Abstract
Oncolytic viruses hold promise as “self-amplifying” cancer therapies wherein a virally killed cell can produce thousands of new viral “drugs” that can kill more cancer cells. Adenoviruses (Ads) are one family of oncolytic viruses. Most human studies have used human Ad serotype 5 (Ad5). Unfortunately, most patients are already immune to Ad5 increasing the likelihood that the agent will be neutralized if used as a cancer therapy. In this work, lower seroprevalence Ad6 was tested as a systemic therapy for prostate cancer. Ad5 and Ad6 were injected intravenously a single time in nude mice bearing human prostate tumors, and toxicity and efficacy were assessed. Ad6 was chemically shielded with polyethylene glycol (PEG) to test if this would further improve its pharmacology. Ad6 produced 30-fold lower liver damage and less toxicity than Ad5. Ad6 significantly repressed the growth of androgen-resistant human DU145 prostate tumors and androgen-sensitive LNCaP tumors after single intravenous injection. PEGylation did not change virus distribution, but blunted liver damage and cytokine production by Ad6. PEGylated Ad6 eradicated LNCaP tumors and maintained body mass, but lost potency against the more challenging DU145 tumors. These and other data suggest that low seroprevalent Ad6 has better efficacy and safety than the benchmark oncolytic virus Ad5 for systemic therapy of prostate cancer. These data also indicate that PEGylation may improve Ad6 safety, but that this shielding may reduce oncolytic efficacy after intravenous treatment.
Introduction
Oncolytic virotherapy is a cancer therapy that utilizes the natural ability of replication-competent viruses to kill cancer cells (reviewed in refs. 1,2). Viruses can be considered self-amplifying drugs. In the process of killing one cancer cell, a virus can generate thousands of progeny viruses that can spread, infect, and kill other cancer cells. This self-amplification is tempered by parallel amplification of the host’s immune response against the virus that can either attenuate oncolytic efficacy or amplify immune responses against tumor antigens as a bystander effect.3
A number of robust viruses are being developed for oncolytic virotherapy including herpes virus, measles virus, reovirus, retrovirus, vesicular stomatitis virus, and vaccinia virus to name a few.1,2 Adenoviruses (Ads) are nonenveloped viruses that cause a variety of respiratory, ocular, and digestive infections.4 Natural human Ads were tested as oncolytic agents in humans as early as the 1950s.5,6 Since then, most oncolytic adenovirus research has focused on human serotype 5 (Ad5) viruses. Indeed, 87 of the 87 human cancer trials that used Ad5 (www.clinicaltrials.gov). For prostate cancer treatment, recent advances with Ad5 have been made with conditionally-replicating Ad5s (CRAds) including Ad5-PPT-E1A,7 with Ad5-∆24-RGD-T1 (ORCA-010)8 and in other applications.9–13
While Ad5 can be potent against an array of cancers, 27–100% of humans are immune to Ad5.14,15 Given this human and nonhuman adenoviruses with lower seroprevalence are being evaluated as oncolytic that can avoid pre-existing immunity in cancer patients.16–21 We recently screened 15 human adenoviruses for oncolytic activity against a range of cancers, with emphasis on the use of serotypes that are less prevalent in humans than Ad5.20–23 In screens against solid tumors with wild-type adenoviruses, lower seroprevalence species C Ad6 had equal to or better efficacy than Ad5 and other viruses in a number of models. For example, species C Ad6 was more effective against DU145 human prostate cancers in immunodeficient mice after single intratumoral or intravenous injections than Ad5 or species B viruses Ad11 and Ad35.20 Ad6 was also most effective when it was compared to Ad5 and Ad11 after intratumoral injection in immunocompetent Syrian hamsters.23
Ad5 and Ad6 are species C human adenoviruses with significant sequence homology.24 Most variation between Ad5 and Ad6 lie in their hexon hypervariable regions and in their E3 immunevasion genes. In addition, the fiber protein of Ad6 has three less β-turn repeats in its shaft than Ad5.24 The early gene 3 (E3) region of Ads encodes a set of genes involved in immune evasion.25 All adenoviruses express E3 10.4K and 14.5K proteins that work in concert to block Fas and TRAIL-mediated apoptotic killing of an Ad-infected cell. Similarly, the 14.7K protein blocks later intracellular apoptosis events when a T-cell attempts to kill the cell. Species B, C, and D Ads express the E3 19K protein that binds to and blocks cell surface display of major histocompatibility I to hide the infected cell from T-cells. 19K also blocks display of minimum inhibitory concentration of antibiotic to avoid NK cell killing. Ironically, in most studies exploring the in vivo biology of Ads, their E3 genes have been deleted make space for exogenous transgenes. Deleting E3 and disabling Ad immune evasion may reduce oncolytic efficacy. Conversely, ablating Ad immune evasion may enhance the ability of Ads to act as immune adjuvants to stimulate antitumor immune responses.
When the tropism of E3-deleted species C Ads were compared in mice after intravenous injection, Ad6 was surprisingly more efficient at gene delivery in the liver than Ad1, Ad2, and more surprisingly the archetype Ad5 vector.24 Subsequent studies demonstrated that this improved tropism was determined by the hexon protein of Ad6, since replacement of Ad5’s hexon with that of Ad6 transferred this phenotype.26 Unlike the Ad5 hexon, the Ad6 hexon evades macrophage and endothelial scavenger receptors thereby avoiding destruction by liver macrophages known as Kupffer cells.26–28
While adenoviruses can be robust oncolytics, they induce side effects after intravenous injection including the production of innate immune system cytokines like IL-6, thrombocytopenia, and liver damage (reviewed in refs. 29,30). For Ad5, these effects can be blunted by “shielding” its surface by covalent conjugation with polyethylene glycol (PEG).31–37 Ad5 PEGylation protects the virus from pre-existing neutralizing antibodies.32–34 PEG also blunts innate immune responses against Ad35,36 and reduces virally-induced thrombocytopenia.37 PEG also reduces uptake of Ad5 by Kupffer cells35 and binding to endothelial cells and platelets.37 Coating Ad5 with 5 kDa or 20 kDa PEG reduced in vitro activity of the virus by inhibiting its interactions with one of its receptors, the coxsackie and adenovirus receptor (CAR).31 However, this did not reduce in vivo oncolytic activity against LNCaP prostate tumors after intravenous injection in mice.
To evaluate the pharmacology of an intact Ad6 vector, we engineered wild-type Ad6 with a luciferase expression cassette in this work. This vector carries all Ad6 genes including the E3 immune evasion cassette. We also test the effects of PEGylation on Ad6 oncolytic efficacy, virus tropism, innate immune responses, and liver damage after intravenous injection in mice bearing human prostate tumors and in permissive Syrian hamsters bearing HaK tumors.
Results
We previously compared the efficacy of Ad5, Ad6, Ad11, and Ad35 in vitro against a number of tumor cells including PC3, LNCaP, and DU145 prostate carcinoma cells.20 In vitro, Ad5 was generally more effective at killing these cells. In vivo, Ad5 and 6 were similar after intratumoral injection. In contrast, Ad6 was more effective after intravenous (i.v.) injection against distant subcutaneous DU145 tumors20 and was more effective than Ad5 against HaK tumors in immunocompetent Syrian hamsters.23
Liver damage in mice after intravenous injection of Ad5 and Ad6
When Ad5, 6, 11, and 35 viruses were compared by i.v. injection in immunocompetent CD46 transgenic C57BL/6 mice, Ad6 provoked far lower levels of liver damage than Ad5.20 To compare liver damage between Ad5 and Ad6 here, groups of 6 immunocompetent C57BL/6 mice were injected i.v. with 3x1010 virus particles (vp) of wild type Ad5 and Ad6 by tail vein and their blood was sampled 3 days later for alanine amino transferase (ALT) levels (Figure 1a). Both Ad5 and Ad6 induced ALT levels that were higher than phosphate-buffered saline (PBS)-injected animals. However, liver damage by Ad5 was 30-fold higher than Ad6 (P < 0.001 by one-way analysis of variance (ANOVA)). The level of liver damage produced by Ad5 required that most of the animals be sacrificed within 4 days of injection (Figure 1b). In contrast, in Ad6-injected animals, ALT levels were transiently elevated, but all Ad6 animals survived after systemic administration of this 3 × 1010 vp dose. Ad6 survival was significantly better than Ad5 (P = 0.004 by log-rank test).
Figure 1.
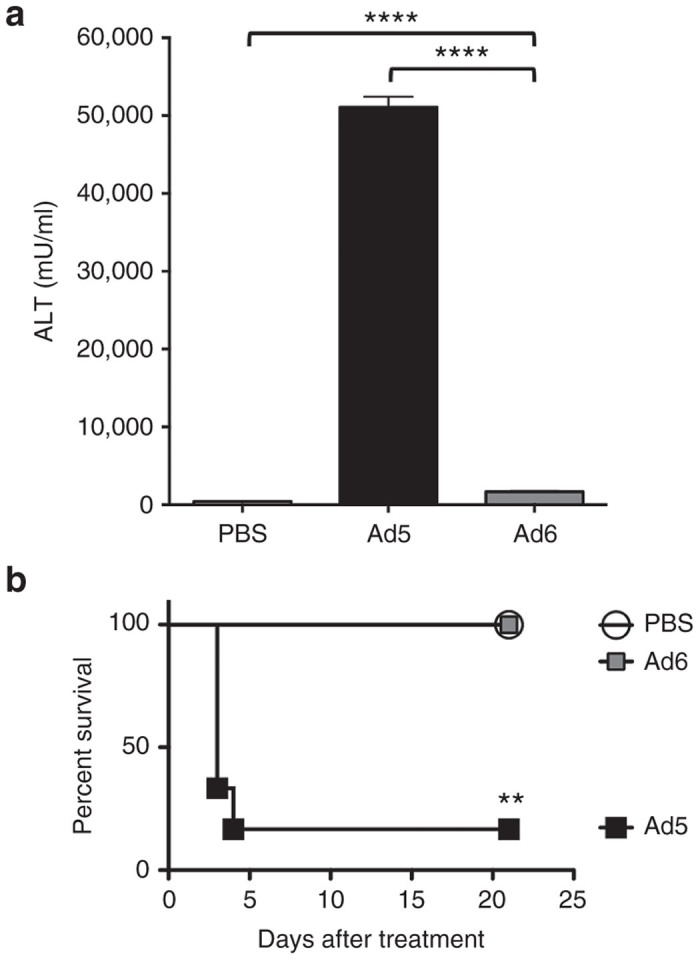
Liver toxicity of species C Ad5 and Ad6 after single intravenous administration. 3 × 1010 vp of the indicated adenovirus was injected intravenously by tail vein into C57BL/6 mice (n = 6 per group). (a) Alanine amino transferase activity was measured from their blood 3 days after treatment. Statistical analysis shows significant difference of the mean values (P < 0.05) and one-way analysis of variance with P < 0.0001. (b) Kaplan-Meier survival curves were plotted and analyzed with the log rank-test, significant difference with P value < 0.003. Ad5-treated mice were moribund and euthanized at indicated days after treatment.
Construction of E3-intact Ad6 expressing Firefly luciferase
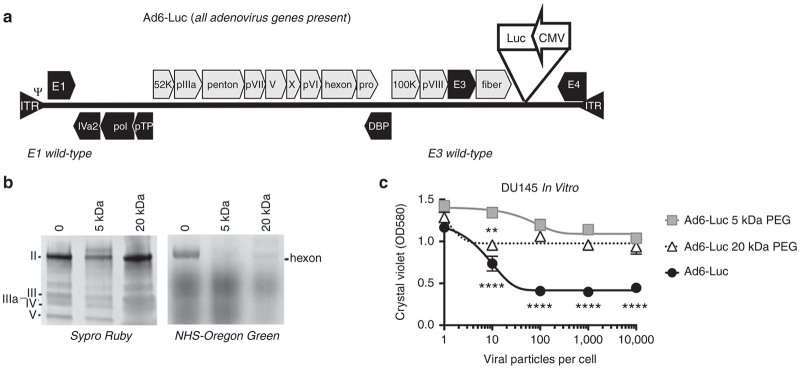
These data and previous efficacy comparisons20,23 suggested that Ad6 might have a more favorable safety window when compared to Ad5 for systemic therapy. To better evaluate its in vivo activity, a minimized firefly luciferase expression cassette driven by the cytomegalovirus (CMV) enhancer/promoter with an SV40 polyadenylation sequence was inserted by red recombination in bacteria between the fiber and E4 genes (Figure 2a). The resulting 38,122 base pair (bp) viral genome was used to rescue the Ad6-Luc virus and this was purified from 293 cells on two sequential CsCl gradients. Purified viral DNA was subjected to Sanger sequencing in E1, hexon, E3, fiber, CMV, and luciferase regions and this verified the identity of the Ad6-Luc virus (data not shown).
Figure 2.
PEGylation of E3-intact Ad6 expressing luciferase. (a) Ad6-Luc vector genome structure. A luciferase expression cassette was inserted into the region between fiber and E4 sequence of Ad6. (b) Unmodified and PEGylated Ad6-Luc were separated on SDS-PAGE, virion hexon was stained with Sypro Ruby (left) and NHS-Oregon Green (right). (c) In vitro testing the viability of human DU145 prostate carcinoma cells with Ad6-Luc, Ad6-Luc 5 kDa-PEG, and Ad6-Luc 20 kDa-PEG. Cells were seeded on 96-well plates, and infected after 24 hours at triplicated multiplicities of infection (MOI) of viral particles/cell. At day 4 after infection, cells were stained with crystal violet protocol and read the absorbance at 580 nm. Statistical significance was analyzed by one-way analysis of variance. Ad6 Luc was significantly more lethal than Ad6 Luc with 5 kDa PEG at MOIs of 10 vp/cell above and was more effective than the 20 kDa PEGylated vector at MOIs of 100 vp/cell and above (P = 0.0001). At 10 vp/cell, Ad6 20 kDa PEG vector was more lethal than Ad6 5 kDa vector (P = 0.01).
PEGylation to blunt antiviral innate immune responses
Adenoviruses can be used as systemic intravenous therapies for cancer.29 However, it has been shown that Ad5 viral capsid and infection induces a number of dose-limiting side effects including the production of cytokines like IL-6 that are released from macrophages and liver Kupffer cells and liver damage due infection and killing of liver hepatocytes.
One approach to blunt Ad5 side effects is to chemically “shield” its surface with the hydrophilic polymer PEG. We previously showed that shielding fully replication-competent Ad5 vectors with 5 or 20 kDa PEG preserved oncolytic efficacy in nude mice bearing human LNCaP prostate tumors.31 Whether Ad6 would benefit from similar shielding was unclear. To test this, Ad6-Luc was purified on CsCl gradients and was reacted with amine-reactive succinimide-activated 5 or 20 kDa PEG and unreacted PEG was removed.
In vitro shielding of Ad6 proteins by PEG
Unmodified and PEGylated Ad6 Luc were separated by SDS-PAGE and viral proteins were stained with SYPRO Ruby. Under these conditions, staining of hexon, the predominant capsomer on Ad6 virions, was markedly reduced by 5 kDa PEG, but not by 20 kDa PEG (Figure 2b). To test to what degree the surface of the virion was still exposed after PEGylation, virions were incubated with succinimide-activated green fluorophore Oregon Green that reacts with the same sites as PEG. When these virions were separated by SDS-PAGE and were imaged for green fluorescence, the hexon of unmodified Ad6 was strongly labeled with the fluorophore (Figure 2b). In contrast, Oregon Green labeling was not detected on the hexon of Ad6 that was shielded with 5 kDa PEG. 20 kDa PEG protected hexon from Oregon green labeling, but not as well as protection by 5 kDa PEG. These data suggest that the smaller 5 kDa PEG shields the Ad6 virion surface against binding (as demonstrated by SyproRuby) and that more lysines are conjugated to and protected by 5 than 20 kDa PEG (as demonstrated by protection against Oregon Green reaction).
Effects of PEGylation on Ad6-Luc in vitro infection
Previous work demonstrated random PEGylation of Ad5 will block its CAR-mediated infection in vitro without negatively impacting liver transduction or killing of LNCaP cells after intravenous injection of Ad5 vectors.31,35,37 Unmodified and PEGylated Ad6-Luc were used to infect DU145 prostate carcinoma cells in vitro at varied multiplicities of infection (MOI) and cell viability was assessed by staining with crystal violet (Figure 2c). Consistent with previous results,31,35,37 conjugation of 5 or 20 kDa PEG to Ad6 virions markedly reduced the ability of the viruses to kill prostate cancer cells in vitro. Ad6 Luc was significantly more lethal than Ad6 Luc with 5 kDa PEG at MOIs of 10 vp/cell and above and was more effective than the 20 kDa PEGylated vector at MOIs of 100 vp/cell and above (P = 0.0001 by one-way ANOVA). At 10 vp/cell, Ad6 20 kDa PEG vector was more lethal than Ad6 5 kDa vector (P = 0.01). These data are consistent with the stronger protection of Ad6 virions by 5 kDa versus 20 kDa PEG (Figure 2b).
In vivo tropism and oncolytic activity of Ad6-Luc after systemic injection in human prostate tumor-bearing mice
We previously showed that Ad6 mediated more potent tumor control against human DU145 prostate tumors than Ad5, 11, and 35 after intravenous injection in nude mice.20 In this previous study, each virus was injected twice 4 hours apart to “predose” and eliminate liver Kupffer cells to improve the efficacy of the second injection.
Given that Ad6 can avoid Kupffer cells, in these studies, predosing was not performed and Ad6-Luc vectors injected only a single time. Groups of 10 nude mice were engrafted with DU145 tumors and were injected a single time by tail vein with 3 × 1010 vp or 1.2 × 1012 vp/kg of Ad6-Luc (Figure 3). Ad6-Luc was tracked by luciferase imaging over 42 days after single intravenous injection. Within 1–4 days strong luciferase signals were observed in the livers of most of the mice demonstrating the natural liver tropism of this and all species C adenoviruses. Luciferase was observed in some of the tumors as early as day 1. By day 14, all tumors had luciferase activity while the liver activity was no longer observed. The tumors in PBS-treated animals grew steadily over 40 days (Fig. 4A). In contrast, tumor growth was significantly delayed in Ad6-Luc treated mice for 70 days after single intravenous injection (P = 0.003 by one-way ANOVA). Kaplan-Meier analysis demonstrated Ad6-Luc mediated significantly better survival than PBS-treated animals (P < 0.002). 60% of Ad6-treated mice survived through 200 days. Of the 40% that did not survive, 20% were sacrificed due to tumor size and the rest were sacrificed due to tumor ulceration.
Figure 3.
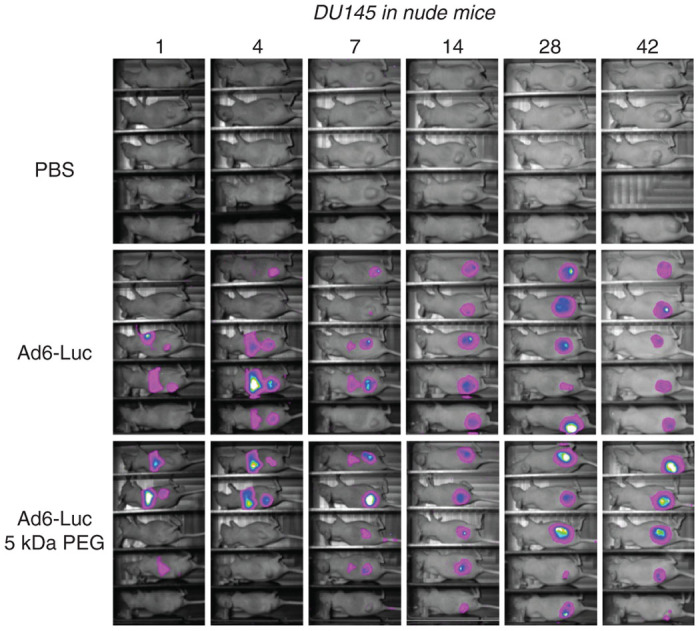
Luciferase imaging after intravenous injection of unmodified and 5 kDa PEGylated Ad6-Luc 5 kDa PEGylated. Luciferase activity of nude mice treated with Ad6-Luc and Ad6-Luc 5 kDa PEG compared to the PBS control group at days 1, 4, 7, 14, 28, and 42 after treatment. Nude mice were implanted with 107 human DU145 prostate cancer cells subcutaneously in their right hind flank. When tumor volumes reached 200 mm3, groups of 9 or 10 mice were randomized and administered with a single intravenous dose of 3 × 1010 vp of the indicated viruses or with PBS. PBS, phosphate-buffered saline.
Figure 4.
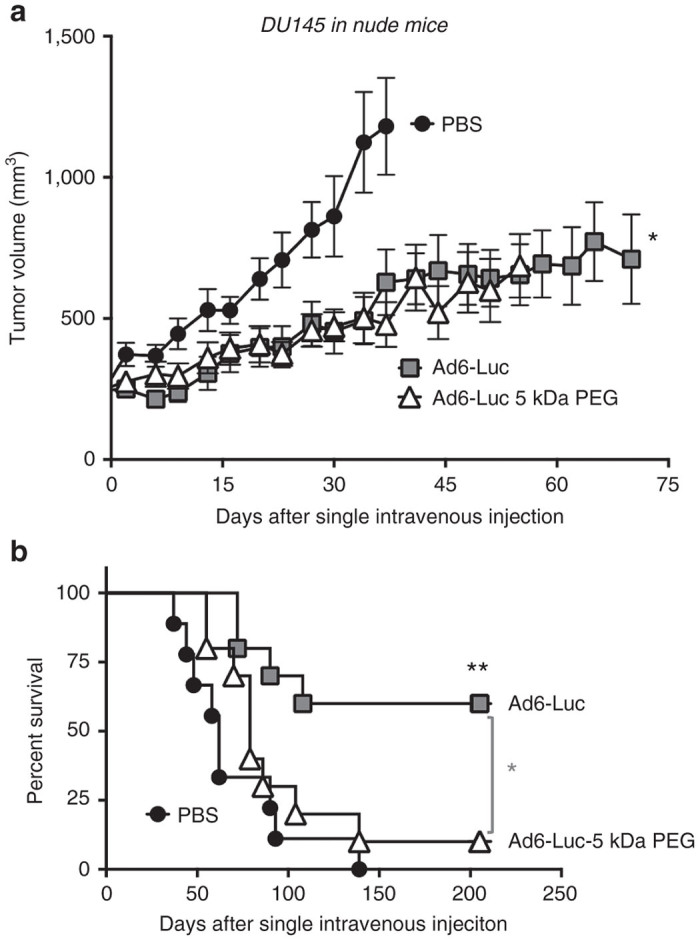
Oncolytic activity of Ad6-Luc and PEGylated Ad6-Luc in DU145 tumor bearing nude mice. (a) Effect of a single intravenous injection of Ad6-Luc and Ad6-Luc 5 kDa PEG on tumor growth. Nude mice (n = 9 or 10 per group) bearing established DU145 tumors in their right hind flank were injected i.v. with a single dose 3 × 1010 vg of the indicated viruses or with PBS. Tumor dimensions were measured with calipers and tumor volume was calculated as width2 × length × 1/2. The data are shown as mean ± standard error. One-way analysis of variance analysis indicated a P < 0.003. (b) Effect of single intravenous injection of Ad6-Luc and Ad6-Luc 5 kDa PEG on survival. Animals from panel a were euthanized when the tumor volume reached 2,000 mm3. Kaplan-Meier survival curves were plotted and analyzed. Significantly better survival was seen with Ad6-Luc-treated group compared to the PBS (P < 0.002) and the Ad6-Luc- 5 kDa PEG (P < 0.03). PBS, phosphate-buffered saline.
Ad6-Luc-5 kDa PEG tropism appeared similar to that of Ad6-Luc with early liver infection followed by predominant luciferase activity in the tumors at later time points (Figure 3). The tumor growth curve for PEGylated Ad6 was similar to unmodified Ad6 (Figure 4a). However, at day 55 and beyond, a larger fraction of PEGylated Ad6 animals had to be euthanized resulting in significantly worse survival than in the Ad6 group (P < 0.002). In this case, 30% were sacrificed due to large tumor size. 50% of the Ad6-PEG mice were sacrificed not due to tumor size, but instead because their tumors had ulcerated.
Testing unmodified and PEGylated Ad6-Luc against human LNCaP prostate tumors
DU145 human prostate carcinoma cells were isolated from a brain metastasis, but are not androgen sensitive and do not express prostate-specific antigen.38 In contrast, LNCaP human prostate adenocarcinoma cells are hormone sensitive and may serve to represent earlier prostate cancers.39
Nude mice were engrafted subcutaneously with LNCaP cells and these animals were treated a single time by the intravenous route with 3 × 1010 vp of unmodified or PEGylated Ad6-Luc (Figure 5). In this case, Ad6-Luc was mock treated or was conjugated with 5 and 20 kDa PEG molecules prior to injection. Single intravenous injection of 3 × 1010 vp of unmodified or PEGylated Ad6-Luc induced significant reductions in tumors size in all virally treated groups. Tumors were eradicated in the virus-treated groups with only a residual small bump < 10 mm3 remaining to mark the sites (data not shown). In contrast, the tumors of PBS-treated animals grew steadily over the same time period. The body weights of the young animals treated with unmodified and PEGylated Ad6 climbed in conjunction with tumor control (Figure 5). In contrast, the body weights of the PBS-treated animals remained flat suggesting the strain of tumor burden on their systems.
Figure 5.
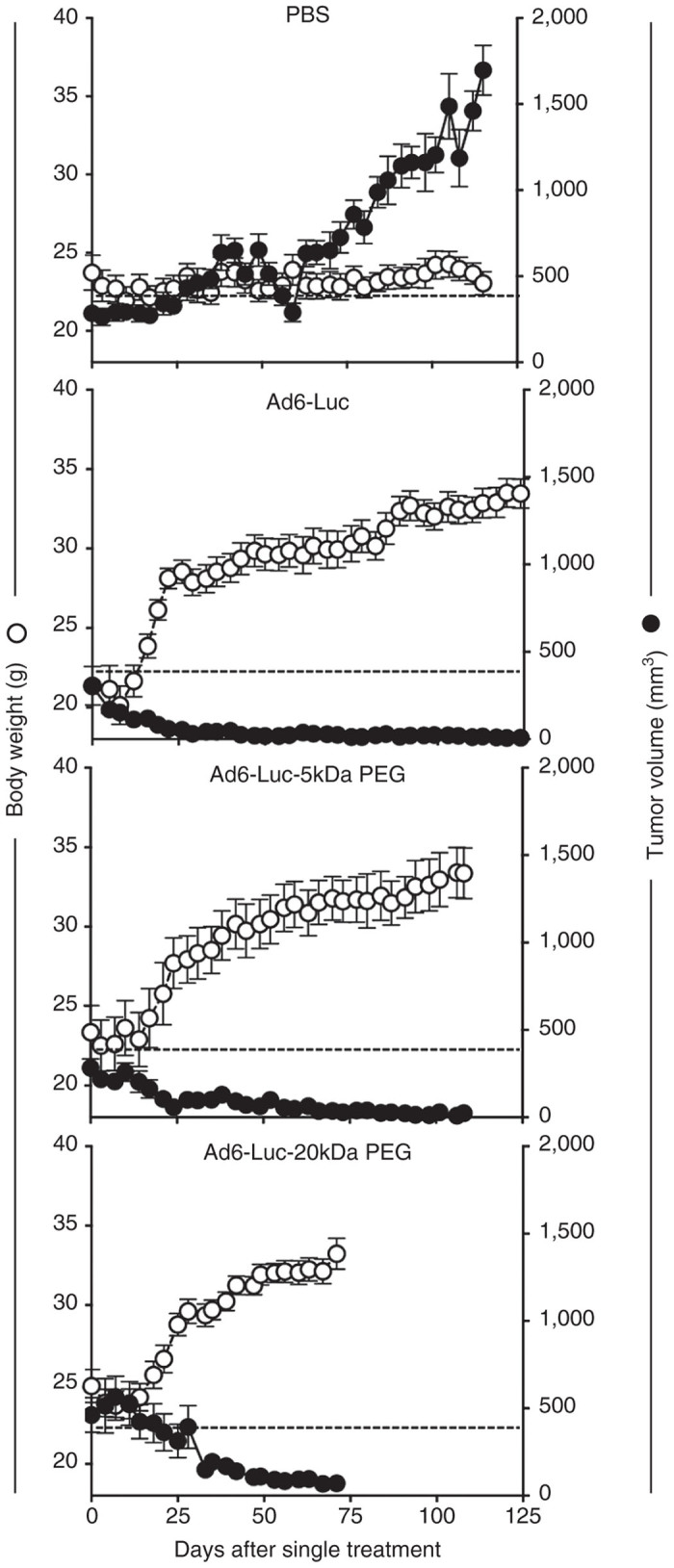
Oncolytic activity of Ad6-Luc and PEGylated Ad6-Luc in LNCaP xenograft nude mice. 107 human LNCaP cells were subcutaneously implanted onto the right hind flank of mice. Mouse groups (n = 5 per group) were randomized and administered with a single intravenous dose of 3 × 1010 vp/m indicated viruses or PBS at day 0 when the established tumor volume reaches 200 mm3. Body weight (g) and tumor volume (mm3) were used to monitor the efficacy of treatment. Data were collected twice a week.
Effects of PEGylation on systemic side effects after intravenous injection
3 × 1010 vp dose of Ad6 equals 1.2 × 1012 vp/kg. If this was used in a 70 kg human, it would equate to a very high dose of virus of 8.4 × 1013 vp. To evaluate if PEGylation affected the relatively low toxicity of Ad6, mice were injected with a single 3 × 1010 vp dose of unmodified or PEGylated Ad6-Luc viruses and IL-6 levels were measured 6 hours later (Figure 6a). All of the Ad6-injected animals produced low, but significant levels of IL-6 after intravenous injection. IL-6 levels were only slightly, but insignificantly blunted by PEGylation. To test liver damage, increasing doses of unmodified and PEGylated Ad6-Luc were injected a single time into mice and ALT levels were measured 3 days later (Figure 6b). At the same 3 × 1010 vp dose used in Figure 1, Ad6-Luc induced similarly low ALT levels. As doses were increased up to 2.5 × 1011 vp, ALT levels increased only 2.5-fold for Ad6-Luc. In contrast, the 5 and 20 kDa PEGylated vectors blunted ALT levels and held them to around 1,000 mU/ml up to the highest 2.5 × 1011 vp dose. This ALT level generated with 2.5 × 1011 vp of PEGylated Ad6 was comparable to the liver damage produced with nearly twofold less unshielded Ad6. This 2.5 × 1011 vp/mouse or 1013 vp/kg dose of this fully replication-competent virus would extrapolate to a dose of 7 × 1014 vp in a 70 kg human.
Figure 6.
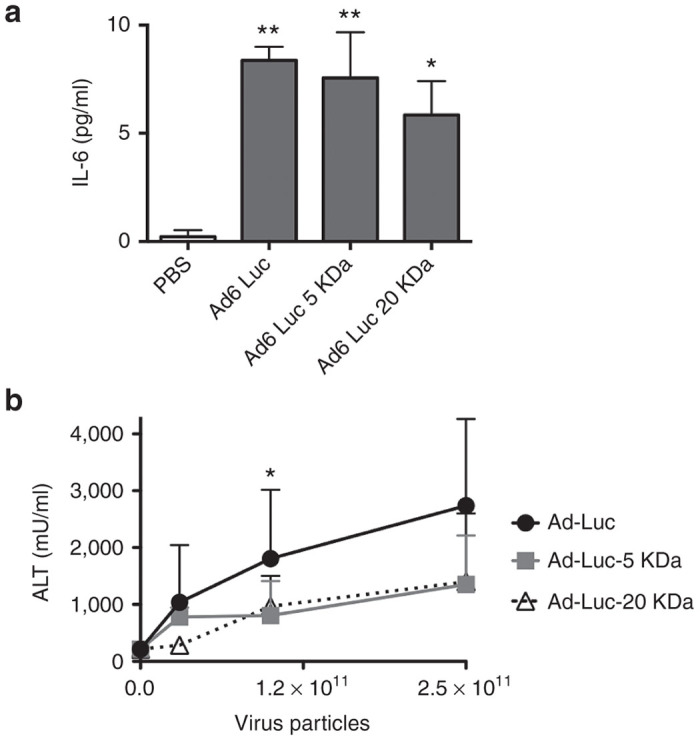
The Effects of PEGylation on liver damage and cytokine release by Ad6-Luc. (a) IL-6 activity was measured from serum 6 hours after intravenous treatment of mice with 3 × 1010 vp of unmodified or PEGylated Ad6-Luc. When compared to PBS by analysis of variance, Ad6-Luc and Ad6-Luc 5 kDa PEG had P < 0.01. Ad6-Luc 20 kDa PEG had a P of < 0.1 compared to the PBS control group. (b) Effects of treatment doses on the alanine amino transferase activity. C57/BL mice (n = 5 per group) were administered with one indicated dose of viruses or PBS. At day 3, serum was collected and tested for alanine amino transferase activity.
Ad6 and PEGylated Ad6 in immunocompetent hamsters bearing HaK tumors
These studies tested oncolytic activity against human prostate tumors in immunodeficient mice. To test the utility of Ad6 and PEGylated Ad6 in an immunocompetent animal model, both viruses and a PBS group were tested by single intravenous treatment in Syrian hamsters bearing subcutaneous HAK kidney tumors. Hamsters were injected a single time by the intravenous jugular route with PBS or 1.5 × 1011 vp of unmodified or 5 kDa PEGylated Ad6-Luc. This dose equaled the 1.2 × 1012 vp/kg dose used in DU145 and LNCaP tumor-bearing mice. Tumor size and survival was measured over time (Figure 7). In this immunocompetent model, HAK tumors in the PBS- and Ad6-Luc PEG-treated animals increased similarly in size over 50 days whereas tumor growth was delayed in the Ad6-Luc group (Figure 7a). Tumor growth in the PBS and 5 kDa PEG group resulted in reduced survival within 60–70 days (Figure 7b). In contrast, all Ad6-Luc-treated hamsters survived through 100 days. While Ad6-Luc mediated better delays in tumor growth and survival, all animals in all groups eventually succumbed with 125 days of treatment after single intravenous treatment.
Figure 7.
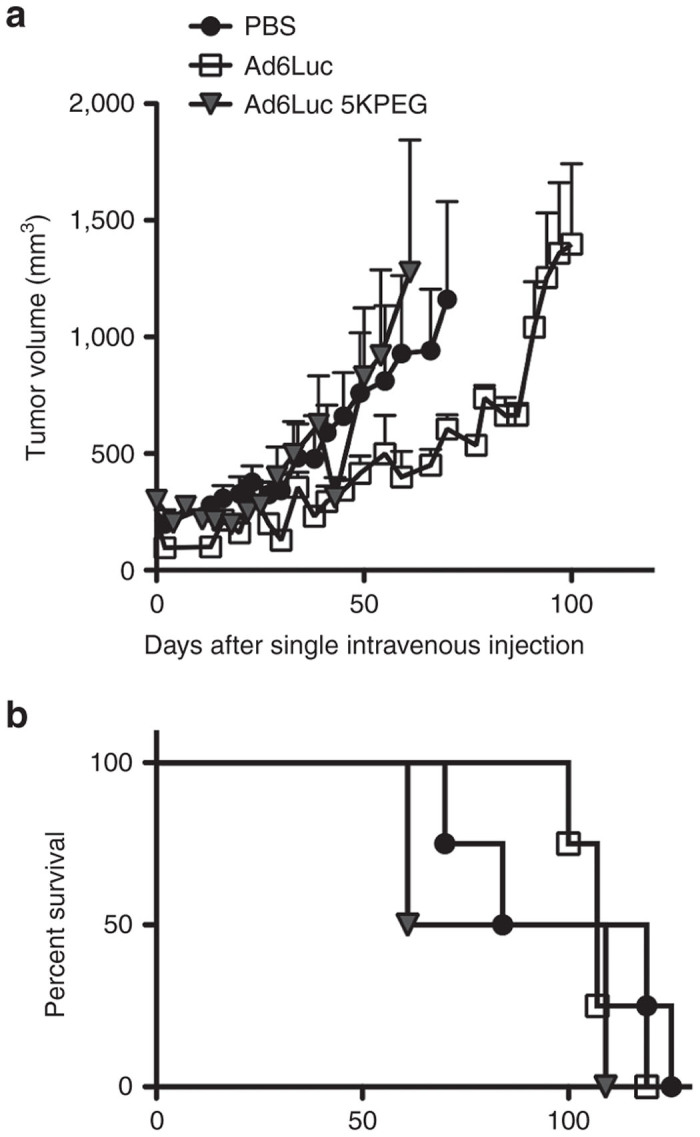
Oncolytic activity of Ad6-Luc and PEGylated Ad6-Luc in immunocompetent hamsters bearing HaK tumors. HaK tumors were inoculated subcutaneously into Syrian hamsters and groups of four animals were treated by single intravenous injection by the jugular vein. 1.5 × 1011 vp of unmodified or 5 kDa PEGylated Ad6-Luc was injected equaling the 1.2 × 1012 vp/kg dose used in DU145 and LNCaP tumor-bearing mice. (a) Tumor growth and (b) survival were monitored after injection.
Discussion
This study was directed at evaluating the efficacy and safety of replication-competent Ad6 as a systemic therapy for prostate cancer. Defining the efficacy and safety of this fully replication-competent adenovirus as an oncolytic agent lays the foundation for subsequent engineering approaches to convert it into a conditionally-replicating Ad (CRAd) or to retarget its tropism.
Patients with pre-existing antibodies to an oncolytic virus can neutralize the virus and render it ineffective. Direct tumor injection may avoid this to some degree, but high levels of antibodies in the blood are likely to inactivate intravenously administered viruses. Considering that 27–100% of humans are already immune to Ad5,15 it is perhaps not surprising that Ad5 has not produced impressive clinical effects when applied as a cancer therapy in patients.
Ad6 is a lower seroprevalent virus than the benchmark oncolytic virus Ad5. Most neutralizing antibodies against adenoviruses target the hexon hypervariable regions of their hexon proteins.40 Seroprevalence screening in Belgium indicated that 78% of volunteers had neutralizing antibodies against Ad5 and 42% had antibodies to Ad6.41 In the United States, 27% of adults were positive for Ad5 antibodies.14 In contrast, only 3% had antibodies against Ad6. These data suggest that more prostate cancer patients can be treated with Ad6 than Ad5 without direct and immediate neutralization of the oncolytic.
When wild-type Ad5, 6, 11, and 35 viruses were previously tested as intratumoral or intravenous therapies against human DU145 prostate tumors in nude mice, species C Ad6 and Ad5 were marked more effective than Ad11 and 35.20 Interestingly, Ad6 was more potent by the intravenous route than by direct intratumoral injection suggesting it may have utility for systemic therapy against advanced prostate cancer. In these and all other tests against tumors in mouse and in immunocompetent hamster models, Ad6 has been more effective than Ad5.20–23 Ad6 may therefore not only have a lower likelihood of frank neutralization in prostate cancer patients, but may also have better efficacy than Ad5.
Liver damage caused by replication-competent Ad5 limits its therapeutic window (reviewed in refs. 29,30). Liver damage generally manifests in the days immediately after intravenous injection due to direct lysis of hepatocytes by the virus or weeks later due to T-cell killing of infected cells.42,43 When liver damage by wild-type Ad5, 6, 11, and 35 viruses were compared in CD46 transgenic C57BL/6 mice, Ad6 provoked by far lower levels of liver damage than Ad5.20 This result was confirmed here. When we attempted to test high doses of 1 × 1011 vp and above, Ad5-injected animals became moribund within 1–2 days and had to be sacrificed. When doses were reduced to 3 × 1010 vp (1.2 × 1012 vp/kg) Ad5-injected animals survived to the desired 3-day time point for ALT measurement, but became moribund after this and had to be sacrificed. In contrast, all Ad6-injected animals survived this dose. ALT measurements at this lower dose demonstrated that Ad6 produced 30-fold less ALT release than Ad5. This suggests that Ad6 has a better safety window for the liver than Ad5 for systemic intravenous treatment even when applied as a fully replication competent virus. This safety profile can likely be further improved by conversion of Ad6 to a CRAd or by detargeting it from the liver.
Modifying an oncolytic virus with a reporter gene makes tracking its in vivo tropism and pharmacology easier as one can perform in life imaging with genes like luciferase or the sodium iodide symporter (NIS) or identify infected cells by detecting fluorescent proteins like GFP. Adding a gene to adenovirus usually comes with a cost, since this icosahedral virus packages only a narrow window of genome sizes. Therefore, in most cases, when reporter genes are added to Ads, something has to be removed. In most cases, it is the early gene 3 (E3) region that is deleted to make space for exogenous genes. E3 is considered “dispensable” for the virus, since E3 deleted Ad5 can still be grown in vitro. Deleted species C E3 frees up approximately 3–4 kb of genome “space” to allow that addition of other genes.
While E3-deleted virus do grow in vitro, it should be noted that this growth is slower and the yields of virus are reduced when compared to an E3-intact viruses.44,45 Therefore, E3 is not entirely dispensable even in vitro. The E3 region of all Ads encodes a set of genes involved in immune evasion25,44,45). The E3 regions of Ads can express proteins that detection and killing by T cells and NK by reducing major histocompatibility I and minimum inhibitory concentration of antibiotic display on the cells, by blocking Fas and Trail-mediated apoptosis, and blocking intrinsic apoptosis signaling. In addition, species C Ads including Ad5 and Ad6 also express the adenovirus death protein that accelerates cell killing.45 If the virus evolved several thousand bases of sequence to evade the immune system naturally, deleting these in an oncolytic version of the virus that also has to survive in the immune system may blunt efficacy. Direct testing of this for Ad5 has shown that E3-deleted oncolytic viruses are generally less effective than E3-intact viruses.46,47 Therefore, E3-intact oncolytic viruses may be more effective for human therapy. Conversely, if the intent is to use Ads to lyse tumors to release tumor antigens and stimulate antitumor immune responses, deleting E3 may have benefits.
Considering the pivotal role of E3 in the in vivo pharmacology and persistence of Ads, in this work, we aimed to generate an Ad6 that could carry a reporter gene without deleting any viral gene including E3. We were able to introduce a minimal CMV-luciferase cassette into intact Ad6 using precision bacterial red recombination.48 By this approach, a minimal CMV promoter luciferase cassette was introduced into Ad6 increasing its size to 106%, a size that still allowed it to be packaged into virions with no loss of viral sequences. The resulting Ad6-Luc virus was rescued and was used here to evaluate viral pharmacology by in vivo luciferase imaging.
Consistent with previous work with Ad5 and replication-defective Ad6,49,50 Ad6-Luc infected the livers of intravenously injected mice immediately and spread later to distant subcutaneous prostate cancer tumors. Peak liver infection detected by luciferase activity was observed at day 4 and this corresponded well ALT elevations at day 3.
After one intravenous dose, Ad6-Luc luciferase activity appeared in distant subcutaneous DU145 tumors within 4 days. Since mice do not support the full viral life cycle, this luciferase activity most likely occurred from viruses that infected the tumors immediately after injection and spread in situ rather than from viruses produced elsewhere. Luciferase activity “bloomed” over time in the animals and this tracked with the ability of the virus to repress tumor growth over time. These data demonstrate the ability to track E3-intact Ad6 with the luciferase transgene and lays the foundation for using this feature to track E3 modified Ad6 vectors in future work.
When Ad6-Luc was tested by single intravenous injection in mice bearing androgen-sensitive LNCaP prostate tumors as subcutaneous tumors, the virus eradicated these distant tumors. LNCaP tumor sites remained tumor-free until the end of studies (9–12 months) and were only evident as a residual small bump marking the site. In contrast, the same single intravenous treatment of androgen-insensitive DU145 tumors markedly reduced tumor growth, but did not eradicate the tumors. Considering that these treatments consisted of only one single intravenous injection, increasing the number doses over multiple days may well improve systemic efficacy.
Intravenous injection of fully replication-competent Ad6 provoked relatively mild side effects when compared to benchmark oncolytic virus Ad5. Innate cytokine responses to Ads are likely mediated by immune cells, particularly by liver Kupffer cells and macrophages. Ad6 may provoke relatively mild IL-6 responses by virtue of its natural ability to avoid macrophage and endothelial cell scavenger receptors and natural antibody-mediated destruction by liver Kupffer cells and macrophages.26,27 We tested if covalent “shielding” of Ad6 could blunt these relatively mild Ad6 side effects. Consistent with previous work with replication-defective and oncolytic Ad5, PEGylation reduced IL-6 and ALT generated by Ad6. PEG’s protective effects were relatively mild, likely due to the already relatively low toxicity of Ad6.
Covalent reaction of 5 kDa PEG with Ads results in the attachment of a linear 35 nm polymer to the virion. Therefore, each 5 kDa PEG is about the same length as Ad fiber itself. Attachment of 20 kDa PEG results in an even longer polymer being attached. Once PEG is attached to Ad, it not only “shields” the reaction site and adjacent surfaces from interactions with problematic proteins, but can also block useful interactions like CAR binding. Previous studies have shown that random PEGylation blocks in vitro Ad5 infection, but has little impact on liver transduction or tumor killing in the LNCaP model.31,35,37 For Ad6, we have verified that 5 or 20 kDa PEGylation reduces in vitro infection, but does not reduce efficacy after intravenous injection in the LNCaP model. In contrast, when PEGylated viruses were tested in more stringent DU145 and HaK mouse and hamster models, PEGylation reduced viral efficacy. This loss of efficacy in these models may be due in part loss of CAR binding and reduced infection after PEGylation (Figure 2c).
Considering that most in vitro activity is lost, it is interesting that there is any efficacy after PEGylation. In vitro, 5 and 20 kDa PEG both significantly reduced Ad6 infection at MOIs of 100 vp/cell and above. This is consistent with loss of CAR binding and infection by Ad5 after 5 kDa PEGylation.35 While CAR binding by Ad5 was markedly reduced by 5 kDa PEG, this notably did not inhibit αv integrin binding.35 Therefore, some oncolytic efficacy by the PEGylated vectors may be mediated by tumor cell infection via integrins rather than by CAR binding.
Oncolytic activity could also be maintained by FX binding to hexon acting as a “bridge” to bind heparin sulfate proteoglycans on cells.51–53 Each PEG attachment on Ad can shield against nearby interactions with proteins and cells on the surface of the virus. However, each attachment likely also shields against nearby PEG conjugation as the reaction continues. Each linear PEG attached to a lysine on the virus likely creates a “shadow” over nearby lysines that sterically inhibits new PEG molecules from reacting with these sites. If so, 20 kDa PEG likely creates a much larger shadow than 5 kDa PEG and therefore is less effective at preventing smaller molecules from interacting with the virion surface. For example, 5 kDa PEG blocked binding of 0.5 kDa Oregon green and 1.5 kDa SYPRO Ruby, but 20 kDa was less able to prevent binding. By extension, binding of globular 4 × 9 nm FX likely inhibited more effectively by 5 kDa PEG than by larger PEGs. Previous work supports this, since 5 kDa PEG reduces FX-mediated infection by Ad5 30-fold in vitro, but larger 35 kDa PEG does not.53 This difference in shielding by small and large PEGs is consistent with our observations of differential protection of hexon to SYPRO ruby and succinimide-Oregon green binding and reaction. If FX bridge effects were involved in maintaining oncolytic activity after PEGylation, we would predict that 5 kDa PEGylated vector would have worse activity than the 20 kDa modified virus. This was not observed, so we speculate that FX bridging is not involved in vivo.
Ad6 is consistently more effective than Ad5 against a number of cancers in mouse and immunocompetent hamster models in our hands.20–23 This may be due in part to Ad6’s ability to evade natural antibody-mediated destruction of the virus by liver Kupffer cells and macrophages.26,27 Data in this study demonstrate that Ad6 has a better safety profile than the archetype oncolytic adenovirus, Ad5. We show that Ad6 generates relatively mild innate immune cytokine responses and lower liver damage even as a fully replication-competent virus. While PEGylation was able to blunt these side effects, this modification was at a cost and Ad6 efficacy was reduced. Considering the already relatively low side effects for fully replication-competent Ad6, the cost/benefit ratio of PEGylation argues against adding this modification to this virus at this time. This is perhaps fortunate as adding PEG as a second test article to this experimental therapy would also complicate gaining regulatory IND approval for phase 1 testing. Perhaps better strategies for Ad6 will be to modify this current fully replication-competent Ad6 platform to be a CRAd, for liver detargeting, and cancer retargeting.
Materials and Methods
Cell lines
DU145 and LNCaP human prostate carcinoma and HaK hamster kidney cancer cells were obtained from ATCC (Manassas, VA). 293 cells were obtained from Microbix, Toronto, Ontario, Canada. Cells were maintained in Dulbecco’s Modified Eagle’s medium with 10% fetal bovine serum (HyClone, Logan, UT).
Viruses
Wild-type Ad5 and Ad6 were purchased from ATCC. The genome of wild-type Ad6 Tonsil 99 strain (ATCC VR-1083) was cloned previously into a low copy plasmid with kanamycin resistance.54 A CMV-luciferase-SV40 polyA followed by an FRT-Zeocin Resistance gene-FRT cassette was recombined into the genome by red recombination48 between the poly-adenylation sites of the fiber and E4 genes (Figure 2a). The FRT-Zeocin-FRT cassette was removed by transformation in FLP expressing bacteria. This produced an Ad6-Luc viral genome in the plasmid of 38,122 bp equal to 106.5% of the 35,770 bp wild-type Ad6 genome.49 The virus was rescued by transfection into 293 cells followed by serial passage up through a 10 plate CellStack (Corning Life Sciences, Lowell, MA). Viruses were purified by banding sequentially on two CsCl gradients and viral particle numbers were calculated by OD260. Viral identity was confirmed by sequencing the E1, hexon, E3, fiber, E4, and luciferase cassette region.
Ad6 PEGylation
Ad6-Luc was PEGylated as in ref. 35. Following CsCl centrifugation, the preparation was desalted on EconoPac 10-DG columns (BioRad, Hercules, CA) into 0.5 M sucrose in PBS (136 mmol/l NaCl, 2.6 mmol/l KCl, 1.7 mmol/l KH2PO4, 10 mmol/l K2HPO4). 5 or 20 kDa succinimide-activated PEG (NOF America, White Plains, NY) was reacted with Ad6 at 10 mg/ml PEG for 1 hour at room temperature with rotation. Unreacted PEG was removed on a Sephadex G50 (GE Healthcare, Piscataway, NJ) size exclusion column. Mock-treated Ad5 was treated with no addition of PEG and treated in parallel.
PEGylation analysis
Unmodified and PEGylated viruses were analyzed by labeling and SDS-PAGE gel separation. 1010 vp of each CsCl-banded and desalted virus was separated on 7–15% gradient SDS-PAGE gels (BioRad, Hercules, CA) and total protein was detected by staining with SYPRO Ruby (Thermo Fisher Scientific, Grand Island, NY). After PEGylation, the viruses were postreacted with Oregon Green 488 Carboxylic Acid, Succinimidyl Ester, 5-isomer (Invitrogen) to detect remaining reactive lysines on the virus. Each of these was then separated on SDS-PAGE gels and green fluorescently label capsomers were visualized on a Kodak In Vivo F Imaging system.
Cell culture studies
DU145 cells were infected with the indicated viruses in 96-well plates at varied MOI in virus particles/cell (vp/cell). Four days later, media was removed and the cells were stained with crystal violet solution (0.05% crystal violet, 1% formaldehyde, 1% methanol) for 20 minutes, and the cells were gently washed with water. The dye was solubilized with 1% SDS and the absorbance of each well was measured at 580 nm.
Animal tumor models
Animals were housed under the Association for Assessment and Accreditation of Laboratory Animal Care (AALAC) guidelines in the Mayo Clinic Animal Facility. Experiments were performed under animal use protocols approved by the Mayo Clinic Animal Use and Care Committee. All experiments were performed under the provisions of the Animal Welfare Act, PHS Animal Welfare Policy and the principles of the NIH Guide for the Care and Use of Laboratory Animals. Four-week-old nude mice (Harlan Sprague Dawley, Indianapolis, IN) were injected subcutaneously (s.c.) with 1 × 107 DU145 or LNCaP cells in 100 µl of Dulbecco’s Modified Eagle’s medium/50% Matrigel (BD Biosciencies, San Jose, CA). Tumor size was measured with calipers and tumor volumes were calculated using the equation ½ length × width × width. When tumors reached 200 mm3 in volume, mice were randomized to different groups and were treated a single time by intravenous (i.v.) injection by tail vein with 3 × 1010 vp of the indicated virus or with PBS. Mice were euthanized when the tumor volume reached 10% body weight, if animals were moribund, in distress, or if the skin ruptured over the tumor. Four- to 5-week-old female Syrian hamsters were obtained from Harlan Sprague Dawley (Indianapolis, IN) and were injected with 2 × 107 HAK cells subcutaneously into the hind flank. When tumor volume reached 200 mm3, the animals were injected intravenously a single time by the jugular route with the indicated number of viral particles. Tumors were measured every 7 days. Volume was calculated as ½ length × width × width. Animals were sacrificed when tumor volume exceeded 2000 mm3 or were observed with ulcerated tumors.
Statistical analysis
Statistical analysis was performed with Prism (Graphpad). Statistical significance was calculated with repeated measures ANOVA or one-way ANOVA followed by Tukey’s honest significant difference test and two sample t-tests. Kaplan-Meier survival curves were plotted and analyzed with the log rank test.
Acknowledgments
This work was supported by NIH R01 CA136945 and from the generosity of the Walter and Lucille Rubin Fund in Infectious Diseases Honoring Michael Camilleri, M.D. at Mayo Clinic.
The authors declare no conflict of interest.
References
- Post, DE, Khuri, FR, Simons, JW and Van Meir, EG (2003). Replicative oncolytic adenoviruses in multimodal cancer regimens. Hum Gene Ther 14: 933–946. [DOI] [PubMed] [Google Scholar]
- Liu, TC, Galanis, E and Kirn, D (2007). Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol 4: 101–117. [DOI] [PubMed] [Google Scholar]
- Prestwich, RJ, Errington, F, Diaz, RM, Pandha, HS, Harrington, KJ, Melcher, AA et al. (2009). The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum Gene Ther 20: 1119–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison, AJ, Benko, M and Harrach, B (2003). Genetic content and evolution of adenoviruses. J Gen Virol 84(Pt 11): 2895–2908. [DOI] [PubMed] [Google Scholar]
- Pereira, HG and Kelly, B (1957). Dose-response curves of toxic and infective actions of adenovirus in HeLa cell cultures. J Gen Microbiol 17: 517–524. [DOI] [PubMed] [Google Scholar]
- Huebner, RJ, Rowe, WP, Schatten, WE, Smith, RR and Thomas, LB (1956). Studies on the use of viruses in the treatment of carcinoma of the cervix. Cancer 9: 1211–1218. [DOI] [PubMed] [Google Scholar]
- Schenk, E, Essand, M, Kraaij, R, Adamson, R, Maitland, NJ and Bangma, CH (2014). Preclinical safety assessment of Ad[I/PPT-E1A], a novel oncolytic adenovirus for prostate cancer. Hum Gene Ther Clin Dev 25: 7–15. [DOI] [PubMed] [Google Scholar]
- Dong, W, van Ginkel, JW, Au, KY, Alemany, R, Meulenberg, JJ and van Beusechem, VW (2014). ORCA-010, a novel potency-enhanced oncolytic adenovirus, exerts strong antitumor activity in preclinical models. Hum Gene Ther 25: 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, W, Neill, T, Yang, Y, Hu, Z, Cleveland, E, Wu, Y et al. (2015). The systemic delivery of an oncolytic adenovirus expressing decorin inhibits bone metastasis in a mouse model of human prostate cancer. Gene Ther 22: 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja, SS (2015). Re: Prospective randomized phase 2 trial of intensity modulated radiation therapy with or without oncolytic adenovirus-mediated cytotoxic gene therapy in intermediate-risk prostate cancer. J Urol 193: 847. [DOI] [PubMed] [Google Scholar]
- Mao, LJ, Zhang, J, Liu, N, Fan, L, Yang, DR, Xue, BX et al. (2015). Oncolytic virus carrying shRNA targeting SATB1 inhibits prostate cancer growth and metastasis. Tumour Biol 36: 9073–9081. [DOI] [PubMed] [Google Scholar]
- Liu, Y, Chen, L, Gong, Z, Shen, L, Kao, C, Hock, JM et al. (2015). Lovastatin enhances adenovirus-mediated TRAIL induced apoptosis by depleting cholesterol of lipid rafts and affecting CAR and death receptor expression of prostate cancer cells. Oncotarget 6: 3055–3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, H, Munns, S, Freytag, S, Barton, K, Veenstra, J, Bettahi, I et al. (2015). Immunotherapeutic intervention with oncolytic adenovirus in mouse mammary tumors. Oncoimmunology 4: e984523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedra, PA, Poveda, GA, Ramsey, B, McCoy, K and Hiatt, PW (1998). Incidence and prevalence of neutralizing antibodies to the common adenoviruses in children with cystic fibrosis: implication for gene therapy with adenovirus vectors. Pediatrics 101: 1013–1019. [DOI] [PubMed] [Google Scholar]
- Abbink, P, Lemckert, AA, Ewald, BA, Lynch, DM, Denholtz, M, Smits, S et al. (2007). Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol 81: 4654–4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki, A, Kanerva, A, Kremer, EJ, Bauerschmitz, GJ, Smith, BF, Liu, B et al. (2003). A canine conditionally replicating adenovirus for evaluating oncolytic virotherapy in a syngeneic animal model. Mol Ther 7: 163–173. [DOI] [PubMed] [Google Scholar]
- Hoffmann, D, Bayer, W, Heim, A, Potthoff, A, Nettelbeck, DM and Wildner, O (2008). Evaluation of twenty-one human adenovirus types and one infectivity-enhanced adenovirus for the treatment of malignant melanoma. J Invest Dermatol 128: 988–998. [DOI] [PubMed] [Google Scholar]
- Hoffmann, D, Heim, A, Nettelbeck, DM, Steinstraesser, L and Wildner, O (2007). Evaluation of twenty human adenoviral types and one infectivity-enhanced adenovirus for the therapy of soft tissue sarcoma. Hum Gene Ther 18: 51–62. [DOI] [PubMed] [Google Scholar]
- Strauss, R, Sova, P, Liu, Y, Li, ZY, Tuve, S, Pritchard, D et al. (2009). Epithelial phenotype confers resistance of ovarian cancer cells to oncolytic adenoviruses. Cancer Res 69: 5115–5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashkova, EV, May, SM and Barry, MA (2009). Characterization of human adenovirus serotypes 5, 6, 11, and 35 as anticancer agents. Virology 394: 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senac, JS, Doronin, K, Russell, SJ, Jelinek, DF, Greipp, PR and Barry, MA (2010). Infection and killing of multiple myeloma by adenoviruses. Hum Gene Ther 21: 179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, CY, Senac, JS, Weaver, EA, May, SM, Jelinek, DF, Greipp, P et al. (2011). Species D adenoviruses as oncolytics against B-cell cancers. Clin Cancer Res 17: 6712–6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, CY, Weaver, EA, Khare, R, May, SM and Barry, MA (2011). Mining the adenovirus virome for oncolytics against multiple solid tumor types. Cancer Gene Ther 18: 744–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver, EA, Hillestad, ML, Khare, R, Palmer, D, Ng, P and Barry, MA (2011). Characterization of species C human adenovirus serotype 6 (Ad6). Virology 412: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blusch, JH, Deryckere, F, Windheim, M, Ruzsics, Z, Arnberg, N, Adrian, T et al. (2002). The novel early region 3 protein E3/49K is specifically expressed by adenoviruses of subgenus D: implications for epidemic keratoconjunctivitis and adenovirus evolution. Virology 296: 94–106. [DOI] [PubMed] [Google Scholar]
- Khare, R, May, SM, Vetrini, F, Weaver, EA, Palmer, D, Rosewell, A et al. (2011). Generation of a Kupffer cell-evading adenovirus for systemic and liver-directed gene transfer. Mol Ther 19: 1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare, R, Hillestad, ML, Xu, Z, Byrnes, AP and Barry, MA (2013). Circulating antibodies and macrophages as modulators of adenovirus pharmacology. J Virol 87: 3678–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Z, Tian, J, Smith, JS and Byrnes, AP (2008). Clearance of adenovirus by Kupffer cells is mediated by scavenger receptors, natural antibodies, and complement. J Virol 82: 11705–11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry, MA, Hofherr, SE, Chen, CY, Senac, JS, Hillestad, ML and Shashkova, EV (2009). Systemic delivery of therapeutic viruses. Curr Opin Mol Ther 11: 411–420. [PubMed] [Google Scholar]
- Khare, R, Chen, CY, Weaver, EA and Barry, MA (2011). Advances and future challenges in adenoviral vector pharmacology and targeting. Curr Gene Ther 11: 241–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doronin, K, Shashkova, EV, May, SM, Hofherr, SE and Barry, MA (2009). Chemical modification with high molecular weight polyethylene glycol reduces transduction of hepatocytes and increases efficacy of intravenously delivered oncolytic adenovirus. Hum Gene Ther 20: 975–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Riordan, CR, Lachapelle, A, Delgado, C, Parkes, V, Wadsworth, SC, Smith, AE et al. (1999). PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum Gene Ther 10: 1349–1358. [DOI] [PubMed] [Google Scholar]
- Croyle, MA, Chirmule, N, Zhang, Y and Wilson, JM (2001). “Stealth” adenoviruses blunt cell-mediated and humoral immune responses against the virus and allow for significant gene expression upon readministration in the lung. J Virol 75: 4792–4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croyle, MA, Chirmule, N, Zhang, Y and Wilson, JM (2002). PEGylation of E1-deleted adenovirus vectors allows significant gene expression on readministration to liver. Hum Gene Ther 13: 1887–1900. [DOI] [PubMed] [Google Scholar]
- Mok, H, Palmer, DJ, Ng, P and Barry, MA (2005). Evaluation of polyethylene glycol modification of first-generation and helper-dependent adenoviral vectors to reduce innate immune responses. Mol Ther 11: 66–79. [DOI] [PubMed] [Google Scholar]
- Croyle, MA, Le, HT, Linse, KD, Cerullo, V, Toietta, G, Beaudet, A et al. (2005). PEGylated helper-dependent adenoviral vectors: highly efficient vectors with an enhanced safety profile. Gene Ther 12: 579–587. [DOI] [PubMed] [Google Scholar]
- Hofherr, SE, Mok, H, Gushiken, FC, Lopez, JA and Barry, MA (2007). Polyethylene glycol modification of adenovirus reduces platelet activation, endothelial cell activation, and thrombocytopenia. Hum Gene Ther 18: 837–848. [DOI] [PubMed] [Google Scholar]
- Stone, KR, Mickey, DD, Wunderli, H, Mickey, GH and Paulson, DF (1978). Isolation of a human prostate carcinoma cell line (DU 145). Int J Cancer 21: 274–281. [DOI] [PubMed] [Google Scholar]
- Horoszewicz, JS, Leong, SS, Kawinski, E, Karr, JP, Rosenthal, H, Chu, TM et al. (1983). LNCaP model of human prostatic carcinoma. Cancer Res 43: 1809–1818. [PubMed] [Google Scholar]
- Sumida, SM, Truitt, DM, Lemckert, AA, Vogels, R, Custers, JH, Addo, MM et al. (2005). Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J Immunol 174: 7179–7185. [DOI] [PubMed] [Google Scholar]
- Vogels, R, Zuijdgeest, D, van Rijnsoever, R, Hartkoorn, E, Damen, I, de Béthune, MP et al. (2003). Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J Virol 77: 8263–8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashkova, EV, Spencer, JF, Wold, WS and Doronin, K (2007). Targeting interferon-alpha increases antitumor efficacy and reduces hepatotoxicity of E1A-mutated spread-enhanced oncolytic adenovirus. Mol Ther 15: 598–607. [DOI] [PubMed] [Google Scholar]
- Yang, Y, Ertl, HC and Wilson, JM (1994). MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity 1: 433–442. [DOI] [PubMed] [Google Scholar]
- Horwitz, MS (2004). Function of adenovirus E3 proteins and their interactions with immunoregulatory cell proteins. J Gene Med 6 Suppl 1: S172–S183. [DOI] [PubMed] [Google Scholar]
- Lichtenstein, DL, Toth, K, Doronin, K, Tollefson, AE and Wold, WS (2004). Functions and mechanisms of action of the adenovirus E3 proteins. Int Rev Immunol 23: 75–111. [DOI] [PubMed] [Google Scholar]
- Suzuki, K, Alemany, R, Yamamoto, M and Curiel, DT (2002). The presence of the adenovirus E3 region improves the oncolytic potency of conditionally replicative adenoviruses. Clin Cancer Res 8: 3348–3359. [PubMed] [Google Scholar]
- Wang, Y, Hallden, G, Hill, R, Anand, A, Liu, TC, Francis, J et al. (2003). E3 gene manipulations affect oncolytic adenovirus activity in immunocompetent tumor models. Nat Biotechnol 21: 1328–1335. [DOI] [PubMed] [Google Scholar]
- Campos, SK and Barry, MA (2004). Rapid construction of capsid-modified adenoviral vectors through bacteriophage lambda Red recombination. Hum Gene Ther 15: 1125–1130. [DOI] [PubMed] [Google Scholar]
- Weaver, EA, Palmer, D, Ng, P and Barry, MA (2011). Characterization of species C human adenovirus serotype 6 (Ad6). Virology 412: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashkova, EV, May, SM, Doronin, K and Barry, MA (2009). Expanded anticancer therapeutic window of hexon-modified oncolytic adenovirus. Mol Ther 17: 2121–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayakhmetov, DM, Gaggar, A, Ni, S, Li, ZY and Lieber, A (2005). Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol 79: 7478–7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, AL, Waddington, SN, Nicol, CG, Shayakhmetov, DM, Buckley, SM, Denby, L et al. (2006). Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood 108: 2554–2561. [DOI] [PubMed] [Google Scholar]
- Hofherr, SE, Shashkova, EV, Weaver, EA, Khare, R and Barry, MA (2008). Modification of adenoviral vectors with polyethylene glycol modulates in vivo tissue tropism and gene expression. Mol Ther 16: 1276–1282. [DOI] [PubMed] [Google Scholar]
- Weaver, EA and Barry, MA (2013). Low seroprevalent species D adenovirus vectors as influenza vaccines. PLoS One 8: e73313. [DOI] [PMC free article] [PubMed] [Google Scholar]