Summary
Exposure to a plethora of environmental challenges commonly triggers pathological type 2 cell-mediated inflammation. Here we report the pathological role of the Wnt antagonist Dickkopf-1 (Dkk-1) upon allergen challenge or non-healing parasitic infection. The increased circulating amounts of Dkk-1 polarized T cells to T helper 2 (Th2) cells, stimulating a marked simultaneous induction of the transcription factors c-Maf and Gata-3, mediated by the kinases p38 MAPK and SGK-1, resulting in Th2 cell cytokine production. Circulating Dkk-1 was primarily from platelets, and the increase of Dkk-1 resulted in formation of leukocyte-platelet aggregates (LPA) that facilitated leukocytes infiltration to the affected tissue. Functional inhibition of Dkk-1 impaired Th2 cell cytokine production and leukocyte infiltration, protecting mice from house dust mite (HDM)-induced asthma or Leishmania major infection. These results highlight that Dkk-1 from thrombocytes is an important regulator of leukocyte infiltration and polarization of immune responses in pathological type 2 cell-mediated inflammation.
Graphical Abstract
Introduction
The primary goal of immune responses is to eliminate the main trigger of inflammation, contributing to the structural and functional repair of the affected tissue. During the process, the key roles for circulating blood leukocytes are to migrate to sites of infection or injury and to develop polarized immune responses (Abbas and Janeway, 2000; Ley et al., 2007). The canonical Wnt signaling pathway which induces cell proliferation is utilized for tissue repair processes, and a Wnt antagonist may inhibit or delay such events in chronic inflammatory diseases (Whyte et al., 2012). Among the quintessential Wnt inhibitory ligands, Dickkopf-1 (Dkk-1) was originally found regulating head formation of Xenopus Laevis and is known to inhibit the canonical Wnt signaling pathway (Cruciat and Niehrs, 2013; Glinka et al., 1998). The inhibition of canonical Wnt pathway activation by Dkk-1 is achieved by its competitive binding of the receptor LRP (low density lipoprotein receptor)-5 and 6 complex with markedly higher affinity than its counterpart agonist Wnt3a (Cheng et al., 2011; Joiner et al., 2013).
Regarding its possible role in chronic inflammation, elevated Dkk-1 amounts in circulating blood have been reported in various types of cancers and bone diseases that are characterized by unhealed tissue lesions (Diarra et al., 2007; Sato et al., 2010; Tian et al., 2003). In addition, a previous study reports that the proliferation of intestinal epithelial cells in a DSS-colitis model is enhanced in Dkk-1 hypomorphic doubleridge mice (Dkk1d/d), suggesting an inhibitory role of Dkk-1 in wound repair in a pro-inflammatory microenvironment (Koch et al., 2011). A diverse array of environmental stimuli also causes tissue damage and they commonly trigger type 2 cell-mediated inflammation or immune responses both in acute and chronic inflammation (Pulendran and Artis, 2012; Whyte et al., 2012). Epithelial cell-derived factors (e.g., TSLP, IL-25, and IL-33) can induce type 2 cell-mediated immune responses (Divekar and Kita, 2015). Cytokines such as IL-4, IL-5, IL-10 and IL-13 are the key features of type 2 cell-mediated immune responses following T helper 2 (Th2) cell differentiation (Allen and Wynn, 2011; Pulendran and Artis, 2012).
The role of the canonical Wnt pathway in T cell biology including T cell development, CD8+ memory T cell formation, and regulatory T cell function has been demonstrated in multiple experimental systems with varying results, depending on the model systems used (Ding et al., 2008; Guo et al., 2007; van Loosdregt et al., 2013; Xie et al., 2005). While the role of canonical Wnt pathway components such as β-catenin or adenomatous polyposis coli (Apc) have been analyzed in these studies, the role of Wnt antagonists including Dkk-1 in the course of type 2 cell-mediated immune responses is poorly understood.
In recent years, the crucial role of platelets in inflammation has been widely emphasized (Morrell et al., 2014; Yeaman, 2014). Platelets are involved in coagulation upon various pathogenic challenges that both protect surrounding tissues from injury or invasion by microorganisms, and they also act as the reservoir of growth factors to recruit cells to repair tissue damage (Li et al., 2012). More recent studies show that platelets are important players in chronic inflammatory diseases by interacting with leukocytes in various pathological inflammatory conditions (Jenne and Kubes, 2015; Rondina and Garraud, 2014) and also selectively secreting immunomodulators to control T cell responses (Shi et al., 2014) and regulating leukocyte migration (Diacovo et al., 1996). It has been reported that activated platelets induce P-selectin expression, and it binds to P-selectin glycoprotein ligand (PSGL-1) in mature leukocytes to form leukocyte-platelet aggregates (Leon and Ardavin, 2008; Ley et al., 2007; Pitchford et al., 2005). Acute bacterial or viral infection can often lead to ITP (Idiopathic thrombocytopenic purpura) with low platelet counts with the destruction of platelets (Cines et al., 2014; Zhang et al., 2009).
Here we demonstrate that the Wnt antagonist Dkk-1 induced Th2 cell polarization in physiologic models of type 2 cell-mediated inflammation or mmune responses, and utilized MAPK and mTOR pathway components to achieve potent Th2 cytokine production. Platelets were primarily responsible for the circulating Dkk-1 and its concentration was elevated by allergen or non-healing parasite challenge. Dkk-1 promoted interaction between leukocytes and platelets, facilitating the migration of leukocytes to the affected tissue.
Results
Dkk-1 hypomorphic mice are protected from house dust mite-induced asthma
Th2-cell-mediated immune responses typically involve tissue damage and repair that requires the regulation of Wnt signaling (Pulendran and Artis, 2012; Whyte et al., 2012). We first explored the possibility that Dkk-1 functions as an immunomodulatory ligand in a standard model of house dust mite (HDM) extract allergen challenge, using the Dkk-1 hypomorphic doubleridge (Dkk1d/d) mouse strain in which Dkk-1 expression is reduced by 90%. We first characterized hematological features of doubleridge mice. Of note, CD4+ T cells from doubleridge mice showed comparable cytokine production upon activation to those from WT littermate controls and the splenic CD4+ T cell population did not show significant differences in their cell surface markers of activation (Supplementary Figure 1A-E). We also characterized platelets from Dkk1d/d mice and WT mice and determined that platelets from Dkk1d/d mice were functionally comparable to WT platelets (Supplementary Figure 2A-G). We confirmed the reduced amounts of Dkk-1 in plasma from doubleridge mice (Figure 1A) and challenged them with HDM extract (Figure 1B). CD45+ leukocytes including neutrophils, eosinophils, and CD4+ T cells were significantly increased in the lung and broncho-alveolar lavage (BAL) fluid of WT mice but this was substantially decreased in Dkk1d/d mice, suggesting that the reduced expression of Dkk-1 following allergen challenge inhibited the infiltration of leukocytes to the lung (Figure 1C, 1D, 1E). Th2 cytokines (IL-5, IL-10 and IL-13) were markedly induced in WT mice but notably decreased in Dkk1d/d mice in ex vivo stimulation of mediastinal lymph node (medLNs) cells with HDM allergen extract (Figure 1F). Hematoxylin and eosin (H&E) and periodic acid-Schiff base (PAS) staining and scoring of lung tissues also showed that Dkk1d/d mice had reduced inflammation and leukocyte infiltration compared to WT mice (Figure 1G, 1H and 1I). We also showed that airway resistance increase by HDM was notably decreased by using the Dkk-1 inhibitor, (1-(4-(naphthalen-2-yl)pyrimidin-2-yl)piperidin-4-yl)methanamine) (Pelletier et al., 2009) (WAY-262611) or in Dkk1d/d mice (Supplementary Figure 1F). Taken together, our results demonstrate that lack of Dkk-1 protects the host from chronic type 2 cell-mediated immune responses in the HDM-induced asthma model.
Figure 1. Reduced expression of Dkk-1 protects the host from house dust mite (HDM)-induced asthma.
(A) Circulating concentrations of Dkk-1 in eight-week-old female Dkk1d/d mice (n=5) and their wildtype (WT) littermate control mice (n=5) (B) A scheme of HDM challenge protocol (10 μg/mouse/time) (C, D) nine to twelve-week old Dkk1d/d (n=7) and their littermate controls (n=7) were analyzed. Total leukocytes (CD45+), neutrophils eosinophils from BAL fluid (BALF) and lung tissue homogenates were quantitated. (E) CD4+ T cells from lung tissue homogenates were quantitated. (F) Supernatants from mediastinal lymph node cells that were stimulated with HDM extract for 4 days were analyzed by ELISA. (G,H,I) Lung tissues from each mouse were scored after H&E and PAS staining. For F, G, and H, controls were challenged with PBS (n=3), and One-Way ANOVA analysis with Dunnet's post-hoc test was performed. Original magnification is 10×. Scale bar in lower right panel represents 10 μm. Small horizontal lines indicate the mean (± s.e.m.). A representative of two independent experiments is shown. Student's t-test was performed. ***, p<0.0005, **, p<0.005, *, p<0.05. See also supplementary Figures 1 and 2.
Functional inhibition of Dkk-1 protects mice from Leishmania (L.) major infection
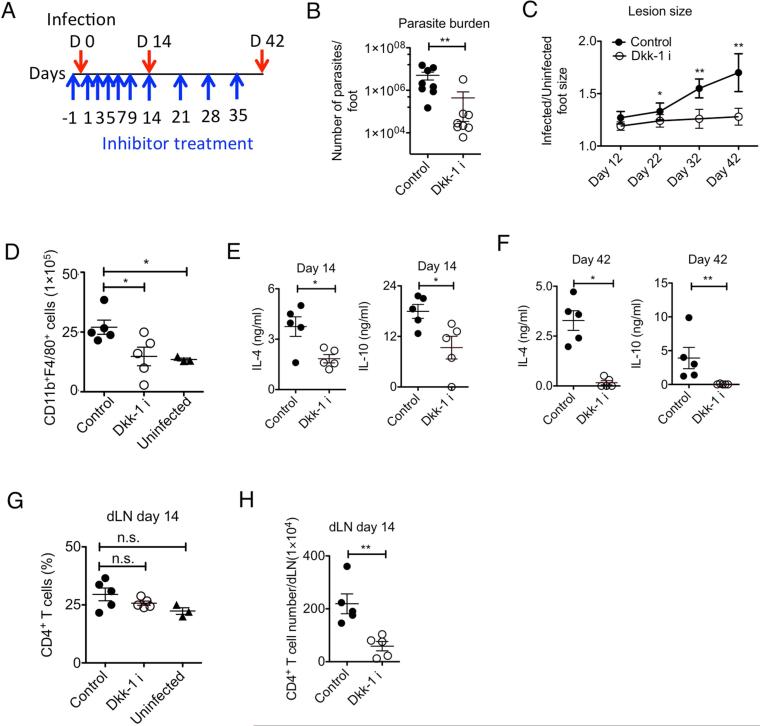
We further questioned whether functional inhibition of Dkk-1 could impair chronic inflammation by different types of environmental pathogens via a different route of challenge such as skin. Parasite infections challenge the immune system to temper its activity with highly evolved immune evasion strategies (Redpath et al., 2014). Infection with the parasite Leishmania (L.) major causes chronic skin lesion formation with unresolved inflammation by Th2 cell-mediated immune responses in a murine model (Belkaid et al., 2001; Tacchini-Cottier et al., 2012). Many inbred mouse strains such as C57BL/6, C3H, and CBA/J are genetically resistant to L. major and spontaneously resolve infection because they mount a protective Th1 cell-type response. In contrast, susceptible BALB/c mice develop large nonhealing chronic lesions and mount a Th2 cell-type response that is associated with the production of the cytokines IL-4 and IL-10 (Reiner and Locksley, 1995; Scott, 1991). We assessed whether the inhibition of Dkk-1 function impairs Th2 cell polarization, ameliorating the development of cutaneous leishmaniasis. A Dkk-1 inhibitor, WAY-262611 was administered intraperitoneally during the course of L. major infection (Figure 2A). Both lesion size and parasite burden were greatly reduced at 42 days post-infection, showing a 91% reduction of parasite survival in the Dkk-1 inhibitor-treated group (Figure 2B and 2C). Macrophage accumulation following parasite infection was also diminished by 14 days after infection (Figure 2D). IL-4 and IL-10 production was notably reduced by Dkk-1 inhibitor treatment at days 14 and 42 post-infection following ex vivo stimulation of draining lymph node cells from the infected mice with soluble leishmania antigen (sLMAG), suggesting that type 2 cell cytokine production was promoted by Dkk-1 (Figure 2E and 2F). It has been known that L.major infection induces CD4+ T cell recruitment and proliferation with lymph node expansion as well as Th2 cell differentiation (Carvalho et al., 2012; Hsu and Scott, 2007). Dkk-1 inhibitor treatment did not reduce the percentage of CD4+ T cells in draining lymph nodes (dLN) but reduced the number of CD4+ T cells in the dLN (Figure 2G and 2H), indicating that CD4+ T cell proliferation or recruitment is markedly reduced by Dkk-1 inhibition and this is concomitantly associated with reduced IL-4 and IL-10 production in draining lymph nodes. Taken together, these results suggest that different types and routes of environmental challenges induced elevation of circulating Dkk-1 to promote chronic tissue inflammation with robust Th2 cytokine production.
Figure 2. Functional inhibition of Dkk-1 protects the host from chronic inflammation caused by L. major.
(A) A schematic diagram for Dkk-1 inhibitor treatment (10 μg/kg/time) in six-week-old female BALB/c mice. (B) The infected foot from each mouse was analyzed for parasite burden at day 42 and (C) Lesion size was measured (n=8/group). (D) Two weeks after vehicle (n=5) Dkk-1 inhibitor treatment (n=5) based on (A), macrophages in the infected hindfoot were counted by flow cytometry and compared with infected (untreated) and uninfected BALB/c mice (n=3). (E, F) Two weeks and 6 weeks after infection, draining lymph node cells from each mouse (n=5/group) were stimulated with sLMAG. (G, H) CD4 T cell numbers and percentage in draining lymph nodes from each group was determined by flow cytometry. Small horizontal lines indicate the mean (± s.e.m.). A representative of two independent experiments is shown. Student's t-test (B, C, E-H) and One-Way-ANOVA analysis with Dunnet's post-hoc test (D,G) were performed. **, p<0.005, *, p<0.05.
Dkk-1 polarizes CD4+ T cells to Th2 cell lineage
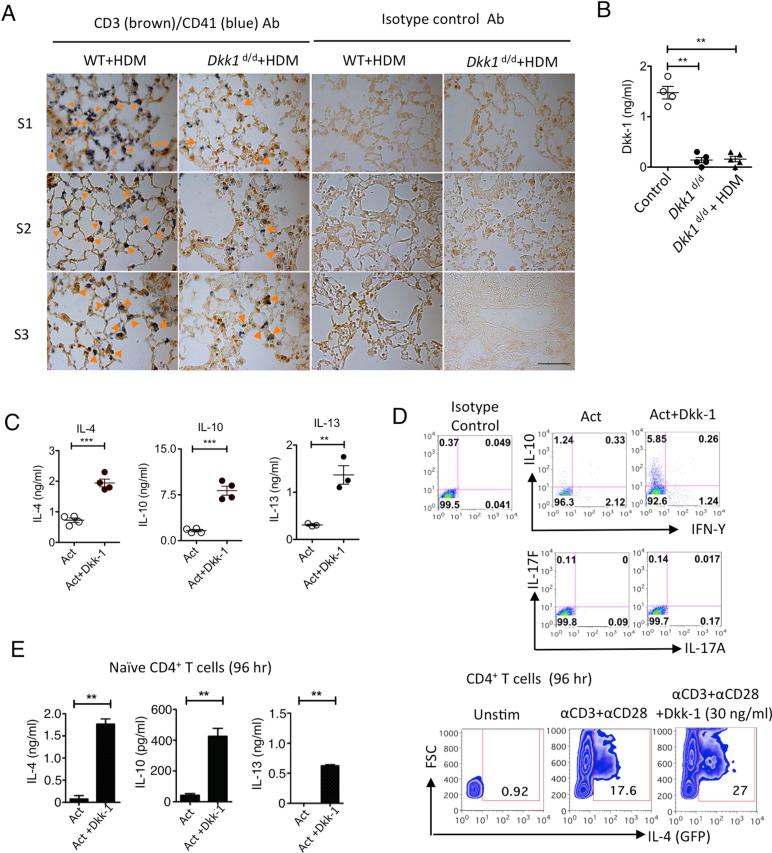
From the allergen exposure model, we observed that platelets and T cells were co-localized in the lung in WT mice after recurrent allergen challenges but this was reduced in Dkk1d/d mice (Figure 3A). This increased T cell infiltration in the lung in WT mice compared to Dkk1d/d mice was consistent with the increased CD4+ T cell numbers and the H&E score in the lung (Figure 1E and 1G). We further checked whether any residual Dkk-1 might be induced in Dkk1d/d mice, but we could not detect any elevation of Dkk-1 after HDM challenge in Dkk1d/d mice (Figure 3B). Next, we tested whether Dkk-1 induces Th2 cytokines from CD4+ T cells; Dkk-1 induced IL-4, IL-10 and IL-13 in total CD4+ T cell activation in vitro (Figure 3C). Other important effector T cell cytokines including Th1 and Th17–associated cytokines (e.g. IFN-γ, IL-17A and IL-17F) were not induced by Dkk-1, suggesting that Dkk-1 preferentially induces Th2 cytokines (Figure 3D). This induction of Th2 cytokines was also observed when naïve CD4+ T cells were activated with Dkk-1 in vitro (Figure 3E).
Figure 3. Dkk-1 induces Th2 cell polarization.
(A) Dkk1d/d mice and their wildtype littermate controls were challenged with HDM extract allergen shown in Figure 1B. Mice were sacrificed and lungs were harvested for immunohistochemistry for CD3 (dark brown), CD41 (blue) and isotype control antibodies. Orange arrowheads in the images indicate co-localized CD3+ T cells with CD41+ platelets. S1, S2, and S3 indicate individual animal in each group. Original magnification is 63×. Scale bar in lower right panel represents 10 μm. (B) Dkk1d/d mice (n=5) were challenged with 50 μg HDM extract per mouse and plasma samples were collected 24 hours later for ELISA. (C, D) Splenic CD4 T cells from 6-8 week-old C57BL/6 mice were activated with anti-CD3 and anti-CD28 antibodies (Act) with or without Dkk-1 (30 ng/ml unless indicated) for 4 days. Supernatants were analyzed by ELISA (C) and flow cytometry (D). For IL-4 in (D), splenic CD4 T cells from 8 week-old IL-4-GFP reporter mice were used. (E) Naïve CD4+ T cells were stimulated anti-CD3 and anti-CD28 antibodies for 96 hr with or without Dkk-1 for flow cytometry analysis and ELISA. Small horizontal lines and error bars indicate the mean (± s.e.m.). A representative of three independent experiments is shown. Student's t-test was performed. ***, p < 0.0001, **, p< 0.005, *, p<0.05.
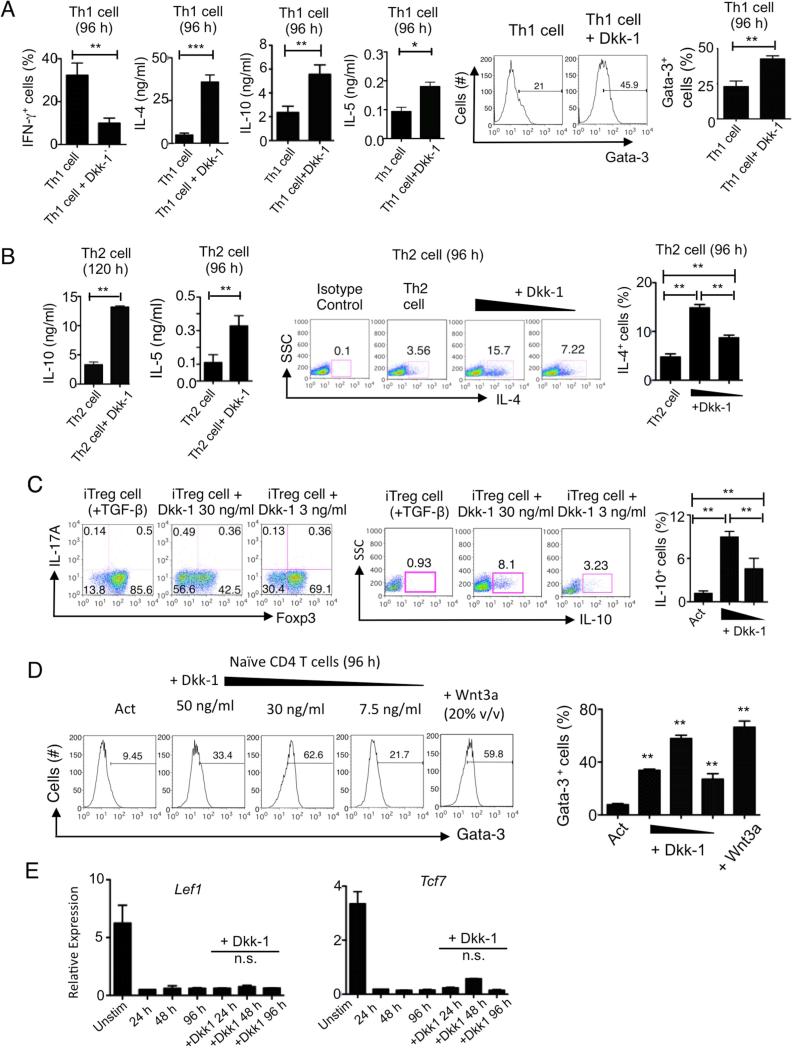
We further examined whether Dkk-1 could antagonize Th1 cell polarization. Dkk-1 suppressed IFN-γ expression under Th1 polarization conditions while still elevating Gata-3 expression and IL-4, IL-5, IL-10, IL-13 secretion, suggesting its strong ability to drive Th2 cell polarization (Figure 4A). Finally, Dkk-1 enhanced IL-4 expression in a dose-dependent manner and also potentiated IL-4, IL-5 and IL-10 production from cells undergoing Th2 cell differentiation (Figure 4B). We also observed that Dkk-1 could induce IL-10 in the presence of TGF-β while inhibiting TGF-β-mediated Foxp3 expression (Figure 4C). Of note, we tested the dose dependent response of Dkk-1 on Gata-3 expression and compared it with Wnt3a. Interestingly, up to 30 ng/ml of Dkk-1 could induce Gata-3 expression similar to Wnt3a; however at higher amounts, Dkk-1 inhibited Gata-3 expression (Figure 4D). It has been shown that the canonical Wnt pathway transcription factors such as Lef and TCF-1 (also known as Tcf7) act as repressors of Gata-3 transcription in naïve CD4+ T cells, and IL-4 inhibits TCF-1 expression (Hebenstreit et al., 2008; Maier et al., 2011). As previously reported, naïve CD4+ T cell activation downregulated Lef and T cell factor-1 (TCF-1) expression. Dkk-1 did not induce TCF-1 or Lef expression (Figure 4E). Collectively, these results show that Dkk-1 induces Th2 cell polarization and potentiate Th2 cell-type cytokine expression.
Figure 4. Dkk-1 induces Th2 cytokines in various T cell differentiation conditions.
(A,B) Naïve CD4 T cells were stimulated under Th1 (A), Th2 (B) polarization conditions for 96 hours with or without Dkk-1 for flow cytometry analysis and ELISA. (C) Splenic naïve CD4+ T cells were isolated and differentiated into iTreg for 5 days with 100 U/ml IL-2 and 1.5 ng/ml TGF-β with or without 30 ng/ml and 3 ng/ml of Dkk-1 was treated during the culture. Foxp3 and IL-17A expression (left panels) and IL-10 expression (right panels) were measured by flow cytometry. (D) Splenic naïve CD4+ T cells from 8 week-old C57BL/6 mice were treated with Dkk-1 with varying doses of Dkk-1 (50, 30, 7.5 ng/ml) or Wnt3a (20% v/v) for 96 h in the presence of anti-CD3 and anti-CD28 antibody stimulation to measure Gata-3 expression amounts. (E) Splenic naïve CD4+ T cells from 8 week-old C57BL/6 mice were stimulated with or without Dkk-1 (30 ng/ml) for 96 hr. RNA was harvested and analyzed by real time qPCR. All results were statistically non-significant. All experiments are a representative of two to three independent experiments. For (A), student's t-test was performed. For (B-E), One way-ANOVA analysis with Bonferonni's post-hoc test was performed. Small horizontal lines and error bars indicate the mean (± s.e.m.). ***, p < 0.0001, **, p< 0.005, *, p<0.05..
Dkk-1 utilizes SGK-1 and p38 MAPK to potentiate Th2 cell cytokine production
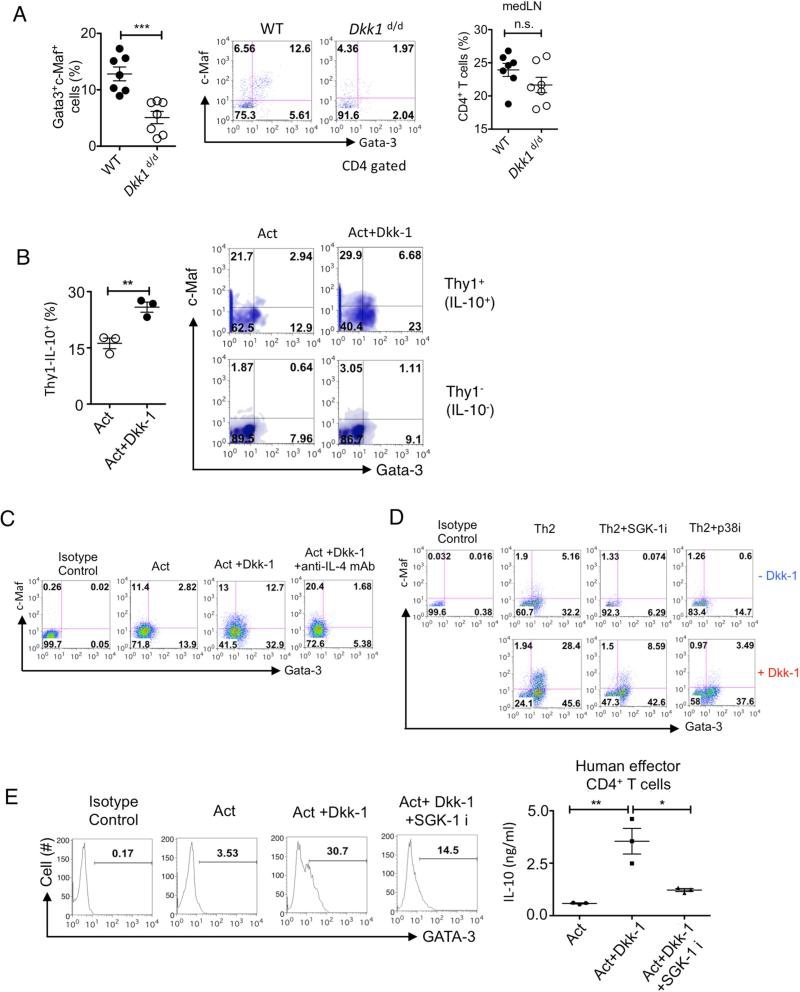
The Th2 differentiation program is mediated by Gata-3 and c-Maf (Farrar et al., 2001; Ho et al., 1996; Lee et al., 2001). Since Figure 4D shows that Dkk-1 could induce Gata-3 expression from naïve CD4+ T cells in a dose-dependent manner and hence Th2 cell-associated cytokines, we further investigated how Dkk-1 potentiated Th2 cell cytokine production. Recent studies show that helper T cell differentiation signaling is mediated by mTOR (mammalian or mechanistic target of rapamycin) and MAPK signaling pathways in response to diverse extracellular cues including Wnts (Chi, 2012; Heikamp et al., 2014). In vivo, a CD4+ T cell population in medLN that concomitantly expressed Th2 cell polarization transcription factors Gata-3 and c-Maf was significantly increased after ex vivo stimulation in WT mice after 2 weeks of recurrent allergen challenges. However, this population was only minimally present in Dkk1d/d mice (Figure 5A), suggesting the role of Dkk-1 in inducing c-Maf. The decrease of Th2 cytokines and the Gata-3+c-Maf+ CD4+ T cell population in Dkk1d/d mice was not related to a decreased percentage of CD4+ T cells in the medLNs (Figure 5A, right panel).
Figure 5. Dkk-1 directs TH2 cell polarization by p38 MAPK and SGK-1 to induce c-Maf and Gata-3.
(A) Mediastinal lymph node cells from Figure 1D were stimulated with HDM extract for 4 days and the percentages of Gata-3+ and c-Maf+ in CD4+ T cells were quantitated by flow cytometry. (B) Splenic CD4+ T cells from 12-week old Thy1-IL-10 reporter mice were activated with or without Dkk-1 (30 ng/ml) for 84 hr. Cells were gated for Thy1+ and Thy1−. (C) Splenic CD4+ T cells were activated with or without Dkk-1 or anti-IL-4 mAb (10 μg/ml) for 4 days and analyzed by flow cytometry. (D) Naïve CD4+ T cells were stimulated under Th2 polarization conditions for 96 hr with or without Dkk-1 for flow cytometry analysis. SGK-1 inhibitor (2 μM) or p38 inhibitor (10 μM) was added at 0 h. (E) PBMCs from healthy volunteers were used to isolate human CD4+ T cells, and then stimulated with human anti-CD3 mAb (OKT3) and anti-CD28 mAb for 4 days with or without Dkk-1 (30 ng/ml) and SGK-1 inhibitor GSK653094 (2 μM). Cells were analyzed by flow cytometry. Supernatants were harvested and analyzed by ELISA (right panel). A representative of three independent experiments is shown. Student's t-test was performed. ***, p < 0.0001, **, p< 0.005, *, p<0.05, n.s., not significant. For (E), One-way ANOVA analysis with Bonferroni's post-hoc test was performed. See also supplementary Figure 3.
We observed that Dkk-1 increased the Gata-3+ and c-Maf+ CD4+ T cell population in vitro, and it increased the expression of IL-10 (Figure 5B). Blockade of IL-4R inhibited the expression of Gata-3 but not c-Maf, revealing that Dkk-1 can induce c-Maf independently of IL-4 signaling (Figure 5C). In the absence of Dkk-1, Th2 cell polarization with exogenous IL-4 was mainly induced by Gata-3, and both p38 MAPK and SGK-1 inhibition reduced Gata-3 expression (Figure 5D, upper panels). The addition of Dkk-1 induced both Gata-3 and c-Maf expressions were markedly. The Inhibition of p38 MAPK regulated c-Maf induction, while SGK (serum glucocorticoid kinase)-1 inhibition primarily reduced Gata-3 expression in c-Maf+Gata-3+ population (Figure 5D, lower panels). The marked potentiation of IL-10 secretion by Dkk-1 in Th2 cell polarization conditions was blocked either by SGK-1 or p38 MAPK inhibition, confirming that both kinases are required for Dkk-1-mediated Th2 cytokine secretion (Supplementary Figure 3A). Although c-Maf has been known to be predominantly involved in IL-4 expression (Kim et al., 1999), our results suggest that Dkk-1 could potentiate other Th2 cell-type cytokines such as IL-10 production in a c-Maf-dependent manner. Based on high homology of the Dkk-1 amino acid sequence (>90%) between mice and humans, human CD4+ T cells were treated with mouse Dkk-1 with or without SGK-1 inhibitor. The inhibition of SGK-1 substantially reduced Gata-3 and IL-10 secretion (Figure 5E). Inhibition of SGK-1 expression by lentiviral shRNA transduction also diminished IL-10 induction by Dkk-1 in human CD4+ T cells (Supplementary Figure 3B). Unlike the SGK-1 inhibition, the phosphorylation of S6 kinase or 4E-BP-1 was not altered by Dkk-1, indicating that the mTORC1 pathway was not affected by Dkk-1 (Supplementary Figures 3C and 3D). In addition, we found that Stat6 was also required in Dkk-1-driven Th2 cell polarization (Supplementary Figures 3E, 3F and 3G). These data, together with inhibition of Gata-3 expression (by IL-4R blocking antibody) demonstrated in Figure 4C, suggests that Dkk-1 promotes IL-4 induction, and naïve CD4+ T cells-derived IL-4 suppresses TCF-1 and/or Lef expression. Thus, our results shows that Dkk-1 utilizes p38 MAPK and SGK-1 to potentiate Th2 cell polarization by increasing expression of c-Maf and Gata-3, respectively.
Dkk-1 is selectively secreted from platelets upon allergen challenge or parasite infection
Based on the pro-inflammatory effect of Dkk-1 in the HDM-induced asthma and the L. major infection model, we investigated the primary source of Dkk-1 in HDM-induced asthma. Initially, we found a marked increase of circulating amounts of Dkk-1 following recurrent allergen challenges (Figure 6A). We further tested whether a single intranasal allergen challenge could induce the elevation of circulating Dkk-1, and found that Dkk-1 was readily elevated in plasma of C57BL/6 mice (Figure 6B). This increase of Dkk-1 was also detected in the infiltrated lungs from WT littermate control mice while it was markedly reduced in Dkk1d/d mice, suggesting that the elevation of Dkk-1 does occur both locally and systemically (Figure 6C). It has been implied that an increase of circulating Dkk-1 in multiple myeloma might be due to platelets (Trikalinos et al., 2009). While we were collecting plasma from C57BL/6 mice, we observed high Dkk-1 amounts even in the homeostatic conditions when samples were collected without anti-coagulant by cardiac puncture (Figure 6D). Since platelets sense mechanical shear stress by aggregating to form clots and release soluble mediators (Butler, 1995; McGrath et al., 2011), we questioned whether the elevated amounts of Dkk-1 are released from platelets. We depleted platelets in intact C57BL/6 mice, and found that depletion of platelets abolished circulating amounts of Dkk-1 in 12 hours (Figure 6E). This marked reduction of Dkk-1 by platelet depletion was also observed with HDM allergen challenge (as in Figure 1B), suggesting that the primary circulating source of Dkk-1 in during both homeostatic and allergen-challenged conditions is platelets (Figure 6F). Next, we investigated whether the elevation of Dkk-1 by HDM is a specific response to the allergen. To this end, we challenged various types of compounds known as Pattern-Associated Molecular Patterns (PAMPs) intranasally. Unlike the PAMPs that were tested, HDM could uniquely increase Dkk-1 in circulating blood (Figure 6G).
Figure 6. Circulating Dkk-1 is from platelets upon allergen challenge or parasitic infection.
(A) Female C57BL/6 mice were challenged with PBS (n=3) or HDM extract (10 μg/challenge/mouse, n=5) as in Figure 1B. (B) Eight-week old female C57BL/6 mice were challenged with HDM extract (30 μg/challenge, n=4) or PBS (n=5) for 4 and 24 hours. Plasma samples were analyzed by ELISA. (C) Dkk1d/d mice (n=7) and their wildtype littermate controls (n=7) were challenged with HDM extract as in Figure 1B. Harvested lungs were analyzed by immunohistochemistry. Original magnification is 10×. Scale bar in lower right panel represents 10 μm. (D) Peripheral blood was collected by cardiac puncture with or without EDTA. (E) Eight-week-old female C57BL/6 mice were injected with platelet depletion antibody (n= 5) or isotype control antibody (n=4). Twenty-four hours later, plasma samples were measured. (F) HDM allergen was challenged as in Figure 1B, and platelets were depleted for 12 hours. (G) Bacterial ds DNA from E.coli (B-ds DNA)(30 μg/mouse,n=4), LPS (3 μg/mouse, n=5), CpG-ODN 1585 (25 μg/mouse, n=4), and House Dust Mite (HDM) allergen extract (50 μg/mouse, n=4) were challenged intranasally in 6 week-old C57BL/6 mice. Twenty-four hours later, plasma samples were collected and Dkk1 concentrations were determined by ELISA. Control (n= 7) mice were given 20 μl of 0.9% NaCl saline. (H) At each time point plasma from female BALB/c mice (6-week old) that were infected with L. major was measured. (I) Platelets were depleted 4 hours prior to parasite infection. Plasma samples from 5-week old female BALB/c mice were analyzed 72 hours after infection. (J) Ten weeks after infection, platelets were depleted for 12 hours in BALB/c mice. (K) Human platelets from four healthy volunteers were activated with sLMAG for 1 hr. S1-S4 designates each healthy donor. X-axis shows dilution of sLMAG in the culture. 1:50 is equivalent to 1×106 parasites. (L) Human platelets (1×108/ml, n=5) were activated with sLMAG (1:50) in the presence of PKCα inhibitor, or PKCβ inhibitor for 1 hour. A representative of two independent experiments is shown. Small horizontal lines indicate the mean (± s.e.m.). Student's t-test (A, D, E), One-way ANOVA with Dunnett's post-hoc test (B, I) and One-way ANOVA with Bonferroni's post-hoc test (F,G,H,J,L) was performed. ***, p< 0.0005, **, p<0.005, *, p<0.05. See also Supplementary Figure 4.
We further checked whether systemic concentrations of Dkk-1 were increased by different types of environmental pathogens via skin to promote Th2 responses. Subcutaneous infection of the parasite, L. major, in the hindfoot of BALB/c mice increased circulating amounts of Dkk-1 which persisted 42 days post-infection (Figure 6H). Upon parasitic infection, depletion of platelets using platelet-specific anti-mouse GPIbα (CD42b) antibody markedly ablated elevation of circulating Dkk-1 amounts, even below homeostatic amounts of Dkk-1 in 72 hours (Figure 6I). At 10 weeks after infection, we showed that Dkk-1 is still maintained at a high concentration, and platelet depletion resulted in the complete loss of Dkk-1 in 12 hours, suggesting that platelets are continuously secreting Dkk-1 (Figure 6J). Activation of human platelets with sLMAG induced Dkk-1 within 1 hour in a dose-dependent manner (Figure 6K). Inhibition of PKCα (Figure 6L) blocked secretion of Dkk-1 upon sLMAG stimulation of human platelets, confirming that activated platelets secrete Dkk-1 from α-granules (Moncada de la Rosa et al., 2013). In contrast, stimulation of human peripheral blood mononuclear cells (PBMCs) or vascular endothelial cells with sLMAG or HDM extract failed to induce secretion of Dkk-1 (Supplementary Figure 4). Taken together, our results suggest that Dkk-1 is primarily secreted from platelets upon allergen challenge or non-healing parasite infection, promoting type 2 cell-mediated immune responses.
Dkk-1 facilitates leukocyte infiltration and plays an important role in chronic type 2 cell-mediated inflammation
Given that the source of circulating Dkk-1 was platelets and Dkk1d/d mice showed reduced leukocyte infiltration in the lung, we considered the possibility that Dkk-1 may regulate leukocyte-platelet interactions and leukocyte migration into the lung upon HDM extract challenge. LPA (leukocyte-platelet aggregate) formation in human and WT mouse peripheral blood has been considered as a prerequisite for the increases of infiltrating immune cells to the affected tissues to develop immune responses (Ley et al., 2007; Li et al., 1997; Pitchford et al., 2005; Tamagawa-Mineoka et al., 2007; Zarbock et al., 2006). We observed that infiltration of CD45+ immune cells into the lung was significantly increased in WT mice but this was impaired in Dkk1d/d mice at 72 h following a single allergen challenge (Figure 7A). Consistent with the marked decrease of leukocyte migration into the lung, a single challenge of allergen induced elevation of LPA (CD45+CD41+ population) in the peripheral blood of WT mice at 4 and 24 hours from the steady state amounts but not in Dkk1d/d mice (Figure 7B and supplementary Figure 5A, 5B). Pretreatment with Dkk-1 inhibitor 24 hours prior to infection with the parasite L. major similarly reduced the elevation of LPA formation at 4 h post-infection (Supplementary Figure 5C). We further tested whether exogenous Dkk-1 could induce LPA formation and increase leukocyte infiltration in the lung. Exogenous administration of Dkk-1 did not increase the number of infiltrated leukocytes in the lung 72 hours after Dkk-1 protein administration (Figure 7C) although increased LPA formation was observed without allergen or parasite challenge (Supplementary Figure 5D). Combined with results from Figure 7A, this suggests that infiltration of leukocytes to the lung requires both Dkk-1 and further innate immune responses for leukocyte migration upon allergen challenge. In addition, in contrast to WT mice, we observed that leukocytes from peripheral blood in Dkk1d/d mice failed to sustain the elevation of PSGL-1 expression and its ligand P-selectin on platelets upon HDM allergen challenges. Similarly, inhibition of Dkk-1 in L. major infection blocked the increase of PSGL-1 expression (Supplementary Figure 5E-5G).
Figure 7. Dkk-1 facilitates leukocyte migration and regulates leukocyte-platelet aggregate formation in type 2 cell-mediated inflammation.
(A) Nine-week old Dkk1d/d mice and wildtype littermate controls (n=5-6/group) were challenged with 30 μg HDM allergen extract intranasally. Lung homogenates were quantitated and analyzed by flow cytometry 72 hours after HDM challenge and CD45+ cells were analyzed by flow cytometry. (B) Peripheral blood from nine-week old Dkk1d/d mice and wildtype littermate controls were collected at 4 and 24 hours after allergen challenge, and analyzed for the percentages of CD45+CD41+ cells. (C) Dkk-1 protein (300 ng) was injected intraperitoneally. CD45 cells were analyzed by flow cytometry. NE, neutrophils, EO, eosinophils. (D) Schematic diagram of Dkk-1 inhibitor treatment protocol for each group (n=5 for each group) in HDM-induced asthma model. HDM allergen extract (10 μg) was challenged intranasally at the indicated time points. CD45+ leukocytes numbers in BALF and Lung (E), neutrophils, eosinophils, and CD4 T cell numbers in the lungs (F) and the number of CD4+ T cells in mediastinal LNs were counted (G). After 4 days of stimulation of med LN cells, cytokines were measured by ELISA (H). H&E and PAS staining were scored (I, J). ET, early treatment; LT, late treatment; FT, full treatment; PC, positive control. Small horizontal lines and error bars indicate the mean (± s.e.m.). A representative of two independent experiments is shown. One-way ANOVA analysis with Dunnet's post-hoc test was performed. ***, p < 0.0005, **, p< 0.005, *, p<0.05, n.s., not significant. See also Supplementary Figures 5 and 6.
Next, we evaluated the importance of Dkk-1 either at the challenge stage or at the sensitization stage in the HDM-induced asthma model. To this end, we treated Dkk-1 inhibitor either only at the challenge stage (ET, early treatment) or at the sensitization stage (LT, late treatment) (Figure 7D). Leukocyte infiltration including CD4+ T cells, neutrophils, and eosinophils was all markedly decreased in BAL fluid and lung tissues in both the ET and LT treatment protocols (Figure 7E, 7F, and supplementary Figure 6A). The number of CD4+ T cells was also reduced in mediastinal lymph nodes by inhibiting Dkk-1, but the percentage of CD4+ T cells in the lymph nodes did not change (Figure 7G, supplementary Figure 6B). Th2 cytokine secretion from mediastinal lymph nodes was diminished (Figure 7H). Consistent with these results, histopathological scores also showed that the functional inhibition of Dkk-1 in either the challenge stage or the sensitization stage resulted in inhibition of type 2 cell inflammatory responses in HDM-induced asthma model (Figure 7I and 7J, supplementary Figure 6C). Taken together, our results suggest that Dkk-1 plays an important proinflammatory role for Th2 cell differentiation and leukocyte migration in each stage of HDM allergen challenge to promote chronic type 2 cell-mediated inflammation.
Discussion
The increased amounts of Dkk-1 in peripheral blood have been reported in chronic inflammatory diseases such as various types of cancers, rheumatoid arthritis and lupus (Diarra et al., 2007; Sato et al., 2010; Wang et al., 2014), and the role of Dkk-1 has been studied as a Wnt antagonist in various model systems. Our results demonstrate an important immunomodulatory role of Dkk-1 in type 2 cell-mediated immune responses caused by environmental challenges.
Although multiple roles of the canonical Wnt signaling pathway have been studied in the context of intracellular mediators such as Apc or β-catenin in T cells and thymocytes (Gounari et al., 2005; Guo et al., 2007; Staal et al., 2008), it is unknown whether Dkk-1 has a role in innate and adaptive immunological responses. Our data show that Dkk-1 utilizes the MAPK and mTOR signaling pathway components to induce type 2 cell-mediated immune responses and/or inflammation upon allergen or non-healing parasite infection.
A previous study has addressed the role of the canonical Wnt pathway that is mediated by β-catenin in Th2 cell differentiation (Notani et al., 2010), focusing on early progenitor T cells (e.g., thymocytes and naïve CD4 T cells) in vitro. The prediction based on these in vitro studies utilizing high concentrations of Dkk-1 or Wnt3a would have been inhibition of Th2-cell differentiation by Dkk-1, and hence the inhibition of HDM-induced asthma or L. major infection. However, we have shown that the function of Dkk-1 in vivo at physiological concentrations is, in fact, the opposite and that Dkk-1 potentiates Th2 cell polarization. Physiologic concentrations of Dkk-1 (up to 30 ng/ml) in fact promote type 2 cell-mediated inflammation and Th2 cell differentiation primarily through SGK-1 and p38 MAPK. Of note, we also observed Wnt3a induced Gata-3 expression and non-physiologic high concentrations of Dkk-1 (>50 ng/ml) inhibited Gata-3 expression similar to the previous report. There are signaling pathways identified which Dkk-1 utilizes other than the well-known canonical Wnt pathway (Fukuda et al., 2010; Krause et al., 2014). It is notable that Dkk-1 has a colipase domain in its carboxy-terminal region that is responsible for Wnt antagonist activity, but the function and role of the N-terminal domain of Dkk-1 is largely unknown (Brott and Sokol, 2002). More detailed characterization of Dkk-1 molecular structure affecting Th2 cell differentiation is warranted in the future. Collectively, our findings suggest that physiological concentrations of Dkk-1 utilized SGK-1 and p38 MAPK under type 2 cell-mediated immune responses or inflammation.
Recent studies point out the specificity of platelet activation and its immunomodulatory role in addition to its traditional role in coagulation events and hemostasis (Rondina and Garraud, 2014). Platelets do express a variety of receptors for PAMPs, and recent studies identify more receptors and ligands that are expressed in immune cells (Semple et al., 2011). We demonstrated that platelets are an important source of Dkk-1 in our models and the release of Dkk-1 is specific to environmental challenges that lead to chronic type 2 cell-mediated immune responses. Platelets are the most abundant source of TGF-β (Kim et al., 2013). Although the release of TGF-β could be assumed in platelet activation and thus further assumed to increase Foxp3+ CD4+ T cells, it should be noted that platelets also possess ligands such as PF4 (CXCL4) which is abundantly present and can inhibit TGF-β-cell-mediated T cell differentiation (Shi et al., 2014). The inhibition of TGF-β-cell-mediated Foxp3 expression by Dkk-1 suggests that the immunosuppressive function of TGF-β in a given microenvironment would be potently inhibited, and hence Dkk-1 would be able to generate robust Th2 cytokines. Together with the inhibitory role of Dkk-1 on TGF-β-cell-mediated Foxp3+ T cell differentiation, it is unlikely that TGF-β would affect T cell differentiation in our models. Additionally, we did not observe any increased Foxp3+ T cells in doubleridge mice, confirming that platelet activation and subsequent Dkk-1 release from platelets is specific to a given environmental challenge.
Our findings may suggest that immunomodulation of Dkk-1 from platelets is an example of the development of the mammalian immune system from the hemocyte system that exists in Drosophila (Lavine and Strand, 2002; Wood and Jacinto, 2007). Our results from exogenous Dkk-1 injection and HDM-induced asthma and Leishmania major infection model suggest that platelet activation and subsequent release of Dkk-1 facilitates leukocytes migration to the site of environmental challenges, and this should be coordinated with the recognition of such challenges by innate immune cells. Since hemocytes play both roles of platelets and innate immune cells, our results may show the specialized role of platelets and leukocytes to coordinate type 2 cell-mediated immune responses in mammals. It is notable that the potent induction of IL-10 by Dkk-1 in the L. major infection model demonstrates that the parasite utilizes Dkk-1 as a strategy to reconcile immune-activation and immunosuppression to favor parasite survival, avoiding complete eradication and maintaining lesions.
Taken together, our findings highlight the importance of the orchestrating role of Dkk-1 from platelets to facilitate leukocyte migration and further to polarize immune responses by inducing Th2 cell polarization, placing Dkk-1 as a highly attractive target for controlling type 2 cell-mediated immune responses.
Experimental Procedures
Mice
C57BL/6J were purchased from the Jackson Laboratory and have been bred in our mouse facility. BALB/c mice were purchased from NCI. The animals were kept under normal light and and dark cycle (12/12). Doubleridge mice (Dkk1d/d) were kindly provided by Asma Nusrat (Emory University). Foxp3-IRES-RFP mice and Stat6-deficient mice were bred in our mouse facility. Thy1-IRES-IL-10 reporter mice were provided by Casey Weaver (University of Alabama). IL-4-GFP reporter (4Get) mice on the BALB/c background were kindly provided by Ruslan Medzhitov (Yale University). All mouse protocols were approved by the Yale University Institutional Animal Care and Use Committee (IACUC) in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC).
Flow cytometry, cell culture, cytokine ELISA, Immunohistochemistry
For CD4 T cell differentiation, naïve CD4 T cells (CD4+CD62LhiCD44loFoxp3(RFP)-) were isolated from 6-7 week-old Foxp3-IRES-RFP mice. For Th1 cell differentiation, cells were activated with plate bound anti-CD3 (2 μg/ml), anti-CD28 (2 μg/ml) in the presence of IL-2 (25 U/ml), and IL-12 (20 ng/ml) for 96 hours in RPMI-1640 media (5% FBS, 1% penicillin/streptomycin and 10 mM HEPES). For Th2 cell differentiation, cells were activated with plate bound anti-CD3 (2 μg/ml), anti-CD28 (2 μg/ml) in the presence of IL-2 (25U/ml), anti-IFN-γ (XMG1.2) mAb (10 μg/ml), and IL-4 (100 U/ml) for 96 hours. For the isolation of CD4 T cells, spleens from 6-7 week-old male Foxp3-IRES-RFP mice were prepared as single cell suspensions. After RBC (red blood cell) lysis, cells were stained with CD16/CD32 FcR (Fc Receptor) blocking antibody (clone 2.4G2). For intracellular staining, cells were stimulated with cell stimulation cocktail with protein transport inhibitors (eBioscience) for 5 hr. Intracellular staining was performed as described in the manufacturer's protocol (eBioscience). FACS Calibur or Stratedigm (BD Biosciences) was used for flow cytometry and data were analyzed by FlowJo software (Treestar). Human and Mouse Dkk-1 ELISA kits were purchased from R&D systems. The human IL-10 ELISA, mouse Th1 and Th2 cytokine panel ELISA kit, and mouse IL-5/IL-13 ELISA kit were purchased from eBioscience. Cytokines were measured by ELISA according to the manufacturer's protocol.
House dust mite asthma model
House dust mite extract (Dermatophagoides pteronyssinus; Greer Labs) (10 μg) was dissolved in 20 μl of PBS for each mouse per intranasal challenge. Six to ten week old doubleridge mice and their littermate controls were challenged intranasally on day 0, 7, 8, 9, 10, 11. Mice were sacrificed on day 14. Broncho-alveolar lavage (BAL) fluid was collected and BAL cells were counted and analyzed by flow cytometry. Mediastinal lymph nodes (medLNs) were harvested and a single cell suspension was prepared. For histological analysis, lungs were fixed with 10% formalin at least for 24 hrs, embedded in paraffin and stained with hematoxylin and eosin (H&E), or periodic acid–Schiff (PAS) reagent and were assigned scores by established methods. For lymphocyte-platelet aggregation assay, 50 μg of HDM extract was dissolved in 25 μl PBS and then intranasal challenge was performed. Lymphocyte-platelet aggregation was measured at 4 and 24 hours after the challenge. At 72 hour, lung homogenate was prepared by collagenase digestion. Cells were counted and lymphocytes were analyzed by flow cytometry. For more details, see supplementary experimental procedures.
Leishmania infection model
L. major WR309 strain (MHOM/IL/79/LRC-L251) was originally isolated from a human case of cutaneous leishmaniasis in Israel and has been maintained through culture and frequent passages in mice to maintain virulence. BALB/c mice were infected with 2×106 late stationary promastigotes isolated from a Percoll gradient. For monitoring 2 week and 6 week experiments, Dkk-1 inhibitor (10 mg/kg) or vehicle (50 μl DMSO) was injected on day −1, +1, +3, +5, +7, +9, +11 and weekly afterwards till harvest. Draining lymph nodes, non-draining lymph nodes, and plasma were collected. For ex vivo analysis, soluble Leishmania major antigen (sLMAg) was employed. For depleting platelets, 80 μg of anti-CD42 mAb (Emfret Analytical) was injected intravenously in 100 μl PBS. For more details, see supplementary experimental procedures.
Human or mouse platelet isolation and functional characterization, lymphocyte-platelet aggregate assay
Human platelets were isolated as described from healthy volunteers (Tang et al., 2011). All procedures were in accordance with HIC protocol (protocol # 1005006865). All volunteers consented to donate their blood. Briefly, 3.2% citrate buffer was used to prevent coagulation. Blood was centrifuged at 250 g for 15 min. The upper layer was taken as platelet rich plasma (PRP). The Hemavet 950FS (Drew Scientific) was used to count platelet numbers. Platelets were washed twice with wash buffer and resuspended in Tyrode's buffer. 100 million platelets/ml were used for activation. House dust mite extract (50 μg) added for activation of platelets for 1 hr. For soluble leishmania antigen (sLMAG), L.major parasites were prepared in 5×108 parasites/ml Schneider's culture medium. sLMAG was prepared by repeated freeze and thaw cycles. This stock was added to platelet culture indicated ratio for 1 hr. Platelets were resuspended in Tyrode's buffer. Supernatant was collected for human Dkk-1 ELISA. For details, see supplemental experiment procedures.
Statistical significance
Statistically significant differences were determined by One-way ANOVA with Bonferroni's post-hoc test, Dunnet's post-hoc test, or student t-test with Graph Pad Prism software (GraphPad Software).
Supplementary Material
Highlights.
Reduced expression of Dkk-1 protects the host from house dust mite induced asthma
Dkk-1 induces Th2 cell polarization and potentiates Th2 cell cytokine expression
Platelets are the major source of Dkk-1 in pathological type 2 inflammation
Pharmacological inhibition of Dkk-1 protects host from cutaneous leishmaniasis
Acknowledgments
We thank Gouzel Tokmulina and Tom Taylor for cell sorting, and Christine Cote for technical help in AMNIS operation. This work was supported by NIH 1R21AI107957-01 and NIH 1R01CA168670-01 (both awarded to A.L.M.B), AI093775 (D.Mc-P.), R01 AI089824 (C.V.R) and T32 AI007019 (P.Y.C.). AMNIS work was supported by the Shared Instrument Grant (1-S10-RR-026526-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contribution
W-J.C, A.K.E, D. McP, G-P.K. performed L. major experiments and analyzed results. W-J.C and P.Y.C. performed HDM asthma experiments and analyzed results. W-J.C, and A.M.T performed mouse platelet experiments. L.H. scored histology samples. W.-H. T., J.H. D.S.K. and A.M.T contributed experimental advice for human platelet experiments. Shan. P and Lee. PJ advised and helped with the Flexivent assay. J.H.S. performed immunohistochemistry. S-T.K and J-H.P assisted in vitro experiments. S.M. gave technical help. C.V.R. provided experimental materials and advised writing manuscript. D.McP conceived Leishmania study, provided experimental materials, and wrote the paper. O.H. analyzed results and provided experimental advice with discussion. W.-J.C. and A.L.M.B conceived all experimental studies and wrote the paper.
Authors declare no conflict of interest.
References
- Abbas AK, Janeway CA., Jr. Immunology: improving on nature in the twenty-first century. Cell. 2000;100:129–138. doi: 10.1016/s0092-8674(00)81689-x. [DOI] [PubMed] [Google Scholar]
- Allen JE, Wynn TA. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathog. 2011;7:e1002003. doi: 10.1371/journal.ppat.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Hoffmann KF, Mendez S, Kamhawi S, Udey MC, Wynn TA, Sacks DL. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. The Journal of experimental medicine. 2001;194:1497–1506. doi: 10.1084/jem.194.10.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brott BK, Sokol SY. Regulation of Wnt/LRP signaling by distinct domains of Dickkopf proteins. Molecular and cellular biology. 2002;22:6100–6110. doi: 10.1128/MCB.22.17.6100-6110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J. Shear stress platelet activation. Lancet. 1995;346:841. doi: 10.1016/s0140-6736(95)91653-9. [DOI] [PubMed] [Google Scholar]
- Carvalho LP, Petritus PM, Trochtenberg AL, Zaph C, Hill DA, Artis D, Scott P. Lymph node hypertrophy following Leishmania major infection is dependent on TLR9. Journal of immunology. 2012;188:1394–1401. doi: 10.4049/jimmunol.1101018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Biechele T, Wei Z, Morrone S, Moon RT, Wang L, Xu W. Crystal structures of the extracellular domain of LRP6 and its complex with DKK1. Nat Struct Mol Biol. 2011;18:1204–1210. doi: 10.1038/nsmb.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nature reviews. Immunology. 2012;12:325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cines DB, Cuker A, Semple JW. Pathogenesis of immune thrombocytopenia. Presse medicale. 2014;43:e49–59. doi: 10.1016/j.lpm.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Cruciat CM, Niehrs C. Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb Perspect Biol. 2013;5:a015081. doi: 10.1101/cshperspect.a015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diacovo TG, Puri KD, Warnock RA, Springer TA, von Andrian UH. Platelet-mediated lymphocyte delivery to high endothelial venules. Science. 1996;273:252–255. doi: 10.1126/science.273.5272.252. [DOI] [PubMed] [Google Scholar]
- Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, Scheinecker C, et al. Dickkopf-1 is a master regulator of joint remodeling. Nature medicine. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- Ding Y, Shen S, Lino AC, Curotto de Lafaille MA, Lafaille JJ. Beta-catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nature medicine. 2008;14:162–169. doi: 10.1038/nm1707. [DOI] [PubMed] [Google Scholar]
- Divekar R, Kita H. Recent advances in epithelium-derived cytokines (IL-33, IL-25, and thymic stromal lymphopoietin) and allergic inflammation. Current opinion in allergy and clinical immunology. 2015;15:98–103. doi: 10.1097/ACI.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar JD, Ouyang W, Lohning M, Assenmacher M, Radbruch A, Kanagawa O, Murphy KM. An instructive component in T helper cell type 2 (Th2) development mediated by GATA-3. The Journal of experimental medicine. 2001;193:643–650. doi: 10.1084/jem.193.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Kokabu S, Ohte S, Sasanuma H, Kanomata K, Yoneyama K, Kato H, Akita M, Oda H, Katagiri T. Canonical Wnts and BMPs cooperatively induce osteoblastic differentiation through a GSK3beta-dependent and beta-catenin-independent mechanism. Differentiation; research in biological diversity. 2010;80:46–52. doi: 10.1016/j.diff.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Gounari F, Chang R, Cowan J, Guo Z, Dose M, Gounaris E, Khazaie K. Loss of adenomatous polyposis coli gene function disrupts thymic development. Nature immunology. 2005;6:800–809. doi: 10.1038/ni1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Dose M, Kovalovsky D, Chang R, O'Neil J, Look AT, von Boehmer H, Khazaie K, Gounari F. Beta-catenin stabilization stalls the transition from double-positive to single-positive stage and predisposes thymocytes to malignant transformation. Blood. 2007;109:5463–5472. doi: 10.1182/blood-2006-11-059071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebenstreit D, Giaisi M, Treiber MK, Zhang XB, Mi HF, Horejs-Hoeck J, Andersen KG, Krammer PH, Duschl A, Li-Weber M. LEF-1 negatively controls interleukin-4 expression through a proximal promoter regulatory element. The Journal of biological chemistry. 2008;283:22490–22497. doi: 10.1074/jbc.M804096200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikamp EB, Patel CH, Collins S, Waickman A, Oh MH, Sun IH, Illei P, Sharma A, Naray-Fejes-Toth A, Fejes-Toth G, et al. The AGC kinase SGK1 regulates TH1 and TH2 differentiation downstream of the mTORC2 complex. Nature immunology. 2014;15:457–464. doi: 10.1038/ni.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho IC, Hodge MR, Rooney JW, Glimcher LH. The proto-oncogene c-maf is responsible for tissue-specific expression of interleukin-4. Cell. 1996;85:973–983. doi: 10.1016/s0092-8674(00)81299-4. [DOI] [PubMed] [Google Scholar]
- Hsu AC, Scott P. Leishmania mexicana infection induces impaired lymph node expansion and Th1 cell differentiation despite normal T cell proliferation. Journal of immunology. 2007;179:8200–8207. doi: 10.4049/jimmunol.179.12.8200. [DOI] [PubMed] [Google Scholar]
- Jenne CN, Kubes P. Platelets in inflammation and infection. Platelets. 2015;26:286–292. doi: 10.3109/09537104.2015.1010441. [DOI] [PubMed] [Google Scholar]
- Joiner DM, Ke J, Zhong Z, Xu HE, Williams BO. LRP5 and LRP6 in development and disease. Trends Endocrinol Metab. 2013;24:31–39. doi: 10.1016/j.tem.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Falet H, Hoffmeister KM, Hartwig JH. Wiskott-Aldrich syndrome protein (WASp) controls the delivery of platelet transforming growth factor-beta1. The Journal of biological chemistry. 2013;288:34352–34363. doi: 10.1074/jbc.M113.459750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JI, Ho IC, Grusby MJ, Glimcher LH. The transcription factor c-Maf controls the production of interleukin-4 but not other Th2 cytokines. Immunity. 1999;10:745–751. doi: 10.1016/s1074-7613(00)80073-4. [DOI] [PubMed] [Google Scholar]
- Koch S, Nava P, Addis C, Kim W, Denning TL, Li L, Parkos CA, Nusrat A. The Wnt antagonist Dkk1 regulates intestinal epithelial homeostasis and wound repair. Gastroenterology. 2011;141:259–268. 268, e251–258. doi: 10.1053/j.gastro.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause U, Ryan DM, Clough BH, Gregory CA. An unexpected role for a Wnt-inhibitor: Dickkopf-1 triggers a novel cancer survival mechanism through modulation of aldehyde-dehydrogenase-1 activity. Cell death & disease. 2014;5:e1093. doi: 10.1038/cddis.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine MD, Strand MR. Insect hemocytes and their role in immunity. Insect biochemistry and molecular biology. 2002;32:1295–1309. doi: 10.1016/s0965-1748(02)00092-9. [DOI] [PubMed] [Google Scholar]
- Lee GR, Fields PE, Flavell RA. Regulation of IL-4 gene expression by distal regulatory elements and GATA-3 at the chromatin level. Immunity. 2001;14:447–459. doi: 10.1016/s1074-7613(01)00125-x. [DOI] [PubMed] [Google Scholar]
- Leon B, Ardavin C. Monocyte migration to inflamed skin and lymph nodes is differentially controlled by L-selectin and PSGL-1. Blood. 2008;111:3126–3130. doi: 10.1182/blood-2007-07-100610. [DOI] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature reviews. Immunology. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Li C, Li J, Li Y, Lang S, Yougbare I, Zhu G, Chen P, Ni H. Crosstalk between Platelets and the Immune System: Old Systems with New Discoveries. Adv Hematol. 2012;2012:384685. doi: 10.1155/2012/384685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Goodall AH, Hjemdahl P. A sensitive flow cytometric assay for circulating platelet-leucocyte aggregates. Br J Haematol. 1997;99:808–816. doi: 10.1046/j.1365-2141.1997.4993305.x. [DOI] [PubMed] [Google Scholar]
- Maier E, Hebenstreit D, Posselt G, Hammerl P, Duschl A, Horejs-Hoeck J. Inhibition of suppressive T cell factor 1 (TCF-1) isoforms in naive CD4+ T cells is mediated by IL-4/STAT6 signaling. The Journal of biological chemistry. 2011;286:919–928. doi: 10.1074/jbc.M110.144949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath B, Mealing G, Labrosse MR. A mechanobiological investigation of platelets. Biomechanics and modeling in mechanobiology. 2011;10:473–484. doi: 10.1007/s10237-010-0248-0. [DOI] [PubMed] [Google Scholar]
- Moncada de la Rosa C, Radziwon-Balicka A, El-Sikhry H, Seubert J, Ruvolo PP, Radomski MW, Jurasz P. Pharmacologic protein kinase Calpha inhibition uncouples human platelet-stimulated angiogenesis from collagen-induced aggregation. J Pharmacol Exp Ther. 2013;345:15–24. doi: 10.1124/jpet.112.200881. [DOI] [PubMed] [Google Scholar]
- Morrell CN, Aggrey AA, Chapman LM, Modjeski KL. Emerging roles for platelets as immune and inflammatory cells. Blood. 2014;123:2759–2767. doi: 10.1182/blood-2013-11-462432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notani D, Gottimukkala KP, Jayani RS, Limaye AS, Damle MV, Mehta S, Purbey PK, Joseph J, Galande S. Global regulator SATB1 recruits beta-catenin and regulates T(H)2 differentiation in Wnt-dependent manner. PLoS biology. 2010;8:e1000296. doi: 10.1371/journal.pbio.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier JC, Lundquist J.T.t., Gilbert AM, Alon N, Bex FJ, Bhat BM, Bursavich MG, Coleburn VE, Felix LA, Green DM, et al. (1-(4-(Naphthalen-2-yl)pyrimidin-2-yl)piperidin-4-yl)methanamine: a wingless beta-catenin agonist that increases bone formation rate. J Med Chem. 2009;52:6962–6965. doi: 10.1021/jm9014197. [DOI] [PubMed] [Google Scholar]
- Pitchford SC, Momi S, Giannini S, Casali L, Spina D, Page CP, Gresele P. Platelet P-selectin is required for pulmonary eosinophil and lymphocyte recruitment in a murine model of allergic inflammation. Blood. 2005;105:2074–2081. doi: 10.1182/blood-2004-06-2282. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Artis D. New paradigms in type 2 immunity. Science. 2012;337:431–435. doi: 10.1126/science.1221064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redpath SA, Fonseca NM, Perona-Wright G. Protection and pathology during parasite infection: IL-10 strikes the balance. Parasite immunology. 2014;36:233–252. doi: 10.1111/pim.12113. [DOI] [PubMed] [Google Scholar]
- Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Annual review of immunology. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- Rondina MT, Garraud O. Emerging evidence for platelets as immune and inflammatory effector cells. Frontiers in immunology. 2014;5:653. doi: 10.3389/fimmu.2014.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Yamabuki T, Takano A, Koinuma J, Aragaki M, Masuda K, Ishikawa N, Kohno N, Ito H, Miyamoto M, et al. Wnt inhibitor Dickkopf-1 as a target for passive cancer immunotherapy. Cancer Res. 2010;70:5326–5336. doi: 10.1158/0008-5472.CAN-09-3879. [DOI] [PubMed] [Google Scholar]
- Scott P. IFN-gamma modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. Journal of immunology. 1991;147:3149–3155. [PubMed] [Google Scholar]
- Semple JW, Italiano JE, Jr., Freedman J. Platelets and the immune continuum. Nature reviews. Immunology. 2011;11:264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- Shi G, Field DJ, Ko KA, Ture S, Srivastava K, Levy S, Kowalska MA, Poncz M, Fowell DJ, Morrell CN. Platelet factor 4 limits Th17 differentiation and cardiac allograft rejection. The Journal of clinical investigation. 2014;124:543–552. doi: 10.1172/JCI71858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nature reviews. Immunology. 2008;8:581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- Tacchini-Cottier F, Weinkopff T, Launois P. Does T Helper Differentiation Correlate with Resistance or Susceptibility to Infection with L. major? Some Insights From the Murine Model. Frontiers in immunology. 2012;3:32. doi: 10.3389/fimmu.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagawa-Mineoka R, Katoh N, Ueda E, Takenaka H, Kita M, Kishimoto S. The role of platelets in leukocyte recruitment in chronic contact hypersensitivity induced by repeated elicitation. Am J Pathol. 2007;170:2019–2029. doi: 10.2353/ajpath.2007.060881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WH, Stitham J, Gleim S, Di Febbo C, Porreca E, Fava C, Tacconelli S, Capone M, Evangelista V, Levantesi G, et al. Glucose and collagen regulate human platelet activity through aldose reductase induction of thromboxane. The Journal of clinical investigation. 2011;121:4462–4476. doi: 10.1172/JCI59291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, Shaughnessy JD., Jr. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- Trikalinos NA, Soupir CP, Dey BR. Lineage switch of acute lymphocyctic leukaemia with t(4;11)(q21;q23) into acute myeloid leukaemia in an adult patient after allogeneic stem cell transplantation. Br J Haematol. 2009;145:262–264. doi: 10.1111/j.1365-2141.2009.07586.x. [DOI] [PubMed] [Google Scholar]
- van Loosdregt J, Fleskens V, Tiemessen MM, Mokry M, van Boxtel R, Meerding J, Pals CE, Kurek D, Baert MR, Delemarre EM, et al. Canonical Wnt signaling negatively modulates regulatory T cell function. Immunity. 2013;39:298–310. doi: 10.1016/j.immuni.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Wang XD, Huang XF, Yan QR, Bao CD. Aberrant activation of the WNT/beta-catenin signaling pathway in lupus nephritis. PLoS One. 2014;9:e84852. doi: 10.1371/journal.pone.0084852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte JL, Smith AA, Helms JA. Wnt signaling and injury repair. Cold Spring Harb Perspect Biol. 2012;4:a008078. doi: 10.1101/cshperspect.a008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W, Jacinto A. Drosophila melanogaster embryonic haemocytes: masters of multitasking. Nature reviews. Molecular cell biology. 2007;8:542–551. doi: 10.1038/nrm2202. [DOI] [PubMed] [Google Scholar]
- Xie H, Huang Z, Sadim MS, Sun Z. Stabilized beta-catenin extends thymocyte survival by up-regulating Bcl-xL. Journal of immunology. 2005;175:7981–7988. doi: 10.4049/jimmunol.175.12.7981. [DOI] [PubMed] [Google Scholar]
- Yeaman MR. Platelets: at the nexus of antimicrobial defence. Nat Rev Microbiol. 2014;12:426–437. doi: 10.1038/nrmicro3269. [DOI] [PubMed] [Google Scholar]
- Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. The Journal of clinical investigation. 2006;116:3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Nardi MA, Borkowsky W, Li Z, Karpatkin S. Role of molecular mimicry of hepatitis C virus protein with platelet GPIIIa in hepatitis C-related immunologic thrombocytopenia. Blood. 2009;113:4086–4093. doi: 10.1182/blood-2008-09-181073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.