Abstract
Bipolar disorder (BD) is a chronic psychiatric disorder of public health importance affecting >1% of the Swedish population. Despite progress, patients still suffer from chronic mood switches with potential severe consequences. Thus, early detection, diagnosis and initiation of correct treatment are critical. Cultured adipocytes from 35 patients with BD and 38 healthy controls were analysed using signal pathway reporter assays, that is, protein kinase C (PKC), protein kinase A (PKA), mitogen-activated protein kinases (extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK)), Myc, Wnt and p53. The levels of activated target transcriptional factors were measured in adipocytes before and after stimulation with lithium and escitalopram. Variations were analysed in the loci of 25 different single-nucleotide polymorphisms (SNPs). Activation of intracellular signals in several pathways analysed were significantly higher in patients than in healthy controls upon drug stimulation, especially with escitalopram stimulation of PKC, JNK and Myc, as well as lithium-stimulated PKC, whereas no meaningful difference was observed before stimulation. Univariate analyses of contingency tables for 80 categorical SNP results versus diagnoses showed a significant link with the ANK3 gene (rs10761482; likelihood ratio χ2=4.63; P=0.031). In a multivariate ordinal logistic fit for diagnosis, a backward stepwise procedure selected ANK3 as the remaining significant predictor. Comparison of the escitalopram-stimulated PKC activity and the ANK3 genotype showed them to add their share of the diagnostic variance, with no interaction (15% of variance explained, P<0.002). The study is cross-sectional with no longitudinal follow-up. Cohorts are relatively small with no medication-free patients, and there are no ‘ill patient' controls. It takes 3 to 4 weeks of culture to expand adipocytes that may change epigenetic profiles but remove the possibility of medication effects. Abnormalities in the reactivity of intracellular signal pathways to stimulation and the ANK3 genotype may be associated with pathogenesis of BD. Algorithms using biological patterns such as pathway reactivity together with structural genetic SNP data may provide opportunities for earlier detection and effective treatment of BD.
Introduction
Almost 1% of the population in the world is affected by bipolar disorder (BD), a mood disorder characterised by switches between depression and hypomanic/manic episodes. Key signs and symptoms manifest not only as mood-related phenomena, but also as more general disturbances in circadian rhythms.1,2 Clinically, the overall suicide risk in patients with BD is high, with ∼15% of patients taking their own lives.3 Despite syndrome remission, the premorbid functional level rarely fully recovers.4,5 BD has been shown to comprise neurodegenerative features in which relapses gradually lead to deterioration of cognitive functions, underlining the importance of early detection in order to prevent an otherwise negative prognosis.6
Evidence for heritability of BD has been recognised in family and monozygotic twin studies, but causal genes have not been identified.7,8 Several single-nucleotide polymorphisms (SNP) have been linked to susceptibility for BD by genome-wide association studies.9 However, it is still unclear how and to what extent different combinations of SNPs affect the susceptibility for BD.
The treatment strategy for BD has focussed mainly on lithium, valproate, lamotrigine and quetiapine—thus, drugs not primarily targeting noradrenaline or serotonin synaptic reuptake mechanisms. Nevertheless, imbalanced neurotransmitter function has been posited to be important in the underlying pathophysiology. However, data support the idea that there exists other endogenous, as well as exogenous factors triggering BD. Pro- and anti-inflammatory cytokines may play a role, and selective serotonin reuptake inhibitors help decrease interleukins and cortisol, at least in major depressive disorder.10 Decreased neurogenesis or increased apoptosis is also thought to be involved in the pathophysiology of this disease.11 Neurotrophic factors are clearly involved—meta-analyses and a large cohort study have reported significantly lower brain-derived neurotrophic factor (BDNF) levels in major depressive disorder patients compared with healthy controls,12 and BDNF has been shown to possess antidepressant effects in animals, effects appearing to be mediated by the mitogen-activated protein (MAP) pathway.13
Furthermore, serum concentrations of mature BDNF and the ratio between mature BDNF and proBDNF are significantly higher in BD patients than in controls.14 Cyclic adenosine monophosphate (cAMP)-dependent protein kinase A (PKA) and protein kinase C (PKC) appear to be potential therapeutic regulatory candidates in mood disorders, although their specific role in BD has yet to be elucidated.15, 16, 17, 18 Abnormalities in Ca2+ signalling have been reported.19 Interestingly, lithium-responsive BD patients had longer telomeres than those not responding well, irrespective of age.20 These reports do support the notion that several pathways are affected in BD.
To study postsynaptic signalling pathways we established adipocyte-derived cell cultures from abdominal fat samples from patients with BD and healthy controls. These adipocytes were exposed to several drugs of interest. We selected escitalopram, which effects neurones in more ways than only inhibiting serotonin synaptic reuptake, and lithium, being the mood stabiliser of choice in BD.
The aims of the study were (1) to test whether a simple drug test shows different patterns in reactivity of postsynaptic signalling pathways in primary cultured adipocytes between patients with BD and healthy controls, and (2) to elucidate whether an algorithm using biological patterns such as pathway reactivity together with structural genetic SNP data could be used for clinical predictions.
Subjects and methods
Bipolar patients
A total of 35 mood-stabilised Caucasian research patients (12 males and 23 females) were recruited from the Bipolar Outpatient Department at the Psychiatric Clinic, Sahlgrenska University Hospital in Gothenburg, Sweden. Mean age was 38±10 years. To validate their diagnoses they were interviewed with the structured psychiatric interview, Mini-International Neuropsychiatric Interview (M.I.N.I.), version 6,21 in an authorised Swedish translation, yielding DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition) diagnoses. Subdiagnoses were bipolar I (n=11), bipolar II (n=7) and bipolar not otherwise specified or spectrum (n=17) diagnoses. The patients with bipolar I diagnoses all had suffered from psychotic symptoms at least once during a previous depressive or manic episode. All patients were above 18 years of age. Mean age was 40.2±10.3 years. Table 1 shows the subdiagnostic composition.
Table 1. Subdiagnostic composition of patients.
| Males | Females | |
|---|---|---|
| Bipolar I, psychotic | 6 | 5 |
| Bipolar I, nonpsychotic | 0 | 0 |
| Bipolar II | 3 | 4 |
| Bipolar not otherwise specified (NOS) | 3 | 14 |
| Healthy controls | 18 | 20 |
The Regional Research Ethics Board in Gothenburg approved the study (172-08).
Healthy controls
As controls, 38 psychiatrically healthy Caucasian individuals (18 men and 20 women) were used. They were interviewed and presented a nondiagnostic result on M.I.N.I. version 6. All controls were above 18 years of age. Mean age was 30±12 years.
Before commencement of the study and signing the written consent, all subjects were provided with verbal and written information about the study and about potential risks and benefits of study participation.
Assessment of clinical variables
Age, height, weight, sagittal abdominal diameter and waist circumference were measured, and the body mass index was calculated. Age, age at first diagnosis, the latency before diagnosis, years with diagnosis, number of suicide attempts and numbers of depressive, manic and mixed episodes were noted. For dimensional assessments, the Montgomery–Åsberg Depression Rating Scale (MADRS)22 and Young Mania Rating Scale (YMRS)23 were used to assess mood states, and Mini-Mental State Examination (MMSE)24 for cognitive impairment. Disability was assessed by Global Assessment of Functioning (GAF).25 To assess addiction, Alcohol Use Disorder Test (AUDIT)26 and the Drug Use Disorder Test (DUDIT)27 were used. When needed, supplementary information was collected from medical records. Healthy controls were assessed by the AUDIT, DUDIT, GAF, YMRS, MADRS and MMSE. All clinical measurements are listed in Table 2.
Table 2. Description of measured clinical variables.
| Arithmetic means (±s.d.) | Patients | Healthy controls |
|---|---|---|
| n | 35 | 38 |
| Age (yrs) | 38±10 | 30±12 |
| Height (cm) | 173±20 | 174±8 |
| Weight (kg) | 83±18 | 71±12 |
| Sagittal diameter (cm) | 19.4±3.5 | 16.3±2.5 |
| Waist circumference (cm) | 93.3±14.6 | 79±11.1 |
| BMI | 25.5±4.8 | 23.5±3.3 |
| Age at first symptoms | 18±6.5 | |
| Age at diagnosis | 30.6±8.9 | |
| Diagnostic latency | 12.5±8.3 | |
| Yrs with diagnosis | 7.4±8.3 | |
| No. of suicide attempts | 1±2.5 | |
| No. of depressive episodes | 12±14 | |
| No. of manic episodes | 7.6±8.2 | |
| No. of mixed episodes | 0.125±0.3 | |
| AUDIT | 4.1±3.8 | 5.6±3.3 |
| DUDIT | 1.3±3.4 | 0.6±1.3 |
| GAF | 71±14 | 89±4 |
| YMRS | 0.8±2.2 | 0.2±0.6 |
| MADRS | 9.1±8.2 | 1.3±2.5 |
| MMSE | 28.5±1.7 | 29.6±0.6 |
Abbreviations: AUDIT, Alcohol Use Disorder Test; BMI, body mass index; DUDIT, Drug Use Disorder Test; GAF, Global Assessment of Functioning; MADRS, Montgomery–Åsberg Depression Rating Scale; MMSE, Mini-Mental State Examination; YMRS, Young Mania Rating Scale.
Individuals with active metabolic disorders and/or alcohol/drug addiction were excluded. Before sampling, the participants underwent a series of biochemical and somatic tests, as well as routine diagnostic screening and clinical estimates.
Adipocyte isolation and primary cell culture establishment
Abdominal adipose tissue (2–3 ml) was isolated by liposuction and transported to the laboratory within 30 min. The tissue was washed several times with Hanks' balanced salt solution (Life Technologies, Carlsbad, CA, USA) to remove any remaining blood residues. The tissue was mechanically disrupted by using sterile action scissors, followed by enzymatically digestion in buffer consisting of 150 mM collagenase I (Sigma-Aldrich, St Louis, MO, USA), bovine serum albumin and phosphate buffer saline. The tissue was incubated for 1 h at 37 °C. The reaction was stopped by adding Dulbecco's modified Eagle's medium (Lonza, Basel, Switzerland) supplemented with 20% fetal bovine serum, 2 mM glutamine and 1% of penicillin/streptomycin, and the disassociated tissue was filtered through a 100 μm Nylon mesh cloth (Cell strainer, 100 mm; BD Biosciences, San José, CA, USA). The isolated adipocytes were grown as described above at 37 °C and 5% CO2. The initial primary cell culture is referred to ‘passage 0.' Cell medium was changed every 2 to 3 days, and cells were passaged every 5–7 days, depending on the cell density. Adipocytes were harvested between passages four and six in order to perform the experiments.
Luciferase assay
In order to analyse possible difference in various signalling pathways, we compared the degree of activation of their target transcription factors as read-out by using luciferase assays. The analyses were performed under three different conditions, that is, untreated cells and cells treated with escitalopram or with lithium. The intracellular signalling molecules, such as kinases that are activated by the drugs, phosphorylate the target transcription factors. Then, they bind the consensus binding sites of different promoters, inducing the expression of the reporter luciferase, used as read-out for the activity. The promoter reporter constructs have different consensus binding sites for transcription factors, that is, cAMP response element-binding protein (CREB; downstream of PKA), nuclear factor of activated T-cells (NFAT; PKC), Elk/SRF (extracellular signal-regulated kinase (ERK)), activation protein 1 (c-Jun N-terminal kinase (JNK)), MycMax (Myc), TCF/LEF (Wnt) and p53/p63/p73 (p53), that were compared with positive and negative controls. The experiment was performed according to the vendor's instructions. Briefly, cells were reverse transfected at 80% confluence in a 24-well plate with Cignal™ Reporter Assays DNA-based reporter for each pathway (Qiagen, Valencia, CA, USA) by using FuGENE HD transfection reagent (Promega, Madison, WI, USA) in a 0.4:6 ratio (plasmid to reagent). The culture medium used upon transfection was Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 2 mM glutamine without antibiotics. After 24 h, the medium was replaced with fresh one with lithium (5 mM, Sigma-Aldrich) or escitalopram (5 μM, H Lundbeck A/S Co, Valby, Denmark) or medium alone for the nonstimulated cells. On the fourth day, 72 h after transfection, the expression of luciferase was read on a Victor X light luminometer (Perkin-Elmer, Waltham, MA, USA) with the use of the Dual-Luciferase© Reporter Assay System (Promega).
Genotyping
DNA extraction from blood samples was done by using DNeasy Blood and Tissue kit (Qiagen). Genes to be analysed were selected from earlier findings reported in recent genetic studies on bipolar disorder and schizophrenia.28,29 Twenty-five selected genes (see Table 5 for chromosome locations and SNP numbers) were analysed in 41 patients and 39 controls with TaqMan SNP genotyping assay from Applied Biosystems (ABI, Foster City, CA, USA) on ABI StepOne plus thermocycler using TaqMan Genotyping Master Mix according to the manufacturer's instructions.
Table 5. Univariate comparisons of contingency tables of 25 selected SNPs.
Statistical analysis of the results
To analyse the results obtained by quantitative PCR analysis (see Supplementary Information), relative expression values of protein kinase A catalytic subunit-α (PRKACA), protein kinase A regulatory subunit, type Iα (PRKAR1A) and protein kinase A regulatory subunit, type IIα (PRKAR2A) and protein kinase C-α (PRKCA) were acquired by normalising the Cq values of each gene of interest to the geometric average of the reference genes ACTB, GAPDH and TBP. As two different reverse transcription reactions were tested for each clinical sample, the arithmetic average of these was considered the final mean relative expression value for each sample assayed.
IBM SPSS Statistics 20 (Armonk, NY, USA) and SAS JMP (Cary, NC, USA) software were used for analyses, where P<0.05 was established as statistically significant.
Results
Screening differences in drug response of intracellular signalling pathways by using adipocytes from patients and controls.
First, 10 inflammation pathways were screened, that is, nuclear factor-κB (NF-κB), PKC, cAMP/PKA, type-I interferon, interferon-γ, MAP/ERK, MAP/JNK, transforming growth factor-β, CCAAT-enhancer-binding proteins and glucocorticoid receptor. As drugs of interest, escitalopram, lithium, certain neurotransmitters (5-hydroxytryptamine, L-glutamine and dopamine) and dexamethasone were tested. The purpose was to compare differences of signal responses between cells derived from patients and healthy controls, even though adipocytes probably lack certain receptors and transporters that neuronal cells would express. Dopamine also yielded some discriminative responses, but less frequently than the pathways mentioned above. We also tested other pathways not examined in the context of pathology of depression, such as Notch, Wnt, HRE, p53 and Myc, that are involved in development, stress, apoptosis and proliferation.
After several tests on two control cell lines established from volunteers, we decided to evaluate the following seven clearly responsive pathways – PKC, cAMP/PKA, MAP/ERK, MAP/JNK, Wnt, Myc and p53. We screened 15 patients and 15 controls, and after repeated screening of drugs in these pathways, we chose the selective serotonin reuptake inhibitor escitalopram and the mood stabiliser lithium. Both elicited clear responses in several of 10 immune pathways, although selective serotonin reuptake inhibitors are not routinely prescribed to bipolar patients. These results suggested increased activities in BD patient cells as compared with controls, especially after drug stimulations.
Gene expression of PKA and PKC
Previous studies have shown an increased level of basal PKA activity in BD lymphoblasts.30,31 Both untreated unipolar and bipolar patients with psychotic depression have significantly lower levels of platelet regulatory type I and higher levels of catalytic subunits of PKA than controls, whereas the levels of regulatory type II are higher only in psychotic unipolar patients.32 In contrast, other reports claim no difference in PKA basal levels between bipolars and controls, but a significant increase in cAMP-stimulated PKA activity in platelets.33
A majority of previous results on the basal mRNA expression of PKA and PKC indicate no significant difference in bipolar patients and controls. However, as it is not fully known if PKA or PKC signalling is affected at the mRNA level of their regulatory and catalytic subunits, we examined their levels by quantitative PCR analysis on randomly selected samples from both groups. A description of the method is described in the Supplementary Information. The basal relative expression of each gene of interest was obtained by quantitative PCR analysis for PRKACA, protein kinase A catalytic subunit-β (PRKACB), PRKAR1A and PRKAR2A, as well as for PRKCA. They were analysed for both patient and control groups (14 randomly selected subjects in each) with error bars set at a 95% confidence interval. Independent-samples t tests were run giving P-values of 0.072 for PRKACA, 0.123 for PRKAR1A, 0.151 for PRKAR2A and 0.121 for PRKCA, revealing no significant differences at transcriptional level in drug-naive samples. Nonparametric Kruskal–Wallis tests also yielded nonsignificant findings.
PKC and PKA signalling pathways
As shown in Table 3, the basal activities of the PKC pathway are nonsignificantly higher in the bipolar group compared with the controls (P=0.10 to 0.12). However, the difference becomes highly significant after stimulation with escitalopram (P=0.00052) or lithium (P=0.004). The PKA pathway shows diagnostic significant differences similar to those of PKC, but they are weaker.
Table 3. Univariate comparisons of intracellular signalling pathways in bipolar patients and healthy controls, showing significant differences in activities stimulated by escitalopram and lithium.
| Pathways | Diagnosis |
Escitalopram |
Lithium |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Basal level | P | Escitalopram stimulated | P | Esc/Basal | P | Basal level | P | Lithium stimulated | P | Li/Basal | P | ||
| PKC pathway (NFAT) | Bipolars (n=35) | 1.90±0.55 | 2.54±0.63 | 1.42±0.50 | 1.92±0.59 | 1.88±0.65 | 0.99±0.27 | ||||||
| Controls (n=35) | 1.70±0.49 | 0.10 | 2.01±0.63 | 0.00052 | 1.20±0.26 | 0.020 | 1.72±0.52 | 0.12 | 1.47±0.52 | 0.004 | 0.85±0.13 | 0.011 | |
| PKA pathway (CREB) | Bipolars (n=35) | 1.91±0.60 | 2.49±0.87 | 1.32±0.36 | 1.91±0.68 | 1.88±0.81 | 0.99±0.30 | ||||||
| Controls (n=35) | 1.76±0.53 | 0.27 | 2.03±0.61 | 0.011 | 1.20±0.40 | 0.15 | 1.77±0.55 | 0.35 | 1.57±0.49 | 0.050 | 0.88±0.14 | 0.044 | |
| JNK pathway | Bipolars (n=35) | 2.57±0.62 | 2.35±0.56 | 0.94±0.22 | 2.55±0.61 | 2.65±0.67 | 1.04±0.14 | ||||||
| Controls (n=35) | 2.31±0.48 | 0.048 | 1.95±0.29 | 0.00034 | 0.89±0.28 | 0.35 | 2.40±0.55 | 0.28 | 2.35±0.59 | 0.047 | 0.98±0.09 | 0.025 | |
| ERK pathway | Bipolars (n=35) | 2.10±0.57 | 2.55±0.73 | 1.24±0.30 | 2.11±0.57 | 2.10±0.63 | 1.00±0.23 | ||||||
| Controls (n=35) | 1.94±0.53 | 0.21 | 2.18±0.75 | 0.034 | 1.11±0.18 | 0.037 | 1.95±0.57 | 0.21 | 1.88±0.67 | 0.15 | 0.96±0.19 | 0.40 | |
| myc pathway | Bipolars (n=35) | 2.04±0.57 | 2.64±0.73 | 1.41±0.78 | 2.06±0.59 | 2.09±0.63 | 1.03±0.17 | ||||||
| Controls (n=35) | 1.86±0.43 | 0.13 | 2.18±0.61 | 0.0055 | 1.18±0.19 | 0.084 | 1.84±0.45 | 0.085 | 1.80±0.51 | 0.033 | 0.98±0.15 | 0.13 | |
| p53 pathway | Bipolars (n=33) | 2.56±0.62 | 2.76±0.78 | 1.24±0.21 | 2.26±0.61 | 2.22±0.73 | 0.98±0.19 | ||||||
| Controls (n=35) | 2.24±0.64 | 0.90 | 2.51±0.76 | 0.16 | 1.13±0.16 | 0.013 | 2.27±0.65 | 0.95 | 2.32±0.59 | 0.56 | 1.04±0.18 | 0.17 | |
| Wnt pathway | Bipolars (n=25) | 1.75±0.60 | 2.54±0.78 | 1.53±0.43 | 1.76±0.59 | 1.71±0.80 | 0.93±0.29 | ||||||
| Controls (n=30) | 1.92±0.62 | 0.30 | 2.16±0.74 | 0.066 | 1.16±0.25 | 0.00064 | 1.94±0.27 | 0.27 | 1.72±0.68 | 0.92 | 0.87±0.13 | 0.34 | |
Abbreviations: CREB, cAMP response element-binding protein; ERK, extracellular signal-regulated kinase; Esc, escitalopram; JNK, c-Jun N-terminal kinase; Li, lithium; NFAT, nuclear factor of activated T-cells; PKA, protein kinase A; PKC, protein kinase C.
Pathway data are raw activity measures (±s.d.), normalised by 10log transformations and expressed as logged counts per second. Bipolar and control adipocytes were transfected with CREB or NFAT reporter plasmids, and stimulated 24 h after with escitalopram (5 μM) or lithium (5 mM). Luminiscence was measured 48 h after stimulation with Victor X light luminometer (Perkin-Elmer) and Dual-Luciferase® Reporter Assay System (Promega). P-values refer to univariate comparisons between bipolar patients and controls using two-tailed t-tests.
Raw data were 10log transformed. P values less than 0.05 are marked in bold.
JNK and ERK signalling pathways
Table 3 shows that the basal activities of the JNK pathway are higher in the bipolar group compared with the controls (P=0.048 to 0.28) that becomes highly significant after stimulation with escitalopram (P=0.00034) or, less so, with lithium (P=0.047). Weaker but still significant differences for ERK activation were found after escitalopram (P=0.034), but no difference was found by lithium stimulation.
Wnt, Myc and p53 signalling pathways
Lithium, serotonin and dopamine all affect glycogen synthase kinase 3 (GSK-3) phosphorylation, a Wnt target.34, 35, 36 C-myc is known as a proto-oncogene associated with cell proliferation, but it is also known as a pro-apoptotic molecule in certain conditions.37,38 In addition, Myc is a target of Wnt and stabilises β-catenin.39 p53 has been considered to act negatively on neuronal development, promoting neurodegeneration,40 probably by its repressive functions on cell cycle and survival. Lithium has been shown to possess survival effects by antagonising p53.41
Table 3 shows that in the Myc pathway, a significantly higher response was seen in the patient group after escitalopram (P=0.0055) and lithium (P=0.033). In the p53 pathway, patients show a significantly higher level only in the escitalopram/basal ratio (P=0.013). There is also higher response in the Wnt escitalopram/basal ratio (P<0.0006). However, p53 and Wnt pathways did not render any significant differences after lithium stimulation.
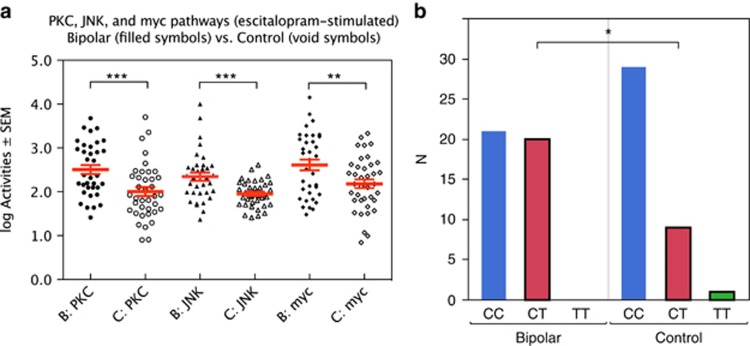
Figure 1a illustrates the significant differences in escitalopram-stimulated activities of PKC, JNK and Myc between bipolar patients and healthy controls.
Figure 1.
(a) Escitalopram-stimulated protein kinase C (PKC), c-Jun N-terminal kinase (JNK) and myc activities vs diagnosis. (b) ANK3 gene (rs10761482) vs diagnosis. ***P<0.001; **P<0.01; *P<0.05.
Multivariate comparisons of seven pathways predicting diagnosis
Log transformed values of PKC, PKA, JNK, ERK, Myc, p53 and Wnt were used as independent variables in nominal logistic fits, predicting the diagnostic dichotomy. Sex was used as a covariate. Table 4a shows results for basal values, after stimulation of escitalopram and the ratios between stimulated and basal activities. Table 4b shows the same for lithium.
Table 4. Multivariate nominal logistic fits for diagnosis (bipolars vs controls), using seven pathways plus sex as independent variables, demonstrating diagnostic power.
| χ2 | P | χ2 | P | χ2 | P | |||
|---|---|---|---|---|---|---|---|---|
| (a) Stimulation with escitalopram | ||||||||
| PKC basal | 6.50 | 0.090 | PKC+esc | 8.99 | 0.030 | PKC esc/basal | 2.87 | 0.41 |
| PKA basal | 10.52 | 0.015 | PKA+esc | 2.62 | 0.45 | PKA esc/basal | 4.68 | 0.20 |
| JNK basal | 7.27 | 0.064 | JNK+esc | 7.22 | 0.065 | JNK esc/basal | 2.43 | 0.49 |
| ERK basal | 0.83 | 0.84 | ERK+esc | 8.23 | 0.041 | ERK esc/basal | 13.23 | 0.0042 |
| Myc basal | 1.95 | 0.58 | Myc+esc | 0.48 | 0.92 | Myc esc/basal | 5.84 | 0.12 |
| p53 basal | 1.66 | 0.64 | p53+esc | 3.70 | 0.30 | p53 esc/basal | 1.51 | 0.68 |
| Wnt basal | 8.02 | 0.046 | Wnt+esc | 3.42 | 0.33 | Wnt esc/basal | 5.61 | 0.13 |
| Sex | 2.80 | 0.42 | Sex | 4.26 | 0.24 | Sex | 1.18 | 0.76 |
| Overall R2 | 0.26 | Overall R2 | 0.31 | Overall R2 | 0.39 | |||
| n | 58 | n | 58 | n | 52 | |||
| P= | 0.071 | P= | 0.018 | P= | 0.0023 | |||
| (b) Stimulation with lithium | ||||||||
| PKC basal | 7.92 | 0.048 | PKC+Li | 13.44 | 0.0038 | PKC Li/basal | 13.45 | 0.0038 |
| PKA basal | 10.38 | 0.016 | PKA+Li | 4.54 | 0.21 | PKA Li/basal | 12.49 | 0.0059 |
| JNK basal | 8.26 | 0.041 | JNK+Li | 15.15 | 0.0017 | JNK Li/basal | 6.61 | 0.085 |
| ERK basal | 2.62 | 0.45 | ERK +Li | 2.37 | 0.50 | ERK Li/basal | 4.86 | 0.18 |
| Myc basal | 1.25 | 0.74 | Myc+Li | 1.41 | 0.70 | Myc Li/basal | 8.77 | 0.033 |
| p53 basal | 2.54 | 0.49 | p53+Li | 12.17 | 0.0068 | p53 Li/basal | 6.72 | 0.081 |
| Wnt basal | 8.89 | 0.031 | Wnt+Li | 13.63 | 0.0035 | Wnt Li/basal | 4.10 | 0.25 |
| Gender | 4.34 | 0.23 | Gender | 9.08 | 0.028 | Gender | 7.91 | 0.048 |
| Overall R2 | 0.26 | Overall R2 | 0.32 | Overall R2 | 0.34 | |||
| n | 59 | n | 59 | n | 59 | |||
| P= | 0.070 | P= | 0.0076 | P= | 0.0040 | |||
Abbreviations: ERK, extracellular signal-regulated kinase; esc, escitalopram; JNK, c-Jun N-terminal kinase; Li, lithium; PKA, protein kinase A; PKC, protein kinase C.
Raw data were 10log transformed before division. Numbers in bold denote significant probabilities (P<0.05).
The best predictions were obtained with the stimulated/basal ratio (block 3 in Table 4a and b). For the escitalopram/basal data, Myc was the strongest individual predictor (P=0.0084), and for the lithium data three individual predictors were significant: PKC (P=0.014), JNK (P=0.0045) and p53 (P=0.030). Maximal variance explained (overall R2) was 39% for the escitalopram/basal data set.
The power to predict diagnosis from these equations was high, with a specificity of 81% and a sensitivity of 85% for the ratios between escitalopram-stimulation/basal level (χ2 test, P<0.0001).
Genetic analyses of 25 SNPs, selected for relevance
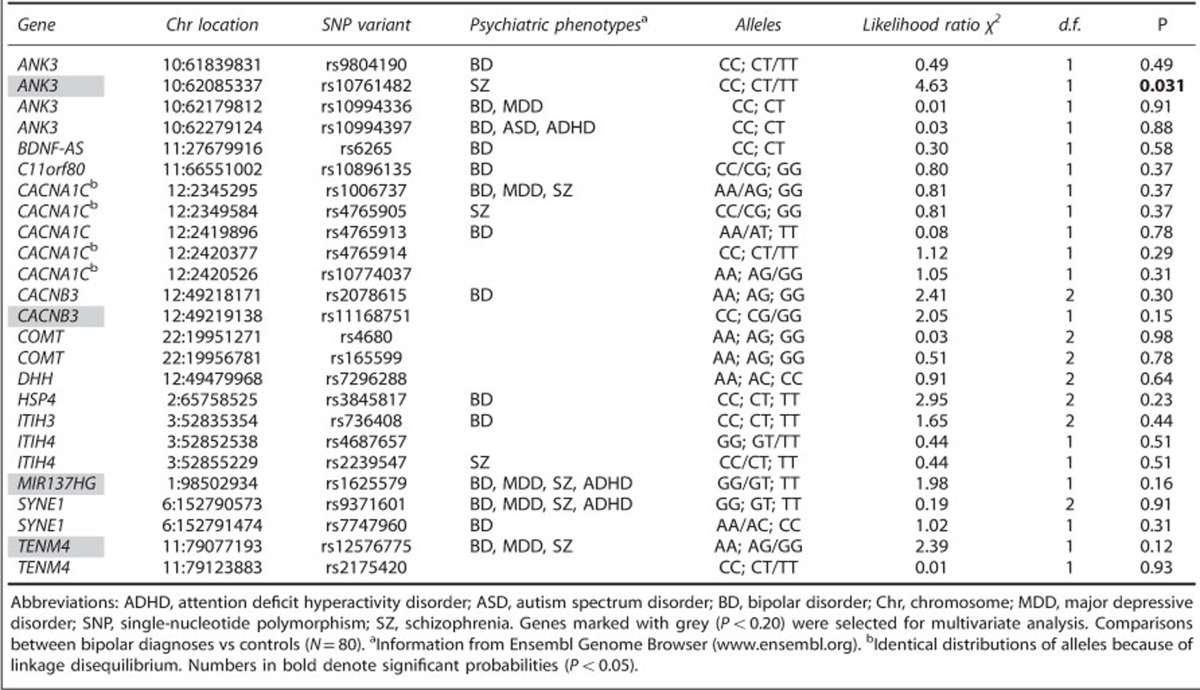
Selection of the 25 SNPs to be included in our statistical analysis was driven by published hypotheses concerning relevance to bipolar and other severe psychiatric disorders. Univariate χ2 analyses of the 80 individuals with contingency tables for categorical SNP results (2 to 3 levels) versus diagnosis (2 levels) are shown in Table 5. The strongest and only significant link to diagnosis was seen with ANK3 (location 10: 62085337; rs10761482; likelihood ratio χ2=4.63; P=0.031). Figure 1b shows the sample distribution of the ANK3 gene (SNPs CC, CT and TT), split for diagnosis. Rare homozygotic alleles were assigned to the heterozygotic variety before statistical calculations (for example, TT was assigned to CT, if TT was represented by only one to three individuals—the minor allele frequency being <5%).
Table 6 shows a multivariate, ordinal logistic fit for diagnosis, using in a backward stepwise selection procedure, starting with four genes with univariate P<0.20. The SNPs that showed obvious linkage disequilibrium were omitted from statistical analysis. These genes explained 9% of the diagnostic variation with a diagnostic specificity of 74% and sensitivity of 71% (χ2=14.47, P<0.0001). In the final backward step, only ANK3 remained significant.
Table 6. Multivariate stepward logistic regressions with genes as independent variables, predicting diagnosis (N=80).
| Gene | Chr | SNP | χ2 | P | χ2 | P |
|---|---|---|---|---|---|---|
| Multivariate logistic regression: | ||||||
| ANK3 | 10 | rs10761482 | 4.05 | 0.044 | 4.63 | 0.031 |
| CACNB3 | 12 | rs11168751 | 1.89 | 0.17 | — | |
| MIR137HG | 1 | rs1625579 | 1.34 | 0.25 | — | |
| TENM4 | 11 | rs12576775 | 2.04 | 0.15 | — | |
| Overall statistics: | ||||||
| R2 | 0.094 | 0.042 | ||||
| n | 80 | 80 | ||||
| P | 0.034 | 0.031 | ||||
| Classification power: | ||||||
| χ2 | 14.47 | 3.63 | ||||
| P | <0.0001 | 0.056 | ||||
| Specificity | 74% | 58% | ||||
| Sensitivity | 71% | 67% | ||||
Abbreviations: Chr, chromosome; SNP, single-nucleotide polymorphism.
Adding sex as an independent variable produced a nonsignificant weight (P=0.35; P=0.29). Numbers in bold denote significant probabilities (P<0.05).
Comparing the powers of PKC activity and ANK3 alleles to predict diagnosis
Two independent variables—the quantitative escitalopram-stimulated PKC activity and the qualitative information on ANK3 alleles—were compared in predicting the dependent bipolar | control dichotomy (Table 7). PKC turned out significant (P=0.0005) and ANK3 was at trend level (P=0.073), overall R2=0.15, P=0.0016. An interactive term was clearly nonsignificant, suggesting the contributions of the PKC pathway and the ANK3 gene to be additive, not synergistic. Diagnostic specificity was 70% and sensitivity 67%.
Table 7. Nominal logistic fits for three dependent variables (diagnosis, heredity and psychotic ever).
| Dependent variables → |
Bipolar|control |
Bipolar heredity (Y|N) |
Psychotic ever (Y|N) |
|||
|---|---|---|---|---|---|---|
| Independent variables ↓ | χ2 | P | χ2 | P | χ2 | P |
| logPKC_esc | 12.24 | 0.0005 | 7.62 | 0.0058 | 2.19 | 0.14 |
| ANK3 (CC, CT) | 3.21 | 0.073 | 4.30 | 0.038 | 2.04 | 0.15 |
| logPKC × ANK3 | 1.19 | 0.28 | 0.04 | 0.84 | 0.07 | 0.79 |
| Overall R2 | 0.15 | 0.14 | 0.05 | |||
| n | 73 | 73 | 71 | |||
| P= | 0.0016 | 0.0067 | 0.21 | |||
| Classification χ2 | 8.48 | 8.24 | 0.00 | |||
| P= | 0.0036 | 0.0041 | 0.98 | |||
| Sensitivity | 70% | 70% | 40% | |||
| Specificity | 67% | 79% | 71% | |||
Independent variables: escitalopram-stimulated protein kinase C activity (logPKC_esc) and the ANK3 (rs10761482) genotype. Simple effects are additive; no significant interactions were detected. Numbers in bold denote significant probabilities (P<0.05).
Predicting bipolar heredity and psychotic episode(s) in patients' psychiatric history
Table 7 also demonstrates presence of bipolar heredity in first-degree relatives to be predictable from the PKC and ANK3 data, but at a somewhat weaker level than that for diagnosis. Prediction of psychotic qualities in any previous manic or depressive episode proved not possible.
Discussion
The relevance of intracellular signalling cascades in mood disorders has become accepted during the past decade, and ever more molecules have been discovered. In this report, escitalopram and lithium were used to stimulate patient-derived primary cultured adipocytes in order to compare their responses in a battery of different signalling pathways.
PKC and PKA
There is evidence that selective serotonin reuptake inhibitors activate presynaptic 5-HT3 receptors, increasing Ca2+ concentration, that activates sigma-1 receptors and efflux of Ca2+ from the endoplasmatic reticulum. This increases intrasynaptosomal Ca2+, resulting in activation of PKC.42 Here, escitalopram was shown to also activate postsynaptic signals.
In contrast, lithium inhibits inositol phosphate hydrolysis and PKC. Increased PKC activity in manic subjects was reported by Friedman et al.43 in 1993, whose work showed higher activity in membrane-bound PKC in platelets derived during mania. When stimulated with lithium for some weeks, both cytosolic and membrane activity decreased. When PKC activity was analysed in post-mortem BD patient brains, cytosolic PKCα and membrane-bound PKCγ and PKCζ activities were elevated, whereas PKCɛ was diminished.16 Activation of PKC was shown to impair the cognitive function of the prefrontal cortex in rat.44
These findings suggested a strong relationship between increased PKC activity and mania; subsequently, considerable effort has been put into finding suitable PKC inhibitors. Lithium, valproate and tamoxifen have proven therapeutic values, decreasing PKC activity.17,43,45 It might be difficult to compare in vivo and in vitro data, but our results showed that the basal activity between cells of patients and controls did not differ. After stimulation with escitalopram or lithium, significantly higher responses were detected in patient-derived cells.
PKA signalling is involved in α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor trafficking by phosphorylating the GluR1 subunit at the S845 site that has currently been shown to be of importance in psychiatric disorders.45 Chang et al.46 analysed the mRNA expression of PKA in post-mortem brains, finding no differences in expression of PKA RIIβ and Cα subunits between patients and controls. We found no differences in the mRNA levels either. Following chronic antidepressant treatments, CREB becomes phosphorylated by PKA but more markedly by a nuclear calmodulin-II (CaMII) kinase and increases the expression of ERK1/2.47 Calcium influx activates CaMII kinase and phosphorylates CREB.48 In our univariate comparisons of PKA, we found significantly higher responses in patients than in controls after drug stimulation, but no difference was seen in basal activity. In agreement, our PCR results showed no difference at the mRNA levels of both the regulatory and catalytic subunits of PKA and PKC, comparing patients and controls in drug-deprived conditions. We did not perform the PCR analysis in other pathways as we focus on intracellular signalling molecules that are often activated by protein modification.
Our study adds to previous reports of differences in PKA and PKC activity in patients suffering from BD.49,50 Lithium and valproic acid treatments reduce PKC activation and receptor-G protein coupling in platelets of bipolar manic patients.50 The luciferase reporter assay showed that these pathways undergo clear changes in activity in vitro after 48 h upon stimulation with lithium or escitalopram, but no differences could be seen at basal level. These responses were stronger in patients, supporting the idea of increase in PKA, CaMKII (both target CREB) as well as PKC signalling in this disorder. However, the methods used in other publications have been diverse as for used cell types as well as the status of the patients. Our method focusses on transcription factors, the most downstream targets next to mRNA and induced proteins.
ERK and JNK
Both ERK and JNK reporter assays showed higher activities in BD patients. Particularly for JNK, a significant difference was noted even at the basal level, stronger after stimulation with both escitalopram and lithium. However, for ERK only escitalopram-stimulated activities yielded significance, and only in univariate comparisons. JNK is activated by various conditions, such as oxidative, mitochondrial and endoplasmatic reticulum stress. Independent transcriptome studies on the frontal cortex of BD patients demonstrated upregulation of immune response genes51,52 and this has also been described in patients with schizophrenia.53
Furthermore, decreases of mitochondrial mRNA and proteins important for certain functions, that is, oxidative phosphorylation, have been found in the prefrontal cortex from patients with BD.54, 55, 56 Oxidative phosphorylation regulates adenosine triphosphate (ATP) levels and affects ATP-dependent ion pumps maintaining the membrane potential in mitochondria. Failure leads to apoptotic cell death.57 This decreased mitochondrial function might contribute to the response seen in Myc and p53 pathways.
p53
Our results on p53 are less clear, as the escitalopram-stimulated/basal ratio was significantly higher in patients in the univariate analysis, but the lithium-stimulated/basal ratio was underlined in the multivariate analysis.
The p53 pathway has been discussed in the context of schizophrenia, where an association of pro72arg variation with abnormal myelin integrity in the frontal lobe has been reported.58 Furthermore, the p53 genomic locus is in proximity with Lis-1.59 A p53 family member, p73, colocalises with reelin in developing human cortex,60 and deletion of 31 bp of reelin causes lissencephaly in sheep.61 These relatively new pathways active in BD need to be further investigated. Our results suggest that there is a difference in the sensitivity to drugs stimulating intracellular signalling molecules. Lithium has been recognised to exert its neuroprotective effects62,63 especially through induction of Bcl-2, possibly via upregulation of BDNF,64,65 as well as inhibition of GSK-3,34 calpain66,67 and Ca2+ influx. Escitalopram has not been reported to possess such activities, and this might explain the fact that it did not affect Myc or p53 pathways.
Long-term lithium treatment decreases p53 and its downstream Bax, whereas anti-apoptotic Bcl-2 is increased; lithium also prevents a glutamate-induced increase of p53 and Bax in cerebellar granule cells.41 However, in our analysis (where lithium was added only for 48 h) it might have induced stress to more vulnerable patients' cells, leading to stabilisation of p53. Consequently, pro-apoptotic BH3 molecules, induced by p53, bind anti-apoptotic Bcls on the mitochondrial membrane, leading to the release of cytochrome c from mitochondria.68 Decreased ATP production will render mitochondria dysfunctional, leading to decreased energy metabolism in brain that is associated with BD.69
It has been shown that both c-Myc and p53 are upregulated by intrastriatal infusion of the N-methyl-D-aspartate receptor agonist quinolinic acid via NF-κB activation, leading to DNA fragmentation of striatal cells.70 In our study, it is not obvious whether the induction of p53 and c-Myc leads to apoptosis, as we did not see any morphological characteristics of apoptosis. This can only be examined by biochemical methods.
Wnt
The escitalopram-stimulated/basal Wnt activity was clearly higher in our patients, both uni- and multivariately.
Wnt has earlier been reported to be dysregulated in monozygotic twins discordant for bipolar disorder.71 Not only the Wnt canonical pathway targets β-catenin, but also some noncanonical pathways.72,73 Recently, β-catenin was found to bind the promoters for many genes (being known candidates for schizophrenia, autism spectrum disorder and BD), including CACNA1B, NRNG, SNAP29, FGFR1, PCDH9 and others being identified in published genome-wide association studies.74 The Wnt/Ca2+ signal activates the transcription factors NF-κB, NFAT and CREB, through calcineurin, CamKII and PKC.73 Some of the other noncanonical Wnt pathways, that is, planer cell polarity, JNK, Ror, GSK3MT, aPKC, RYK and mTOR, are shown to activate or interfere with Ca2+ signalling. Ca2+ influx through the L-type voltage-activated channels activates CREB and MEF-2. Thus, Ca2+-dependent signalling could be activated by several pathways.
ANK3
Of the 25 genes analysed by us in a hypothesis-driven endeavour, only the ANK3 gene (rs10761482) yielded significance in both univariate and multivariate comparisons. Two other genes were at trend significance when studied univariately. Our sample is small, and no conclusions can be reached as for the biological impact of the genes chosen. Nevertheless, the ANK3 gene turned out to carry a diagnostic value.
The genome-wide association studies and meta-analyses have identified ANK3 and CACNA1C (alpha 1C subunit of the L-type voltage-activated channels) as the most notable risk genes for BD.75, 76, 77 ANK3 encodes Ankyrin-G isoforms that anchor membrane protein complexes, such as Na+-gated channels, to the cytoskeleton at the axonal initial segment and nodes of Ranvier to facilitate action potential firing and the synapse targeting process at the axonal initial segment.78, 79, 80, 81 ANK3 polymorphisms have also been associated with decreased integrity of white matter.82 We could not find significant associations for rs10994336 or rs984190 as in previously reported genome-wide association studies.75,76
In the present study with the small number of samples, ANK3 rs10761482 showed a significant association with BD. Previously, this SNP has been reported to be associated with schizophrenia in a large European sample.83 However, a German study on rs10761482, including 400 BD and 920 schizophrenia samples, showed an association with BD but not with schizophrenia.84 In a recent study, cognitive functional effects of two risk ANK3 variants (rs10761482 and rs10994336) were examined on 7 neurocognitive domains in 49 Japanese BD patients and 633 controls. The risk C-allele of rs10761482, but not of rs10994336 (linkage disequilibrium with 10994397), was significantly associated with worse performance.85 In another Canadian study, the G-allele of rs1938526 was found to be associated with cognitive impairment on 6 domains and cortical thinning in 173 patients with first-episode psychosis.86 As such endophenotypes are shared between schizophrenia and BD, more studies are needed in order to distinguish these entities.
State or trait?
By logistic regression, we compared the classification power of escitalopram-stimulated activity of the PKC pathway and the ANK3 gene in predicting diagnosis, presence of bipolar heredity and whether individuals had suffered from any psychotic episode in their lives.
First, an interaction term was clearly nonsignificant, suggesting that the contributions of these two independent variables are additive, not synergistic. Second, the diagnostic dichotomy was better predicted than the presence of bipolar heredity in a first-degree relative, even though the heritability of BD is very high. We have evidence that quantitative clinical measures such as age, body size, MADRS, YMRA and GAF are not related to any of the variables measured in this study (not shown).
This offers some support that the signalling deviations detected are more trait than state like. Only a longitudinal study may bring clarity.
Limitations
The study is cross-sectional with no longitudinal follow-up. Cohorts are relatively small with no medication-free patients, and there are no ‘ill patient' controls. It takes 3 to 4 weeks to expand adipocytes that may change epigenetic profiles. However, adipocytes are easier to handle compared with lymphoblastoid cells immortalised by transfection of exogenous genes. Another issue might be that we do not target neuronal cells, which should be the proper target, although BD manifests with diverse symptoms in the whole body. Yet, our analysis resulted in clear differences between the responses to drugs between BD and controls.
Conclusion
To our knowledge, this is the first time postsynaptic signalling analyses have been performed on adipocytes, an easily accessible source of cells. We report several instances of significantly increased activity in BD of calcium-dependent postsynaptic signals, such as escitalopram- and lithium-stimulated PKC, JNK and myc activitites, as well as a genetic allelic disproportion of the ANK3 gene. Using multivariate logistic equations, correct classification of bipolar patients and healthy controls was possible, with high degrees of specificity and sensitivity.
More studies are needed to ascertain these results, and to do so we are in the process of creating induced pluripotent stem cell lines from adipocytes and inducing neurones and astrocytes. In order to confirm these findings and study signalling activities in a more native environment, we will use neural progenitors and generated neuronal cells from induced pluripotent stem cells from patient and control adipocytes.
Acknowledgments
For expert help in administration and tests we thank Richard Brodd. We also thank the H Lundbeck A/S Company for a generous gift of escitalopram. This work was supported by grants and scholarships from Swedish Science Council, Västra Götaland Region County Council (ALF), Swedish Childhood Cancer Foundation, Swedish Cancer Society, Gothenburg Psychiatric Research Fund, Uppsala University, Wilhelm and Martina Lundgren Foundation, Adlerbertska Forskningsstiftelsen, Åhlén Foundation, Assar Gabrielsson Foundation, Fredrik and Ingrid Thuring Foundation, Söderström-König Foundation and Teodor Nerander Foundation (Fysiografen).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Supplementary Material
References
- 1Milhiet V, Etain B, Boudebesse C, Bellivier F. Circadian biomarkers, circadian genes and bipolar disorders. J Physiol Paris 2011; 105: 183–189. [DOI] [PubMed] [Google Scholar]
- 2McClung CA. Clock genes and bipolar disorder: implications for therapy. Pharmacogenomics 2007; 8: 1097–1100. [DOI] [PubMed] [Google Scholar]
- 3Ösby U, Brandt L, Correia N, Ekbom A, Sparén P. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry 2001; 58: 844–850. [DOI] [PubMed] [Google Scholar]
- 4Goldstein TR, Keller M, Birmaher B, Axelson D, Goldstein BI, Gill MK et al. Psychosocial functioning among bipolar youth. J Affect Disord 2009; 114: 174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5Zarate CA Jr, Tohen M, Land M, Cavanagh S. Functional impairment and cognition in bipolar disorder. Psychiatr Q 2000; 71: 309–329. [DOI] [PubMed] [Google Scholar]
- 6Ekman CJ, Lind J, Rydén E, Ingvar M, Landén M. Manic episodes are associated with grey matter volume reduction - a voxel-based morphometry brain analysis. Acta Psychiatr Scand 2010; 122: 507–515. [DOI] [PubMed] [Google Scholar]
- 7Cardno AG, Marshall EJ, Coid B, Macdonald AM, Ribchester TR, Davies NJ et al. Heritability estimates for psychotic disorders: the Maudsley twin psychosis series. Arch Gen Psychiatry 1999; 56: 162–168. [DOI] [PubMed] [Google Scholar]
- 8McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry 2003; 60: 497–502. [DOI] [PubMed] [Google Scholar]
- 9Craddock N, Sklar P. Genetics of bipolar disorder: successful start to a long journey. Trends Genet 2009; 25: 99–105. [DOI] [PubMed] [Google Scholar]
- 10Hernandez ME, Mendieta D, Perez-Tapia M, Bojalil R, Estrada-Garcia I, Estrada-Parra S et al. Effect of selective serotonin reuptake inhibitors and immunomodulator on cytokines levels: an alternative therapy for patients with major depressive disorder. Clin Dev Immunol 2013; 2013: 267871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev 2011; 35: 804–817. [DOI] [PubMed] [Google Scholar]
- 12Bocchio-Chiavetto L, Bagnardi V, Zanardini R, Molteni R, Nielsen MG, Placentino A et al. Serum and plasma BDNF levels in major depression: a replication study and meta-analyses. World J Biol Psychiatry 2010; 11: 763–773. [DOI] [PubMed] [Google Scholar]
- 13Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci 2002; 22: 3251–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Södersten K, Pålsson E, Ishima T, Funa K, Landén M, Hashimoto K et al. Abnormality in serum levels of mature brain-derived neurotrophic factor (BDNF) and its precursor proBDNF in mood-stabilized patients with bipolar disorder: a study of two independent cohorts. J Affect Disord 2014; 160: 1–9. [DOI] [PubMed] [Google Scholar]
- 15Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry 1997; 54: 597–606. [DOI] [PubMed] [Google Scholar]
- 16Hahn CG, Friedman E. Abnormalities in protein kinase C signaling and the pathophysiology of bipolar disorder. Bipolar Disord 1999; 1: 81–86. [DOI] [PubMed] [Google Scholar]
- 17Ali SM, Ahmad A, Shahabuddin S, Ahmad MU, Sheikh S, Ahmad I. Endoxifen is a new potent inhibitor of PKC: a potential therapeutic agent for bipolar disorder. Bioorg Med Chem Lett 2010; 20: 2665–2667. [DOI] [PubMed] [Google Scholar]
- 18Koenigsberg HW, Yuan P, Diaz GA, Guerreri S, Dorantes C, Mayson S et al. Platelet protein kinase C and brain-derived neurotrophic factor levels in borderline personality disorder patients. Psychiatry Res 2012; 199: 92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Kato T. Molecular neurobiology of bipolar disorder: a disease of 'mood-stabilizing neurons'? Trends Neurosci 2008; 31: 495–503. [DOI] [PubMed] [Google Scholar]
- 20Martinsson L, Wei Y, Xu D, Melas PA, Mathé AA, Schalling M et al. Long-term lithium treatment in bipolar disorder is associated with longer leukocyte telomeres. Transl Psychiatry 2013; 3: e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59(Suppl 20): 22–33, quiz 34-57. [PubMed] [Google Scholar]
- 22Asberg M, Montgomery SA, Perris C, Schalling D, Sedvall G. A comprehensive psychopathological rating scale. Acta Psychiatr Scand Suppl 1978; 127: 5–27. [DOI] [PubMed] [Google Scholar]
- 23Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133: 429–435. [DOI] [PubMed] [Google Scholar]
- 24Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state'. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 25Hall RC. Global assessment of functioning. A modified scale. Psychosomatics 1995; 36: 267–275. [DOI] [PubMed] [Google Scholar]
- 26Conigrave KM, Hall WD, Saunders JB. The AUDIT questionnaire: choosing a cut-off score. Alcohol Use Disorder Identification Test. Addiction 1995; 90: 1349–1356. [DOI] [PubMed] [Google Scholar]
- 27Cassidy CM, Schmitz N, Malla A. Validation of the alcohol use disorders identification test and the drug abuse screening test in first episode psychosis. Can J Psychiatry 2008; 53: 26–33. [DOI] [PubMed] [Google Scholar]
- 28Sklar P, Smoller JW, Fan J, Ferreira MA, Perlis RH, Chambert K et al. Whole-genome association study of bipolar disorder. Mol Psychiatry 2008; 13: 558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Williams HJ, Holmans P, Owen MJ, O'Donovan MC, Craddock N, Russo G et al. Most genome-wide significant susceptibility loci for schizophrenia and bipolar disorder reported to date cross-traditional diagnostic boundaries. Hum Mol Genet 2011; 20: 387–391. [DOI] [PubMed] [Google Scholar]
- 30Karege F, Schwald M, El Kouaissi R. Drug-induced decrease of protein kinase a activity reveals alteration in BDNF expression of bipolar affective disorder. Neuropsychopharmacology 2004; 29: 805–812. [DOI] [PubMed] [Google Scholar]
- 31Karege F, Schwald M, Papadimitriou P, Lachausse C, Cisse M. The cAMP-dependent protein kinase A and brain-derived neurotrophic factor expression in lymphoblast cells of bipolar affective disorder. J Affect Disord 2004; 79: 187–192. [DOI] [PubMed] [Google Scholar]
- 32Perez J, Tardito D, Racagni G, Smeraldi E, Zanardi R. cAMP signaling pathway in depressed patients with psychotic features. Mol Psychiatry 2002; 7: 208–212. [DOI] [PubMed] [Google Scholar]
- 33Tardito D, Mori S, Racagni G, Smeraldi E, Zanardi R, Perez J. Protein kinase A activity in platelets from patients with bipolar disorder. J Affect Disord 2003; 76: 249–253. [DOI] [PubMed] [Google Scholar]
- 34Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR et al. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci USA 2004; 101: 5099–5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 2005; 122: 261–273. [DOI] [PubMed] [Google Scholar]
- 36Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V et al. A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell 2008; 132: 125–136. [DOI] [PubMed] [Google Scholar]
- 37Packham G, Cleveland JL. c-Myc and apoptosis. Biochim Biophys Acta 1995; 1242: 11–28. [DOI] [PubMed] [Google Scholar]
- 38Nakagomi T, Asai A, Kanemitsu H, Narita K, Kuchino Y, Tamura A et al. Up-regulation of c-myc gene expression following focal ischemia in the rat brain. Neurol Res 1996; 18: 559–563. [DOI] [PubMed] [Google Scholar]
- 39Coyle-Rink J, Del Valle L, Sweet T, Khalili K, Amini S. Developmental expression of Wnt signaling factors in mouse brain. Cancer Biol Ther 2002; 1: 640–645. [DOI] [PubMed] [Google Scholar]
- 40Duan W, Zhu X, Ladenheim B, Yu QS, Guo Z, Oyler J et al. p53 inhibitors preserve dopamine neurons and motor function in experimental parkinsonism. Ann Neurol 2002; 52: 597–606. [DOI] [PubMed] [Google Scholar]
- 41Chen RW, Chuang DM. Long term lithium treatment suppresses p53 and Bax expression but increases Bcl-2 expression. A prominent role in neuroprotection against excitotoxicity. J Biol Chem 1999; 274: 6039–6042. [DOI] [PubMed] [Google Scholar]
- 42Fu Y, Yu S, Guo X, Li X, Li T, Li H et al. Fluvoxamine increased glutamate release by activating both 5-HT(3) and sigma-1 receptors in prelimbic cortex of chronic restraint stress C57BL/6 mice. Biochim Biophys Acta 2012; 1823: 826–837. [DOI] [PubMed] [Google Scholar]
- 43Friedman E, Hoau Yan W, Levinson D, Connell TA, Singh H. Altered platelet protein kinase C activity in bipolar affective disorder, manic episode. Biol Psychiatry 1993; 33: 520–525. [DOI] [PubMed] [Google Scholar]
- 44Birnbaum SG, Yuan PX, Wang M, Vijayraghavan S, Bloom AK, Davis DJ et al. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science 2004; 306: 882–884. [DOI] [PubMed] [Google Scholar]
- 45Du J, Gray NA, Falke CA, Chen W, Yuan P, Szabo ST et al. Modulation of synaptic plasticity by antimanic agents: the role of AMPA glutamate receptor subunit 1 synaptic expression. J Neurosci 2004; 24: 6578–6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46Chang A, Li PP, Warsh JJ. cAMP-Dependent protein kinase (PKA) subunit mRNA levels in postmortem brain from patients with bipolar affective disorder (BD). Brain Res Mol Brain Res 2003; 116: 27–37. [DOI] [PubMed] [Google Scholar]
- 47Tiraboschi E, Tardito D, Kasahara J, Moraschi S, Pruneri P, Gennarelli M et al. Selective phosphorylation of nuclear CREB by fluoxetine is linked to activation of CaM kinase IV and MAP kinase cascades. Neuropsychopharmacology 2004; 29: 1831–1840. [DOI] [PubMed] [Google Scholar]
- 48Chawla S, Bading H. CREB/CBP and SRE-interacting transcriptional regulators are fast on-off switches: duration of calcium transients specifies the magnitude of transcriptional responses. J Neurochem 2001; 79: 849–858. [DOI] [PubMed] [Google Scholar]
- 49Akin D, Manier DH, Sanders-Bush E, Shelton RC. Signal transduction abnormalities in melancholic depression. Int J Neuropsychopharmacol 2005; 8: 5–16. [DOI] [PubMed] [Google Scholar]
- 50Hahn CG, Umapathy C, Wang HY, Koneru R, Levinson DF, Friedman E. Lithium and valproic acid treatments reduce PKC activation and receptor-G protein coupling in platelets of bipolar manic patients. J Psychiatric Res 2005; 39: 355–363. [DOI] [PubMed] [Google Scholar]
- 51Ryan MM, Lockstone HE, Huffaker SJ, Wayland MT, Webster MJ, Bahn S. Gene expression analysis of bipolar disorder reveals downregulation of the ubiquitin cycle and alterations in synaptic genes. Mol Psychiatry 2006; 11: 965–978. [DOI] [PubMed] [Google Scholar]
- 52Rao JS, Harry GJ, Rapoport SI, Kim HW. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol Psychiatry 2010; 15: 384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53Arion D, Unger T, Lewis DA, Levitt P, Mirnics K. Molecular evidence for increased expression of genes related to immune and chaperone function in the prefrontal cortex in schizophrenia. Biol Psychiatry 2007; 62: 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54Iwamoto K, Bundo M, Kato T. Altered expression of mitochondria-related genes in postmortem brains of patients with bipolar disorder or schizophrenia, as revealed by large-scale DNA microarray analysis. Hum Mol Genet 2005; 14: 241–253. [DOI] [PubMed] [Google Scholar]
- 55Pennington K, Beasley CL, Dicker P, Fagan A, English J, Pariante CM et al. Prominent synaptic and metabolic abnormalities revealed by proteomic analysis of the dorsolateral prefrontal cortex in schizophrenia and bipolar disorder. Mol Psychiatry 2008; 13: 1102–1117. [DOI] [PubMed] [Google Scholar]
- 56Sun X, Wang JF, Tseng M, Young LT. Downregulation in components of the mitochondrial electron transport chain in the postmortem frontal cortex of subjects with bipolar disorder. J Psychiatry Neurosci 2006; 31: 189–196. [PMC free article] [PubMed] [Google Scholar]
- 57Calabresi P, Pisani A, Mercuri NB, Bernardi G. On the mechanisms underlying hypoxia-induced membrane depolarization in striatal neurons. Brain 1995; 118(Pt 4): 1027–1038. [DOI] [PubMed] [Google Scholar]
- 58Molina V, Papiol S, Sanz J, Rosa A, Arias B, Fatjo-Vilas M et al. Convergent evidence of the contribution of TP53 genetic variation (Pro72Arg) to metabolic activity and white matter volume in the frontal lobe in schizophrenia patients. Neuroimage 2011; 56: 45–51. [DOI] [PubMed] [Google Scholar]
- 59Tabares-Seisdedos R, Mata I, Escamez T, Vieta E, Lopez-Ilundain JM, Salazar J et al. Evidence for association between structural variants in lissencephaly-related genes and executive deficits in schizophrenia or bipolar patients from a Spanish isolate population. Psychiatr Genet 2008; 18: 313–317. [DOI] [PubMed] [Google Scholar]
- 60Meyer G, Perez-Garcia CG, Abraham H, Caput D. Expression of p73 and Reelin in the developing human cortex. J Neurosci 2002; 22: 4973–4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61Suarez-Vega A, Gutierrez-Gil B, Cuchillo-Ibanez I, Saez-Valero J, Perez V, Garcia-Gamez E et al. Identification of a 31-bp deletion in the RELN gene causing lissencephaly with cerebellar hypoplasia in sheep. PLoS One 2013; 8: e81072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62Manji HK, Moore GJ, Chen G. Lithium at 50: have the neuroprotective effects of this unique cation been overlooked? Biol Psychiatry 1999; 46: 929–940. [DOI] [PubMed] [Google Scholar]
- 63Manji HK, Lenox RH. Signaling: cellular insights into the pathophysiology of bipolar disorder. Biol Psychiatry 2000; 48: 518–530. [DOI] [PubMed] [Google Scholar]
- 64Hashimoto R, Takei N, Shimazu K, Christ L, Lu B, Chuang DM. Lithium induces brain-derived neurotrophic factor and activates TrkB in rodent cortical neurons: an essential step for neuroprotection against glutamate excitotoxicity. Neuropharmacology 2002; 43: 1173–1179. [DOI] [PubMed] [Google Scholar]
- 65Machado-Vieira R, Manji HK, Zarate CA Jr.. The role of lithium in the treatment of bipolar disorder: convergent evidence for neurotrophic effects as a unifying hypothesis. Bipolar Disord 2009; 11(Suppl 2): 92–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66Camins A, Verdaguer E, Folch J, Pallas M. Involvement of calpain activation in neurodegenerative processes. CNS Drug Rev 2006; 12: 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67Camins A, Crespo-Biel N, Junyent F, Verdaguer E, Canudas AM, Pallas M. Calpains as a target for therapy of neurodegenerative diseases: putative role of lithium. Curr Drug Metabol 2009; 10: 433–447. [DOI] [PubMed] [Google Scholar]
- 68Love S. Apoptosis and brain ischaemia. Prog Neuropsychopharmacol Biol Psychiatry 2003; 27: 267–282. [DOI] [PubMed] [Google Scholar]
- 69Nierenberg AA, Kansky C, Brennan BP, Shelton RC, Perlis R, Iosifescu DV. Mitochondrial modulators for bipolar disorder: a pathophysiologically informed paradigm for new drug development. Austr N Z J Psychiatry 2013; 47: 26–42. [DOI] [PubMed] [Google Scholar]
- 70Qin ZH, Chen RW, Wang Y, Nakai M, Chuang DM, Chase TN. Nuclear factor kappaB nuclear translocation upregulates c-Myc and p53 expression during NMDA receptor-mediated apoptosis in rat striatum. J Neurosci 1999; 19: 4023–4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71Matigian N, Windus L, Smith H, Filippich C, Pantelis C, McGrath J et al. Expression profiling in monozygotic twins discordant for bipolar disorder reveals dysregulation of the WNT signalling pathway. Mol Psychiatry 2007; 12: 815–825. [DOI] [PubMed] [Google Scholar]
- 72Camins A, Verdaguer E, Junyent F, Yeste-Velasco M, Pelegri C, Vilaplana J et al. Potential mechanisms involved in the prevention of neurodegenerative diseases by lithium. CNS Neurosci Ther 2009; 15: 333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73De A. Wnt/Ca2+ signaling pathway: a brief overview. Acta Biochim Biophys Sin (Shanghai) 2011; 43: 745–756. [DOI] [PubMed] [Google Scholar]
- 74Pedrosa E, Shah A, Tenore C, Capogna M, Villa C, Guo X et al. β-catenin promoter ChIP-chip reveals potential schizophrenia and bipolar disorder gene network. J Neurogenet 2010; 24: 182–193. [DOI] [PubMed] [Google Scholar]
- 75Ferreira MA, O'Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet 2008; 40: 1056–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76Schulze TG, Detera-Wadleigh SD, Akula N, Gupta A, Kassem L, Steele J et al. Two variants in Ankyrin 3 (ANK3) are independent genetic risk factors for bipolar disorder. Mol Psychiatry 2009; 14: 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77Psychiatric GWAS Consortium Bipolar Disorder Working Group1. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet 2011; 43: 977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78Kuba H, Oichi Y, Ohmori H. Presynaptic activity regulates Na(+) channel distribution at the axon initial segment. Nature 2010; 465: 1075–1078. [DOI] [PubMed] [Google Scholar]
- 79Bennett V, Chen L. Ankyrins and cellular targeting of diverse membrane proteins to physiological sites. Curr Opin Cell Biol 2001; 13: 61–67. [DOI] [PubMed] [Google Scholar]
- 80Zhou D, Lambert S, Malen PL, Carpenter S, Boland LM, Bennett V. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol 1998; 143: 1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell 2004; 119: 257–272. [DOI] [PubMed] [Google Scholar]
- 82Linke S, Hampton-Smith RJ, Peet DJ. Characterization of ankyrin repeat-containing proteins as substrates of the asparaginyl hydroxylase factor inhibiting hypoxia-inducible transcription factor. Methods Enzymol 2007; 435: 61–85. [DOI] [PubMed] [Google Scholar]
- 83Athanasiu L, Mattingsdal M, Kahler AK, Brown A, Gustafsson O, Agartz I et al. Gene variants associated with schizophrenia in a Norwegian genome-wide study are replicated in a large European cohort. J Psychiatr Res 2010; 44: 748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84Gella A, Segura M, Durany N, Pfuhlmann B, Stober G, Gawlik M. Is Ankyrin a genetic risk factor for psychiatric phenotypes? BMC Psychiatry 2011; 11: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85Hori H, Yamamoto N, Teraishi T, Ota M, Fujii T, Sasayama D et al. Cognitive effects of the ANK3 risk variants in patients with bipolar disorder and healthy individuals. J Affect Disord 2014; 158: 90–96. [DOI] [PubMed] [Google Scholar]
- 86Cassidy C, Buchy L, Bodnar M, Dell'elce J, Choudhry Z, Fathalli F et al. Association of a risk allele of ANK3 with cognitive performance and cortical thickness in patients with first-episode psychosis. J Psychiatry Neurosci 2014; 39: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.