Abstract
Background
We hypothesized that endothelial cells having distinct mitochondrial genetic backgrounds would show variation in mitochondrial function and oxidative stress markers concordant with known differential cardiovascular disease susceptibilities. To test this hypothesis, mitochondrial bioenergetics were determined in endothelial cells from healthy individuals with African versus European maternal ancestries.
Methods and Results
Bioenergetics and mitochondrial DNA (mtDNA) damage were assessed in single donor human umbilical vein endothelial cells (HUVECs) belonging to mtDNA haplogroups H and L, representing West Eurasian and African maternal ancestry, respectively. HUVECs from haplogroup L utilized less oxygen for ATP production and had increased levels of mtDNA damage compared to those in haplogroup H. Differences in bioenergetic capacity were also observed in that HUVECs belonging to haplogroup L had decreased maximal bioenergetic capacities compared to haplogroup H. Analysis of peripheral blood mononuclear cells from age-matched healthy controls with West Eurasian or African maternal ancestries showed that haplogroups sharing an A to G mtDNA mutation at nucleotide pair (np) 10,398 had increased mtDNA damage compared to those lacking this mutation. Further study of angiographically proven coronary artery disease patients and age-matched healthy controls revealed that mtDNA damage was associated with vascular function and remodeling, and that age of disease onset was later in individuals from haplogroups lacking the A to G mutation at np 10,398.
Conclusions
Differences in mitochondrial bioenergetics and mtDNA damage associated with maternal ancestry may contribute to endothelial dysfunction and vascular disease.
Keywords: endothelial cell, mitochondria, genetics
Introduction
Cardiovascular disease (CVD) has been the leading cause of mortality and morbidity in the United States for the past century 1. While racial differences in the prevalence of CVD have been well documented, the mechanisms that underlie these differential susceptibilities have yet to be determined. Endothelial dysfunction is generally considered to be the initiating factor in CVD development, and increased cellular oxidant stress has been linked to endothelial dysfunction. In this regard, there have been multiple endogenous sources of reactive oxygen species (ROS) implicated in endothelial dysfunction and CVD. Among them, the mitochondrion has been noted to be both a primary source and target of oxidants.
The mitochondrion is a multifunctional organelle, serving as the site for electron transport, oxidative phosphorylation (OXPHOS), the citric acid cycle, β-oxidation, steroidogenesis, and many other important cellular functions including growth, oxidant generation and programmed cell death. Mitochondria also have their own genome (mtDNA), which encodes the translational machinery (2 rRNAs and 22 tRNAs) necessary for the generation of polypeptides from 13 mtDNA – encoded genes that are essential for electron transport and OXPHOS. During OXPHOS, mitochondria couple electron transport with proton translocation, which in turn enables the production of ATP. Mitochondrial ROS generation occurs when electrons “leak” from electron transport (estimated to account for ~4–5% of total oxygen consumption) resulting in the formation of superoxide (O2•−) 2, 3. It has been proposed that variation in mitochondrial energy and oxidant production exists in normal cells that would translate into differences in mitochondrial oxygen utilization between individuals. Moreover, these changes are attributed to the known inherent genetic variability of the mtDNA that characterize mtDNA haplogroups 4–6.
Indeed, epidemiologic studies have indicated that associations exist between certain mtDNA haplogroups and a number of age related diseases including ischemic CVD, hypertension, type-2 diabetes, obesity, metabolic syndrome, cancer and neurodegenerative diseases 7–12. Similarly, animal studies indicate that mtDNA haplotype can influence longevity, risk for type-2 diabetes, cardiovascular disease, cancer, and fatty liver disease development 13–21. Although cellular studies utilizing animal models of mtDNA variation or immortalized cybrid cell lines have yielded conflicting results in terms of mitochondrial function and ROS production22–24, to our knowledge no studies have compared mitochondrial function or integrity in normal cells from healthy individuals that represent different mtDNA haplogroups. In fact, all published data have utilized systems that are reliant upon immortalized cell lines, thereby potentially confounding the interpretation of these data within the normal cellular context.
In the present study, endothelial cells harboring either mitochondrial haplogroup H or L (representing West Eurasian or African mtDNAs, respectively) demonstrated distinct bioenergetic profiles. These profiles indicate that cells belonging to haplogroup L were more economical in terms of energy generation (utilized less oxygen for ATP production), but also had higher basal levels of mtDNA damage. MtDNA damage was also quantified from peripheral blood mononuclear cells (PBMCs) collected from age-matched healthy controls and compared between individuals belonging to West Eurasian and African haplogroups. Results show that mtDNA damage is significantly higher in groups belonging to African and West Eurasian maternal ancestries which share a single nucleotide polymorphism (SNP) at nucleotide pair (np) 10,398. Furthermore, additional studies comparing CVD patients and healthy, age-matched individuals found that mtDNA damage significantly correlated with vascular function and remodeling. These data demonstrate that (i) mitochondrial bioenergetics can vary in normal, primary cells having distinct mtDNA haplogroups, (ii) mtDNA damage varies by mtDNA haplogroup, and (iii) increased mtDNA damage segregates with both CVD and differences in vascular function and remodeling. Thus, our results provide new insights into the mechanisms that govern CVD susceptibility in humans.
Methods
Primary endothelial cells
Primary human umbilical vein endothelial cells (HUVECs) were chosen for these studies due to the desire to minimize the possibility of pre-existent factors influencing endothelial function (age, CVD risk factor exposure, etc.) and obtain normal, naive endothelial cells. Single donor HUVECs were obtained from individual cords from male infants (Lonza - Walkersville, MD, USA) and mtDNA haplotype was determined by direct sequencing as previously described 25, 26. For these studies, HUVEC samples belonging to haplogroups H (West Eurasian, N = 5) or L (African, N = 9) were selected and maintained in a humidified incubator at 37°C with 5% CO2 in EGM2 growth medium (Lonza, Walkersville, MD, USA). All experiments utilized cells between passages 5 and 9. HUVECs within haplogroups H and L were utilized because their mtDNAs represent the most common maternal lineages in North American Caucasians and African Americans, respectively. All HUVEC studies were performed in triplicate.
Measurement of mitochondrial function and estimation of oxygen utilization
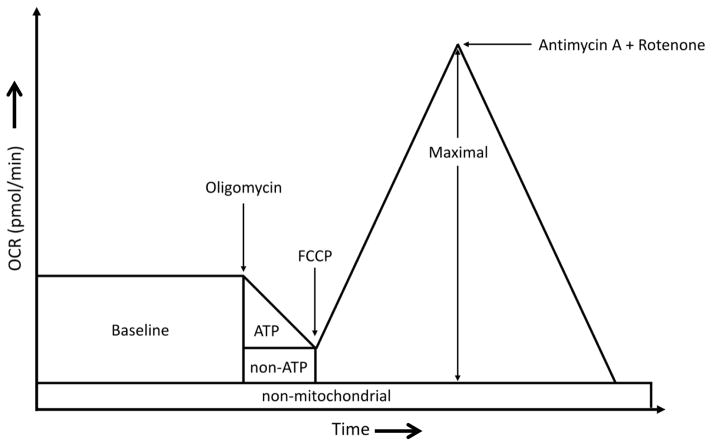
A Seahorse Bioscience XF24 extracellular flux analyzer was used to measure mitochondrial function in intact HUVECs. The XF24 creates a transient, 7-μl chamber in specialized microplates that allows for the determination of oxygen and proton concentrations in real time 27, 28. Thus, the rates of oxygen consumption and proton production can be measured across several samples at a time. Measurements of extracellular flux were made in unbuffered media. For these experiments, cells were seeded at 30,000 cells/well onto Seahorse Bioscience (North Billerica, MA, USA) V7 tissue culture plates in culture media and allowed to adhere and grow for 24 hours. The following day, the media was changed 1 h before the start of the extracelluar flux assay to DMEM supplemented with 25 mM D-glucose, 2 mM L-glutamine, and 1 mM pyruvate. The pH of the medium was adjusted to 7.4 with NaOH. To allow comparison between experiments, data are expressed as the rate of oxygen consumption in pmol/min/3 × 104 cells. To assay mitochondrial function, oligomycin, FCCP, and a combination of antimycin A / rotenone were injected through ports in the Seahorse Flux Pak cartridges to final concentrations of 4 μg/ml, 4 μM, and 10 μM / 1 μM respectively29. Figure 1 illustrates how basal (baseline), ATP, non-ATP, and non-mitochondrial levels of oxygen consumption rates (OCR) are evaluated. Baseline OCR (OCRbaseline) is represented by the total oxygen consumption rate of the cells prior to addition of mitochondrial inhibitors. Because oligomycin inhibits mitochondrial ATP synthase (complex V), non-ATP linked OCR is equal to the rate observed in the presence of oligomycin (OCRoligo). Consequently, ATP linked OCR (OCRATP) is determined by subtracting the non-ATP OCR from the baseline OCR (OCRATP = OCRbaseline − OCRoligo). Maximal OCR (OCRmax) is determined in the presence of FCCP, an ionophore that uncouples the mitochondrion, leading to maximal oxygen consumption in an attempt to re-establish a membrane potential. Finally, addition of antimycin A and rotenone blocks electron entry into the electron transport chain, inhibiting mitochondrial oxygen consumption, which allows estimation of non-mitochondrial OCR, which is subtracted out as background for the mitochondrial OCR calculations. By using these OCR calculations, oxygen utilization can be estimated by determining the percentage of oxygen dedicated for ATP production using the formula OCRATP/OCRbaseline, and percent oxygen consumed for non-ATP purposes by using the formula OCRoligo/OCRbaseline.
Figure 1.
Experimental profile of basal, oligomycin, FCCP, and antimycin A + rotenone (BOFA) induced oxygen consumption rates (OCR): For these studies, 30,000 HUVEC per well from single donors were cultured 24 hours onto V7 tissue culture plates and oxygen consumption rates (OCR) determined in the Seahorse XF24 Bioanalyzed. First, baseline OCR (portion indicated by “baseline”) were determined. Second, oligomycin was added to inhibit mitochondrial respiration at ATP synthase and thus, decrease OCR by the amount of oxygen consumed for mitochondrial ATP production (indicated by “ATP”). The OCR remaining in the presence of oligomycin represents the oxygen consumed by the mitochondrion for non-ATP related consumption (indicated by “non-ATP”) and non-mitochondrial oxygen consumption. Third, [carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone] or FCCP, an ionophore, uncouples mitochondrial respiration and dissipates mitochondrial membrane potential which induces maximal mitochondrial oxygen consumption (indicated by “Maximal”). Finally, cells are treated with the combination of antimycin A and rotenone, inhibitors of cytochromes b and c and NADH dehydrogenase respectively, which inhibits electron entry into electron transport and thus, provides an estimate of non-mitochondrial oxygen consumption (indicated by “non-mitochondrial”).
Determination of intracellular ATP
For determination of intracellular ATP, HUVECs were seeded at 30,000 cells/well in 96-well tissue culture plates and ATP levels determined using the Adenosine 5′-triphosphate (ATP) bioluminescent somatic cell assay kit (Sigma, MO, USA) following the manufacturer’s instructions. ATP production rates were determined by measuring intracellular ATP under both basal and oligomycin (4 μg/ml) treated conditions.
Flow cytometry
Cultured HUVECs grown to ~85–90% confluence in T-75 flasks were washed twice with PBS followed by a 5 minute incubation with 3 ml of 0.25% trypsin/EDTA at 37°C. Following trypsin treatment, cells were resuspended in PBS at a final concentration of 300,000 cells/ml. MitoTracker Red® was added to a final concentration of 200 nM and the mitochondria were labeled for 30 min at 37°C. After labeling, cells were washed once with PBS containing 5% FBS and then resuspended in 200 ml of PBS with 5% FBS for fluorescence activated cell sorting (FACS) analysis. Cells were analyzed with the assistance of the Analytical and Preparative Flow Core Facility on a SORP-LSRII digital cell analyzer and MitoTracker Red® fluorescence was monitored using the 535 nm excitation laser. Live cells were gated using FSC v. SSC and comparisons on the amount of MitoTracker Red® staining compared to a non-stained negative control.
DNA isolation and mtDNA damage assays
DNA was isolated from HUVECs or peripheral blood mononuclear cells (PBMCs). MtDNA copy number, quantitative PCR (QPCR) conditions, and calculation of DNA lesion frequencies have been previously described 30, 31. Briefly, genomic DNAs were extracted using QIAamp® DNA Mini kits (Qiagen), and mtDNA damage assessed via amplification of a 16.2 kb qPCR product (L) from mtDNA templates which was normalized by mtDNA copy number as determined by a smaller qPCR product (S), yielding a L/S ratio 30, 31. For the studies herein, mtDNA lesion frequencies were determined relative to either average L/S of haplogroup H or DdeI − (10398A) haplogroups, which represented “zero-class” lesions as previously described31.
Aconitase assay
Aconitase activity was determined by measuring the transformation of isocitrate to cis-aconitate at 240 nm in 50 mM TrisHCl (pH 7.4) containing MnCl2 and 20 mM isocitrate at 25°C. Aconitase is specifically inactivated by superoxide (O2•−) and peroxynitrite (ONOO−) and hence, decreased activity correlates with increased oxidative stress associated with these oxidants32–34. Because ONOO− formation is related to O2.− production, decreased aconitase activity can be associated indirectly with increased O2•− generation. Decreases in enzymatic activity were interpreted as consistent with increased oxidant stress associated with O2•−.
Human Subjects
To investigate the relations between mtDNA haplogroups in terms of mtDNA damage in normal, healthy individuals, peripheral blood mononuclear cells (PBMCs) from healthy, age-matched individuals (23 males, 22 females) were collected and mtDNA haplogroup was determined. MtDNA haplogroups were then segregated by presence (+) or absence (−) of a DdeI restriction site at np 10,394. An ancient transitional mutation, the guanosine (DdeI +) to adenosine (DdeI −) switch at np 10,398 alters the last nucleotide of the DdeI recognition sequence (CTGAG) that begins at np 10,394. This site is present in maternal lineages which are ubiquitous in African populations (L0 – L7) and also in a specific subset of West Eurasian mtDNAs (I, J, K) proposed to have Middle Eastern origins, but is absent in the remaining West Eurasian maternal lineages (haplogroups H, T, U, V, W, X) 35, 36. Although this SNP has also been proposed to increase risk of invasive breast cancer in African American women when cancer exists, many studies investigating this issue have been conflicting37–41. Its use in the study herein was simply to segregate haplogroups into “northern” (DdeI −) or “southern” (DdeI +) latitudes. PBMCs were isolated from whole blood by density gradient centrifugation (BD™ Vacutainer CPT tubes Becton, Dickerson and Company, Franklin, NJ). The mean ages for individuals representing the DdeI − (N = 29) and DdeI + (N = 16) groups were 52.2 ± 1.4 and 50.4 ± 2.0 years, respectively (p = 0.47).
To determine whether a relationship existed between mtDNA damage, cardiovascular disease and vascular function, an additional age-matched 56 subjects (23 healthy volunteers and 33 angiographically proven coronary artery disease) receiving care at Boston Medical Center were enrolled (mean age 55.0 ± 10.0 years, 37 males, 19 females). Among these, 23 were healthy volunteers with no history of cardiovascular disease, diabetes mellitus, hypertension, dyslipidemia, or cigarette smoking (10 males, 13 females) and 33 had angiographically proven coronary artery disease (27 males, 6 females). A blood sample was collected for assessment of mtDNA damage, haplogroup status, flow-mediated dilation (a measure of endothelial vasodilator function) and brachial artery diameter (a measure of chronic remodeling) as previously described 42–44. All participants were provided written informed consent. The protocol was approved by the Institutional Review Boards of the Boston Medical Center and the University of Alabama at Birmingham.
Vascular measurements in human subjects
We examined the correlation between mtDNA damage and flow-mediated dilation (a measure of endothelial vasodilator function) and brachial artery diameter (a measure of chronic remodeling). Both variables have been shown to be related to cardiovascular risk45. Subjects rested for 10 minutes in a recumbent position and endothelium-dependent brachial artery flow-mediated dilation was assessed as previously described 43, 44. Briefly, two-dimensional images and Doppler ultrasound signals were recorded from the brachial artery before and 1 minute after induction of reactive hyperemia by 5 minute cuff occlusion of the upper arm.
Statistical Analysis
Study results are expressed as mean ± SEM. ANOVA was used to test the null hypothesis that all samples were drawn from a single population. If this test revealed significant differences (P<0.05), then a Student-Newman-Keuls test was used for group comparisons. The non-parametric Mann-Whitney Rank Sum test was used in certain cases (aconitase activity and mtDNA damage in HUVEC). We calculated Pearson correlations between the measures of vascular function and mtDNA damage. Statistical analyses were performed with SigmaStatR statistical software (Systat Software, Inc).
Results
Single donor HUVECs from haplogroups H or L have distinct oxygen consumption profiles
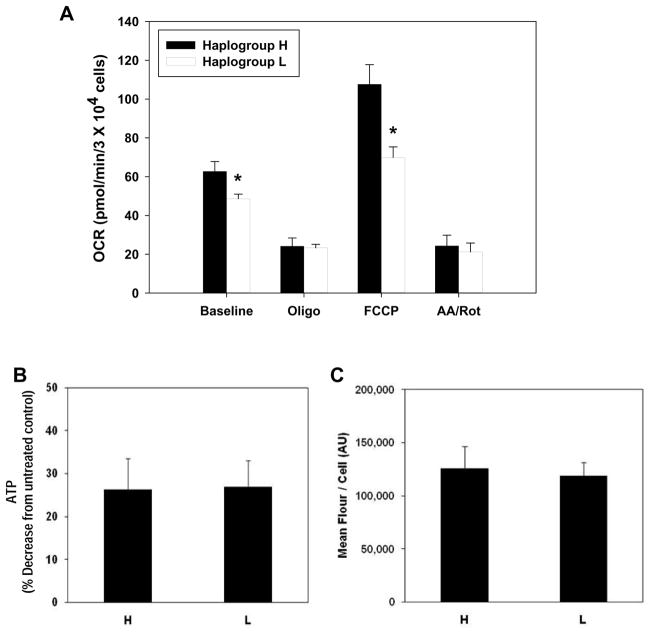
Oxygen consumption rates were monitored in single donor HUVECs representing haplogroups H and L under basal conditions and following treatment with oligomycin, FCCP, and a combination of antimycin A + rotenone (design illustrated in Figure 1). Figure 2A shows that HUVECs from haplogroup H had significantly higher basal oxygen consumption rates compared to those from haplogroup L (62.78 ± 5.05 versus 48.60 ± 2.40 pmol/min, respectively). Treatment with oligomycin, an inhibitor of complex V (ATP synthase), decreased oxygen consumption rates in both haplogroups (Figure 2A; 24.17 ± 4.19 vs 23.28 ± 1.80 pmol/min); decreases in intracellular ATP levels were also observed for haplogroups H and L under these conditions (Figure 2B; 26.33 ± 7.15% vs 26.93 ± 6.07% in H vs L, respectively), suggesting similar patterns of ATP utilization for them. Dissipation of mitochondrial membrane potential by the addition of FCCP revealed that HUVECs from haplogroup H had a significantly higher maximal oxygen consumption rate compared to those from haplogroup L (Figure 2A; 107.68 ± 10.06 vs 69.89 ± 5.49 pmol/min, respectively), while the addition of antimycin A + rotenone (which blocks mitochondrial electron flow) revealed no significant differences in non-mitochondrial oxygen consumption between groups (Figure 2A; 24.36 ± 5.43 vs 21.17 ± 4.63 pmol/min, respectively). No differences in mitochondrial number per cell were observed between haplogroups H and L (Figure 2C), suggesting that the differences in oxygen consumption were not due to organelle number per cell between the two lineages.
Figure 2.
Oxygen consumption rates, ATP utilization, and mitochondrial number in HUVECs from haplogroups H and L. (A) HUVECs from haplogroup H (West Eurasian; n=5 single donors) and haplogroup L (African; n=9 single donors) were seeded on Seahorse XF24 plates (30,000 cells/well) and oxygen consumption rates (OCR) were monitored at baseline and following treatment with oligomycin (4 μg/ml), FCCP (4μM), or antimycin A + rotenone (10 μM and 1 μM) over a period of 18 minutes in unbuffered minimal essential media. (B) Under identical conditions, the decrease in intracellular ATP due to oligomycin treatment (4 μg/ml) was determined relative to untreated controls. (C) Mitochondrial number was assessed by staining mitochondria from both groups with MitoTracker Red (200nM) for 30 min and determining the average fluorescence per cell via flow cytometry. Values are means ± SEM of ≥ 3 independent experiments per single donor cell line. * p < 0.05.
Oxygen utilization differs in HUVECs from haplogroups H and L
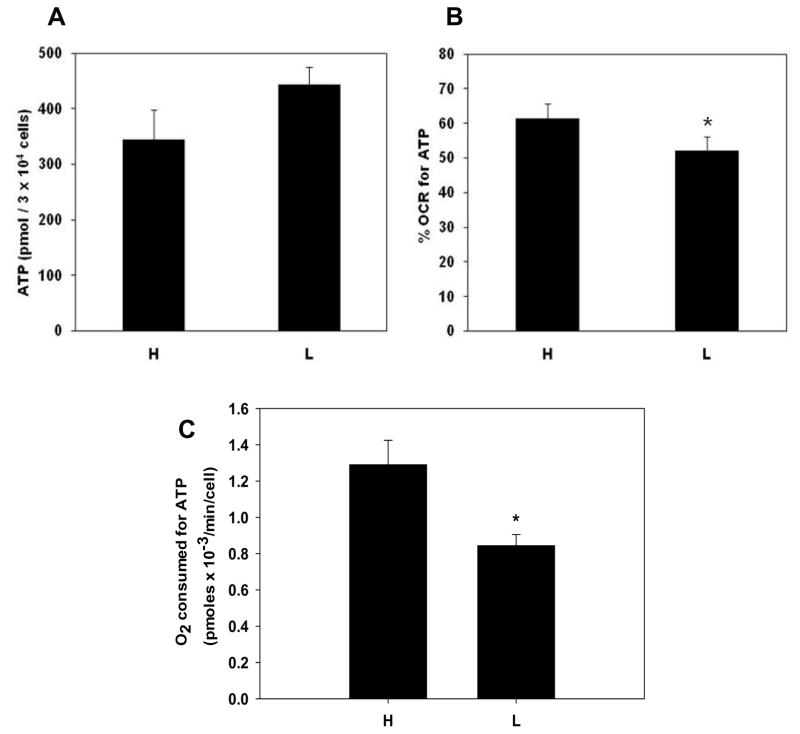
HUVECs from haplogroups H and L maintained similar steady-state levels of intracellular ATP (Figure 3A) under basal conditions, although those from haplogroup L trended toward increased levels of intracellular ATP (344.12 ± 53.56 pmol/3 × 104 cells vs 442.98 ± 31.71 pmol/3 × 104 cells in H and L, respectively). Because HUVECs from the two haplogroups had significantly different oxygen consumption rates yet maintained similar levels of intracellular ATP, we postulated that they had different oxygen utilization profiles. Consequently, the percentage of oxygen consumed for ATP production relative to total oxygen consumption was calculated in HUVECs from haplogroups H and L. Utilizing oligomycin as specific inhibitor of ATP synthase, the percentage of oxygen dedicated for ATP production is calculated by subtracting the oligomycin inhibited oxygen consumption rate from the baseline oxygen consumption rate (OCRATP = OCRbaseline − OCRoligo)and dividing by the baseline rate (OCRATP/OCRbaseline, see methods). This estimates oxygen consumption directly linked to mitochondrial ATP synthesis relative to all other oxygen consuming processes in the mitochondria (e.g. proton leak and oxidant generation). Figure 3B shows that HUVECs from the two haplogroups had significant differences in the percentage of basal oxygen utilized for ATP production: haplogroup H committed 61.5 ± 4.2% of total oxygen consumed under basal conditions for ATP production whereas HUVECs in haplogroup L utilized 52.1 ± 4.1% of total oxygen consumed for ATP production. These results translated into haplogroup H HUVECs utilizing more oxygen for ATP production than haplogroup L HUVECs under basal conditions (1.29 × 10−3 pmoles oxygen/min/cell versus 8.44 × 10−4 pmoles oxygen/min/cell, respectively, Figure 3C). Thus, haplogroup H HUVECs consumed more oxygen to maintain similar steady-state levels of ATP indicating a lower mitochondrial economy with respect to ATP production when compared to those from haplogroup L.
Figure 3.
Oxygen consumption devoted to ATP maintenance differs in HUVECs from haplogroups H and L. (A) HUVECs from haplogroup H (West Eurasian) and haplogroup L (African) were seeded on 96 well plates (30,000 cells/well) and steady state concentrations of ATP were determined via bioluminescent detection (see methods). (B) Percent oxygen utilization for ATP production was calculated in HUVECs by subtracting oligomycin associated oxygen consumption from baseline consumption and dividing by baseline consumption [(baseline – oligomycin)/baseline. (C) Cellular oxygen consumption rate for ATP production was determined by multiplying the basal oxygen consumption rates by the proportion of oxygen utilized for ATP generation for each sample. Bar graph depicted the mean and S.E.M. for all samples. Values presented as means ± SEM of ≥ 3 independent experiments per single donor cell line (n = 5 haplogroup H single donors and n = 9 haplogroup L single donors). * p< 0.05.
Mitochondrial damage is increased in HUVECs from haplogroup L
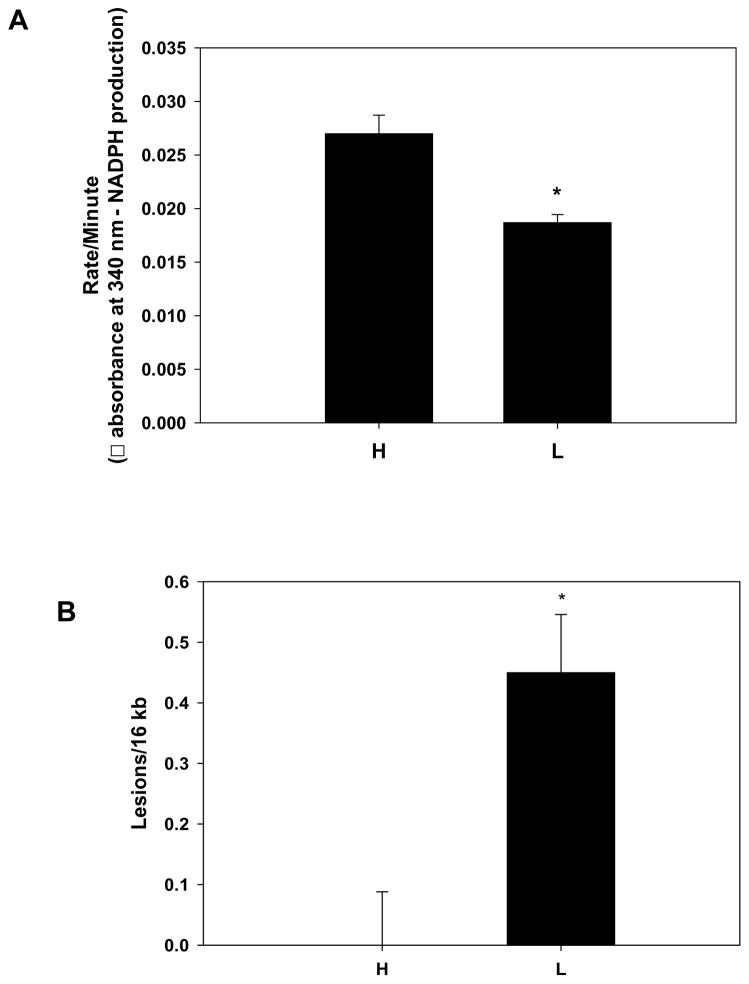
As these studies suggested that HUVECs from haplogroup L were more economical in generating ATP than those from haplogroup H, we were curious to know whether levels of mitochondrial damage were different between the two lineages. Consequently, activity of aconitase, a citric acid cycle enzyme in the mitochondrial matrix which is specifically inactivated by superoxide or peroxynitrite33, was quantified to determine overall levels of oxidative stress within the mitochondrion. Figure 4A shows that aconitase activity was significantly decreased in HUVECs from haplogroup L compared to those in haplogroup H, indicating greater oxidative stress in the mitochondria from the former group. Next, aconitase protein levels were determined via immunoblot, and no significant differences between H and L haplogroups were observed (data not shown), indicating the differences in enzyme activity were not due to differences in protein levels. To more specifically examine the degree of mtDNA damage in both groups, the level of mtDNA damage was determined in HUVECs from both haplogroups H and L. Consistent with the aconitase results, Figure 4B shows that HUVECs from haplogroup L had significantly more mtDNA damage relative to those from haplogroup H (0.45 ± 0.096 lesions / 16 kb vs 0.0 ± 0.088 lesions / 16 kb respectively). Collectively, these results suggest that HUVEC belonging to haplogroup L are exposed to higher levels of oxidant stress compared to those in haplogroup H, as reflected by differences in enzymatic activity of aconitase, and the levels of mtDNA damage.
Figure 4.
Mitochondrial DNA damage in HUVECs. (A) The activity of the citric acid cycle enzyme aconitase, was quantified in HUVEC belonging to haplogroup H (West Eurasian; n=3 single donors) and haplogroup L (African; n=3 single donors) to determine relative levels of oxidative stress – aconitase is specifically inactivated by superoxide or peroxynitrite in the mitochondrial matrix. Decreased activity indicates increased oxidant stress. Data is presented as means + SEM from 3 independent determinations per cell line. * p < 0.05. (B) Baseline mtDNA damage was assessed in HUVECs from haplogroup H (West Eurasian; n=3 single donors) and haplogroup L (African; n=3 single donors) via quantitative PCR. MtDNA damage is expressed relative to haplogroup H (zero class lesions), and are presented as means ± SEM and are representative of ≥ 3 independent determinations per cell line. * p < 0.05.
Mitochondrial DNA damage differs in PBMCs collected from individuals with haplogroups having African or West Eurasian origins
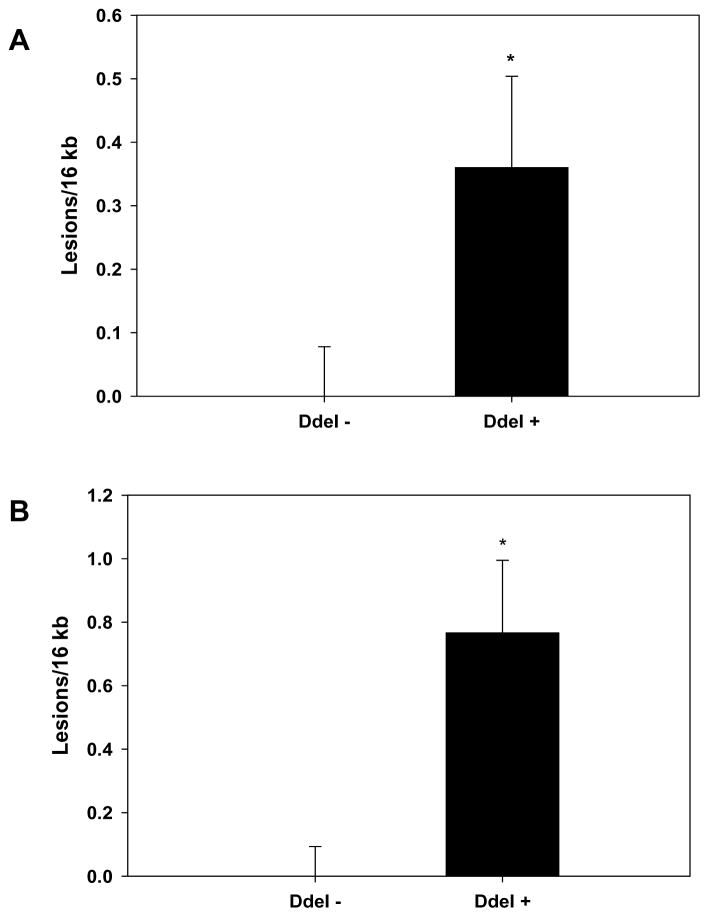
To further investigate whether differences in mtDNA damage levels existed between humans having West Eurasian or African maternal origins, peripheral blood mononuclear cell (PBMC) mtDNAs from 45 age-matched healthy individuals were sequenced and then placed into either African or West Eurasian maternal lineages. Interestingly no differences in mtDNA damage between these two groups were initially observed (data not shown). However, additional analysis revealed that differences in mtDNA damage existed when haplogroups were segregated using the 10398 SNP resulting in the presence (+) or absence (−) of the DdeI 10,394 site (Figure 5A). When the ancestral nucleotide (G) is present at 10398, it creates the DdeI+ 10,394 site. African lineages (DdeI+) are distinguished from the bulk of West Eurasian (DdeI−) haplogroups (H, T, U, V, W, and X) by this site (the latter having 10398A). However, the site has also independently recurred in three West Eurasian haplogroups (I, J, K) that are phylogenetically distinctive from each other, arose at different times in Europe or the Middle East and have a more southern latitude distribution 46. Further analysis showed that differences in mtDNA damage were more significantly associated with gender. DdeI+ 10,394 males had significantly higher levels of mtDNA damage relative to DdeI− 10,394 males (Figure 5B, 0.0 ± 0.094 lesions/16kb versus 0.766 ± 0.229 lesions/16kb), whereas no significant differences were observed in healthy females (data not shown).
Figure 5.
Mitochondrial DNA damage in healthy humans (A) MtDNA damage was quantified from genomic DNA extracted from PBMCs collected from 45 age-matched, healthy controls that were positive (Dde +) or negative (DdeI −) for a DdeI 10,394 restriction site, that delineates African (N = 16) or West Eurasian (N=29) mtDNA haplogroups. MtDNA damage is expressed relative to the DdeI − haplogroups (zero class lesions), and is presented as mean ± SEM. (B) MtDNA damage was quantified from genomic DNA extracted from PBMCs collected from 23 age-matched, healthy male controls that were positive (Dde +) or negative (DdeI −) for the DdeI 10,394 restriction site, indicative of mtDNA haplogroups of African (N = 8) or West Eurasian (N=15) origin. MtDNA damage is expressed relative to the DdeI − haplogroups (zero class lesions), and is presented as means ± SEM.* p < 0.05.
Vascular function, remodeling and mtDNA damage in humans
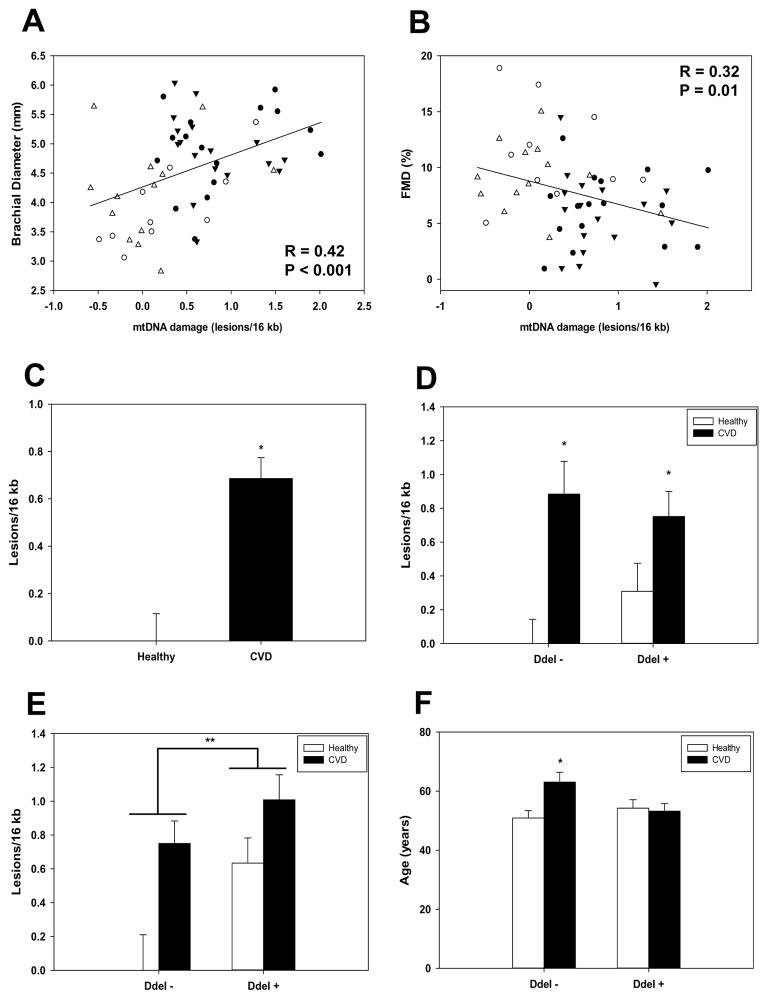
Because increased ROS production contributes to both endothelial dysfunction and mtDNA damage, we were interested in determining whether a relationship existed between mtDNA damage, vascular function, and CVD. Consequently, mtDNA damage was assayed in PBMCs collected from 33 CVD patients and 23 age-matched controls in which vascular function had also been assessed by flow-mediated dilation (FMD). Changes in brachial artery diameter were monitored in response to reactive hyperemia while vascular remodeling was assessed by quantifying the brachial diameter in the conduit arteries of the upper extremity through pulse wave velocity. Figure 6A shows that a significantly positive relationship between brachial diameter and mtDNA damage was observed in all subjects (r = 0.42, p = 0.001). Consistent with pathological changes in vascular function, a negative relationship between FMD and mtDNA damage was also observed in all subjects (Figure 6B, r = −0.32, p = 0.01).
Figure 6.
Relationship between mitochondrial DNA damage and markers of vascular dysfunction. Brachial diameter and FMD were determined in 34 CVD patients (filled symbols) and 23 age matched control (open symbols) human subjects and the relationship with PBMC mtDNA damage was examined. Circles and triangles indicate DdeI + (open circles - healthy N = 10; filled circles – CVD N = 17) and DdeI − (open triangles - healthy N = 13; filled triangles – CVD N = 17) mtDNA haplogroups, respectively. (A) A significant positive relationship was found between brachial diameter and mtDNA damage. (B) A significant negative relationship was identified between FMD and mtDNA damage. (C) Mean PBMC mtDNA damage levels were significantly different between CVD patients and age matched controls. MtDNA damage is expressed relative to healthy controls (zero class lesions). * P < 0.001. (D) MtDNA damage was quantified from genomic DNA extracted from PBMCs collected from CVD age-matched, healthy controls that were positive (Dde +) or negative (DdeI −) for a DdeI 10,394 restriction site. MtDNA damage is expressed relative to the DdeI − healthy controls (zero class lesions), and is presented as mean ± SEM. * P < 0.05 from matched healthy control. (E) MtDNA damage in DdeI− 10,394 (healthy N = 6; CVD = 15) and DdeI+ 10,394 (healthy N = 4; CVD = 13) male CVD and healthy controls. Data are expressed relative to the DdeI − healthy control group (zero class lesions). * P < 0.05 between CVD and matched healthy control, ** P < 0.05 between DdeI 10,394 (−) versus (+) groups (healthy + CVD combined). (F) Mean age of controls and CVD patients in DdeI − (N=13 healthy, 17 CVD) and DdeI + (N=10 healthy, 17 CVD) individuals. * P < 0.05 from healthy control.
To further determine whether PBMC mtDNA damage was also associated with CVD, levels of mtDNA damage were compared between patients and age-matched, healthy controls. The results showed that CVD patients had significantly higher levels of mtDNA damage in their PBMCs compared to age-matched controls (Figure 6C; 0.686 ± 0.0882 vs 0.00 ± 0.115 respectively). These differences also appeared to be maintained between the DdeI 10,394 (−) and (+) haplogroups compared to their corresponding age-matched controls (Figure 6D). As found previously, an interaction between latitude and gender was also observed in that mtDNA damage was significantly higher in the healthy male control DdeI+ 10,394 lineages compared to those with DdeI− 10,394 haplogroups (P = 0.018). In addition, these differences were also seen when comparing overall haplogroups irrespective of health status (e.g. healthy + CVD DdeI− 10,394 versus healthy + CVD DdeI+ 10,394, p = 0.021, Figure 6E). While differences between healthy and CVD groups were maintained within DdeI− 10,394 and Dde+ 10,394 haplogroups in females, there was no significant association was observed for females regarding latitude within the healthy or CVD groups (data not shown).
Finally, while the mean ages of healthy and CVD groups were not significantly different when undifferentiated by mtDNA haplogroup, further analysis revealed an interesting relationship between the ages of healthy controls, CVD patients and DdeI 10,394 status. Figure 6F shows that the age of CVD patients was significantly older in individuals that belonged to DdeI− 10,394 haplogroups relative to (DdeI−) healthy controls (12.15 years older) and their DdeI+ CVD counterparts (9.82 years older), consistent with the notion of a delayed onset of CVD for DdeI− 10,394 haplogroup individuals.
Discussion
Numerous epidemiologic studies have demonstrated that associations exist between mitochondrial haplogroup and the incidence of disease 7–9, 47–49, and more recent studies in animals have shown that mtDNA background can play a significant role in aspects of disease susceptibility in heart and liver 18, 19. Whether differences exist in cellular bioenergetics and mitochondrial damage between normal individuals having different mtDNA backgrounds has not been studied in primary cells. In this study, primary endothelial cells having mtDNAs belonging to either H (West Eurasian) or L (African) haplogroups exhibited significant differences in mitochondrial function, oxygen utilization, and damage consistent with the observed differences in CVD susceptibility between individuals with West Eurasian (e.g. European) and African ancestry.
Classically, the identification of mtDNA haplogroups was based upon the presence or absence of different diagnostic SNPs that had been identified in numerous molecular anthropology and evolutionary studies36, 50, 51. The reckoning of these SNPs, and the haplogroup classifications they facilitated, were made through comparisons with the “Cambridge Reference Sequence” (CRS) for the human mtDNA 52, which was later revised to correct for sequencing errors and haplogroup specific markers (rCRS) 53. A more refined haplogroup classification was achieved through the comparison of whole mtDNA genome sequences from various world populations 26, 54–57 and the comparison of control region (D-loop) sequences from previous mtDNA studies to these new whole mtDNA genome data sets 58. In this context, it was demonstrated that populations harboring mtDNA haplogroups from more northern latitudes had a greater proportion of missense mutations relative to those living in Africa 35. Based on this observation, it was hypothesized that the increase in missense mutations in certain East and West Eurasian haplogroups allowed for better adaptation and survival of human populations in colder climates, due to increased heat production resulting from less efficient energy coupling. By contrast, haplogroups arising in populations living in sub-Saharan Africa were predicted to be more economical in the use of caloric energy for generation of molecular energy (ATP) 35, 59, 60. The data presented in this study are consistent with the latter view in that cells having L mtDNAs generate equal amounts of ATP compared to those harboring H mtDNA yet use less oxygen. These findings are also consistent with the prediction that individuals with more economical mitochondria will be more prone to oxidative stress compared to those with less economical mitochondria (e.g. haplogroup H). They are further consistent with mitochondrial paradigms which propose that different mtDNA lineages convey distinct mitochondrial bioenergetics capacities due to prehistoric selection events for features of mitochondrial function 61.
Interestingly, women of African descent have generally lower values of total and resting daily energy expenditure (REE) rates compared to Caucasian females 62–64, and in this respect, uncoupling protein (UCP) gene variation has been interrogated as a potential basis for these differences 65. However, no significant variation in REE has been linked with UCP gene variants with exception of an exon 5 variant for UCP3, and while this variant is found in both females of African and Eurasian descent, the association is only found in those of African ancestry 65. Although UCP expression and genetic variability was not investigated herein, no differences in OCR were observed between or within the H and L haplogroups in the presence of oligomycin or antimycin A + rotenone (Figure 2A), suggesting little or no role of UCP’s in the differences seen in HUVEC experiments herein – differential UCP expression should manifest in differences in oxygen consumption rates in the presence of oligomycin – further, the lack of differences in OCR between oligomycin and antimycin A + rotenone rates suggests that the observed differences in HUVEC are not consistent with differential UCP expression. Finally, the 10398 SNP utilized to segregate mtDNA haplogroups into “northern and southern” latitudes has been previously associated certain neurodegenerative diseases and breast cancer 38, 66. However, these findings are controversial 37, 40, 41 and whether this SNP is directly related to the differences observed in these studies is not yet known. Direct comparisons between closely related mtDNA haplogroups within the West Eurasia may provide additional insights into this question.
Subtle differences in mitochondrial function in endothelial cells could potentially contribute to CVD initiation and progression through the production of ROS. Measures of mtDNA damage in both cultured cells (in vitro) and tissues (in vivo) are consistent with this notion 19, 42, 67–69. Furthermore, these differences in function are likely to be exacerbated under conditions of positive energy balance (excess substrate - high caloric intake and low energy demand - physical inactivity), cellular challenges (e.g. inflammation) which increase the propensity for mitochondrial ROS production. The observations of increased oxidative stress (aconitase activity) and mtDNA damage in HUVECs harboring haplogroup L mtDNAs relative to those having haplogroup H mtDNAs support this concept. However, under conditions of chronic stress, even individuals having haplogroup H mtDNAs would sustain significant damage over time, and therefore be also predisposed to eventual CVD development. Our observations that the average age of CVD patients is significantly older in DdeI− individuals are consistent with this notion, as are the findings of greater levels of mtDNA damage in healthy individuals harboring DdeI+ haplogroups compared to those with DdeI− lineages. Consequently, it follows that differences in mitochondrial function and damage may only be observed in “healthy” controls and will not be easily observable in individuals with advanced CVD belonging to different mtDNA haplogroups. Interestingly, comparison of vascular function (flow-mediated dilation – FMD) with the level of mtDNA damage present in lymphocytes collected from CVD patients and healthy controls revealed a significant relationship between mtDNA damage and decreased vessel relaxation (Figure 6B), accompanied by significant changes in vascular remodeling (Figure 6A). These findings suggest that perhaps mtDNA integrity of the circulating blood cells can serve as an indirect and early biomarker of vascular function and condition. Accordingly, information regarding an individual’s haplotype (haplogroup), bioenergetic profile, and mtDNA integrity (mtDNA damage) will be required for a more complete assessment of vascular disease risk. Future studies that pursue understanding the interrelationship between genetics, bioenergetics, and cellular integrity will provide a clear basis for making informed decisions regarding individual risk for cardiovascular disease development.
Studies illustrating the mitochondrion’s role in a number of disease processes continue to grow. The data presented here demonstrate that endothelial cell mtDNA from haplogroups H and L, which are maternal lineages with distinct geographic origins (West Eurasia and Africa), have unique mitochondrial bioenergetic profiles. We show that these differences also appear to associate with levels of mtDNA damage and oxidant stress. Additional analyses in blood samples from healthy humans showed that mtDNA damage was higher in DdeI+ haplogroups compared to DdeI− haplogroups and also higher in CVD patients compared to healthy controls. Consequently, when making comparisons between “healthy” and “diseased” groups it seems advisable perform haplogroup matching between age-matched controls and disease cohorts whenever possible. Increases in mtDNA damage were also associated with decreased FMD and increased vascular remodeling in humans, and was also observed to be highest in CVD patients. This work demonstrates that subtle differences in bioenergetics between mitochondrial haplogroups occur in primary cells. These effects may have a significant influence on endothelial cell function which, in turn, can affect systemic vascular function. The molecular mechanisms behind these effects are currently unclear; it is unlikely however, that a singular molecular pathway will be the basis due to the nature of mitochondrial metabolism which is at the hub of all cellular metabolism and therefore even subtle changes have the potential for impacting multiple aspects of cellular function. Studies using animal models that examine the impact of different “normal” mtDNA backgrounds have shown effects upon adaptive immunity, cognition, heart failure susceptibility, fatty liver disease, and most recently cancer 13, 18, 19, 70, 71. The diversity of these phenotypes suggests that a single mechanism is unlikely and instead argues that a complex interaction of mitochondrial and nuclear genetic backgrounds guides cellular metabolism in response to environmental cues and challenges which influences cellular function in response.
Supplementary Material
Clinical Perspective.
Studies illustrating the mitochondrion’s role in a number of disease processes continue to grow – the data herein demonstrate that endothelial cells derived from maternal lineages with distinct geographic origins (West Eurasia and Africa), have unique bioenergetic profiles that also associate with differential levels of mitochondrial DNA (mtDNA) damage and oxidant stress. Analyses using blood samples from healthy humans showed that mtDNA damage was higher in individuals having mtDNAs representative of southern geographic origins (Africa) compared to those having northern origins (West Eurasia), and that CVD patients had increased mtDNA damage compared to matched, healthy controls. Increases in mtDNA damage were also associated with decreased FMD and increased vascular remodeling in humans, and was also observed to be highest in CVD patients. This work demonstrates that subtle differences in bioenergetics between mitochondrial haplogroups occur in primary cells that may have a significant influence on vascular function. The molecular etiology behind these effects are currently unclear; however, it is unlikely that a single mechanism is causative and instead we propose that a complex interaction of mitochondrial and nuclear genetic backgrounds guide cellular metabolism in response to environmental cues and challenges which influence cellular function in response. This concept of “Mito-Mendelian” interactions could explain the genetics of common disease susceptibility and therefore may be a potentially important clinical consideration when assessing risk.
Acknowledgments
Funding Sources: Support for this project came from NIH grants RO1 HL94518, HL103859 (SWB), HL083801, HL081587, HL083269, HL75795 and HL102299 (JV), NIH postdoctoral training grant in Vascular Biology and Hypertension (T-32 HL007457) (DMK), an American Heart Association Predoctoral Fellowship 11PRE7650033 (KJD), University of Alabama at Birmingham Postbaccalaureate Research Experience Program Scholarship, supported by NIH grant 5R25GM086256 (JB), a Bridge to Doctorate fellowship, supported by National Science Foundation grant HRD-1304515 (JB), NHLBI Pre-Doctoral Training Grant T32HL007918 in Cardiovascular Pathophysiology (AWB), and funding from the Center for Clinical and Translational Science (LJD), and by the NIH-funded Diabetes Research and Training Center Bioanalytical Redox Biology Core (P60 DK079626) located at the University of Alabama at Birmingham.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forman HJ, Boveris A. Superoxide radical and hydrogen peroxide in mitochondria. In: Prior WA, editor. Free Radicals In Biology. Orlando: Academic Press; 1982. pp. 65–90. [Google Scholar]
- 3.Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J. 1980;191:421–427. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruiz-Pesini E, Wallace DC. Evidence for adaptive selection acting on the tRNA and rRNA genes of human mitochondrial DNA. Hum Mutat. 2006;11:1072–1081. doi: 10.1002/humu.20378. [DOI] [PubMed] [Google Scholar]
- 5.Wallace DC. A Mitochondrial Paradigm of Metabolic and Degenerative Diseases, Aging, and Cancer: A Dawn for Evolutionary Medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krzywanski DM, Moellering DR, Fetterman JL, Dunham-Snary KJ, Sammy MJ, Ballinger SW. The mitochondrial paradigm for cardiovascular disease susceptibility and cellular function: a complementary concept to Mendelian genetics. Lab Invest. 2011;91:1122–1135. doi: 10.1038/labinvest.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu HY, Wang SW, Martin LJ, Liu L, Li YH, Chen R, et al. The role of mitochondrial genome in essential hypertension in a Chinese Han population. Eur J Hum Genet. 2009;11:1501–1506. doi: 10.1038/ejhg.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuku N, Park KS, Yamada Y, Nishigaki Y, Cho YM, Matsuo H, et al. Mitochondrial haplogroup N9a confers resistance against type 2 diabetes in Asians. Am J Hum Genet. 2007;80:407–415. doi: 10.1086/512202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo LJ, Oshida Y, Fuku N, Takeyasu T, Fujita Y, Kurata M, et al. Mitochondrial genome polymorphisms associated with type-2 diabetes or obesity. Mitochondrion. 2005;5:15–33. doi: 10.1016/j.mito.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka M, Fuku N, Nishigaki Y, Matsuo H, Segawa T, Watanabe S, et al. Women with mitochondrial haplogroup N9a are protected against metabolic syndrome. Diabetes. 2007;56:518–521. doi: 10.2337/db06-1105. [DOI] [PubMed] [Google Scholar]
- 11.Darvishi K, Sharma S, Bhat AK, Rai E, Bamezai RN. Mitochondrial DNA G10398A polymorphism imparts maternal Haplogroup N a risk for breast and esophageal cancer. Cancer Lett. 2007;249:249–255. doi: 10.1016/j.canlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Pyle A, Foltynie T, Tiangyou W, Lambert C, Keers SM, Allcock LM, et al. Mitochondrial DNA haplogroup cluster UKJT reduces the risk of PD. Ann Neurol. 2005;57:564–567. doi: 10.1002/ana.20417. [DOI] [PubMed] [Google Scholar]
- 13.Feeley KP, Bray AW, Westbrook DG, Johnson LW, Kesterson RA, Ballinger SW, et al. Mitochondrial Genetics Regulate Breast Cancer Tumorigenicity and Metastatic Potential. Cancer Res. 2015;75:4429–4436. doi: 10.1158/0008-5472.CAN-15-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houstek J, Vrbacky M, Hejzlarova K, Zidek V, Landa V, Silhavy J, et al. Effects of mtDNA in SHR-mtF344 versus SHR conplastic strains on reduced OXPHOS enzyme levels, insulin resistance, cardiac hypertrophy, and systolic dysfunction. Physiol Genomics. 2014;46:671–678. doi: 10.1152/physiolgenomics.00069.2014. [DOI] [PubMed] [Google Scholar]
- 15.Kumarasamy S, Gopalakrishnan K, Shafton A, Nixon J, Thangavel J, Farms P, et al. Mitochondrial polymorphisms in rat genetic models of hypertension. Mamm Genome. 2010;21:299–306. doi: 10.1007/s00335-010-9259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumarasamy S, Gopalakrishnan K, bdul-Majeed S, Partow-Navid R, Farms P, Joe B. Construction of two novel reciprocal conplastic rat strains and characterization of cardiac mitochondria. Am J Physiol Heart Circ Physiol. 2013;304:H22–H32. doi: 10.1152/ajpheart.00534.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roubertoux PL, Sluyter F, Carlier M, Marcet B, Maarouf-Veray F, Cherif C, et al. Mitochondrial DNA modifies cognition in interaction with the nuclear genome and age in mice. Nat Genet. 2003;35:65–69. doi: 10.1038/ng1230. [DOI] [PubMed] [Google Scholar]
- 18.Betancourt AM, King AL, Fetterman JL, Millender-Swain T, Finley RD, Oliva CR, et al. Mitochondrial-nuclear genome interactions in nonalcoholic fatty liver disease in mice. Biochem J. 2014;46:223–232. doi: 10.1042/BJ20131433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fetterman JL, Zelickson BR, Johnson LW, Moellering DR, Westbrook DG, Pompilius M, et al. Mitochondrial genetic background modulates bioenergetics and susceptibility to acute cardiac volume overload. Biochem J. 2013;455:157–167. doi: 10.1042/BJ20130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pravenec M, Hyakukoku M, Houstek J, Zidek V, Landa V, Mlejnek P, et al. Direct linkage of mitochondrial genome variation to risk factors for type 2 diabetes in conplastic strains. Genome Res. 2007;17:1319–1326. doi: 10.1101/gr.6548207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sethumadhavan S, Vasquez-Vivar J, Migrino RQ, Harmann L, Jacob HJ, Lazar J. Mitochondrial DNA variant for complex I reveals a role in diabetic cardiac remodeling. J Biol Chem. 2012;287:22174–22182. doi: 10.1074/jbc.M111.327866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amo T, Yadava N, Oh R, Nicholls DG, Brand MD. Experimental assessment of bioenergetic differences caused by the common European mitochondrial DNA haplogroups H and T. Gene. 2008;411:69–76. doi: 10.1016/j.gene.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez-Duran A, Pacheu-Grau D, Lopez-Gallardo E, Diez-Sanchez C, Montoya J, Lopez-Perez MJ, et al. Unmasking the causes of multifactorial disorders: OXPHOS differences between mitochondrial haplogroups. Hum Mol Genet. 2010;19:3343–3353. doi: 10.1093/hmg/ddq246. [DOI] [PubMed] [Google Scholar]
- 24.Moreno-Loshuertos R, Acin-Perez R, Fernandez-Silva P, Movilla N, Perez-Martos A, de Cordoba SR, et al. Differences in reactive oxygen species production explain the phenotypes associated with common mouse mitochondrial DNA variants. Nat Genet. 2006;38:1261–1268. doi: 10.1038/ng1897. [DOI] [PubMed] [Google Scholar]
- 25.Kong QP, Yao YG, Sun C, Bandelt HJ, Zhu CL, Zhang YP. Phylogeny of East Asian mitochondrial DNA lineages inferred from complete sequences. Am J Hum Genet. 2003;73:671–676. doi: 10.1086/377718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palanichamy MG, Sun C, Agrawal S, Bandelt HJ, Kong QP, Khan F, et al. Phylogeny of mitochondrial DNA macrohaplogroup N in India, based on complete sequencing: Implications for the peopling of South Asia. Am J Hum Genet. 2004;75:966–978. doi: 10.1086/425871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrick DA, Neilson A, Beeson C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov Today. 2008;13:268–274. doi: 10.1016/j.drudis.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Gerencser AA, Neilson A, Choi SW, Edman U, Yadava N, Oh RJ, et al. Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal Chem. 2009;81:6868–6878. doi: 10.1021/ac900881z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dranka BP, Hill BG, Darley-Usmar VM. Mitochondrial reserve capacity in endothelial cells: The impact of nitric oxide and reactive oxygen species. Free Radic Biol Med. 2010;48:905–914. doi: 10.1016/j.freeradbiomed.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballinger SW, Van Houten B, Jin GF, Conklin CA, Godley BF. Hydrogen peroxide causes significant mitochondrial DNA damage in human RPE cells. Exp Eye Res. 1999;68:765–772. doi: 10.1006/exer.1998.0661. [DOI] [PubMed] [Google Scholar]
- 31.Ballinger SW, Patterson C, Knight-Lozano CA, Burow DL, Conklin CA, Hu Z, et al. Mitochondrial integrity and function in atherogenesis. Circulation. 2002;106:544–549. doi: 10.1161/01.cir.0000023921.93743.89. [DOI] [PubMed] [Google Scholar]
- 32.Gardner PR. Superoxide-driven aconitase FE-S center cycling. Biosci Rep. 1997;17:33–42. doi: 10.1023/a:1027383100936. [DOI] [PubMed] [Google Scholar]
- 33.Gardner PR. Aconitase: sensitive target and measure of superoxide. Methods Enzymol. 2002;349:9–23. doi: 10.1016/s0076-6879(02)49317-2. [DOI] [PubMed] [Google Scholar]
- 34.Hausladen A, Fridovich I. Measuring nitric oxide and superoxide: rate constants for aconitase reactivity. Methods Enzymol. 1996;269:37–41. doi: 10.1016/s0076-6879(96)69007-7. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz-Pesini E, Mishmar D, Brandon M, Procaccio V, Wallace DC. Effects of purifying and adaptive selection on regional variation in human mtDNA. Science. 2004;303:223–226. doi: 10.1126/science.1088434. [DOI] [PubMed] [Google Scholar]
- 36.Ballinger SW, Schurr TG, Torroni A, Gan YY, Hodge JA, Hassan K, et al. Southeast Asian mitochondrial DNA analysis reveals genetic continuity of ancient mongoloid migrations. Genetics. 1992;130:139–152. doi: 10.1093/genetics/130.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blein S, Berndt S, Joshi AD, Campa D, Ziegler RG, Riboli E, et al. Factors associated with oxidative stress and cancer risk in the Breast and Prostate Cancer Cohort Consortium. Free Radic Res. 2014;48:380–386. doi: 10.3109/10715762.2013.875168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canter JA, Kallianpur AR, Parl FF, Millikan RC. Mitochondrial DNA G10398A polymorphism and invasive breast cancer in African-American women. Cancer Res. 2005;65:8028–8033. doi: 10.1158/0008-5472.CAN-05-1428. [DOI] [PubMed] [Google Scholar]
- 39.Kulawiec M, Owens KM, Singh KK. mtDNA G10398A variant in African-American women with breast cancer provides resistance to apoptosis and promotes metastasis in mice. J Hum Genet. 2009;54:647–654. doi: 10.1038/jhg.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pezzotti A, Kraft P, Hankinson SE, Hunter DJ, Buring J, Cox DG. The mitochondrial A10398G polymorphism, interaction with alcohol consumption, and breast cancer risk. PLoS One. 2009;4:e5356. doi: 10.1371/journal.pone.0005356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Setiawan VW, Chu LH, John EM, Ding YC, Ingles SA, Bernstein L, et al. Mitochondrial DNA G10398A variant is not associated with breast cancer in African-American women. Cancer Genet Cytogenet. 2008;181:16–19. doi: 10.1016/j.cancergencyto.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ballinger SW, Patterson WC, Yan C-N, Doan R, Burow DL, Young CG, et al. Hydrogen peroxide and peroxynitrite induced mitochondrial DNA damage and dysfunction in vascular endothelial and smooth muscle cells. Circ Res. 2000;86:960–966. doi: 10.1161/01.res.86.9.960. [DOI] [PubMed] [Google Scholar]
- 43.McMackin CJ, Vita JA. Update on nitric oxide-dependent vasodilation in human subjects. Methods Enzymol. 2005;396:541–553. doi: 10.1016/S0076-6879(05)960-16-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vita JA. Nitric oxide-dependent vasodilation in human subjects. Methods Enzymol. 2002;359:186–200. doi: 10.1016/s0076-6879(02)59183-7. [DOI] [PubMed] [Google Scholar]
- 45.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell SL, Goodloe R, Brown-Gentry K, Pendergrass SA, Murdock DG, Crawford DC. Characterization of mitochondrial haplogroups in a large population-based sample from the United States. Hum Genet. 2014;133:861–868. doi: 10.1007/s00439-014-1421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bai RK, Leal SM, Covarrubias D, Liu A, Wong LJ. Mitochondrial genetic background modifies breast cancer risk. Cancer Res. 2007;67:4687–4694. doi: 10.1158/0008-5472.CAN-06-3554. [DOI] [PubMed] [Google Scholar]
- 48.Shen L, Wei J, Chen T, He J, Qu J, He X, et al. Evaluating mitochondrial DNA in patients with breast cancer and benign breast disease. J Cancer Res Clin Oncol. 2011;137:669–675. doi: 10.1007/s00432-010-0912-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lakatos A, Derbeneva O, Younes D, Keator D, Bakken T, Lvova M, et al. Association between mitochondrial DNA variations and Alzheimer’s disease in the ADNI cohort. Neurobiol Aging. 2010;31:1355–1363. doi: 10.1016/j.neurobiolaging.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen YS, Torroni A, Excoffier L, Santachiara-Benerecetti AS, Wallace DC. Analysis of mtDNA variation in African populations reveals the most ancient of all human continent-specific haplogroups. Am J Hum Genet. 1995;57:133–149. [PMC free article] [PubMed] [Google Scholar]
- 51.Torroni A, Huoponen K, Francalacci P, Petrozzi M, Morelli L, Scozzari R, et al. Classification of European mtDNAs from an analysis of three European populations. Genetics. 1996;144:1835–1850. doi: 10.1093/genetics/144.4.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderson S, Bankier AT, Barrell BG, de Bruin MH, Coulson AR, Drouin J, et al. Sequence and organization of the human miochonrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 53.Andrews RM, Kubacka I, Chinnery PF, Lightowlers RN, Turnbull DM, Howell N. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat Genet. 1999;23:147. doi: 10.1038/13779. [DOI] [PubMed] [Google Scholar]
- 54.Hudjashov G, Kivisild T, Underhill PA, Endicott P, Sanchez JJ, Lin AA, et al. Revealing the prehistoric settlement of Australia by Y chromosome and mtDNA analysis. Proc Natl Acad Sci USA. 2007;104:8726–8730. doi: 10.1073/pnas.0702928104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kivisild T, Tolk HV, Parik J, Wang Y, Papiha SS, Bandelt HJ, et al. The emerging limbs and twigs of the East Asian mtDNA tree. Mol Biol Evol. 2002;19:1737–1751. doi: 10.1093/oxfordjournals.molbev.a003996. [DOI] [PubMed] [Google Scholar]
- 56.Tamm E, Kivisild T, Reidla M, Metspalu M, Smith DG, Mulligan CJ, et al. Beringian standstill and spread of Native American founders. PLoS One. 2007;2:e829. doi: 10.1371/journal.pone.0000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonder MK, Mortensen HM, Reed FA, de SA, Tishkoff SA. Whole-mtDNA genome sequence analysis of ancient African lineages. Mol Biol Evol. 2007;24:757–768. doi: 10.1093/molbev/msl209. [DOI] [PubMed] [Google Scholar]
- 58.van OM, Kayser M. Updated comprehensive phylogenetic tree of global human mitochondrial DNA variation. Hum Mutat. 2009;30:E386–E394. doi: 10.1002/humu.20921. [DOI] [PubMed] [Google Scholar]
- 59.Mishmar D, Ruiz-Pesini E, Golik P, Macaulay V, Clark AG, Hosseini S, et al. Natural selection shaped regional mtDNA variation in humans. Proc Natl Acad Sci USA. 2003;100:171–176. doi: 10.1073/pnas.0136972100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kivisild T, Shen P, Wall DP, Do B, Sung R, Davis K, et al. The role of selection in the evolution of human mitochondrial genomes. Genetics. 2006;172:373–387. doi: 10.1534/genetics.105.043901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wallace DC, Ruiz-Pesini E, Mishmar D. mtDNA variation, climatic adaptation, degenerative diseases, and longevity. Cold Spring Harb Symp Quant Biol. 2003;68:479–86. doi: 10.1101/sqb.2003.68.471. [DOI] [PubMed] [Google Scholar]
- 62.Carpenter WH, Fonong T, Toth MJ, Ades PA, Calles-Escandon J, Walston JD, et al. Total daily energy expenditure in free-living older African-Americans and Caucasians. Am J Physiol. 1998;274:E96–E101. doi: 10.1152/ajpendo.1998.274.1.E96. [DOI] [PubMed] [Google Scholar]
- 63.Chitwood LF, Brown SP, Lundy MJ, Dupper MA. Metabolic propensity toward obesity in black vs white females: responses during rest, exercise and recovery. Int J Obes Relat Metab Disord. 1996;20:455–462. [PubMed] [Google Scholar]
- 64.Weyer C, Snitker S, Bogardus C, Ravussin E. Energy metabolism in African Americans: potential risk factors for obesity. Am J Clin Nutr. 1999;70:13–20. doi: 10.1093/ajcn/70.1.13. [DOI] [PubMed] [Google Scholar]
- 65.Kimm SY, Glynn NW, Aston CE, Damcott CM, Poehlman ET, Daniels SR, et al. Racial differences in the relation between uncoupling protein genes and resting energy expenditure. Am J Clin Nutr. 2002;75:714–719. doi: 10.1093/ajcn/75.4.714. [DOI] [PubMed] [Google Scholar]
- 66.Wallace DC, Shoffner JM, Trounce I, Brown MD, Ballinger SW, Corral-Debrinski M, et al. Mitochondrial DNA mutations in human degenerative diseases and aging. Biochim Biophys Acta. 1995;1271:141–151. doi: 10.1016/0925-4439(95)00021-u. [DOI] [PubMed] [Google Scholar]
- 67.Yang Z, Knight CA, Mamerow MM, Vickers K, Penn A, Postlethwait EM, et al. Prenatal environmental tobacco smoke exposure promotes adult atherogenesis and mitochondrial damage in apolipoprotein E−/− mice fed a chow diet. Circulation. 2004;110:3715–3720. doi: 10.1161/01.CIR.0000149747.82157.01. [DOI] [PubMed] [Google Scholar]
- 68.Harrison CM, Pompilius M, Pinkerton KE, Ballinger SW. Mitochondrial oxidative stress significantly influences atherogenic risk and cytokine-induced oxidant production. Environ Health Perspect. 2011;119:676–681. doi: 10.1289/ehp.1002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fetterman JL, Pompilius M, Westbrook DG, Uyeminami D, Brown J, Pinkerton KE, et al. Developmental exposure to second-hand smoke increases adult atherogenesis and alters mitochondrial DNA copy number and deletions in apoE(−/−) mice. PLoS One. 2013;8:e66835. doi: 10.1371/journal.pone.0066835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deuse T, Wang D, Stubbendorff M, Itagaki R, Grabosch A, Greaves LC, et al. SCNT-derived ESCs with mismatched mitochondria trigger an immune response in allogeneic hosts. Cell Stem Cell. 2015;16:33–38. doi: 10.1016/j.stem.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 71.Sharpley MS, Marciniak C, Eckel-Mahan K, McManus M, Crimi M, Waymire K, et al. Heteroplasmy of mouse mtDNA is genetically unstable and results in altered behavior and cognition. Cell. 2012;151:333–343. doi: 10.1016/j.cell.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.