Abstract
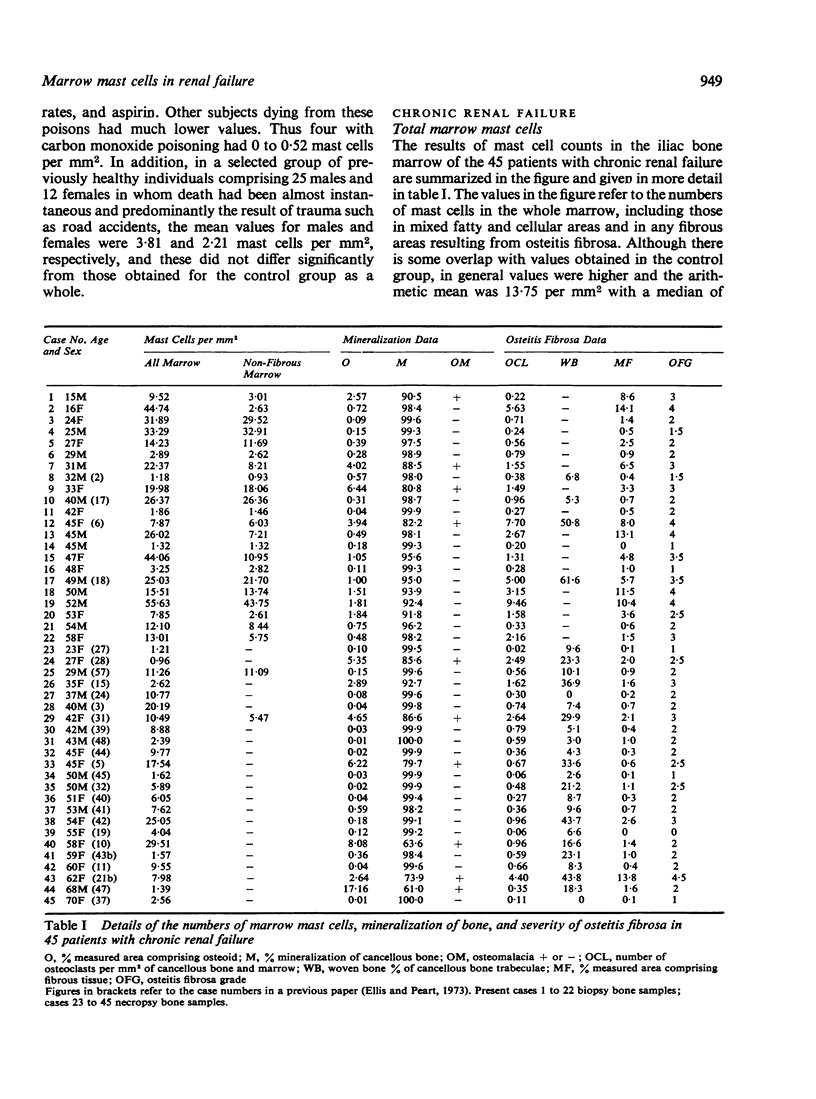
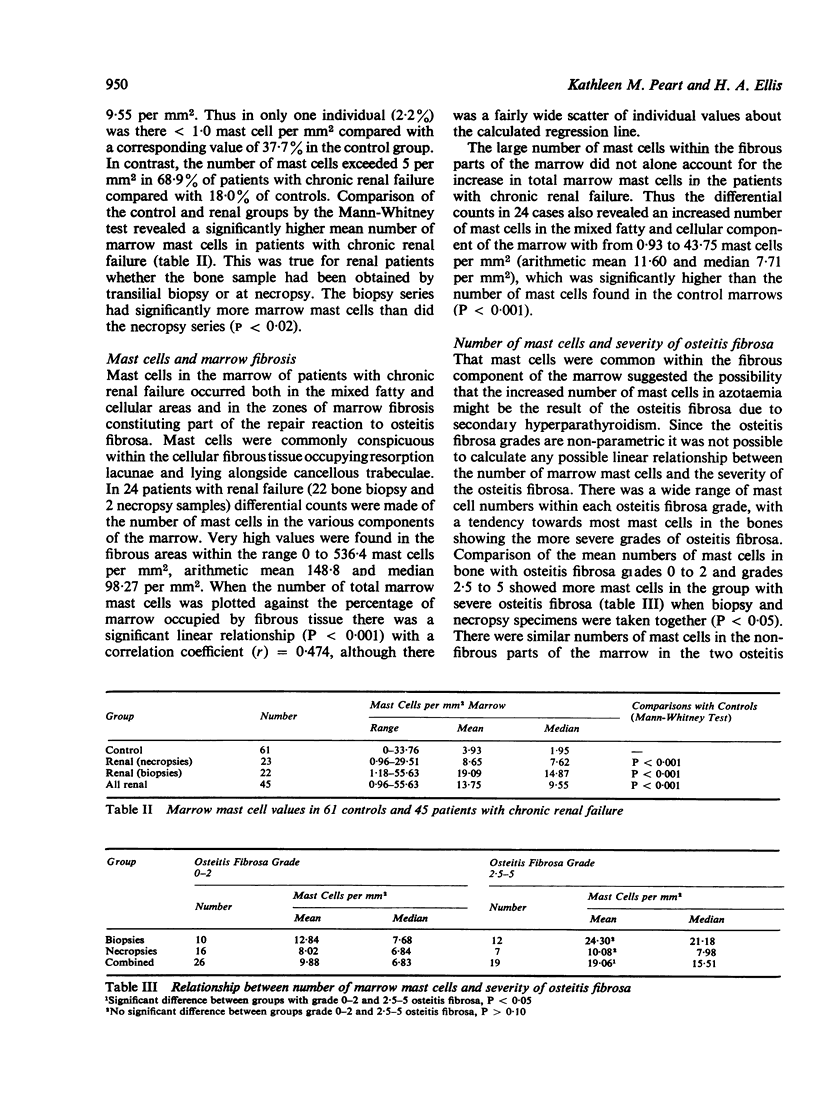
Mast cells have been counted in sections of iliac bone from 61 control subjects at necropsy. Mast cells were found in all but three, and the range was 0-33-7, median 1-95 per mm2 marrow. The majority (82%) had less than 4-99 mast cells per mm2 marrow; in 37-7% there was less than 1 mast cell per mm2 marrow. In a group of 45 patients with chronic renal failure there was a significant increase in the numbers of mast cells (P less than 0-001) with a range of 0-96-55-63, median 9-55 per mm2 marrow. Mast cells were common in the areas of marrow fibrosis associated with osteitis fibrosa but this was not the sole cause of the increase since there was also an excess of mast cells in the non-fibrous parts of the marrow. There was a tendency towards greater numbers of mast cells in those cases with most marked osteitis fibrosa in association with the prominent marrow fibrosis, but there was no significant relationship between mast cell numbers and other features of oesteitis fibrosa such as the number of osteoclasts and the amount of woven bone formation. There was no relationship between the numbers of mast cells and the amounts of total bone, ostoid, percentage mineralization of cancellous bone, or the presence of osteomalacia.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CASS R., MARSHALL P. B., RILEY J. F. 5-Hydroxytryptamine and histamine in mast cells of the mouse and rat. J Physiol. 1958 May 28;141(3):510–519. doi: 10.1113/jphysiol.1958.sp005992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALY J. F., STARK E., VAN BUSKIRK F. W. Radiologic and pathologic bone changes associated with urticaria pigmentosa; report of a case. AMA Arch Pathol. 1956 Aug;62(2):143–148. [PubMed] [Google Scholar]
- ELLIS H. A. EFFECTS OF THE LONG-TERM ADMINSTRATION TO ANIMALS OF DEXTRAN SULPHATE. J Pathol Bacteriol. 1965 Apr;89:437–460. [PubMed] [Google Scholar]
- Ellis H. A. Bone disease in end-stage renal failure. Br Med J. 1973 Jul 28;3(5873):232–233. doi: 10.1136/bmj.3.5873.232-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis H. A., Peart K. M. Azotaemic renal osteodystrophy: a quantitative study on iliac bone. J Clin Pathol. 1973 Feb;26(2):83–101. doi: 10.1136/jcp.26.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis H. A., Peart K. M. Dextran sulphate osteopathy in parathyroidectomized rats. Br J Exp Pathol. 1971 Dec;52(6):684–695. [PMC free article] [PubMed] [Google Scholar]
- Ellis H. A., Peart K. M. Quantitative observations on mineralized and non-mineralized bone in the iliac crest. J Clin Pathol. 1972 Apr;25(4):277–286. doi: 10.1136/jcp.25.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis H. A., Peart K. M. The effects of heparin and dextran sulphate on cultured mouse limb bones. Br J Exp Pathol. 1970 Feb;51(1):43–52. [PMC free article] [PubMed] [Google Scholar]
- FADEM R. S. Tissue mast cells in human bone marrow. Blood. 1951 Jul;6(7):614–630. [PubMed] [Google Scholar]
- GILLMAN T. Mast cell increases after calciferol intoxication and in experimental odoratism. Acta Haematol. 1958 Mar;19(3):179–186. doi: 10.1159/000205430. [DOI] [PubMed] [Google Scholar]
- GILLMAN T., NAIDOO S. S. In vitro effects of heparin and calcium ions on lipaemic serum. Nature. 1957 May 4;179(4566):904–905. doi: 10.1038/179904a0. [DOI] [PubMed] [Google Scholar]
- GOLDHABER P. HEPARIN ENHANCEMENT OF FACTORS STIMULATING BONE RESORPTION IN TISSUE CULTURE. Science. 1965 Jan 22;147(3656):407–408. doi: 10.1126/science.147.3656.407. [DOI] [PubMed] [Google Scholar]
- GRIFFITH G. C., NICHOLS G., Jr, ASHER J. D., FLANAGAN B. HEPARIN OSTEOPOROSIS. JAMA. 1965 Jul 12;193:91–94. doi: 10.1001/jama.1965.03090020005001. [DOI] [PubMed] [Google Scholar]
- HAVARD C. W., SCOTT R. B. Urticaria pigmentosa with visceral and skeletal lesions. Q J Med. 1959 Oct;28:459–470. [PubMed] [Google Scholar]
- HIGGINBOTHAM R. D., DOUGHERTY T. F., JEE W. S. Fate of shed mast cell granules. Proc Soc Exp Biol Med. 1956 Jun;92(2):256–261. doi: 10.3181/00379727-92-22445. [DOI] [PubMed] [Google Scholar]
- JAFFE M. D., WILLIS P. W., 3rd MULTIPLE FRACTURES ASSOCIATED WITH LONG-TERM SODIUM HEPARIN THERAPY. JAMA. 1965 Jul 12;193:158–160. doi: 10.1001/jama.1965.03090020072024. [DOI] [PubMed] [Google Scholar]
- JENSEN W. N., LASSER E. C. Urticaria pigmentosa associated with widespread sclerosis of the spongiosa of bone. Radiology. 1958 Dec;71(6):826–832. doi: 10.1148/71.6.826. [DOI] [PubMed] [Google Scholar]
- JOHNSTONE J. M. The appearance and significance of tissue mast cells in human bone marrow. J Clin Pathol. 1954 Nov;7(4):275–280. doi: 10.1136/jcp.7.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAMER H., WINDRUM G. M. The metachromatic staining reaction. J Histochem Cytochem. 1955 May;3(3):227–237. doi: 10.1177/3.3.227. [DOI] [PubMed] [Google Scholar]
- LERNER M. R., LERNER A. B. Urticaria pigmentosa with systemic lesions and otosclerosis. Arch Dermatol. 1960 Feb;81:203–204. doi: 10.1001/archderm.1960.03730020039006. [DOI] [PubMed] [Google Scholar]
- Lalich J. J., Groupner K., Jolin J. The influence of copper and molybdate salts on the production of bony deformities in rats. Lab Invest. 1965 Aug;14(8):1482–1493. [PubMed] [Google Scholar]
- Lindholm R. V., Lindholm T. S. Mast cells in endosteal and periosteal bone repair. A quantitative study on callus tissue of healing fractures in rabbits. Acta Orthop Scand. 1970;41(2):129–133. doi: 10.3109/17453677008991500. [DOI] [PubMed] [Google Scholar]
- Lindholm R., Lindholm S., Liukko P., Paasimäki J., Isokäntä S., Rossi R., Autio E., Tamminen E. The mast cell as a component of callus in healing fractures. J Bone Joint Surg Br. 1969 Feb 1;51(1):148–155. [PubMed] [Google Scholar]
- Neiman R. S., Bischel M. D., Lukes R. J. Uraemia and mast-cell proliferation. Lancet. 1972 Apr 29;1(7757):959–959. doi: 10.1016/s0140-6736(72)91523-1. [DOI] [PubMed] [Google Scholar]
- Rasmussen P. An experimental study of mast cells in the rat metaphysis. Calcif Tissue Res. 1972;9(4):325–330. doi: 10.1007/BF02061971. [DOI] [PubMed] [Google Scholar]
- Rebel A., Riberi P., Bregeon P., Malkani K. Mastocytes et remaniements du tissu osseux. Pathol Biol (Paris) 1974 Mar;22(3):213–220. [PubMed] [Google Scholar]
- Rockoff S. D., Armstrong J. D., Jr Parathyroid hormone as a stimulus to mast cell accumulation in bone. Calcif Tissue Res. 1970;5(1):49–55. doi: 10.1007/BF02017533. [DOI] [PubMed] [Google Scholar]
- Sakamoto S., Goldhaber P., Glimcher M. J. Mouse bone collagenase. The effect of heparin on the amount of enzyme released in tissue culture and on the activity of the enzyme. Calcif Tissue Res. 1973;12(3):247–258. doi: 10.1007/BF02013739. [DOI] [PubMed] [Google Scholar]
- Schuster J., Meier-Ruge W., Egli F. Zur Pathologie der Osteopathie nach Heparinbehandlung. Dtsch Med Wochenschr. 1969 Nov 7;94(45):2334–2338. doi: 10.1055/s-0028-1110442. [DOI] [PubMed] [Google Scholar]
- Severson A. R. Mast cells in areas of experimental bone resorption and remodelling. Br J Exp Pathol. 1969 Feb;50(1):17–21. [PMC free article] [PubMed] [Google Scholar]
- Sledge C. B., Blackburn W. W. Heparin, dextran and dextran sulfate: effect on lysosomes from embryonic cartilage. Calcif Tissue Res. 1968;(Suppl):65–65a. doi: 10.1007/BF02065247. [DOI] [PubMed] [Google Scholar]
- URIST M. R., MCLEAN F. C. Accumulation of mast cells in endosteum of bones of calcium-deficient rats. AMA Arch Pathol. 1957 Mar;63(3):239–251. [PubMed] [Google Scholar]
- WICHMANN B. E. The mast cell count during the process of wound healing; an experimental investigation on rats. Acta Pathol Microbiol Scand Suppl. 1955;(Suppl 108):1–35. [PubMed] [Google Scholar]


