Abstract
Purpose
This study aimed to derive heteroparental normal karyotypic human embryonic stem cells (hESCs) from microsurgically corrected tripronuclear (3PN) zygotes.
Methods
After sequential culture for 5–6 days, embryos developed from microsurgically corrected 3PN zygotes were analyzed by fluorescence in situ hybridization (FISH) using probes for chromosomes 17, X and Y. Intact 3PN zygotes from clinical in vitro fertilization (IVF) cycles were cultured as the control group. The inner cell mass (ICM) of blastocysts that developed from microsurgically corrected 3PN zygotes was used to derive hESC lines, and the stem cell characteristics of these lines were evaluated. G-banding analysis was adopted to identify the karyotype of the hESC line, and the heteroparental inheritance of the hESC line was analyzed by DNA fingerprinting analysis.
Results
The blastocyst formation rate (13.5 %) of the microsurgically corrected 3PN zygotes was significantly higher (P < 0.05) than that of intact 3PN zygotes (8.7 %). The diploid rate of the blastocysts (55.0 %) was significantly higher (P < 0.05) than that of the arrested cleavage-stage embryos (18.4 %) in microsurgically corrected 3PN zygotes. The triploid rate of the microsurgically corrected 3PN zygotes (5.7 %) was significantly lower (P < 0.01) than that of intact 3PN zygotes (19.4 %). Furthermore, we established one heteroparental normal karyotypic hESC line from the microsurgically corrected tripronuclear zygotes.
Conclusions
Pronuclear removal can effectively remove the surplus chromosome set of 3PN zygotes. A combination of pronuclear removal and blastocyst culture enables the selection of diploidized blastocysts from which heteroparental normal karyotypic hESC lines can be derived.
Keywords: Tripronuclear zygotes, Pronuclear removal, Blastocyst culture, hESCs, Heteroparental inheritance
Introduction
Tripronuclear (3PN) zygotes occur at a rate of 5–8.1 % after conventional in vitro fertilization (IVF) [1–3], and at least one 3PN zygote occurs in 60 % of IVF cycles [4]. 3PN zygotes can be either diandric (dispermic or diploid spermic fertilization of normal haploid oocytes) [5] or digynic (the retention of a second polar body or the fertilization of a giant diploid oocyte by a haploid sperm) [2]. 3PN embryos have very complicated chromosomal constitution [6–9] and are at high risk for an abnormal pregnancy [10–12]. Many infants with mosaic trisomy may be the result of 3PN embryos [8], and thus, 3PN embryos are considered not suitable for clinical transfer.
Several studies have attempted to restore the diploid status of 3PN zygotes by microsurgical removal of the extra pronucleus [13–19]. Kattera et al. reported a birth of a healthy boy after transferring three embryos that developed from microsurgically corrected 3PN zygotes. However, cytogenetic studies of microsurgically corrected zygotes are very rare [18, 20], and the chromosomal constitution of microsurgically corrected 3PN embryos remains uncertain.
As a routine method in clinical IVF, blastocyst culture and transfer significantly increases implantation [21], facilitating the selection of embryos with high developmental potential and that are chromosomally normal [22]. The inner cell mass (ICM) of blastocysts can be isolated to establish human embryonic stem cells (hESCs), which can differentiate into the three germ layers of the human body [23, 24]. The pluripotency of hESCs makes them a useful tool in basic scientific research and in regenerative medicine. The blastocysts used to derive hESCs are usually obtained from surplus normal or poor-quality embryos donated by IVF patients [25] or from chromosomally abnormal embryos diagnosed by preimplantation genetic screening [26]. If microsurgically corrected 3PN embryos can reach the blastocyst stage by blastocyst culture and be applied to derive normal karyotypic hESCs, the utilization efficiency of these embryos, which were destined to be discarded, will be improved, with favorable ethical implications.
This study aimed to determine if chromosomally normal embryos can be reliably selected by a combination of pronuclear removal and blastocyst culture and to attempt to derive hESCs from microsurgically corrected blastocysts.
Materials and methods
The experiment was approved by the ethical committee of the CITIC-Xiangya Reproductive and Genetic Hospital (the reference number of the approval: LL-SC-SG-2006-004), and the 3PN zygotes used in this study were donated by couples who underwent conventional IVF and signed consent forms.
IVF treatment
Ovarian stimulation was performed by our routine downregulation protocol using gonadotropin-releasing hormone agonist (GnRHa). Gonadotropin was administered from days 2 to 3 of the menstrual cycle to stimulate follicle growth. Human chorionic gonadotropin (hCG) was injected when there were at least 3 dominant follicles with diameters of ≥18 mm. Then, 34–36 h after the administration of hCG, oocyte retrieval was performed by ultrasound-guided transvaginal aspiration. Sperm was prepared using the swim-up or density-gradient centrifugation method.
Microsurgical removal of the extra pronucleus from 3PN zygotes
At 16–18 h after insemination (day 1), the fertilization status was evaluated. The 3PN zygotes were identified by the presence of three distinct pronuclei and two polar bodies (PBs). The 3PN zygotes were then placed in G-MOPS containing 5 % HSA (Vitrolife, Sweden) and examined under an inverted microscope (Nikon Ti-S, Japan). First, the 3PN zygotes were rotated by a holding pipette until the three pronuclei and PBs were present clearly in the same plane. The extra pronucleus was identified by the distance between the pronucleus and the second PB (larger and more integrated than the first PB and always connected to the ooplasmic membrane), and the farthest pronucleus from the second PB was removed as the extra male pronucleus [13, 16]. The extra pronucleus was removed using an intracytoplasmic sperm injection (ICSI) needle (Humagen, USA). The tip of the needle was inserted directly into the zona pellucida and oolemma, stopping near the extra pronucleus. The pronucleus and a small amount of cytoplasm (approximately 2 pl) [20] in its vicinity were gently aspirated. The remaining two pronuclei and the cytoplasmic membrane were examined 4–6 h later to confirm successful pronuclear removal.
Blastocyst culture
Next, 3PN zygotes for which pronuclear removal was successful were transferred to G1.5 (Vitrolife, Sweden) and cultured to day 3 in an incubator at 37.5 °C and 6 % CO2. The 3PN zygotes were then transferred to G2.5 (Vitrolife, Sweden) and cultured to days 5–6 in an incubator at 37.5 °C and 6 % CO2. Intact 3PN zygotes that directly underwent blastocyst culture served as the control group.
hESC derivation and cultivation
When the microsurgically corrected zygotes reached the blastocyst stage, the ICM of these blastocysts was used to derive hESCs. The method for deriving hESCs has been described previously [27]. The isolated ICM from the blastocyst was plated on mitotically inactivated human embryonic fibroblasts (HEFs) that were cultured in our laboratory from an aborted fetus of 2–3 months of age. The cells were first cultured in DMEM/F12-serum replacement (DFSR) medium containing DMEM/F12 supplemented with 15 % knockout serum replacement, 2 mM non-essential amino acids, 2 mM l-glutamine, 0.1 mM β-mercaptoethanol, and 50 ng/ml basic fibroblast growth factor (bFGF) (all from Invitrogen, Carlsbad, CA). The medium was changed every 2 days, and the cells were passaged every 7 days by mechanical cutting. Once established and propagated through several passages, the cells were transferred back to DFSR medium containing 4 ng/ml bFGF for long-term propagation.
Identification of human embryonic stem cell characteristics
Human embryonic stem cell characteristics were identified using the methods described previously [28].
Immunohistochemistry and alkaline phosphatase staining
Undifferentiated and spontaneously differentiated cells were fixed in 4 % paraformaldehyde at room temperature for 20 min, followed by blocking with 4 % goat serum in PBS and incubating overnight at 4 °C with primary antibodies diluted in blocking solution. To detect intracellular antigens, the cells were permeabilized for 10 min in 0.1 % Triton-X 100 before blocking. Mouse anti-Tra-1–60, mouse anti-Tra-1–81, mouse anti-SSEA4, rat anti-SSEA3, and mouse anti-Oct-3/4 were used to detect undifferentiated molecular markers of hESCs; mouse anti-β-tubulin, mouse anti-AFP, mouse anti-SMA were used for the in vitro differentiation assay. Unbound antibody was removed by extensive washing, and localization of the antigens was visualized using an Alexa Fluor 488-conjugated secondary antibody. Nuclei were counterstained with DAPI. Alkaline phosphatase activity was detected with the Fast Red Substrate Pack (Zymed Laboratories, South San Francisco, USA) according to the manufacturer’s recommendations.
RT-PCR analysis of pluripotency-related genes and telomerase activity
Total RNA was isolated with TRIzol (Invitrogen), and complementary DNA (cDNA) was synthesized using 1 g of total RNA in a 20-μl reaction using the Revert AidTM first strand cDNA synthesis kit (Fermentas Life Sciences; Ontario, USA) according to the manufacturer’s instructions. The reverse transcription polymerase chain reaction (RT-PCR) was conducted under the following conditions: 95 °C for 2 min, 30 cycles of amplification (95 °C for 30 s, 54∼64 °C for 30 s, and 72 °C for 30 s), and a final extension at 72 °C for 7 min. The PCR products were separated on a 1.5 % agarose gel, stained with ethidium bromide, and visualized and photographed on a UV transilluminator. The product sizes, annealing temperatures, and primer sequences were described previously [28]. The telomerase activity of the hESCs was measured using a TRAPeze Telomerase detection kit (Chemicon) according to the manufacturer’s instructions.
In vitro differentiation assay
hESC colonies cultured on HEF feeders were mechanically dissociated into small clumps and detached to grow as aggregates in suspension for 7 days to form embryoid bodies in DFSR medium without bFGF. The embryoid bodies were then transferred to a gelatin-coated six-well plate for adherent culture for 2 weeks in the same medium, followed by immunocytochemistry analysis.
DNA fingerprinting analysis
hESCs were transferred to Matrigel-coated dishes for further expansion as well as to exclude contamination from HEFs. Total genomic DNA was extracted from undifferentiated hESCs using a DNeasy Blood & Tissue kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. The parents’ genomic DNA was extracted from the father’s semen and mother’s follicular fluid. Then, 15 short tandem repeat (STR) loci and amelogenin were amplified by the PowerPlex® 16 system (Promega, Madison, USA) according to the manufacturer’s instructions and detected by a 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
Cytogenetic analysis
Fluorescence in situ hybridization
Embryos that developed to blastocyst stage and embryos that arrested in the cleavage stage were placed in acid Tyrode’s solution for 1–2 min to remove the zona pellucida. Then, all blastomeres were analyzed by fluorescence in situ hybridization (FISH). The blastomeres were placed in hypotonic solution (6 mg/ml BSA + 1 % NaAc) for 5 min and transferred to slides. Then, 0.01 N HCL + 0.01 % Tween was added, and the disappearance of the blastomeres was observed under a stereoscope. The slides were maintained at 60 °C for 2 h, followed by digestion by ribonuclease for 1 h. The slides were then rinsed in 2× SSC, 70 % ethanol, 90 % ethanol, and absolute ethanol for 2 min. After air-drying, the slides were placed in denaturation solution (70 % formamide, 2× SSC, 0.1 mM EDTA) at 75 °C for 5 min, followed by rinsing in 70 % ethanol, 90 % ethanol, and absolute ethanol for 2 min each. After denaturation at 75 °C for 5 min, three chromosome-specific probes (D17Z1, CEPX, and CEPY, Abbott Molecular Inc.) were hybridized to the nuclei at room temperature overnight. The slides were rinsed three times in WS-I (50 % formamide, 10 % 20× SSC, 40 % distilled water) at 45 °C for 5 min each, three times in WS-II (4× SSC/0.05 % Tween20) at room temperature for 5 min each, and once in WS-III (4× SSC), 70 % ethanol, 90 % ethanol, and absolute ethanol for 2 min each, followed by air-drying. The nuclei were counterstained with DAPI and then examined using an Olympus fluorescence microscope with DAPI, FITC, and Texas fluorescence filters.
G-banding analysis
hESCs were treated with 100 ng/ml demecolcine (Sigma, St. Louis, MO) for 4 h and dissociated with 0.05 % trypsin/EDTA (Invitrogen) into single cells for standard G-banding analysis. Typically, more than 20 metaphases were analyzed for every sample.
Statistics
SPSS10.0 was used to perform χ2 tests to analyze differences between groups. P < 0.05 was considered significant.
Results
A total of 261 3PN zygotes were microsurgically corrected, of which 208 zygotes survived (79.7 %) after manipulation and presented two distinct pronuclei. Of these zygotes, 94.2 % (196/208) cleaved. After 5–6 days of blastocyst culture, 28 microsurgically corrected 3PN zygotes developed to the blastocyst stage and 168 were arrested in the cleavage stage. A total of 772 intact 3PN zygotes were blastocyst cultured as the control group, of which 96.1 % (742/772) intact 3PN zygotes cleaved and 67 reached the blastocyst stage. The blastocyst formation rate of the microsurgically corrected 3PN zygotes (13.5 %, 28/208) was significantly higher (P < 0.05) than that of the control group (8.7 %, 67/772) (Table 1).
Table 1.
The developmental potential of microsurgically corrected 3PN zygotes (experimental) and intact 3PN zygotes (control)
| Number of zygotes cultured | Cleavage (%) | Blastocyst (%) | |
|---|---|---|---|
| Experimental group | 208 | 196 (94.2) | 28 (13.5)a |
| Control group | 772 | 742 (96.1) | 67 (8.7)a |
a P < 0.05
The chromosomal constitution of 103 arrested cleavage-stage embryos and 20 trophoblasts of the blastocysts that developed from microsurgically corrected 3PN zygotes were analyzed successfully by FISH (Table 2, Fig. 1). Based on the signal numbers of autosome chromosome 17 and sex chromosomes X and Y, six types of chromosomal constitution were observed in the arrested cleavage-stage embryos: 19 diploid (18.4 %), 6 triploid (5.8 %), 61 mosaic (59.2 %), 7 haploid (6.8 %), 9 aneuploid (8.7 %), and 1 tetraploid (1.0 %).
Table 2.
Chromosomal constitution of arrested cleavage-stage embryos and blastocysts from microsurgically corrected 3PN zygotes
| Arrested cleavage-stage embryo | Blastocyst | |
|---|---|---|
| n = 103 | n = 20 | |
| Diploid | 19 | 11 |
| 18.4 %a | 55.0 %a | |
| Abnormal karyotype | ||
| Tetraploid | 1 | 1 |
| 1.0 % | 5.0 % | |
| Triploid | 6 | 1 |
| 5.8 % | 5.0 % | |
| Mosaic | 61 | 6 |
| 59.2 %b | 30.0 %b | |
| Haploid | 7 | 0 |
| 6.8 % | 0 | |
| Aneuploid | ||
| 1717XO | 4 | 1 |
| 171717XO | 1 | 0 |
| 1717YY | 2 | 0 |
| 1717XYY | 1 | 0 |
| 17XY | 1 | 0 |
| 8.7 % | 5.0 % | |
a P < 0.05, b P < 0.05
Fig. 1.
a A cleavage-stage embryo developing from a microsurgically corrected 3PN zygote; b a blastocyst developing from a microsurgically corrected 3PN zygote; c FISH analysis of a diploid cleavage-stage embryo: two 17 chromosome signals (white), one X chromosome signal (green), and one Y chromosome signal (red) in each nucleus; d FISH analysis of the trophoblast of a diploid blastocyst: two 17 chromosome signals (white), one X chromosome signal (green) and one Y chromosome signal (red) in each nucleus
The diploid rate was 55.0 % (11/20) in the blastocysts that developed from microsurgically corrected 3PN zygotes. Four types of chromosomal abnormalities were observed: 1 triploid (5.0 %), 6 mosaic (30.0 %), 1 tetraploid (5.0 %), and 1 aneuploid (5.0 %). No haploid embryos were observed among the blastocysts. The diploid rate of the blastocysts (55.0 %) was significantly higher (P < 0.05) than that of the arrested cleavage-stage embryos (18.4 %), and the mosaic rate of the blastocysts (30.0 %) was significantly lower (P < 0.05) than that of the arrested cleavage-stage embryos (59.2 %).
A total of 195 intact 3PN zygotes were analyzed by FISH; of these, 90 arrested cleavage-stage embryos and 13 blastocysts were analyzed successfully. Seven types of chromosomal constitution were observed in the arrested cleavage-stage embryos: 13 diploid (14.4 %), 16 triploid (17.8 %), 46 mosaic (51.1 %), 6 haploid (6.7 %), 6 aneuploid (6.7 %), 2 pentaploid (2.2 %), and 1 tetraploid (1.1 %). Four types of chromosomal constitutions were observed in the blastocysts that developed from intact 3PN zygotes: 4 diploid (30.8 %), 4 triploid (30.8 %), 4 mosaic (30.8 %), and 1 tetraploid (7.7 %).
The triploid rate of the microsurgically corrected 3PN zygotes (5.7 %) was significantly lower (P < 0.01) than that of the intact 3PN zygotes (19.4 %) (Table 3).
Table 3.
Chromosomal constitution of embryos developing from microsurgically corrected 3PN zygotes and intact 3PN zygotes
| Microsurgically corrected 3PN zygotes | Intact 3PN zygotes | |
|---|---|---|
| n = 123 | n = 103 | |
| Diploid | 30 | 17 |
| 24.4 %a | 16.5 %a | |
| Abnormal karyotype | ||
| Pentaploid | 0 | 3 |
| 0 | 2.9 % | |
| Tetraploid | 2 | 1 |
| 1.6 % | 1.0 % | |
| Triploid | 7 | 20 |
| 5.7 %b | 19.4 %b | |
| Mosaic | 67 | 50 |
| 54.5 % | 48.5 % | |
| Haploid | 7 | 6 |
| 5.7 % | 5.8 % | |
| Aneuploid | ||
| 1717XO | 5 | 1 |
| 171717XO | 1 | 0 |
| 1717YY | 2 | 0 |
| 1717XYY | 1 | 0 |
| 17XX | 0 | 2 |
| 17XY | 1 | 2 |
| 17YY | 0 | 1 |
| 8.1 % | 5.8 % | |
aNS, b P < 0.01
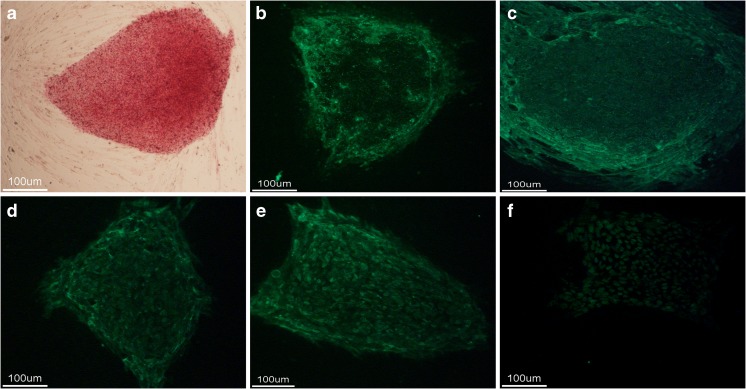
The ICMs of the 28 blastocysts that developed from microsurgically corrected 3PN zygotes were used to derive hESCs, and one hESC line was established successfully and named chHES-249. The stem cells stained positively for alkaline phosphatase (AKP), SSEA-3, SSEA-4, OCT-4, TRA-1–60, and TRA-1–81 (Fig. 2). RT-PCR analysis revealed high expression levels of pluripotency-related genes, including Oct4, Nanog, SOX2, TDGF1, TERF1, Thy’s-1, Rex1, LEFTB, and FGF4. High levels of telomerase activity were also observed (Fig. 3). An in vitro differentiation assay was performed to evaluate the pluripotency of chHES-249; after differentiation in vitro for 3 weeks, the derived cells immunostained positively for β-tubulin-III, SMA, and AFP (Fig. 4). G-banding analysis demonstrated that chHES-249 maintained a normal karyotype (46, XX) (Fig. 3). Furthermore, DNA fingerprinting analysis of 15 short tandem repeat (STR) loci indicated that chHES-249 was genetically heteroparental (Table 4).
Fig. 2.
Characterization of chHES-249 cells with hESC-specific markers. The undifferentiated hES cells stained positively for a AKP, b SSEA-3, c SSEA-4, d OCT-4, e TRA-1-60, and f TRA-1-81
Fig. 3.
a Telomerase activities of chHES-249 cells. 249, chHES-249 cell sample; 249H, heated chHES-249 cell sample. b RT-PCR analysis of the expression of pluripotency-related genes in undifferentiated ES cells: 1 Oct4, 2 Nanog, 3 SOX2, 4 TDGF1, 5 TERF1, 6 Thy-1, 7 Rex1, 8 LEFTB, 9 FGF4, and 10 β-actin. c G-banding analysis of chHES-249-P8 cells: 46, XX
Fig. 4.
In vitro differentiation of chHES-249 cells. After differentiation in vitro for 3 weeks, the derived cells immunostained positively for a β-tubulin-III, b SMA, and c AFP
Table 4.
DNA fingerprinting analysis of chHES-249
| D3S1358 | TH01 | D21S11 | D18S51 | Penta_E | D5S818 | D13S317 | D7S820 | |
| chHES-249 | 17 | 7,9 | 31,32.2 | 13 | 13,15 | 11 | 9,10 | 12,13 |
| Mother | 17 | 7,9 | 29,32.2 | 13,15 | 15,17 | 10,11 | 9,10 | 10,12 |
| Father | 15,17 | 7,9 | 29,31 | 13,21 | 11,13 | 11,12 | 10,11 | 10,13 |
| D16S539 | CSF1PO | Penta_D | vWA | D8S1179 | TPOX | FGA | Amelogenin | |
| chHES-249 | 12 | 10,12 | 9 | 18,19 | 11,14 | 9,11 | 22,24 | X,X |
| Mother | 10,12 | 10,12 | 9 | 18,19 | 12,14 | 9 | 22,24 | X,X |
| Father | 9,12 | 9,12 | 9,11 | 18,19 | 11 | 8,11 | 22.2,24 | X,Y |
Discussion
In this study, the blastocyst formation rate of the microsurgically corrected 3PN zygotes (13.5 %) was significantly higher than that of the control group (8.7 %). These results indicate that the developmental potential of 3PN zygotes can be improved by pronuclear removal of the surplus chromosome set. However, the diploid rate of the microsurgically corrected 3PN zygotes was similar to that of the control group. The normal development of an embryo requires not only a correct diploid genome but also a normal bipolar spindle, which allows the two chromosome sets to depart equally to two daughter blastomeres [16]; abnormal spindle formation will result in multiple chromosomal abnormalities in the blastomeres [1]. Therefore, in addition to restoring the diploid genome by removing the extra pronucleus, we attempted to remove the surplus centrosome by drawing off a small amount of cytoplasm around the extra pronucleus to facilitate the formation of a normal bipolar spindle [18, 20] and thus improve the developmental potential of the microsurgically corrected 3PN zygotes. Parlermo et al. demonstrated that the removal of the extra pronucleus does not correct the abnormal division of the zygote [16]. Gu et al. observed that the abnormal centrioles and abnormal patterns in the first mitosis are not corrected by pronuclear removal [19].
The triploid rate of intact 3PN zygotes ranges from 24.1 to 50 % [6, 29–31]. Escriba et al. analyzed three 3PN blastocysts by FISH and determined that they were all triploid [18]. Furthermore, Lin et al. derived 33 hESCs from intact 3PN zygotes and observed that 42.4 % of these hESC lines were triploid [28]. These studies indicate that the three chromosome sets in the 3PN zygotes can gather on the metaphase plate of the first division and divide regularly into two cells, resulting in complete triploid embryos [6]. The triploid embryos have good developmental potential because they can reach the blastocyst stage and establish hESC lines, consistent with the results of many clinical studies demonstrating that triploidy is one of the most common chromosomal abnormalities in human pregnancies, with an incidence rate of 1–3 % [10, 11]. Few triploid fetuses survive until birth [32]. Therefore, triploid embryos cannot be eliminated merely by blastocyst culture, and the pronuclear removal is essential to restore 3PN zygotes to a diploid state. In the present study, only 5.7 % of the embryos that developed from the microsurgically corrected 3PN zygotes were triploid, which was significantly lower than the proportion of triploid zygotes that developed from intact 3PN zygotes (19.4 %). This finding demonstrates that pronuclear removal is effective in excluding the surplus chromosome set from 3PN zygotes.
During blastocyst culture, the diploid rate of blastocysts from the microsurgically corrected 3PN zygotes increased to 55.0 %, significantly higher than the diploid rate of the arrested cleavage-stage embryos (18.4 %), and the mosaic rate of the blastocysts (30.0 %) was significantly lower than that of the arrested cleavage-stage embryos (59.2 %), although chromosomal abnormalities, such as mosaic, polyploidy, and aneuploidy, remained. These results indicate that blastocyst culture is an effective method for selecting against embryos with abnormal chromosomal constitution, consistent with previous studies that have observed that the developmental potential of chromosomal abnormal embryos to the morula and blastocyst stage is significantly lower than that of normal karyotypic embryos [33–37]. The possible mechanism of selection is apoptosis, a common mechanism by which human embryos eliminate abnormal cells [38]. Chromosomally abnormal embryos or cells probably undergo apoptosis, which leads to the developmental block against chromosome abnormal embryos at the compaction [36] or cavitation stage [39] and, consequently, results in an increased normal diploid rate among the blastocysts. These results demonstrate that blastocyst culture can be further used to identify corrected normal diploid embryos after diploidization of 3PN zygotes by pronuclear removal.
Previous studies in which diploid hESCs were established from microsurgically corrected 3PN zygotes employed G-banding analysis to examine karyotypes; this method cannot confirm that the diploid is heteroparental [40–42]. In this study, we first demonstrated that the karyotype of chHES-249 was heteroparental normal by DNA fingerprinting analysis. This result demonstrates that a 3PN zygote can be reliably corrected by removing the exact extra pronucleus and develop into a genetically normal embryo. Thus, microsurgically corrected blastocysts can be used as abundant materials for deriving hESC lines. This achievement has extensive applications in regenerative medicine and basic science research. Moreover, the normal blastocysts can be applied in clinical transfers in cycles in which there were no normal fertilized zygotes but 3PN zygotes were present. A case report described the birth of a healthy boy by transferring three corrected 3PN cleavage-stage embryos [20]. However, to eliminate possible mosaic, polyploid and aneuploid anomalies, preimplantation genetic diagnosis (PGD), and prenatal diagnosis are necessary.
In conclusion, this study demonstrates that pronuclear removal can effectively improve the developmental potential of 3PN zygotes. A combination of pronuclear removal and blastocyst culture can effectively be used to identify normal diploid embryos but cannot reliably eliminate mosaic, polyploid, and aneuploid anomalies. The microsurgically corrected blastocysts can be used to derive heteroparental normal karyotypic hESC lines, which will improve the efficiency of ART treatment.
Acknowledgments
This work was supported by grants from the Major State Basic Research Development Program of China (no. 2012CB944901) and the National Science Foundation of China (no. 81222007), and by the Program for New Century Excellent Talents in University.
Footnotes
Capsule
Pronuclear removal can effectively remove the surplus chromosome set of 3PN zygotes. A combination of pronuclear removal and blastocyst culture enables the selection of diploidized blastocysts from which heteroparental normal karyotypic hESC lines can be derived.
Hong-Qing Liao and Qi OuYang contributed equally to this work.
Contributor Information
Hong-Qing Liao, Phone: +86-734-8288086, Email: potlhq@gmail.com.
Ge Lin, Email: linge_csu@yahoo.com.
References
- 1.Pieters MH, Dumoulin JC, Ignoul-Vanvuchelen RC, Bras M, Evers JL, Geraedts JP. Triploidy after in vitro fertilization: cytogenetic analysis of human zygotes and embryos. J Assist Reprod Genet. 1992;9:68–76. doi: 10.1007/BF01204118. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbusch BE. Selective microsurgical removal of a pronucleus from tripronuclear human oocytes to restore diploidy: disregarded but valuable? Fertil Steril. 2009;92:897–903. doi: 10.1016/j.fertnstert.2008.07.1740. [DOI] [PubMed] [Google Scholar]
- 3.Porter R, Han T, Tucker MJ, Graham J, Liebermann J, Sills ES. Estimation of second polar body retention rate after conventional insemination and intracytoplasmic sperm injection: in vitro observations from more than 5000 human oocytes. J Assist Reprod Genet. 2003;20:371–6. doi: 10.1023/A:1025481011680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang HJ, Rosenwaks Z. Triploidy—the breakdown of monogamy between sperm and egg. Int J Dev Biol. 2008;52:449–54. doi: 10.1387/ijdb.082602hk. [DOI] [PubMed] [Google Scholar]
- 5.Plachot M, Crozet N. Fertilization abnormalities in human in-vitro fertilization. Hum Reprod. 1992;7(Suppl 1):89–94. doi: 10.1093/humrep/7.suppl_1.89. [DOI] [PubMed] [Google Scholar]
- 6.Staessen C, Van Steirteghem AC. The chromosomal constitution of embryos developing from abnormally fertilized oocytes after intracytoplasmic sperm injection and conventional in-vitro fertilization. Hum Reprod. 1997;12:321–7. doi: 10.1093/humrep/12.2.321. [DOI] [PubMed] [Google Scholar]
- 7.Grossmann M, Calafell JM, Brandy N, Vanrell JA, Rubio C, Pellicer A, et al. Origin of tripronucleate zygotes after intracytoplasmic sperm injection. Hum Reprod. 1997;12:2762–5. doi: 10.1093/humrep/12.12.2762. [DOI] [PubMed] [Google Scholar]
- 8.Golubovsky MD. Postzygotic diploidization of triploids as a source of unusual cases of mosaicism, chimerism and twinning. Hum Reprod. 2003;18:236–42. doi: 10.1093/humrep/deg060. [DOI] [PubMed] [Google Scholar]
- 9.Rosenbusch BE. Mechanisms giving rise to triploid zygotes during assisted reproduction. Fertil Steril. 2008;90:49–55. doi: 10.1016/j.fertnstert.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 10.Zaragoza MV, Surti U, Redline RW, Millie E, Chakravarti A, Hassold TJ. Parental origin and phenotype of triploidy in spontaneous abortions: predominance of diandry and association with the partial hydatidiform mole. Am J Hum Genet. 2000;66:1807–20. doi: 10.1086/302951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McFadden DE, Robinson WP. Phenotype of triploid embryos. J Med Genet. 2006;43:609–12. doi: 10.1136/jmg.2005.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berkowitz RS, Goldstein DP. Clinical practice molar pregnancy. N Engl J Med. 2009;360:1639–45. doi: 10.1056/NEJMcp0900696. [DOI] [PubMed] [Google Scholar]
- 13.Rawlins RG, Binor Z, Radwanska E, Dmowski WP. Microsurgical enucleation of tripronuclear human zygotes. Fertil Steril. 1988;50:266–72. doi: 10.1016/s0015-0282(16)60071-7. [DOI] [PubMed] [Google Scholar]
- 14.Gordon JW, Grunfeld L, Garrisi GJ, Navot D, Laufer N. Successful microsurgical removal of a pronucleus from tripronuclear human zygotes. Fertil Steril. 1989;52:367–72. doi: 10.1016/s0015-0282(16)60901-9. [DOI] [PubMed] [Google Scholar]
- 15.Malter HE, Cohen J. Embryonic development after microsurgical repair of polyspermic human zygotes. Fertil Steril. 1989;52:373–80. doi: 10.1016/s0015-0282(16)60902-0. [DOI] [PubMed] [Google Scholar]
- 16.Palermo G, Munne S, Cohen J. The human zygote inherits its mitotic potential from the male gamete. Hum Reprod. 1994;9:1220–5. doi: 10.1093/oxfordjournals.humrep.a138682. [DOI] [PubMed] [Google Scholar]
- 17.Ivakhnenko V, Cieslak J, Verlinsky Y. A microsurgical technique for enucleation of multipronuclear human zygotes. Hum Reprod. 2000;15:911–6. doi: 10.1093/humrep/15.4.911. [DOI] [PubMed] [Google Scholar]
- 18.Escriba MJ, Martin J, Rubio C, Valbuena D, Remohi J, Pellicer A, et al. Heteroparental blastocyst production from microsurgically corrected tripronucleated human embryos. Fertil Steril. 2006;86:1601–7. doi: 10.1016/j.fertnstert.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 19.Gu Y-F, Lin G, Lu C-F, Lu G-X. Analysis of the first mitotic spindles in human in vitro fertilized tripronuclear zygotes after pronuclear removal. Reprod BioMed Online. 2009;19:745–54. doi: 10.1016/j.rbmo.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Kattera S, Chen C. Normal birth after microsurgical enucleation of tripronuclear human zygotes: case report. Hum Reprod. 2003;18:1319–22. doi: 10.1093/humrep/deg262. [DOI] [PubMed] [Google Scholar]
- 21.Gardner DK, Schoolcraft WB. No longer neglected: the human blastocyst. Hum Reprod. 1998;13:3289–92. doi: 10.1093/oxfordjournals.humrep.a019677. [DOI] [PubMed] [Google Scholar]
- 22.Jones GM, Trounson AO, Gardner DK, Kausche A, Lolatgis N, Wood C. Evolution of a culture protocol for successful blastocyst development and pregnancy. Hum Reprod. 1998;13:169–77. doi: 10.1093/humrep/13.1.169. [DOI] [PubMed] [Google Scholar]
- 23.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 24.Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 25.Mitalipova M, Calhoun J, Shin S, Wininger D, Schulz T, Noggle S, et al. Human embryonic stem cell lines derived from discarded embryos. Stem Cells. 2003;21:521–6. doi: 10.1634/stemcells.21-5-521. [DOI] [PubMed] [Google Scholar]
- 26.Lavon N, Narwani K, Golan-Lev T, Buehler N, Hill D, Benvenisty N. Derivation of euploid human embryonic stem cells from aneuploid embryos. Stem Cells. 2008;26:1874–82. doi: 10.1634/stemcells.2008-0156. [DOI] [PubMed] [Google Scholar]
- 27.Lin G, OuYang Q, Zhou XY, Gu YF, Yuan D, Li W, et al. A highly homozygous and parthenogenetic human embryonic stem cell line derived from a one-pronuclear oocyte following in vitro fertilization procedure. Cell Res. 2007;17:999–1007. doi: 10.1038/cr.2007.97. [DOI] [PubMed] [Google Scholar]
- 28.Lin G, Xie Y, OuYang Q, Qian X, Xie P, Zhou X, et al. HLA-matching potential of an established human embryonic stem cell bank in China. Cell Stem Cell. 2009;5:461–5. doi: 10.1016/j.stem.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Angell RR, Templeton AA, Messinis IE. Consequences of polyspermy in man. Cytogenet Cell Genet. 1986;42:1–7. doi: 10.1159/000132242. [DOI] [PubMed] [Google Scholar]
- 30.Kola I, Trounson A, Dawson G, Rogers P. Tripronuclear human oocytes: altered cleavage patterns and subsequent karyotypic analysis of embryos. Biol Reprod. 1987;37:395–401. doi: 10.1095/biolreprod37.2.395. [DOI] [PubMed] [Google Scholar]
- 31.Rosenbusch B, Schneider M, Sterzik K. The chromosomal constitution of multipronuclear zygotes resulting from in-vitro fertilization. Hum Reprod. 1997;12:2257–62. doi: 10.1093/humrep/12.10.2257. [DOI] [PubMed] [Google Scholar]
- 32.Iliopoulos D, Vassiliou G, Sekerli E, Sidiropoulou V, Tsiga A, Dimopoulou D, et al. Long survival in a 69, XXX triploid infant in Greece. Genet Mol Res. 2005;4:755–9. [PubMed] [Google Scholar]
- 33.Clouston HJ, Fenwick J, Webb AL, Herbert M, Murdoch A, Wolstenholme J. Detection of mosaic and non-mosaic chromosome abnormalities in 6- to 8-day old human blastocysts. Hum Genet. 1997;101:30–6. doi: 10.1007/s004390050581. [DOI] [PubMed] [Google Scholar]
- 34.Evsikov S, Verlinsky Y. Mosaicism in the inner cell mass of human blastocysts. Hum Reprod. 1998;13:3151–5. doi: 10.1093/humrep/13.11.3151. [DOI] [PubMed] [Google Scholar]
- 35.Magli MC, Jones GM, Gras L, Gianaroli L, Korman I, Trounson AO. Chromosome mosaicism in day 3 aneuploid embryos that develop to morphologically normal blastocysts in vitro. Hum Reprod. 2000;15:1781–6. doi: 10.1093/humrep/15.8.1781. [DOI] [PubMed] [Google Scholar]
- 36.Sandalinas M, Sadowy S, Alikani M, Calderon G, Cohen J, Munne S. Developmental ability of chromosomally abnormal human embryos to develop to the blastocyst stage. Hum Reprod. 2001;16:1954–8. doi: 10.1093/humrep/16.9.1954. [DOI] [PubMed] [Google Scholar]
- 37.Liao H, Zhang S, Cheng D, Ouyang Q, Lin G, Gu Y, et al. Cytogenetic analysis of human embryos and embryonic stem cells derived from monopronuclear zygotes. J Assist Reprod Genet. 2009;26:583–9. doi: 10.1007/s10815-009-9355-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hardy K. Cell death in the mammalian blastocyst. Mol Hum Reprod. 1997;3:919–25. doi: 10.1093/molehr/3.10.919. [DOI] [PubMed] [Google Scholar]
- 39.Janny L, Menezo YJ. Maternal age effect on early human embryonic development and blastocyst formation. Mol Reprod Dev. 1996;45:31–7. doi: 10.1002/(SICI)1098-2795(199609)45:1<31::AID-MRD4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 40.Fan Y, Li R, Huang J, Yu Y, Qiao J. Diploid, but not haploid, human embryonic stem cells can be derived from microsurgically repaired tripronuclear human zygotes. Cell Cycle. 2013;12:302–11. doi: 10.4161/cc.23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang C, Cai L, Huang B, Dong J, Chen A, Ning S, et al. Normal human embryonic stem cell lines were derived from microsurgical enucleated tripronuclear zygotes. J Cell Biochem. 2013;114:2016–23. doi: 10.1002/jcb.24547. [DOI] [PubMed] [Google Scholar]
- 42.Fan Y, Li R, Huang J, Zhao HC, Ding T, Sun X, et al. Improved efficiency of microsurgical enucleated tripronuclear zygotes development and embryonic stem cell derivation by supplementing epidermal growth factor, brain-derived neurotrophic factor, and insulin-like growth factor-1. Stem Cells Dev. 2014;23:563–75. doi: 10.1089/scd.2013.0420. [DOI] [PMC free article] [PubMed] [Google Scholar]