Abstract
There has been increasing interest in the lateral habenula (LHb) given its potent regulatory role in many aversion-related behaviors. Interestingly, ethanol can be rewarding as well as aversive, we therefore investigated whether ethanol exposure alters pacemaker firing or glutamate receptor signaling in LHb neurons in vitro, and also whether LHb activity in vivo might contribute to the acquisition of conditioned place aversion to ethanol. Surprisingly, in epithalamic slices, low doses of ethanol (1.4 mM) strongly accelerated LHb neuron firing (by ~60%), and ethanol's effects were much reduced by blocking glutamate receptors. Ethanol increased presynaptic glutamate release, and about half of this effect was mediated by dopamine subtype 1 receptors (D1Rs) and cAMP-dependent signaling pathways. In agreement with these findings, c-Fos immunoreactivity in LHb regions was enhanced after a single administration of a low dose of ethanol (0.25 g/kg i.p.). Importantly, the same dose of ethanol in vivo also produced strong conditioned place aversion, and this was prevented by inhibiting D1Rs or neuronal activity within the LHb. By contrast, a higher dose (2 g/kg) led to ethanol conditioned place preference, which was enhanced by inhibiting neuronal activity or D1Rs within the LHb, and suppressed by infusing AMPA or the D1R agonist SKF38393 within the LHb. Our in vitro and in vivo observations show, for the first time, that ethanol increases LHb excitation, mediated by D1R- and glutamate receptors, may underlie a LHb aversive signal that contributes to ethanol-related aversion.
Keywords: alcohol abuse, conditioned place aversion and preference, cyclic AMP signaling, epithalamic slices from rats
INTRODUCTION
Like many addictive drugs, ethanol has both rewarding and aversive effects, which modulate drug seeking and addiction (Koob and Le Moal, 2008; Verendeev and Riley, 2013). Ethanol's rewarding property may be associated with its ability to increase the firing of dopamine neurons in the ventral tegmental area (VTA) and cause dopamine release in target areas such as the nucleus accumbens (Tupala and Tiihonen, 2004), although ethanol activates many signaling systems within diverse brain regions that could also contribute to its rewarding effects (Koob, 2013).
The mechanisms that mediate ethanol-related aversion remain much less clear. Recently, the lateral habenula (LHb) has emerged as a key brain region for regulating aversion-related behavior (Root et al., 2014). LHb neurons are activated by aversive events, including stress, disappointment, fear or anticipation of a negative reward (Lecca et al., 2014; Shabel et al., 2014a). LHb neurons receive glutamatergic afferents from various structures (Lecca et al., 2014; Meye et al., 2013), that include lateral hypothalamus (Meye et al., 2013), basal ganglia (Shabel et al., 2014b) and VTA (Root et al., 2014), and sends outputs to several structures. Recent work has focused on LHb activation of GABAergic cells in the rostromedial tegmental nucleus (RMTg), which in turn inhibit a subpopulation of midbrain dopamine neurons (Jhou et al., 2009a; Jhou et al., 2009b), a pathway which is a central mediator of aversive conditioning (Lammel et al., 2012; Stamatakis and Stuber, 2012). Thus, the LHb might contribute importantly to the aversive effects of ethanol, and recent evidence shows that LHb lesions increase ethanol self-administration and block ethanol-induced conditional taste aversion (Haack et al., 2014). However, the cellular and molecular mechanisms through which the LHb might contribute to the aversive effects of ethanol remain largely unknown.
We hypothesized that LHb activation by ethanol contributes to its aversive properties, and tested this idea in experiments on rats, by combining electrophysiological, pharmacological, immunocytochemical and behavioral methods. We provide here the first evidence that ethanol strongly enhances spike firing and synaptic activity in LHb in vitro, by an action mediated in part by glutamate receptors, D1-type dopamine receptors (D1Rs) and cAMP-dependent pathways. Importantly, our findings suggest that the mechanisms we identified in vitro also regulate ethanol-induced conditioned place aversion and preference.
MATERIALS AND METHODS
Animals
All procedures were in accordance with National Institutes of Health guidelines and with the approval of the Animal Care and Utilization Committee of Rutgers, the State University of New Jersey, New Jersey Medical School. To minimize the number of animals used, we made electrical recordings from 270 juvenile Sprague-Dawley (SD, PN20-30) rats of either sex, as well as 30 adolescent males (PN35-45) and 30 adult males (PN90-110). Data from juvenile males and females did not differ significantly and were pooled. Male SD rats (180–300g) were used for c-Fos immunohistochemistry (n = 20) and behavior (n = 190). Rats were housed with a standard 12-h light/12-h dark cycle with ad libitum food and water.
Brain slice preparation and electrophysiology
Coronal epithalamic slices (250 μm) were cut in ice-cold glycerol-based artificial cerebrospinal fluid (GaCSF) containing (in mM): 252 glycerol, 2.5 KCl, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2, 25 NaHCO3, 1 L-ascorbate, and 11 glucose, and saturated with 95%O2/5%CO2 (carbogen)(Ye et al., 2006). Slices were then incubated >1-hr at 24-25°C in carbogenated aCSF of similar composition as GaCSF, but with 126 mM NaCl replacing glycerol. Experiments were performed at ~33°C, with aCSF perfused at 1.5-2 ml/min. Electrophysiological recordings (from ~350 LHb neurons) were performed as described (Zuo et al., 2013). Since LHb neurons have heterogeneous morphological but similar physiological properties (Weiss and Veh, 2011), recordings were performed throughout the entire LHb. Patch pipettes (6-8 MΩ) were filled with internal solutions containing (in mM) 140 cesium methanesulfonate, 5 KCl, 2 MgCl2, 10 HEPES, 2 MgATP, 0.2 GTP for recordings under voltage-clamp; and 140 potassium gluconate, 5 KCl, 2 MgCl2, 10 HEPES, 2 MgATP, and 0.2 GTP for current-clamp. Spontaneous firing was recorded by the loose-patch cell-attached technique, allowing long-lasting recordings without perturbing the cytoplasmic contents, and in whole-cell mode to measure membrane potential and input resistance. Dopamine neurons in the posterior VTA were identified as previously described (Guan et al., 2012).
Immunohistochemistry
One hour after systemic administration of saline or ethanol, rats were perfused, and sections prepared and immunostained for c-Fos, as previously described (Li et al., 2011c), with the following differences: for secondary antibody treatment, sections were incubated in biotinylated goat anti-rabbit IgG secondary antibody (1:400, Vector Laboratories, Burlingame, CA) diluted in 0.3% (v/v) Triton X-100 in 0.1 M PBS for 2 h at room temperature, then treated with avidinbiotin-peroxidase complex (1:400, Vectastain ABC Elite, Vector Laboratories) and visualized with nickel-intensified DAB (0.02% (w/v) 3,30-diaminobenzidine, 2.5% (w/v) nickel ammonium sulfate and 0.000083% (v/v) H2O2, in 0.175 M sodium acetate). The reaction was halted with 0.175 M sodium acetate.
Histological analysis
We counted c-Fos+ nuclei bilaterally in the entire LHb, between −3.0 and −4.5 mm from the bregma, analyzing a 40 μm section every 120 μm. Blind counting was done under a Nikon Eclipse 90i microscope with the NIS Elements BR 3.0 software (Micron Optics). Data are expressed as positive nuclei per mm2.
Implantation of cannulae
Stereotaxic surgery was performed on adult rats as in (Li et al., 2011c). Bilateral guide cannulae (FIT 5MM C232G-1.5W-1MM PROJ, 28 gauge; Plastics One, Roanoke, VA) were inserted dorsally to the LHb (−3.8 AP, ±0.75 ML, −5.2 DV) (Li et al., 2011a). Histological verification was done as described (Li et al., 2011c). Data obtained at injection sites outside the LHb were excluded from further analysis.
Conditioning place preference (CPP) experiments
Apparatus
The CPP apparatus (homemade) consisted of a rectangular cage with a left (net) and right (grid) chamber (each 50L×40W×30H, in cm) and an interconnecting area (20L×40W×30H, in cm); guillotine doors separated the chambers from the connecting area. The chambers were illuminated by a 40 W light. The two chambers differed by wall pattern (black and white vertical or horizontal stripes) and floor type (net vs grid). The rat's location, defined by the position of its two front paws was monitored with a video camera situated above the cage.
Experimental procedure and treatment
Rats were allowed 7-10 days after surgery before place conditioning. During this period, rats were brought to the experimental room and handled for 5 min each day. The CPP experiment, lasting six consecutive days, had three distinct phases: pre-conditioning, conditioning and postconditioning.
Pre-conditioning
On day 1, a rat was placed in the connecting area with the guillotine doors removed to allow access to the entire apparatus for 15 min. Most rats spent roughly equal time in the two compartments (net = 307.1±10.7 s, grid = 322.8±11.2 s, n = 168), indicating no unconditioned baseline preference for either chamber, and supporting our unbiased method. However, 22 rats showing strong initial preference for one chamber (>75% time) were excluded from the analysis to avoid an initial bias or “ceiling” or “floor” effects.
Conditioning
Starting 1 day after the pre-conditioning phase, conditioning took place on days 2-5. This consisted of 4 days with one 15-min saline and one 15-min ethanol pairing per day, with a 6-8 h interval between the saline and ethanol pairing. Ethanol was paired with one compartment and saline with the other (left or right, randomly chosen), with an ethanol or saline injection immediately before confinement in one of the two chambers. The connecting area was not accessible during conditioning and was blocked by guillotine doors.
Post-conditioning test day
On day 6, 24 h after the last conditioning, guillotine doors were kept open, and the time spent in each chamber was recorded during 15 min. The preference score was defined as the difference (in seconds) between the time spent in the drug-paired chamber on test day (i.e. drug-free state), and the time spent in this same chamber during the pre-conditioning (Pascual et al., 2012). Positive scores indicate preference for the drug-paired side, and negative scores an aversion to the drug-paired side.
Intra-LHb microinjections of drugs
Drugs were administered into the LHb as described earlier (Li et al., 2011c): the injector extended 1.0 mm beyond the guide cannulae tip, infusion lasted 120 sec, and injectors were left in place for an additional 60 sec to allow for diffusion. A given compound was infused into the LHb twice per day, 10 min before injecting ethanol or saline (i.p.) on the conditioning days (d2-5). The compounds include: 10μg/side of the glutamate receptor agonist AMPA, 4μg/side of the D1R agonist SKF38393, 10μg/side of the sodium channel blocker lidocaine, or 0.5μg/side of D1R antagonist SCH23390. A limitation of the CPP study is that a high number of bilateral intracranial injections (4 infusions of a particular compound and 4 aCSF) were infused per rat.
Drugs
Most reagents were from Sigma (St Louis, MO): cadmium chloride (CdCl2), 6,7-dinitroquinoxaline-2,3-dione (DNQX), DL-2-amino-5-phosphono-valeric acid (DL-AP5), aminomethylphosphonic acid (AMPA), tetrodotoxin (TTX), forskolin, lidocaine, strychnine, SKF38393, SCH23390, gabazine and Rp-cAMP. Ethanol, stored in a glass bottle, was from Pharmco Products (Brookfield, CT). PKI-5-24 was from Santa Cruz Biotechnology (Santa Cruz, CA).
Data analysis and statistics
All data are expressed as means ± SEM. To calculate the percent change in EPSC or firing frequency for a given cell, recordings during the 5 min baseline before ethanol addition were averaged and normalized to 100%. Statistical significance was assessed using an unpaired or paired two-tailed Student's t test, a one-way ANOVA with Tukey's post hoc test for multiple group comparisons, or a Kolmogorov-Smirnov test. Dose-response data were fitted to the logistic equation: y = 100xα/(xα + xoα), where y was the percentage change, x the ethanol concentration, α the slope parameter, and xo the ethanol concentration which induced a half-maximal change. CPA/CPP scores were compared by one-way ANOVA or unpaired t test; values of P < 0.05 were considered significant.
RESULTS
Ethanol activates LHb neurons in juvenile animals
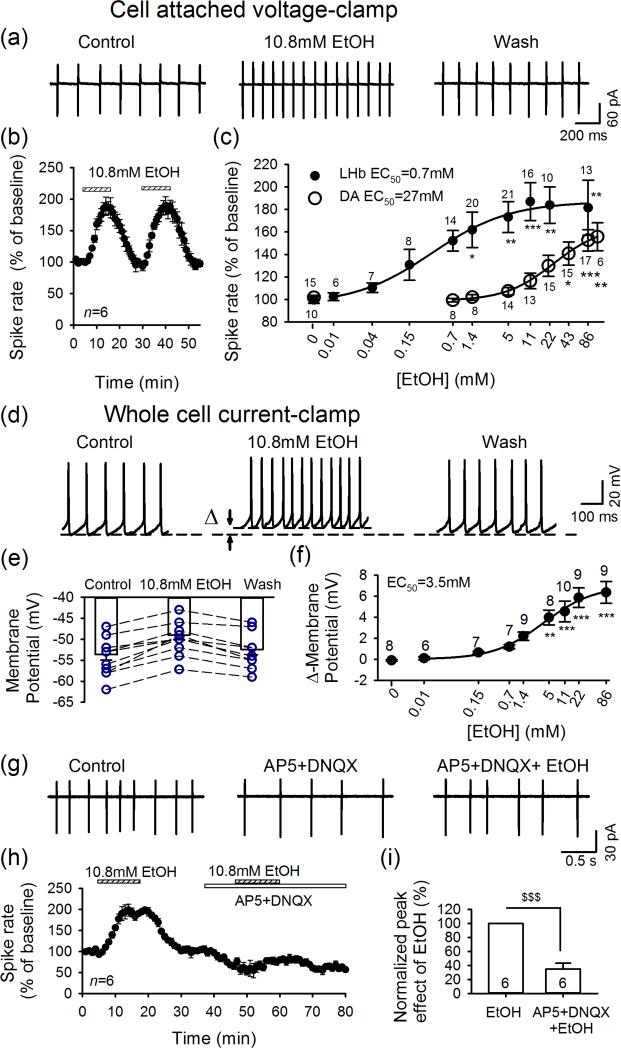
Ethanol induced a concentration-dependent increase in pacemaker firing of LHb neurons (Fig. 1a-d), in 115/131 cells. Firing changes recorded in cell-attached mode or whole-cell current-clamp mode were not significantly different, and thus were pooled. Surprisingly, ethanol enhanced LHb firing at low concentrations, with an EC50 of only 0.7 mM (F9,111 = 3.7, P < 0.001; Fig. 1c, black dots), and a near-maximal increase to 187±16.7% of baseline with 10.8 mM ethanol (P < 0.001). Firing returned to baseline levels after washout (Fig. 1a and b), and repeated applications of ethanol were equally effective (Fig. 1b). Thus, ethanol produced a reliable and pronounced increase in firing of most LHb neurons. As expected, ethanol depolarized LHb neurons (F8,64 = 11.9, P < 0.001) concentration-dependently in current-clamp mode, with an EC50 of 3.5 mM (Fig. 1d-f). In contrast, in slices from the same animals, ethanol accelerated firing of dopamine neurons in the posterior VTA only at much higher concentrations (≥ 43.2mM, F8,89 = 6.5, P < 0.001), with an EC50 of 27 mM (Fig. 1c, dark open dot), in keeping with previous reports (Guan et al., 2012).
Figure 1.
Ethanol depolarizes lateral habenula (LHb) neurons and accelerates their firing. (a) Ethanol enhanced LHb firing recorded in cell-attached mode. (b) Repeated applications of 10.8-mM ethanol induced similar acceleration of ongoing discharge (n = 6). (c) Markedly different dose–response relations of ethanol-induced increases in firing of LHb (□) and ventral tegmental area dopamine (DA) neurons (′), with EC50s of 0.7 and 27 mM, respectively. *P<0.05, **P<0.01, ***P<0.001, ethanol application versus baseline, one-way ANOVA. (d, e) Increased discharge and membrane depolarization during ethanol application (n = 8). (f) Dose–response relation of ethanol-induced depolarization. Glutamate antagonists 2-amino-5-phosphono-valeric acid (AP5) and 6,7-dinitroquinoxaline-2,3-dione (DNQX) partly blocked the ongoing discharge of LHb neurons (g) and largely suppressed ethanol's enhancement of firing (h, i; n=6)
LHb neuron stimulation by ethanol is mediated partly by glutamate receptors
LHb receives strong glutamatergic synaptic inputs (Lecca et al., 2014; Meye et al., 2013). To determine whether glutamate receptors are involved in ethanol's action, we compared its effect in the absence and presence of glutamate antagonists: AP5 (50μM) and DNQX (20μM) to block NMDARs and AMPARs, respectively. These agents lowered ongoing firing (to 56.4 ± 13.5% of baseline, n = 8, P = 0.014), indicating tonic glutamatergic activation of LHb neurons. Moreover, glutamate antagonists significantly decreased the enhancement of firing induced by 10.8 mM ethanol (to 34.7 ± 8.5% of the effect of ethanol alone, n = 6, P < 0.001; Fig. 1g-i).
Ethanol facilitates glutamatergic transmission in juvenile LHb neurons
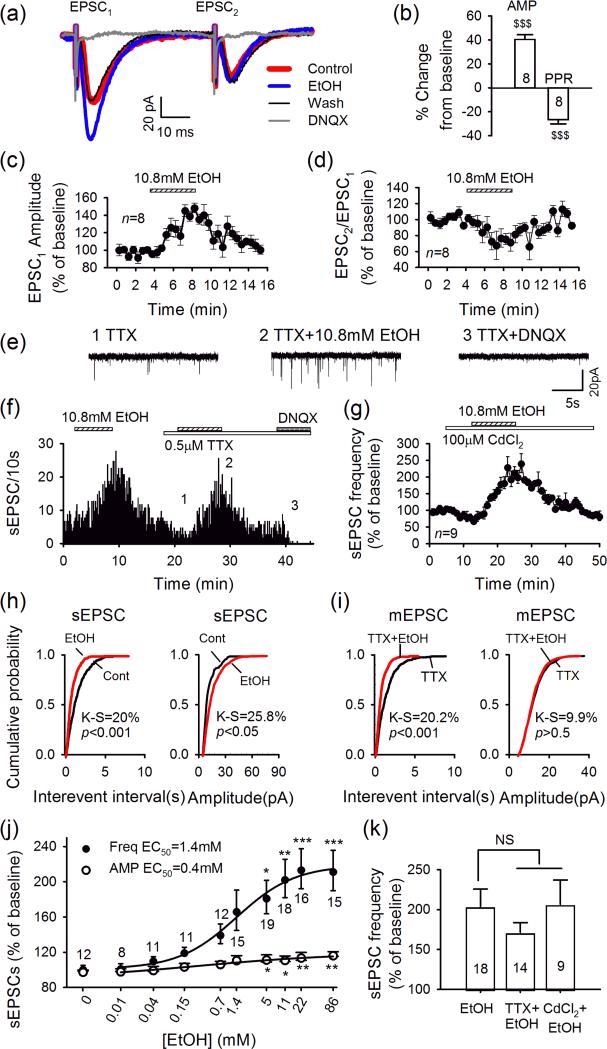
We next examined whether ethanol altered glutamate release by measuring the paired-pulse ratio (PPR), observed when pairs of excitatory postsynaptic currents (EPSCs) are evoked 50 msec apart by local electrical stimulation, in the presence of gabazine (10 μM) and strychnine (1 μM) to block GABAA and glycine receptors. These EPSCs were abolished by DNQX (Fig. 2a), indicating mediation by AMPARs as in previous studies (Li et al., 2011a; Zuo et al., 2013). Ethanol (10.8mM) enhanced the amplitude of the first EPSC (EPSC1) of each pair (to 140.4 ± 4.0% of baseline, n = 8, P < 0.001; Fig. 2a-c), but not the second EPSC (EPSC2). Thus, ethanol significantly reduced the PPR (PPR = EPSC2/EPSC1, to 73.6 ± 3.6% of baseline, n = 8, P < 0.001, Fig. 2b and d), suggesting enhancement of presynaptic glutamate release onto LHb neurons.
Figure 2.
Ethanol enhances glutamatergic transmission in LHb by increasing presynaptic glutamate release. (a) When two excitatory postsynaptic currents (EPSCs) were evoked (50-millisecond apart) by paired-pulse stimulation, ethanol reversibly increased the first (EPSC1) but not the second(EPSC2) of each pair. (b) Percent change in EPSC1 amplitude (AMP) and paired-pulse ratio (PPR = EPSC2/EPSC1). $$$P<0.001, ethanol application versus baseline, paired t-test. (c, d) Time course of ethanol effects on EPSC1AMP(n = 8) (c) and PPR (d). (e) Miniature EPSCs (mEPSCs) recorded in the presence of tetrodotoxin (TTX), gabazine and strychnine at times indicated in (f), which illustrates the time course of ethanol's effect in the absence and presence of TTX. (g) Ethanol enhancement of spontaneous EPSC (sEPSC) frequency was not depressed by calcium channel blocker cadmium chloride (CdCl2) (n = 9). (h) Cumulative probability plots show higher incidence of shorter inter-sEPSC intervals and larger sEPSCs during ethanol applications (red line). (i) Incidence of shorter inter-mEPSC intervals, but no change in mEPSC AMP during ethanol applications. (j) Concentration dependence of increases in frequency (□) and AMP (′) of sEPSCs. *P<0.05, **P<0.01, ***P<0.001, one-wayANOVA. (k) Mean potentiation of sEPSC frequency induced by 10.8-mM ethanol in the absence and presence of TTX or CdCl2 (unpaired t-test)
To further investigate possible changes in glutamate release, we next examined ethanol's effects on spontaneous EPSCs (sEPSCs). In most LHb cells (116/141), ethanol robustly increased sEPSC frequency (10.8 mM: by 203.8 ± 21.8% of baseline; Fig. 2f, h and j). This effect, also indicated by the increased incidence of shorter inter-sEPSC intervals (Fig. 2h), was reversible by washout. Ethanol's action was concentration-dependent (F9,127 = 5.4, P < 0.001; Fig. 2j) with an EC50 of 1.4 mM. Ethanol also increased the incidence of larger amplitude sEPSCs (P < 0.001, K-S test; Fig. 2h and j) in a concentration-dependent manner (F9,127 = 3.7, P < 0.001, EC50 of 0.4 mM).
We then examined ethanol's action on miniature EPSCs (mEPSCs) recorded in the presence of TTX (0.5 μM). TTX lowered sEPSC frequency (to 60.3 ± 5.1% of pre-TTX baseline, n = 14, P < 0.001, Fig. 2f) and significantly reduced sEPSC amplitude (to 76.1 ± 6.2% of baseline, n = 14, P < 0.001), indicating ongoing activity in glutamatergic inputs to LHb cells. Importantly, ethanol (10.8 mM) substantially increased mEPSC frequency (to 171.4 ± 12.1% of TTX baseline, n = 14, P < 0.001; Fig. 2e, f and i) and shifted the cumulative frequency distribution to shorter inter-event intervals (P < 0.001, K-S test). As there was no consistent change in mEPSC amplitudes, ethanol appears to have had no effect on postsynaptic AMPAR function (P > 0.5, K-S test; Fig. 2i). Finally, in the presence of 100 μM CdCl2, a non-selective blocker of voltage-gated calcium channels, ethanol (10.8 mM) robustly enhanced sEPSC frequency (to 206.5 ± 30.4% of CdCl2 alone, n = 9, P = 0.003, Fig. 2g and k) and amplitude (to 112.6 ± 4.4% of CdCl2 baseline, n = 9, P = 0.011), suggesting that the action of ethanol was not dependent on voltage-gated calcium channels. Together, these observations indicate that ethanol strongly increases glutamate release.
Dopamine receptors (D1Rs) mediate ethanol effects on glutamatergic transmission and neuronal firing
D1Rs mediate cocaine-induced enhancement of glutamatergic transmission in LHb (Zuo et al., 2013). To determine whether D1Rs contribute to ethanol's action in LHb, we recorded firing and mEPSCs in the absence and presence of SCH23390, a D1R antagonist. SCH23390 (10 μM) alone did not alter the frequency of mEPSCs or firing, but did reduce ethanol-induced increases in mEPSC frequency (to 33.6 ± 9.3% of ethanol control, n = 14, P = 0.001; Fig. 3b and d) and firing (to 44.6 ± 10.2% of ethanol control; n = 14, P < 0.001; Fig. 3c and d). In addition, bath application of SKF38393, a D1R agonist, concentration-dependently enhanced mEPSC frequency (F3,30=5.7, P < 0.001; Fig. 3e). Importantly, after pretreating neurons with 30 μM SKF38393, the ability of 10.8 mM ethanol to facilitate mEPSCs (P < 0.001) and firing (P < 0.05) was substantially reduced (Fig. 3f and g).
Figure 3.
Dopamine type 1 receptors (D1Rs) and cAMPprotein kinaseAmediate ethanol's action on the frequency of excitatory postsynaptic currents (EPSCs) and firing in LHb. (a) Repeated applications of ethanol caused similar increases in miniature EPSC (mEPSC) frequency (n=7). (b, c) Ethanol's effects on mEPSC frequency(n=14) (b) and neuronal firing rate (n=14) (c) were significantly reduced by D1R antagonist SCH23390. (d) Summary of these results, normalized to control effects of ethanol. $$P<0.01, $$$P<0.001, ethanol versus ethanol plus SCH23390, paired t-test. (e) Concentration dependence of increases in the frequency of sEPSCs induced by D1R agonist SKF38393. (f) Time course of ethanol's effect on mEPSCs and firing in the presence of 30-μM SKF38393. (g) Mean potentiation of mEPSC and firing frequency induced by 10.8-mM ethanol in the absence and presence of SKF38393. #P<0.05, ###P<0.001, unpaired t-test. (h, i) Bath-applied Rp-cAMP reduced ethanol's effect on sEPSC frequency. $$$P<0.001, ethanol versus ethanol plus Rp-cAMP, paired t-test. (j, k) Intracellular PKI-5-24 (in recording pipette) did not prevent ethanol's effect on sEPSC frequency. (l) Bath-applied forskolin (10 or 30μM) increased the frequency of sEPSCs and firing. $P<0.05, $$$P<0.001, forskolin application versus baseline. (m) Forskolin's effect on neuronal firing rate was significantly reduced by glutamate antagonists 6,7-dinitroquinoxaline-2,3-dione (DNQX) and 2-amino-5-phosphono-valeric acid (AP5). (n) Time course of ethanol's effect on sEPSCs and firing in the presence of 10-μM forskolin. (o) Mean potentiation of sEPSC and firing frequency induced by ethanol in the absence and presence of forskolin. #P<0.05, unpaired t-test
cAMP inhibition reduces ethanol's effect on glutamatergic transmission in LHb
Since both ethanol and D1Rs activate cAMP-dependent signaling within the mesolimbic system (Self, 2004), we examined whether the cAMP-dependent pathway mediated ethanol effects in the LHb. Bath perfusion of Rp-cAMP (10 μM), a membrane-permeable, selective antagonist of cAMP-dependent signaling alone decreased baseline sEPSC frequency (to 77.3 ± 7.8 % of baseline, n = 8, P < 0.05), indicating tonic modulation of glutamatergic transmission by cAMP. In addition, Rp-cAMP significantly reduced ethanol's effect on sEPSC frequency (to 53.1 ± 7.7% of ethanol alone, n = 7, P < 0.001; Fig. 3h and i). To determine whether postsynaptic cAMP-PKA-dependent pathways contributed to the acceleration of sEPSCs, we added to the recording pipette PKI-5-24 (5 μM), a membrane impermeable PKA antagonist. Under these conditions, ethanol (10.8 mM) still robustly increased the mean sEPSC frequency (to 204.6 ± 35.3% of baseline, n = 12, P = 0.007; Fig. 3j and k); this effect was not different from that recorded with the control internal solution (to 203.8 ± 21.8% of baseline, n = 18, P = 0.98 comparing with and without PKI; Fig. 3k). Direct activation of adenylyl cyclase with bath-applied forskolin (10 or 30 μM, 10 min) increased the frequency of both sEPSCs and firing (Fig. 3l). In addition, forskolin-induced facilitation on firing was substantially attenuated in the presence of glutamate receptors antagonists DNQX and AP5 (from 152.5 ± 16.9 to 87.6 ± 5.1% of baseline, P = 0.007; Fig. 3m). Ethanol (10.8 mM)-induced facilitation of sEPSCs and firing was significantly attenuated in the presence of forskolin (Fig.3 n and o). Together, these results support the idea that ethanol-induced facilitation of firing and glutamate release was partially mediated by the activity of presynaptic cAMP-dependent signaling pathways.
Ethanol and a D1 receptor agonist also stimulate the LHb in adolescent and adult rats
To determine whether the effects of ethanol and a D1R agonist in the LHb might change with development, and our results in Fig. 1-3 were from juvenile rats (PN20-30), we studied LHb cells in slices from adolescent (PN35-45, ~80g) and adult (PN90-110, ~300g) rats. Acute ethanol (10.8 mM) caused a similar acceleration of firing in slices from adolescents (to 169.9 ± 10.4% of baseline, n = 18) and adults (to 174.1 ± 20.2% of baseline, n = 12; P = 0.8, adolescents versus adults) and a similar increase in mEPSC frequency (adolescent: to 161.8 ± 13.4%, n = 12; adult: to 171.4 ± 17.6%, n = 11; P = 0.67 adolescents versus adults). Likewise, applications of SKF38393 (10 μM) similarly elicited an acceleration of firing in slices from adolescents and adults (to 137.7 ± 6.2% of baseline, n = 9 for adolescent slices; to 135.7 ± 6.6% of baseline, n = 9 for adult slices; P = 0.8, adolescents versus adults) and mEPSC frequency (to 177.8 ± 12.8%, n = 12 for adolescents; and to 166.9 ± 12.9%, n = 18 for adults; P = 0.57, adolescents versus adults). These data suggested that ethanol's effect on LHb neurons do not change during development.
Ethanol increases c-Fos protein expression in the adult LHb
Since ethanol enhanced LHb activity in vitro, we looked for a comparable effect in vivo by measuring c-Fos expression in the LHb. As shown in Fig. 4, c-Fos positive neurons were observed between −3.0 and −4.5 mm from bregma, and we counted all the labelled cells across the entire LHb in each section and expressed data as means ± SEM of positive nuclei per mm2. LHb c-Fos was significantly elevated one hour after a single injection of ethanol (0.25 or 2 g/kg i.p., n = 5-7 rats/group) (F2,14 = 24.6, P < 0.001 compared with saline controls), suggesting that systemically administered ethanol also activates LHb neurons in vivo.
Figure 4.
One hour after systemic ethanol injection, c-Fos expression in lateral habenula is much increased. Upper: bright field photomicrographs illustrating c-Fos expression in lateral habenula after injections of saline, or 0.25- or 2-g/kg ethanol. Numbers at the left indicate the distance from bregma (mm). Scale bar = 200 μm. Lower: mean numbers (±standard error of mean) of c-Fos positive cells per square millimeter of tissue (n=5–7 rats/group). ***P<0.001, one-way ANOVA
Place conditioning with ethanol in adults rats
To assess the possible behavioral relevance of ethanol's effect on LHb activity in vitro, we next examined the contribution of LHb activity to the acquisition of ethanol-induced conditioned place aversion (CPA) or preference (CPP) in adult male SD rats (see Methods). We first determined whether rats could develop a CPA or CPP when ethanol administration (12.5 % (v/v); 0.25, 0.5, 1, or 2 g/kg, i.p., n = 12-13 rats/group) was paired with the rat's location in a particular chamber. Control rats, initially ‘conditioned’ with saline injections (2 ml/kg i.p., n = 13), showed no preference for either chamber on the test day (Fig. 5a), confirming the validity of the counterbalancing procedure. In strong contrast, four days of conditioning with 0.25 g/kg ethanol led to CPA, while conditioning with 2.0 g/kg ethanol led to CPP (F4,58 = 9.7, P < 0.001; P = 0.045 for 0.25 g/kg and P = 0.011 for 2.0 g/kg by Turkey's post test; Fig. 5a).
Figure 5.
Lateral habenula (LHb) neuronal activity and D1-type dopamine receptors (D1Rs) regulate the acquisition of ethanol-conditioned place aversion (CPA) and preference (CPP). (a) Preference score was defined as the difference (in seconds) between the time spent in drugpaired chamber on the testing day and the time spent in the same chamber during the pre-conditioning session. After 4 days of conditioning with 0.25-g/kg ethanol, time spent in ethanol-paired chamber on the testing day was significantly decreased but was greatly increased on the test day after previous place conditioning with 2-g/kg ethanol. *P<0.05, one-way ANOVA. (b) Schematic of injection sites. Red dots represent the locations of injector tips. Numbers at the right indicate the distance from bregma (mm). (c) In control rats, LHb activation by local aminomethylphosphonic acid (AMPA) (glutamate agonist) injections produces CPA, but inactivating LHb neurons by lidocaine or the D1R antagonist SCH23390 had no effect on place preference. (d, e) By contrast, in rats conditioned with ethanol, LHb infusions had significant effects: CPA induced by low doses of ethanol (0.25 g/kg) was reversed to CPP after LHb infusions of lidocaine or D1R antagonist SCH23390 (d), whereas CPP induced by high doses of ethanol (2 g/kg) was suppressed by LHb infusions of these blockers but was strikingly enhanced by LHb infusions of AMPA or D1R agonist SKF38393 (e). #P<0.05, ##P<0.01, unpaired t-test for AMPA, SKF3839 or SCH23390 versus vehicle application
Influence of neuronal activity and D1Rs in the LHb on the acquisition of ethanol-induced place conditioning
Having discovered that ethanol enhances LHb activity in vitro via D1Rs (Fig. 3), we hypothesized that this mechanism may contribute to ethanol place conditioning. We therefore microinjected various agents into the LHb bilaterally 10 min before the systemic administrations of ethanol or saline in place conditioning sessions. Thus, we injected AMPA or SKF38393 to activate LHb neurons, and lidocaine or SCH23390 to reduce ethanol induced activation of LHb neurons. To determine whether these compounds alone would have any effect on place preference, in a separate group of ethanol-naïve rats, a given compound was infused into the LHb 10 min before the rat was placed in one chamber in the morning, and aCSF was infused into the LHb 10 min before the rat was placed in another chamber in the afternoon. As shown in Fig. 5c, activating LHb neurons by AMPA administration without ethanol pairing can produce significant CPA in ethanol-naive animals (P = 0.027); but inactivating LHb with lidocaine had no significant effect on place preference. Correct cannula placement was confirmed after all experiments (Fig. 5b)(Paxinos and Watson, 2007). The cannula tips of 7/132 rats were located outside of the LHb, and therefore corresponding data were excluded from analysis.
Given the role of the LHb in promoting aversion, our working hypothesis was that lowering LHb activity would reduce acquisition of ethanol-induced CPA. Indeed, inhibiting LHb activity with lidocaine, or blocking LHb D1Rs with SCH23390, prevented the development of CPA induced by 0.25 g/kg ethanol (lidocaine: P = 0.006; SCH23390: P = 0.009; Fig. 5d), and these agents actually enhanced CPP produced by 2 g/kg ethanol (lidocaine: P = 0.042; SCH23390: P = 0.04; Fig. 5e). By contrast, AMPA or the D1R agonist SKF38393 significantly attenuated CPP produced by 2.0 g/kg ethanol (AMPA: P = 0.024; SKF38393: P = 0.004, Fig. 5e), which presumably reflects increased aversive effects of ethanol after LHb activation (Stamatakis et al., 2013). Taken together, these results suggest that neuronal activity and D1Rs within the LHb can strongly regulate ethanol-related conditioned behaviors (Fig. 6).
Figure 6.
Cartoon demonstrating that ethanol and dopamine 1 receptor(D1R) can interact to regulate glutamate (Glut) release in the lateral habenula (LHb) and modulate place conditioning. With lower doses of ethanol (0.25 g/kg), the primary effect of ethanol was to stimulate LHb neuron activity in a Glut-dependent manner via D1Rs, which mediated the development of conditioned place aversion (CPA) to ethanol. At higher doses of ethanol (2 g/kg), ethanol activated the LHb and ventral tegmental area dopamine (VTA-DA) neurons, which we propose then mediates the observed ethanol conditioned place preference (CPP). Activation of LHb with 2-g/kg ethanol also provides a conditioned aversion that decreases the level of CPP
DISCUSSION
We report here the first electrophysiological evidence that ethanol accelerates the firing of LHb neurons in epithalamic slices from juvenile or adult rats by enhancing glutamate release, an action partly mediated by D1Rs and presynaptic cAMP signaling. Accordingly, systemic administration of ethanol increased c-Fos expression in the LHb in vivo. Importantly, in concurrence with our in vitro findings, inhibiting LHb activity or D1Rs suppressed ethanol-related conditioned place aversion and enhanced place preference, whereas stimulating AMPARs or D1Rs in the LHb suppressed ethanol-related place preference.
Low concentrations of ethanol robustly activated most LHb neurons
Our finding that ethanol enhanced LHb activity with an EC50 of 0.7 mM is perhaps unexpected, since ethanol can stimulate VTA-DA neurons (Brodie et al., 1990) and LHb activity in vivo tends to be the inverse of that of midbrain dopamine neurons during aversive and rewarding states (Matsumoto and Hikosaka, 2007). Interestingly, the concentration response curve is essentially flat from 1 to 86 mM, even though this is the pharmacologically relevant concentration range. While the underlying mechanism requires further investigation, one possible interpretation is that the LHb neurons are very sensitive to ethanol, perhaps owing to the need to detect and signal aversion to what might be a toxin, and thus at 1 mM ethanol already had near maximal effects. By contrast, at low concentrations ethanol had no effect on posterior VTA-DA neurons in vitro (Fig. 1c), in agreement with previous reports that only higher ethanol concentrations (> 40 mM) increase VTA-DA neuron firing in brain slices (Brodie et al., 1990; Guan et al., 2012). We demonstrated that ethanol-induced increase in glutamate release in the LHb is mediated by D1Rs. Although we do not know where the dopamine was coming from during in vitro experiments, the LHb receives dense projections from midbrain dopamine neurons ((Gruber et al., 2007) but see (Root et al., 2015)) and has a significant amount of dopamine (Phillipson and Pycock, 1982). In addition, there is evidence from other brain regions such as the nucleus accumbens that there can be some ongoing dopamine release in brain slice, for example that cocaine in vitro can acutely modulate evoked glutamate currents in a dopamine-receptor dependent manner (Nicola et al., 1996). Also, dopamine release in some terminal regions can occur independently of dopamine neuron activity, including through activation of glutamate receptors (Floresco et al., 1996). It is also important to understand the relationship between ethanol concentrations that enhance LHb activity in vitro and brain ethanol levels after various systemic injections. According to a previous study (Yoshimoto and Komura, 1993), 40 min after 0.5 g/kg ethanol i.p. injections, brain ethanol levels peak at 5 mM; therefore 0.25 g/kg injections should yield brain ethanol levels of ~2-3 mM, a concentration which sharply enhanced LHb activity in vitro, and also strongly increased c-Fos expression in the LHb in vivo (Fig. 4).
Ethanol-induced LHb excitation was mediated by glutamate receptors, D1Rs and cAMP
LHb neurons receive many glutamatergic inputs (Lecca et al., 2014), which can promote aversive conditioning (Root et al., 2014). Here, the glutamate receptor blockers DNQX and AP5 substantially attenuated the ethanol-induced acceleration of LHb firing, and ethanol significantly increased presynaptic glutamate release. However, as DNQX/AP5 did not completely abolish the ethanol enhancement of LHb firing, other mechanisms may also be involved.
D1Rs have been found in the LHb (Weiner et al., 1991), but so far there is no direct evidence that D1Rs are expressed in glutamatergic afferents to the LHb. Here, we found that inhibiting D1Rs blocked about half of ethanol's enhancement on LHb firing and mEPSC frequency. This suggests that D1Rs may be located on presynaptic glutamatergic terminals in the LHb, as observed in other brain regions (Xiao et al., 2009). Interestingly, inhibiting D1Rs in vivo completely suppressed CPA produced by low doses of ethanol, and enhanced CPP caused by higher concentrations of ethanol. Taken together, our in vitro observations suggest that even partial block of ethanol's action by D1R inhibitors can significantly alter the development of ethanol place conditioning in vivo. We also note that the inhibition of ethanol-induced glutamate release by D1R antagonists was partial, while antagonists of cAMP signaling had a greater inhibition of the ethanol enhancement of glutamate release. Thus, it is possible that ethanol may also interact with other receptors that activate cAMP signaling, e.g. such as β-adrenergic receptors (Ferrero et al., 2013), to enhance glutamate release within the LHb. In addition. the LHb contains both D1Rs and D2-like receptors (Wamsley et al., 1989). D2-family receptors mediate cocaine-related LHb excitation and aversive conditioning (Jhou et al., 2013) and cue-induced reinstatement of nicotine-seeking behavior (Khaled et al., 2014). Here, we focused on D1R regulation of LHb and ethanol-related behaviors, since in vitro studies found that D2Rs can either inhibit (Jhou et al., 2013; Maroteaux and Mameli, 2012) or excite LHb activity (Good et al., 2013; Zuo et al., 2013). Future studies are needed to clarify the behavioral roles of LHb D2Rs.
In agreement with previous reports that ethanol and D1Rs can activate PKA signaling (Self, 2004), we found that the ethanol-induced increase in glutamate release was mediated in part by enhanced cAMP-dependent activity. This may have occurred within presynaptic glutamatergic terminals, since PKA inhibition within postsynaptic cells did not inhibit ethanol's enhancement of sEPSC frequency (Fig. 3k). It is also possible that D1R and cAMP-dependent effects occurred through postsynaptic activation of cAMP pathways without involvement of PKA, although an effect on glutamate release could suggest that a presynaptic action of cAMP is perhaps more likely.
Behavioral implications of ethanol's action on LHb activity
The aversive effects of ethanol are associated with many factors, such as species, age, sex, drug dose and drug history (Riley, 2011). It is reported that after acute ethanol administration, especially in naive users, people often have cognitive impairment and feel anxiety and panic. If the user fails to have a positive, euphoric experience, and even worse, is sensitive to ethanol's aversive effects, it may act as a limiting or protecting factor against continued consumption, especially by suppressing formation of a conditioned reward for ethanol. The LHb is considered critical for a conditioned aversive memory (Tomaiuolo et al., 2014), and lesioning or inactivating the LHb impairs aversion memory (Goutagny et al., 2013; Lecourtier et al., 2004). It is also interesting that low and high concentration of ethanol similarly activated the LHb, measured using c-fos, but induced opposite preference behavior; CPP with higher ethanol concentrations (> 1.5 g/kg) has been reported by others (Groblewski et al., 2008; Morales et al., 2012). Ethanol at 0.25 g/kg should give a blood level of about ~2-3 mM (Yoshimoto and Komura, 1993) at which stimulation of LHb firing approaches a ceiling effect; conversely, ethanol-related conditioned reward at higher concentrations of ethanol may involve activation of VTA dopamine neurons (Brodie et al., 1990). Ethanol at 2.0 g/kg gives a blood level of ~38 mM (Yoshimoto and Komura, 1993), close to the EC50 for VTA dopamine cells. Thus, the marked difference in ethanol sensitivity between LHb and the VTA dopamine neurons may contribute to the opposing behavioral consequences. Interestingly, a previous study failed to find CPA/CPP for ethanol at 0.3 g/kg i.p. in adult male SD rats (Matsuzawa et al., 1998). The discrepancy between these results and our findings probably reflect differences in conditioning sessions, since differences in session duration can affect the development of ethanol-CPP/CPA (Wrobel, 2011).
D1Rs within many brain regions can mediate aversive associative learning (Katche et al., 2013). Here, inhibiting LHb neuronal activity or blocking LHb D1Rs completely abolished CPA induced by low concentrations of ethanol (0.25 g/kg), in agreement with our finding that D1Rs mediate ethanol's action on LHb in vitro, and that the low dose of ethanol induced c-fos expression. This also concurs with a recent report that lesioning the LHb results in faster recovery from taste aversion to a low ethanol dose (0.7 g/kg) (Haack et al., 2014). Furthermore, inhibiting LHb activity or D1Rs actually increased the ethanol-CPP produced by a higher ethanol concentration (2 g/kg), while LHb activation with AMPA or D1R agonists suppressed this ethanol CPP. Notably, exciting LHb neuron with AMPA but without ethanol pairing can produce CPA as well, further confirming that LHb is involved in aversive processes. Taken together, our findings underscore the importance of LHb in ethanol-related CPA and CPP. Since conditioning to ethanol may contribute to ethanol addiction (Little et al., 2005), our results suggest that LHb could be a promising target for novel therapeutic interventions.
Conclusion
We provide here several lines of new information about the impact of ethanol on the LHb. Low ethanol concentrations, acting through D1Rs and cAMP-dependent signaling pathways, enhanced LHb activity by increasing glutamate release from excitatory synaptic inputs. Both LHb activity and D1Rs were also critical for developing ethanol CPA. Collectively, these data suggest that LHb neurons encode the aversive effects of ethanol, which may prevent the development of ethanol addiction and thus represent a pivotal element in the neurobiological processes underlying ethanol addiction. Manipulating LHb activity, including through D1R and glutamate receptors, may be of therapeutic value in the prevention and treatment of alcohol abuse.
Acknowledgements
This research is supported by NIH-NIAAA AA021657, AA022292 (JHY). The authors thank Dr. Hong Nie for advice on statistical data analysis.
Footnotes
Authors Contribution
JHY elaborated the study design. WHZ, RF, GQX and JL collected data. WHZ and RF contributed to data analysis and interpretation. FWH, KK and JHY drafted the article. All authors critically reviewed content and approved final version for publication.
Disclosure/Conflict of Interest
The authors declare no conflicts of interest, financial or otherwise.
References
- Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- Ferrero JJ, Alvarez AM, Ramirez-Franco J, Godino MC, Bartolome-Martin D, Aguado C, Torres M, Lujan R, Ciruela F, Sanchez-Prieto J. beta-Adrenergic receptors activate exchange protein directly activated by cAMP (Epac), translocate Munc13-1, and enhance the Rab3A-RIM1alpha interaction to potentiate glutamate release at cerebrocortical nerve terminals. J Biol Chem. 2013;288:31370–31385. doi: 10.1074/jbc.M113.463877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. A selective role for dopamine in the nucleus accumbens of the rat in random foraging but not delayed spatial win-shift-based foraging. Behav Brain Res. 1996;80:161–168. doi: 10.1016/0166-4328(96)00031-9. [DOI] [PubMed] [Google Scholar]
- Good CH, Wang H, Chen YH, Mejias-Aponte CA, Hoffman AF, Lupica CR. Dopamine D4 receptor excitation of lateral habenula neurons via multiple cellular mechanisms. J Neurosci. 2013;33:16853–16864. doi: 10.1523/JNEUROSCI.1844-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutagny R, Loureiro M, Jackson J, Chaumont J, Williams S, Isope P, Kelche C, Cassel JC, Lecourtier L. Interactions between the lateral habenula and the hippocampus: implication for spatial memory processes. Neuropsychopharmacology. 2013;38:2418–2426. doi: 10.1038/npp.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groblewski PA, Bax LS, Cunningham CL. Reference-dose place conditioning with ethanol in mice: empirical and theoretical analysis. Psychopharmacology (Berl) 2008;201:97–106. doi: 10.1007/s00213-008-1251-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber C, Kahl A, Lebenheim L, Kowski A, Dittgen A, Veh RW. Dopaminergic projections from the VTA substantially contribute to the mesohabenular pathway in the rat. Neurosci Lett. 2007;427:165–170. doi: 10.1016/j.neulet.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Guan Y, Xiao C, Krnjevic K, Xie G, Zuo W, Ye JH. GABAergic actions mediate opposite ethanol effects on dopaminergic neurons in the anterior and posterior ventral tegmental area. J Pharmacol Exp Ther. 2012;341:33–42. doi: 10.1124/jpet.111.187963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack AK, Sheth C, Schwager AL, Sinclair MS, Tandon S, Taha SA. Lesions of the lateral habenula increase voluntary ethanol consumption and operant self-administration, block yohimbine-induced reinstatement of ethanol seeking, and attenuate ethanol-induced conditioned taste aversion. PLoS One. 2014;9:e92701. doi: 10.1371/journal.pone.0092701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009a;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009b;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Good CH, Rowley CS, Xu SP, Wang H, Burnham NW, Hoffman AF, Lupica CR, Ikemoto S. Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. J Neurosci. 2013;33:7501–7512. doi: 10.1523/JNEUROSCI.3634-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katche C, Dorman G, Gonzalez C, Kramar CP, Slipczuk L, Rossato JI, Cammarota M, Medina JH. On the role of retrosplenial cortex in long-lasting memory storage. Hippocampus. 2013;23:295–302. doi: 10.1002/hipo.22092. [DOI] [PubMed] [Google Scholar]
- Khaled MA, Pushparaj A, Di Ciano P, Diaz J, Le Foll B. Dopamine D3 receptors in the basolateral amygdala and the lateral habenula modulate cue-induced reinstatement of nicotine seeking. Neuropsychopharmacology. 2014;39:3049–3058. doi: 10.1038/npp.2014.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Addiction is a Reward Deficit and Stress Surfeit Disorder. Front Psychiatry. 2013;4:72. doi: 10.3389/fpsyt.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca S, Meye FJ, Mameli M. The lateral habenula in addiction and depression: an anatomical, synaptic and behavioral overview. Eur J Neurosci. 2014;39:1170–1178. doi: 10.1111/ejn.12480. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Neijt HC, Kelly PH. Habenula lesions cause impaired cognitive performance in rats: implications for schizophrenia. Eur J Neurosci. 2004;19:2551–2560. doi: 10.1111/j.0953-816X.2004.03356.x. [DOI] [PubMed] [Google Scholar]
- Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D, Henn F, Malinow R. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011a;470:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Bian W, Dave V, Ye JH. Blockade of GABA(A) receptors in the paraventricular nucleus of the hypothalamus attenuates voluntary ethanol intake and activates the hypothalamic-pituitary-adrenocortical axis. Addict Biol. 2011c;16:600–614. doi: 10.1111/j.1369-1600.2011.00344.x. [DOI] [PubMed] [Google Scholar]
- Little HJ, Stephens DN, Ripley TL, Borlikova G, Duka T, Schubert M, Albrecht D, Becker HC, Lopez MF, Weiss F, Drummond C, Peoples M, Cunningham C. Alcohol withdrawal and conditioning. Alcohol Clin Exp Res. 2005;29:453–464. doi: 10.1097/01.alc.0000156737.56425.e3. [DOI] [PubMed] [Google Scholar]
- Maroteaux M, Mameli M. Cocaine evokes projection-specific synaptic plasticity of lateral habenula neurons. J Neurosci. 2012;32:12641–12646. doi: 10.1523/JNEUROSCI.2405-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Matsuzawa S, Suzuki T, Misawa M, Nagase H. Involvement of mu- and delta-opioid receptors in the ethanol-associated place preference in rats exposed to foot shock stress. Brain Res. 1998;803:169–177. doi: 10.1016/s0006-8993(98)00679-9. [DOI] [PubMed] [Google Scholar]
- Meye FJ, Lecca S, Valentinova K, Mameli M. Synaptic and cellular profile of neurons in the lateral habenula. Front Hum Neurosci. 2013;7:860. doi: 10.3389/fnhum.2013.00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Varlinskaya EI, Spear LP. Evidence for conditioned place preference to a moderate dose of ethanol in adult male Sprague-Dawley rats. Alcohol. 2012;46:643–648. doi: 10.1016/j.alcohol.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Kombian SB, Malenka RC. Psychostimulants depress excitatory synaptic transmission in the nucleus accumbens via presynaptic D1-like dopamine receptors. J Neurosci. 1996;16:1591–1604. doi: 10.1523/JNEUROSCI.16-05-01591.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Do Couto BR, Alfonso-Loeches S, Aguilar MA, Rodriguez-Arias M, Guerri C. Changes in histone acetylation in the prefrontal cortex of ethanol-exposed adolescent rats are associated with ethanol-induced place conditioning. Neuropharmacology. 2012;62:2309–2319. doi: 10.1016/j.neuropharm.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th edn. Elsevier/Academic Press; New York: 2007. [Google Scholar]
- Phillipson OT, Pycock CJ. Dopamine neurones of the ventral tegmentum project to both medial and lateral habenula. Some implications for habenular function. Exp Brain Res. 1982;45:89–94. doi: 10.1007/BF00235766. [DOI] [PubMed] [Google Scholar]
- Riley AL. The paradox of drug taking: the role of the aversive effects of drugs. Physiol Behav. 2011;103:69–78. doi: 10.1016/j.physbeh.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Root DH, Hoffman AF, Good CH, Zhang S, Gigante E, Lupica CR, Morales M. Norepinephrine activates dopamine D4 receptors in the rat lateral habenula. J Neurosci. 2015;35:3460–3469. doi: 10.1523/JNEUROSCI.4525-13.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Mejias-Aponte CA, Qi J, Morales M. Role of glutamatergic projections from ventral tegmental area to lateral habenula in aversive conditioning. J Neurosci. 2014;34:13906–13910. doi: 10.1523/JNEUROSCI.2029-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW. Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. Neuropharmacology 47 Suppl. 2004;1:242–255. doi: 10.1016/j.neuropharm.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Shabel SJ, Murphy RT, Malinow R. Negative learning bias is associated with risk aversion in a genetic animal model of depression. Front Hum Neurosci. 2014a;8:1. doi: 10.3389/fnhum.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabel SJ, Proulx CD, Piriz J, Malinow R. Mood regulation. GABA/glutamate co-release controls habenula output and is modified by antidepressant treatment. Science. 2014b;345:1494–1498. doi: 10.1126/science.1250469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Jennings JH, Ung RL, Blair GA, Weinberg RJ, Neve RL, Boyce F, Mattis J, Ramakrishnan C, Deisseroth K, Stuber GD. A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron. 2013;80:1039–1053. doi: 10.1016/j.neuron.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci. 2012;15:1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaiuolo M, Gonzalez C, Medina JH, Piriz J. Lateral Habenula determines long-term storage of aversive memories. Front Behav Neurosci. 2014;8:170. doi: 10.3389/fnbeh.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupala E, Tiihonen J. Dopamine and alcoholism: neurobiological basis of ethanol abuse. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1221–1247. doi: 10.1016/j.pnpbp.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Verendeev A, Riley AL. The role of the aversive effects of drugs in self-administration: assessing the balance of reward and aversion in drug-taking behavior. Behav Pharmacol. 2013;24:363–374. doi: 10.1097/FBP.0b013e32836413d5. [DOI] [PubMed] [Google Scholar]
- Wamsley JK, Gehlert DR, Filloux FM, Dawson TM. Comparison of the distribution of D-1 and D-2 dopamine receptors in the rat brain. J Chem Neuroanat. 1989;2:119–137. [PubMed] [Google Scholar]
- Weiner DM, Levey AI, Sunahara RK, Niznik HB, O'Dowd BF, Seeman P, Brann MR. D1 and D2 dopamine receptor mRNA in rat brain. Proc Natl Acad Sci U S A. 1991;88:1859–1863. doi: 10.1073/pnas.88.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss T, Veh RW. Morphological and electrophysiological characteristics of neurons within identified subnuclei of the lateral habenula in rat brain slices. Neuroscience. 2011;172:74–93. doi: 10.1016/j.neuroscience.2010.10.047. [DOI] [PubMed] [Google Scholar]
- Wrobel M. Acquisition and expression of ethanol-induced conditioned place preference in mice is inhibited by naloxone. Pharmacol Rep. 2011;63:79–85. doi: 10.1016/s1734-1140(11)70401-7. [DOI] [PubMed] [Google Scholar]
- Xiao C, Shao XM, Olive MF, Griffin WC, 3rd, Li KY, Krnjevic K, Zhou C, Ye JH. Ethanol facilitates glutamatergic transmission to dopamine neurons in the ventral tegmental area. Neuropsychopharmacology. 2009;34:307–318. doi: 10.1038/npp.2008.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JH, Zhang J, Xiao C, Kong JQ. Patch-clamp studies in the CNS illustrate a simple new method for obtaining viable neurons in rat brain slices: glycerol replacement of NaCl protects CNS neurons. J Neurosci Methods. 2006;158:251–259. doi: 10.1016/j.jneumeth.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, Komura S. Monitoring of ethanol levels in the rat nucleus accumbens by brain microdialysis. Alcohol Alcohol. 1993;28:171–174. [PubMed] [Google Scholar]
- Zuo W, Chen L, Wang L, Ye JH. Cocaine facilitates glutamatergic transmission and activates lateral habenular neurons. Neuropharmacology. 2013;70:180–189. doi: 10.1016/j.neuropharm.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]