Abstract
Human Immunodeficiency Virus (HIV)-infected individuals are at increased risk for developing neurocognitive disorders and depression. These conditions collectively affect more than 50% of people living with HIV/AIDS and adversely impact adherence to HIV therapy. Thus, identification of early markers of neurocognitive impairment could lead to interventions that improve psychosocial functioning and slow or reverse disease progression through improved treatment adherence. Evidence has accumulated for the role and function of microRNAs in normal and pathological conditions. We have optimized a protocol to profile microRNAs in body fluids. Using this methodology, we have profiled plasma microRNA expression for 30 age-matched, HIV-infected (HIV+) patients and identified highly sensitive and specific microRNA signatures distinguishing HIV+ patients with cognitive impairment from those without cognitive impairment. These results justify follow-on studies to determine whether plasma microRNA signatures can be used as a screening or prognostic tool for HIV+ patients with neurocognitive impairment.
Keywords: microRNA, HIV, neurological disorders
Introduction
Neurocognitive impairments affect more than 50% of HIV+ individuals, with negative consequences for antiretroviral therapy (ART) adherence and high-risk behaviors, including drug and alcohol abuse (Hinkin et al., 2007; Tozzi et al., 2003). The introduction of ART greatly improved the lifespan of people with HIV/AIDS. However, for many patients immune fitness is not fully restored, and persistent viral replication increases the risk for chronic cognitive impairment (Deeks et al., 2013). Since diagnosis of cognitive disorders of the central nervous system (CNS) often cannot be achieved through direct assessment (tissue biopsies or spinal fluid analyses), they are determined through evaluation of symptoms which are often evident only at later stages of disease. Thus, the discovery of circulating (plasma or cell-based) biomarkers for early diagnosis is a top priority for CNS disorders, including HIV-associated neurocognitive disorders (HAND). Critical obstacles in identifying biomarkers for those slowly progressing/chronic conditions are the adaptive responses that take place to re-balance acutely activated factors and networks. Nevertheless, in the past few years the discovery of cell-free microRNAs, together with the development of new technologies for their detection in body fluids, has permitted formulation of new hypotheses for biomarker discovery. For example, microRNA signatures related to cancer have been proposed for identifying patients at risk for disease progression (Erkan et al., 2011; Gibcus et al., 2009; O'Hara et al., 2008; Pedranzini et al., 2010; Robertus et al., 2010; Shah et al., 2010), and diagnostic and prognostic utility has been identified for microRNA quantification within body fluids for both cancers and neurocognitive disorders (Alevizos and Illei, 2010; Cho, 2010; De Smaele et al., 2010; Ferracin et al., 2010; Heneghan et al., 2010; Ju, 2010; Kosaka et al., 2010; Shafi et al., 2010; Taft et al., 2009; Wittmann and Jack, 2010; Xie et al., 2010). In addition, existing data strongly implicate brain-enriched microRNAs in pathogenesis for HIV+ individuals with encephalopathy (HIVE) (Eletto et al., 2008; Noorbakhsh et al., 2010; Rom et al., 2010; Tatro et al., 2010; Yelamanchili et al., 2010), and two independent studies enrolling HIV-negative patients have identified plasma microRNAs which are enriched in brain tissue and may identify patients with mild cognitive impairment (Sheinerman et al., 2013a; Sheinerman et al., 2012). These latter data and others indicate that because of their high stability and resistance to RNases, plasma microRNAs may be good candidate biomarkers for CNS diseases (De Felice et al., 2012; Gandhi et al., 2013; Jin et al., 2013; Rao et al., 2013; Sheinerman and Umansky, 2013a; Witwer et al., 2011). We have recently identified two microRNAs, miR-23a-3p and miR-23b-3p, which are consistently expressed at high levels within plasma from HIV+ patients and, therefore, can serve as reference genes for normalization and indicators of RNA quality. Taking advantage of this advance, we sought to identify microRNA-pairs that can distinguish, with high sensitivity and specificity, HIV+ individuals with and without cognitive impairment.
Materials and Methods
Whole blood collection
Following informed consent, 30 ml whole blood was collected from HIV-infected patients at the Louisiana State University Health Sciences Center (LSUHSC) HIV Outpatient (HOP) Clinic in New Orleans, Louisiana in the context of routine health assessment visits to this clinic. Samples were de-identified using an alphanumeric coding system, and Whole blood was transported twice daily (within two hours of collection) to the HIV Specimen Biorepository housed within the Stanley S. Scott Cancer Center (SSSCC) where plasma was immediately separated from cell fractions and stored at −80°C. Patients with opportunistic infections involving the CNS, existing CNS tumors, history of significant head injury, multiple sclerosis, and other dementing disorders (Alzheimer’s disease) were excluded from the study. All studies were performed with approval from the LSUHSC Institutional Review Board and in conjunction with national guidelines for protection of patient confidentiality and safety.
Cognitive testing
Cognitive functioning was assessed using a modified version of the Multicenter AIDS Cohort Study (MACS) screening battery, which measures memory, attention, processing speed, language, and motor skill. Participants completed six measures of cognitive functioning: WAIS-IV Digit Span, Controlled Oral Word Association Test, Rey Auditory Verbal Learning Test, Trail Making Test, Symbol Digit Modalities Test, and Grooved Pegboard Test. Raw scores on these measures were converted to t-scores using demographically-adjusted normative data. T-scores were converted to deficit scores and averaged to develop Global Deficit Scores (GDS) using methods previously reported by Heaton and Carey (Carey et al., 2004; Heaton et al., 1995). GDS scores range from 0 to 5 with higher scores indicative of greater cognitive impairment. A GDS of 0.5 or higher is considered a positive predictive value in establishing HIV-associated cognitive impairment (Carey et al., 2004). Accordingly, patients were categorized as either cognitively impaired (CI; GDS ≥ 0.5) or unimpaired (nonCI; GDS < 0.5) based on their performance across these six measures. The categories of cognitively impaired and unimpaired were used to determine between-group differences in expression of microRNAs.
RNA extraction, quality control, and miRNA profiling
RNA extraction and microRNA profiling were performed as previously reported (Pacifici et al., 2014). RNA was obtained from 200 μl of plasma using the miRCURY RNA extraction kit (Exiqon, Woburn, MA). To increase the RNA recovery, 1 μg of MS2 carrier RNA were added to each plasma sample. 8 μl of total RNA was subjected to retro-transcription using the Universal cDNA synthesis kit (Exiqon, Woburn, MA), followed by qRT-PCR using microRNA ready-to-use PCR human Panels I and II V3.0 (Exiqon, Woburn, MA). qRT-PCR was carried out on a Roche LightCycler 480 Real-Time PCR System according to the Exiqon recommended protocol. Cycling conditions were as follows: 95°C for 10 minutes, 40 cycles of 15 seconds at 95°C, and 60 seconds at 60°C. Fluorescent data were converted into cycle threshold (Ct) measurements by the Roche LyghtCycler system software (Version 1.5; Roche). Quantification using 2nd derivative maximum were further calculated with Roche Lightcycler 480 software. qPCR data were analyzed in GenEx Professional 5 software (MultiD Analyses AB, Goteborg, Sweden). Degree of hemolysis was determined as the difference in Ct of miR-23a-3p (a microRNA not affected by hemolysis) and miR-451a (an indicator of hemolysis); this calculation was performed in GenEx. All samples had a ΔCt < 5, which indicated no hemolysis. After inter-plate calibration, the amount of target microRNAs was normalized relative to the amount of reference genes, determined using the GeNorm algorithm, an application of GenEx software.
Statistical Analyses
Statistical calculations were performed in GenEx Professional software. Mann-Whitney tests were two-sided and set at 5% level and 95% confidence intervals (CIs). Bonferroni correction was applied to determine statistically significant microRNA pairs. The potential of microRNA pairs for use in diagnosis of neurocognitive impairment was assessed by estimating sensitivity and specificity based on an ROC analysis using GraphPad Prism 5. Pearson’s correlations were performed in GraphPad Prism 5.
Results
We profiled plasma microRNAs from 30 individual patients recruited from an existing cohort of >400 individuals participating in the HOP Clinic Cancer Prevention Study (HOP CPS Cohort). Participants enrolled in the HOP CPS Cohort exhibit characteristics similar to those receiving care at other academic centers: 94% are on ART with a mean CD4+ T-cell count of 458 cells/mm3 (range 12 to 1773); 14% exhibit undetectable HIV viral loads (< 20 copies/ml), and 62% have viral loads < 400 copies/ml; African Americans comprise 88% of the cohort. Patients in the subsample underwent neurocognitive evaluations and were categorized as either cognitively impaired (CI, n=17) (Global Deficit Score, GDS ≥ 0.5) or unimpaired (nonCI, n=13, GDS < 0.5) based on their performance across six measures, as detailed in Methods. The mean age for the two groups was 50. Demographics, neurocognitive status and other relevant parameters are shown in Table I.
Table I.
Demographic and medical parameters of HIV+ patients.
| CI (n=17) | nonCI (n=13) | ||
|---|---|---|---|
| Age | Avg | 50.64 | 49.38 |
| Min-Max | 41–59 | 42–57 | |
| Education | ≤ 8 grade | 5 | 1 |
| > 8 grade | 12 | 12 | |
| Gender | Males | 9 | 10 |
| Females | 8 | 4 | |
| Ethnicity | African-American | 10 | 12 |
| Caucasian | 6 | 1 | |
| Multiracial | 1 | 0 | |
| GDS | ≥ 0.5 | < 0.5 | |
| HCV | 1 | 1 | |
| VL | Avg | 978 | 610 |
| Min-Max | 20–7083 | 20–3925 | |
| N/A | 2 | 1 | |
| <400 | 10 (58.8%) | 8 (61.5%) | |
| <20 | 1 | 2 | |
| CD4 | Avg | 548 | 453 |
| Min-Max | 194–1034 | 202–806 | |
| N/A | 1 | 1 |
n= number of patients; GDS= Global Deficit Score; VL= viral load/ml; CD4= CD4 count/ml; N/A= number of samples in which the parameter is not available
Use of MiR-23a-3p and MiR-23b-3p for Normalization and for RNA Quality
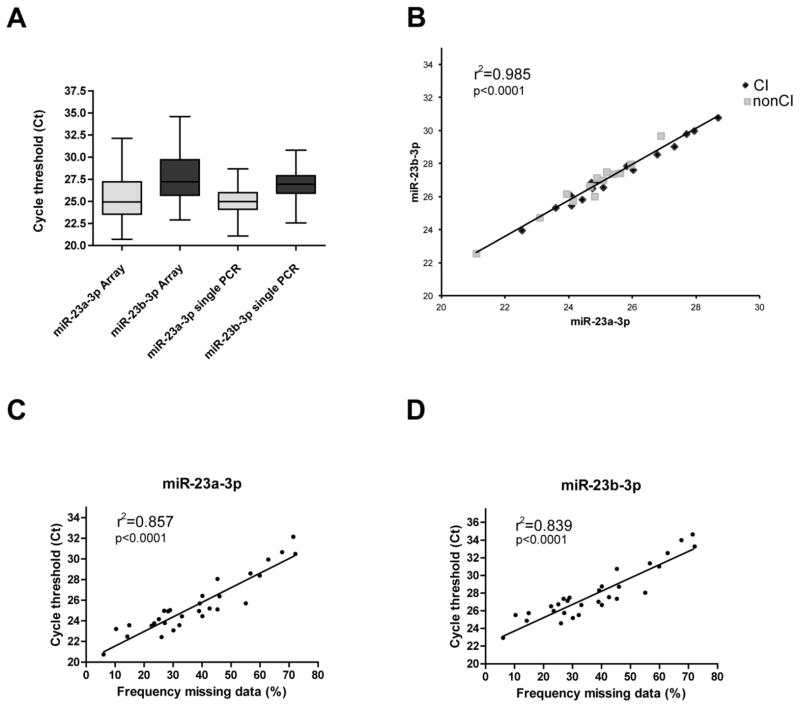
A total of 752 unique microRNAs were profiled for each sample. After interplate calibration, a cycle threshold (Ct) cutoff of 39 was applied, and 230 microRNAs were discarded. The pre-processing also included a 60% validation of the data, in which the microRNAs that were not detected in at least 60% of the samples and were discarded. This accounted for the additional removal of 281 microRNAs. The remaining 241 microRNAs were considered for further analysis. First, we determined the frequency of missing data (valid Ct values). 57 microRNAs were detected in all (100%) plasma samples; a similar number (61 microRNAs) was detected in 60 to 70% of samples. The remaining microRNAs (a total of 110) were almost equally represented among 70 to 99% of the samples. Next, the 57 microRNAs detected in all samples were tested for their possible use as reference genes, using geNORM algorithm (incorporated in the GenEx software, see Materials and Methods). MiR-23a-3p and miR-23b-3p had the best M-value (0.3), which indicates the variation of a microRNA compared to all other candidate microRNAs (Vandesompele et al., 2002). The range of detection (Ct) of those microRNAs across the samples was between 20.7 and 32.1 for miR-23a-3p and between 24.5 and 30.4 for miR-23b-3p, with a standard deviation of 2.74 and 2.90, respectively (Fig. 1A, left two plots). We also recognized that samples which had the highest Cts for miR-23a-3p and miR-23b-3p were also those with the highest number of missing values in the arrays. Indeed, the Ct values of both microRNAs correlated very well with the percentage of missing data (Fig. 1C–D). Therefore for additional validation, we repeated RNA extraction from plasma samples with missing data to specifically reassess expression levels of miR-23a-3p and miR-23b-3p, and found improved Ct values for both microRNAs in the newly prepared RNA samples (Fig. 1A, right two plots). The relationship between the expression levels of miR-23a-3p and miR-23b-3p across our samples was further assessed using Pearson’s correlation, which indicated a strong positive correlation (R2= 0.985, Fig. 1B). Overall, these results indicate that miR-23a-3p and miR-23b-3p can be utilized to assess the quality of RNA/cDNA in plasma samples and can serve as reference genes.
Figure 1. Use of miR-23a-3p and miR-23b-3p as reference gene and for sample quality.
(A) Box plots represent cycle threshold (Ct) for miR-23a-3p and miR-23b-3p in the arrays (left two plots) and in single qRT-PCRs (right two plots). The mean value is indicated in each box plot. (B) Pearson’s correlation of miR-23a-3p and miR-23b-3p as determined by individual qRT-PCRs. (C-D). Scattered plots show the analysis of correlation between the frequency of missing data in arrays and Ct values for miR-23a-3p (C) or miR-23b-3p (D). Pearson’s rank correlation coefficient r2 and p values are indicated.
MicroRNA Pairs Associate with CI in HIV+ Patients
To identify microRNAs that could distinguish CI from nonCI in HIV+ patients, we performed microRNA pair-wise analyses (Hennessey et al., 2012; Sheinerman et al., 2012), as described in detail in Materials and Methods. After unequal variance t-test analysis, we subjected microRNA-pairs to the Receiver-Operating Characteristic (ROC) curve analyses to evaluate the discriminating value of microRNAs as biomarkers of cognitive impairment. This method resulted in over 30 microRNA pairs with an AUC > 0.8. Table II shows the microRNA pairs with a p value < 0.03. Interestingly, the most heavily represented microRNA among pairs with a low p value was miR-495-3p. It is important to note that although the miR-495-3p/miR-19b-5p pairing exhibited a perfect AUC, one or both microRNAs were not detected in 5 and 6 samples within CI and nonCI groups, respectively. In contrast, miR-376a-3p/miR-16-5p and miR-495-3p/miR-29a-5p were detected in almost every sample from each group, and therefore despite lower AUC, rationalized these pairings as stronger candidates for further validation. Comparisons are depicted in Figure 2, showing the relative expression of the three microRNA pairs including miR-495-3p.
Table II.
List of microRNA pairs that discriminate cognitively impaired from non-impaired HIV+ patients obtained with qRT-PCR arrays.
| miRNA pairs | AUC | Sensitivity | Specificity | P-Value | CI (n=17) | nonCI (n=13) |
|---|---|---|---|---|---|---|
| miR-495-3p/miR-151a-5p | 0.97 | 1.00 | 0.82 | 1.37E-06 | 15 | 9 |
| miR-495-3p/miR-194-5p | 0.98 | 0.93 | 0.91 | 3.16E-06 | 15 | 10 |
| miR-495-3p/miR-30d-3p | 0.99 | 1.00 | 0.89 | 0.000167 | 12 | 8 |
| miR-495-3p/miR-130b-5p | 0.96 | 0.92 | 0.90 | 0.00020 | 13 | 9 |
| miR-495-3p/miR-942 | 0.98 | 1.00 | 0.89 | 0.000222 | 12 | 8 |
| miR-495-3p/miR-19b-1-5p | 1.00 | 1.00 | 0.86 | 0.000389 | 12 | 7 |
| miR-221-5p/miR-19b-1-5p | 1.00 | 1.00 | 0.86 | 0.000389 | 12 | 7 |
| miR-495-3p/miR-135a-5p | 0.97 | 1.00 | 0.88 | 0.000391 | 13 | 7 |
| miR-495-3p/miR-16-5p | 0.99 | 1.00 | 0.89 | 0.000491 | 9 | 8 |
| miR-495-3p/miR-92b-3p | 0.95 | 0.92 | 0.89 | 0.000501 | 12 | 8 |
| miR-495-3p/miR-139-3p | 0.95 | 0.80 | 0.90 | 0.000675 | 10 | 9 |
| miR-495-3p/miR-1271-5p | 0.98 | 0.92 | 0.86 | 0.000729 | 12 | 6 |
| miR-32-3p/miR-19b-1-5p | 0.99 | 1.00 | 0.86 | 0.000913 | 10 | 6 |
| hsa-miR-376a-3p/miR-16-5p | 0.89 | 0.67 | 0.90 | 0.001381 | 12 | 11 |
| miR-376b-3p/miR-19b-1-5p | 0.95 | 0.82 | 0.86 | 0.001791 | 11 | 6 |
| miR-376c-3p/miR-19b-1-5p | 1.00 | 1.00 | 0.83 | 0.001957 | 8 | 6 |
| miR-411-5p/miR-19b-1-5p | 0.92 | 0.83 | 0.86 | 0.00271 | 12 | 6 |
| miR-127-3p/miR-19b-1-5p | 1.00 | 1.00 | 0.83 | 0.002713 | 7 | 6 |
| miR-495-3p/miR-29a-5p | 0.92 | 0.92 | 0.86 | 0.003111 | 12 | 6 |
| miR-495-3p/miR-744-5p | 0.88 | 0.90 | 0.70 | 0.003115 | 11 | 10 |
| miR-22-5p/miR-19b-1-5p | 0.86 | 0.89 | 0.86 | 0.01727 | 9 | 7 |
| miR-495-3p/let-7b-5p | 0.80 | 0.67 | 0.89 | 0.02095 | 12 | 9 |
| miR-379-3p/miR-19b-1-5p | 0.83 | 0.90 | 0.87 | 0.02096 | 11 | 7 |
The area under the curve (AUC) is indicated together with specificity, sensitivity and p-values. The last two columns indicate the number of samples in which the two microRNAs were detected.
Figure 2. Differentially regulated plasma microRNA pairs in HIV-patients with neurocognitive impairment (CI) compared to non-cognitively impaired patients (nonCI).
For each microRNA-pair, relative expression in the two groups is represented on the left graphs as 2−ΔCt. Actual p-values are reported for each experiment. Receiver-operator Characteristic (ROC) curves (right panels) were constructed and the area under the curves was calculated to evaluate sensitivity and specificity of the indicated biomarker sets (see also Table II).
Validation of microRNA pairs with individual qRT-PCRs
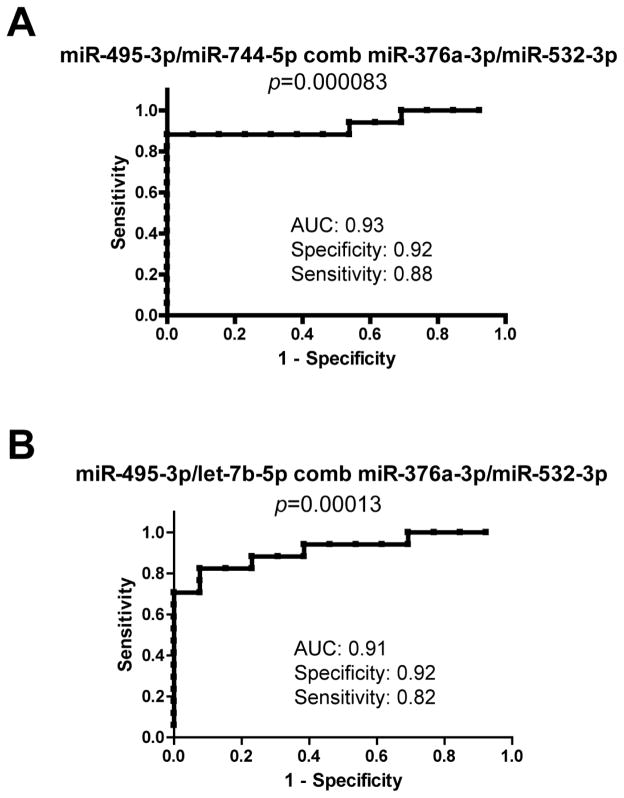
Next, we performed qRT-PCR for individual microRNAs and their respective pairs for further validation. Selected microRNAs were assayed from each of the 30 plasma samples, and miR-23a-3p and miR-23b-3p used to normalize Ct values prior to determining the microRNA pairs that best distinguished CI and nonCI groups (Table III). Ten microRNA pairs were confirmed differentially expressed, and the pair miR-495-3p/let-7b-5p actually exhibited improved sensitivity using individual qRT-PCR relative to the array approach (compare Tables II and III). Four microRNA pairs best distinguished CI from nonCI groups (miR-495-3p/let-7b-5p, miR-495-3p/miR-151-5p, miR-495-3p/miR-744-5p and miR-376a-3p/miR-16-5p), and there was no association between expression of these microRNA-pairs and any of the clinical and demographic variables listed in Table I. Finally, combinations of microRNA–pairs were tested for their ability to further distinguish CI from nonCI groups. ROC analyses revealed two sets of microRNA-pairs exhibiting improved sensitivity and specificity for distinguishing CI from nonCI groups: miR-376a-3p/miR-532-3p combined with either miR-495-3p/miR-744-5p (Fig. 4A) or miR-495-3p/let-7b-5p (Fig. 4B).
Table III.
List of microRNA pairs that discriminate cognitively impaired from non-impaired HIV+ patients confirmed with individual qRT-PCRs.
| microRNA pairs | AUC | Sensitivity | Specificity | P-value |
|---|---|---|---|---|
| miR-495-3p/let-7b-5p | 0.89 | 0.71 | 0.92 | 0.000322 |
| miR-532-3p/miR-377-3p | 0.89 | 0.63 | 0.92 | 0.000326 |
| miR-495-3p/miR-744-5p | 0.89 | 0.76 | 0.92 | 0.000349 |
| miR-495-3p/miR-194-5p | 0.89 | 0.82 | 0.92 | 0.000400 |
| miR-495-3p/miR-151a-5p | 0.89 | 0.76 | 0.92 | 0.000473 |
| miR-495-3p/miR-16-5p | 0.87 | 0.53 | 0.92 | 0.000653 |
| miR-495-3p/miR-106a-5p | 0.87 | 0.76 | 0.92 | 0.000653 |
| miR-376a-3p/miR-16-5p | 0.87 | 0.76 | 0.92 | 0.000704 |
| miR-376a-3p/miR-532-3p | 0.86 | 0.59 | 0.92 | 0.000884 |
| miR-431-5p/miR-16-5p | 0.85 | 0.76 | 0.93 | 0.000992 |
Figure 4. Diagnostic value of combined microRNA pairs.
Plots showing ROC curve analysis resulting from the combination of the pair miR-495-3p/miR-744-5p with miR-376a-3p/miR-532-3p (A), and miR-495-3p/let-7b-5p with miR-376a-3p/miR-532-3p (B). Actual p-values, AUC, specificity and sensitivity are shown.
Discussion
A minimally invasive test for the early detection and monitoring of CI in HIV+ patients is not currently available. MicroRNAs regulate gene expression, and changes in their concentration may reflect changes in cellular function. MicroRNAs are also secreted and remarkably stable in the extracellular environment, making them attractive molecules for biomarker discovery. The current view of microRNAs as biomarkers relates to their increased expression and secretion as a result of cellular/organ injury, resulting in their detection in plasma. Although advances in microRNA technology have allowed increased detection of minute amounts of circulating microRNAs, their application as predictive or prognostic tools for neurodegenerative diseases remains underdeveloped. A growing need exists to identify chronic neurodegeneration during it’s early phases, and only a small number of reports show correlation between dysregulated plasma microRNAs and neuropsychological conditions [reviewed in (Jin et al., 2013; Sheinerman and Umansky, 2013a)]. To the best of our knowledge, of the putative role of microRNAs as biomarkers for HIV-associated neurocognitive impairment has not been reported.
In this study, we utilized our published protocol for isolating microRNAs from body fluids (Pacifici et al., 2013; Pacifici et al., 2014) and optimized this protocol for profiling plasma microRNAs from HIV+ individuals. As methodologies are often overlooked in the literature (Moldovan et al., 2014), we have curated critical procedural aspects, such as blood collection and processing, that can affect stability of microRNAs. To avoid introducing technical variations we used a standardized procedure for sampling in which EDTA-anticoagulated blood was collected from patients and processed for plasma separation within two hours of collection.
While new technologies have greatly improved the recovery and measurement of circulating microRNAs, less attention has been given to standardization procedures for data analysis that would allow comparison across different studies (Kroh et al., 2010; Mitchell et al., 2008; Moldovan et al., 2014). We have identified two microRNAs, miR-23a-3p and miR-23b-3p, that were consistently expressed across all samples from HIV+ patients and that could be used as reference genes. We also determined that these microRNAs may serve as indicators of RNA/cDNA quality. In fact, high threshold values for miR-23a-3p and miR-23b-3p correlated with poor performance of samples using the array approach, resulting in a high rate of missing cycle threshold values (Fig. 1C-D). Importantly, the identification of reference genes for HIV+ samples could pave the way to standardized protocols that would enable investigators to compare profiles across different cohorts of patients.
For data analysis we used a microRNA pairing approach (Hennessey et al., 2012; Sheinerman et al., 2012) based on empirical search of microRNA pairs that differentiate cognitively impaired from unimpaired control patients with the best sensitivity and specificity. The miRNA-pairing approach is becoming the preferred method for circulating microRNA biomarker discovery (Hennessey et al., 2012; Hsu et al., 2014; Sheinerman et al., 2012; Sheinerman and Umansky, 2013b). A modification of this approach, in which only organ-enriched microRNAs are screened in plasma, has been succesfully applied for the discovery of microRNA-pairs that discriminate mild cognitive impairments from controls (Sheinerman et al., 2013a; Sheinerman et al., 2012; Sheinerman and Umansky, 2013a; Sheinerman and Umansky, 2013b) or other pathologies like pulmonary and gastrointestinal cancers (Hennessey et al., 2012; Sheinerman et al., 2013b). In this study, we applied the microRNA pair-wise approach to array data, resulting in discovery of more than 30 microRNA-pairs potentially distinguishing CI from nonCI patients with good sensitivity and specificity (Table II). After validation though individual qRT-PCR, a total of 10 microRNA pairs were confirmed to distinguish CI from nonCI (Table III). We observed a loss in the sensitivity for some microRNA-pairs using the latter approach, due likely to the high number of undetected microRNAs in the arrays, while individual qRT-PCR, and in some instances isolation of fresh RNA, eliminated this problem. The microRNA-pairs best dsitinguishing CI from nonCI in our HIV+ cohort were: miR-495-3p in combination with let-7b-5p, miR-151a-5p or miR-744-5p, and the pair miR-376a-3p/miR-16-5p (Figure 3). Improved sensitivity (0.88) was achieved with the combination of two microRNA pairs, miR-495-3p/miR-744-5p and miR-376a-3p/miR-532-3p. Finally, we wanted to assess whether other patient’s characteristics might account for observed differences in miRNAs expression in CI/nonCI groups. Thus, we compared expression of the four microRNA pairs, miR-495-3p/let-7b-5p, miR-495-3p/miR-151-5p, miR-495-3p/miR-744-5p and miR-376a-3p/miR-16-5p, with other clinical and demographic variables, as shown in Table I. However, this type of analysis did not return any significant association.
Figure 3. Validated microRNA pairs that distinguish CI from nonCI.
Left panels show expression levels of the indicated microRNA pairs as determined by individual qRT-PCR assays in the HIV+ samples. Exact p-values are shown. Right panels show the results from ROC analysis. Area under the curve (AUC), specificity and sensitivity are indicated in each graph.
The microRNAs identified as potentially distinguishing CI from nonCI in this study are either brain-enriched, or have validated neuronal functions (Im and Kenny, 2012; Landgraf et al., 2007; Liang et al., 2007). For example, miR-495, miR-16, and miR-30 target brain-derived neurotrophic factor (BDNF) (Mellios et al., 2008), a factor downregulated in several neurodegenerative disorders (Durany et al., 2000; Ferrer et al., 2000; Zuccato et al., 2001), including HAND (Bachis et al., 2012), and known not only to support neuronal survival but also promote synaptic transmission, growth and plasticity [reviewed by (Lu et al., 2013)]. Expression of miR-495 appears to be restricted to layer II of the pre-frontal cortex, suggesting its critical role in neurogenesis and synaptic regulation during adulthood in this layer (Mellios et al., 2008). Notably, six distinguishing microRNAs in the array (miR-495, miR-376a, miR-376b, miR-376c, miR-127, and miR-379; Table II) and four from individual qRT-PCR (miR-495, miR-376a, miR-431, and miR-377; Table III) are clustered in the chromosome 14q32 region. This region contains paternally imprinted/maternally expressed DLK1-DIO3 domain and two large microRNA clusters that encode a population of brain enriched microRNAs (Benetatos et al., 2013; Gardiner et al., 2012). Much of the 14q32-derived microRNAs are involved in the maintenance of the white matter where they are implicated in cell adhesion, differentiation, neuronal myelination, and oligodendrocyte maturation (Manzardo et al., 2013). Therefore, detection of this cluster in the periphery may reflect white matter pathology observed in HIV+ patients by MRI imaging (Pomara et al., 2001), although MRIs were not available for patients in our cohort. Mir-19b-5p, which demonstrated distinguishing capacities in combination with other microRNAs (Tables II and III), associates with Argonaute 2 (Ago2) in the amigdala following chronic stress and modulates behavioral responses (Volk et al., 2014), and is upregulated in ischemic models of stroke (Dhiraj et al., 2013). Furthermore, miR-532 is an axon-enriched microRNA which localizes in granules at distal axons and growth cones (Sasaki et al., 2014).
In summary, using novel approaches validated using plasma samples from HIV+ patients we have identified potential microRNA-pairs that may distinguish CI from nonCI in HIV+ patients with good sensitivity and specificity. While further validation studies are needed in other cohorts of HIV+ patients, these data establish a framework for longitudinal testing to determine whether plasma microRNA profiling can be used to predict the onset and/or progression of neurocognitive impairment among HIV+ individuals.
Acknowledgments
We would like to thank the clinical research associates at the HOP clinic. We thank Kira Sheinerman for methodological insights. This work was supported in part by 1 U54 GM104940 from the National Institute of General Medical Sciences of the NIH which funds the Louisiana Clinical and Translational Science Center (FP, CP), by the HOP Clinic Cancer-Prevention Study (HOP CPS Cohort (NIH CA142362 to CP), and by NIH P20 GM103501 (FP, CP).
Footnotes
This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: [10.1002/jcp.25131]
The Authors declare no conflict of interests.
References
- Alevizos I, Illei GG. MicroRNAs as biomarkers in rheumatic diseases. Nat Rev Rheumatol. 2010;6(7):391–398. doi: 10.1038/nrrheum.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Avdoshina V, Zecca L, Parsadanian M, Mocchetti I. Human immunodeficiency virus type 1 alters brain-derived neurotrophic factor processing in neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(28):9477–9484. doi: 10.1523/JNEUROSCI.0865-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetatos L, Hatzimichael E, Londin E, Vartholomatos G, Loher P, Rigoutsos I, Briasoulis E. The microRNAs within the DLK1-DIO3 genomic region: involvement in disease pathogenesis. Cellular and molecular life sciences : CMLS. 2013;70(5):795–814. doi: 10.1007/s00018-012-1080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004;26(3):307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Cho WC. MicroRNAs: potential biomarkers for cancer diagnosis, prognosis and targets for therapy. Int J Biochem Cell Biol. 2010;42(8):1273–1281. doi: 10.1016/j.biocel.2009.12.014. [DOI] [PubMed] [Google Scholar]
- De Felice B, Guida M, Guida M, Coppola C, De Mieri G, Cotrufo R. A miRNA signature in leukocytes from sporadic amyotrophic lateral sclerosis. Gene. 2012;508(1):35–40. doi: 10.1016/j.gene.2012.07.058. [DOI] [PubMed] [Google Scholar]
- De Smaele E, Ferretti E, Gulino A. MicroRNAs as biomarkers for CNS cancer and other disorders. Brain research. 2010;1338:100–111. doi: 10.1016/j.brainres.2010.03.103. [DOI] [PubMed] [Google Scholar]
- Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382(9903):1525–1533. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhiraj DK, Chrysanthou E, Mallucci GR, Bushell M. miRNAs-19b, -29b-2* and -339-5p show an early and sustained up-regulation in ischemic models of stroke. PloS one. 2013;8(12):e83717. doi: 10.1371/journal.pone.0083717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durany N, Michel T, Kurt J, Cruz-Sanchez FF, Cervas-Navarro J, Riederer P. Brain-derived neurotrophic factor and neurotrophin-3 levels in Alzheimer's disease brains. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2000;18(8):807–813. [PubMed] [Google Scholar]
- Eletto D, Russo G, Passiatore G, Del Valle L, Giordano A, Khalili K, Gualco E, Peruzzi F. Inhibition of SNAP25 expression by HIV-1 Tat involves the activity of mir-128a. Journal of cellular physiology. 2008;216(3):764–770. doi: 10.1002/jcp.21452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkan EP, Breakefield XO, Saydam O. miRNA signature of schwannomas: Possible role(s) of "tumor suppressor" miRNAs in benign tumors. Oncotarget. 2011:265–270. doi: 10.18632/oncotarget.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferracin M, Veronese A, Negrini M. Micromarkers: miRNAs in cancer diagnosis and prognosis. Expert Rev Mol Diagn. 2010;10(3):297–308. doi: 10.1586/erm.10.11. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Goutan E, Marin C, Rey MJ, Ribalta T. Brain-derived neurotrophic factor in Huntington disease. Brain research. 2000;866(1–2):257–261. doi: 10.1016/s0006-8993(00)02237-x. [DOI] [PubMed] [Google Scholar]
- Gandhi R, Healy B, Gholipour T, Egorova S, Musallam A, Hussain MS, Nejad P, Patel B, Hei H, Khoury S, Quintana F, Kivisakk P, Chitnis T, Weiner HL. Circulating microRNAs as biomarkers for disease staging in multiple sclerosis. Annals of neurology. 2013;73(6):729–740. doi: 10.1002/ana.23880. [DOI] [PubMed] [Google Scholar]
- Gardiner E, Beveridge NJ, Wu JQ, Carr V, Scott RJ, Tooney PA, Cairns MJ. Imprinted DLK1-DIO3 region of 14q32 defines a schizophrenia-associated miRNA signature in peripheral blood mononuclear cells. Molecular psychiatry. 2012;17(8):827–840. doi: 10.1038/mp.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibcus JH, Tan LP, Harms G, Schakel RN, de Jong D, Blokzijl T, Moller P, Poppema S, Kroesen BJ, van den Berg A. Hodgkin lymphoma cell lines are characterized by a specific miRNA expression profile. Neoplasia. 2009;11(2):167–176. doi: 10.1593/neo.08980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, McCutchan JA, Taylor MJ, Kelly MD, Ellis RJ, et al. The HNRC 500--neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. Journal of the International Neuropsychological Society : JINS. 1995;1(3):231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Heneghan HM, Miller N, Kerin MJ. MiRNAs as biomarkers and therapeutic targets in cancer. Curr Opin Pharmacol. 2010;10(5):543–550. doi: 10.1016/j.coph.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Hennessey PT, Sanford T, Choudhary A, Mydlarz WW, Brown D, Adai AT, Ochs MF, Ahrendt SA, Mambo E, Califano JA. Serum microRNA biomarkers for detection of non-small cell lung cancer. PloS one. 2012;7(2):e32307. doi: 10.1371/journal.pone.0032307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Barclay TR, Castellon SA, Levine AJ, Durvasula RS, Marion SD, Myers HF, Longshore D. Drug use and medication adherence among HIV-1 infected individuals. AIDS and behavior. 2007;11(2):185–194. doi: 10.1007/s10461-006-9152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu A, Chen SJ, Chang YS, Chen HC, Chu PH. Systemic approach to identify serum microRNAs as potential biomarkers for acute myocardial infarction. BioMed research international. 2014;2014:418628. doi: 10.1155/2014/418628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im HI, Kenny PJ. MicroRNAs in neuronal function and dysfunction. Trends in neurosciences. 2012;35(5):325–334. doi: 10.1016/j.tins.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin XF, Wu N, Wang L, Li J. Circulating microRNAs: a novel class of potential biomarkers for diagnosing and prognosing central nervous system diseases. Cellular and molecular neurobiology. 2013;33(5):601–613. doi: 10.1007/s10571-013-9940-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju J. miRNAs as biomarkers in colorectal cancer diagnosis and prognosis. Bioanalysis. 2010;2(5):901–906. doi: 10.4155/bio.10.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, Ochiya T. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010;101(10):2087–2092. doi: 10.1111/j.1349-7006.2010.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroh EM, Parkin RK, Mitchell PS, Tewari M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR) Methods. 2010;50(4):298–301. doi: 10.1016/j.ymeth.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M, Lin C, Socci ND, Hermida L, Fulci V, Chiaretti S, Foa R, Schliwka J, Fuchs U, Novosel A, Muller RU, Schermer B, Bissels U, Inman J, Phan Q, Chien M, Weir DB, Choksi R, De Vita G, Frezzetti D, Trompeter HI, Hornung V, Teng G, Hartmann G, Palkovits M, Di Lauro R, Wernet P, Macino G, Rogler CE, Nagle JW, Ju J, Papavasiliou FN, Benzing T, Lichter P, Tam W, Brownstein MJ, Bosio A, Borkhardt A, Russo JJ, Sander C, Zavolan M, Tuschl T. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129(7):1401–1414. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Ridzon D, Wong L, Chen C. Characterization of microRNA expression profiles in normal human tissues. BMC genomics. 2007;8:166. doi: 10.1186/1471-2164-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Nagappan G, Guan X, Nathan PJ, Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nature reviews Neuroscience. 2013;14(6):401–416. doi: 10.1038/nrn3505. [DOI] [PubMed] [Google Scholar]
- Manzardo AM, Gunewardena S, Butler MG. Over-expression of the miRNA cluster at chromosome 14q32 in the alcoholic brain correlates with suppression of predicted target mRNA required for oligodendrocyte proliferation. Gene. 2013;526(2):356–363. doi: 10.1016/j.gene.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellios N, Huang HS, Grigorenko A, Rogaev E, Akbarian S. A set of differentially expressed miRNAs, including miR-30a-5p, act as post-transcriptional inhibitors of BDNF in prefrontal cortex. Human molecular genetics. 2008;17(19):3030–3042. doi: 10.1093/hmg/ddn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldovan L, Batte KE, Trgovcich J, Wisler J, Marsh CB, Piper M. Methodological challenges in utilizing miRNAs as circulating biomarkers. Journal of cellular and molecular medicine. 2014;18(3):371–390. doi: 10.1111/jcmm.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorbakhsh F, Ramachandran R, Barsby N, Ellestad KK, LeBlanc A, Dickie P, Baker G, Hollenberg MD, Cohen EA, Power C. MicroRNA profiling reveals new aspects of HIV neurodegeneration: caspase-6 regulates astrocyte survival. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24(6):1799–1812. doi: 10.1096/fj.09-147819. [DOI] [PubMed] [Google Scholar]
- O'Hara AJ, Vahrson W, Dittmer DP. Gene alteration and precursor and mature microRNA transcription changes contribute to the miRNA signature of primary effusion lymphoma. Blood. 2008;111(4):2347–2353. doi: 10.1182/blood-2007-08-104463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici M, Delbue S, Ferrante P, Jeansonne D, Kadri F, Nelson S, Velasco-Gonzalez C, Zabaleta J, Peruzzi F. Cerebrospinal fluid miRNA profile in HIV-encephalitis. Journal of cellular physiology. 2013;228(5):1070–1075. doi: 10.1002/jcp.24254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici M, Delbue S, Kadri F, Peruzzi F. Cerebrospinal fluid MicroRNA profiling using quantitative real time PCR. Journal of visualized experiments : JoVE. 2014;(83):e51172. doi: 10.3791/51172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedranzini L, Mottadelli F, Ronzoni S, Rossella F, Ferracin M, Magnani I, Roversi G, Colapietro P, Negrini M, Pelicci PG, Larizza L. Differential cytogenomics and miRNA signature of the Acute Myeloid Leukaemia Kasumi-1 cell line CD34+38- compartment. Leuk Res. 2010;34(10):1287–1295. doi: 10.1016/j.leukres.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Pomara N, Crandall DT, Choi SJ, Johnson G, Lim KO. White matter abnormalities in HIV-1 infection: a diffusion tensor imaging study. Psychiatry research. 2001;106(1):15–24. doi: 10.1016/s0925-4927(00)00082-2. [DOI] [PubMed] [Google Scholar]
- Rao P, Benito E, Fischer A. MicroRNAs as biomarkers for CNS disease. Frontiers in molecular neuroscience. 2013;6:39. doi: 10.3389/fnmol.2013.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertus JL, Kluiver J, Weggemans C, Harms G, Reijmers RM, Swart Y, Kok K, Rosati S, Schuuring E, van Imhoff G, Pals ST, Kluin P, van den Berg A. MiRNA profiling in B non-Hodgkin lymphoma: a MYC-related miRNA profile characterizes Burkitt lymphoma. Br J Haematol. 2010;149(6):896–899. doi: 10.1111/j.1365-2141.2010.08111.x. [DOI] [PubMed] [Google Scholar]
- Rom S, Rom I, Passiatore G, Pacifici M, Radhakrishnan S, Del Valle L, Pina-Oviedo S, Khalili K, Eletto D, Peruzzi F. CCL8/MCP-2 is a target for mir-146a in HIV-1-infected human microglial cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24(7):2292–2300. doi: 10.1096/fj.09-143503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Gross C, Xing L, Goshima Y, Bassell GJ. Identification of axon-enriched microRNAs localized to growth cones of cortical neurons. Developmental neurobiology. 2014;74(3):397–406. doi: 10.1002/dneu.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafi G, Aliya N, Munshi A. MicroRNA signatures in neurological disorders. Can J Neurol Sci. 2010;37(2):177–185. doi: 10.1017/s0317167100009902. [DOI] [PubMed] [Google Scholar]
- Shah AA, Leidinger P, Blin N, Meese E. miRNA: small molecules as potential novel biomarkers in cancer. Curr Med Chem. 2010;17(36):4427–4432. doi: 10.2174/092986710794182980. [DOI] [PubMed] [Google Scholar]
- Sheinerman KS, Tsivinsky VG, Abdullah L, Crawford F, Umansky SR. Plasma microRNA biomarkers for detection of mild cognitive impairment: biomarker validation study. Aging. 2013a;5(12):925–938. doi: 10.18632/aging.100624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinerman KS, Tsivinsky VG, Crawford F, Mullan MJ, Abdullah L, Umansky SR. Plasma microRNA biomarkers for detection of mild cognitive impairment. Aging. 2012;4(9):590–605. doi: 10.18632/aging.100486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinerman KS, Tsivinsky VG, Umansky SR. Analysis of organ-enriched microRNAs in plasma as an approach to development of Universal Screening Test: feasibility study. Journal of translational medicine. 2013b;11:304. doi: 10.1186/1479-5876-11-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinerman KS, Umansky SR. Circulating cell-free microRNA as biomarkers for screening, diagnosis and monitoring of neurodegenerative diseases and other neurologic pathologies. Frontiers in cellular neuroscience. 2013a;7:150. doi: 10.3389/fncel.2013.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinerman KS, Umansky SR. Early detection of neurodegenerative diseases: circulating brain-enriched microRNA. Cell cycle. 2013b;12(1):1–2. doi: 10.4161/cc.23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taft RJ, Pang KC, Mercer TR, Dinger M, Mattick JS. Non-coding RNAs: regulators of disease. J Pathol. 2009;220(2):126–139. doi: 10.1002/path.2638. [DOI] [PubMed] [Google Scholar]
- Tatro ET, Scott ER, Nguyen TB, Salaria S, Banerjee S, Moore DJ, Masliah E, Achim CL, Everall IP. Evidence for Alteration of Gene Regulatory Networks through MicroRNAs of the HIV-infected brain: novel analysis of retrospective cases. PloS one. 2010;5(4):e10337. doi: 10.1371/journal.pone.0010337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Galgani S, Murri R, Bellagamba R, Narciso P, Antinori A, Giulianelli M, Tosi G, Costa M, Sampaolesi A, Fantoni M, Noto P, Ippolito G, Wu AW. Neurocognitive performance and quality of life in patients with HIV infection. AIDS research and human retroviruses. 2003;19(8):643–652. doi: 10.1089/088922203322280856. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk N, Paul ED, Haramati S, Eitan C, Fields BK, Zwang R, Gil S, Lowry CA, Chen A. MicroRNA-19b associates with Ago2 in the amygdala following chronic stress and regulates the adrenergic receptor beta 1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(45):15070–15082. doi: 10.1523/JNEUROSCI.0855-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann J, Jack HM. Serum microRNAs as powerful cancer biomarkers. Biochimica et biophysica acta. 2010;1806(2):200–207. doi: 10.1016/j.bbcan.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Witwer KW, Sarbanes SL, Liu J, Clements JE. A plasma microRNA signature of acute lentiviral infection: biomarkers of central nervous system disease. Aids. 2011;25(17):2057–2067. doi: 10.1097/QAD.0b013e32834b95bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Qian X, Liu B. MicroRNAs: novel biomarkers for gastrointestinal carcinomas. Mol Cell Biochem. 2010;341(1–2):291–299. doi: 10.1007/s11010-010-0463-0. [DOI] [PubMed] [Google Scholar]
- Yelamanchili SV, Chaudhuri AD, Chen LN, Xiong H, Fox HS. MicroRNA-21 dysregulates the expression of MEF2C in neurons in monkey and human SIV/HIV neurological disease. Cell death & disease. 2010;1(9):e77. doi: 10.1038/cddis.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C, Ciammola A, Rigamonti D, Leavitt BR, Goffredo D, Conti L, MacDonald ME, Friedlander RM, Silani V, Hayden MR, Timmusk T, Sipione S, Cattaneo E. Loss of huntingtin-mediated BDNF gene transcription in Huntington's disease. Science. 2001;293(5529):493–498. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]