Abstract
Background
Small cell lung cancer (SCLC) is an aggressive and invasive variant of lung tumors. SCLC, especially in advanced stages, is distinguished by extremely high numbers of circulating tumor cells (CTCs) in comparison to other malignancies. CTCs are operative in tumor spread and are currently enumerated to assess prognosis and response to cytotoxic therapy. Chemosensitivity of SCLC CTCs compared to primary tumors and metastases is not known.
Methods
Establishment of two SCLC CTC cell lines, namely BHGc7 and BHGc10, allowed the in vitro characterization of their chemosensitivity to the second-line chemotherapeutics topotecan and epirubicin in comparison to a range of SCLC cell lines.
Results
The SCLC CTC cell lines exhibited an approximately 7- and 12-fold increased chemosensitivity to epirubicin compared to topotecan, respectively, in in vitro cytotoxicity assays. In comparison to a panel of six SCLC cell lines, the two CTC lines showed a significantly higher chemosensitivity to epirubicin (range, 3- to 16-fold) and topotecan (range, 2.2- to 14.4-fold), respectively.
Conclusions
CTC cell lines derived from SCLC patients with recurrent disease exhibit high chemosensitivity to epirubicin vs. topotecan and show considerable more cytotoxicity in response to both compounds in comparison to a panel of SCLC cell lines. Thus, a decrease in the number of CTCs in response to second-line chemotherapy in SCLC patients may overestimate the effect on resident SCLC lesions and metastases.
Keywords: Small cell lung cancer (SCLC), circulating tumor cells (CTCs), chemotherapy, epirubicin, topotecan, chemosensitivity
Introduction
Small cell lung cancer (SCLC) accounts for approximately 15% of lung cancers and has a poor prognosis due to rapid growth and early tumor spread in combination with unavoidably drug resistance (1,2). Standard care for SCLC patients is platinum-based chemotherapy and (prophylactic cranial) irradiation due to the fact that most of them exhibit disseminated disease at first presentation (3). Despite high initial responses, SCLC usually recurs within approximately 1 year as chemoresistant tumor which is further treated with topotecan or combinations including anthracyclines. Topotecan, which is the only FDA-approved drug for second-line treatment of SCLC, targets topoisomerase I eventually resulting in DNA strand breaks and apoptosis (4). Topoisomerase II-targeting anthracyclines, such as epirubicin may be used as alternative. This drug represents a 4-epimer of doxorubicin/adriamycin which is a chemotherapeutic included in the cyclophosphamide/adriamycin/vincristine (CAV) regimen used for treatment of SCLC (5). A host of other drugs and attempts of targeted therapy for SCLC were not successful so far (3). SCLC is distinguished by the appearance of a large number of circulating tumor cells (CTCs) in the periphery which seems to be linked to metastasis and a poor prognosis (6,7). Hou et al. reported the presence of CTCs in 85% of SCLC patients at a mean count of approximately 1,600 cells/7.5 mL blood (8). The number of pretreatment CTCs and change in count after one cycle of chemotherapy constituted independent prognostic factors. In a study by Huang et al. a mean reduction greater than 95% in CTCs from baseline to post-treatment correlating with improved survival was found for 15/24 SCLC patients with extended disease in response to platinum/etoposide chemotherapy (9). The CTCs may interact with cells of the immune system to induce cachexia and tumor-associated death at final stages (10). The chemosensitivity of SCLC CTCs has not been known due to the lack of sufficient number of these cells for such investigations. We have succeeded in obtaining several permanent cultures of CTCs from advanced SCLC patients, which could be employed for in vitro investigations of their drug sensitivity profiles (11,12). In the present study, SCLC BHGc7 and BHGc10 CTC cell lines were treated with chemotherapeutics in vitro and their chemosensitivities compared to drug effects on a panel of permanent SCLC cell lines derived from lung as well as different metastatic sites.
Material and methods
Materials
Epirubicin, topotecan, cisplatin and etoposide were obtained from Sigma-Aldrich, St. Louis, MO, USA. Stock solutions (100×) were prepared in dimethyl sulfoxide and stored frozen, except for cisplatin which was dissolved in 0.9% sodium chloride.
Cell lines and culture conditions
SCLC26A was established in our lab from pleural effusion of an SCLC patient before treatment. GLC14 and GLC16 stem from the same patient before and after first cycles of chemotherapy, DMS153 was cultured from a liver metastases and NCI-H526 from bone metastases, respectively, whereas NCI-H417 was established from a primary lung SCLC before treatment. All cell lines, with exception of SCLC26A, were obtained from the Finsen Center, Copenhagen, Denmark. The SCLC CTC cell lines BHGc7 and BHGc10 were established from peripheral blood of SCLC patients with extended disease at our institution (9). Cells were cultured in RPMI-1640 medium (Seromed, Berlin, Germany) supplemented with 10% fetal bovine serum (Seromed) and antibiotics (Sigma-Aldrich).
Cytotoxicity assays
1×104 cells in 100 µL medium were distributed to wells of 96-well microtiter plates (TPP, Trasadingen Switzerland) and ten 2-fold dilutions of the chemotherapeutics added in triplicate. Assays were at least performed in triplicate. The plates were incubated for four days under tissue culture conditions and viable cells detected using a modified MTT assay (EZ4U, Biomedica, Vienna, Austria). IC50 values were determined using Origin 9.1 software (OriginLab, Northampton, MA, USA).
Statistics
Statistical significance was tested by t-tests and P<0.05 regarded as significant difference.
Results
Dose-response curves of epirubicin and topotecan for two SCLC CTC lines
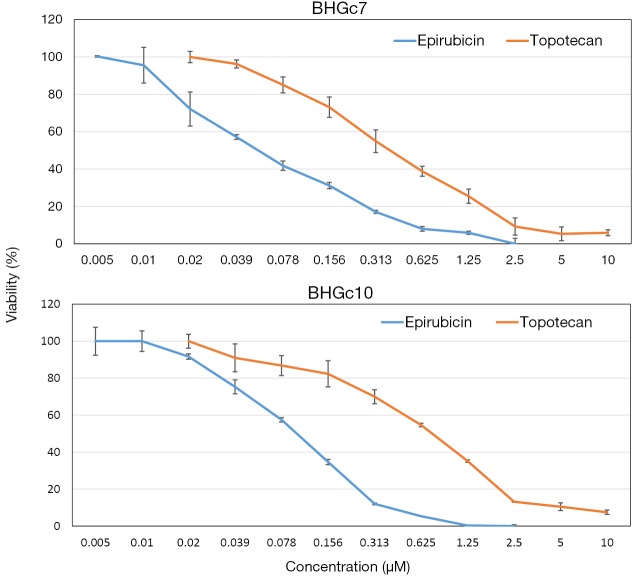
The cytotoxic activity of epirubicin and topotecan against BHGc7 and BHGc10 CTC cell lines was tested in MTT assays (Figure 1). The dose-response curves revealed that, on a molar basis, epirubicin was approximately 7.2- to 12.3-fold more cytotoxic for BHGc7 and BHGc10, respectively, compared to topotecan.
Figure 1.
Chemosensitivity of the SCLC CTC lines to epirubicin and topotecan. BHGc7 and BHGc10 CTC cell lines were treated with the indicated concentrations of epirubicin and topotecan, respectively, for four days. Dose response curves are shown for ten 2-fold dilutions of the drugs (mean values ± SD). SCLC, small cell lung cancer; CTC, circulating tumor cells.
Chemosensitivity of the two CTC lines compared to a panel of SCLC cell lines
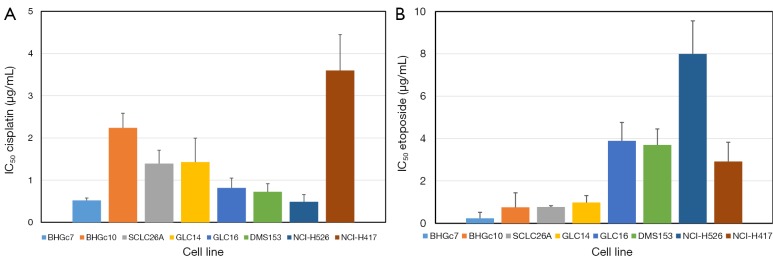
Dose response curves for cisplatin, etoposide, epirubicin and topotecan were obtained for both CTC and a panel of SCLC cell lines by MTT assays and the corresponding IC50 values are summarized in Figure 2 for cisplatin and etoposide as well in Figure 3 for epirubicin and topotecan, respectively. Whereas BHGc7 is sensitive to cisplatin, BHGc10 showed approximately 4-fold higher IC50 values. The SCLC cell lines exhibited varying cisplatin sensitivities with exception of the marked chemoresistance of the NCI-H417 cells (Figure 2A). Half of the cell lines, including BHGc7 and 10, proved to be highly etoposide-sensitive but GLC14, DMS153, NCI-H526 and NCI-H417 displayed elevated IC50 values (Figure 2B).
Figure 2.
Comparison of the chemosensitivities of SCLC CTCs and tumor cell lines to cisplatin and etoposide. IC50 values (mean values ± SD) as obtained from dose-response curves are shown for both SCLC CTC and tumor cell lines as well as cisplatin (A) and etoposide (B), respectively. The chemosensitivity of the BHGc7 CTC cell line is significantly different from all other cell lines, except NCI-H526. For etoposide, the CTC cell lines as well as the SCLC26A and GLC14 SCLC cell lines exhibit high drug sensitivity which is significantly different from the higher IC50 values of the remaining cell lines. SCLC, small cell lung cancer; CTC, circulating tumor cells.
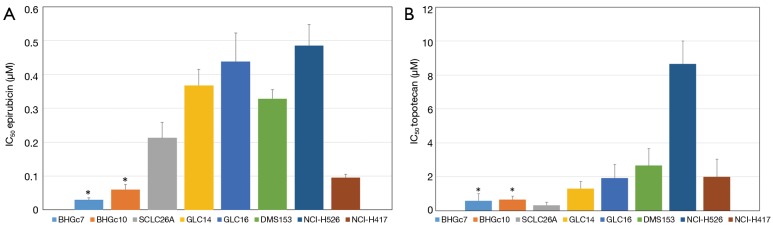
Figure 3.
Comparison of the chemosensitivities of SCLC CTCs and tumor cell lines to epirubicin and topotecan. IC50 values (mean values ± SD) as obtained from dose-response curves are shown for both SCLC CTC and tumor cell lines as well as epirubicin and topotecan, respectively. The chemosensitivities of the CTC cell lines are significantly different from all SCLC tumor cell lines for epirubicin (A) and topotecan (B), except for the topotecan-sensitive SCLC26A pleural effusion cell line. SCLC, small cell lung cancer; CTC, circulating tumor cells.
Both CTC lines proved to be significantly more sensitive to epirubicin compared to the panel of SCLC cell lines tested (Figure 3A). The partial chemosensitive NCI-H417 line was established from primary untreated SCLC of the lung. On average, the SCLC cell lines are 10- and 5-fold more resistant to epirubicin than BHGc7 and BHGc10, respectively and approximately 5.5-fold more resistant to topotecan, excluding the topotecan-sensitive SCLC26A cell line (Figure 3B). NCI-H526 exhibited distinct resistance to treatment with topotecan.
Discussion
Combination chemotherapy has been the mainstay of treatment for disseminated SCLC over the last decades, even though it only achieves a short prolongation in survival time (2). Although platinum-based combination chemotherapy regimens have been shown to increase complete response rates, tumors recur in the majority of patients within 1–2 years (4,13). Patients are categorized into relapsed (progression after 3 months) and refractory patients (no response or relapse within 3 months) and especially for the latter group efficacy of second-line chemotherapy is poor (14). Topotecan monotherapy improves survival and quality of life as well as cancer-related symptoms and is the single approved drug for this setting. Alternatively, doxorubicin-based combination therapy can be administered with a similar outcome but a slightly lower rate of symptom control (15).
Permanent CTC cultures of SCLC patients were established at our institution for the first time (11). Relapsing patient BHGc7 responded to initial treatment with several cycles of carboplatin/etoposide but the tumor recurred 10 months later in form of multiple bone metastases. This patient did not respond to further therapy and died shortly after tumor progression. The BHGc10 CTC line was established from a blood sample of a refractory patient before initiation of the second-line therapy, consisting of CEV. This patient had received three cycles of carboplatin/etoposide but progressed under therapy obviously exhibiting chemoresistance to the platinum drug in conformance with our subsequent in vitro cytotoxicity tests. Permanent CTC cultures were obtained from both patients and checked for their chemosensitivity to cisplatin, etoposide, epirubicin and topotecan. BHGc10 showed an IC50 value for cisplatin in the range of the maximal achievable peak plasma concentration of approximately 3 µg/mL, whereas BHGc7 is chemosensitive. All cell lines seem to be sensitive to etoposide since this drug exhibits peak plasma concentrations up to 20 µg/mL in vivo, clearly exceeding the IC50 values determined. The CTC lines express typical SCLC markers and show slow proliferation with doubling times of approximately 60 hrs (data not shown). Both CTC cultures are highly sensitive to epirubicin compared to a panel of SCLC cell lines of diverse metastatic origin and are much more sensitive on a molar basis to this anthracycline in comparison to topotecan.
Anthracyclines are among the most effective chemotherapeutics for several types of cancer. The combination regimens etoposide-cisplatin (EP) and CAV can be considered equivalently effective therapies in extensive SCLC (16,17). Doxorubicin/Adriamycin may be replaced by epirubicin which exhibits reduced cardiotoxicity due to faster elimination (18). Topotecan and epirubicin regimens yield matchable peak plasma concentrations of approximately 0.2 µM (18,19). Topotecan was at least as effective as CAV in the treatment of patients with recurrent SCLC and resulted in improved control of several symptoms (20). This was the only study to report improvement, although not statistically significant in symptom management with topotecan compared with CAV.
Many patients with SCLC relapse due to spread of cancer cells which evades detection by conventional methods. CTCs are tumor cells that have disseminated from primary and metastatic sites and circulate in the bloodstream. Their analysis is considered as a liquid biopsy approach for early diagnosis, risk stratification, evaluation of curative efficacy, and early detection of lung cancer relapse (6). For example, in one study CTCs were present in 85% of SCLC patients and were abundant with a mean count of 1,600 CTCs/7.5 mL of blood (8). Pretreatment CTCs and change in numbers after one cycle of chemotherapy were independent prognostic factors. Overall survival was 5.4 months for patients with ≥50 CTCs/7.5 mL of blood and 11.5 months for patients with less than 50 CTCs/7.5 mL of blood before chemotherapy, indicating just a delay of approximately 6 months in final tumor progression. Higher baseline CTCs and percentage change in post-treatment CTCs were associated with decreased survival in another small trial (9). The widely differing mean number of 75 CTCs/7.5 mL blood of CTCs in ED-SCLC reported in this study seems to indicate different results in dependence of patients and technologies employed. Of course, the best prognostic group being patients without CTCs before and after therapy, indicating localized SCLC (21). A metaanalysis covering 440 SCLC patients revealed that CTCs were significantly associated with shorter progression-free and overall survival (22). CTCs may overlap with CD133+ cancer stem cells (CSCs) which were reported to have predictive value in lung adenocarcinoma (23).
In other malignancies, CTCs are associated with poor response to chemotherapy in metastatic gastrointestinal cancer patients (24). The ability to determine resistance-mediating androgen receptor modifications or other mutations in CTCs has the potential to considerably improve treatment of prostate and other cancers (25). Furthermore, CTCs are correlated with prognosis in colorectal cancer patients treated with chemotherapy (26). CTCs could provide additional prognostic information to tumor radiographic imaging and might be used as a surrogate and novel predictive marker for the response to chemotherapy. However, in metastatic breast cancer enumeration of CTCs had prognostic but not predictive significance for chemotherapy (27). Cluster formation in the post-treatment short-term CTC culture of breast cancer patients correlated with shorter overall survival of 9.8 months versus a mean overall survival of 16.6 months for cluster-negative patients (28).
In the present study, both CTC cell lines exhibited higher sensitivity to epirubicin and topotecan compared to SCLC lines derived from lung and distinct metastases. Thus, a drop in CTC counts in patients may not exactly reflect response of primary and metastatic SCLC lesions to chemotherapeutic treatment in general. Furthermore, CTCs are freely accessible in the bloodstream but chemoresistance of tumors in vivo may be further enhanced by adverse microenvironmental conditions, such as poor vascularization, increased intratumoral pressure and acidosis (29). Nevertheless, monitoring of CTCs in SCLC has the potential to detect tumor spread at an early phase before clinically appearance. Removal of CTCs by chemotherapeutics is expected to impair tumor spread and in advanced disease with high CTC counts these tumor cells may contribute to morbidity through their interactions with the immune system. We have demonstrated recently that SCLC CTCs recruit macrophages via differentiation of monocytes and stimulate release of inflammatory cytokines and mediators possibly involved in triggering cachexia (10).
Conclusions
In conclusion, application of CTC counts as surrogate marker of tumor response may be impaired by different drug sensitivities of circulating versus primary and metastatic SCLC cells. Furthermore, chemosensitive CTCs may survive through homing to special niches or formation of resistant spheroids for subsequent generation of secondary lesions.
Acknowledgements
We thank the Molecular Oncology Group of the Department of Obstetrics and Gynecology/Medical University of Vienna and Mr. G. Hassknecht for support.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer 2015;121:664-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pillai RN, Owonikoko TK. Small cell lung cancer: therapies and targets. Semin Oncol 2014;41:133-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalemkerian GP. Advances in pharmacotherapy of small cell lung cancer. Expert Opin Pharmacother 2014;15:2385-96. [DOI] [PubMed] [Google Scholar]
- 4.Asai N, Ohkuni Y, Kaneko N, et al. Relapsed small cell lung cancer: treatment options and latest developments. Ther Adv Med Oncol 2014;6:69-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.López-González A, Diz P, Gutierrez L, et al. The role of anthracyclines in small cell lung cancer. Ann Transl Med 2013;1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu N, Zhou J, Cui F, et al. Circulating tumor cells in lung cancer: detection methods and clinical applications. Lung 2015;193:157-71. [DOI] [PubMed] [Google Scholar]
- 7.Hodgkinson CL, Morrow CJ, Li Y, et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat Med 2014;20:897-903. [DOI] [PubMed] [Google Scholar]
- 8.Hou JM, Krebs MG, Lancashire L, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol 2012;30:525-32. [DOI] [PubMed] [Google Scholar]
- 9.Huang CH, Wick JA, Sittampalam GS, et al. A multicenter pilot study examining the role of circulating tumor cells as a blood-based tumor marker in patients with extensive small-cell lung cancer. Front Oncol 2014;4:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamilton G, Rath B, Klameth L, et al. Small cell lung cancer: recruitment of macrophages by circulating tumor cells. OncoImmunology 2015. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton G, Burghuber O, Zeillinger R. Circulating tumor cells in small cell lung cancer: ex vivo expansion. Lung 2015;193:451-2. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton G, Rath B, Burghuber O. Chitinase-3-like-1/YKL-40 as marker of circulating tumor cells. Transl Lung Cancer Res 2015;4:287-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pujol JL, Carestia L, Daurès JP. Is there a case for cisplatin in the treatment of small-cell lung cancer? A meta-analysis of randomized trials of a cisplatin-containing regimen versus a regimen without this alkylating agent. Br J Cancer 2000;83:8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmittel A. Second-line therapy for small-cell lung cancer. Expert Rev Anticancer Ther 2011;11:631-7. [DOI] [PubMed] [Google Scholar]
- 15.Schmittel A. Controversies in the treatment of advanced stages of small cell lung cancer. Front Radiat Ther Oncol 2010;42:193-7. [DOI] [PubMed] [Google Scholar]
- 16.Giaccone G, Dalesio O, McVie GJ, et al. Maintenance chemotherapy in small-cell lung cancer: long-term results of a randomized trial. European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group. J Clin Oncol 1993;11:1230-40. [DOI] [PubMed] [Google Scholar]
- 17.Roth BJ, Johnson DH, Einhorn LH, et al. Randomized study of cyclophosphamide, doxorubicin, and vincristine versus etoposide and cisplatin versus alternation of these two regimens in extensive small-cell lung cancer: a phase III trial of the Southeastern Cancer Study Group. J Clin Oncol 1992;10:282-91. [DOI] [PubMed] [Google Scholar]
- 18.Robert J. Clinical pharmacokinetics of epirubicin. Clin Pharmacokinet 1994;26:428-38. [DOI] [PubMed] [Google Scholar]
- 19.O'Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol 2006;24:5441-7. [DOI] [PubMed] [Google Scholar]
- 20.von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol 1999;17:658-67. [DOI] [PubMed] [Google Scholar]
- 21.Khan MS, Kirkwood AA, Tsigani T, et al. Early Changes in Circulating Tumor Cells Are Associated with Response and Survival Following Treatment of Metastatic Neuroendocrine Neoplasms. Clin Cancer Res 2016;22:79-85. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Wang HT, Li BG. Prognostic significance of circulating tumor cells in small--cell lung cancer patients: a meta-analysis. Asian Pac J Cancer Prev 2014;15:8429-33. [DOI] [PubMed] [Google Scholar]
- 23.Luo YH, Tseng PC, Lee YC, et al. A prospective study of the use of circulating markers as predictors for epidermal growth factor receptor-tyrosine kinase inhibitor treatment in pulmonary adenocarcinoma. Cancer Biomark 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 24.Lee SJ, Lee J, Kim ST, et al. Circulating tumor cells are predictive of poor response to chemotherapy in metastatic gastric cancer. Int J Biol Markers 2015;30:e382-6. [DOI] [PubMed] [Google Scholar]
- 25.Steinestel J, Luedeke M, Arndt A, et al. Detecting predictive androgen receptor modifications in circulating prostate cancer cells. Oncotarget 2015. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang X, Gao P, Song Y, et al. Relationship between circulating tumor cells and tumor response in colorectal cancer patients treated with chemotherapy: a meta-analysis. BMC Cancer 2014;14:976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathew A, Brufsky AM, Davidson NE. Can circulating tumor cells predict resistance in metastatic breast cancer? Clin Cancer Res 2015;21:2421-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khoo BL, Lee SC, Kumar P, et al. Short-term expansion of breast circulating cancer cells predicts response to anti-cancer therapy. Oncotarget 2015;6:15578-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khawar IA, Kim JH, Kuh HJ. Improving drug delivery to solid tumors: priming the tumor microenvironment. J Control Release 2015;201:78-89. [DOI] [PubMed] [Google Scholar]