Abstract
Purpose
In in vitro maturation (IVM) cycles primed with human chorionic gonadotropin (hCG), both immature and mature oocytes are retrieved from antral follicles sized 8–12 mm. Using time-lapse microscopy, we compared the morphokinetic behavior of embryos developed from oocytes matured in vivo and in vitro, testing the hypothesis that IVM affects preimplantation development. Furthermore, we extended the morphokinetic analysis of these embryos by a comparison with embryos obtained in stimulated assisted reproduction technology (ART) cycles.
Methods
In IVM cycles primed with follicle-stimulating hormone (FSH)/hCG, prior to sperm microinjection, oocytes surrounded by an expanded cumulus at retrieval and presumably mature (EC-MII) were incubated for 6 h, while immature oocytes enclosed in a compact cumulus (CC) were matured in vitro for 30 h. The morphokinetics of embryos selected for transfer or cryopreservation, derived from EC-MII and CC oocytes, were comparatively and retrospectively analyzed in terms of cleavage times (t2, t3, t4, t5, and t8) and intervals (cc2, cc3, s2, s3). For further comparison, the morphokinetics of embryos selected for transfer or cryopreservation (ICSI) or giving rise to ongoing pregnancies (model) in stimulated ART cycles was also assessed.
Results
The morphokinetic behavior of EC-MII and CC embryos was entirely comparable, as suggested by the absence of statistical differences in the averages of all cleavage times and intervals. Almost all cleavage and interval times were also similar between EC-MII, CC, ICSI, and model groups, with the exception of t4 and s2, which were delayed and longer, respectively, in embryos generated in IVM cycles (EC-MII and CC).
Conclusions
These findings do not support the hypothesis that maturation in vitro affects embryo morphokinetics, while they suggest only marginal differences in the morphokinetics of embryos developed from oocytes matured in vivo and in vitro in IVM cycles and embryos developed from mature oocytes recovered in stimulated cycles.
Keywords: Oocytes, In vitro maturation, Embryos, Time-lapse microscopy, Preimplantation development
Introduction
Oocyte in vitro maturation (IVM) is an alternative approach to assisted reproduction technology (ART) treatment in which no or minimal doses of follicle-stimulating hormone (FSH) are administered and oocytes are retrieved from medium-sized (6–12 mm) follicles. Under such conditions, oocytes are recovered while still arrested at the meiotic prophase and cultured for 30–32 h to allow meiotic resumption and progression to the metaphase II (MII) stage [1]. The clinical efficiency of IVM is constrained by various factors, not least the fact that the rate of maturation in vitro is only approximately 50 % [2]. For such a reason, to facilitate the initiation of oocyte maturation in vivo, IVM cycles may include the administration of human chorionic gonadotropin (hCG) before the leading follicle exceeds the size of 12 mm [3]. In this fashion, a fraction of oocytes not only initiates but also completes the maturation process in vivo [4–6]. For such a reason, it is intensely debated whether hCG-primed cycles should be technically considered as a genuine IVM approach.
Doubts have been raised on the developmental competence of oocytes derived from IVM cycles. In fact, it is plausible that in vitro maturation, which is unable to precisely reproduce in vivo conditions, can affect the fidelity of the process of chromosome segregation or crucial cytoplasmic changes occurring during the transition from the germinal vesicle (GV) to the MII stage [7, 8], with possible deleterious consequences for preimplantation and postimplantation development [9]. In addition, it cannot be ruled out a priori that also in vivo matured oocytes collected in IVM cycles primed with hCG are developmentally compromised, considering that they derive from medium-sized, but not large or preovulatory, follicles [10–12]. Full characterization of the developmental ability of immature and mature oocytes obtained in IVM cycles is therefore a priority in order to better determine the possible margins of improvement of IVM as a clinical treatment.
The ultimate proof of oocyte developmental potential is clearly its ability to establish a viable pregnancy. While universally valid, nevertheless this concept is difficult to apply to the aim of establishing the competence of IVM oocytes relative to those collected in stimulated ART cycles.
Analysis of embryo development in vitro can offer an answer to the question of the developmental competence of oocytes collected in IVM cycles, being independent from possible endometrial factors.
For decades, embryo developmental competence has been classically evaluated by static morphological criteria (number of blastomeres, proportion of anucleated fragments, multinucleation, etc.) elaborated at few isolated time points. Adopting this approach, only limited information concerning the quality of embryos produced in IVM cycles has been reported [13]. Therefore, more evidence is needed on embryo development in vitro in IVM cycles.
The relatively recent introduction of time-lapse microscopy in the IVF laboratory offers novel opportunities to assess embryo development. In fact, sequential observation at short intervals (10–15 min) has allowed the identification of temporal parameters, such as cleavage times and intervals, that are able to predict blastocyst development and quality as well as embryo implantation [14–16].
Hence, by time-lapse microscopy, in this study, we pursued the goal of comparing the morphokinetic behavior of embryos, selected for transfer or cryopreservation, developed from oocytes matured in vivo and in vitro in IVM cycles. Furthermore, we compared the morphokinetics of embryos derived from in vivo and in vitro matured oocytes with those generated from stimulated ART cycles, to better assess the possible morphokinetic differences between embryos generated in different treatment approaches.
Materials and methods
This study is based on retrospective data derived from ART treatments performed at Biogenesi, Reproductive Medicine Centre, Monza, Italy. Couples included in the analysis had an indication for an IVF procedure because of infertility due to male factor, tubal factor, or polycystic ovary without chronic anovulation. Approval was obtained by the competent ethical committee. Written informed consent was obtained from all couples before starting treatment.
Study design
Data were analyzed in order to compare the morphokinetics of embryos developed from oocytes matured in vivo or in vitro in IVM cycles primed with hCG. Moreover, the morphokinetics of these two groups of embryos was further analyzed in comparison with two alternative groups: (a) embryos obtained from conventional ICSI cycles selected for transfer or cryopreservation (ICSI group), and (b) embryos obtained from conventional ICSI cycles that gave rise to ongoing pregnancies (model group). Embryos generated in standard IVF cycles were not used for comparison because it is known that the insemination technique can influence embryo morphokinetics [15, 17].
IVM cycles
Patient inclusion and exclusion criteria
Patients were selected for IVM treatment according to previously described criteria [18–20]. Women presenting with chronic anovulation associated with polycystic ovary syndrome (PCOS), poor ovarian reserve, or endometriosis as a cause of infertility were excluded, as were those suffering from very severe male factor (less than 0.1 × 106 spermatozoa/ml after semen preparation) or azoospermia.
Cycle preparation
IVM cycles were carried out with a 3-day gonadotropin priming by using 150 IU recombinant follicle-stimulating hormone (rFSH; MSD, Milano, Italy) from the third day of cycle, as previously reported [3, 21]. Transvaginal ultrasonography was initially performed by day 3 of the menstrual cycle in order to assess the presence of ovarian cysts, endometrial thickness, and antral follicle count. Further ultrasound examinations were planned after the 3-day FSH administration, until a 10–12-mm leading follicle was measured. At such stage, a trigger with human chorionic gonadotropin (hCG) 10,000 IU (MSD, Milano, Italy) was administered subcutaneously and oocyte retrieval was scheduled 38 h later [4–6, 22].
Oocyte handling
Retrieved cumulus cell–oocyte complexes (COCs) were examined and classified according to cumulus oophorus morphology and stage of oocyte maturation as previously illustrated [7–9]. On such basis, COCs were classified as having an expanded cumulus (EC) or a compact cumulus (CC).
COCs were transferred to petri dishes containing 0.5 ml of IVM medium (Origio, Måløv, Denmark) supplemented with rFSH 0.075 IU/ml (MSD, Italy), hCG 0.10 IU/ml (MSD, Italy), and 10 % synthetic serum (Irvine, USA).
EC COCs, presumed to enclose mature oocytes, were cultured at 37 °C in a 6 % CO2 humidified atmosphere for 6 h and then treated with culture medium containing 80 IU/ml cumulase (ICSI Cumulase®, Origio, Måløv, Denmark). Metaphase II stage oocytes (EC-MII, representing 60 % of all cultured EC COCs) isolated by such EC COCs, were directly microinjected.
CC COCs, most likely enclosing an immature GV-stage oocyte, were kept in the maturation medium for 30 h. Afterward, they were treated with culture medium containing 80 IU/ml cumulase (ICSI Cumulase®, Origio, Måløv, Denmark) to remove cumulus cells. Denuded oocytes showing the first polar body and therefore matured in vitro (representing 48 % of all cultured CC COCs) were microinjected (CC).
Semen preparation
Semen samples were prepared by discontinuous gradients (47.5 and 90 %) of Sil-Select (Sil-Select Stock™, Ferti-Pro, Belgium), and spermatozoa were washed and re-suspended in Universal IVF Medium (Universal IVF Medium, Origio, Måløv, Denmark) and stored in an incubator at 37 °C in a 6 % CO2 humidified atmosphere until use [9, 23].
Embryo culture
After ICSI, microinjected oocytes were moved to Embryoslide™ Culture slides (Unisense, Aarhus, Denmark), a specific 12-well plate prepared with a 30-μl microdrop of medium covered in paraffin oil. The plates were equilibrated overnight before use. Prezygotes and embryos up to 48 h were cultured in ISM1 (ISM1™, Origio, Måløv, Denmark). Afterward, they were cultured in Blast Assist (BlastAssist®, Origio, Måløv, Denmark) until embryo transfer or cryopreservation. In all cases, a N2/CO2/O2 (89:6:5, v/v) atmosphere without humidity control and 37 °C temperature were adopted as culture conditions. Embryos were cultured and analyzed for morphokinetic parameters in an integrated embryo culture time-lapse microscopy system, i.e., the EmbryoScope™ Time-lapse System (Unisense, Aarhus, Denmark).
Acquisition of morphokinetic data
Image acquisition was set every 15 min at seven different focal planes for each embryo. Images (1280 × 1024 pixels) were acquired by a Leica 20 × 0.40 LWD Hoffman Modulation contrast objective specialized for 635-nm illumination. Illumination for image acquisition was <0.5 s per image, using single 1-W red LED. Only two embryologists were in charge of the annotation of embryo parameters, in order to reduce bias due to inter-operator differences. Embryos were classified according to morphological parameters, such as fragmentation and blastomere shape and number [10–12, 24]. Embryos showing grossly abnormal cleavage behaviors, such as direct cleavage from one to three cells, were not analyzed, being the study object the dynamics of cleavage through well-defined stages.
Morphokinetic parameters
The time of the oocyte microinjection was considered as the starting time for assessing embryo kinetics. Cleavage times (t) corresponding to the two-, three-, four-, five-, and eight-cell stages (i.e., t2, t3, t4, t5, and t8) were annotated for each embryo. Cell cycle duration was calculated considering the time elapsed between the formation of the first blastomere of a cell cycle (cc) and the formation of the first blastomere of the following cycle (cc2 = t3 − t2 and cc3 = t5 − t3). Cell cycle synchrony (s) was measured as the time for transition from the first to the last blastomere stages of a cell cycle (s2 = t4 − t3 and s3 = t8 − t5). Morphokinetic annotations were not blinded, therefore representing a study limitation.
ICSI and model groups
The couples included in the ICSI and model groups were attentively matched with the IVM cases for age, ovarian reserve, causes of infertility, number of previous procedures, and semen characteristics. In both groups, pituitary downregulation, gonadotropin stimulation, and hCG trigger were carried out according to women’s characteristics as described elsewhere [13, 23]. Methods of microinjection, embryo culture, and generation morphokinetic data were the same as those applied in the IVM group. Ongoing pregnancies were defined as those pregnancies reaching the 12th week of gestation.
Statistical analysis
Mean age, cleavage times, and intervals were statistically compared by the Student’s t test or non-parametric sum rank test, depending on assessment of the normality of data distribution. Categorical/dichotomic variables were analyzed by chi-square test. Stata 9.0 software was used for performing the statistical analysis (1999, Stata Corporation, Texas, USA). A level of P < 0.05 was adopted for significance.
Results
Two hundred embryos were analyzed in terms of cleavage times (t2, t3, t4, t5, and t8) and intervals (cc2–cc3, s2–s3). Embryos were divided into two groups: in vivo matured (expanded cumulus mass at retrieval, EC-MII; n = 149; 39 cycles, 38 patients) and in vitro matured (compact cumulus at retrieval, CC; n = 51; 80 cycles, 74 patients).
As a control group, 365 embryos (obtained from 158 cycles, 146 patients) transferred or cryopreserved in ICSI cycles were considered. To further comparatively assess the developmental dynamics of EC-MII and CC embryos, the morphokinetic analysis was extended to 65 embryos (obtained from 44 cycles, 44 patients), referred to as model group, that gave rise to viable pregnancies in ICSI cycles.
In all groups, the average age was 33.0 ± 3.0 years. Other demographic parameters, such as antimüllerian hormone (AMH), antral follicle count (AFC), and FSH, were also comparable among groups (data not shown) and consistent with selection criteria identified for IVM treatment by our group in previous studies [18, 19].
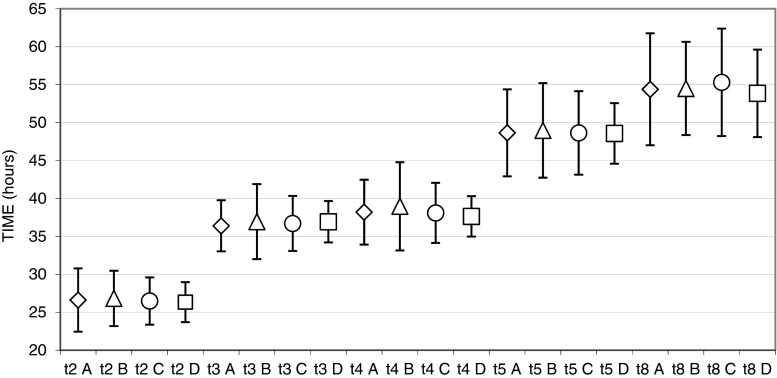
The morphokinetic behavior of EC-MII and CC embryos was entirely comparable, as suggested by the absence of statistical differences in the averages of all cleavage times and intervals (please see Fig. 1 for cleavage times and Table 1 for time intervals).
Fig. 1.
Graphical representation of cleavage times (t2, t3, t4, t5, t8) of EC-MII (A, diamond), CC (B, triangle), ICSI (C, circle), and model (D, square) groups. Values are mean ± SD times (hours) from microinjection (t4 A vs. t4 C, P = 0.026; t4 A vs. t4 D, P = 0.039)
Table 1.
Comparison of time intervals (cc2, cc3, s2, s3) of EC-MII, CC, ICSI, and model groups
| Interval | EC-MII (n = 149) | CC (n = 51) | ICSI (n = 365) | Model (n = 65) |
|---|---|---|---|---|
| cc2 | 10.2 ± 2.8 (0.5–18.6) |
10.2 ± 3.0 (0.8–14.3) |
10.3 ± 2.1 (1.3–16.3) |
10.6 ± 1.3 (4.8–13.1) |
| cc3 | 12.5 ± 3.1 (0.5–20.9) |
12.3 ± 4.2 (2.7–21.2) |
12.1 ± 3.4 (0.5–28.2) |
11.8 ± 2.4 (1.0–15.9) |
| s2 | 2.0 ± 3.5 a,b
(0.2–18.7) |
2.0 ± 3.2 c
(0.3–15.1) |
1.4 ± 2.3 b
(0.2–16.1) |
0.7 ± 0.6 b,c
(0.3–3.0) |
| s3 | 6.5 ± 6.1 (0.7–28.7) |
7.1 ± 6.2 (0.8–18.3) |
6.9 ± 6.2 (0.8–28.0) |
5.4 ± 5.1 (0.8–21.3) |
Superscripts indicate statistically significant differences. Values are mean ± SD (range) times in hours
a P = 0.009; b P = 0.001; c P = 0.001
Comparison of the EC-MII and CC groups with the ICSI and model groups is also shown in Fig. 1 and Table 1. The large majority of cleavage and interval times were similar between the EC-MII and ICSI groups and between the EC-MII and model groups. Exceptions were for t4, whose values were 39.0 ± 5.8 h (EC-MII), 38.1 ± 4.0 h (ICSI), and 37.6 ± 2.7 h (model) (EC-MII vs. ICSI, P = 0.026; EC-MII vs. model, P = 0.039), and for s2, whose times were 2.0 ± 3.5 h (EC-MII), 1.4 ± 2.3 h (ICSI), and 0.7 ± 0.6 h (model) (EC-MII vs. ICSI, P = 0.009; EC-MII vs. model, P = 0.001).
The only statistically different parameter between CC and model groups was s2 (2.0 ± 3.2 and 0.7 ± 0.6 h, P = 0.001). It should be noted that most of the above differences emerged only after paired comparison, while analysis of variance indicated only a difference in s2 among groups (data not shown). Also, as a general note of caution relevant to the above morphokinetic analysis, we estimated that power calculation might limit the significance of the comparison of t8 between ICSI and model (statistical power = 40.9 %) and t3 among all groups (minimal statistical power = 27.6 %).
Abnormal direct cleavage (i.e., division of the fertilized egg into three blastomeres within a cleavage time to t3 of less than 5 h) was also assessed. The proportions of embryos displaying such a behavior were 9.1 % (15/165), 11.9 % (7/59), and 7.8 (31/398) in the EC-MII, CC, and ICSI groups, respectively (P = not significant (NS)). In the same groups, the rate of cleaved embryos was very high, i.e., 99.4 % (164/165), 98.3 % (58/59), and 99.5 % (396/398), respectively (P = NS). Embryo fragmentation was also assessed. On day 2, the percentages of embryo exhibiting less than 20 % of their volume fragmented were 90.6 % (135/149), 88.2 % (45/51), and 92.6 (338/365) in the EC-MII, CC, and ICSI groups, respectively. On day 3, such proportions were 91.1 % (123/135), 96.5 % (55/57), and 93.9 % (303/329), respectively (P = NS). Data on abnormal direct cleavage, embryo arrest, and fragmentation were not applicable and therefore not generated for the model group.
Discussion
With unprecedented precision gained through time-lapse microscopy, in this study, we compared the morphokinetic behavior of embryos developed from immature and mature oocytes retrieved from antral follicles sized 8–12 mm, in IVM cycles primed with hCG, testing the hypothesis that IVM affects preimplantation development.
In hCG-primed IVM cycles, a fraction of retrieved oocytes, amounting to approximately 20 %, is found mature at retrieval [2, 25].
Embryos developed from such in vivo matured oocyte implant with higher frequency in comparison to embryos derived from genuinely in vitro matured oocytes in cycles without hCG priming [9]. Therefore, concerns have been raised on the possibility that in vitro matured oocytes may be developmentally compromised. However, it is disputable to conclude that IVM compromises oocyte developmental ability, because exposure to hCG not only triggers the processes of oocyte maturation and ovulation but also positively influences endometrial receptivity.
Our morphokinetic analysis is not consistent with the assumption that oocyte IVM impacts early embryo development. This conclusion is in striking contrast with the widespread belief that suboptimal performance of IVM cycles can be explained by developmental anomalies generated by inadequate conditions under which maturation occurs in vitro. In addition, our data are in agreement with a recent study in which oocytes matured in vitro in IVM cycles developed into embryos with normal morphokinetic behavior [26]. Our study did not include morphokinetic parameters that were found to be associated to blastocyst development and embryo viability, such as continued observation of multinucleation and reverse cleavage [27]. Therefore, our data represent a useful set of observations that, however, require confirmation through analysis of a more complete array of morphokinetic parameters.
To extend our analysis of embryos generated in IVM cycles (EC-MII and CC), we adopted comparatively two reference groups, i.e., embryos selected for transfer or preservation (ICSI) or that gave rise to ongoing pregnancies (model) in stimulated cycles. The model group represents a true known implantation data (KID) population. On the contrary, the ICSI group does not respond to the KID criteria, representing as such a limitation of the study.
Overall, such comparisons indicated that the morphokinetics of embryos produced in IVM cycles is only moderately different from that of embryos generated in stimulated cycles. In particular, in both IVM groups, the cleavage synchrony of blastomeres at the two-cell stage (s2, t4 − t3) was reduced and in the EC-MII the t4 timing was delayed in comparison to the morphokinetics of embryos of stimulated cycles giving rise to an ongoing pregnancy. The same isolated difference in the s2 interval was observed between the ICSI and model groups (data not shown), suggesting again that IVM and ICSI embryos have a remarkably similar morphokinetic behavior. As mentioned above, longer s2 intervals (i.e., reduced synchrony) have been hypothesized to be associated to reduced implantation rates [13]. It was also observed that time intervals and relative ratios defining synchronicity are highly predictive of blastocyst development and quality [28]. Therefore, the difference in s2 emerged in our study from the comparison of embryos generated in IVM and stimulated cycles should not be overlooked but rather further investigated. Doubts remain on the reason why delayed cleavage times and extended cleavage intervals express differences in implantation ability, also in consideration of a clear lack of association between embryo morphokinetics and embryo aneuploidy [29]. It is plausible that such a difference reflects an inherently different implantation ability between oocytes collected in IVM and ICSI cycles, for the reason that the former are retrieved from follicles of smaller size (6–12 mm). However, it may be interesting to note that in a recently published study, we observed comparable implantation rates between embryos developed from in vivo matured oocytes in IVM and ICSI cycles [30].
Assessment of embryo performance in vitro can provide at least a partial answer to the question of the competence of in vitro matured oocytes, being several embryo morphological parameters associated to developmental ability, even when measured at isolated time points [24]. Previous studies generated only marginal and imprecise information on possible differences in the development of embryos derived from in vivo and in vitro matured oocytes in IVM cycles. In particular, Son and colleagues reported that in vivo matured oocytes produce a higher proportion of good quality embryos [13]. However, the criteria of “good quality” adopted by these authors are rather disputable, including characteristics such as “at least a 3-cell embryo on day 2 and a 6-cell embryo on day 3,” in contrast with an established consensus on embryo morphological evaluation [24]. In a recent study, we investigated the morphological quality and implantation potential of embryos developed from EC-MII in hCG-primed IVM cycles, but comparative data are presently lacking [30]. Introduction of time-lapse microscopy equipment among the options of the IVF laboratory has opened new horizons for the study of human preimplantation development in vitro. Continued observation at short intervals has allowed the observation of previously unrecognized phenomena, and above all the determination of morphokinetic parameters able to predict embryo development [31]. In particular, absolute times of cleavage (t2 to t5, t8), duration of cell cycles (cc2, t3 − t2), and synchrony of blastomere cleavage (s2, t4-t3; s3, t8-t5) have been identified as parameters associated with the ability to develop to the blastocyst stage [15, 32], while some of such parameters (t5, t8, cc2, s2) were also found to predict implantation [15, 32].
Interestingly, from a clinical standpoint, our suggestion that IVM does not compromise embryo development in vitro prompts the interesting consideration that a reduced efficiency of IVM treatments may be caused by factors not related to oocyte quality, such as endometrial receptivity. Consistent with this, embryos produced in IVM cycles implant with a higher rate after cryopreservation and transfer in frozen embryo replacement cycles [33].
Overall, based on precise annotation of temporal parameters made possible by time-lapse microscopy, the present data argue against the notion that maturation in vitro affects embryo morphokinetics.
In addition, our findings suggest that embryo morphokinetics is largely comparable between embryos derived from oocytes collected from antral or preovulatory follicles. These conclusions will require independent confirmation from larger studies, but nevertheless, they suggest attractive reflections about in vitro maturation and the relationship between oocyte competence and follicle development. Lack of data on blastocyst development in vitro and on implantation limits the significance of our study. Therefore, we suggest that future research directions should include such aspects.
Authors’ contributions
MDC was responsible for the design, database elaboration, and critical reading; PVN for the embryology, morphokinetic annotation, database elaboration, and critical reading; GC for the design, data assessment, critical reading and manuscript writing; FB for the embryology and morphokinetic annotation; CB for the clinical work; MMR for the design and clinical work; EDP for the statistical analysis; and RF for the design and critical reading.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Capsule These findings do not support the hypothesis that maturation in vitro affects embryo morphokinetics, while they suggest only marginal differences in the morphokinetics of embryos developed from oocytes matured in vivo and in vitro in IVM cycles and embryos developed from mature oocytes recovered in stimulated cycles.
Contributor Information
Mariabeatrice Dal Canto, Email: dalcanto.biogenesi@grupposandonato.it.
Paola V. Novara, Email: pvnovara@gmail.com
Giovanni Coticchio, Phone: +39 039 8383314, Email: coticchio.biogenesi@grupposandonato.it.
Mario Mignini Renzini, Email: mariomigninirenzini@centrosofia.it.
Fausta Brambillasca, Email: fbrambillasca@hotmail.com.
Claudio Brigante, Email: claudio.brigante@grupposandonato.it.
Elena De Ponti, Email: e.deponti@hsgerardo.org.
Rubens Fadini, Email: rfadini@libero.it.
References
- 1.Smith SD, Mikkelsen A, Lindenberg S. Development of human oocytes matured in vitro for 28 or 36 hours. FNS. 2000;73(3):541–4. doi: 10.1016/s0015-0282(99)00574-9. [DOI] [PubMed] [Google Scholar]
- 2.Fadini R, Dal Canto MB, Mignini Renzini M, Brambillasca F, Comi R, Fumagalli D, et al. Effect of different gonadotrophin priming on IVM of oocytes from women with normal ovaries: a prospective randomized study. Reprod Biomed Online Reproductive Healthcare Ltd. 2008;19(3):343–51. doi: 10.1016/S1472-6483(10)60168-X. [DOI] [PubMed] [Google Scholar]
- 3.Chian RC, Buckett WM, Tulandi T, Tan SL. Prospective randomized study of human chorionic gonadotrophin priming before immature oocyte retrieval from unstimulated women with polycystic ovarian syndrome. Hum Reprod. 2000;15(1):165–70. doi: 10.1093/humrep/15.1.165. [DOI] [PubMed] [Google Scholar]
- 4.Son WY, Tan SL. Laboratory and embryological aspects of hCG-primed in vitro maturation cycles for patients with polycystic ovaries. Hum Reprod Update. 2010;16(6):675–89. doi: 10.1093/humupd/dmq014. [DOI] [PubMed] [Google Scholar]
- 5.Fadini R, Dal Canto MB, Mignini Renzini M, Brambillasca F, Comi R, Fumagalli D, et al. Effect of different gonadotrophin priming on IVM of oocytes from women with normal ovaries: a prospective randomized study. Reprod Biomed Online. 2009;19(3):343–51. doi: 10.1016/S1472-6483(10)60168-X. [DOI] [PubMed] [Google Scholar]
- 6.Fadini R, Mignini Renzini M, Dal Canto M, Epis A, Crippa M, Caliari I, et al. Oocyte in vitro maturation in normo-ovulatory women. Fertil Steril. 2013;99(5):1162–9. doi: 10.1016/j.fertnstert.2013.01.138. [DOI] [PubMed] [Google Scholar]
- 7.Coticchio G, Dal Canto M, Guglielmo M-C, Mignini Renzini M, Fadini R. Human oocyte maturation in vitro. Int J Dev Biol. 2012;56(10–12):909–18. doi: 10.1387/ijdb.120135gv. [DOI] [PubMed] [Google Scholar]
- 8.Smitz JEJ, Thompson JG, Gilchrist RB. The promise of in vitro maturation in assisted reproduction and fertility preservation. Semin Reprod Med. 2011;29(1):24–37. doi: 10.1055/s-0030-1268701. [DOI] [PubMed] [Google Scholar]
- 9.Dal Canto M, Brambillasca F, Mignini Renzini M, Coticchio G, Merola M, Lain M, et al. Cumulus cell-oocyte complexes retrieved from antral follicles in IVM cycles: relationship between COCs morphology, gonadotropin priming and clinical outcome. J Assist Reprod Genet. 2012;29(6):513–9. doi: 10.1007/s10815-012-9766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ectors FJ, Vanderzwalmen P, Van Hoeck J, Nijs M, Verhaegen G, Delvigne A, et al. Relationship of human follicular diameter with oocyte fertilization and development after in-vitro fertilization or intracytoplasmic sperm injection. Hum Reprod. 1997;12(9):2002–5. doi: 10.1093/humrep/12.9.2002. [DOI] [PubMed] [Google Scholar]
- 11.Bergh C, Broden H, Lundin K, Hamberger L. Comparison of fertilization, cleavage and pregnancy rates of oocytes from large and small follicles. Hum Reprod. 1998;13(7):1912–5. doi: 10.1093/humrep/13.7.1912. [DOI] [PubMed] [Google Scholar]
- 12.Salha O, Nugent D, Dada T, Kaufmann S, Levett S, Jenner L, et al. The relationship between follicular fluid aspirate volume and oocyte maturity in in-vitro fertilization cycles. Hum Reprod. 1998;13(7):1901–6. doi: 10.1093/humrep/13.7.1901. [DOI] [PubMed] [Google Scholar]
- 13.Son W-Y, Chung J-T, Demirtas E, Holzer H, Sylvestre C, Buckett W, et al. Comparison of in-vitro maturation cycles with and without in-vivo matured oocytes retrieved. Reprod Biomed Online. 2008;17(1):59–67. doi: 10.1016/S1472-6483(10)60294-5. [DOI] [PubMed] [Google Scholar]
- 14.Meseguer M, Herrero J, Tejera A, Hilligsøe KM, Ramsing NB, Remohí J. The use of morphokinetics as a predictor of embryo implantation. Hum Reprod. 2011;26(10):2658–71. doi: 10.1093/humrep/der256. [DOI] [PubMed] [Google Scholar]
- 15.Dal Canto M, Coticchio G, Mignini Renzini M, De Ponti E, Novara PV, Brambillasca F, et al. Cleavage kinetics analysis of human embryos predicts development to blastocyst and implantation. Reprod Biomed Online. 2012;25(5):474–80. doi: 10.1016/j.rbmo.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Campbell A, Fishel S, Bowman N, Duffy S, Sedler M, Thornton S. Retrospective analysis of outcomes after IVF using an aneuploidy risk model derived from time-lapse imaging without PGS. Reprod Biomed Online Reproductive Healthcare Ltd. 2013;27(2):140–6. doi: 10.1016/j.rbmo.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Cruz MA, Garrido N, Gadea B, Muñoz M, Pérez-Cano I, Meseguer M. Oocyte insemination techniques are related to alterations of embryo developmental timing in an oocyte donation model. Reprod Biomed Online Reproductive Healthcare Ltd. 2013;27(4):367–75. doi: 10.1016/j.rbmo.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Fadini R, Dal Canto MB, Renzini MM, Brambillasca F, Comi R, Fumagalli D, et al. Predictive factors in in-vitro maturation in unstimulated women with normal ovaries. Reprod Biomed Online. 2009;18(2):251–61. doi: 10.1016/S1472-6483(10)60263-5. [DOI] [PubMed] [Google Scholar]
- 19.Fadini R, Comi R, Mignini Renzini M, Coticchio G, Crippa M, De Ponti E, et al. Anti-mullerian hormone as a predictive marker for the selection of women for oocyte in vitro maturation treatment. J Assist Reprod Genet. 2011;28(6):501–8. doi: 10.1007/s10815-011-9589-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guzman L, Ortega-Hrepich C, Polyzos NP, Anckaert E, Verheyen G, Coucke W, et al. A prediction model to select PCOS patients suitable for IVM treatment. Hum Reprod. 2013;28:1261–6. doi: 10.1093/humrep/det034. [DOI] [PubMed] [Google Scholar]
- 21.Dal Canto MB, Mignini Renzini M, Brambillasca F, Cepparo H, Comi R, Villa A, et al. IVM—the first choice for IVF in Italy. Reprod Biomed Online. 2006;13(2):159–65. doi: 10.1016/S1472-6483(10)60610-4. [DOI] [PubMed] [Google Scholar]
- 22.Son WY, Chung JT, Chian RC, Herrero B, Demirtas E, Elizur S, et al. A 38 h interval between hCG priming and oocyte retrieval increases in vivo and in vitro oocyte maturation rate in programmed IVM cycles. Hum Reprod. 2008;23(9):2010–6. doi: 10.1093/humrep/den210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fadini R, Brambillasca F, Renzini MM, Merola M, Comi R, De ponti E, et al. Human oocyte cryopreservation: comparison between slow and ultrarapid methods. Reprod Biomed Online. 2009;19(2):171–80. doi: 10.1016/S1472-6483(10)60069-7. [DOI] [PubMed] [Google Scholar]
- 24.Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. Balaban B, Brison D, Calderon G, Catt J, Conaghan J. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26(6):1270–83. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 25.Chian RC, Buckett WM, Too LL, Tan SL. Pregnancies resulting from in vitro matured oocytes retrieved from patients with polycystic ovary syndrome after priming with human chorionic gonadotropin. Fertil Steril. 1999;72(4):639–42. doi: 10.1016/S0015-0282(99)00323-4. [DOI] [PubMed] [Google Scholar]
- 26.Walls ML, Ryan JP, Keelan JA, Hart R. In vitro maturation is associated with increased early embryo arrest without impairing morphokinetic development of useable embryos progressing to blastocysts. Hum Reprod. 2015;30(8):1842–9. doi: 10.1093/humrep/dev125. [DOI] [PubMed] [Google Scholar]
- 27.Desai N, Ploskonka S, Goodman LR, Austin C, Goldberg J, Falcone T. Analysis of embryo morphokinetics, multinucleation and cleavage anomalies using continuous time-lapse monitoring in blastocyst transfer cycles. Reprod Biol Endocrinol. 2014;12(1):1–10. doi: 10.1186/1477-7827-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cetinkaya M, Pirkevi C, Yelke H, Colakoglu YK, Atayurt Z, Kahraman S. Relative kinetic expressions defining cleavage synchronicity are better predictors of blastocyst formation and quality than absolute time points. J Assist Reprod Genet. 2014;32(1):27–35. doi: 10.1007/s10815-014-0341-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ottolini C, Rienzi L, Capalbo A. A cautionary note against embryo aneuploidy risk assessment using time-lapse imaging. Reprod Biomed Online Reproductive Healthcare Ltd. 2014;28(3):273–5. doi: 10.1016/j.rbmo.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 30.Fadini R, Coticchio G, Brambillasca F, Mignini Renzini M, Novara PV, Brigante C, et al. Clinical outcomes from mature oocytes derived from preovulatory and antral follicles: reflections on follicle physiology and oocyte competence. J Assist Reprod Genet. 2015;32(2):255–61. doi: 10.1007/s10815-014-0386-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaser DJ, Racowsky C. Clinical outcomes following selection of human preimplantation embryos with time-lapse monitoring: a systematic review. Hum Reprod Update. 2014;20(5):617–31. doi: 10.1093/humupd/dmu023. [DOI] [PubMed] [Google Scholar]
- 32.Cruz MA, Garrido N, Herrero J, Pérez-Cano I, Muñoz M, Meseguer M. Timing of cell division in human cleavage-stage embryos is linked with blastocyst formation and quality. Reprod Biomed Online Reproductive Healthcare Ltd. 2012;25(4):371–81. doi: 10.1016/j.rbmo.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Ortega-Hrepich C, Stoop D, Guzman L, Van Landuyt L, Tournaye H, Smitz J, et al. A “freeze-all” embryo strategy after in vitro maturation: a novel approach in women with polycystic ovary syndrome? Fertil Steril. 2013;100(4):1002–7. doi: 10.1016/j.fertnstert.2013.06.018. [DOI] [PubMed] [Google Scholar]