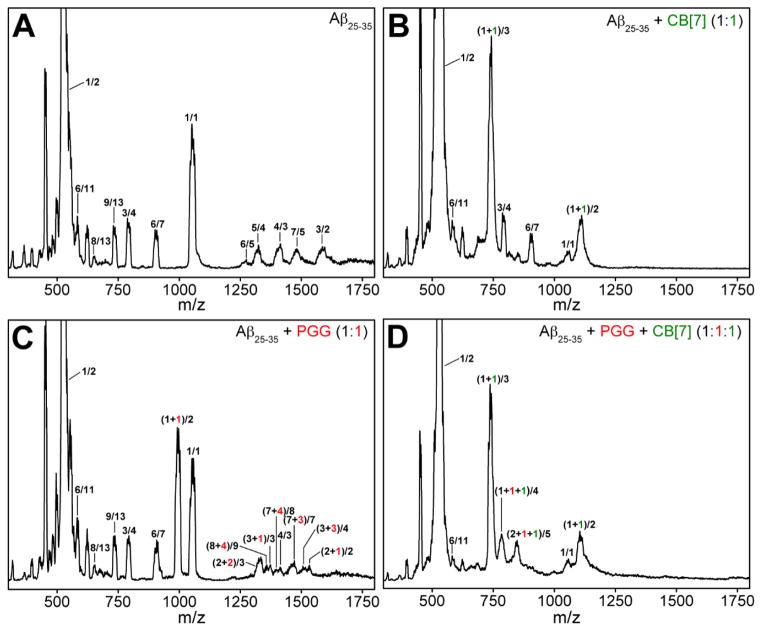
The nano-ESI-Q mass spectrum of Aβ25–35 is given in Figure 1A. Numerous peaks are reported with respect to their n/z ratios, where n is the oligomer number and z the charge, including n/z = 1/2 (m/z = 530) and n/z = 1/1 (m/z = 1060) and numerous less intense peaks for higher order oligomers. Since these are relatively low resolution spectra a given n/z can contain several oligomeric states. For example n/z = 1/1 can also be n/z = 2/2. The various contributions of different oligomeric states to each peak can be obtained from the arrival time distributions (ATDs) given in Figure S2 in Supporting Information.
Figure 1.
ESI-Q mass spectra of (A) pure Aβ25–35, (B) Aβ25–35 incubated with CB[7] at 1:1, (C) Aβ25–35 incubated with PGG at 1:1, and (D) Aβ25–35 incubated with PGG and CB[7] at 1:1:1 molar ratio. The Aβ25–35 concentration was 100 μM in acidified water (0.1% (v/v) of formic acid) in all cases. The peaks of the Aβ25–35 homo-oligomers are annotated as ratios of the oligomer size n and the charge z, whereas the hetero-oligomer peaks of Aβ25–35 with ligands are annotated by (n+p)/z, (n+k)/z and (n+p+k)/z where p and k are the numbers of PGG and CB[7] molecules, respectively.
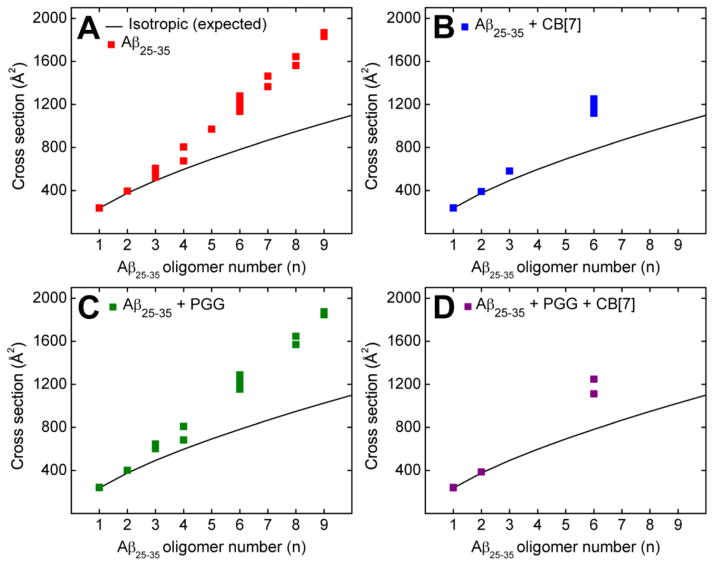
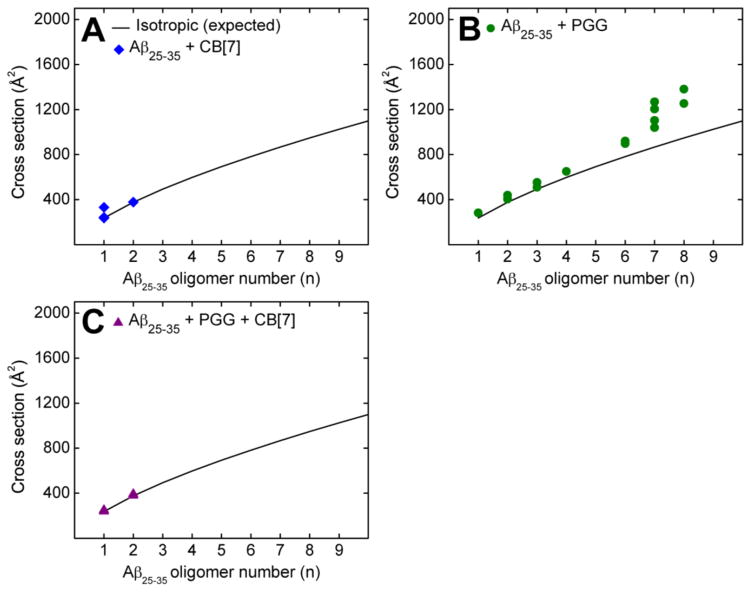
The experimental collisional cross sections (CCS) (reported in Figures 2A and Supporting Information, Figure S2 and Table S1) are obtained from measuring ATDs at different pressure to voltage (P/V) ratios from a plot of arrival time (tA) versus P/V (Equations S3 and S4). To analyze the transient states of the soluble oligomers, the experimental data were compared to the ideal isotropic growth model (Figure 2), in which the isotropic growth approximates the cross section of an oligomer whose structure is equally distributed in all spatial dimensions (σn = σ1 × n2/3).31,61 For Aβ25–35 a positive deviation from the ideal isotropic growth model is observed at the dimer and increasingly significant deviations are observed for trimers and larger oligomeric species (Figure 2A). Recent studies31,61,62 have convincingly shown that significant and persistent positive deviations of oligomers above the isotropic growth curve are reliable indicators of β-sheet formation. This occurs because β-sheet growth occurs in a linear manner and hence the CCSs of β-sheet oligomers increase linearly with n, the oligomer number, not as n2/3 which describes isotropic growth. More specifically, previous work has shown that the formation of β-sheet-like trimers and tetramers is a crucial step in the process of Aβ25–35 aggregation.31,47,63 In summary, the IMS-MS data indicate that extended β-sheet conformations are populated more abundantly as the Aβ25–35 oligomer number increases, a clear signature of eventual amyloid fibril formation. These results are consistent with TEM images (Figure 3A) that shows the formation of amyloid fibrils and were obtained from the same Aβ25–35 sample used in the IMS-MS experiments. Our data here are also consistent with previous studies on the same system.31
Figure 2.
Oligomer growth curve data of the Aβ25–35 homo-oligomers of (A) pure Aβ25–35, (B) Aβ25–35 incubated with CB[7] at 1:1, (C) Aβ25–35 incubated with PGG at 1:1, and (D) Aβ25–35 incubated with PGG and CB[7] at 1:1:1 molar ratio.
Figure 3.
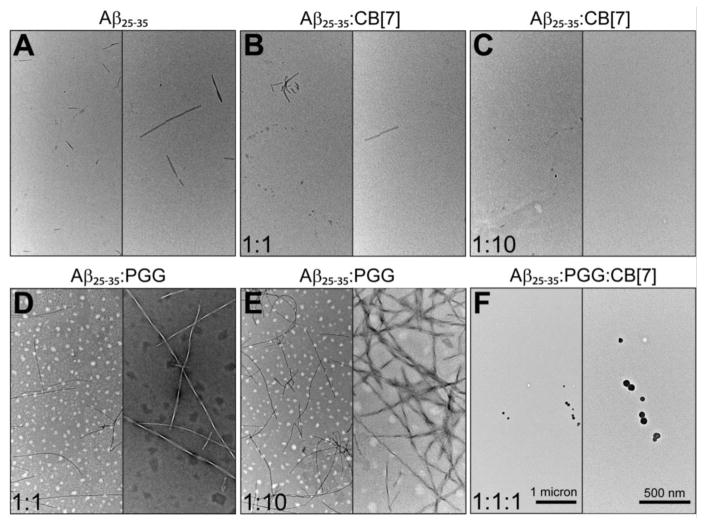
Representative TEM images of (A) pure Aβ25–35, (B, C) Aβ25–35 incubated with CB[7] at 1:1 and 1:10, (D, E) Aβ25–35 incubated with PGG at 1:1 and 1:10, and (F) Aβ25–35 incubated with PGG and CB[7] at 1:1:1 molar ratio. The scale bars for all images are given in the two panels of section F.
Inhibitory effect of cucurbit[7]uril (CB[7]) on Aβ25–35 amyloid aggregation
As shown in Figures 1B and 2B, the presence of an equimolar concentration of CB[7] remodels and dramatically reduces the steady-state oligomer distribution of Aβ25–35. In the presence of CB[7], the only identified Aβ25–35 homo-oligomers are monomers at n/z = 1/2 (m/z = 530) and n/z = 1/1 (m/z = 1060), dimer at n/z = 2/2 (m/z = 1060), trimer at n/z = 3/4 (m/z = 795) and hexamers at n/z = 6/7 (m/z = 908) and n/z = 6/11 (m/z = 578). The low charge state mass spectral peaks at high m/z are absent, indicating that CB[7] not only modulated oligomer structures (see discussion below), but also prevented the formation of large oligomers.
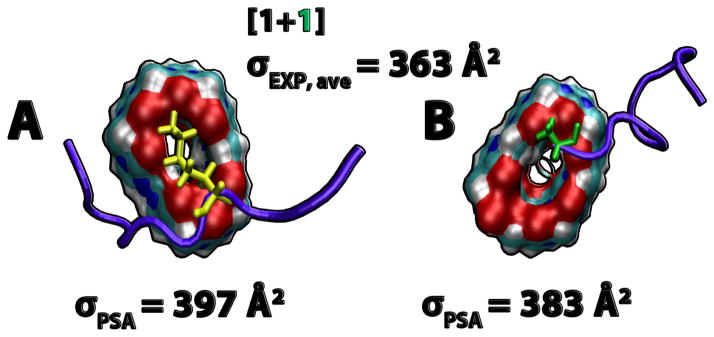
Two new mass spectral peaks are recognized in Figure 1B as Aβ25–35:CB[7] hetero-oligomers, in which the most abundant peak at m/z = 741 is a triply charged heterodimer of an Aβ25–35 monomer and one CB[7] molecule ((n+k)/z = (1+1)/3), where k is the number of CB[7] molecules, and the peak at m/z = 1111 is a doubly charged heterodimer (see Figure S3 and Table S2). The CCSs obtained for Aβ25–35:CB[7] hetero-oligomers (Figure 4A) suggest that the dimer is globular but the monomer has two conformations, one globular and one extended conformer.
Figure 4.
Oligomer growth curve data of hetero-oligomers of (A) Aβ25–35 incubated with CB[7] at 1:1, (B) Aβ25–35 incubated with PGG at 1:1, and (C) Aβ25–35 incubated with PGG and CB[7] at 1:1:1 molar ratio. The cross sections of Aβ25–35 oligomers reported here were extracted from the hetero-oligomer cross sections (see Supporting Information Tables S2, S3 and S4).
At 1:10 Aβ25–35:CB[7] molar ratio, there are only three remaining homo-oligomers: Aβ25–35 monomers and dimers at n/z = 1/2 (m/z = 530), n/z = 1/1 (m/z = 1060) and n/z = 2/2 (m/z = 1060) (Figure S1). The remaining peaks are Aβ25–35:CB[7] hetero complexes previously described. The higher intensities of these Aβ25–35:CB[7] complex peaks at 1:10 ratio suggests that CB[7] competitively binds to Aβ25–35 species and suppresses Aβ25–35 oligomer growth. Our data indicate that CB[7] is a very effective inhibitor that intervenes in the aggregation cascade by blocking self-assembly at the dimer stage. TEM imaging (Figures 3B, C) shows that CB[7] reduces the formation of macroscopic fibrillar aggregates compared to the TEM image of the pure Aβ25–35, supporting the IMS-MS data.
Computational modeling provides evidence supporting an inhibitory effect of CB[7] on the amyloid formation of Aβ25–35. The simulations reveal that CB[7] ligands preferentially capture the amino-groups of lysines (Lys28), and to a lesser extent, the positively charged N-termini of Aβ25–35 monomers (see Figure 5). The binding of CB[7] specifically to lysines is also observed with the 38-residue islet amyloid polypeptide whose aggregation is a pathological hallmark of Type 2 Diabetes.41 The interaction motif is similar to the molecular tweezer ligands that possess torus-shaped binding pockets.32,64,65 The polar pockets serve as specific hosts for charged residues, and the strong binding between protein:ligand complexes can modulate the aggregation cascade. Each CB[7] molecule has only one binding site for Aβ25–35 and the bulkiness of CB[7] helps to isolate the Aβ25–35 monomers from each other and prevent large oligomer formation.
Figure 5.
Representative structures of Aβ25–35:CB[7] complexes obtained from simulations. (1+1) complexes extracted from the simulation, and their theoretical cross sections. The experimental cross section is an average of the (1+1)/2 and (1+1)/3 complexes. The CB[7] molecules are represented in filled surface with oxygens in red, carbons in cyan, nitrogens in blue and hydrogens in white. The Aβ25–35 peptides are shown in cartoon representation and colored in violet. Lysine (yellow) and N-terminus (green) are shown in licorice representation.
1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose (PGG) enhances Aβ25–35 amyloid aggregation
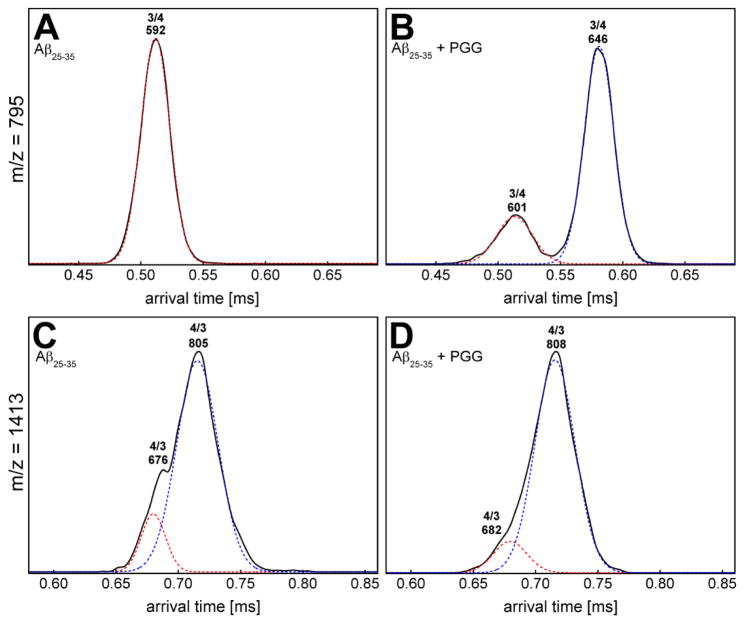
In the mass spectrum of Aβ25–35 incubated with PGG at a 1:1 molar ratio (Figure 1C) significant homo- and hetero-oligomers are present. This is in contrast to the mixture of Aβ25–35 with CB[7] where very few oligomers are observed. The CCS data (Figures 2C and 4B, and Supporting Information, Figure S4 and Table S3) indicate the formation of extended Aβ25–35 homo-oligomers and Aβ25–35:PGG hetero-oligomers, which correlates with a conformational conversion from isotropic to β-sheet structures. It has been previously shown that the conformational transition of Aβ25–35 trimer and tetramer from native to β-sheet is a crucial step for Aβ25–35 fibril formation.31,47 Therefore we examined the ATDs of the mass spectral peaks corresponding to these oligomers in the presence of PGG. The ATD at 795 m/z for the pure Aβ25–35 sample displays only a compact trimer at ~0.51 ms (Figure 6A). However, the ATD of the same m/z in the Aβ25–35:PGG mixture shows two distinct features corresponding to a compact structure (at ~0.51 ms) and a new dominant feature for an extended trimer at ~0.58 ms (Figure 6B). For the Aβ25–35 tetramer (n/z = 4/3) at m/z = 1413, the ATDs show two features for both Aβ25–35 and Aβ25–35:PGG (Figure 6C, D), however, there is an increase in the population of the extended species when the ligand is added. The enhanced formation of extended Aβ trimers and tetramers in the presence of PGG unambiguously demonstrates that the ligand facilitates the conformational transition of Aβ25–35 oligomers from compact to extended. This result is very surprising and intriguing because PGG was originally thought to be an amyloid inhibitor.33,35 The TEM images (Figure 3D, E) of the same samples used in IMS-MS experiments show a concentration-dependent increase in the abundance of fibrillar Aβ25–35 aggregates in the presence of PGG. The fibrils in the mixed system show a twisted morphology, whereas the fibrils obtained from the pure Aβ25–35 sample showed a combination of straight and twisted morphologies. Lastly, the fibrils become more abundant in the presence of excess PGG (1:10 ratio), further suggesting that this ligand is not an aggregation inhibitor for Aβ25–35, but rather an aggregation accelerator.
Figure 6.
Representative arrival time distributions (ATDs) for m/z = 795 and m/z = 1413 of (A, C) pure Aβ25–35 and (B, D) Aβ25–35 incubated with PGG at 1:1 molar ratio.
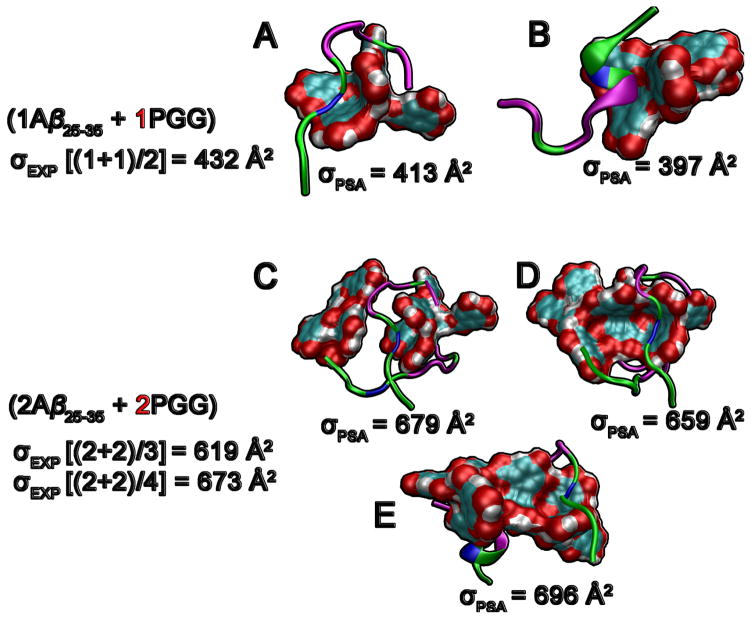
The MD simulations provide insight into Aβ25–35 behavior in the presence of PGG. Since PGG is a large polyphenol, Aβ25–35 is unable to wrap around this molecule like it could with EGCG (Figure 7).31 Unlike the case of CB[7], there are no specific binding sites between the ligand and peptide. The PGG core formed by one or more ligand molecules provides a template surface with both hydrophobic and hydrophilic sites to which one or more amphipathic Aβ25–35 peptides can weakly attach and undergo structural conversion. This observation from the MD simulations is consistent with the experimental detection of complexes made of multiple PGG molecules and Aβ25–35 in which the Aβ25–35 oligomers adopt extended conformations. Lastly, the theoretical cross sections of hetero-oligomers extracted from the simulation show good agreement with experimental data.
Figure 7.
Representative structures of Aβ25–35:PGG complexes obtained from simulations. (A, B) Hetero-dimers of one Aβ25–35 and one PGG. Aβ25–35 monomers adopt a wide range of structures from random coil to partial helix. (C, D, E) Hetero-tetramers of two Aβ25–35 and two PGG molecules. The PGG molecules are represented in filled surface with oxygens in red, carbon in cyan and hydrogen in white. The Aβ25–35 peptides are shown in cartoon representation where non-polar residues are shown in pink, charged residues in blue and polar residues in green.
Cucurbit[7]uril (CB[7]) overrides the effect of 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose (PGG) on Aβ25–35 amyloid aggregation
Interestingly, when fresh Aβ25–35 peptide is incubated together with both PGG and CB[7] at a 1:1:1 molar ratio, most Aβ25–35 homo-oligomers become depleted (Figures 1D and 2D) and Aβ25–35:CB[7] complexes dominate. No Aβ25–35:PGG complexes are found in the mass spectrum. Instead, two new peaks appear at m/z = 791 and m/z = 845, which are accounted for by the “triple” complexes formed from Aβ25–35, PGG and CB[7] molecules (i.e., (n+p+k)/z = (1+1+1)/4 and (2+1+1)/5), respectively).
The dominance of binary complexes between Aβ25–35 and CB[7] in the 1:1:1 mixture indicates that Aβ25–35 forms more stable complexes with CB[7] than with PGG. In addition, the formation of triple complexes between the peptide and the two ligands is intriguing. From the cross section data (Figure S5 and Table S4), the hetero-oligomer topologies are consistent with an isotropic conformation (Figure 4C). The data indicate that the strong inhibitory effect of CB[7] overrides the conformational conversion from isotropic to β-sheet structures and the multimeric Aβ25–35 homo-oligomer formation promoted by the polyphenol. In a good agreement with the IMS-MS results, the TEM imaging in the presence of both CB[7] and PGG (Figure 3F) shows the formation of globular aggregates, and very few short and irregular fibers (data not shown).
In a further experiment, CB[7] was added to an Aβ25–35 sample previously incubated with PGG (for 1 week at 1:1 ratio) to give final molar ratios of 1:1:5 and 1:1:10 of Aβ25–35:PGG:CB[7], and the samples were incubated an additional two days. The TEM images (Figure S6) indicate that addition of CB[7] to the aged Aβ25–35:PGG sample reduces the amount of fibrillar aggregates. Although some of the remaining fibers retain a typical amyloid fiber morphology, most exhibit some degree of irregularity, with regions that are thicker and more heavily stained. Indeed, the irregular fibers were similar to those few fibers that were observed when PGG and CB[7] were both present from the onset of aggregation (not shown). Collectively, these results demonstrate that CB[7] inhibits the assembly of fibrillar Aβ25–35 aggregates and also triggers their disassembly in a concentration-dependent manner.
SUMMARY and CONCLUSIONS
In the current study, IMS-MS data reveal distinctive effects of two assembly modulators, CB[7] and PGG, on the amyloid aggregation system, as well as on the structures of Aβ25–35 homo- and hetero-oligomers. The self-assembly of pure Aβ25–35 (Figures 1A, 2A) proceeds from compact monomers into β-rich oligomers emerging at the dimer and trimer stages. In the presence of CB[7], one and two CB[7] molecules selectively bind to Aβ25–35 monomer and dimer, respectively. The most probable binding site for CB[7] on Aβ25–35 is the lysine residue (Lys28). This observation expands our knowledge of the action mechanism of CB[7], since its inhibitory effect on β-amyloid fibrillation was previously understood to involve the capture of only aromatic residues: The hydrophilic rims of CB[7] created by oxygen atoms are a potential site to capture polar and charged residues, while the hydrophobic cavity hosts aromatic residues. These two possible host sites reveal the versatility of CB[7] as a possible novel therapeutic agent for AD. The IMS-MS data reveal that CB[7] suppresses Aβ25–35 aggregation by preventing homo-oligomer formation. Complex formation between CB[7] and Aβ25–35 monomers and dimers depletes the population of Aβ25–35 oligomers larger than dimer. Consequently, fewer fibrillar Aβ25–35 aggregates are found by TEM imaging at a 1:1 Aβ25–35:CB[7] molar ratio and aggregates are only very rarely observed at a 1:10 Aβ25–35:CB[7] ratio (Figure 3B, C).
In contrast to CB[7], PGG significantly enhances the formation of Aβ25–35 aggregates (Figures 1C, 2C and 4B). Complex formation between PGG and Aβ25–35 promotes extended Aβ25–35 conformations in both homo- and hetero-oligomers (Figures 6 and 7). Consequently, long fibrillar Aβ25–35 aggregates are found by TEM in the presence of PGG at a 1:1 Aβ25–35:PGG ratio, and these become remarkably more abundant with an increase in PGG molar ratio (1:10 of Aβ25–35:PGG) (Figure 3D, E). This is the first time a polyphenol has been shown to accelerate amyloid formation. These experimental results diverge from previous reports in the literature,33 which describe a strong inhibitory effect of PGG on the fibril formation of Aβ1–40 and Aβ1–42 (of which Aβ25–35 is a fragment). The chemical structure of PGG possesses both hydrophobic (aromatic rings) and hydrophilic (hydroxyl groups) sites for Aβ binding. In fact, both IMS-MS and MD data indicate that the binding of this ligand to the peptide is non-selective. The opposing effects of PGG on Aβ25–35 and full-length Aβ1–40/Aβ1–42 may arise from the difference in the conformations required for aggregation. Short peptides such as Aβ25–35 can aggregate when adopting extended conformations31,47 whereas the monomers of Aβ1–40/Aβ1–42 in the fibril state exist in strand-loop-strand conformations.66,67 Another possibility is that PGG suppresses fibril formation in Aβ1–40/Aβ1–42 by interacting with segments other than Aβ25–35. Aβ16–22 [KLVFFAE]68 could be a probable target for PGG as it contains an important hydrophobic stretch and aromatic residues that can interact with PGG through π-stacking.
Finally, when both CB[7] and PGG are simultaneously present during aggregation, the dominance of binary complexes of Aβ25–35 and CB[7] over Aβ25–35 and PGG indicates that the inhibitory effect of CB[7] overrides the stimulatory effect of PGG (Figures 1D, 2D and 4C). CB[7] competitively binds to not only Aβ25–35 homo-oligomers, but also hetero-Aβ25–35:PGG complexes, thereby suppressing the formation of large Aβ25–35 oligomers and eliminating the isotropic to β-rich structural conversion induced by PGG.
METHODS
A detailed description of materials and methods is given in Supporting Information (Section S1). The stock solutions were prepared as follows: Aβ25–35 and CB[7] samples were prepared in acidified water (0.1% (v/v) formic acid), whereas PGG was dissolved in water containing 20% methanol (v/v). These stock solutions were used for all experiments (pure Aβ25–35, Aβ25–35 with CB[7], Aβ25–35 with PGG and Aβ25–35 with PGG and CB[7] samples). For IMS-MS analysis, the samples were loaded into gold coated nano-ESI capillaries and electrosprayed in a home-built ion mobility spectrometry-mass spectrometer.69 The theoretical cross sections were calculated using the projected superposition approximation (PSA) method available at http://luschka.bic.ucsb.edu:8080/WebPSA/.70,71 For the TEM analyses, aliquots of the same sample solutions used in IMS-MS experiments were adsorbed onto mesh formvar/carbon copper grids and imaged on a JEOL 123 microscope with an ORCA camera and AMT Image Capture Software v. 5.24.
Supplementary Material
Acknowledgments
N.E.C.A. thanks Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for a post doctoral fellowship (204613/2014-0). We gratefully acknowledge support from the National Science Foundation (NSF) Grants CHE-1301032 (M.T.B.) and MCB-1158577 (J.-E.S.), the National Institutes of Health Grant 1RO1AG047116-01 (M.T.B.), the David and Lucile Packard Foundation (J.-E.S.), and a grant from Santa Barbara Cottage Hospital and the University of California, Santa Barbara (N.E.L). This work used the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by National Science Foundation Grant OCI-1053575. We acknowledge the Texas Advanced Computing Center (TACC) at the University of Texas at Austin for providing HPC resources through XSEDE Grant TG-MCA05S027 (J.- E.S.), and the NRI Microscopy Facility at UCSB. We acknowledge support from the Center for Scientific Computing at the CNSI and MRL via NSF MRSEC (DMR-1121053) and NSF Grant CNS-0960316.
ABREVIATIONS USED
- AD
Alzheimer’s disease
- Aβ
amyloid β-protein
- Aβ1–40
amyloid β-protein (1–40)
- Aβ1–42
amyloid β-protein (1–42)
- Aβ25–35
amyloid β-protein (25–35)
- Aβ16–22
amyloid β-protein (16–22)
- ATD
arrival time distribution
- CB[7]
cucurbit[7]uril
- CCS
collision cross sections
- EGCG
epigallocatechin gallate
- IAPP
islet amyloid polypeptide
- IMS
ion mobility spectrometry
- IMS-MS
ion mobility spectrometry coupled mass spectrometry
- Lys
lysines
- MD
molecular dynamics
- PGG
1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose
- tA
arrival time
- TEM
transmission electron microscopy
Footnotes
Author Contributions
N.E.C.A. and T.D.D. contributed equally.
Notes
The authors declare no competing financial interest.
Supporting Information. Detailed description of materials and methods, sample preparation, ion mobility spectrometry coupled mass spectrometry (IMS-MS), transmission electron microscopy (TEM), molecular dynamic (MD) simulations and cross section calculations. Mass spectra, arrival time distributions (ATDs) and TEM images of pure Aβ25–35 sample and in the presence of cucurbit[7]uril (CB[7]) and 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose (PGG). This information is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 2.Lee HG, Zhu XW, Castellani RJ, Nunomura A, Perry G, Smith MA. Amyloid-β in Alzheimer disease: the null versus the alternate hypotheses. J Pharm Exper Ther. 2007;321:823–829. doi: 10.1124/jpet.106.114009. [DOI] [PubMed] [Google Scholar]
- 3.Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov. 2011;10:698–U1600. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 4.Zaghi J, Goldenson B, Inayathullah M, Lossinsky AS, Masoumi A, Avagyan H, Mahanian M, Bernas M, Weinand M, Rosenthal MJ, Espinosa-Jeffrey A, de Vellis J, et al. Alzheimer disease macrophages shuttle amyloid-β from neurons to vessels, contributing to amyloid angiopathy. Acta Neuropathol. 2009;117:111–124. doi: 10.1007/s00401-008-0481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jakob-Roetne R, Jacobsen H. Alzheimer’s disease: from pathology to therapeutic approaches. Angew Chem Int Ed Engl. 2009;48:3030–3059. doi: 10.1002/anie.200802808. [DOI] [PubMed] [Google Scholar]
- 6.Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, et al. Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasegawa K, Yamaguchi I, Omata S, Gejyo F, Naiki H. Interaction between Aβ(1–42) and Aβ(1–40) in Alzheimer’s β-amyloid fibril formation in vitro. Biochemistry. 1999;38:15514–15521. doi: 10.1021/bi991161m. [DOI] [PubMed] [Google Scholar]
- 9.Jarrett JT, Berger EP, Lansbury PT. The carboxy terminus of the β-amyloid protein is critical for the seeding of amyloid formation - implications for the pathogenesis of Alzheimers-disease. Biochemistry. 1993;32:4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- 10.Kuperstein I, Broersen K, Benilova I, Rozenski J, Jonekheere W, Debulpaep M, Vandersteen A, Segers-Nolten I, Van der Werf K, Subramaniam V, Braeken D, Callewaert G, et al. Neurotoxicity of Alzheimer’s disease Aβ peptides is induced by small changes in the Aβ42 to Aβ40 ratio. EMBO J. 2010;29:3408–3420. doi: 10.1038/emboj.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 12.Chiti F, Dobson CM. Amyloid formation by globular proteins under native conditions. Nat Chem Biol. 2008;5:15–21. doi: 10.1038/nchembio.131. [DOI] [PubMed] [Google Scholar]
- 13.Krafft GA, Klein WL. ADDLs and the signaling web that leads to Alzheimer’s disease. Neuropharmacology. 2010;59:230–242. doi: 10.1016/j.neuropharm.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein SL, Dupuis NF, Lazo ND, Wyttenbach T, Condron MM, Bitan G, Teplow DB, Shea JE, Ruotolo BT, Robinson CV, Bowers MT. Amyloid-β protein oligomerization and the importance of tetramers and dodecamers in the aetiology of Alzheimer’s disease. Nat Chem. 2009;1:326–331. doi: 10.1038/nchem.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teplow DB, Lazo ND, Bitan G, Bernstein S, Wyttenbach T, Bowers MT, Baumketner A, Shea JE, Urbanc B, Cruz L, Borreguero J, Stanley HE. Elucidating amyloid β–protein folding and assembly: a multidisciplinary approach. Acc Chem Res. 2006;39:635–645. doi: 10.1021/ar050063s. [DOI] [PubMed] [Google Scholar]
- 16.Eisenberg D, Nelson R, Sawaya MR, Balbirnie M, Sambashivan S, Ivanova MI, Madsen AO, Riekel C. The structural biology of protein aggregation diseases: fundamental questions and some answers. Acc Chem Res. 2006;39:568–575. doi: 10.1021/ar0500618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenberg D, Jucker M. The amyloid state of proteins in human diseases. Cell. 2012;148:1188–1203. doi: 10.1016/j.cell.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ono K, Condron MM, Teplow DB. Structure-neurotoxicity relationships of amyloid β-protein oligomers. Proc Natl Acad Sci U S A. 2009;106:14745–14750. doi: 10.1073/pnas.0905127106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noguchi A, Matsumura S, Dezawa M, Tada M, Yanazawa M, Ito A, Akioka M, Kikuchi S, Sato M, Ideno S, Noda M, Fukunari A, et al. Isolation and characterization of patient-derived, toxic, high mass amyloid β-protein (Aβ) assembly from Alzheimer disease brains. J Biol Chem. 2009;284:32895–32905. doi: 10.1074/jbc.M109.000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Estrada LD, Soto C. Disrupting β-amyloid aggregation for Alzheimer disease treatment. Curr Top Med Chem. 2007;7:115–126. doi: 10.2174/156802607779318262. [DOI] [PubMed] [Google Scholar]
- 21.Bates KA, Verdile G, Li QX, Ames D, Hudson P, Masters CL, Martins RN. Clearance mechanisms of Alzheimer’s amyloid-β peptide: implications for therapeutic design and diagnostic tests. Mol Psychiatry. 2009;14:469–486. doi: 10.1038/mp.2008.96. [DOI] [PubMed] [Google Scholar]
- 22.Mason JM, Kokkoni N, Stott K, Doig AJ. Design strategies for anti-amyloid agents. Curr Opin Struc Biol. 2003;13:526–532. doi: 10.1016/s0959-440x(03)00100-3. [DOI] [PubMed] [Google Scholar]
- 23.Bartolini M, Andrisano V. Strategies for the inhibition of protein aggregation in human diseases. ChemBioChem. 2010;11:1018–1035. doi: 10.1002/cbic.200900666. [DOI] [PubMed] [Google Scholar]
- 24.Liu TY, Bitan G. Modulating self-assembly of amyloidogenic proteins as a therapeutic approach for neurodegenerative diseases: strategies and mechanisms. ChemMedChem. 2012;7:359–374. doi: 10.1002/cmdc.201100585. [DOI] [PubMed] [Google Scholar]
- 25.Feng BY, Toyama BH, Wille H, Colby DW, Collins SR, May BCH, Prusiner SB, Weissman J, Shoichet BK. Small-molecule aggregates inhibit amyloid polymerization. Nat Chem Biol. 2008;4:197–199. doi: 10.1038/nchembio.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed M, Davis J, Aucoin D, Sato T, Ahuja S, Aimoto S, Elliott JI, Nostrand WEV, Smith SO. Structural conversion of neurotoxic amyloid-β1–42 oligomers to fibrils. Nat Struct Mol Biol. 2010;17:561–567. doi: 10.1038/nsmb.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stroud JC, Liu C, Teng PK, Eisenberg D. Toxic fibrillar oligomers of amyloid-β have cross-β structure. Proc Natl Acad Sci U S A. 2012;109:7717–7722. doi: 10.1073/pnas.1203193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLaurin J, Kierstead ME, Brown ME, Hawkes CA, Lambermon MH, Phinney AL, Darabie AA, Cousins JE, French JE, Lan MF, Chen F, Wong SS, et al. Cyclohexanehexol inhibitors of Aβ aggregation prevent and reverse Alzheimer phenotype in a mouse model. Nat Med. 2006;12:801–808. doi: 10.1038/nm1423. [DOI] [PubMed] [Google Scholar]
- 29.Zheng X, Gessel MM, Wisniewski ML, Viswanathan K, Wright DL, Bahr BA, Bowers MT. Z-Phe-Ala-diazomethylketone (PADK) disrupts and remodels early oligomer states of the Alzheimer disease Aβ42 protein. J Biol Chem. 2012;287:6084–6088. doi: 10.1074/jbc.C111.328575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ehrnhoefer DE, Bieschke J, Boeddrich A, Herbst M, Masino L, Lurz R, Engemann S, Pastore A, Wanker EE. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat Struc Biol. 2008;15:558–566. doi: 10.1038/nsmb.1437. [DOI] [PubMed] [Google Scholar]
- 31.Bleiholder C, Do TD, Wu C, Economou NJ, Bernstein SS, Buratto SK, Shea JE, Bowers MT. Ion mobility spectrometry reveals the mechanism of amyloid formation of Aβ(25–35) and its modulation by inhibitors at the molecular level: epigallocatechin gallate and scyllo-inositol. J Am Chem Soc. 2013;135:16926–16937. doi: 10.1021/ja406197f. [DOI] [PubMed] [Google Scholar]
- 32.Zheng XY, Liu D, Klarner FG, Schrader T, Bitan G, Bowers MT. Amyloid β-protein assembly: the effect of molecular tweezers CLR01 and CLR03. J Phys Chem B. 2015;119:4831–4841. doi: 10.1021/acs.jpcb.5b00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujiwara H, Tabuchi M, Yamaguchi T, Iwasaki K, Furukawa K, Sekiguchi K, Ikarashi Y, Kudo Y, Higuchi M, Saido TC, Maeda S, Takashima A, et al. A traditional medicinal herb Paeonia suffruticosa and its active constituent 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose have potent anti-aggregation effects on Alzheimer’s amyloid β proteins in vitro and in vivo. J Neurochem. 2009;109:1648–1657. doi: 10.1111/j.1471-4159.2009.06069.x. [DOI] [PubMed] [Google Scholar]
- 34.Porat Y, Abramowitz A, Gazit E. Inhibition of amyloid fibril formation by polyphenols: Structural similarity and aromatic interactions as a common inhibition mechanism. Chem Biol Drug Des. 2006;67:27–37. doi: 10.1111/j.1747-0285.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 35.Bruno E, Pereira C, Roman KP, Takiguchi M, Kao PY, Nogaj LA, Moffet DA. IAPP aggregation and cellular toxicity are inhibited by 1,2,3,4,6-penta-O-galloyl-β-D-glucose. Amyloid. 2013;20:34–38. doi: 10.3109/13506129.2012.762761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palhano FL, Lee J, Grimster NP, Kelly JW. Toward the molecular mechanism(s) by which EGCG treatment remodels mature amyloid fibrils. J Am Chem Soc. 2013;135:7503–7510. doi: 10.1021/ja3115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bieschke J, Russ J, Friedrich RP, Ehrnhoefer DE, Wobst H, Neugebauer K, Wanker EE. EGCG remodels mature α-synuclein and amyloid-β fibrils and reduces cellular toxicity. Proc Natl Acad Sci USA. 2010;107:7710–7715. doi: 10.1073/pnas.0910723107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H, Raleigh DP. General amyloid inhibitors? A critical examination of the inhibition of IAPP amyloid formation by inositol stereoisomers. PLoS One. 2014;9:e104023. doi: 10.1371/journal.pone.0104023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu HJ, Li M, Liu GP, Geng J, Wang JZ, Ren JS, Zhao CQ, Qu XG. Metallosupramolecular complex targeting an α/β discordant stretch of amyloid β peptide. Chem Sci. 2012;3:3145–3153. [Google Scholar]
- 40.Lee HH, Choi TS, Lee SJC, Lee JW, Park J, Ko YH, Kim WJ, Kim K, Kim HI. Supramolecular inhibition of amyloid fibrillation by cucurbit[7]uril. Angew Chem Int Ed. 2014;53:7461–7465. doi: 10.1002/anie.201402496. [DOI] [PubMed] [Google Scholar]
- 41.Lopes DH, Attar A, Nair G, Hayden EY, Du Z, McDaniel K, Dutt S, Bravo-Rodriguez K, Mittal S, Klarner FG, Wang C, Sanchez-Garcia E, et al. Molecular tweezers inhibit islet amyloid polypeptide assembly and toxicity by a new mechanism. ACS Chem Biol. 2015;10:1555–1569. doi: 10.1021/acschembio.5b00146. [DOI] [PubMed] [Google Scholar]
- 42.Masson E, Ling XX, Joseph R, Kyeremeh-Mensah L, Lu XY. Cucurbituril chemistry: a tale of supramolecular success. RSC Adv. 2012;2:1213–1247. [Google Scholar]
- 43.Lee JW, Samal S, Selvapalam N, Kim HJ, Kim K. Cucurbituril homologues and derivatives: new opportunities in supramolecular chemistry. Acc Chem Res. 2003;36:621–630. doi: 10.1021/ar020254k. [DOI] [PubMed] [Google Scholar]
- 44.Kim J, Jung IS, Kim SY, Lee E, Kang JK, Sakamoto S, Yamaguchi K, Kim K. New cucurbituril homologues: syntheses, isolation, characterization, and X-ray crystal structures of cucurbit[n]uril (n=5, 7, and 8) J Am Chem Soc. 2000;122:540–541. [Google Scholar]
- 45.Bardelang D, Banaszak K, Karoui H, Rockenbauer A, Waite M, Udachin K, Ripmeester JA, Ratcliffe CI, Ouari O, Tordo P. Probing cucurbituril assemblies in water with TEMPO-like nitroxides: a trinitroxide supraradical with spin-spin interactions. J Am Chem Soc. 2009;131:5402–5404. doi: 10.1021/ja900306m. [DOI] [PubMed] [Google Scholar]
- 46.Lee JW, Lee HHL, Ko YH, Kim K, Kim HI. Deciphering the specific high-affinity binding of cucurbit[7]uril to amino acids in water. J Phys Chem B. 2015;119:4628–4636. doi: 10.1021/acs.jpcb.5b00743. [DOI] [PubMed] [Google Scholar]
- 47.Larini L, Shea JE. Role of β-hairpin formation in aggregation: the self-assembly of the amyloid-β(25–35) peptide. Biophys J. 2012;103:576–586. doi: 10.1016/j.bpj.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang Z, Luo Y, Zhang Y, Wei G. Interactions of Aβ25–35 β-barrel-like oligomers with anionic lipid bilayer and resulting membrane leakage: an all-atom molecular dynamics study. J Phys Chem B. 2011;115:1165–1174. doi: 10.1021/jp107558e. [DOI] [PubMed] [Google Scholar]
- 49.Yu X, Wang Q, Zheng J. Structural determination of Aβ25–35 micelles by molecular dynamics simulations. Biophys J. 2010;99:666–674. doi: 10.1016/j.bpj.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.D’Ursi AM, Armenante MR, Guerrini R, Salvadori S, Sorrentino G, Picone D. Solution structure of amyloid β-peptide (25–35) in different media. J Med Chem. 2004;47:4231–4238. doi: 10.1021/jm040773o. [DOI] [PubMed] [Google Scholar]
- 51.Wei GH, Jewett AI, Shea JE. Structural diversity of dimers of the Alzheimer amyloid-β(25–35) peptide and polymorphism of the resulting fibrils. Phys Chem Chem Phys. 2010;12:3622–3629. doi: 10.1039/c000755m. [DOI] [PubMed] [Google Scholar]
- 52.Kittner M, Knecht V. Disordered versus fibril-like amyloid β(25–35) dimers in water: structure and thermodynamics. J Phys Chem B. 2010;114:15288–15295. doi: 10.1021/jp1065264. [DOI] [PubMed] [Google Scholar]
- 53.Kohno T, Kobayashi K, Maeda T, Sato K, Takashima A. Three-dimensional structures of the amyloid β peptide (25–35) in membrane-mimicking environment. Biochemistry. 1996;35:16094–16104. doi: 10.1021/bi961598j. [DOI] [PubMed] [Google Scholar]
- 54.Pike CJ, Walencewiczwasserman AJ, Kosmoski J, Cribbs DH, Glabe CG, Cotman CW. Structure-activity analyses of β-amyloid peptides - contributions of the β25–35 region to aggregation and neurotoxicity. J Neurochem. 1995;64:253–265. doi: 10.1046/j.1471-4159.1995.64010253.x. [DOI] [PubMed] [Google Scholar]
- 55.Gessel MM, Bernstein S, Kemper M, Teplow DB, Bowers MT. Familial Alzheimer’s disease mutations differentially alter amyloid β-protein oligomerization. ACS Chem Neurosci. 2012;3:909–918. doi: 10.1021/cn300050d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bernstein SL, Wyttenbach T, Baumketner A, Shea JE, Bitan G, Teplow DB, Bowers MT. Amyloid β-protein: monomer structure and early aggregation states of Aβ42 and its Pro19 alloform. J Am Chem Soc. 2005;127:2075–2084. doi: 10.1021/ja044531p. [DOI] [PubMed] [Google Scholar]
- 57.Gessel MM, Wu C, Li H, Bitan G, Shea JE, Bowers MT. Aβ(39–42) modulates Aβ oligomerization but not fibril formation. Biochemistry. 2012;51:108–117. doi: 10.1021/bi201520b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Young LM, Saunders JC, Mahood RA, Revill CH, Foster RJ, Tu LH, Raleigh DP, Radford SE, Ashcroft AE. Screening and classifying small-molecule inhibitors of amyloid formation using ion mobility spectrometry-mass spectrometry. Nat Chem. 2015;7:73–81. doi: 10.1038/nchem.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee S, Zheng XY, Krishnamoorthy J, Savelieff MG, Park HM, Brender JR, Kim JH, Derrick JS, Kochi A, Lee HJ, Kim C, Ramamoorthy A, et al. Rational design of a structural framework with potential use to develop chemical reagents that target and modulate multiple facets of Alzheimer’s disease. J Am Chem Soc. 2014;136:299–310. doi: 10.1021/ja409801p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soper MT, DeToma AS, Hyung SJ, Lim MH, Ruotolo BT. Amyloid-β-neuropeptide interactions assessed by ion mobility-mass spectrometry. Phys Chem Chem Phys. 2013;15:8952–8961. doi: 10.1039/c3cp50721a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bleiholder C, Dupuis NF, Wyttenbach T, Bowers MT. Ion mobility-mass spectrometry reveals a conformational conversion from random assembly to β-sheet in amyloid fibril formation. Nat Chem. 2011;3:172–177. doi: 10.1038/nchem.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Do TD, Economou NJ, LaPointe NE, Kincannon WM, Bleiholder C, Feinstein SC, Teplow DB, Buratto SK, Bowers MT. Factors that drive peptide assembly and fibril formation: experimental and theoretical analysis of Sup35 NNQQNY mutants. J Phys Chem B. 2013;117:8436–8446. doi: 10.1021/jp4046287. [DOI] [PubMed] [Google Scholar]
- 63.Do TD, Economou NJ, Chamas A, Buratto SK, Shea JE, Bowers MT. Interactions between amyloid-β and Tau fragments promote aberrant aggregates: implications for amyloid toxicity. J Phys Chem B. 2014;118:11220–11230. doi: 10.1021/jp506258g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Attar A, Bitan G. Disrupting self-assembly and toxicity of amyloidogenic protein oligomers by “molecular tweezers” - from the test tube to animal models. Curr Pharm Des. 2014;20:2469–2483. doi: 10.2174/13816128113199990496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Acharya S, Safaie BM, Wongkongkathep P, Ivanova MI, Attar A, Klarner FG, Schrader T, Loo JA, Bitan G, Lapidus LJ. Molecular basis for preventing α-synuclein aggregation by a molecular tweezer. J Biol Chem. 2014;289:10727–10737. doi: 10.1074/jbc.M113.524520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luhrs T, Ritter C, Adrian M, Riek-Loher D, Bohrmann B, Doeli H, Schubert D, Riek R. 3D structure of Alzheimer’s amyloid-β(1–42) fibrils. Proc Natl Acad Sci U S A. 2005;102:17342–17347. doi: 10.1073/pnas.0506723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Petkova AT, Ishii Y, Balbach JJ, Antzutkin ON, Leapman RD, Delaglio F, Tycko R. A structural model for Alzheimer’s β-amyloid fibrils based on experimental constraints from solid state NMR. Proc Natl Acad Sci USA. 2002;99:16742–16747. doi: 10.1073/pnas.262663499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Balbach JJ, Ishii Y, Antzutkin ON, Leapman RD, Rizzo NW, Dyda F, Reed J, Tycko R. Amyloid fibril formation by Aβ16–22, a seven-residue fragment of the Alzheimer’s beta-amyloid peptide, and structural characterization by solid state NMR. Biochemistry. 2000;39:13748–13759. doi: 10.1021/bi0011330. [DOI] [PubMed] [Google Scholar]
- 69.Wyttenbach T, Kemper PR, Bowers MT. Design of a new electrospray ion mobility mass spectrometer. Int J Mass Spectrom. 2001;212:13–23. [Google Scholar]
- 70.Bleiholder C, Wyttenbach T, Bowers MT. A novel projection approximation algorithm for the fast and accurate computation of molecular collision cross sections (I). Method. Int J Mass Spectrom. 2011;308:1–10. [Google Scholar]
- 71.Bleiholder C, Contreras S, Do TD, Bowers MT. A novel projection approximation algorithm for the fast and accurate computation of molecular collision cross sections (II). Parameterization and application to biomolecules. Int J Mass Spectrom. 2013;345–347:89–96. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.