Abstract
Introduction
An ideal cranioplasty material is one which adequately restores calvarial continuity, contour and esthetics, provides adequate cerebral protection, is biocompatible and corrosion resistant, lightweight yet strong, easy to manipulate and possesses long term stability. The 3-D Titanium mesh implant fulfills most of these criteria, and offers certain added advantages, as exemplified in this case series. Four patients with post craniectomy osseous defects of varying sizes and at different locations of the calvarium were studied. In addition to the obvious cosmetic deformity, the patients also exhibited various subjective and objective features of neurosensory and motor deficits characteristic of the motor trephine syndrome (MTS), that often develops secondary to large cranial defects.
Objective
There have been no documented reports so far on the effect of Titanium mesh cranioplasty on features of the MTS in patients with large cranial defects. It was the objective of this study to see if any specific therapeutic goals such as reversal of the neurological deterioration and sensorimotor deficits associated with the syndrome could be achieved by performing Titanium mesh cranioplasty to reconstruct the missing part of the cranial shield in these patients. Any added benefits of using 3-D Titanium mesh as a cranioplasty material were also recorded.
Materials and Methods
The cranial defects in all four patients were reconstructed using different dimensions of Titanium mesh implants. Two of the cases were early cranioplasties (performed within 3 months of craniectomy) and two were late cranioplasties (performed after 3 months of craniectomy), one of them even being a previous autologous bone flap cranioplasty failure. The patients were followed up for a period ranging from 3 to 4 years and observed carefully for cosmetic, functional and neurological improvements following the cranioplasty.
Results
There was achieved gratifying cosmetic correction of the cranial deformities, and remarkable functional recovery from the sensorimotor deficits, reversal of the neurological deterioration as well as resolution of most of the symptoms of MTS in all the four patients, following the Titanium mesh cranioplasty. Many added benefits were noted, such as quick post operative recovery, absence of any early or late complications and a ready means to aspirate any epidural collections, if they developed post operatively, through the mesh structure of the implant.
Conclusion
Apart from the cosmetic, functional and protective roles, Titanium mesh cranioplasty demonstrated a definite therapeutic role in all the cases presented, making it preferable to even replacement with natural bone (either re-implanted excised autologous bone flaps, or cortico-cancellous bone autografts). It is an extremely safe and reliable alternative to autografts, and is even preferable to them, especially when the size of the cranial defect is large.
Keywords: Decompressive craniectomy (DC), Subdural hematoma (SDH), Cranioplasty, Motor trephine syndrome (MTS), Sinking skin flap syndrome (SSFS), 3-Dimensional titanium mesh implant
Introduction
A large bony defect of the cranial vault can result from trauma, infection, ablative resection of a cranial tumor or a cerebral decompression procedure. The resulting absence of a cranial shield or barrier makes the intracranial structures such as the brain, vulnerable to injury.
Cranioplasty is the surgical repair, replacement and restoration of a skull defect, thus achieving morphological and functional rehabilitation of the cranial vault [1]. In addition to the cosmetic and protective roles, cranioplasty has also been reported to have a functional and therapeutic role as it sometimes helps to reverse features of a condition called the motor trephine syndrome (MTS) [2], which is characterized by Neurosensory and Motor deficits, that develop due to derangements in Cerebral hemodynamics, Cerebrospinal fluid (CSF) hydrodynamics and shrinkage and displacement of intracranial structures, secondary to large cranial defects [3].
Since the end of the 19th century, a wide array of materials has been be used for the closure of cranial defects. These days, it is mostly osteoplastic reconstruction using autografts [4, 5] and restoration with alloplastic implants, which are in vogue. Although autografting, that is, either reimplantation of the excised bone flap [6, 7], or transfer of cortical, cancellous or corticocancellous bone from any anatomic site to the defect site in the same subject, is obviously the best choice owing to the lack of immune or foreign body reactions, absence of a risk of transmission of disease, and the potential of the graft to be incorporated as biologically active and dynamic living tissue it has certain inherent limitations and disadvantages. Bone flap/graft resorption and infection [8, 9], donor site morbidity, inadequate quantity of graft harvestable to bridge large cranial defects, prolonged intra-operative time, intraoperative blood loss and requirement of transfusions, need for surgical expertise and delayed post operative recovery [10] are definite drawbacks of autografting. Advanced age, presence of comorbidities, poor general condition of the patient, difficulties with bone flap storage, acute case scenarios and anatomical constraints can lead to difficulty in autografting or to the loss of the excised bone flap in a large number of cases [11, 12]. At times, complications may result following Autogenous bone grafting, such as surgical site infection (SSI), graft/bone flap exposure, resorption, migration, hematomas and seromas [13].
On the other hand, alloplastic materials, particularly the Titanium mesh implant as a skull-repairing material is convenient for calvarial defect reconstruction because it is readily available, inert, lightweight, sufficiently strong and rigid to reconstruct large cranial defects, undergoes no resorption, and entails no donor site morbidity [14]. It is malleable and pliable making it easy to mould and manipulate intraoperatively based on the shape of the skull defect, at the same time having adequate impact resistance to offer reasonable cerebral protection. The Titanium mesh implant, by virtue of its excellent biocompatibility, corrosion resistance and ability to osseointegrate, makes it a very safe and reliable alternative to autografts and is sometimes even preferred over them [14]. Titanium mesh has been proved to have greater strength and provide a superior cranial contour restoration than the thin split thickness calvarial bone grafts [15]. Being a non-ferromagnetic metal, that is relatively radiolucent, Titanium induces no major artifacts, causes no significant scatter or image degradation on CT scans or on MRI and permits safe and accurate imaging of the brain in the long-term post operative follow up period [16].
A series of four cases is reported, in which cranioplasties of large cranial defects, 3 resulting from decompressive craniectomies and one from excision of a cranial tumor, were carried out, using Titanium mesh implants. All the four patients had exhibited varying degrees of neurological and sensorimotor deficits characteristic of the MTS which developed following the craniectomy, most of which were successfully reversed following the Titanium mesh cranioplasty. This was achieved by the Titanium mesh replacing the lost cranial structure, allowing restoration of the size, shape and position of the shrunken and displaced brain, normalizing intracranial pressure and volume relationships and restoring cerebral hemodynamics. There was also a good restoration of the protective shield and barrier at the calvarial defect site, with gratifying cosmetic, functional and therapeutic outcomes in all the cases.
Case Series
Case 1
A 49 year old male patient had undergone an emergency fronto-temporo-parietal craniectomy a year ago for the evacuation of a spontaneous intracerebral hematoma. At the time, he had presented with severe headache of sudden onset with episodes of vomiting, three episodes of tonic–clonic seizures and bladder and bowel incontinence since the preceding 36 h. Non Contrast Computed Tomography (NCCT) scans of the head had revealed intraventricular haemorrhage and a 5 cm × 6 cm hematoma in the right Temporo-parietal region. Cerebral angiography had revealed a multilobulated fusiform aneurysm at the bifurcation of the right middle cerebral artery (MCA). An emergency right fronto-temporo-parietal craniectomy was carried out with clipping of the aneurysm and evacuation of the intracerebral hematoma.
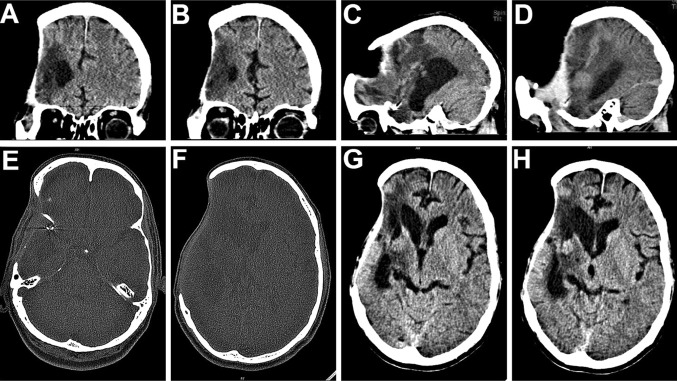
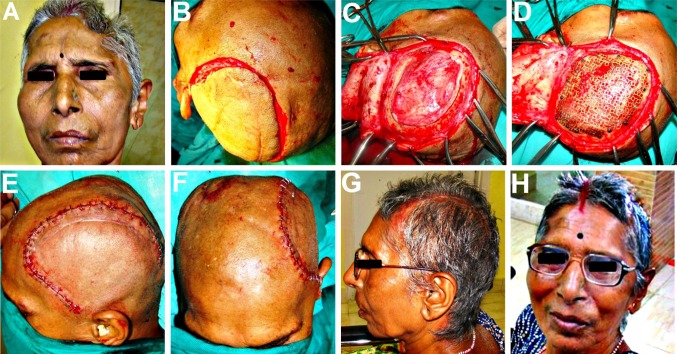
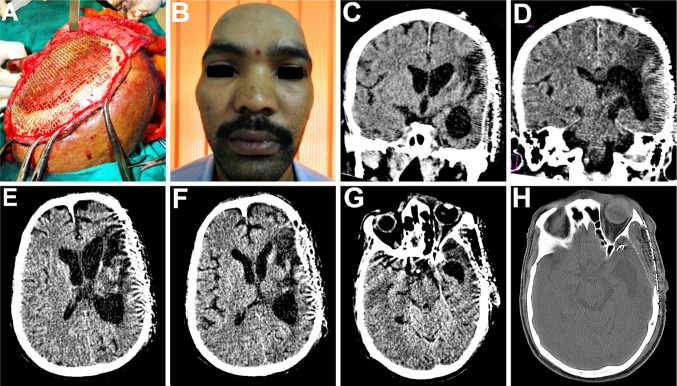
Following the craniectomy, the patient had developed a left sided hemiparesis and was confined to a wheelchair (Fig. 1A). He also suffered from headache, dizziness, seizures, and episodes of anxiety attacks, depression, memory loss and mood swings, all typical objective and subjective symptoms of the motor trephine syndrome. The scalp flap overlying the bony defect site was indrawn, sucked in producing a gorge like appearance (Fig. 1B, C), typical of the “sinking skin flap syndrome (SSFS)”. On the CT scans, the cranial contents appeared to have a kidney bean shape with a concavo-convex surface, with a midline shift to the left (Fig. 2A–H). As the excised calvarial bone flap of the patient had not been preserved and due the large size of the cranial defect, a Titanium mesh cranioplasty was planned for reconstructing it (Fig. 1D).
Fig. 1.
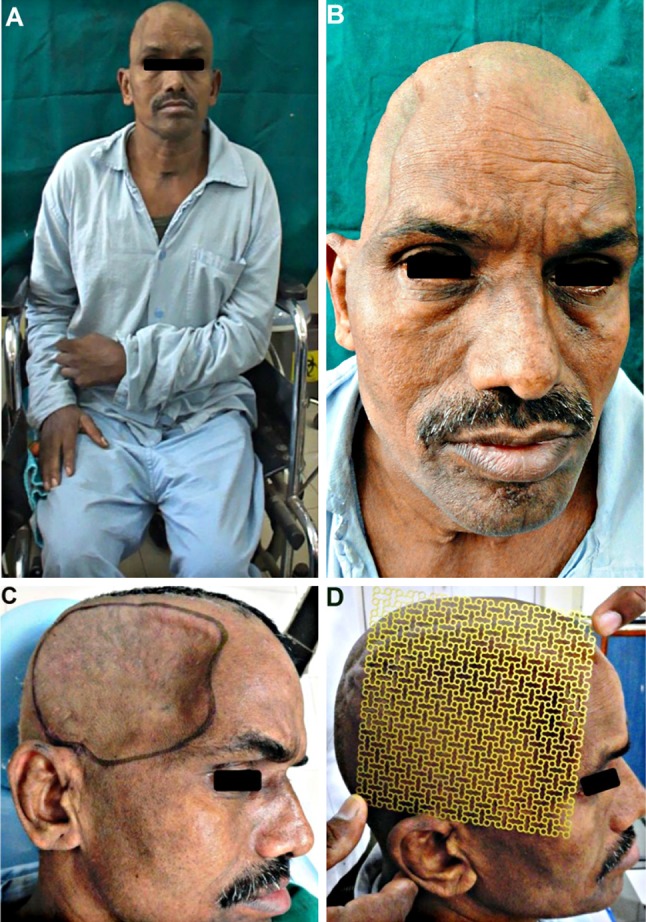
A A 49 year old patient who developed left sided hemiparesis following a right sided decompressive craniectomy carried out a year ago. B, C The large right sided fronto-temporo-parietal post craniectomy defect. The scalp flap overlying the cranial defect appeared disfigured, indrawn and “sucked- in”, creating a tense, non-pulsatile, gorge-like pit, typical of the “sinking skin flap syndrome” (“SSFS”). D Reconstruction of the cranial defect planned using a 3-D titanium mesh implant
Fig. 2.
A–D Coronal and sagittal sections of CT scans of the head post craniectomy, revealing the large calvarial defect. E–H On axial section, the cranial contents appeared to have a “kidney bean” shape with a concavo-convex surface, and a midline shift to the left
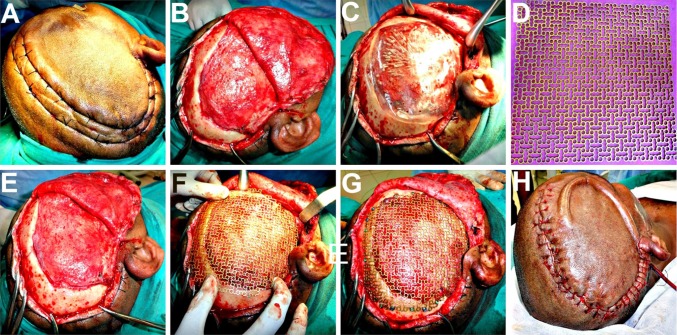
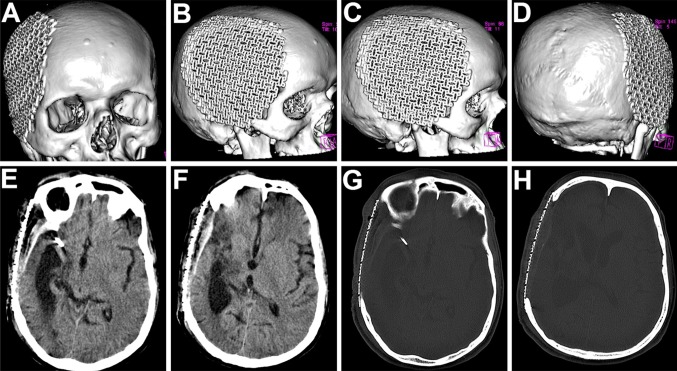
After part preparation, Adrenaline (1:100,000) was injected along the pre-existing scar and haemostatic sutures were placed on either side to reduce the intraoperative bleeding (Fig. 3A). A full thickness scalp flap was raised in a sub-pericranial plane around the defect and along the avascular subgaleal plane between the myocutaneous scalp flap and the underlying dura-like layer over the defect (Fig. 3B). The “sunken in” appearance of dura and underlying brain tissue was immediately evident. A prefabricated acrylic plate that had been prepared and kept as a standby was tried for fit (Fig. 3C) and kept aside. The Titanium mesh (Fig. 3D) was moulded to attain the appropriate curvature of the skull (Fig. 3E), adapted over the defect, cut into shape, contoured and fixed using Titanium micro-screws (Fig. 3F, G). A drain was placed and the scalp flap was closed in layers (Fig. 3H). Post operative radiographs and CT scans showed a good restoration of calvarial contour (Fig. 4A–D) as well as continuity. There was also observed an expansion of the brain and other intracranial contents to their proper dimensions and positions (Fig. 4E–H).
Fig. 3.
A Haemostatic sutures placed on either side of the proposed incision line, to reduce the intraoperative bleeding. B Full thickness scalp flap raised in a sub-pericranial plane around the defect and along the avascular subgaleal plane over the defect. C A prefabricated acrylic plate kept as a standby, was tried for fit. D–F Titanium mesh implant moulded to attain the appropriate curvature of the skull and adapted over the defect. G Implant cut into shape, contoured and fixed using titanium micro-screws. H A vacuum assisted closed suction drain placed prior to scalp flap closure in layers
Fig. 4.
A–D 3-D reformatted CT scan images of the skull showing an excellent restoration of calvarial continuity, contour and symmetry following the titanium mesh cranioplasty. E–H The titanium mesh implant effectively restored a symmetrical cranial outline at the defect site, allowing a distinctly appreciable expansion of the intracranial contents back to their original dimensions
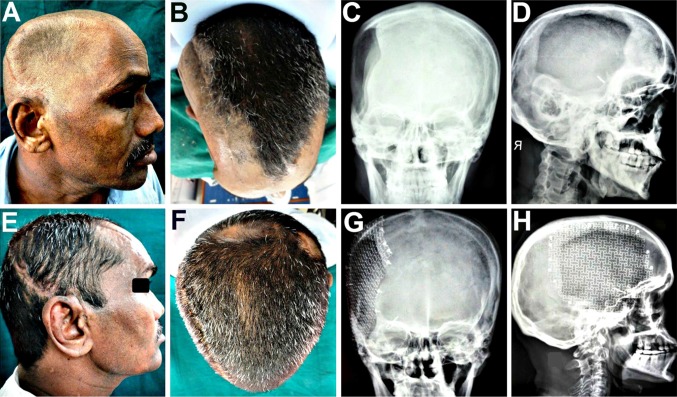
The esthetic as well as functional results following cranioplasty were quite gratifying (Fig. 5) with reversal of most of the symptoms of the MTS syndrome. The left sided hemiparesis improved drastically following the Titanium mesh cranioplasty and the patient was no longer confined to the wheel chair, but was able to walk comfortably with the help of a crutch by the 15th postoperative day. He experienced relief from the persistent discomfort and pain at the defect site he had suffered from, ever since the craniectomy. The episodes of dizziness, seizures, and anxiety attacks all showed a drastic reduction in both frequency and intensity. His family members confirmed that he no longer suffered from mood swings, depression and memory loss.
Fig. 5.
A–D Clinical and radiographic appearance prior to cranioplasty, revealing the unsightly cranial deformity, and the size and extent of the craniectomy defect. E–H Gratifying cosmetic correction and esthetic results achieved, with restoration of cranial form, contour and symmetry following the Titanium mesh cranioplasty, evident both on clinical as well as on anteroposterior and lateral radiographic examination
Case 2
The second patient was a 65 year old lady (Fig. 6A) who had undergone a left sided temporo-parietal craniectomy 3 months ago, to evacuate an acute subdural hematoma (SDH) sustained in a fall from a two-wheeler. She started developing a vague pain and discomfort at the craniectomy defect site, and became more and more listless, irritable and depressed. She also developed weakness of her right limbs and was unable to walk, and was hence confined to a wheelchair. She was unable to use her right hand for eating or writing. She was taken up for an early cranioplasty using Titanium mesh implant (Fig. 6B–F), immediately following which there was a drastic improvement in her motor and cognitive functions as well as her mood and temperament. She became less irritable and more cheerful and interested in her surroundings (Fig. 6G, H). There was an esthetically satisfactory restoration of the calvarial contour and continuity as well. Within the next 10 days, she was able to walk quite comfortably and could use her right hand normally.
Fig. 6.
A 65 year old patient with a Lt temporo-parietal post craniectomy defect. B Incision line marked approximately 1.5 cm outside the palpable margins of the bony defect. C Full thickness scalp flap raised exposing the defect site. D 3-D titanium mesh implant contoured, cut and adapted over the defect and fixed to the adjacent bony margins using titanium microscrews. E, F Scalp flap closed in layers, using deep interrupted resorbable sutures for the pericranium and galea, followed by surgical staples for the cutaneous closure. G, H A good cosmetic reconstruction of the cranial defect as well as functional improvement in her motor and cognitive functions as well as her mood and temperament, following the titanium mesh cranioplasty
Case 3
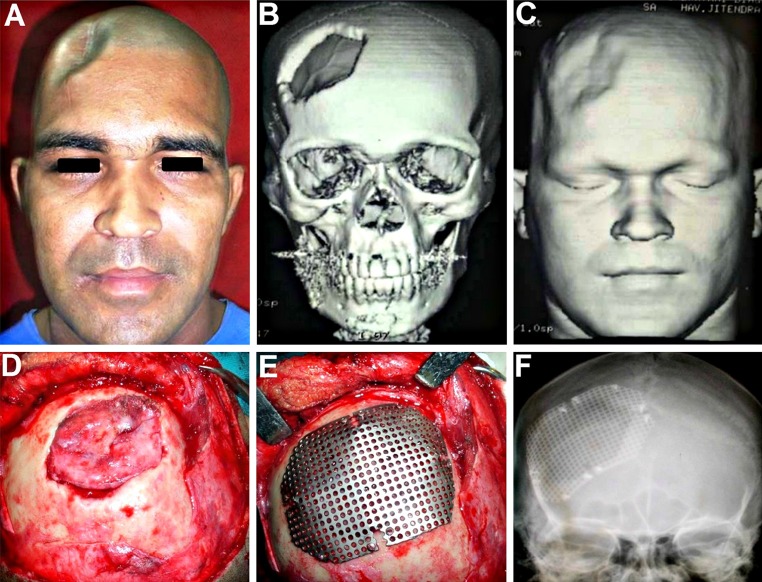
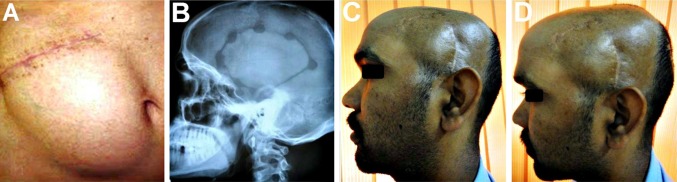
The third patient was a 46 year old male who had undergone a right sided fronto-parietal DC for the evacuation of an acute SDH sustained in road traffic accident (Fig. 7A–C). Following the craniectomy, he developed recurrent episodes of epileptic seizure attacks and a persistent pain and discomfort at the cranial defect site. As the forehead and the frontal region form a dominant part of the face, the defect and irregularity in this region was obvious and easily noticeable, making the patient extremely dissatisfied with his appearance. Cranioplasty of this region is generally both cosmetically as well as functionally challenging as skin of the forehead is thin, delicate, and exposed to view, unlike the scalp which is thick and hidden by the hair. An early cranioplasty using Titanium mesh was carried out (Fig. 7D–F) with excellent cosmetic as well as functional outcomes. It provided a smooth and even contour at the reconstructed site and adequate cerebral protection. The patient was completely free of the seizure episodes and the persistent headache in the region following the cranioplasty, and was completely satisfied with the esthetic results.
Fig. 7.
A–C 46 year old patient who had undergone a right sided fronto-parietal decompressive craniectomy for evacuation of an acute SDH sustained in road traffic accident. Defect was easily noticeable as it was in a prominent part of the forehead which was covered by thin non hair bearing skin. D Defect site exposed by raising a full thickness flap. E Titanium mesh implant contoured over the defect and fixed in place. F A good restoration of form as well as function, with achievement of a smooth even contour of the forehead region, following the cranioplasty
Case 4
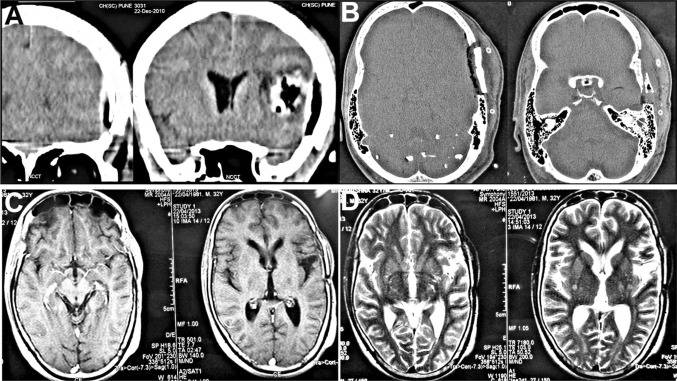
The fourth case was a 32 yr old male patient, in whom a left temporo-parietal craniectomy had been carried out to gain access to treat an Astrocytoma (Fig. 8). The excised bone flap had been preserved in the subcutaneous pocket of his abdominal wall for a period of 7 months (Fig. 8A), during which time he was kept under neurological surveillance, before being finally cleared for cranioplasty. He had developed a weakness of his right arm in the months following the craniectomy, resulting in inability to hold a spoon or to write. The implanted autologous bone flap was retrieved from the abdominal wall and re-implanted at the craniectomy defect site (Fig. 8B). The immediate postoperative recovery was good, however on the 14th postoperative day, the patient developed an epidural effusion and collection at the operated site and a palpable collection of fluid formed beneath the scalp flap in the subgaleal plane (Fig. 8C, D), which was also evident on CT scans of the head (Fig. 9A–D). The bone flap was removed and the epidural collection was aspirated under antibiotic cover. The recipient bed as well as the undersurface of the scalp flap was thoroughly debrided of all granulation tissue and exudate. The cranial defect was then reconstructed using a Titanium mesh implant during the same operative procedure (Fig. 10A) and the patient recovered uneventfully thereafter. There was a good restoration of form as well as function (Fig. 10B–D), with no postoperative SSI. Postoperative CT scans revealed a good restoration of the calvarial continuity and progressive resolution of epidural effusion and collections (Fig. 10E–H). Following the cranioplasty, he regained complete use of his right hand and was able to write as before.
Fig. 8.
A 32 yr old male patient, in whom a left temporo-parietal craniectomy had been carried out to gain access to, and treat an astrocytoma. A The excised bone flap was preserved in his abdominal wall. B 7 months later, the autologous bone flap was retrieved and re-implanted. C, D Palpable collection of fluid which developed beneath the scalp flap, 14 days following the autologous bone cranioplasty
Fig. 9.
CT scans revealed an epidural effusion at the operated site with a collection of fluid beneath the replaced bone flap as well as in the subgaleal plane. There was also some evidence of pneumocephalus beneath the replaced bone flap
Fig. 10.
A Implanted bone flap removed, followed by aspiration of the epidural fluid collection. Surgical bed thoroughly debrided and irrigated followed by immediate reconstruction using titanium mesh implant. B–D An excellent restoration of cranial contour and continuity. E–H Resolution of the epidural as well as subgaleal fluid collection following the titanium mesh cranioplasty
Discussion
The objective of cranioplasty is to obtain a durable and stable reconstruction of a missing part of the cranium, covered with a good skin layer, to restore protection to important visceral components like the brain, improving both aesthetics and function [17]. Literature reports claim that the simplest way to perform a mechanically good cranioplasty is to replace the bone flap that was removed at craniectomy, as it obviously has the most perfect fit [17]. The usual method to preserve an excised bone flap is to place it in a subcutaneous pocket in the abdominal wall or anterolateral thigh of the patient (subcutaneous preservation/intracorporeal storage) [18]. The second method is the Extracorporeal storage by cryopreservation (freezing or freeze drying) of the bone flap in a bone bank at around −70° or so [19]. In this method, there is destruction of bone proteins so the bone flap does not revitalize, and chances of resorption and SSI after cranioplasty is more [20–22]. Studies [9] have documented that implanted bone flaps which are larger than 12 cm and have been preserved for more than 6–9 months (delayed cranioplasties), have a tendency for aseptic resorption resulting in deficiencies at the edges of the bone flap following reimplantation [23], which becomes evident clinically as well as radiographically. Studies have also documented that the longer the delay in cranioplasty, the greater are the chances of autogenous bone flap resorption as well as SSI following cranioplasty [24, 25].
Some data suggest that following DC, a good option would be to consider different alloplastic solutions for the repair, instead of the replacement of the patient’s autogenous flap [26, 27], and that the use of a Titanium mesh carries the lowest risk of infection [27], which makes sense because the patient’s bone is a pabulum for bacteria (being devitalized but organic) [27], whereas Titanium is not. In the fourth patient of the case series presented, the epidural collection which developed beneath the re-implanted bone flap could not be aspirated without removing the flap altogether. The fluid collection in the subgaleal plane could be attributed to infection or an inflammatory exudate surrounding the autologous bone flap, which could have resulted from bacterial growth in the non-vital, yet organic flap. Subsequent cranioplasty using the Titanium mesh implant gave much superior results with ideal restoration of both form and function, and of course, esthetics.
The cranial cavity, unlike other body cavities, is unique in that it resembles a “closed box” [28] the bony structure of its walls restricting any changes in volume, pressure or position of its contents, namely, the brain tissue, blood and CSF, which under normal circumstances exist in a state of equilibrium [29]. Decompressive craniectomy can disrupt this equilibrium by causing a large area of skull defect, destroying integrity of the skull cavity and converting the cranium from a “closed box” to an “open box” [28, 30]. As a result, the intracranial contents may repeatedly develop collapse-bulge shifts with changes in body position [31]. Also, in such patients, absence or lack of a cranial shield or cover at the craniectomy defect site allows the atmospheric pressure to be brought to bear directly upon the unprotected brain causing its displacement and shrinkage [32]. So there occurs a change in the volume, size and shape of the brain, which typically becomes “kidney bean” or “concavo–convex” in form. In addition, factors such as the adhesion between the dura on the brain surface and the margins of the bony defect, cicatricial changes and fibrous adhesions occurring between the brain cortex, dura and the overlying scalp flap, cause further traction, torsion, and compression of the brain and other intracranial contents, impeding local blood supply and venous return [33]. The Barometric pressure of the atmosphere overwhelms the intracranial pressure and causes expulsion of CSF from the ventricular system of the brain, resulting in ventricular deformity; a fall in CSF pressure thus reducing its flow and motion; a reduction in the cerebral blood flow and venous return, thus hampering cerebral hemodynamics, perfusion and metabolism [2]. This is the actual pathophysiology of the “MTS”.
MTS is characterized by objective symptoms such as decline in cognitive behavior and motor functions, including development or worsening of a contralateral Hemiparesis, Neurosensory deficits and altered sensorium, for e.g. a fall in GCS, loss of concentration and memory, and delayed motor deficits. Subjective symptoms of the MTS include vague pain and discomfort at the site of the cranial defect, intolerance to vibrations, undue fatigability, headache and dizziness, anxiety and apprehension, mental depression and mood swings [2, 33]. The scalp flap overlying the Craniectomy defect often becomes disfigured, indrawn and “sucked- in”, creating a tense, non-pulsatile, gorge-like pit, hence the alternative name “SSFS” [34–36].
It is necessary to repair large skull defects following acute phase of craniocerebral injuries and decompressive craniectomies in an attempt not only to meet cosmetic needs and to provide mechanical protection but also to meliorate local blood flow and metabolism of the brain tissues and accordingly facilitate neurofunctional recovery [37]. Cranioplasty using the excised autologous bone flaps have been documented to sometimes help in reversing the MTS by providing a cranial shield or cover [2, 38], thus protecting the brain and cranial contents from the atmospheric pressure. Till date, there have been no documented reports or studies on the effect of Titanium mesh cranioplasty on the functional outcome in patients exhibiting features of the MTS.
In three of the cases presented in this case series, the excised calvarial bone flap had not been preserved at craniectomy, while in one case the flap had been preserved, however, following re-implantation, the patient developed a collection of fluid exudate in the subgaleal plane as well as an epidural effusion and collection beneath the bone flap, which hence had to be removed and discarded and the defect reconstructed using Titanium mesh.
There was an excellent restoration of the calvarial contour and continuity following the Titanium mesh cranioplasty in all the four cases and the Titanium mesh implants displayed satisfactory rigidity, strength and impact resistance, thus providing adequate protection to the intracranial contents. Also, post cranioplasty NCCT confirmed a good restoration of integrity of the cranial vault with a well appreciable expansion of the brain back to its original contour and dimensions. The patients were followed up for a period ranging from 3 to 4½ years, with no development of any complications like wound dehiscence, implant exposure or SSI and the implants maintained their integrity, form and structure. An important and interesting observation in all the four cases was that there was a definite and remarkable reversal of most of the features of the MTS following the Titanium mesh cranioplasty. In the first patient, the left sided hemiparesis improved drastically following the Titanium mesh cranioplasty (“late cranioplasty”) carried out a year after the DC and he was no longer confined to the wheel chair, but was able to walk comfortably with the help of a crutch. He experienced relief from the persistent headache that he had suffered from ever since the craniectomy. The episodes of dizziness, seizures, mood swings and anxiety attacks all showed a drastic reduction in both frequency and intensity. The second patient had become listless, depressed and irritable following the DC and had also developed a right sided hemiparesis, which resolved spontaneously following the Titanium mesh cranioplasty which was carried out at 3 months (“early cranioplasty”). There was a dramatic improvement in her mood and temperament following the cranioplasty. She regained complete use of her right hand (was able to write and hold a spoon while eating, etc.) and was able to walk comfortably. The third patient, with the relatively cosmetically and functionally challenging cranial defect in the fronto-parietal region, exhibited an excellent esthetic outcome following the Titanium mesh cranioplasty in addition to complete recovery from episodes of epileptic seizures that had developed post craniectomy.
The fourth patient, who had undergone craniectomy to gain access to and to remove a large intracranial Astrocytoma, had developed a weakness of his right upper limb, resulting in inability to hold a spoon or to write. Following the replacement of the autologous flap with the 3-D Titanium mesh, he regained complete use of his right hand and arm.
Conclusion
The observations and findings of this series of cases presented, support the fact that Titanium mesh cranioplasty can serve not only as a merely cosmetic procedure, but as a definite therapeutic procedure as well, by reversing the subjective and objective features of the MTS, bringing about a definite reversal in the neurological deterioration and sensorimotor deficits that often develop following large craniectomy procedures. Its malleable and pliable nature makes it easy to handle, manipulate and mould to the desired shape and contour intraoperatively. It provides sufficient cerebral protection by virtue of its reasonable impact resistance. It can be used for large craniectomy defects without fear of resorption or SSI unlike that often seen with autologous bone flaps and grafts. It helps to provide a smooth and even contour at the relatively challenging frontal and forehead region as well, where it lies directly beneath the delicate forehead skin. An added advantage could also be its mesh structure, which can permit aspiration of any epidural collections that might develop in the post operative period, without having to remove the implant altogether. It is thus an extremely safe and reliable alternative to autografts, and is even preferable to them, especially when the size of the cranial defect is large.
References
- 1.Aciduman A, Belen D. The earliest document regarding the history of cranioplasty from the Ottoman era. Surg Neurol. 2007;68:349–353. doi: 10.1016/j.surneu.2006.10.073. [DOI] [PubMed] [Google Scholar]
- 2.Dujovny M, Agner C, Aviles A. Syndrome of the trephined: theory and facts. Crit Rev Neurosurg. 1999;9:271–278. doi: 10.1007/s003290050143. [DOI] [PubMed] [Google Scholar]
- 3.Alibhai MK, Balasundaram I, Bridle C, Holmes SB. Is there a therapeutic role for cranioplasty? Int J Oral Maxillofac Surg. 2013;42:559–561. doi: 10.1016/j.ijom.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Artico M, Ferrante L, Pastore FS, Ramundo EO, Cantarelli D, Scopelliti D, et al. Bone autografting of the calvaria and craniofacial skeleton: historical background, surgical results in a series of 15 patients, and review of the literature. Sur Neurol. 2003;60:71–79. doi: 10.1016/S0090-3019(03)00031-4. [DOI] [PubMed] [Google Scholar]
- 5.Prolo DJ, Oklund SA. The use of bone grafts and alloplastic materials in cranioplasty. Clin Orthop Rel Res. 1991;268:270–278. [PubMed] [Google Scholar]
- 6.Osawa M, Hara H, Ichinose Y, Koyama T, Kobayashi S, Sugita Y. Cranioplasty with a frozen and autoclaved bone flap. Acta Neurochir. 1990;102:38–41. doi: 10.1007/BF01402184. [DOI] [PubMed] [Google Scholar]
- 7.Movassaghi K, Ver Halen J, Ganchi P, Amin-Hanjani S, Mesa J, Yaremchuk M (2006) Cranioplasty with subcutaneously preserved autologous bone grafts. Plast Reconstr Surg 117:202–206 [DOI] [PubMed]
- 8.Kim H, Sung SO, Kim SJ, Kim SR, Park IS. Analysis of the factors affecting graft infection after cranioplasty. Acta Neurochir (Wien) 2013;155:2171–2176. doi: 10.1007/s00701-013-1877-8. [DOI] [PubMed] [Google Scholar]
- 9.Grant GA, Jolley M, Ellenbogen RG, et al. Failure of autologous bone-assisted cranioplasty following decompressive craniectomy in children and adolescents. J Neurosurg. 2004;100:163. doi: 10.3171/ped.2004.100.2.0163. [DOI] [PubMed] [Google Scholar]
- 10.Lee C-H, Chung YS, Lee SH, Yang H-J, Son Y-J. Analysis of the factors influencing bone graft infection after cranioplasty. J Trauma Acute Care Surg. 2012;73:255–260. doi: 10.1097/TA.0b013e318256a150. [DOI] [PubMed] [Google Scholar]
- 11.Matsuno A, Tanaka H, Iwamuro H, Takanashi S, Miyawaki S, Nakashima M, et al. Analyses of the factors influencing bone graft infection after delayed cranioplasty. Acta Neurochir (Wien) 2006;148:535–540. doi: 10.1007/s00701-006-0740-6. [DOI] [PubMed] [Google Scholar]
- 12.Moreira-Gonzalez A, Jackson IT, Miyawaki T, Baqrakat K, DiNick V. Clinical outcome in cranioplasty: critical review in long-term follow-up. J Craniofac Surg. 2003;14:144–153. doi: 10.1097/00001665-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Eom KS, Kim DW, Kang SD. Bilateral diffuse intracerebral hemorrhagic infarction after cranioplasty with autologous bone graft. Clin Neurol Neurosurg. 2010;112:336–340. doi: 10.1016/j.clineuro.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Bogris Elephterios N, Dobrin A Chiriac. Titanium mesh cranioplasty for patients with large cranial defects—technical notes. Roman Neurosurg. 2010;17:456–460. [Google Scholar]
- 15.Sanus GZ, Tanriverdi T, Ulu MO, et al. Use of Cortoss as an alternative material in calvarial defects: the first clinical results in cranioplasty. J Craniofac Surg. 2008;19:88–95. doi: 10.1097/SCS.0b013e3181506477. [DOI] [PubMed] [Google Scholar]
- 16.Cabraja M, Klein M. Long-term results following titanium cranioplasty of large skull defects. Neurosurg Focus. 2009;26:E10–E13. doi: 10.3171/2009.3.FOCUS091. [DOI] [PubMed] [Google Scholar]
- 17. Redfern RM, Pülhorn H (2007) Cranioplasty. 321 ACNR 7(5):32–34
- 18.Schuss P, Vatter H, Oszvald A, Marquardt G, Imöhl L, Seifert V. Bone flap resorption: risk factors for the development of a long-term complication following cranioplasty after decompressive craniectomy. J Neurotrauma. 2013;30:91–95. doi: 10.1089/neu.2012.2542. [DOI] [PubMed] [Google Scholar]
- 19.Yadla S, Campbell PG, Chitale R, Maltenfort MG, Jabbour P, Shara AD. Effect of early surgery, material, and method of flap preservation on cranioplasty infections: a systematic review. Neurosurgery. 2011;68:1124–1129. doi: 10.1227/NEU.0b013e31820a5470. [DOI] [PubMed] [Google Scholar]
- 20.Cheng CH, Lee HC, Chen CC, Cho DY, Lin HL. Cryopreservation versus subcutaneous preservation of autologous bone flaps for cranioplasty: comparison of the surgical site infection and bone resorption rates. Clin Neurol Neurosurg. 2014;124:85–89. doi: 10.1016/j.clineuro.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 21.Honeybul S, Ho KM. How “successful” is calvarial reconstruction using frozen autologous bone? Plast Reconstr Surg. 2012;130:1110–1117. doi: 10.1097/PRS.0b013e318267d4de. [DOI] [PubMed] [Google Scholar]
- 22.Inamasu J, Kuramae T, Nakatsukasa M. Does difference in the storage method of bone flaps after decompressive craniectomy affect the incidence of surgical site infection after cranioplasty? Comparison between subcutaneous pocket and cryopreservation. J Trauma. 2010;68:183–187. doi: 10.1097/TA.0b013e3181c45384. [DOI] [PubMed] [Google Scholar]
- 23.Scloeker B, Trummer M. Prediction parameters of bone flap resorption following cranioplasty with autologous bone. Clin Neurol Neurosurg. 2014;120:64–67. doi: 10.1016/j.clineuro.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Dünisch P, Walter J, Sakr Y, Kalff R, Waschke A, Ewald C. Risk factors of aseptic bone resorption: a study after autologous bone flap reinsertion due to decompressive craniotomy. J Neurosurg. 2013;118:1141–1147. doi: 10.3171/2013.1.JNS12860. [DOI] [PubMed] [Google Scholar]
- 25.Thavarajah D, De Lacy P, Hussien A, Sugar A. The minimum time for cranioplasty insertion from craniectomy is six months to reduce risk of infection—a case series of 82 patients. Br J Neurosurg. 2012;26:78–80. doi: 10.3109/02688697.2011.603850. [DOI] [PubMed] [Google Scholar]
- 26.Alessandro D, Bhaskar et al (2014) From Incan time to today, the unresolved problem of cranioplasty. Commentary on clinical, radiological, and microbiological profile of patients with autogenous cranioplasty infections. World Neurosurg 82(3–4):e439–e441 [DOI] [PubMed]
- 27.Cheng YK. Clinical study factors affecting graft infection after cranioplasty. J Clin Neurosci. 2008;15:1115–1119. doi: 10.1016/j.jocn.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 28.Isago T, Nozaki M, Kikuchi Y. Sinking skin flap syndrome: a case of improved cerebral blood flow after cranioplasty. Ann Plast Surg. 2004;53:288–292. doi: 10.1097/01.sap.0000106433.89983.72. [DOI] [PubMed] [Google Scholar]
- 29.Stiver SL, Wintermark M, Manley GT. Motor trephine syndrome: a mechanistic hypothesis. Acta Neurochir Suppl. 2008;102:273–277. doi: 10.1007/978-3-211-85578-2_51. [DOI] [PubMed] [Google Scholar]
- 30.Yamamura A, Makino H. Neurological deficits in the presence of the sinking skin flap following decompressive craniectomy. Neurol Med Chir (Tokyo) 1997;17:43–53. doi: 10.2176/nmc.17pt1.43. [DOI] [PubMed] [Google Scholar]
- 31.Zhang GL, Yang WZ, Jiang YW, Tao ZENG. Extensive duraplasty with autologous graft in decompressive craniectomy and subsequent early cranioplasty for severe head trauma. Chin J Traumatol. 2010;13:259–264. [PubMed] [Google Scholar]
- 32.Fodstad H, Love JA, Ekstedt J, Friden H, Liliequist B. Effect of cranioplasty on cerebrospinal fluid hydrodynamics in patients with the syndrome of the trephined. Acta Neurochir (Wien) 1984;70:21–30. doi: 10.1007/BF01406039. [DOI] [PubMed] [Google Scholar]
- 33.Akins PT, Guppy KH. Sinking skin flaps, paradoxical herniation and external brain tamponade: a review of decompressive craniectomy management. Neurocrit Care. 2008;9:269–276. doi: 10.1007/s12028-007-9033-z. [DOI] [PubMed] [Google Scholar]
- 34.Schorl M (2009) Sinking skin flap syndrome (SSFS): clinical spectrum and impact on rehabilitation. Cent Eur Neurosurg 70:68–72 [DOI] [PubMed]
- 35.Cecchi PC, Rizzo P, Campello M, Schwarz A. Haemorrhagic infarction after autologous cranioplasty in a patient with sinking flap syndrome. Acta Neurochir (Wien) 2008;150:409–411. doi: 10.1007/s00701-007-1459-8. [DOI] [PubMed] [Google Scholar]
- 36.Bhat AR, Kirmani AR. ‘Sunken brain and scalp flap’’ syndrome following decompressive ‘‘extra-craniectomy’. Indian J Neurotrauma. 2011;8:105–108. doi: 10.1016/S0973-0508(11)80009-2. [DOI] [Google Scholar]
- 37.Liu MY. Practical craniocerebral injuries. 2. Beijing: People’s Military Medical Press; 2003. pp. 403–464. [Google Scholar]
- 38.Dujovny M, Aviles A, Agner C, et al. Cranioplasty: cosmetic or therapeutic? Surg Neurol. 1997;47:238. doi: 10.1016/S0090-3019(96)00013-4. [DOI] [PubMed] [Google Scholar]