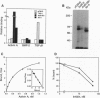
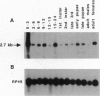
Abstract
Activins are cytokines of the transforming growth factor beta superfamily that control various events during vertebrate embryo development and cell differentiation in the adult, and act through transmembrane receptors that contain a cytoplasmic protein-serine/threonine kinase domain. We describe the identification, deduced primary structure, and expression pattern of Atr-II, a receptor serine/threonine kinase found in Drosophila. With the exception of the spacing of 10 cysteine residues, the extracellular domain of Atr-II is very dissimilar from those of vertebrate activin receptors, yet it binds activin with high affinity and specificity. The kinase domain sequence of Atr-II is 60% identical to those of activin receptors from vertebrates, suggesting similarities in their signaling mechanisms. Maternal Atr-II transcript and its product are abundant in the oocyte. During development, the highest levels of Atr-II transcript and protein are observed in the mesoderm and gut. The possible role of an activin signaling system in Drosophila development is discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attisano L., Wrana J. L., Cheifetz S., Massagué J. Novel activin receptors: distinct genes and alternative mRNA splicing generate a repertoire of serine/threonine kinase receptors. Cell. 1992 Jan 10;68(1):97–108. doi: 10.1016/0092-8674(92)90209-u. [DOI] [PubMed] [Google Scholar]
- Brown N. H., Kafatos F. C. Functional cDNA libraries from Drosophila embryos. J Mol Biol. 1988 Sep 20;203(2):425–437. doi: 10.1016/0022-2836(88)90010-1. [DOI] [PubMed] [Google Scholar]
- Cagan R. L., Ready D. F. The emergence of order in the Drosophila pupal retina. Dev Biol. 1989 Dec;136(2):346–362. doi: 10.1016/0012-1606(89)90261-3. [DOI] [PubMed] [Google Scholar]
- Cavener D. R. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res. 1987 Feb 25;15(4):1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doctor J. S., Jackson P. D., Rashka K. E., Visalli M., Hoffmann F. M. Sequence, biochemical characterization, and developmental expression of a new member of the TGF-beta superfamily in Drosophila melanogaster. Dev Biol. 1992 Jun;151(2):491–505. doi: 10.1016/0012-1606(92)90188-m. [DOI] [PubMed] [Google Scholar]
- Georgi L. L., Albert P. S., Riddle D. L. daf-1, a C. elegans gene controlling dauer larva development, encodes a novel receptor protein kinase. Cell. 1990 May 18;61(4):635–645. doi: 10.1016/0092-8674(90)90475-t. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M. Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods Enzymol. 1991;200:38–62. doi: 10.1016/0076-6879(91)00126-h. [DOI] [PubMed] [Google Scholar]
- Hoffmann F. M. Transforming growth factor-beta-related genes in Drosophila and vertebrate development. Curr Opin Cell Biol. 1991 Dec;3(6):947–952. doi: 10.1016/0955-0674(91)90112-c. [DOI] [PubMed] [Google Scholar]
- Karch F., Bender W., Weiffenbach B. abdA expression in Drosophila embryos. Genes Dev. 1990 Sep;4(9):1573–1587. doi: 10.1101/gad.4.9.1573. [DOI] [PubMed] [Google Scholar]
- Lin H. Y., Wang X. F., Ng-Eaton E., Weinberg R. A., Lodish H. F. Expression cloning of the TGF-beta type II receptor, a functional transmembrane serine/threonine kinase. Cell. 1992 Feb 21;68(4):775–785. doi: 10.1016/0092-8674(92)90152-3. [DOI] [PubMed] [Google Scholar]
- Lyons K. M., Jones C. M., Hogan B. L. The DVR gene family in embryonic development. Trends Genet. 1991 Nov-Dec;7(11-12):408–412. doi: 10.1016/0168-9525(91)90265-r. [DOI] [PubMed] [Google Scholar]
- Massagué J. Receptors for the TGF-beta family. Cell. 1992 Jun 26;69(7):1067–1070. doi: 10.1016/0092-8674(92)90627-o. [DOI] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Mathews L. S., Vale W. W. Expression cloning of an activin receptor, a predicted transmembrane serine kinase. Cell. 1991 Jun 14;65(6):973–982. doi: 10.1016/0092-8674(91)90549-e. [DOI] [PubMed] [Google Scholar]
- Mathews L. S., Vale W. W., Kintner C. R. Cloning of a second type of activin receptor and functional characterization in Xenopus embryos. Science. 1992 Mar 27;255(5052):1702–1705. doi: 10.1126/science.1313188. [DOI] [PubMed] [Google Scholar]
- Padgett R. W., St Johnston R. D., Gelbart W. M. A transcript from a Drosophila pattern gene predicts a protein homologous to the transforming growth factor-beta family. Nature. 1987 Jan 1;325(6099):81–84. doi: 10.1038/325081a0. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Price B. M., Van Nimmen K., Huylebroeck D. Identification of a potent Xenopus mesoderm-inducing factor as a homologue of activin A. Nature. 1990 Jun 21;345(6277):729–731. doi: 10.1038/345729a0. [DOI] [PubMed] [Google Scholar]
- Tautz D., Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989 Aug;98(2):81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Thomsen G., Woolf T., Whitman M., Sokol S., Vaughan J., Vale W., Melton D. A. Activins are expressed early in Xenopus embryogenesis and can induce axial mesoderm and anterior structures. Cell. 1990 Nov 2;63(3):485–493. doi: 10.1016/0092-8674(90)90445-k. [DOI] [PubMed] [Google Scholar]
- Wharton K. A., Thomsen G. H., Gelbart W. M. Drosophila 60A gene, another transforming growth factor beta family member, is closely related to human bone morphogenetic proteins. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9214–9218. doi: 10.1073/pnas.88.20.9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana J. L., Attisano L., Cárcamo J., Zentella A., Doody J., Laiho M., Wang X. F., Massagué J. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992 Dec 11;71(6):1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- van den Eijnden-Van Raaij A. J., van Zoelent E. J., van Nimmen K., Koster C. H., Snoek G. T., Durston A. J., Huylebroeck D. Activin-like factor from a Xenopus laevis cell line responsible for mesoderm induction. Nature. 1990 Jun 21;345(6277):732–734. doi: 10.1038/345732a0. [DOI] [PubMed] [Google Scholar]