Abstract
Clinically, primary and permanent teeth are distinct anatomically and the presentation of caries lesions differs between the two dentitions. However, the possibility exists that genetic contributions to tooth formation of the two dentitions are different. The purpose of this study was to test the hypothesis that genetic associations with an artificial caries model will not be the same between primary and permanent dentitions. Enamel samples from primary and permanent teeth were tested for microhardness at baseline, after carious lesion creation, and after fluoride application to verify association with genetic variants of selected genes. Associations were found between genetic variants of ameloblastin, amelogenin, enamelin, tuftelin, tuftelin interactive protein 11, and matrix metalloproteinase 20 and enamel from permanent teeth but not with enamel from primary teeth. In conclusion, our data continue to support that genetic variation may impact enamel development and consequently individual caries susceptibility. These effects may be distinct between primary and permanent dentitions.
Keywords: dental caries, dentition, enamel microhardness, primary, permanent
Genetic association studies of caries have suggested that caries experience may be influenced by polymorphic variants in ameloblastin (1–3) amelogenin (1, 2, 4–6), enamelin (1, 2, 6, 7), matrix metalloproteinase 20 (8), tuftelin (1, 2, 4, 9), and tuftelin-interacting protein 11 (2, 6). However, these results are not consistent across the studies and differences related to study design (how caries experience is defined, inclusion of covariates such as Streptococcus mutans data, sample sizes, genetic polymorphisms studied, geographic origin of the DNA samples, age and dentition of the population studied, concomitant systemic conditions) likely contribute to the discrepancies seen in the reported findings.
Based on observations of Streptococcus mutans colonization data, our group previously suggested that genetic studies of caries should take into consideration the dentition of the subjects (10). Genome wide association analyses of caries in the primary dentition (11) showed distinct results in comparison to similar analyses in the permanent one (12). We have also used an in vitro approach to create artificial initial caries lesions and have used these data as the phenotype for genetic association analysis (2). The initial results from analyses of a cohort of permanent teeth suggested that results vary depending which tooth surface is tested. However, genetic variation in tuftelin-interacting protein 11 was associated with subclinical demineralization.
Here we expanded this work to a larger sample of permanent teeth and added a cohort of primary teeth to test the hypothesis that genetic associations with our initial caries model will not be the same between primary and permanent dentitions.
Material and Methods
Permanent teeth collection
This portion of the study was approved by the Ethics committee of the Istanbul University, Medical Faculty, Istanbul, Turkey and the University of Pittsburgh Institutional Review Board (IRB # 11070236). Informed consent was obtained from all participating individuals and parents/legal guardians. One hundred orthodontic patients from Istanbul University, Faculty of Dentistry, Department of Orthodontics participated in this study during the period of September 2011 to November 2012.
Participants were seated in a dental chair, and one of the authors (M.B.) carried out the clinical examination after being calibrated by an experienced specialist (F.S.). The intraexaminer agreement was assessed by a second clinical exam in 10% of the sample after 2 wk, with a κ of 1.0. Subjects were examined with a flashlight, dental mirror and probe. The sum of decayed, missing due to caries, and filled teeth (DMFT) was calculated for each subject (13). Teeth, which were extracted for orthodontic reasons, were not included in the DMFT/DMFS scores. Dental photographs and panoramic radiographs were also obtained from all participants. One first premolar extracted for orthodontic reasons was obtained from each participant as a source of enamel samples.
Primary teeth collection
Enamel samples from 108 exfoliated primary teeth (74 molars, 27 incisors, and 7 canines) and genomic DNA were used for this experiment. Biological samples were collected after subjects and their parents provided written informed consent. This portion of the study is approved by the University of Pittsburgh Institutional Review Board (IRB # 11070236) and by the Federal University from Rio de Janeiro (#333.167).
Samples were collected by three examiners (E.C.K., A.L., and H.F.R.) and calibrated by an experienced specialist (M.C.C.). The intraexaminer agreement was assessed by a second clinical exam in 10% of the sample after 2 wk, with a κ of 1.0. Cohen’s kappa values for agreement between examiners were 0.91. The sum of decayed, missing due to caries, and filled teeth (DMFT) was calculated for each subject (13) for both the primary (dmft) and permanent (DMFT) dentitions. Teeth lost to trauma or primary teeth lost to exfoliation were not included in the final DMFT/dmft scores. When records indicated that teeth were extracted for orthodontic reasons, or treatments were performed in sound teeth, these situations were not included in the final DMFT/dmft scores.
Details regarding the characteristics of each studied population are presented in Table 1.
Table 1.
Characteristics of the populations from who samples were obtained.
| Sample Origin | Turkey (100 permanent premolars) |
Brazil (108 primary teeth) |
Brazilian whites |
Brazilian black |
|---|---|---|---|---|
| Mean age in yr (SD) | 17.2 (3.0) | 8.8 (2.5) | 9.2 (3.3) | 8.4 (2.1) |
| Sex | ||||
| Male | 38 | 62 | 43 | 19 |
| Female | 62 | 46 | 23 | 23 |
| Ethnicity | ||||
| White | 100 | 65 | - | - |
| Black | 0 | 43 | - | - |
| Caries Status of the Individuals Studied | ||||
| Caries Free | 6 | 44 | 22 | 22 |
| Caries Affected | 94 | 64 | 43 | 21 |
| Mean DMFT/dmft (standard deviation) | 5.19 (3.4) | 3.17 (3.4) | 3.0 (0.4) | 4.2 (0.6) |
| Enamel Microhardness mean (standard deviation)* Baseline |
||||
| Mesial | 289.52 (48.68) | 210.54 (81.08) | 212.0 (81.16) | 207.7 (83.2) |
| Distal | 280.65 (48.23) | 229.24 (64.95) | 236.0 (71.64) | 216.1 (48.6) |
| Buccal | 284.04 (39.86) | 235.04 (69.26) | 238.2 (66.1) | 230.6 (73.5) |
| Occlusal | 260.27 (50.59) | 235.86 (69.53) | 256.4 (61.56) | 205.8 (71.7) |
| Lingual/Palatine | 281.04 (44.64) | 239.99 (73.12) | 231.7 (74.51) | 248.2 (74.6) |
| After Artificial Caries Creation | ||||
| Mesial | 200.66 (79.16) | 150.03 (76.48) | 166.9 (75.7) | 136.2 (84.3) |
| Distal | 185.68 (73.31) | 152.48 (78.29) | 236.0 (71.6) | 123.8 (73.2) |
| Buccal | 201.82 (74.79) | 143.27 (79.79) | 147.8 (77.87) | 136.5 (80.2) |
| Occlusal | 172.15 (73.69) | 140.31 (64.05) | 134.0 (40.31) | 144.9 (78.6) |
| Lingual/Palatine | 186.88 (75.2) | 151.12 (79.33) | 139.5 (66.23) | 162.4 (95.9) |
| After Fluoride Exposure | ||||
| Mesial | 210.61 (81.47) | 195.75 (89.69) | 209.6 (89.37) | 172.0 (76.07) |
| Distal | 199.78 (81.01) | 194.5 (73.82) | 203.7 (74.3) | 160.3 (85.10) |
| Buccal | 221.1 (77.56) | 161.23 (88.51) | 167.3 (85.7) | 151.5 (94.24) |
| Occlusal | 187.5 (75.26) | 146.5 (80.82) | 160.0 (57.17) | 136.0 (97.54) |
| Lingual/Palatine | 199.33 (78.77) | 179.38 (81.08) | 158.7 (66.08) | 203.0 (92.83) |
All surfaces studied were free of any clinical signs of caries or demineralization. Differences in enamel microhardness in the 3 experimental conditions (at baseline, after artificial caries creation, and after fluoride exposure) are statistically significant (p<0.05).
DNA samples and genotyping
Unstimulated saliva samples were obtained from all participants and stored in Oragene DNA Self-Collection kits (Kanata, ON, Canada) at room temperature until processed. DNA was extracted according to manufacturer’s instructions. Twenty-two single nucleotide polymorphisms (SNPs) were selected including rs7526319, rs4970957, rs3828054, rs3790506, and rs2337360 in tuftelin (TUFT1), rs4694075 and rs34538475 in ameloblastin (AMBN), rs12640848 and rs3796704 in enamelin (ENAM), rs1784418 in matrix metallopeptidase 20 (MMP20), rs5997096 and rs134136 in tuftelin-interacting protein 11 (TFIP11), and rs17878486 and rs946252 in amelogenin (AMELX). These SNPs were chosen based on their locations relative to the genes, linkage disequilibrium relationships, and results of previous studies (1, 2, 4, 8). Table 2 summarizes linkage disequilibrium between markers in the two cohorts studied.
Table 2.
Linkage disequilibrium (D’) between the markers studied.
| Gene | SNP Combinations | Permanent Dentition |
Primary Dentition | |
|---|---|---|---|---|
| AMBN | rs4694075 | rs34538475 | 0.02 | 0.15 |
| AMELX | rs17878486 | rs946252 | 0.01 | 0.68 |
| ENAM | rs3796704 | rs640848 | 0.01 | 0.54 |
| TUFIP11 | rs5997096 | rs134136 | 0.16 | 0.16 |
| TUFT1 | rs7526319 | rs4970957 | 0.13 | 0.01 |
| rs7526319 | rs3828054 | 0.12 | 0.49 | |
| rs7526319 | rs3790506 | 0.14 | 0.13 | |
| rs7526319 | rs2337360 | 0.12 | 0.17 | |
| rs4970957 | rs3828054 | 0.12 | 0.06 | |
| rs4970957 | rs3790506 | 0.13 | 0.01 | |
| rs4970957 | rs2337360 | 0.14 | 0.12 | |
| rs3828054 | rs3790506 | 0.15 | 0.12 | |
| rs3828054 | rs2337360 | 0.17 | 0.12 | |
| rs3790506 | rs2337360 | 0.18 | 0.4 | |
Polymerase chain reactions with TaqMan SNP Genotyping Assays from Applied Biosystems (Valencia, CA, USA) with a total volume of 3 µl/reaction with 3.0 ng of DNA/reaction were used for genotyping all selected markers in a Tetrad PTC225 thermocycler from MJ Research (Waltham, MA, USA). Genotype detection and analysis were performed on the ABI 7900HT with ABI SDS software (Valencia, CA, USA).
Specimen preparation and enamel microhardness analysis
One hundred caries-free extracted premolar teeth were studied, one from each participant. These teeth were extracted for orthodontic reasons. In addition, 108 exfoliated primary teeth were also studied. The tissue remnants were cleaned from the teeth and then teeth were stored in 10% buffered formalin (pH 7.0) solution at 4°C until initial laboratory manipulation. The crowns were separated from the roots, and then each crown was separated buccolingually and mesiodistally with an Isomet low speed saw from Buehler (Lake Bluff, IL, USA) under continuous water-cooling. Five surfaces (mesial, buccal, distal, occlusal, lingual/palatine) were obtained from each crown. The enamel surfaces were sanded using abrasive papers of 320, 400 and 600 grit followed by polishing with 6, 1, and 0.25 µm polycrystalline diamond suspension on a Minimet 1000 grinder/polisher machine (Lake Bluff, IL, USA) under water-cooling. After the polishing step, all samples were sonicated for 1 minute with distilled water in an FS6 ultrasonic cleaner from Fischer Scientific (Waltham, MA, USA).
Samples were submitted to baseline microhardness analysis using an Indentamet 1100 Series microhardness tester (Lake Bluff, IL, USA) with a knoop diamond under a load of 25 g for 5 s. Five indentations spaced 100 µm away from each other were made. Artificial caries lesions were created by immersing each enamel sample in 24 mL of demineralizing solution (1.3 mmol/L Ca, 0.78 mmol/L P, 0.05 mol/L acetate buffer, 0.03 µg F/mL, pH 5.0) at 37°C for h hours (14). Surface microhardness was measured again by another five indentations created right underneath the initial ones. Caries lesions were exposed to a fluoride solution made from Aquafresh Extreme Clean toothpaste (Brentford, Middlesex, UK) containing 0.15% w/v fluoride ion for 10 min. Surface microhardness was measured one more time by creating five indentations underneath the previous ones.
Phenotype definitions and statistical analysis
Based on DMFT/dmft (Decayed, Missing due to caries, Filled Teeth index) distributions, subjects were classified as having “low caries experience” (below the mean DMFT of the 100 Turkish subjects or below the mean dmft of the 108 Brazilian subjects), or having “high caries experience” (above the mean DMFT of the 100 Turkish subjects or above the mean dmft of the 108 Brazilian subjects). The differences in genotype and allele frequencies between the ‘high’ and ‘low’ caries experience groups were tested using the PLINK software package (15) with an established alpha of 0.05. Standard case/control association analysis using Fisher’s exact test, as well as full model association tests (Cochran-Armitage trend test, genotypic 2-degree of freedom test, dominant gene action 1-degree of freedom test, and recessive gene action 1-degree of freedom test) were used to evaluate the data. Finally linear and logistic models were used to allow the inclusion of sex and ethnic background (for the Brazilian cohort) as covariates.
Based on microhardness values, subjects were classified into dichotomous groups (baseline values or rate changes above or below the average of the group). Subjects were classified as having “softer enamel” (below the average of the groups) and “harder enamel” (above the average of the groups) for determination of microhardness phenotypes. Representative examples of the distribution of these values are shown in Figure 1. Data were analyzed by surface since we are aware enamel assessments differ between surfaces within the same tooth (2).
Figure 1.
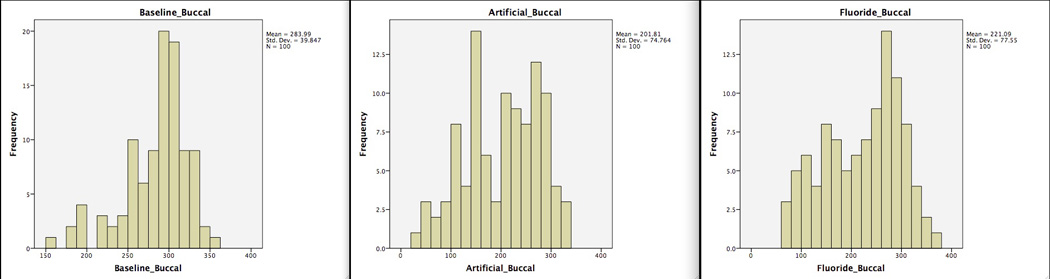
Representative distribution of enamel microhardness values in the permanent teeth cohort (buccal surface) at baseline, after artificial caries creation, and after fluoride exposure. X-axis is the enamel microhardness values, y-axis is the frequency of tooth surfaces.
The following three differences of enamel microhardness values were compared by the Wilcoxon signed-rank test to confirm the in vitro model showed the expected decrease in enamel microhardness between baseline and after artificial caries lesion creation and subsequent increase in enamel microhardness after exposure to a fluoridated solution: between baseline and after artificial caries lesion creation, after artificial caries lesion creation and after fluoride exposure, and baseline and after fluoride exposure (Table 1). Chi-square and Fisher’s exact tests were used to assess association between the SNPs and microhardness values by the use of the PLINK software package (15) with an established alpha of 0.05.
Results
Whereas associations between caries experience and markers in AMBN, AMELX, and TFIP11 (ENAM was borderline associated) were found for the individuals that provided premolars for this study, only one marker in TUFT1 showed association with caries experience among the children who donated their exfoliated primary teeth (Table 3which only lists the models that provided statistical evidence for differences in genotype or allele distributions). Linear and logistic models used to allow the inclusion of sex and ethnic background (for the Brazilian cohort) as covariates and results did not differ from the ones presented in Table 3 and these data is not shown.
Table 3.
Single nucleotide polymorphisms (SNPs) and summary p-values for association tests between caries experience and genetic variants in the two studied samples.
| Minor Allele Frequency | Summary p-value |
||||||
|---|---|---|---|---|---|---|---|
| Gene | Marker | Alleles | Turkey | Brazil | Permanent Teeth |
Primary Teeth |
|
| Black | White | ||||||
| TUFT1 | rs7526319 | CT | 0.338 | 0.481 | 0.469 | NS | NS |
| rs4970957 | AG | 0.241 | 0.133 | 0.152 | NS | 0.009a | |
| rs3828054 | AG | 0.105 | 0.448 | 0.393 | NS | NS | |
| rs3790506 | AG | 0.249 | 0.4 | 0.357 | NS | NS | |
| rs2337360 | AG | 0.25 | 0.366 | 0.414 | NS | NS | |
| AMBN | rs4694075 | CT | 0.478 | 0.264 | 0.424 | 0.004b | NS |
| rs34538475 | GT | 0.187 | 0.255 | 0.387 | NS | NS | |
| ENAM | rs12640848 | AG | 0.357 | 0.451 | 0.471 | 0.06a | NS |
| rs3796704 | AG | 0.12 | 0.173 | 0.133 | NS | NS | |
| MMP20 | rs1784418 | CT | 0.407 | 0.388 | 0.414 | NS | NS |
| TFIP11 | rs5997096 | CT | 0.47 | 0.416 | 0.444 | 0.006a | NS |
| rs134136 | CT | 0.335 | 0.258 | 0.291 | 0.002a | NS | |
| AMELX | rs17878486 | CT | 0.111 | 0.148 | 0.136 | 0.03a | NS |
| rs946252 | AG | 0.3 | 0.183 | 0.2 | 0.025a | NS | |
NS = not statistically significant.
Fisher’s exact test on the distribution of alleles.
Genotypic 2-degree of freedom test.
As expected, enamel microhardness declined after artificial caries creation and increased after fluoride exposure for teeth from both dentitions (Table 1). Also, microhardness values for primary teeth were lower than the values of permanent teeth. When the results of the microhardness of the enamel at baseline, after artificial caries creation, and fluoride exposure were considered, in comparison to genetic variation, statistically significant differences could be seen in the permanent teeth, but no differences were observed in the comparisons of the primary teeth.
Lower baseline microhardness was significantly associated with rs7526319 (p = 0.03 for lingual/palatine surface) and rs2337360 (p = 0.01 for lingual/palatine surface) in TUFT1; rs3796704 (p = 0.04 for distal surface) in ENAM; rs1784418 (p = 0.003 for buccal surface) in MMP20, and rs17878486 (p = 0.02 for mesial surface) in AMELX (Table 4). Softer enamel was significantly associated with the CC genotype (for lingual/palatine surface) in rs7526319 and the AA genotype (for lingual/palatine surface) in rs2337360 in TUFT1; the AG genotype in rs3796704 (for distal surface) in ENAM; the TT genotype in rs1784418 (for buccal surface) in MMP20, and the C allele in rs17878486 (for mesial surface) in AMELX (Table 4). Higher baseline microhardness was significantly associated with rs7526319 (p = 0.01 for occlusal surface) and rs2337360 (p = 0.03 for occlusal surface) in TUFT1; rs1784418 in MMP20 (p = 0.03 for buccal surface), and rs134136 in TFIP11 (p = 0.02 for buccal surface) (Table 4). The harder enamel group was significantly associated with the CC genotype in rs7526319 (for occlusal surface) and the GG genotype in rs2337360 (for occlusal surface); the C allele in rs1784418 (for buccal surface), and the C allele in rs134136 in TUFT1 (for buccal surface) (Table 4).
Table 4.
Summary of the positive associations of the genotype and allele frequency comparisons of baseline enamel microhardness assessments in the permanent teeth.
| Gene | SNP | Above the Mean Enamel Microhardness Enamel [N(%)] |
Below the Mean Enamel Microhardness Enamel [N(%)] |
p-value | |||
|---|---|---|---|---|---|---|---|
| TUFT1 | rs7526319 | Occlusal | |||||
| Genotype | CC | 8(14.2) | 1(2.9) | 0.01 | |||
| CT | 27(48.2) | 27(79.4) | |||||
| TT | 21(37.5) | 6(17.6) | |||||
| Allele | C | 43(38.3) | 29(42.6) | 0.57 | |||
| T | 69(61.6) | 39(57.3) | |||||
| rs7526319 | Lingual/Palatine | ||||||
| Genotype | CC | 2(3.6) | 7(10.7) | 0.03 | |||
| CT | 37(67.2) | 17(48.5) | |||||
| TT | 16(29.09) | 11(31.4) | |||||
| Allele | C | 41(37.2) | 31(44.2) | 0.34 | |||
| T | 69(62.7) | 39(55.7) | |||||
| rs2337360 | Occlusal | ||||||
| Genotype | AA | 9(14.7) | 5(12.8) | 0.03 | |||
| AG | 27(44.2) | 27(69.2) | |||||
| GG | 25(40.9) | 7(17.9) | |||||
| Allele | A | 45(36.8) | 37(47.4) | 0.13 | |||
| G | 77(63.1) | 41(52.5) | |||||
| rs2337360 | Lingual/Palatine | ||||||
| Genotype | AA | 4(6.4) | 10(26.3) | 0.01 | |||
| AG | 38(61.2) | 16(42.1) | |||||
| GG | 20(32.2) | 12(31.5) | |||||
| Allele | A | 46(37.09) | 36(47.3) | 0.15 | |||
| G | 78(62.9) | 40(52.6) | |||||
| ENAM | rs3796704 | Distal | |||||
| Genotype | AA | 0(0) | 0(0) | 0.04 | |||
| AG | 3(7.6) | 7(25) | |||||
| GG | 36(92.3) | 21(75) | |||||
| Allele | A | 3(3.8) | 7(12.5) | 0.06 | |||
| G | 75(96.1) | 49(87.5) | |||||
| MMP20 | rs1784418 | Buccal | |||||
| Genotype | CC | 11(17.7) | 6(15.7) | 0.003 | |||
| CT | 44(70.9) | 17(44.7) | |||||
| TT | 7(11.2) | 15(39.4) | |||||
| Allele | C | 66(53.2) | 29(38.1) | 0.03 | |||
| T | 58(46.7) | 47(61.8) | |||||
| TFIP11 | rs134136 | Buccal | |||||
| Genotype | CC | 9(14.5) | 2(5.2) | 0.9 | |||
| CT | 30(48.3) | 14(36.8) | |||||
| TT | 23(37.09) | 22(57.8) | |||||
| Allele | C | 48(38.7) | 18(23.6) | 0.02 | |||
| T | 76(61.2) | 58(76.3) | |||||
| AMELX | rs17878486 | Mesial | |||||
| Genotype | CC | 1(2.5) | 1(4.5) | 0.05 | |||
| CT | 10(25) | 12(54.5) | |||||
| TT | 29(72.5) | 9(40.9) | |||||
| Allele | C | 12(15) | 14(31.8) | 0.02 | |||
| T | 68(85) | 30(68.1) | |||||
Bold indicates statistically significant differences.
After artificial caries lesion creation, higher microhardness was significantly associated with rs134136 (p = 0.006 for buccal surface) in TFIP11, and rs946252 (p = 0.03 for distal and p = 0.006 for buccal surface) in AMELX (Table 5). More demineralization was significantly associated with the T allele in rs134136 in TFIP11 (for buccal surface), the T allele (for distal and buccal surfaces) and the TT genotype (for buccal surface) in rs946252 in AMELX (Table 5). After artificial caries lesion creation, softer enamel was significantly associated with rs134136 (p = 0.009 for buccal surface) in TFIP11 (Table 4). More demineralization was significantly associated with the CC genotype in rs134136 (for buccal surface) in TFIP11 (Table 5).
Table 5.
Summary of the positive associations of the genotype and allele frequency comparisons of enamel microhardness assessments in the permanent teeth after artificial caries lesion creation.
| Gene | SNP | Above the Mean Enamel Microhardness Enamel [N(%)] |
Below the Mean Enamel Microhardness Enamel [N(%)] |
p-value | |||
|---|---|---|---|---|---|---|---|
| TFIP11 | rs134136 | Buccal | |||||
| Genotype | TT | 6(14.6) | 5(8.4) | 0.009 | |||
| CT | 24(58.5) | 20(33.8) | |||||
| CC | 11(26.8) | 34(57.6) | |||||
| Allele | T | 36(43.9) | 30(25.4) | 0.006 | |||
| C | 46(56.09) | 88(74.5) | |||||
| AMELX | rs946252 | Distal | |||||
| Genotype | TT | 6(21.4) | 2(5.8) | 0.15 | |||
| CT | 8(28.5) | 9(26.4) | |||||
| CC | 14(50) | 23(67.6) | |||||
| Allele | T | 20(35.7) | 13(19.1) | 0.03 | |||
| C | 36(64.2) | 55(80.8) | |||||
| rs946252 | Buccal | ||||||
| Genotype | TT | 7(25.9) | 1(2.8) | 0.02 | |||
| CT | 7(25.9) | 10(28.5) | |||||
| CC | 13(48.1) | 24(68.5) | |||||
| Allele | T | 21(38.8) | 12(17.1) | 0.006 | |||
| C | 33(61.1) | 58(82.8) | |||||
Bold indicates statistically significant differences.
After fluoride treatment, higher enamel microhardness values were significantly associated with rs2337360 (p = 0.03 for lingual/palatine surface) in TUFT1, rs4694075 (p = 0.01 for distal surface) in AMBN, and rs1784418 (p = 0.04 for mesial surface) in MMP20 (Table 5). A larger amount of enamel remineralization was significantly associated with the AA genotype in rs2337360 (lingual/palatine surface) in TUFT1, the T allele in rs4694075 (distal surface) in AMBN, and the TT genotype in rs1784418 (mesial surface) in MMP20 (Table 6). After fluoride treatment, lower microhardness was significantly associated with rs4694075 (p = 0.01 for distal surface) in AMBN, and rs5997096 (p = 0.01 for mesial surface) and rs134136 (p = 0.01 for mesial surface) in TFIP11 (Table 6). A lesser amount of remineralization was associated with the CC genotype in rs4694075 (distal surface) in AMBN, and the T allele in rs5997096 (mesial surface), the TT genotype and T allele in rs134136 (mesial surface) in TFIP11 (Table 6).
Table 6.
Summary of the positive associations of the genotype and allele frequency comparisons of enamel microhardness assessments in the permanent teeth after fluoride exposure.
| Gene | SNP | Above the Mean Enamel Microhardness Enamel [N(%)] |
Below the Mean Enamel Microhardness Enamel [N(%)] |
p-value | ||||
|---|---|---|---|---|---|---|---|---|
| TUFT1 | rs2337360 | Lingual/Palatine | ||||||
| Genotype | AA | 10(20.8) | 4(7.6) | 0.03 | ||||
| AG | 20(41.6) | 34(65.3) | ||||||
| GG | 18(37.5) | 14(26.9) | ||||||
| Allele | A | 40(41.6) | 42(40.3) | 0.85 | ||||
| G | 56(58.3) | 62(59.6) | ||||||
| AMBN | rs4694075 | Distal | ||||||
| Genotype | TT | 4(10.2) | 4(7.5) | 0.01 | ||||
| CT | 24(61.5) | 18(33.9) | ||||||
| CC | 11(28.2) | 31(58.4) | ||||||
| Allele | T | 32(41.02) | 26(24.5) | 0.01 | ||||
| C | 46(58.9) | 80(75.4) | ||||||
| MMP20 | rs1784418 | Mesial | ||||||
| Genotype | CC | 10(21.7) | 7(12.9) | 0.04 | ||||
| CT | 22(47.8) | 39(72.2) | ||||||
| TT | 14(30.4) | 8(14.8) | ||||||
| Allele | C | 42(45.6) | 53(49.07) | 0.62 | ||||
| T | 50(54.3) | 55(50.9) | ||||||
| C | 66(78.5) | 61(64.8) | ||||||
| TFIP11 | rs5997096 | Mesial | ||||||
| Genotype | CC | 16(37.2) | 8(18.1) | 0.06 | ||||
| CT | 19(44.1) | 20(45.4) | ||||||
| TT | 8(18.6) | 16(36.3) | ||||||
| Allele | C | 51(59.3) | 36(40.9) | 0.01 | ||||
| T | 35(40.6) | 52(59.09) | ||||||
| rs134136 | Mesial | |||||||
| Genotype | TT | 3(6.5) | 8(14.8) | 0.03 | ||||
| CT | 16(34.7) | 28(51.8) | ||||||
| CC | 27(58.6) | 18(33.3) | ||||||
| Allele | T | 22(23.9) | 44(40.7) | 0.01 | ||||
| C | 70(76.08) | 64(59.2) | ||||||
Bold indicates statistically significant differences.
Enamel microhardness values (for all surfaces) did not correlate with caries experience of the individuals that provided samples (data not shown).
Discussion
Our data supports the hypothesis that genetic factors affecting dental caries, and involved in the structure of enamel, impact differently the primary and permanent dentitions. This result comes with no surprise since previous genome wide association studies (11, 12) and follow-up fine mapping studies of loci of interest (16) provided very distinct results between primary and permanent dentitions. Additional evidence supporting differences between genetic influences of caries in the permanent versus the primary dentitions comes from the analysis of the keratin75 polymorphism rs2232387 (alanine to threonine substitution at position 161), which is associated with higher number of carious tooth surfaces in adults but not children (17). Also, the clinical patterns we observe in early childhood caries, related to the progression of the disease, are clearly very distinct from the typical chronic development of caries in the permanent dentition. This is true even in more severe cases, suggesting that both dentitions are distinct not only in the number of units and anatomical features, but also at the microscopic level.
While concerned about multiple testing, we avoided to apply the strict Bonferroni correction and increase type II error. If we had used Bonferroni correction, we would have lowered the alpha to 0.0000595 (0.05/840). We have demonstrated before (18) that known true associations are missed when correction for multiple testing is implemented. The results of our work should be considered with caution and serve to generate hypothesis to be directly tested in larger and more homogeneous samples. On the other hand, simply disregarding the nominal associations presented here may delay discovery by misleading the field to believe no true biological relationships exist.
Another limitation of our study is the outcome “dental caries” is first analyzed as caries experience (DMFT/dmft, Tables 1 and 3), which represents the accumulated dental caries over time. This is not the same phenotype as the one analyzed in Tables 4, 5 and 6 concerning genotype associations with enamel microhardness. This phenotype is better characterized as a “subclinical caries lesion”, which is obviously clinically not detectable by the typical dental examination. DMFT/dmft values and experimental variations in enamel microhardness observed here do not correlate, as we expected from our previous preliminary work (2). Also, the few SNPs identified as associated have no clear functional implications and we are assuming they may reflect changes in enamel that are relevant to the mechanism(s) of disease. It is still worth mentioning that the caries process in humans is complex and influenced by a high number of other factors which are not studied here or controlled for.
The present study follows our preliminary work that suggested enamel microhardness might be a more sensitive way to define caries in comparison with the traditional DMFT/dmft scores (2). We collected additional enamel samples from both dentitions and repeated the original studies. Similar to our preliminary data, we found that some individuals had lower enamel microhardness to begin with. In general, enamel microhardness declines after creation of an artificial caries lesion and increases after fluoride exposure. It is not apparent that some individuals have enamel that demineralizes at a faster rate and that caries susceptibility is linked to baseline mineralization levels of the enamel. However, it is not possible to determine if the variation we see in our data is biologically relevant, and to conclude that some individuals may be more susceptible to caries due to their original enamel structure or mineralization levels. Variation in the enamel microhardness data by tooth surface brings an additional layer of complication making it almost impossible to compile the data in any way that can convincingly provide a direction for further analyses. In other words, independent from the innate genetic background that may protect the enamel against acidic conditions, clinically, if the enamel is under unfavorable conditions long enough, it will develop a carious lesion. We recently showed that genetic variation in the genes studied here may influence calcium and magnesium concentrations of teeth (19), and biochemical rather than mechanical analyses of enamel might be more relevant to determine if particular individuals are more susceptible to enamel demineralization due to acidic conditions created by biofilm formation.
In conclusion, our data continue to support that genetic variation may impact enamel development, which might be more prone to demineralization under acidic conditions. These effects may be distinct between primary and permanent dentitions.
Acknowledgements
This study is supported by NIH Grant R01-DE18914. This paper is based in part on a thesis submitted to the graduate faculty, Istanbul University, in partial fulfillment of the requirements for a doctorate degree (M.B.).
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Patir A, Seymen F, Yildirim M, Deeley K, Cooper ME, Marazita ML, Vieira AR. Enamel formation genes are associated with high caries experience in Turkish children. Caries Res. 2008;42:394–400. doi: 10.1159/000154785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimizu T, Ho B, Deeley K, Briseño-Ruiz J, Faraco IMJR, Schupack BI, Brancher JA, Pecharki GD, Küchler EC, Tannure PN, Lips A, Vieira TC, Patir A, Yildirim M, Poletta FA, Mereb JC, Resick JM, Brandon CA, Orioli IM, Castilla EE, Marazita ML, Seymen F, Costa MC, Granjeiro JM, Trevilatto PC, Vieira AR. Enamel formation genes influence enamel microhardness before and after cariogenic challenge. PLoS One. 2012;7:e45022. doi: 10.1371/journal.pone.0045022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ergöz N, Seymen F, Gencay K, Tamay Z, Deeley K, Vinski S, Vieira AR. Genetic variation in ameloblastin is associated with caries in asthmatic children. Eur Arch Paediatr Dent. 2014;15:211–216. doi: 10.1007/s40368-013-0096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deeley K, Letra A, Rose EK, Brandon CA, Resick JM, Marazita ML, Vieira AR. Possible association of amelogenin to high caries experience in a Guatemalan-Mayan population. Caries Res. 2008;42:8–13. doi: 10.1159/000111744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang SW, Yoon I, Lee HW, Cho J. Association between AMELX polymorphisms and dental caries in Koreans. Oral Dis. 2011;17:399–406. doi: 10.1111/j.1601-0825.2010.01766.x. [DOI] [PubMed] [Google Scholar]
- 6.Jeremias F, Koruyucu M, Küchler EC, Bayram M, Tuna EB, Deeley K, Pierri RA, Souza JF, Fragelli CM, Paschoal MA, Gencay K, Seymen F, Caminaga RM, Dos Santos-Pinto L, Vieira AR. Genes expressed in dental development are associated with molar-incisor hypomineralization. Arch Oral Biol. 2013;58:1434–1442. doi: 10.1016/j.archoralbio.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaussain C, Bouazza N, Gasse B, Laffont AG, Opsahl Vital S, Davit-Béal T, Moulis E, Chabadel O, Hennequin M, Courson F, Droz D, Vaysse F, Laboux O, Tassery H, Carel JC, Alcais A, Treluyer JM, Beldjord C, Sire JY. Dental caries and enamelin haplotype. J Dent Res. 2014;93:360–365. doi: 10.1177/0022034514522060. [DOI] [PubMed] [Google Scholar]
- 8.Tannure PN, Küchler EC, Lips A, Costa MDE C, Luiz RR, Granjeiro JM, Vieira AR. Genetic variation in MMP20 contributes to higher caries experience. J Dent. 2012;40:381–386. doi: 10.1016/j.jdent.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slayton RL, Cooper ME, Marazita ML. Tuftelin, mutans streptococci, and dental caries susceptibility. J Dent Res. 2005;84:711–714. doi: 10.1177/154405910508400805. [DOI] [PubMed] [Google Scholar]
- 10.Vieira AR, Deeley KB, Callahan NF, Noel JB, Anjomshoaa I, Carricato WM, Schulhof LP, Desensi RS, Gandhi P, Resick JM, Brandon CA, Rozhon C, Patir A, Yildirim M, Poletta FA, Mereb JC, Letra A, Menezes R, Wendell S, Lopez-Camelo JS, Castilla EE, Orioli IM, Seymen F, Weyant RJ, Crout R, MCNeil DW, Modesto A, Marazita ML. Detection of Streptococcus mutans genomic DNA in human DNA samples extracted from saliva and blood. ISRN Dentistry. 2011;2011:543561. doi: 10.5402/2011/543561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaffer JR, Wang X, Feingold E, Lee M, Begum F, Weeks DE, Cuenco KT, Barmada MM, Wendell SK, Crosslin DR, Laurie CC, Doheny KF, Pugh EW, Zhang Q, Feenstra B, Geller F, Boyd HA, Zhang H, Melbye M, Murray JC, Weyant RJ, Crout R, Mcneil DW, Levy SM, Slayton RL, Willing MC, Broffitt B, Vieira AR, Marazita ML. Genome-wide association scan for childhood caries implicates novel genes. J Dent Res. 2011;90:1457–1462. doi: 10.1177/0022034511422910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Shaffer JR, Zeng Z, Begum F, Vieira AR, Noel J, Anjomshoaa I, Cuenco KT, Lee MK, Beck J, Boerwinkle E, Cornelis MC, Hu FB, Crosslin DR, Laurie CC, Nelson SC, Doheny KF, Pugh EW, Polk DE, Weyant RJ, Crout R, MCNeil DW, Weeks DE, Feingold E, Marazita ML. Genome-wide association scan of dental caries in the permanent dentition. BMC Oral Health. 2012;12:57–67. doi: 10.1186/1472-6831-12-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein H, Palmer CE. Studies on dental caries: a procedure for the recording and statistical processing of dental examination findings. J Dent Res. 1940;19:243–256. [Google Scholar]
- 14.Queiroz CS, Hara AT, Leme AFP, Cury JA. pH-cycling model to evaluate the effect of low fluoride dentifrice on enamel de- and remineralization. Braz Dent J. 2008;19:21–29. doi: 10.1590/s0103-64402008000100004. [DOI] [PubMed] [Google Scholar]
- 15.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, De Bakker PI, Daly MJ, Sham PC. PLINK: a toolset for whole-genome association and population-based linkage analysis. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanley BO, Feingold E, Cooper M, Vanyukov MM, Maher BS, Slayton RL, Willing MC, Reis SE, MCNeil DW, Crout RJ, Weyant RJ, Levy SM, Vieira AR, Marazita ML, Shaffer JR. Genetic Association of MPPED2 and ACTN2 with Dental Caries. J Dent Res. 2014;93:626–632. doi: 10.1177/0022034514534688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duverger O, Ohara T, Shaffer JR, Donahue D, Zerfas P, Dullnig A, Crecelius C, Beniash E, Marazita ML, Morasso MI. Hair keratin mutations in tooth enamel increase dental decay risk. J Clin Invest. 2014;124:5219–5224. doi: 10.1172/JCI78272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vieira AR, MCHenry TB, Daack-Hirsch S, Murray JC, Marazita ML. Candidate gene/loci studies in cleft lip/palate and dental anomalies finds novel susceptibility genes for clefts. Genet Med. 2008;10:668–674. doi: 10.1097/GIM.0b013e3181833793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halusic AM, Sepich VR, Shirley DC, Granjeiro JM, Costa MC, Küchler EC, Vieira AR. Calcium and magnesium levels in primary tooth enamel and genetic variation in enamel formation genes. Ped Dent. 2014;36:384–388. [PubMed] [Google Scholar]


