Abstract
Purpose
The aim of this research is to study whether basic fibroblast growth factor (bFGF) alone or in combination with vascular endothelial growth factor (VEGF) could improve the quality of vitrified-thawed human ovarian tissue xenotransplanted to severe combined immune deficiency (SCID) mice.
Methods
After collection and cryopreservation, thawed human ovarian tissue were cultured in vitro for 2 days and then xenografted to severe combined immune deficiency (SCID) mice for 7 days. The in vitro culture medium was separated into six groups, including (A) the blank control group, (B) the human recombinant bFGF (150 ng/ml) group, (C) the bFGF (150 ng/ml)+human recombinant VEGF (25 ng/ml) group, (D) bFGF (150 ng/ml)+VEGF (50 ng/ml) group, (E) bFGF (150 ng/ml)+ VEGF (75 ng/ml) group and (F) bFGF (150 ng/ml) + VEGF (100 ng/ml) group. In addition, eight pieces of thawed ovarian tissue were transplanted without in vitro culture, which serve as the fresh control group. The effect of transplantation was assessed by histological analysis, immunohistochemical staining for CD34, Ki-67, and AC-3 expression, and microvessel density (MVD).
Results
There was no significant difference between the fresh and blank control group. Compared to the blank control group, the number of follicles, MVD, and rate of Ki-67-positive cells increased significantly in groups B, C, D, E, and F, while apoptosis decreased significantly. Compared to the bFGF treatment group, no significant difference appeared in group C, D, E, and F.
Conclusions
The administration of bFGF alone or in combination with VEGF improved the quality of postgraft human ovarian tissue, though VEGF, regardless of different concentrations, did not influence effect of bFGF.
Keywords: Ovarian preservation, Angiogenesis, Xenotransplantation, VEGF, bFGF, Human
Introduction
As cancer diagnosis and treatment have been greatly improved, the desire of young female cancer survivors to reproduce has become a worldwide concern. However, anticancer treatment including radical operation, radiotherapy, and cytotoxic drugs, especially alkylating agents, often result in great damage to ovarian reserve, losing both steroidogenic and gametogenic functions [1, 2]. For fertility conservation, oocyte, embryo, or ovarian tissue preservation are available options depending on an individual’s status [3]. Ovarian tissue preservation requires neither ovarian stimulation nor sperm fertilization and thus can be carried out whenever the need arises [4]. After ovarian tissue cryopreservation and autologous transplantation, primordial follicles can be reserved, preserving both reproductive and endocrine functions and providing the potential of offspring [5]. There have been 24 live births resulting from 60 orthotopic transplantations of cryopreserved ovarian tissue according to literatures [2] and there has been no retransplantation-related relapse of cancer reported yet [6].
However, cellular and molecular injury resulting from cryopreservation, thawing process, follicular loss and stroma integrity damage resulting from ischemia–reperfusion injury after transplantation [1] are the primary concerns during ovarian tissue preservation.
When comparing the process of ovarian tissue cryopreservation, to the slow cooling process, vitrification was comparable in terms of follicle reserve [7] and was better in terms of stroma morphological integrity [8]. In addition, needle immersed vitrification (NIV) using an acupuncture need (0.18 mm in diameter) as a carrier maximized the cooling rate [9]. For human and mouse ovarian fragments vitrified by NIV, the primordial follicles and the ultrastructure of stromal cells were better preserved than the slow-cooling group or the dropping vitrification group [10–13]. Therefore, needle immersed vitrification improved the quality of ovarian implants in terms of cryopreservation procedure.
After transplantation, small ovarian tissue, without arterial blood vessels and surgical reanastomosis, strongly depend on the angiogenesis process [4]. However, the hypoxic period lasted for 3 days in mice [14] and for 5 days in humans [15] before vascularization process was completed, which resulted in massive follicle loss [1, 16]. Therefore, effective angiogenic factors are needed to induce revascularization for the critical time immediately after transplantation to improve ischemic hypoxia injury [17].
Ovarian angiogenesis is a highly regulated process that involves a balance between multiple vasoactive and angiogenic factors [18]. Vascular endothelial growth factor (VEGF) is a known potent angiogenic factor that affects endothelial cell proliferation, differentiation, and immigration [19]. VEGF was also observed to have regulatory effects on mammalian folliculogenesis, especially for the survival and growth of early and advanced follicles [20]. Basic fibroblast growth factor (bFGF) was the first angiogenic factor identified in the ovary [21] and then proved to localize in granulosa cells, theca cells, and endothelial cells in human [22]. It was reported to play an important role in angiogenesis process, such as stimulation of endothelial cell proliferation and migration [23] and recruit mural cells and monocyte [24]. Moreover, bFGF may influence ovarian stomal cell and follicular growth because [25, 26] because bFGF-induced angiogenesis needs expression of VEGF and bFGF is also capable of regulating other growth factor and chemokine signaling, such as VEGF, hepatocyte growth factor, platelet-derived growth factor, and monocyte chemoattractant protein [24]. However, the effects of VEGF in combination with bFGF on human ovarian tissues have not yet been fully illuminated.
Free bFGF is rapidly degradable and will lose biological functional activity quickly when injected in vivo [27]. Some studies showed that bFGF supplied to an in vitro culture medium for culturing follicles was testified to significantly increase follicle survival [25, 28, 29]. Moreover, previous study demonstrated that in vitro culture for 2 days with 100 or 150 ng/ml bFGF significantly improved angiogenesis in transplanted human ovarian tissues, and proliferation of stromal cells was significantly improved (data not shown). Hence, the method of culturing human ovarian tissues in vitro with bFGF and VEGF before transplantation is feasible.
The aim of this study is to examine if the transplantation effect of vitrified-thawed human ovarian tissue after xenograft to severe combined immune deficiency (SCID) mice can be improved by bFGF or in combination with VEGF added to an in vitro culture system.
Materials and methods
Experimental animals
Male SCID mice (Beijing HFK Bio-Technology. co., LTD; 6–8 weeks old, weight 20.0 ± 2.5 g, N = 28) were housed in groups of four per cage under controlled light and darkness conditions in the Animal Center of Medical College of Sichuan University according to national legislation for animal care. Food and water were freely available. This research project had been approved by the Institutional Animal Care and Use Committee of Sichuan University (Sichuan, China).
Human ovarian tissue collection and preparation
Human ovarian cortical tissues were collected from seven patients (mean age 28 years; range 24–30) undergoing oophorocystectomy because of endometriosis following informed consent and approval of the West China 2nd Hospital, West China Medical Center, Sichuan University Ethics committee. The collected ovarian tissues were transported to laboratory as soon as possible in L-15 medium (Sigma, St Louis, MO, USA) containing 10 % human serum albumin (HSA; LifeGlobal, Guilford, CT, USA) at 2–6 °C. Then the ovarian cortex was minced into about 2 × 2 × 1 mm after the ovarian medulla was removed in fresh L-15 medium with 10 % HSA. Before cryopreservation, one ovarian cortex slice from each patient was fixed in 4 % formaldehyde for morphological assessment. All the samples were verified by pathological examination without malignant transformation or metastasis.
Ovarian tissue cryopreservation and thawing
The prepared ovarian cortical slices were cryopreserved by NIV according to the following procedure and stored in liquid nitrogen for at least 1 week before thawing and transplantation. Three to four pieces of ovarian fragments were held in a row by an acupuncture needle (Cloud & Dragon Medical Device Co. Ltd, China) in L-15 medium with 10 % HSA. Then the needles were immersed into an equilibration solution containing 7.5 % (v/v) DMSO, 7.5 % (v/v) EG, and 20 % HSA in DPBS for 10 min and then a vitrification solution containing 15 % DMSO, 15 % EG, and 0.5 M sucrose for 2 min at room temperature. Then the remaining vitrification solution on the needles holding ovarian slices was removed softly by an aseptic absorbent gauze. And then the needles were plunged in liquid nitrogen rapidly and finally placed into liquid nitrogen-prefilled cryovials.
For thawing, the needles holding ovarian fragments were taken out of the vial and immediately immersed successively into 1 M sucrose solution at 37 °C, 0.5 M, and 0.25 M sucrose solution with room temperature for 5 min, respectively, washed three times by DPBS with 20 % HSA, and incubated for 15–20 min at 37 °C with 5 % CO2 in incubator.
Ovarian tissue culture in vitro
Serum-free culture medium was used for in vitro culture consisting of αMEM (Invitrogen, Carlsbad, CA, USA) with 10 % HSA, 50 μg/ml vitamin C (Sigma Chemical Co., St Louis, MO), 1 % ITS-G (10 μg/ml insulin, 5.5 μg/ml transferrin, and 6.7 ng/ml sodium selenite; Invitrogen), 0.47 mmol/l pyruvic acid (Sigma), 0.5 % antibiotic/antimycotic solution (50 IU/ml penicillin G, 50 μg/ml streptomycin sulfate, and 0.125 μg/ml amphotericin B; Invitrogen), 2 mmol/l L-glutamine (Sigma), and 0.5 IU/ml recombinant human FSH (Gonal-F; Serono Nordic). The thawed ovarian slices were transferred into Millicell CM inserts (12-mm diameter, 0.4-mm pore size; Millipore, Bedford, MA, USA) coated with 100 μl extracellular matrix (1:3 dilution by culture medium; Matrigel, Becton Dickinson, MA, USA) fitted into 24-well plates (Corning). Two ovarian fragments were placed in each well and cultured with half culture medium (400 μl) renewed every second day in a humidified incubator at 37 °C with 5 % CO2.
The thawed human ovarian slices were divided randomly into seven groups (n = 8). Eight pieces were immediately transplanted without in vitro culture, designed as a fresh control group. The other six groups were cultured in vitro for 2 days before transplantation to provide enough local concentration of angiogenic factors in serum-free medium containing different drugs: (A) control group without angiogenic factor, (B) human recombinant bFGF (150 ng/ml, Invitrogen), (C) bFGF (150 ng/ml) + recombinant human VEGF (25 ng/ml, PEPROTECH, USA), (D) bFGF (150 ng/ml) + VEGF (50 ng/ml), (E) bFGF (150 ng/ml) + VEGF (75 ng/ml), and (F) bFGF (150 ng/ml) + VEGF (100 ng/ml).
Ovarian tissue xenotransplantation and grafts recovery
One percent pentobarbital was intraperitoneally injected for anesthesia. Two ovarian tissue grafts from the same group were placed in one subcutaneous incision on the back area for each mouse. The incisions were then sutured with absorbable 5/0 Prolene. After surgery, the host mice were sent back and euthanized by cervical dislocation 1 week after transplantation. Human ovarian grafts were recovered and fixed in 4 % formaldehyde for the following immunohistochemical staining. All procedures above were performed under aseptic conditions.
Immunohistochemistry
The recovered ovarian fragments were paraffin-embedded and sliced, 5 μm in thickness. Sections were deparaffinated and rehydrated by xylene and an decreasing ethanol concentration. After demasking in citrate buffer (pH 6.0) at 98 °C for 20 min, the sections were incubated by 0.3 % H2O2 at room temperature for 10 min to block endogenous peroxidase activity. Subsequently, the slices were incubated with primary antibodies at 48 °C overnight, washed by PBS for 2 min for three times, and incubated with secondary antibodies at room temperature for 30 min, following 3,3′-diaminobenzidine tetrahydrochloride (DAB; 1.2 mg/ml) incubating for 2–5 min to change the color and hematoxylin counterstaining for 5 min. Finally, the slides were dehydrated in an ethanol series of increasing concentrations and xylene and then sealed [30]. The primary antibody was omitted for negative control.
To quantify angiogenesis, anti-CD34 antibody (1:200 dilution; Abcam, Cambridge, UK) was used to stain epithelial cells of microvessels, and microvessel density (MVD) was calculated by counting and taking the mean of CD34-positive-staining vessels at three randomly selected positions in a high-power field (×400) for each sample. Cell proliferation was confirmed by IHC staining against Ki-67 (1:200 dilution; Abcam) in ovarian grafts from various groups while apoptosis was confirmed by anti-active caspase-3 (AC-3) staining (1:200 dilution; Abcam). The primary antibody was omitted as negative control. Red-brown coloring of the cytoplasm/nucleus of the stromal cells, granulosa cells, or oocytes was considered as positive staining (otherwise as negative staining). The number of the positive staining cells and the total number of all the cells were counted by eyeball in the same high-power field (×400) randomly selected. The ratio of positively stained cells was calculated.
Two slices were stained from every 10 serial sections of each graft with Ki-67 antibody and AC-3 antibody, respectively, to assess follicle survival and development. Follicles were counted in a high-power field (×400) in 10 sections when the Ki-67/AC-3 positively staining nucleolus was visualized within the nucleus of the follicles. Follicles with at least one Ki-67-positive were considered to be growing [31]. Follicles were classified to be undynamic when both the oocyte and more than 50 % of granulosa cells were AC-3-positive staining [31]. The total number of Ki-67/AC-3-positive follicles in each section was counted, respectively. The follicles were classified as primordial follicles with one layer of flattened granulosa cells surrounding the oocyte, primary follicles with one layer of cuboid granulosa cells, and secondary follicles with two or three layers of granulosa cells [32].
According to the manufacturers’ instructions for the above primary antibodies, human tonsil tissues were used as positive control for CD34 immunostaining, human colon tissues were used as positive control for Ki-67 immunostaining, and human hepatocellular carcinoma tissues were used as positive control for VEGF immunostaining.
Statistical analysis
Data were described as the mean value for each graft and the mean value ± standard deviation (X ± SD) for each group. All the data were analyzed by analysis of one-way ANOVA with SPSS Statistics 20.0 software, and P < 0.05 was considered to be statistically significant.
Results
Graft recovery and morphologic assay
During the study, all SCID mice undergoing surgery survived to 7 days after transplantation and recovered from the wound well without dehiscence of the surgical wound observed. Human ovarian grafts were collected 7 days after transplantation. The recovery rate was 100 % for each group. All the grafts were attached tightly to the surrounding subcutaneous tissues near the surgical wound. Most of the grafts retained their original size with smooth edge and a reddish color. Especially in groups B–F, serried small vessels were visible on the surface of ovarian fragments. Parts of the grafts were pale and smaller than pretransplantation. After 7 days of xenotransplantation, most of the ovarian fragments retained original tissue structure under microscope. In most fragments, many red blood cells were observed as showed in Fig. 1b. Some neutrophils and macrophages appeared in the peripheral tissue of grafts. A limited number of follicles were observed in pretransplantation grafts, about 0 to 4 follicles in each section at high magnification (×400), most of which were primordial follicles, without antral follicle observed (Fig. 1c).
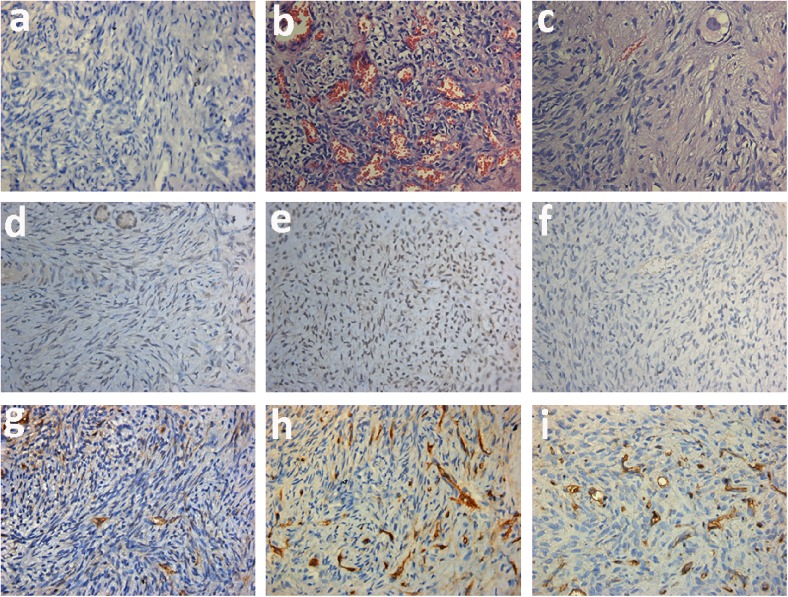
Fig. 1.
Representative photograph of postgraft human ovarian tissues (original magnification ×400). a Negative control for IHC staining. b, c Hematoxylin and eosin stain. d, e Ki-67 signals in follicles and stroma in human ovarian graft. f AC-3 staining in human ovarian tissue after transplantation. g–i CD34 staining in groups A, B, and C, respectively
Analysis for proliferation and apoptosis of ovarian grafts
Follicles and stromal cells were stained with Ki-67 to evaluate proliferation, and with AC-3 to evaluate apoptosis in ovarian tissues. Eight ovarian grafts in each group were evaluated 7 days after transplantation. Ki-67 mainly stained in granulose cells, vascular endothelial cells, and some stromal cells in ovarian tissues, and the signals were strong (Fig. 1d, e). The AC-3-positive signals were weak and mainly detected in the ovarian stromal cells (Fig. 1f). There were few apoptotic cells detected.
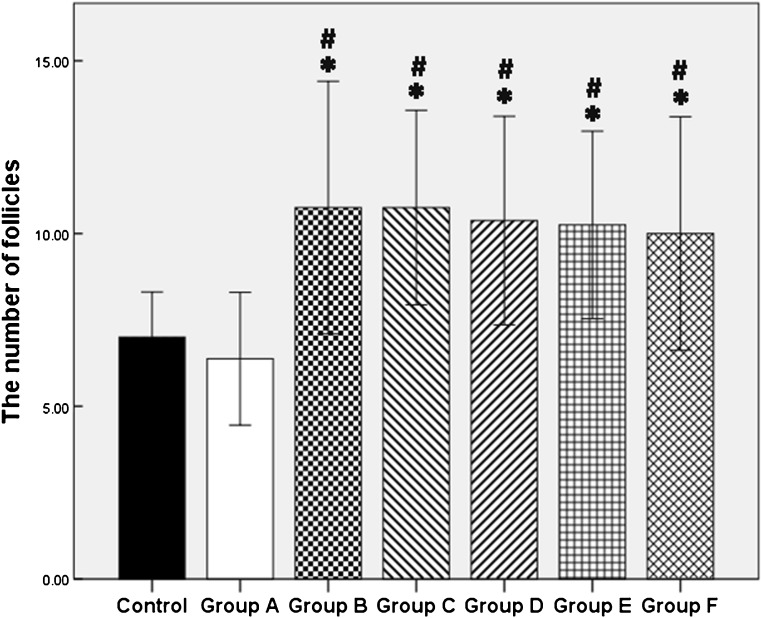
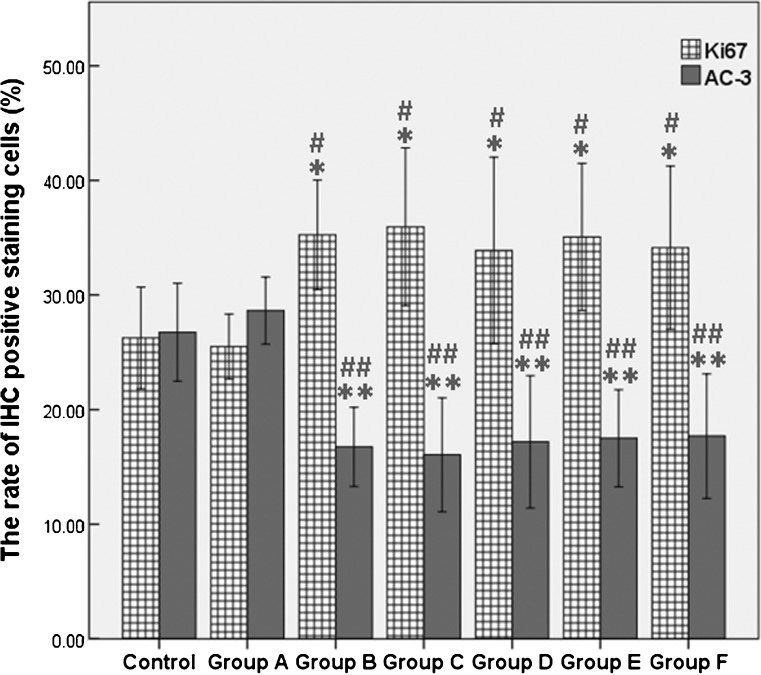
Follicles detected were all Ki-67 positive while no AC-3-positive follicle was observed, all of which were preantral. No significant difference appeared between fresh control group and blank control group (group A) in terms of the number of follicles (7.00 ± 1.31 vs. 6.38 ± 1.92, P = 0.57) and the rate of Ki-67/AC-3-positive cells (P < 0.05). Compared with blank control group, the numbers of Ki-67-positive follicles were significantly increased in group B (bFGF) and groups C–F (bFGF + VEGF) (P < 0.05) (Fig. 2). As demonstrated by Ki-67 staining in ovarian tissues, a low rate of proliferation was presented in both fresh and blank control group, and more positive signals were detected in bFGF and bFGF + VEGF treatment groups (P < 0.05), indicating that bFGF and VEGF treatment could significantly improve graft survival. In addition, bFGF and VEGF decreased the rate of apoptosis cells compared with control groups (P < 0.05) (Fig. 3). However, no significant differences existed between bFGF treatment group and bFGF + VEGF treatment groups (P > 0.05), demonstrating that the addition of different concentrations of VEGF did not further increase follicle and stroma survival nor decrease apoptosis.
Fig. 2.
The number of follicles was presented as mean ± SD. *P < 0.05, significantly higher than fresh control group. #P < 0.05, significantly higher than group A (blank control group)
Fig. 3.
Proliferation and apoptosis was measured by Ki-67 and AC-3 IHC staining, respectively. Results are presented as mean ± SD. The rate of Ki-67 and AC-3-staining cells was counted at high power field (×400). *P < 0.05, the rate of Ki-67-positive staining cells significantly higher than fresh control group. **P < 0.05, the rate of Ki-67-positive staining cells significantly higher than a group A (black control group). #P < 0.05, the rate of AC-3-positive staining cells significantly lower than fresh control group. ##P < 0.05, the rate of AC-3-positive cells significantly lower than group A (black control group)
Identification of angiogenesis in transplanted ovarian tissues
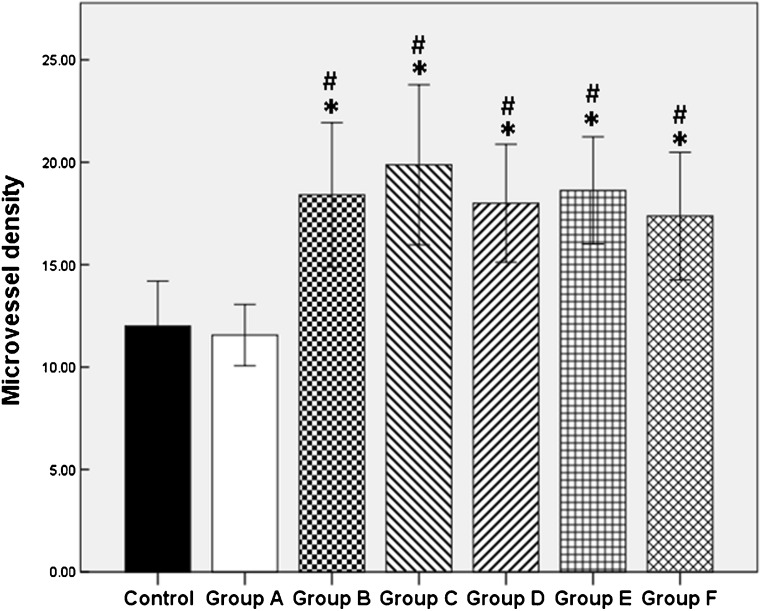
To evaluate the effect of bFGF and VEGF on angiogenesis, CD34 immunostaining was performed (Fig. 1g–i), and microvessel density (MVD) was calculated for each transplant ovarian fragment [30]. Eight ovarian grafts were collected and stained for each group. Positive signal of CD34 was detected mainly in peripheral sites of the ovarian grafts. Figure 4 represented the microvessel density as a bar chart. The average number of CD34-positive cells in fresh control group and blank control group was limited and no significant difference appeared (12.00 ± 2.20 vs. 11.56 ± 1.50, P = 0.77; ×400). The microvessel density was more prominent in the ovarian tissues in group B (bFGF) and groups C–F (bFGF + VEGF) compared to control groups (P < 0.05) (Fig. 4). Though the value of MVD in group C was a little higher than in group B, no significant difference existed between the two groups (P = 0.336).
Fig. 4.
The microvessel density was measured in CD34-labeled vessels. Results are presented as mean ± SD. CD34 staining vessels were counted at high power field (×400). *P < 0.05, significantly higher than fresh control group. #P < 0.05, significantly higher than group A (black control group)
Discussion
In this study, we investigated the effects of bFGF and VEGF on the quality of vitrified-thawed human ovarian tissues after allotransplantation. The results showed that treatment with bFGF alone or in combination with VEGF significantly improved the quality of ovarian grafts by preventing follicle loss, improving angiogenesis and proliferation, and decreasing apoptosis. However, compared to treatment with bFGF alone, and addition of VEGF, did not further improve the quality of transplanted ovarian tissues.
Currently, orthotopic and heterotopic transplantations are available for autotransplantation of human ovarian tissue. Orthotopic transplantation can provide the chance for natural pregnancy, but abdominal surgery is needed and transplanted tissue monitoring may be more difficult with high risk of ovarian metastasis. Focusing on these aspects, heterotopic transplantation is more preferable than orthotopic transplantation [30]. As reported, preantral follicles were successfully retrieved from subcutaneously transplanted marmoset monkey ovarian tissues [33]. Considering where the ovarian tissues should be transplanted, four grafting sites, including ovarian bursa, intraperitoneal, subcutaneous, and i.m. sites, were reported to have equal performance in terms of follicle preservation and stroma integrity following short-term transplantation of cryobanked human ovarian tissues [34] and in terms of endocrine function following long-term transplantation for 7 years [33]. Thus, subcutaneous location was an appropriate heterotopic transplantation site for restoration of ovarian reproduction and endocrine function for women. And it was usually selected as grafting site because it is easier to observe the situation of ovarian tissue, and the transplantation surgery is easy and simple in experiments using murine models [30].
After transplantation, massive follicular loss and stroma damage because of ischemia–reperfusion injury led to shorter duration of ovarian function than expected in many studies in animal experiments and in human [1, 35, 36]. In mice, initial blood perfusion was detected 3 days following transplantation in ovarian autograft [14], and functional blood vessels were observed from day 7 postgraft by MRI and histology analysis [33]. In human, dermal microvascular endothelial cells were found to be organized into empty tubular structures by day 5 and differentiated into functional microvessels within 7–10 days after transplantation to biodegradable polymer matrices [15]. Moreover, rich blood vessels were observed 7 days posttransplantation in stroma of human ovarian tissues [37]. Normally, angiogenesis begins within 48 h following transplantation and may take 7 days to generate functional blood vessels [38–40]. So, 7 days were suggested as an important duration of time after transplantation [30]. Based on these results, human ovarian fragments were retrieved 7 days after transplantation in our study.
The ultimate aim of this study was to improve the quality and function in the process of human ovarian tissue autotransplantation, so the risk of side effect of antigenic factor administration also should be considered. Angiogenesis plays a crucial role in the development of many types of cancer [41]. As a known potent antigenic factor, VEGF is related to many kinds of cancer, such as cervical cancer, breast cancer, lung cancer, liver cancer, melanoma, and osteosarcoma, and specific antibody against VEGF was used for anticancer treatment [42]. According to Lund’s research on small cell lung cancer xenografts, 200 ng per chamber for VEGF and 1000 ng per chamber for bFGF were considered to be the upper limit of physiological level [43]. A dosage of 50 ng/ml VEGF and 100 ng/ml bFGF was used to incubate human ovarian tissues in vitro before heterograft to rabbit model [4]. For culturing follicles in vitro, the reported concentration of bFGF was 40–300 ng/ml [25, 28]. In our study, the upper concentration of VEGF and bFGF was 100 ng/ml and 150 ng/ml, respectively, and is relatively low and safe.
The most widely approach to study changes in angiogenesis in ovary is to stain endothelial cells with a specific marker in ovarian sections and then perform image analysis. CD34 is a transmembrane glycoprotein localized in endothelial cells and is reliably detected in human ovary [44, 45]. And CD34 antibodies were reported to cross-react less in other species [46]. The present study showed that treatment with bFGF and VEGF improved the amount of CD34-staining cells. These results may be explained by the angiogenic effects of bFGF and VEGF or the indirect effect of more conserved follicles.
Our study showed that treatment with bFGF alone or in combination with VEGF significantly improved angiogenesis of ovarian grafts. However, addition of VEGF on the basis of bFGF did not present an additive angiogenic effect. Angiogenesis is a complex process and endothelial cells are the key points. The development of endothelial networks occurred in a series of stages, including migration and clustering, sprouting, cellular alignment, tubule initiation, and finally mature vessel generation [47]. And this network was stimulated by bFGF alone or in combination with VEGF. bFGF mainly increased the size of the endothelial cluster by inducing sprouting and tubule initiation. VEGF mainly increased the number of clusters by promoting EC migration, survival, and proliferation of the initial clusters [46] through two tyrosine kinase receptors, VEGFR-1 and VEGFR-2 [46]. As reported, the combination of bFGF and VEGF promoted endothelial cell network formation in a luteal angiogenesis culture system [48]. And in the same culture system, the EC network decreased to 60 % when VEGFR-2 inhibitor was added, while drastically reduced to 10 % when FGFR1 inhibitor was employed, indicating that bFGF was more important than VEGF for luteal vascular networks [49]. In some researches, it was thought that there were no interactions between the effects of VEGF and bFGF, indicating that their effects were additive and that they have independent and different actions on the angiogenic process itself [50]. But some others held that bFGF probably was an upstream factor VEGF and some other angiogenic factors and regulated their angiogenic effect [24]. bFGF and VEGF are critical angiogenic growth factors; however, their precise roles remain to be elucidated to provide a reasonable explanation for our results.
With respect to the effect of bFGF and VEGF on the course of follicular survival after transplantation, the result of the present study showed that treatment with bFGF alone or in combination with VEGF significantly prevented follicle loss compared with control group. Previous studies demonstrated that bFGF messenger RNA (mRNA) and protein were expressed throughout goat follicular development [26], while VEGF mRNA is first detected in secondary follicles and then a further increase of expression is observed in the late secondary and tertiary stages of follicles [9, 12]. And in this stage, administration of GnRH antagonist did not affect VEGF mRNA expression [9]. Moreover, some studies have shown the effect of bFGF on follicle growth in vitro culture in rat and goat ovarian tissues [25]. And for human follicles in vitro, the diameter and survival rate significantly increased by bFGF intervention, same to the percentage with good viability and preantral follicles [51]. A high dose of bFGF enhanced follicular development and stimulated E2 production for human ovarian tissue cultured in vitro [28]. Though bFGF and VEGF affect follicle growth and development, their angiogenic effect may also play an important part in follicle survival in ovarian tissues postgraft resulting from improving the ischemia–reperfusion injury during the hypoxic period after transplantation.
In this study, a limited number of follicles were observed both in fresh and postgraft tissues by HE and IHC staining. In human ovary, follicles present uneven distribution [52]. The patients involved in our study had ovarian cysts with at least 5 cm in diameter leading to significantly decrease of follicular density [53, 54] and decrease of ovarian reserve by 40 % in volume compared with the healthy ovary [55]. The vast majority of follicles detected were primordial follicles, which were in a dormant state with a lower level of metabolic activity and thus affected less by ischemia [56]. Most follicles were Ki-67 positively stained and few were AC-3 positive, indicating that follicles were viable and could survive well.
In conclusion, the present study demonstrated that bFGF alone or in combination with VEGF improved the effect of transplantation for frozen-thawed human ovarian tissues, though VEGF did not show additive effect on the basis of bFGF. The next phase of our study should be a longer xenotransplantation period for thawed human ovarian fragments following gonadotrophin stimulation to observe follicle growth and development.
Acknowledgments
This work was supported by the National Natural Science Funds of China (31171442 to L. S. W and 31201117 to W. Y.).
Compliance with ethical standards
This research project had been approved by the Institutional Animal Care and Use Committee of Sichuan University (Sichuan, China).
Ethics approval and consent to participate
Human ovarian cortical tissue were collected from seven patients following informed consent and approval of the West China 2nd Hospital, West China Medical Center, Sichuan University Ethics committee.
Footnotes
Capsule
The administration of bFGF alone or in combination with VEGF improved the quality of postgraft human ovarian tissue, though VEGF, regardless of different concentrations, did not influence effect of bFGF.
References
- 1.Friedman O, Orvieto R, Fisch B, Felz C, Freud E, Ben-Haroush A, et al. Possible improvements in human ovarian grafting by various host and graft treatments. Hum Reprod. 2012;27:474–82. doi: 10.1093/humrep/der385. [DOI] [PubMed] [Google Scholar]
- 2.Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Sanchez Serrano M, Schmidt KT, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99(6):1503–13. doi: 10.1016/j.fertnstert.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 3.Donnez J, Dolmans MM. Preservation of fertility in females with haematological malignancy. Br J Haematol. 2011;154:175–84. doi: 10.1111/j.1365-2141.2011.08723.x. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Ying YF, Ouyang YL, Wang JF, Xu J. VEGF and bFGF increase survival of xenografted human ovarian tissue in an experimental rabbit model. J Assist Reprod Genet. 2013;30(10):1301–11. doi: 10.1007/s10815-013-0043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grynberg M, Poulain M, Sebag-Peyrelevade S, le Parco S, Fanchin R, Frydman N. Ovarian tissue and follicle transplantation as an option for fertility preservation. Fertil Steril. 2012;97:1260–8. doi: 10.1016/j.fertnstert.2012.04.042. [DOI] [PubMed] [Google Scholar]
- 6.Rosendahl M, Greve T, Andersen CY. The safety of transplanting cryopreserved ovarian tissue in cancer patients: a review of the literature. J Assist Reprod Genet. 2013;30(1):11–24. doi: 10.1007/s10815-012-9912-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herraiz S, Novella-Maestre E, Rodríguez B, Díaz C, Sánchez-Serrano M, Mirabet V, et al. Improving ovarian tissue cryopreservation for oncologic patients: slow freezing versus vitrification, effect of different procedures and devices. Fertil Steril. 2014;101(3):775–84. doi: 10.1016/j.fertnstert.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Keros V, Xella S, Hultenby K, Pettersson K, Sheikhi M, Volpe A, et al. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod. 2009;24(7):1670–83. doi: 10.1093/humrep/dep079. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Xiao Z, Li L, Fan W, Li SW. Novel needle immersed vitrification: a practical and convenient method with potential advantages in mouse and human ovarian tissue cryopreservation. Hum Reprod. 2008;23:2256–65. doi: 10.1093/humrep/den255. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Cheng KM, Silversides FG. Novel needle-in-straw vitrification can effectively preserve the follicle morphology, viability, and vascularization of ovarian tissue in Japanese quail (Coturnix japonica) Anim Reprod Sci. 2012;134(3–4):197–202. doi: 10.1016/j.anireprosci.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Fatehi R, Ebrahimi B, Shahhosseini M, Farrokhi A, Fathi R. Effect of ovarian tissue vitrification method on mice preantral follicular development and gene expression. Theriogenology. 2014;81(2):302–8. doi: 10.1016/j.theriogenology.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 12.Xiao Z, Wang Y, Li LL, Li SW. In vitro culture thawed human ovarian tissue: NIV versus slow freezing method. Cryo Lett. 2013;34(5):520–6. [PubMed] [Google Scholar]
- 13.Xiao Z, Li SW, Zhang YY, Wang Y, Li LL, Fan W. NIV versus dropping vitrification in cryopreservation of human ovarian tissue. Cryo Lett. 2014;35(3):226–31. [PubMed] [Google Scholar]
- 14.Nugent D, Newton H, Gallivan L, Gosden RG. Protective effect of vitamin E on ischaemia-reperfusion injury in ovarian grafts. J Reprod Fertil. 1998;114:341–6. doi: 10.1530/jrf.0.1140341. [DOI] [PubMed] [Google Scholar]
- 15.Van Eyck AS, Jordan BF, Gallez B, Heilier JF, Van Langendonckt A, Donnez J. Electron paramagnetic resonance as a tool to evaluate human ovarian tissue reoxygenation after xenografting. Fertil Steril. 2009;92:374–81. doi: 10.1016/j.fertnstert.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Orthotopic and heterotopic ovarian tissue transplantation. Hum Reprod Update. 2009;15:649–65. doi: 10.1093/humupd/dmp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imthurn B, Cox SL, Jenkin G, Trounson AO, Shaw JM. Gonadotrophin administration can benefit ovarian tissue grafted to the body wall: implications for human ovarian grafting. Mol Cell Endocrinol. 2000;163:141–6. doi: 10.1016/S0303-7207(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 18.Robinson RS, Woad KJ, Hammond AJ, Laird M, Hunter MG, Mann GE. Angiogenesis and vascular function in the ovary. Reproduction. 2009;138(6):869–81. doi: 10.1530/REP-09-0283. [DOI] [PubMed] [Google Scholar]
- 19.Kaczmarek MM, Schams D, Ziecik AJ. Role of vascular endothelial growth factor in ovarian physiology—an overview. Reprod Biol. 2005;5(2):111–36. [PubMed] [Google Scholar]
- 20.Araújo VR, Duarte AB, Bruno JB, Pinho Lopes CA, de Figueiredo JR. Importance of vascular endothelial growth factor (VEGF) in ovarian physiology of mammals. Zygote. 2013;21(3):295–304. doi: 10.1017/S0967199411000578. [DOI] [PubMed] [Google Scholar]
- 21.Gospodarowicz D, Cheng J, Lui GM, Baird A, Esch F, Bohlen P. Corpus luteum angiogenic factor is related to fibroblast growth factor. Endocrinology. 1985;117(6):2383–91. doi: 10.1210/endo-117-6-2383. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto S, Konishi I, Nanbu K, Komatsu T, Mandai M, Kuroda H, et al. Immunohistochemical localization of basic fibroblast growth factor (bFGF) during folliculogenesis in the human ovary. Gynecol Endocrinol. 1997;11(4):223–30. doi: 10.3109/09513599709152538. [DOI] [PubMed] [Google Scholar]
- 23.Presta M, Dell’Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16(2):159–78. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Murakami M, Simons M. Fibroblast growth factor regulation of neovascularization. Curr Opin Hematol. 2008;15(3):215–20. doi: 10.1097/MOH.0b013e3282f97d98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilsson E, Parrott JA, Skinner MK. Basic fibroblast growth factor induces primordial follicle development and initiates folliculogenesis. Mol Cell Endocrinol. 2001;175:123–30. doi: 10.1016/S0303-7207(01)00391-4. [DOI] [PubMed] [Google Scholar]
- 26.Almeida AP, Saraiva MV, Alves Filho JG, Silva GM, Goncalves RF, Brito IR, et al. Gene expression and immunolocalization of fibroblast growth factor 2 in the ovary and its effect on the in vitro culture of caprine preantral ovarian follicles. Reprod Domest Anim. 2012;47:20–5. doi: 10.1111/j.1439-0531.2011.01793.x. [DOI] [PubMed] [Google Scholar]
- 27.Yun YR, Won JE, Jeon E, Lee S, Kang W, Jo H, et al. Fibroblast growth factors: biology, function, and application for tissue regeneration. J Tissue Eng. 2010;2010:218142. doi: 10.4061/2010/218142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garor R, Abir R, Erman A, Felz C, Nitke S, Fisch B. Effects of basic fibroblast growth factor on in vitro development of human ovarian primordial follicles. Fertil Steril. 2009;91(5 Suppl):1967–75. doi: 10.1016/j.fertnstert.2008.04.075. [DOI] [PubMed] [Google Scholar]
- 29.Peng X, Yang M, Wang L, Tong C, Guo Z. In vitro culture of sheep lamb ovarian cortical tissue in a sequential culture medium. J Assist Reprod Genet. 2010;27(5):247–57. doi: 10.1007/s10815-010-9415-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao JM, Yan J, Li R, Li M, Yan LY, Wang TR, et al. Improvement in the quality of heterotopic allotransplanted mouse ovarian tissues with basic fibroblast growth factor and fibrin hydrogel. Hum Reprod. 2013;28(10):2784–93. doi: 10.1093/humrep/det296. [DOI] [PubMed] [Google Scholar]
- 31.Kedem A, Hourvitz A, Fisch B, Shachar M, Cohen S. Alginate scaffold for organ culture of cryopreserved-thawed human ovarian cortical follicles. J Assist Reprod Genet. 2011;28:761–9. doi: 10.1007/s10815-011-9605-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen SU, Chien CL, Wu MY, Chen TH, Lai SM, Lin CW, et al. Novel direct cover vitrification for cryopreservation of ovarian tissues increases follicle viability and pregnancy capability in mice. Hum Reprod. 2006;21(11):2794–800. doi: 10.1093/humrep/del210. [DOI] [PubMed] [Google Scholar]
- 33.Kim SS. Assessment of long term endocrine function after transplantation of frozen-thawed human ovarian tissue to the heterotopic site: 10 year longitudinal follow-up study. J Assist Reprod Genet. 2012;29(6):489–93. doi: 10.1007/s10815-012-9757-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dath C, Van Eyck AS, Dolmans MM, Romeu L, Delle Vigne L, Donnez J, et al. Xenotransplantation of human ovarian tissue to nude mice: comparison between four grafting sites. Hum Reprod. 2010;25(7):1734–43. doi: 10.1093/humrep/deq131. [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Van der Elst J, Van den Broecke R, Dhont M. Early massive follicle loss and apoptosis in heterotopically grafted newborn mouse ovaries. Hum Reprod. 2002;17(3):605–11. doi: 10.1093/humrep/17.3.605. [DOI] [PubMed] [Google Scholar]
- 36.Israely T, Nevo N, Harmelin A, Neeman M, Tsafriri A. Reducing ischaemic damage in rodent ovarian xenografts transplanted into granulation tissue. Hum Reprod. 2006;21(6):1368–79. doi: 10.1093/humrep/del010. [DOI] [PubMed] [Google Scholar]
- 37.Dath C, Dethy A, Van Langendonckt A, Van Eyck AS, Amorim CA, Luyckx V, et al. Endothelial cells are essential for ovarian stromal tissue restructuring after xenotransplantation of isolated ovarian stromal cells. Hum Reprod. 2011;26(6):1431–9. doi: 10.1093/humrep/der073. [DOI] [PubMed] [Google Scholar]
- 38.Kim SS, Soules MR, Battaglia DE. Follicular development, ovulation, and corpus luteum formation in cryopreserved human ovarian tissue after xenotransplantation. Fertil Steril. 2002;78(1):77–82. doi: 10.1016/S0015-0282(02)03144-8. [DOI] [PubMed] [Google Scholar]
- 39.Kim SS, Hwang IT, Lee HC. Heterotopic autotransplantation of cryobanked human ovarian tissue as a strategy to restore ovarian function. Fertil Steril. 2004;82(4):930–2. doi: 10.1016/j.fertnstert.2004.02.137. [DOI] [PubMed] [Google Scholar]
- 40.Bedaiwy MA, Burlingame JM, Hussein M, Flyckt R, Assad R, Falcone T. Assessment of vascular endothelial growth factor, basic fibroblast growth factor, and transforming growth factor levels in amniotic fluid. J Reprod Med. 2012;57(9–10):405–10. [PubMed] [Google Scholar]
- 41.Tomao F, Papa A, Rossi L, Zaccarelli E, Caruso D, Zoratto F, et al. Angiogenesis and antiangiogenic agents in cervical cancer. Oncol Targets Ther. 2014;7:2237–48. doi: 10.2147/OTT.S68286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramchandani D, Weber GF. Interactions between osteopontin and vascular endothelial growth factor: implications for cancer. Biochim Biophys Acta. 2015;1855(2):202–22. doi: 10.1016/j.bbcan.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Lund EL, Thorsen C, Pedersen MW, Junker N, Kristjansen PE. Relationship between vessel density and expression of vascular endothelial growth factor and basic fibroblast growth factor in small cell lung cancer in vivo and in vitro. Clin Cancer Res. 2000;6:4287–91. [PubMed] [Google Scholar]
- 44.Otani N, Minami S, Yamoto M, Shikone T, Otani H, Nishiyama R, et al. The vascular endothelial growth factor/fms-like tyrosine kinase system in human ovary during the menstrual cycle and early pregnancy. J Clin Endocrinol Metab. 1999;84(10):3845–51. doi: 10.1210/jcem.84.10.6025. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki T, Sasano H, Takaya R, Fukaya T, Yajima A, Nagura H. Cyclic changes of vasculature and vascular phenotypes in normal human ovaries. Hum Reprod. 1998;13(4):953–9. doi: 10.1093/humrep/13.4.953. [DOI] [PubMed] [Google Scholar]
- 46.Fraser HM. Regulation of the ovarian follicular vasculature. Reprod Biol Endocrinol. 2006;4:18. doi: 10.1186/1477-7827-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirschberg RM, Sachtleben M, Plendl J. Electron microscopy of cultured angiogenic endothelial cells. Microsc Res Tech. 2005;67(5):248–59. doi: 10.1002/jemt.20204. [DOI] [PubMed] [Google Scholar]
- 48.Robinson RS, Hammond AJ, Mann GE, Hunter MG. A novel physiological culture system that mimics luteal angiogenesis. Reproduction. 2008;135(3):405–13. doi: 10.1530/REP-07-0370. [DOI] [PubMed] [Google Scholar]
- 49.Woad KJ, Hammond AJ, Hunter M, Mann GE, Hunter MG, Robinson RS. FGF2 is crucial for the development of bovine luteal endothelial networks in vitro. Reproduction. 2009;138:581–8. doi: 10.1530/REP-09-0030. [DOI] [PubMed] [Google Scholar]
- 50.Shirasuna K, Nitta A, Sineenard J, Shimizu T, Bollwein H, Miyamoto A. Vascular and immune regulation of corpus luteum development, maintenance, and regression in the cow. Domest Anim Endocrinol. 2012;43(2):198–211. doi: 10.1016/j.domaniend.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 51.Wang TR, Yan LY, Yan J, Lu CL, Xia X, Yin TL, et al. Basic fibroblast growth factor promotes the development of human ovarian early follicles during growth in vitro. Hum Reprod. 2014;29(3):568–76. doi: 10.1093/humrep/det465. [DOI] [PubMed] [Google Scholar]
- 52.Abir R, Fisch B, Jessel S, Felz C, Ben-Haroush A, Orvieto R. Improving posttransplantation survival of human ovarian tissue by treating the host and graft. Fertil Steril. 2011;95:1205–10. doi: 10.1016/j.fertnstert.2010.07.1082. [DOI] [PubMed] [Google Scholar]
- 53.Schubert B, Canis M, Darcha C, Artonne C, Pouly JL, Dechelotte P, et al. Human ovarian tissue from cortex surrounding benign cysts: a model to study ovarian tissue cryopreservation. Hum Reprod. 2005;20:1786–92. doi: 10.1093/humrep/dei002. [DOI] [PubMed] [Google Scholar]
- 54.Kitajima M, Defrère S, Dolmans MM, Colette S, Squifflet J, Van Langendonckt A, et al. Endometriomas as a possible cause of reduced ovarian reserve in women with endometriosis. Fertil Steril. 2011;96:685–91. doi: 10.1016/j.fertnstert.2011.06.064. [DOI] [PubMed] [Google Scholar]
- 55.Soleimani R, Heytens E, Oktay K. Enhancement of neoangiogenesis and follicle survival by sphingosine-1-phosphate in human ovarian tissue xenotransplants. PLoS One. 2011;6 doi: 10.1371/journal.pone.0019475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu J, Van der Elst J, Van den Broecke R, Dhont M. Live offspring by in vitro fertilization of oocytes from cryopreserved primordial mouse follicles after sequential in vivo transplantation and in vitro maturation. Biol Reprod. 2001;64:171–8. doi: 10.1095/biolreprod64.1.171. [DOI] [PubMed] [Google Scholar]