Abstract
Many pulmonary injuries and pathologies may lead to structural and functional changes in the lungs resulting in measurable sound transmission changes on the chest surface. Additionally, noninvasive imaging of externally driven mechanical wave motion in the chest (e.g., using magnetic resonance elastography) can provide information about lung structural property changes and, hence, may be of diagnostic value. In the present study, a comprehensive computational simulation (in silico) model was developed to simulate sound wave propagation in the airways, lung, and chest wall under normal and pneumothorax conditions. Experiments were carried out to validate the model. Here, sound waves with frequency content from 50 to 700 Hz were introduced into airways of five porcine subjects via an endotracheal tube, and transmitted waves were measured by scanning laser Doppler vibrometry at the chest wall surface. The computational model predictions of decreased sound transmission with pneumothorax were consistent with experimental measurements. The in silico model can also be used to visualize wave propagation inside and on the chest wall surface for other pulmonary pathologies, which may help in developing and interpreting diagnostic procedures that utilize sound and vibration.
Keywords: Computational modeling, Lung acoustics, Sound transmission, Pneumothorax, Animal modeling
1 Introduction
1.1 Motivation
Lung acoustic properties may be altered by various disease or injury states including fibrosis, congestion, consolidation, neoplasm, trauma, and pneumothorax (PTX). These conditions often lead to structural and functional changes of the pulmonary system and measurable variations in sound transmission detectable over the chest surface during auscultation. The presence of air within the lungs limits the utility of some imaging modalities. For example, ultrasound has limited utility in imaging lung structures due to the acoustic impedance mismatch between the soft tissue and air within the lungs. Conventional magnetic resonance imaging (MRI) suffers from poor signal-to-noise ratio (SNR) due to the lack of hydrogen found in air as compared to water. X-ray computed tomography (CT) provides limited contrast for soft biological tissues and also introduces potentially harmful ionizing radiation. Due to these limitations, a preliminary study that utilizes noninvasive measurement of external mechanical wave motion (sound and vibration) for lung injuries was performed in dog and porcine subjects [30, 40]. The study suggested that this method may provide information about lung mechanical property changes, which may have diagnostic value.
Many animal studies have been previously conducted to investigate different aspects of sound transmission in the torso [11, 22, 30, 42, 52]. In the current study, porcine subjects were used due to the similarity of their torso to humans as compared to other options. A three-dimensional (3D) model was developed and compared with experimental findings in the control and pneumothorax states. The ability to build realistic 3D models has been useful for various biomechanical and physiological simulations, including mechanics [36, 41, 35], electromagnetics [48], and pathology [38]. However, challenges still exist especially in having accurate biological material property data for the computational models and validating the models for more general applications. An experimentally validated acoustic computational model could aid in enhancing our understanding of sound transmission in the chest. Recently, a diagnostic technique for measuring tissue stiffness based on MRI imaging known as magnetic resonance elastography (MRE) has been applied to the lungs in pilot studies with some success [15, 32]. This technique is able to provide a map of the viscoelastic properties within the region of interest (ROI) [46]. These maps may correlate with injuries, the progression of diseases, and/or the response to therapy. Validated computational models would help predict transmission patterns of the mechanical waves used in MRE, which may help interpret and understand MRE images.
1.2 Measurements of sound transmission in lung and thorax
Mechanical compression waves (sound) travel in the lung parenchyma significantly more slowly than in the air and soft tissue of which it is comprised. Sound speeds in the human lung [2, 23, 24, 28, 31, 37] and animal lung [21, 22, 42, 60] have been studied. In human studies, sound was usually introduced into the mouth. In animal studies, sound was usually applied and measured at the lung surface. All these animal studies concluded that the sound speed depended on the lung volume, which is known to change throughout the respiratory cycle. That volume change leads to an alteration of lung density and air volume fraction, which affect the sound speed. This is consistent with theoretical models [42].
To measure the response of the thorax to acoustic excitation with known spectral content, a number of investigations have focused on studying the transmission of sound that was introduced at the mouth and detected on the chest surface. In this manner, the static and even dynamic properties of the system can be measured and compared with the computational models. Chest surface responses relative to a reference measurement over the extrathoracic trachea have been used to determine the amplitude and phase delay of transmission. The frequency-dependent decrease in amplitude agrees with models of the thorax that account for parenchymal losses [56, 58]. A strong spatial dependence of sound transmission from the mouth to the chest wall was reported by Kraman et al. [23–25], and later confirmed by Wodicka et al. [59] and Pasterkamp et al. [39]. They found that the amplitude at low frequencies at sites overlying the right lung was significantly greater than that measured at corresponding locations over the left lung.
Changes in lung structure that occur in disease affect the amplitude and timing of sound transmission from the airways to the chest surface. In patients with emphysema [5] and in dogs with pneumothorax [30], a decrease in transmitted amplitude at low frequencies was observed [31], which is qualitatively consistent with the common auscultatory finding of decreased lung sound intensity. In contrast, cardiogenic pulmonary edema was found to increase the amplitude of sound transmitted to the chest wall in dogs in a linear fashion over a wide frequency range relative to postmortem wet-to-dry weight ratios (a measure of the water content of the lungs) [11], a finding consistent with that of bronchial breathing heard over consolidated lung.
1.3 Poroviscoelastic modeling of lung
In 1956, Biot developed a theory [3, 4] for the stress wave propagation in a porous elastic solid containing compressible viscous fluid, which predicts the existence of two types of compression waves in fluid-saturated porous media. This theory has been extended to poroviscoelastic media and used in several studies for poroelastic and poroviscoelastic modeling of soft tissues. Mow et al. [33] first applied the biphasic theory to the articular cartilage which is a biphasic material composed of the solid matrix and interstitial fluid. Simon et al. [50, 51] extended the poroelastic model to include transport and swelling in the tissue. Currently, there have been limited studies on poroviscoelasticity modeling of lung acoustics. Siklosi et al. [49] used the theory to model the lung parenchyma as a porous solid with air-filled pores. Dai et al. [8] compared the Biot theory with the “effective medium” theory for modeling sound transmission in the lung parenchyma. In that study, measurements of compression wave speed and attenuation in freshly excised pig lungs matched theoretical predictions of Biot theory significantly better than effective medium theory predictions. Hence, the current study has adopted the Biot theory approach.
1.4 Chest wall material properties
To quantify material properties of soft biological tissue in the human body, Van Loocke et al. [54] and Wang et al. [57] conducted experiments by using stress relaxation technique to investigate the mechanical properties of passive skeletal muscle of animals. Von Gierke et al. [55] carried out experiments on the human thigh and upper arm, in which the skin surface was excited by a vibrating piston with different frequencies, and propagation of surface waves was measured using stroboscopy. The soft tissue in many previous studies was considered a viscoelastic medium [10, 16], and the viscous and elastic material parameter values were estimated based on experimental measurements. On the other hand, Garner et al. [14] studied human bone viscoelastic properties over a wide range of frequencies. Experiments were conducted by using torsional and bending methods, which were previously used by Lakes et al. [26] on human dry and wet compact bone to investigate their material properties, especially Young’s modulus and shear modulus. According to these studies, the bone material properties were found to be frequency dependent due to bone viscoelasticity.
1.5 Objectives of this study
The main objective of this study is to develop and experimentally validate a comprehensive computational acoustic model that simulates sound propagation in the airways, lungs, and chest wall, and how propagation is changed under the pathological condition of pneumothorax (PTX). Experimental measurements and methods of generating a 3D computational model are described in Sect. 2. The parenchymal tissue is modeled as a poroviscoelastic material based on Biot theory. A finite element (FE) model of the pig chest that includes the underlying organ details is constructed and used in the FE software package to simulate the vibroacoustic response of the chest surface caused by the sound input at the trachea. In Sect. 3 the results from the FE simulation are compared with experimental measurements for cases of normal and PTX states. A discussion of the results is provided in Sect. 4, followed by conclusions in Sect. 5.
2 Materials and methods
Section 2.1 describes experimental acoustic studies conducted on porcine subjects, which serve to experimentally validate the developed computer simulation model. Section 2.2 describes experiments to identify some of the material properties needed for the computer model, which were not already available from other studies. In Sect. 2.3, the computer simulation model is detailed, with subsections describing construction of the 3D model from X-ray CT images (Sect. 2.3.1) and implementation of the material parameters and FE model structure for the lung parenchyma (Sect. 2.3.2), larger airways (Sect. 2.3.3), and the surrounding soft and hard tissue of the chest wall (Sect. 2.3.4).
2.1 Experimental porcine studies
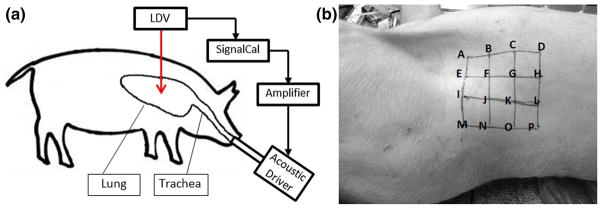
Experiments were conducted on five freshly killed female Landrace and Yorkshire cross pig subjects (weight 30–35 kg) after getting approval of the institutional animal care and use committee (IACUC). The experimental setup is shown in Fig. 1a. Immediately after the pig was euthanized, it was secured in the left lateral decubitus position (right-side-up) with the skin hair of the measurement area shaved completely. In addition, the pig lungs were kept inflated with air at an airway pressure of 5 cm H2O through an endotracheal tube. The lungs are located roughly between the first and tenth rib. During the experiment, the airway pressure was adjusted to 20 cm H2O to help fully open the airways inside the lung. A laser Doppler vibrometer (LDV) (PDV-100, Polytec, Irvine, CA) was used to measure the normal velocity of the skin surface at 16 evenly spaced points in an array located in the area from the fifth rib to the ninth rib (Fig. 1b). In order to enhance the laser reflectivity for improved signal-to-noise ratio, P-RETRO-250 glass beads (45–63 μm dia., Polytec, Irvine, CA) were applied to the skin surface. A broad-band periodic chirp signal with spectral content from 50 to 800 Hz was generated from a dynamic signal analyzer (SignalCalc ACE, Data Physics, San Jose, CA) and sent to an amplifier (P 3500S, Yamaha, Buena Park, CA) to drive a 3.5 inch acoustic speaker (PDWR30 W, PylePro, Brooklyn, NY). The sound wave generated from the speaker was sent into the pig lung through the endotracheal tube. As the sound pressure generated by the speaker is frequency dependent, a 1/4-inch microphone (378C01, PCB Piezotronics, Depew, NY) was inserted into a drill hole in the proximal endotracheal tube. This measured acoustic pressure was used for the FE simulation as the acoustic pressure input described in Sect. 2.3.
Fig. 1.
a Experimental setup. b The location of array of measuring points
The above measurements were performed for the normal and pneumothorax (PTX) states for each animal. The PTX state was purposefully created by making a small incision in the seventh intercostal space in the right mid-clavicular line, which allowed air to enter into the plural space. The airway pressure was set to “0” in the PTX state to allow for full lung collapse. A 5-mm thoracoscopic trocar (Endopath Dilating Tip, model 355, Ethicon, Cincinnati, OH) was inserted into the chest, and an endoscope was used to visually confirm the PTX state.
2.2 Material property measurement of pig muscle tissue
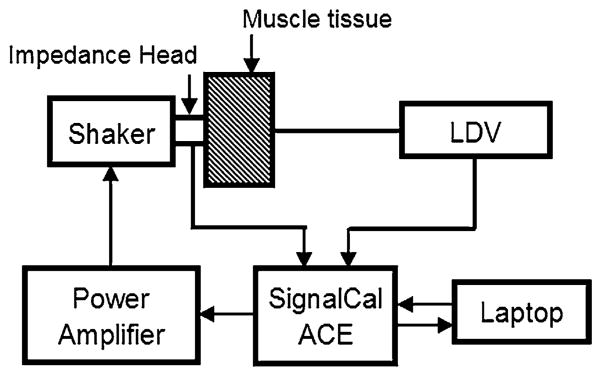
To get the compression wave speed and attenuation of porcine muscle tissue in our frequency range of interest, an experiment was implemented on a piece of pig muscle tissue obtained from the posterior chest of another Landrace and Yorkshire cross pig that had similar size and weight to the pigs in Sect. 2.1. The schematic diagram of the experimental setup is shown in Fig. 2. A sinusoidal chirp signal containing frequencies from 50 to 4000 Hz was generated from a dynamic signal analyzer (SignalCalc ACE, Data Physics, San Jose, CA) and was sent into a power amplifier (P 3500S, Yamaha, Buena Park, CA) that was connected to an electromagnetic shaker (ET-132, Lab-Works Inc., Mesa Costa, CA). An impedance head (288D01, PCB Piezotronics, Depew, NY) was mounted on the shaker and was placed in contact with the muscle tissue to measure the input acceleration signal to the tissue sample. In the testing experiment, it showed that with the same fixtures and rectangular size, compression wave speed on tissue samples with thickness ranging from 3 to 6 cm was consistent. The sample thickness was L = 5.8 cm and contact area with the input from the shaker was circular with a diameter of 2 cm. The out-of-plane velocity on the other side of sample was measured by a laser Doppler vibrometer (LDV) (PDV-100, Polytec, Irvine, CA). The acceleration and velocity measurements were recorded by the same signal analyzer with a sampling frequency of 102.4 kHz, and data were stored in a laptop computer. The input acceleration signal measured by the impedance head was denoted by x(t). The output acceleration signal y(t) was the derivative (with respect to time) of the velocity measured by the LDV. Consequently, the compression wave speed can be calculated from the phase angle θxy of the cross-spectral transfer function between x(t) and y(t). The transit time τ can be calculated from the slope of the θxy frequency curve by
Fig. 2.
Schematic of experimental setup
| (1) |
For a linear viscoelastic material, the complex-valued compression wave number kp is given by
| (2a) |
Here, cp is the complex-valued compression wave speed and ω is the angular frequency. The real part of the compression wave number kpR is related to the phase speed cph (calculated by dividing the thickness L by the transit time τ) [9] by
| (2b) |
The attenuation of the compression wave is governed by the imaginary part of the wave number kpI [9]. To estimate the attenuation of the compression wave in the tissue, a continuous sinusoidal input with a single frequency was used. That signal was generated from the dynamic signal analyzer, sent to the amplifier, and delivered into the sample by the shaker. The input acceleration a1 was measured by the impedance head, and the output acceleration a2 was derived from the velocity measured by the LDV. The experiment was repeated for different frequencies from 50 to 1000 Hz. Assuming planar compression waves, the input and output accelerations (a1 and a2, respectively) can be expressed by:
| (3) |
| (4) |
Here, A0 is the amplitude of acceleration. Then
| (5) |
| (6) |
After evaluating kpR and kpI, the complex compression wave speed is determined from Eq. (2a). Experimentally determined values of these material properties will be used in the following sections.
2.3 Computational simulations
2.3.1 Three-dimensional geometry model construction
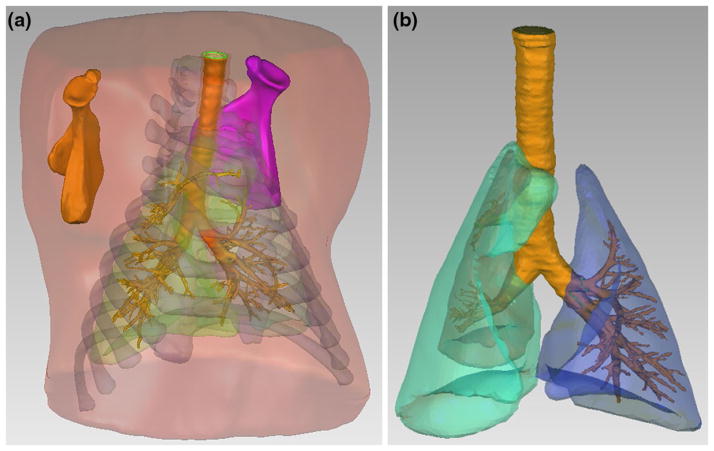
To generate the 3D porcine geometries for computational studies, comprehensive geometrical details of the torso structures were constructed from CT images obtained from scans with 1-mm slice steps and pixel matrix size 512 × 512 (Brilliance 64, Philips Electronics). The pig used for scanning was similar in size (weighted 34 kg) to those studied in the acoustic experiments described in Sect. 2.1. CT image sets were imported and processed using Mimics V14 (Materialise, Plymouth, MI), which is a biomedical image processing software for the segmentation of 3D models. Different density of the contrast regions (lungs, bone regions, and muscle tissue regions) can be distinguished in the software to assign layers at each slice of the CT images. Then, all of the 2D layers can be combined to create a 3D model for each part. The 3D torso model includes different parts such as ribcage, scapulae, soft tissue (skin, muscle and fat are regarded as soft tissue with same material properties for simiplification) and lungs as shown in Fig. 3a.
Fig. 3.
a 3D model of porcine upper torso and internal organs. b 3D model of porcine PTX lung and airways
To build the 3D model of airways inside the lung, an open-source medical image segmentation software ITK-SNAP version 2.4 [61] was used to construct the geometry of the different chest structures. CT images were imported for automatic segmentation using the snake algorithm in ITK-SNAP. The automatic segmentation was used as a first step in generating the geometry, which can later be improved manually. Due to the contrast resolution quality of the CT image sets, there were twenty airway segments created by the automatic segmentation method; the quality of the segmentation is largely dependent on the quality of the images. Because of the complexity and small size of the distal branches of the airways, these airways were completed manually by marking contrasted regions slice by slice. This was achieved by creating the airway segments with the marker tool in the local contrasted regions. By combining the above automatic and manual approaches, a more intricate airway tree with about 100 segments was constructed.
The main stem bronchi and trachea were the starting point of the snake algorithm and were the first to be automatically segmented. With the completion of all the segmentations, a surface tessellation algorithm was applied by ITK-SNAP for capturing the 3D geometrical data, and these data were exported as a stereolithography (STL) CAD file so that it could be meshed properly in ANSYS ICEM CFD.
Once the airways and trachea geometries were imported to ANSYS ICEM CFD 12.1 (Ansys Inc., Canonsburg, PA), a meshing tool in ANSYS, they were combined with the files containing the geometries of the torso, lungs, ribcage, and scapulae built in Mimics V14. The geometries were checked in ANSYS for mesh quality and intersecting errors. Once the geometry passed that test, it was volume meshed to create a FE model. In addition, the surface of the trachea and mainstem bronchi was extruded in the radial direction to construct the thickness of the airways, creating two volumes for the airways: one for the air and another for the thickness of the mainstem bronchi and trachea walls. To generate a 3D model of PTX, the right lung was reduced by a factor of 71 % in volume which corresponds to a PTX of 95 %; it was fit inside of the original right lung space, while keeping the chest cavity geometry unchanged; the remaining volume inside the chest cavity was assumed to be filled with air. The PTX percentage is defined by the ratio of volume of air which occupies the chest cavity outside the lung and the air volume in normal lungs [42, 58].
2.3.2 Material properties of lung parenchyma
Sound propagation into the lungs and transmission to the chest surface was modeled in a FE environment COMSOL Multiphysics® 4.3b (COMSOL Inc, Burlington, MA) using the acoustic-solid interaction module [34] for frequency domain analysis. The entire volume mesh of the relevant parts, such as chest wall, lungs, ribcage, scapulae, and airways mentioned above, was imported into COMSOL for simulation. Acoustic excitation at the inlet of the trachea was applied in the simulation by using the frequency-dependent sound pressure measured in the experiment in Sect. 2. In the COMSOL simulation, air regions (within the lumen of the larger explicitly modeled airways and within the air cavity created by a pneumothorax) were set as “pressure acoustics” elements. All other regions—the lung parenchyma, soft/muscle tissue and bone—were set as “linear viscoelastic solid” elements. A free boundary condition was applied on the torso surface, while an “acoustic-structure boundary” was applied at the air–tissue interfaces. At these interfaces, there is continuity of normal velocity and pressure as the air is assumed to be inviscid in the simulation. At solid–solid interfaces, such as the airway wall—lung parenchyma interface, there is continuity of displacement and normal stress.
Material properties of each organ need to be provided to the modeling software before running the simulation. For the lung parenchyma, a recent study [9] demonstrated that the Biot theory of wave propagation in poroviscoelastic media [3, 4] was a more accurate model than the previously used effective medium theory [9] to model sound transmission in the lung parenchyma. The following set of coupled differential equations (written in the frequency domain neglecting initial conditions and denoting a derivative with respect to time as multiplication by jω) [47] can be used to describe the steady-state dynamic oscillatory displacement u of the lung parenchyma and dynamic pressure p of the air in the lung:
| (7) |
| (8) |
Here, μ is the shear modulus of the lung tissue, Kb is the bulk modulus of the lung tissue when it is inflated, ρ is the lung parenchyma density, ρf is the air density, ϕ is the air volume fraction in the lung, and α, β, and R are the coupling parameters between the lung parenchyma and the air. Based on Eq. (7), the complex shear wave speed is given by
| (9) |
The coupled Eqs. (7) and (8) lead to two compression waves, a fast wave and a slow wave with much larger attenuation. The compression wave speeds are given by
| (10a) |
| (10b) |
Here, cpf and cps are the fast and slow compression wave speeds, respectively. Their corresponding wave numbers, kpf and kps, are derived from Eq. (7). In our frequency range of interest (50–700 Hz), the slow compression wave cannot propagate for noticeable distances as the relative motion between the lung parenchyma and air is impeded by viscous drag [6]. Hence, only the fast compression wave propagation may be detectable in the parenchyma. As the lung parenchyma is viscoelastic, the shear wave speed is frequency dependent. The phase speed and attenuation are expressed in the complex-valued shear wave speed. The compression wave attenuation is mainly due to the friction between the air and the lung parenchyma. The complex-valued fast compression wave speed is given by Eq. (10a). By implementing the above equations, the frequency-dependent compression wave speed and total attenuation of compression wave and shear wave in the lung parenchyma can be calculated. The information is then input into COMSOL as the lung material properties to perform the simulations. The lung compression wave and shear wave speeds are assumed to be the same throughout the parenchymal region (assumption of homogeneity). COMSOL calculates the displacement, velocity, acceleration, stress and strain of the soft tissue, rib cage, cartilage, and lung. It also calculates the acoustic pressure inside the airways and inside the air cavity created by a pneumothorax.
The value of ϕ approximates the average percentage of air volume in a normal lung. The experiment was done on euthanized pigs; hence, ϕ was constant. Previous studies [42, 58] suggest that the lung air volume fraction ϕ = 75 % [1], the lung tissue density ρp = 1000 kg/m3, and the air density in the lung ρg = 1.21 kg/m3. Hence, the composite lung parenchymal density would be given by:
| (11) |
These properties result in a density of the normal lung ρ = 250.9 kg/m3. The lung shear modulus was measured by a surface wave method and fit by a fractional Voigt model as μ = [3.67 × 103 + 235.09(jω)0.43] Pa [9] Other parameters in Eq. 7 are ρf = 1.2 kg/m3, Kb = 8.26 × 103 Pa, α =1, and R = 7.74 × 104 Pa [9].
Figure 3a shows the chest structure under normal conditions. When a PTX is introduced in the right chest, the right lung collapses and thus will have a lower air volume fraction depending on the level of collapse. In this case, the chest region in the 3D model that was originally occupied by the right lung was split into two portions, the collapsed parenchymal region and the air outside the lung. For the current simulation, the 3D model was made to create a large PTX state with ϕ = 11 %. This value of air volume fraction corresponds to a PTX ~= 95 %, which presents the extreme state of PTX similar to that induced in the experiment. The lung compression wave speed, shear wave speed, and attenuation were then calculated using Eq. (10) for this air volume fraction.
2.3.3 Airway acoustics
In this study, the distal airway segments are un-modeled because of the limit of the resolution of the CT image. However, the terminal impedance (ratio of acoustic pressure to particle velocity as a function of frequency) of the terminal segments needs to be calculated to represent the effect of the downstream airways that are un-modeled on the acoustic field in the modeled larger airways. The Horsfield airway model [19, 20] was utilized to perform the impedance calculations. The pig appears to have morphology close to the human airway tree, especially the segments with small diameters. Hence, in these calculations, the structural parameters of the human airway were used to calculate the pig airway terminal acoustic impedance.
Using the fractal asymmetric Horsfield airway scheme, previous studies have developed an acoustic impedance model of the airways that incorporates non-rigid airway wall motion caused by changing acoustic pressure and terminal (alveoli) acoustic impedance [17, 18]. The starting step is to calculate the acoustic impedance at a terminal bronchiole, n = 1, and then “march up” the recursion ladder to n = 2, 3, 4 until reaching n = 35, the trachea [9]. For the nth-order airway segment of length l(n), the input acoustic impedance (taken at the end closer to the trachea) is given by
| (12) |
The terms and are the characteristic impedance and propagation coefficient of the nth airway segment, respectively, and are given by Royston et al. [45]. The term denotes the acoustic impedance at the far end of each segment, which is given by
| (13) |
Here, NT denotes the total number of terminal bronchiole segments. For the Horsfield model, this can be calculated using the following recursion formula, taking and ,
| (14) |
The result is NT ≈ 2.35 million for human lungs. The term Cg (0.003 L/cm H2O) denotes the alveolar gas compression compliance based on the Dubois et al. [12] six-element terminal airway model where Rt (3.8 cm H2O/L/s), It (0.0057 cm H2O/L/s2), and Ct (0.13 L/cm H2O) denote the terminal tissue resistance, inertia, and compliance, respectively.
To calculate the acoustic impedance of the un-modeled terminal airways, an order number needs to be specified for the terminal segments. The standard deviation of the terminal segment diameter is small such that the terminal segments are regarded as the same and their order number is found by relating their mean diameter to the closest value in Table 9–2 in Royston et al. [43]. For the normal airways of the current model, the terminal segment mean diameter is 0.1568 cm, which corresponds to the airway order number 14. For the PTX state, the lung is collapsed, and this causes a decrease in the small airway diameters. Assuming the airway segment is a thin-walled elastic tube (airway wall viscosity and inertia do not affect airway segment diameter under steady-state conditions), the relationship between airway radius and transpulmonary pressure is [13]
| (15) |
where da(n) is the change of radius of the airway segment with order n and dp is the transpulmonary pressure change. Also
| (16) |
Here a(n), h(n), and E(n) are the radius, wall thickness, and Young’s modulus of the airway segment with order n, respectively. E(n) is calculated from the airway cartilage Young’s modulus Ec and airway soft tissue Young’s modulus Es by
| (17) |
From studies by Suki et al. [52], Ec and Es are taken to be 392 kPa and 58.1 kPa, respectively. Here, c(n) is the cartilage percentage of the airway segment with order number n, and it is listed in Table 9–2 of Royston et al. [43]. Here, dp decreases by 20 cm H2O when going from the normal to the PTX state. Changes in transpulmonary pressure lead to noticeable diameter change for small airway segments that have little to no cartilage content as compared to the main stem bronchi and the larger few branches below the main stem bronchi. From Eq. (15), for airway segments with order number from n = 15 to 27, the segment diameter decreases an average of 32 %, and this is close to but only a bit more than experimental studies on airway diameters under different transpulmonary pressures in dogs [53]. However, as the Young’s moduli of the trachea, main stem bronchi, and lobar segments (n = 31–35) are at least 20 times larger than that of the surrounding lung parenchyma [47], the diameter decrease in these airways is small and is neglected. Finally, the acoustic impedance of the terminal airway segment in the PTX state was calculated for the decreased airway segment diameters. The calculated corresponding terminal impedance is applied to branch ends of the airways as boundary conditions in the simulations. And, the material properties of airways from Royston et al. [43] are used in the FE simulations.
2.3.4 Material properties of muscle tissue and bone
The values of material properties of muscle tissue were based on previous studies [10, 44, 46, 55]. According to those studies, the soft muscle tissue was considered viscoelastic [16], and the Voigt model was used. The shear modulus can be expressed as:
| (18) |
Here, the subscript “t” denotes the soft tissue, and μt1 and μt2 denote shear elasticity and shear viscosity, respectively: μt1 = 2.5 × 103 Pa and μt2 = 15 Pa S [44, 55]. With the reported soft tissue density of 1100 kg/m3, the complex shear wave speed can be calculated by using Eq. 9 in Sect. 2.3. With the compression wave speed and attenuation of muscle tissue measured in the Sect. 2.2, the muscle tissue mechanical properties can be completely defined in the simulation.
The complex young’s modulus of the bone can be represented by E = Eb1 + j · Eb2 with tan δE = Eb2/Eb1 [7, 14] where subscript “b” denotes the bone. The loss tangent tan δE is frequency dependent and was experimentally measured by Garner et al. [14]. For the current frequency range of interest, the real part of E is taken to be Eb1 = 12.7 GPa in the simulation [7]. The complex shear modulus is expressed as μb = μb1 + jωμb2 with tan δμ = μb2/μb1. The shear loss tangent tan δμ was determined from previous experimental measurements [14, 26], and the real part of shear modulus is μb1 = 3.15 GPa [7].
3 Results
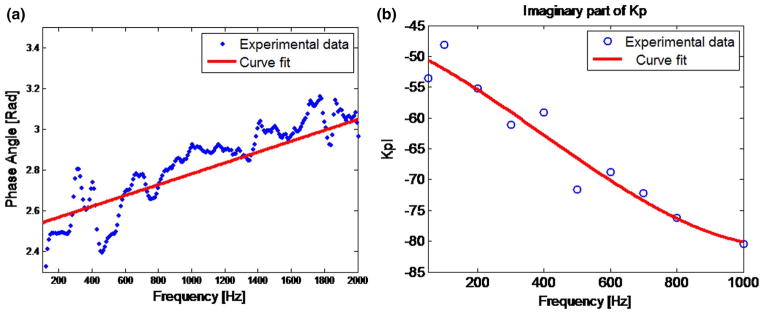
Figure 4a shows the phase angle of the cross-spectral transfer function of the accelerations on two sides of the pig muscle. A linear trend between phase angle and frequency can be seen between 600 and 2000 Hz. The estimated phase speed is 1345.5 m/s. (This is a low compression wave speed for soft biological tissue as compared to 1483 m/s assuming that tissue bulk modulus is close to that of water. Compared to the compression wave speed differences between the lung parenchyma and other soft biological tissues, the relative differences between 1345.5 and 1483 m/s are an order of magnitude less with regard to the salient properties that will affect the acoustic simulations reported in this study. Test simulations carried out using 1345 and 1500 m/s for the soft tissue of the chest wall yielded negligible differences; 1345.5 m/s was used in this study.) The measured imaginary part of the compression wave number is shown in Fig. 4b. Using these measurements and Eq. (2a) and (2b), the frequency-dependent complex-valued compression wave speeds are obtained and input into COMSOL together with complex-valued shear wave speeds as material properties of the soft tissue in the chest.
Fig. 4.
Experimental results and curve fitting of (a) phase angle (b) the imaginary part of wave number
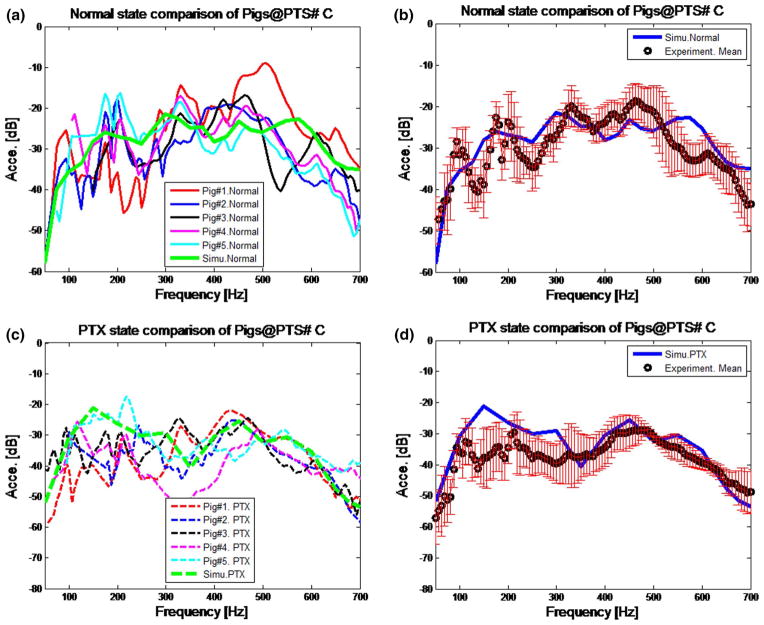
The acceleration amplitude of the normal and PTX states at point #C on the chest surface (see Fig. 1b for point location) is shown in Fig. 5a, c. Experimental results for all five pigs and simulation results are shown. The experimental acceleration was calculated from the velocity (measured by LDV). The experimental data are only plotted at frequencies where the coherence (between measured input acoustic pressure and chest velocity) exceeded 0.85. There were very few frequencies that did not meet this cutoff. It can be seen in the figure that the variability in the measured values is noticeable for the normal and PTX states. Figure 5b, d shows the mean acceleration of five pigs for the normal and PTX state along with the simulation results. In these two figures, the simulation prediction is comparable to the experiment mean, and similar trends can be observed. The root-mean-square error (RMSE) of the simulation prediction and mean of experimental results of Fig. 5b, d are 5.526 and 6.538 dB, respectively. The locations of spectral peaks are a slightly different between experiment and simulation, especially below 250 Hz. The simulation predictions were compared with experimental results at other measurement locations and results were similar to those presented here.
Fig. 5.
Acceleration on the chest surface for normal state at point C: a comparison of simulation and experiments on five pigs; b comparison of mean acceleration of experiment and simulation. The error bars indicate standard deviation of five pigs. Acceleration on the chest surface for PTX state at point C: c comparison of simulation and experimental acceleration of five pigs; d comparison of mean acceleration of experiment and simulation. The error bars indicate standard deviation. Units (m/s2 in dB scale)
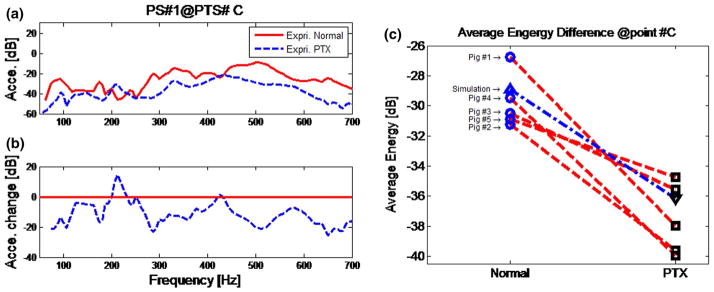
In Fig. 6a experimental results of the normal state and PTX state are compared at a specific measurement point #C. It can be seen that the acceleration amplitudes of the PTX state are consistently lower than those of the normal state for most frequencies in the range from 250 to 700 Hz. For frequencies from 50 to 250 Hz, the amplitude differences between the normal and PTX state appear smaller. The maximum change of amplitude between the normal and PTX states is about 20 dB. The average reduction over the frequency range of 250 to 700 Hz for the five pig subjects is 9.92 dB with a standard deviation of 1.88 dB. Figure 6c shows the average energy for the normal and PTX state of experimental results of the five pig subjects and the results of the simulation. It can be seen that in the frequency range under consideration, the average transmitted energy in the normal state is higher than that in the PTX state for every subject. Comparison at other points also showed a drop in torso surface acceleration amplitude and average energy for the PTX state.
Fig. 6.
Experimental comparison of normal and PTX state. a The comparison of normal and PTX. b The acceleration amplitude change between PTX and normal states (change = PTX-Normal). c Average measured energy of normal and PTX state at point #C for five pig subjects in the 50- to 700-Hz frequency band. Experimental result (normal): blue circle; experimental result (PTX): square; simulation result (normal): blue triangle; simulation results (PTX): inverse triangle; red line connects the results of the same pig subject; blue dashed line connects the results of simulation (color figure online)
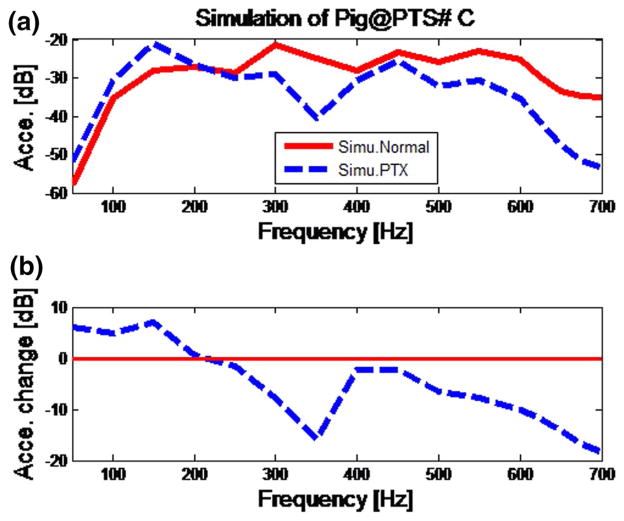
In Fig. 7a, b, simulation results of the normal state and PTX state are compared at the surface measured point #C. The results at other points are similar. Simulation results match experimental trends in that the acceleration amplitudes of the PTX state are lower than those of the normal state in the frequency range from 250 to 700 Hz. The average reduction predicted by the simulation over the frequency range from 250 to 700 Hz for the presented points is 9.05 dB with a standard deviation of 3.16 dB (Fig. 6c). The drop in the acceleration amplitude with PTX is close to the experimental result of 9.92 dB.
Fig. 7.
Simulation results for normal versus PTX state at point #C. a The simulation results of normal and PTX state; b the normalized PTX results
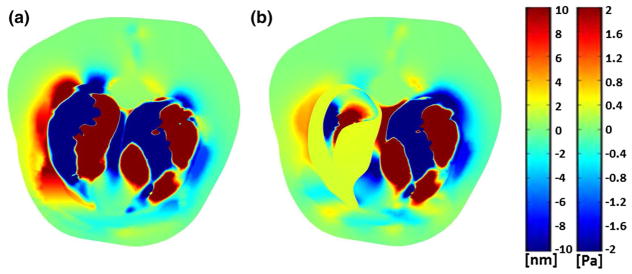
The cross-sectional image of torso displacement in the left-to-right direction and air acoustic pressure at a frequency of 600 Hz are shown in Fig. 8a, b for the normal and PTX states of the right lung. This frequency was chosen since it resulted in sufficiently short wavelengths that are completely observable in the cross-sectional image. The unit Pa for the acoustic pressure is applicable to the air inside and outside the lungs. Patterns of compression waves emanating from the airways and propagating into the chest are evident and are shown as red and blue (indicating positive and negative displacement) bands. These patterns are changed in the collapsed lung side due to change in lung acoustic properties and the acoustic impedance mismatch between the lung and the air in the chest. Simulation images like those shown in Fig. 8 helps in understanding displacement images obtained from elastography imaging modalities.
Fig. 8.
Simulation at 600 Hz: cross section of torso showing dynamic displacement at an instant in time in the left–right direction for a normal state and b PTX state. Color bar shows tissue displacement in [nm] (left color bar) and air acoustic pressure in [Pa] (right color bar) (color figure online)
4 Discussion
The experimental technique (described in the Sect. 2) is similar to methods presented and applied to cats and dogs in previous studies [11, 30]. In Mansy et al. [30], an electronic stethoscope was used to measure the transmitted acoustic waves on the chest skin surface. Instead, a LDV was used for measurements in the current study. As the electronic stethoscope is attached to the skin surface, it may alter the dynamics of the system. A benefit of using a non-contacting LDV is that it does not alter the system it is measuring.
One objective of the present study is to investigate whether the technique of airway insonification can be successfully implemented on relatively large animals like a pig as studies on animals with pulmonary injuries or diseases can be carried out more easily than on human subjects. Another objective of the study is to develop a computational model of sound transmission in the pig chest.
To the best knowledge of the authors, the material properties of bone are close to those of human within the frequency range of interest [7, 26]. So, the complex young’s modulus and shear modulus of material properties of human bone were used in the simulation. As we are mainly interested in the wave motion in the lung parenchyma (solid part of the lung), not the air motion in the subglottal airway tree and alveolar sacs, it suffices to input the complex-valued lung compression wave and shear wave speed derived from the Biot model and applies these parameters to the lung region defined as a viscoelastic solid.
Pig CT scans were used to build realistic 3D geometries for FE simulation. The results of simulation and experiments on five pig subjects in the normal and PTX states are compared in Fig. 5. In Fig. 5b, d the mean of experimental results of five pigs for both states and the simulation prediction are plotted together for further comparison. Simulation results capture the main trends seen in the experiments. Inter-subject variability is seen in Fig. 5a, c for normal and PTX state, respectively. In Fig. 5d the computational results were higher than the mean of the experimental data between 100 and 300 Hz. However, by examining Fig. 5c, we can see that the computational results are within the range of experimental data. The experimental data had variability due to subject variability [30]. Even with these simplifications, several experimental trends could be predicted using the computational model. A shift of peaks and dips can be observed especially in Fig. 5b, d. This may be because of slight differences in size between the simulation model and the five experimental subjects. Chest surface accelerations from model predictions are higher than the experimental results at most of the frequencies of interest. A possible reason is underestimation of the tissue damping in the simulation. This is more obvious in the frequency range from 50 to 200 Hz for the PTX state comparison (Fig. 5b).
In the current experimental studies, measurements were taken on a limited portion of the chest surface due to the time it took for the LDV to measure each point consecutively. In future studies, a sensor pad that covers most or all of the chest surface and measures all the points simultaneously or an entire chest measurement by scanning laser Doppler vibrometer could provide more comprehensive experimental information [27]. In the current study, the porcine subjects were euthanized before the experiment. Thus, the effect of lung volume change during the respiration cycle was not considered. For human subject studies, participants could be requested to hold their breath for a short time to avoid lung volume change.
Figure 6a, c suggests that the PTX results in lower amplitude of transmitted sound in the frequency range from 250 to 700 Hz. Similar findings were reported in previous studies on canine subjects [30]. Transmitted sound amplitude decreases with a PTX, likely due to the acoustic impedance mismatch at the boundaries of the air in the chest and collapsed lung [29, 30]. This can be seen more clearly in Fig. 6c where the average transmitted energy over all frequencies in the experiment for the PTX state was lower in all pig subjects. In Fig. 7, simulation predictions of PTX were found to be similar to experimental measurements presented in Fig. 6a. Chest surface acceleration for the PTX condition is slightly higher than that in normal case between 50 and 200 Hz. It is speculated that there may be a resonance response in this frequency range. The observed decrease in the transmitted acoustic energy with increasing frequency is consistent with the results of the experiment. For both simulation and experiment, the acceleration amplitudes decrease by about 2–20 dB in the 250- to 700-Hz frequency range. Simulation also suggests that that acceleration amplitude drop may continue above the frequencies in the current study. The maximum frequency in the current experimental study was limited to 700 Hz to maintain a sufficiently high SNR.
As shown in the current study, detecting PTX was achieved by measuring transmitted sound on the chest surface. In Fig. 8 the model was used to predict torso displacement caused by transmitted sound. Visualization of wave motion inside the torso measured by an elastographic imaging technique may also help interpret tissue viscoelasticity based on wavelength estimates. An accurate computer simulation of sound transmission through the chest and lungs under normal and pathologic conditions may help to enhance our understanding of auscultation results and our ability to use the sounds for diagnosis. The in silico model developed here may also prove useful in the development of a more effective educational platform for acoustic-based diagnostic methods.
5 Conclusion
A computational model was developed for simulating acoustic transmission from airways to the chest surface in a pig. The computer model was validated by experimental measurements on five porcine subjects before and after pneumothorax induction. Simulation and experimental findings suggest that certain characteristics of acoustic transmission patterns may be useful in detecting pulmonary abnormalities such as PTX. Model predictions were found to be consistent with experimental measurements and the literature. The developed computational models can also predict wave propagation inside internal organs which may be of use in assessing the performance of other acoustic diagnostic approaches.
Acknowledgments
Financial support of the National Institutes of Health (Grant No. EB012142) is acknowledged.
Biographies

Ying Peng received his B.Sc and M.Sc in Mechanical engineering in 2006 and 2009, respectively. And recently he received his Ph.D in Mechanical Engineering with focusing on acoustic and vibration in lungs from University of Illinois at Chicago. His research interests include medical diagnostics based on vibration and acoustics, biological response to vibration, pulmonary modeling, and finite element analysis.

Zoujun Dai received his B.Sc in Mechanical engineering in 2006 and his Ph.D in Mechanical Engineering with focusing on acoustic and vibration in lungs from University of Illinois at Chicago (UIC) in 2013. He is a postdoctoral researcher in bio-engineering department at UIC. His research interests include medical diagnostics based on vibration and acoustics (development of novel sonic/ultrasonic techniques, MR elastography, lung acoustic imaging) and biological response to vibration.

Hansen A. Mansy received his Ph.D in Aerospace Engineering with a focus on oscillating fluid flows from Illinois Institute of Technology. He was the director of the Biomedical Acoustics Research Center at Rush Medical College, and a research associate professor at the Illinois Institute of Technology. He is now an associate professor and the director of Biomedical Acoustics Research Laboratory in Department of Mechanical and Aerospace Engineering in University of Central Florida.

Brian M. Henry received his B.Sc in Bioengineering at the University of Illinois at Chicago. And now his is doing his Ph.D in Bioengineering Engineering in University of Illinois at Chicago. His research interests include pulmonary modeling, programming, and finite element analysis.

Richard H. Sandler completed medical school and a Pediatrics residency at Michigan State University and a sub-specialty fellowship in Pediatric Gastroenterology at Harvard Medical School. He is a pediatric gastroenterologist in Orlando, Florida, and is affiliated with multiple hospitals in the area, including Plantation General Hospital and Rush University Medical Center.

Robert A. Balk completed medical school and Internal Medicine residency from University of Missouri and a sub-specialty fellowship in Pulmonary and Critical Care Medicine residency from University of Arkansas. His is the director of the Section of Pulmonary and Critical Care Medicine and professor of Medicine at Rush Medical College.

Thomas J. Royston received his Ph.D in Mechanical Engineering from The Ohio State University in 1995. He is a professor of Mechanical Engineering, Head of Bioengineering department, and the Director of the Acoustics and Vibrations Laboratory at the University of Illinois at Chicago.
References
- 1.Armstrong J, Gluck E, Crapo R. Lung tissue volume estimated by simultaneous radiographic and helium dilution methods. Thorax. 1982;37(9):676–679. doi: 10.1136/thx.37.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergstresser T, Ofengeim D, Vyshedskiy A, et al. Sound transmission in the lung as a function of lung volume. J Appl Physiol. 2002;93:667–674. doi: 10.1152/japplphysiol.00050.2002. [DOI] [PubMed] [Google Scholar]
- 3.Biot MA. Theory of propagation of elastic waves in a fluid-saturated porous solid. I. low-frequency range. J Acoust Soc Am. 1956;28:168. [Google Scholar]
- 4.Biot MA. Theory of Propagation of Elastic Waves in a Fluid-Saturated Porous Solid. II. Higher Frequency Range. J Acoust Soc Am. 1956;28:179. [Google Scholar]
- 5.Böhme HRBH. Variable low-frequency sound conduction of the lung in pulmonary emphysema. Z Gesamte Inn Med. 1972;27:765–770. [PubMed] [Google Scholar]
- 6.Bourbié T, Coussy O. Acoustics of porous media. Editions TECHNIP; 1987. [Google Scholar]
- 7.Cowin SC. Bone mechanics handbook. 2. Gulf Publishing Company; Huston, TX: 2001. [Google Scholar]
- 8.Dai Z, Peng Y, Henry B, et al. A comprehensive computational model of sound transmission through the porcine lung. J Acoust Soc Am. 2014;136(3):1419–1429. doi: 10.1121/1.4890647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai Z, Peng Y, Mansy HA, et al. Comparison of poroviscoelastic models for sound and vibration in the lungs. J Vib Acoust. 2014;136:051012. doi: 10.1115/1.4026436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai Z, Peng Y, Mansy HA, et al. A model of lung parenchyma stress relaxation using fractional viscoelasticity. Med Eng Phys. 2015 doi: 10.1016/j.medengphy.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnerberg RL, Druzgalski CK, Hamlin RL, et al. Sound transfer function of the congested canine lung. Br J Dis Chest. 1980;74:23–31. [PubMed] [Google Scholar]
- 12.DuBois AB, Brody AW, Lewis DH, Burgess BFJ. Oscillation mechanics of lungs and chest in man. J Appl Physiol. 1956;8:587–594. doi: 10.1152/jappl.1956.8.6.587. [DOI] [PubMed] [Google Scholar]
- 13.Fung Y. Biomechanics: circulation. Springer; New York: 1997. [Google Scholar]
- 14.Garner E, Lakes R, Lee T, et al. Viscoelastic dissipation in compact bone: implications for stress-induced fluid flow in bone. J Biomech Eng. 2000;122:166. doi: 10.1115/1.429638. [DOI] [PubMed] [Google Scholar]
- 15.Goss BC, McGee KP, Ehman EC, et al. Magnetic resonance elastography of the lung: technical feasibility. Magn Reson Med. 2006;56:1060–1066. doi: 10.1002/mrm.21053. [DOI] [PubMed] [Google Scholar]
- 16.Haas C, Best T, Wang Q. In vivo passive mechanical properties of skeletal muscle improve with massage-like loading following eccentric exercise. J Biomech. 2012 doi: 10.1016/j.jbiomech.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habib RH, Chalker RB, Suki B, Jackson AC. Airway geometry and wall mechanical properties estimated from sub-glottal input impedance in humans. J Appl Physiol. 1994;77:441–451. doi: 10.1152/jappl.1994.77.1.441. [DOI] [PubMed] [Google Scholar]
- 18.Habib RH, Suki B, Bates JH, Jackson AC. Serial distribution of airway mechanical properties in dogs: effects of histamine. J Appl Physiol. 1994;77:554–566. doi: 10.1152/jappl.1994.77.2.554. [DOI] [PubMed] [Google Scholar]
- 19.Horsfield K, Dart G, Olson DE, et al. Models of the human bronchial tree. J Appl Physiol. 1971;31:207–217. doi: 10.1152/jappl.1971.31.2.207. [DOI] [PubMed] [Google Scholar]
- 20.Horsfield K, Kemp W, Phillips S. An asymmetrical model of the airways of the dog lung. J Appl Physiol. 1982;52:21–26. doi: 10.1152/jappl.1982.52.1.21. [DOI] [PubMed] [Google Scholar]
- 21.Jahed M, Lai-Fook SJ. Stress wave velocity measured in intact pig lungs with cross-spectral analysis. J Appl Physiol. 1994;76:565–571. doi: 10.1152/jappl.1994.76.2.565. [DOI] [PubMed] [Google Scholar]
- 22.Jahed M, Lai-Fook SJ, Bhagat PK, Kraman SS. Propagation of stress waves in inflated sheep lungs. J Appl Physiol. 1989;66:2675–2680. doi: 10.1152/jappl.1989.66.6.2675. [DOI] [PubMed] [Google Scholar]
- 23.Kraman SS. Speed of low-frequency sound through lungs of normal men. J Appl Physiol. 1983;55:1862–1867. doi: 10.1152/jappl.1983.55.6.1862. [DOI] [PubMed] [Google Scholar]
- 24.Kraman SS, Austrheim O. Comparison of lung sound and transmitted sound amplitude in normal men. Am Rev Respir Dis. 1983;128:451–454. doi: 10.1164/arrd.1983.128.3.451. [DOI] [PubMed] [Google Scholar]
- 25.Kraman SS, Bohadana AB. Transmission to the chest of sound introduced at the mouth. J Appl Physiol. 1989;66:278–281. doi: 10.1152/jappl.1989.66.1.278. [DOI] [PubMed] [Google Scholar]
- 26.Lakes RS, Katz JL, Sternstein SS. Viscoelastic properties of wet cortical bone—I. Torsional and biaxial studies. J Biomech. 1979;12:657–678. doi: 10.1016/0021-9290(79)90016-2. [DOI] [PubMed] [Google Scholar]
- 27.Li B, You JH, Kim Y-J. Low frequency acoustic energy harvesting using PZT piezoelectric plates in a straight tube resonator. Smart Mater Struct. 2013;22:055013. [Google Scholar]
- 28.Mahagnah M, Gavriely N. Gas density does not affect pulmonary acoustic transmission in normal men. J Appl Physiol. 1995;78:928–937. doi: 10.1152/jappl.1995.78.3.928. [DOI] [PubMed] [Google Scholar]
- 29.Mansy HA, Royston TJ, Sandler RH. Acoustic characteristics of air cavities at low audible frequencies with application to pneumoperitoneum detection. Med Biol Eng Comput. 2001;39:159–167. doi: 10.1007/BF02344798. [DOI] [PubMed] [Google Scholar]
- 30.Mansy HA, Royston TJ, Balk RA, Sandler RH. Pneumothorax detection using pulmonary acoustic transmission measurements. Med Biol Eng Comput. 2002;40:520–525. doi: 10.1007/BF02345449. [DOI] [PubMed] [Google Scholar]
- 31.Mansy HA, BalK RA, Warren WH, et al. Pneumothorax effects on pulmonary acoustic transmission. J Appl Physiol. 2015 doi: 10.1152/japplphysiol.00148.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mariappan YK, Glaser KJ, Hubmayr RD, et al. MR elastography of human lung parenchyma: technical development, theoretical modeling and in vivo validation. J Magn Reson Imaging. 2011;33:1351–1361. doi: 10.1002/jmri.22550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mow VC, Kuei SC, Lai WM, Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression: theory and experiments. J Biomech Eng. 1980;102:73. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 34.Multiphysics C. User’s manual. 2011. Acoustics module user guide version 4.2. [Google Scholar]
- 35.Nedel LP, Thalmann D. Proceedings of computer graphics international (Cat. No.98EX149) IEEE computer society; Real time muscle deformations using mass-spring systems; pp. 156–165. [Google Scholar]
- 36.Niu Y, Shen W, Stuhmiller JH. Finite element models of rib as an inhomogeneous beam structure under high-speed impacts. Med Eng Phys. 2007;29:788–798. doi: 10.1016/j.medengphy.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 37.Paciej R, Vyshedskiy A, Shane J, Murphy R. Transpulmonary speed of sound input into the supraclavicular space. J Appl Physiol. 2003;94:604–611. doi: 10.1152/japplphysiol.00568.2002. [DOI] [PubMed] [Google Scholar]
- 38.Panzer MB, Myers BS, Capehart BP, Bass CR. Development of a finite element model for blast brain injury and the effects of CSF cavitation. Ann Biomed Eng. 2012;40:1530–1544. doi: 10.1007/s10439-012-0519-2. [DOI] [PubMed] [Google Scholar]
- 39.Pasterkamp H, Patel S, Wodicka GR. Asymmetry of respiratory sounds and thoracic transmission. Med Biol Eng Comput. 1997;35:103–106. doi: 10.1007/BF02534138. [DOI] [PubMed] [Google Scholar]
- 40.Peng Y, Dai Z, Mansy HA, et al. Sound transmission in the chest under surface excitation: an experimental and computational study with diagnostic applications. Med Biol Eng Comput. 2014;52:695–706. doi: 10.1007/s11517-014-1172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng Y, Khavari R, Stewart JN, et al. Computational study of the dynamics of the single-incision sling in stress urinary incontinence treatment: outcome and risk factors. J Biomech Eng. 2015 doi: 10.1115/1.4030978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rice DA. Sound speed in pulmonary parenchyma. J Appl Physiol. 1983;54:304–308. doi: 10.1152/jappl.1983.54.1.304. [DOI] [PubMed] [Google Scholar]
- 43.Royston T, Acikgoz S. Biomedical Applications of Vibration and Acoustics in Therapy, Bioeffect and Modeling. ASME Press; 2008. Advances in computational modeling of sound propagation in the lungs and torso with diagnostic applications. [Google Scholar]
- 44.Royston TJ, Zhang X, Mansy HA, Sandler RH. Modeling sound transmission through the pulmonary system and chest with application to diagnosis of a collapsed lung. J Acoust Soc Am. 2002;111:1931. doi: 10.1121/1.1452742. [DOI] [PubMed] [Google Scholar]
- 45.Royston TJ, Acikgoz S, Ozer MB, et al. Biomedical Applications of Vibration and Acoustics in Therapy, Bioeffect and Modeling. ASME Press; 2008. Advances in computational modeling of sound propagation in the lungs and torso with diagnostic applications; p. 32. [Google Scholar]
- 46.Royston TJ, Dai Z, Chaunsali R, et al. Estimating material viscoelastic properties based on surface wave measurements: a comparison of techniques and modeling assumptions. J Acoust Soc Am. 2011;130:4126–4138. doi: 10.1121/1.3655883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schanz M. Wave propagation in viscoelastic and poroelastic continua: a boundary element approach. Springer; New York: 2012. [Google Scholar]
- 48.Schmidt S, Cela C, Singh V, Weiland J. Computational modeling of electromagnetic and thermal effects for a dual-unit retinal prosthesis: inductive telemetry, temperature increase, and current densities in the. Artif, Sight 2008 [Google Scholar]
- 49.Siklosi M, Jensen O, Tew R, Logg A. Multiscale modeling of the acoustic properties of lung parenchyma. ESAIM Proceedings. 2008:78–97. [Google Scholar]
- 50.Simon BR. Multiphase poroelastic finite element models for soft tissue structures. Appl Mech Rev. 1992;45:191. [Google Scholar]
- 51.Simon BR, Liable JP, Pflaster D, et al. A poroelastic finite element formulation including transport and swelling in soft tissue structures. J Biomech Eng. 1996;118:1. doi: 10.1115/1.2795941. [DOI] [PubMed] [Google Scholar]
- 52.Suki B, Habib RH, Jackson AC. Wave propagation, input impedance, and wall mechanics of the calf trachea from 16 to 1600 Hz. J Appl Physiol. 1993;75:2755–2766. doi: 10.1152/jappl.1993.75.6.2755. [DOI] [PubMed] [Google Scholar]
- 53.Tisi GM, Minh VD, Friedman PJ. In vivo dimensional response of airways of different size to transpulmonary pressure. J Appl Physiol. 1975;39:23–29. doi: 10.1152/jappl.1975.39.1.23. [DOI] [PubMed] [Google Scholar]
- 54.Van Loocke M, Lyons CG, Simms CK. Viscoelastic properties of passive skeletal muscle in compression: stress-relaxation behaviour and constitutive modelling. J Biomech. 2008;41:1555–1566. doi: 10.1016/j.jbiomech.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Von Gierke HE, Oestreicher HL, Franke EK, et al. Physics of vibrations in living tissues. J Appl Physiol. 1952;4:886–900. doi: 10.1152/jappl.1952.4.12.886. [DOI] [PubMed] [Google Scholar]
- 56.Vovk IV, Grinchenko VT, Oleinik VN. Modeling the acoustic properties of the chest and measuring breath sounds. Acoust Phys. 1995;41:667–676. [Google Scholar]
- 57.Wang Q, Zeng H, Best TM, et al. A mechatronic system for quantitative application and assessment of massage-like actions in small animals. Ann Biomed Eng. 2014;42:36–49. doi: 10.1007/s10439-013-0886-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wodicka GR, Stevens KN, Golub HL, et al. A model of acoustic transmission in the respiratory system. IEEE Trans Biomed Eng. 1989;36:925–934. doi: 10.1109/10.35301. [DOI] [PubMed] [Google Scholar]
- 59.Wodicka GR, DeFrain PD, Kraman SS. Bilateral asymmetry of respiratory acoustic transmission. Med Biol Eng Comput. 1994;32:489–494. doi: 10.1007/BF02515306. [DOI] [PubMed] [Google Scholar]
- 60.Yen RT, Fung YC, Ho HH, Butterman G. Speed of stress wave propagation in lung. J Appl Physiol. 1986;61:701–705. doi: 10.1152/jappl.1986.61.2.701. [DOI] [PubMed] [Google Scholar]
- 61.Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]