Abstract
Accumulation of β-amyloid (Aβ) in the brain is associated with memory decline in healthy individuals as a prelude to Alzheimer's disease (AD). Genetic factors may moderate this decline. We examined the role of apolipoprotein E (ɛ4 carrier[ɛ4+], ɛ4 non-carrier[ɛ4−]) and brain-derived neurotrophic factor (BDNFVal/Val, BDNFMet) in the extent to which they moderate Aβ-related memory decline. Healthy adults (n=333, Mage=70 years) enrolled in the Australian Imaging, Biomarkers and Lifestyle study underwent Aβ neuroimaging. Neuropsychological assessments were conducted at baseline, 18-, 36- and 54-month follow-ups. Aβ positron emission tomography neuroimaging was used to classify participants as Aβ− or Aβ+. Relative to Aβ−ɛ4−, Aβ+ɛ4+ individuals showed significantly faster rates of cognitive decline over 54 months across all domains (d=0.40–1.22), while Aβ+ɛ4− individuals showed significantly faster decline only on verbal episodic memory (EM). There were no differences in rates of cognitive change between Aβ−ɛ4− and Aβ−ɛ4+ groups. Among Aβ+ individuals, ɛ4+/BDNFMet participants showed a significantly faster rate of decline on verbal and visual EM, and language over 54 months compared with ɛ4−/BDNFVal/Val participants (d=0.90–1.02). At least two genetic loci affect the rate of Aβ-related cognitive decline. Aβ+ɛ4+/BDNFMet individuals can expect to show clinically significant memory impairment after 3 years, whereas Aβ+ɛ4+/BDNFVal/Val individuals can expect a similar degree of impairment after 10 years. Little decline over 54 months was observed in the Aβ− and Aβ+ ɛ4− groups, irrespective of BDNF status. These data raise important prognostic issues in managing preclinical AD, and should be considered in designing secondary preventative clinical trials.
Introduction
In healthy individuals, high β-amyloid (Aβ) levels suggest that preclinical Alzheimer's disease (AD) has begun.1,2 However, variability in the extent of cognitive and clinical impairment in Aβ+ healthy individuals suggests other factors influence Aβ-related cognitive decline.3,4 The major genetic risk factor for sporadic AD is the apolipoprotein E (APOE) ɛ4 allele:5,6 apoE may be involved in AD pathogenesis directly, through increasing Aβ accumulation, reducing clearance of Aβ or modifying Aβ-synaptic toxicity,5, 6, 7 or indirectly, through reducing synaptic plasticity, increasing neuroinflammation or affecting concurrence of cerebrovascular events.3,8 In accord with this, a recent study of 490 healthy individuals aggregated from the Australian Imaging, Biomarkers and Lifestyle (AIBL) Study, the Alzheimer's Disease Neuroimaging Initiative (ADNI) and the Harvard Aging Brain Study showed that carriage of the ɛ4 allele increased substantially the rate of memory decline in healthy individuals with high Aβ levels (Aβ+ɛ4+) over a median follow-up period of 1.5 years.9 This analysis also showed that individuals who were ɛ4 carriers but with low Aβ (Aβ−ɛ4+) showed no memory decline compared with ɛ4 non-carriers with low Aβ (Aβ−ɛ4−), suggesting that, by itself, the APOE ɛ4 allele is not associated with memory decline. However, as the APOE ɛ4 allele is associated with increased cognitive decline in healthy individuals,10 and earlier diagnosis of AD,11 the effect of APOE ɛ4 on Aβ-related cognitive decline warrants further investigation over time intervals greater than 18 months.
Another strong genetic candidate for moderating Aβ-related memory decline is the brain-derived neurotrophic factor (BDNF)Val66Met polymorphism. BDNF is important in the biological basis of learning and memory in animals and humans.4,12, 13, 14 Prospective studies show that in healthy and mild cognitive impairment groups from both the AIBL and ADNI cohorts, BDNFMet carriage is associated with faster Aβ-related memory decline and hippocampal atrophy over 3 years but is unrelated to Aβ accumulation,15, 16, 17 suggesting that BDNFVal66Met moderates the effects of Aβ on synaptic integrity in preclinical AD.17 To our knowledge, no study has examined the interaction between BDNF, APOE and Aβ-related memory decline.
The overarching aim of this study was to explore potential interactions between Aβ, APOE and BDNF on cognitive decline in 333 healthy individuals who had undergone Aβ neuroimaging, genetic testing and 54-month clinical follow-up as part of the AIBL study. First, we examined whether episodic memory (EM) and other aspects of cognition would remain stable over 54 months in healthy Aβ−ɛ4+ participants, where any cognitive decline would provide an estimate of Aβ-independent effects of ɛ4. We then examined whether the Aβ+ɛ4+ group, compared with Aβ−ɛ4− healthy individuals, would show faster rates of decline in EM. Finally, we explored whether BDNFMet moderated any relationship between Aβ, ɛ4 and cognition.
Materials and methods
Participants
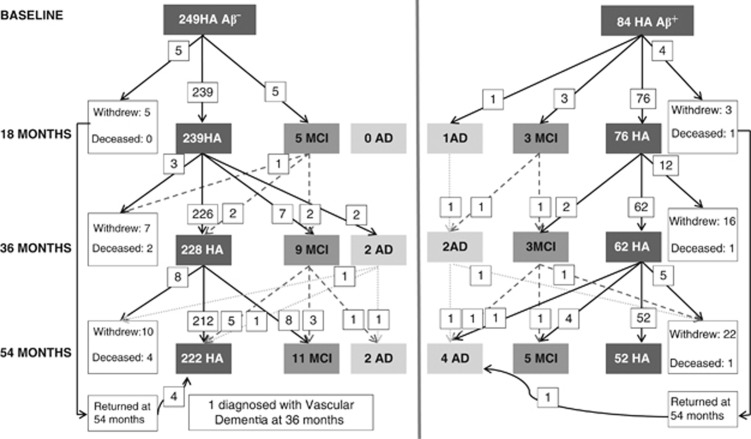
Participants were recruited from the AIBL healthy adult group, the recruitment of which has been described previously.18,19 Briefly, exclusion criteria included the following: schizophrenia, depression (15-item Geriatric Depression Score ⩾6), Parkinson's disease, symptomatic stroke, uncontrolled diabetes and alcohol use exceeding two standard drinks per day for women or four per day for men. Participants underwent medical, psychiatric and neuropsychological assessments at baseline, 18-, 36- and 54-month follow-up.19 At each assessment, a clinical review panel considered all available medical, psychiatric and neuropsychological information to classify clinical status.19 Clinical classification was blinded to neuroimaging results. Group demographic and clinical characteristics are provided in Table 1, with the number of participants whose diagnostic classification changed or who withdrew from the study shown in Figure 1. The study was approved by and complied with the regulations of three institutional research and ethics committees.19 All participants provided written informed consent.
Table 1. Demographic and clinical characteristics of the full sample and of study groups.
| Full sample (n=333) | Aβ− ɛ4− (n=188) | Aβ− ɛ4+ (n=61) | Aβ+ ɛ4− (n=36) | Aβ+ ɛ4+ (n=48) | P-value | |
|---|---|---|---|---|---|---|
| N (%) or mean (s.d.) | N (%) or mean (s.d.) | N (%) or mean (s.d.) | N (%) or mean (s.d.) | N (%) or mean (s.d.) | ||
| N (%) Female | 173 (52.0%) | 95 (50.5%) | 33 (54.1%) | 19 (52.8%) | 26 (54.2%) | 0.947 |
| Age (years) | 69.95 (6.80) | 69.22 (6.28) | 66.98 (5.20) | 76.06 (7.27) | 72.04 (7.03) | 0.000 |
| Premorbid IQ | 108.59 (7.07) | 108.51 (6.84) | 106.98 (7.75) | 111.47 (6.55) | 108.75 (6.93) | 0.000 |
| HADS depression subscale | 2.58 (2.24) | 2.58 (2.26) | 2.73 (2.11) | 1.83 (1.42) | 2.92 (2.70) | 0.151 |
| HADS anxiety subscale | 4.20 (2.78) | 4.18 (2.72) | 4.12 (2.82) | 3.26 (1.99) | 5.10 (3.21) | 0.026 |
| CDR sum of boxes | 0.04 (0.16) | 0.04 (0.18) | 0.04 (0.14) | 0.06 (0.16) | 0.02 (0.10) | 0.777 |
| MMSE | 28.87 (1.19) | 28.94 (1.18) | 28.84 (1.23) | 28.69 (1.26) | 28.75 (1.14) | 0.578 |
| N (%) high Aβ+ | 44 (13.2%) | n.a. | n.a. | 18 (50.0%) | 26 (54.2%) | 0.705 |
| N (%) progressed at 54 months | 23/296 (7.8%) | 11/178 (6.2%) | 2/57 (3.5%) | 3/26 (11.5%) | 7/35 (20.0%) | 0.020 |
Abbreviations: CDR, Clinical Dementia Rating scale; HADS, Hospital Anxiety and Depression Scale; MMSE, Mini Mental State Examination; PET, positron emission tomography; SUVr, standardized uptake value ratio.
Bolded values are significant at the P<0.05 or the P<0.001 level; of the 333 healthy older adults who underwent PET neuroimaging, 183 were scanned using 11C Pittsburgh Compound B, 76 using 18F florbetapir and 74 using 18F flutemetamol; high Aβ+ was classified when SUVr PiB >1.9, flutemetamol >0.82 and florbetapir >1.29.
Figure 1.
Clinical classification and disease progression of β-amyloid negative (Aβ−) and β-amyloid positive (Aβ+) participants over 54 months.
Measures
Neuroimaging
Aβ imaging with positron emission tomography (PET) was conducted using either 11C-Pittsburgh Compound B (PiB), 18F-florbetapir or 18F-flutemetamol. PET methodology has been described in detail previously.18,20,21 A 30-min acquisition was started 40 min post injection of PiB and a 20-min acquisition was performed 50 min post injection of florbetapir and 90 min post injection of flutemetamol. For PiB-PET, standardized uptake value (SUV) data were summed and normalized to the cerebellar cortex SUV, resulting in a region-to-cerebellar ratio termed SUV ratio (SUVr). The whole cerebellum was the reference region for florbetapir,21 while for flutemetamol the reference region was the pons. Consistent with these studies, SUVr was classified dichotomously as either negative (Aβ−) or positive (Aβ+). PiB studies were classified Aβ+ when SUVr⩾1.5,18 florbetapir, when SUVr⩾1.11 ref. 21 and for flutemetamol when SUVr⩾0.62.20 Aβ+ levels were further classified as being ‘high' Aβ+ (SUVr PiB>1.9; flutemetamol>0.82; florbetapir>1.29) or ‘low' Aβ+ (SUVr PiB=1.5–1.9; flutemetamol=0.62–0.82; florbetapir=1.11–1.29).22,23
Genotyping
A blood sample was taken from each participant for genotyping. The BDNFVal66Met polymorphism (rs6265) was included in a custom Illumina GoldenGate assay, which included 1536 single-nucleotide polyorphisms, and was performed by the Beijing Genomics Institute. Of the 333 healthy individuals who had undergone PET neuroimaging for Aβ, BDNFVal66Met data was available for 314 healthy individuals, of which 191 were BDNFVal/Val homozygotes and 123 were BDNFMet carriers (111 BDNFMet/Val heterozygotes and 12 BDNFMet/Met homozygotes).
Cognitive assessments
Composite cognitive scores were computed by standardizing outcome measures for each neuropsychological test against the baseline mean and s.d. for the Aβ− group. Standardized scores were averaged to form composite scores for verbal EM (Logical Memory delayed recall, California Verbal Learning Test, Second Edition [CVLT-II] long delay, CVLT-II d'); visual EM (Rey Complex Figure Test [RCFT ] 3-min delayed recall, RCFT 30-min delayed recall, CogState One-Card Learning); executive function (CogState One-Back, Letter Fluency, Category Fluency Switching [Fruit/Furniture]); language (Category Fluency [Animals/Boys' Names], Boston Naming Test); and attention (Digit Symbol, CogState Detection, CogState Identification). The development and validation of each cognitive composite score has been described previously.23,24
Data analysis
For each composite cognitive score, three planned comparisons were constructed using repeated-measures linear mixed-effects model with maximum likelihood estimation and an unstructured covariance matrix. Linear mixed modeling was employed because of its ability to model both fixed and random effects, which accounts for multiple sources of variability in longitudinal studies. In addition, both empirical and theoretical models of AD show that once the threshold for Aβ positivity is reached, there is a linear trend in cognitive decline, neurodegeneration and amyloid accumulation until a clinical diagnosis of AD is reached.1,2,25 In these analyses, Aβ status (Aβ−, Aβ+), APOE status (ɛ4+, ɛ4−), time, APOE × Aβ interaction, APOE × time interaction, Aβ × time interaction and APOE × Aβ × time interaction were entered as fixed factors; participant as a random factor; age, premorbid intelligence and anxiety levels as covariates; and cognitive composite score as the dependent variable. Within the model, the magnitude of difference from the Aβ−ɛ4− group was expressed using Cohen's d.26 Where the planned comparisons indicated differences between group trajectories, group means (95% confidence intervals (95% CIs)) for each assessment were estimated from the model and differences in these between Aβ+ɛ4− and Aβ+ɛ4+ groups at each assessment were determined by the extent of overlap between the 95% CIs associated with those means.
To examine the effect of BDNFVal66Met, separate linear mixed-effects models were conducted for each composite score in Aβ+ individuals. In these analyses, APOE status, BDNF status (BDNFVal/Val, BDNFMet), time, APOE × BDNF interaction, APOE × time interaction, BDNF × time interaction and APOE × BDNF × time interaction were entered as fixed factors; participant as a random factor; age, premorbid intelligence and anxiety levels as covariates and composite cognitive test score as the dependent variable. Within this model, rate of change over 18-month intervals in each group (ɛ4− BDNFMet; ɛ4+BDNFVal/Val; ɛ4+ BDNFMet) was compared with that for the ɛ4− BDNFVal/Val group. For each comparison, the magnitude of difference from the ɛ4− BDNFVal/Val group was expressed using Cohen's d.26 Group means (95% CIs) at each assessment were estimated from the linear mixed-effects model, and differences in performance between ɛ4+ BDNFVal/Val and ɛ4+ BDNFMet groups at each assessment was determined by the extent of overlap of 95% CIs associated with those means. Bonferonni correction was applied to all pairwise comparisons.
To estimate the clinical meaning for the effect of each genetic risk factor on decline in cognition, a group mean of 1.5 s.d. below the Aβ−ɛ4− group was defined as clinically important cognitive impairment.27 The time to reach this criterion was estimated for each group based on linear mixed-effects model-derived linear functions.
Results
Demographic and clinical characteristics
At baseline, statistically significant differences between groups were observed for age, premorbid intelligence and anxiety symptoms (Table 1). No other demographic or clinical characteristics differed between groups. There was no difference in the proportion of individuals in the ɛ4+ or ɛ4− groups who were classified as high Aβ+ (Table 1).
Effect of Aβ levels and ɛ4 on cognitive change
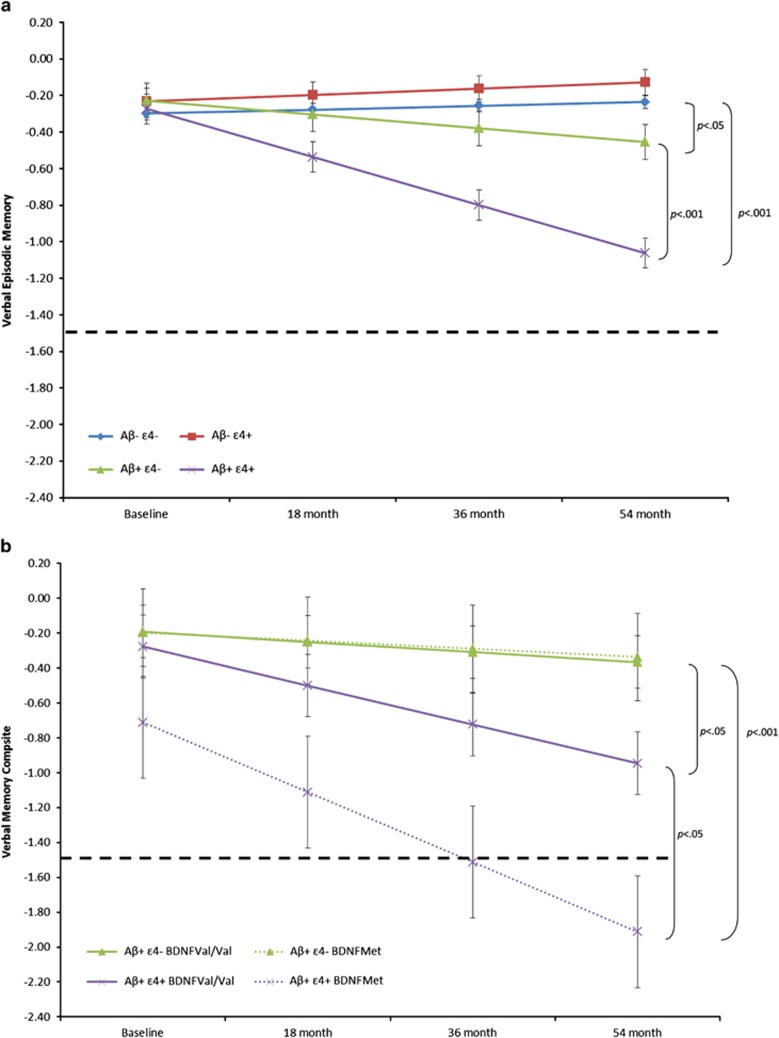
Group mean slopes for each Aβ-ɛ4 group for each composite cognitive score are summarized in Table 2. Relative to Aβ−ɛ4−, the Aβ+ɛ4+ group showed a significantly faster decline on all cognitive composites, with these differences moderate to large in magnitude (Table 2). Extrapolation of the rate of decline in verbal EM in the Aβ+ɛ4+ group indicated it would meet criterion for clinically significant impairment (<1.5 s.d. from controls) in ~9 years (Supplementary Table 1). Compared with Aβ−ɛ4−, the Aβ+ɛ4− group showed a faster rate of decline only for verbal EM composite (Table 2). Inspection of performance on each assessment indicated no overlap between 95% CIs for group mean verbal and visual EM composites between Aβ+ɛ4+ and Aβ+ɛ4− groups at 18-, 36- and 54-month assessments (Supplementary Table 1). Extrapolation of the rate of verbal EM decline in the Aβ+ɛ4− group indicated it would meet criterion for clinically significant impairment in 27 years (Supplementary Table 1). Group mean slopes did not differ significantly for any cognitive composite between the Aβ−ɛ4− and Aβ−ɛ4+ groups.
Table 2. Mean slopes (s.d.) per 18-month interval for each cognitive composite score and magnitudes of difference (Cohen's d) in slopes.
|
Mean slope (s.d.) |
Cohen's d (95% CIs) (vs Aβ−
ɛ4−) |
||||||
|---|---|---|---|---|---|---|---|
| Aβ− ɛ4− (n=188) | Aβ− ɛ4+ (n=61) | Aβ+ ɛ4− (n=36) | Aβ+ ɛ4+(n=48) | Aβ− ɛ4+ | Aβ+ ɛ4− | Aβ+ ɛ4+ | |
| Verbal EM | 0.021 (0.239) | 0.034 (0.206) | −0.075 (0.197) | −0.263 (0.206) | −0.06 (−0.34, 0.23) | 0.41 (0.05, 0.77) | 1.22 (0.88, 1.55) |
| Visual EM | 0.026 (0.276) | 0.030 (0.238) | −0.001 (0.229) | −0.198 (0.237) | −0.01 (−0.30, 0.27) | 0.10 (−0.26, 0.46) | 0.83 (0.51, 1..16) |
| Executive Function | −0.011 (0.220) | −0.003 (0.190) | −0.051 (0.180) | −0.103 (0.188) | −0.04 (−0.33, 0.25) | 0.19 (−0.17, 0.54) | 0.43 (0.11, 0.75) |
| Language | −0.033 (0.252) | −0.035 (0.217) | −0.086 (0.206) | −0.176 (0.216) | 0.01 (−0.28, 0.30) | 0.22 (−0.14, 0.57) | 0.58 (0.26, 0.90) |
| Attention | −0.101 (0.201) | −0.125 (0.174) | −0.100 (0.164) | −0.180 (0.177) | 0.12 (−0.17, 0.41) | −0.01 (−0.36, 0.35) | 0.40 (0.08, 0.72) |
Abbreviations: CI, confidence interval; EM, episodic memory.
Bolded values are significant at the P<0.05 or P<0.001 level; values are adjusted for age, premorbid intelligence and anxiety.
Effect of BDNFVal66Met on the relationship between Aβ, ɛ4 and cognitive change
In Aβ− participants, mean slopes between ɛ4− BDNFVal/Val and the three subgroups (ɛ4− BDNFMet, ɛ4+ BDNFVal/Val and ɛ4+BDNFMet) did not differ for any other composite (data not shown).
In Aβ+participants, relative to the ɛ4− BDNFVal/Val group, the ɛ4+ BDNFMet group showed a faster decline on the verbal and visual EM and language composites, and differences between slopes were moderate to large in magnitude (Table 3). Inspection of group means for individual assessments indicated no overlap between 95% CIs for the mean verbal and visual EM and language composite between ɛ4+ BDNFMet and ɛ4+BDNFVal/Val groups at the 36- and 54-month assessments (Supplementary Table 1). The rate of verbal EM decline in the ɛ4+ BDNFMet group indicated it met criterion for clinically significant impairment within 3 years from enrolment (Figure 2b). In contrast, extrapolation of the rate of verbal EM decline suggested that ɛ4+ BDNFVal/Val group would meet criterion for clinically significant impairment within 10 years. Groups did not differ in the rate of decline on the executive function and attention composites (Table 3). Finally, relative to the ɛ4− BDNFVal/Val group, the ɛ4− BDNFMet group did not show a significantly faster decline on any cognitive composite.
Table 3. Mean slopes (s.d.) per 18-month interval for each cognitive composite score and magnitudes of difference (Cohen's d) in slopes in Aβ+ healthy individuals.
| Mean slope (s.d.) | Cohen's d (95% CIs) (vs ɛ4− BDNFVal/Val) | ||||||
|---|---|---|---|---|---|---|---|
| ɛ4− BDNFVal/Val (n=19) | ɛ4− BDNFMet (n=11) | ɛ4+ BDNFVal/Val (n=27) | ɛ4+ BDNFMet (n=14) | ɛ4− BDNFMet | ɛ4+ BDNFVal/Val | ɛ4+ BDNFMet | |
| Verbal EM | −0.058 (0.341) | −0.046 (0.326) | −0.223 (0.451) | −0.400 (0.423) | −0.04 (−0.76, 0.69) | 0.40 (−0.16, 0.96) | 0.91 (0.19, 1.59) |
| Visual EM | 0.039 (0.325) | −0.091 (0.315) | −0.146 (0.428) | −0.328 (0.407) | 0.40 (−0.32, 1.12) | 0.48 (−0.09, 1.03) | 1.02 (0.29, 1.71) |
| Executive Function | −0.017 (0.262) | −0.087 (0.249) | −0.018 (0.345) | −0.181 (0.323) | 0.27 (−0.46, 0.99) | 0.00 (−0.55, 0.56) | 0.57 (−0.12, 1.24) |
| Language | −0.063 (0.282) | −0.141 (0.269) | −0.130 (0.372) | −0.341 (0.349) | 0.28 (−0.45, 1.00) | 0.20 (−0.36, 0.75) | 0.90 (0.18, 1.58) |
| Attention | −0.028 (0.218) | −0.143 (0.200) | −0.207 (0.279) | −0.134 (0.263) | 0.54 (−0.21, 1.26) | 0.70 (0.12, 1.26) | 0.45 (−0.24, 1.12) |
Abbreviations: CI, confidence interval; EM, episodic memory.
Bolded values are significant at the P<0.05 or P<0.001 level; values are adjusted for age, premorbid intelligence and anxiety.
Figure 2.
(a) Trajectories of change over 54 months on the Verbal Episodic Memory composite for Aβ−ɛ4−, Aβ−ɛ4+, Aβ+ɛ4− and Aβ+ɛ4+groups, with age and premorbid IQ as covariates (error bars represent 95% confidence intervals). Dotted line indicates 1.5 s.d. decline for clinically significant memory impairment. (b) Trajectories of change over 54 months in Aβ+ healthy individuals on the Verbal Episodic Memory composite for ɛ4−/BDNFVal/Val, ɛ4−/BDNFMet, ɛ4+/BDNFVal/Val and ɛ4+/BDNFMet groups, with age and premorbid IQ as covariates (error bars represent 95% confidence intervals). Dotted line indicates 1.5 s.d. decline for clinically significant memory impairment.
Discussion
EM and all other aspects of cognition remained stable over 54 months in Aβ− individuals, irrespective of ɛ4 status, which replicates and extends previous observations from AIBL23,28 and other cohorts.29, 30, 31 The absence of any ɛ4-related cognitive decline in the current Aβ− individuals is also consistent with findings of a recent study9 and supports the hypothesis that there are no Aβ-independent effects of APOE on cognitive decline in healthy individuals, even when studied over more than 4 years. Compared with the Aβ−ɛ4− group, Aβ+ individuals showed faster decline in EM, and this decline was increased by ɛ4+ (Figure 2a). Recently, the exacerbation of Aβ-related memory decline by ɛ4 carriage in healthy individuals was shown over 1.5 years.9 The current findings support and extend this report by demonstrating that APOE ɛ4 carriage does exacerbate Aβ-related cognitive decline in healthy individuals, and persists over more than 4 years. Neurologically, APOE can affect both intrinsic (for example, synaptic plasticity and neuroinflammation) and extrinsic (for example, cerebrovascular disease) factors.3,8,32 As all participants in the AIBL study have well-controlled risk factors for cardiovascular disease,19 the risk of concomitant cerebrovascular events over the period of observation was reduced. Further, as there is increasing experimental evidence that APOE isoforms have a direct effect on Aβ deposition, clearance and Aβ-mediated synaptotoxicity,5, 6, 7 a likely explanation for the cognitive decline seen in the Aβ+ɛ4+ group is that APOE moderates the direct effects of Aβ accumulation. These results differ from previous reports, where we and others found no effect of ɛ4 carriage on Aβ-related cognitive decline.23,28,30,33 The most likely reason for this lack of effect was that the smaller sample sizes used in these studies resulted in reduced power to detect any effects of ɛ4 on Aβ-related cognitive decline.23,28,30,33
We reported previously that BDNFVal66Met moderated Aβ-related changes in cognition and neurodegeneration in both healthy older and mild cognitive impairment groups in AIBL and ADNI, although it did not moderate the rate of Aβ deposition.15, 16, 17 Consistent with evidence indicating that BDNF is necessary for synaptic plasticity in the hippocampus,4 we proposed that BDNFVal66Met affects the clinical manifestation of early AD by influencing the ability of the brain to tolerate Aβ toxicity.16,17 In the current study, we explored whether BDNFVal66Met moderated any relationship between Aβ, ɛ4 and cognition. The Aβ+ɛ4+BDNFMet group showed faster decline in EM and language compared with both the ɛ4− BDNFVal/Val and ɛ4− BDNFMet groups (Figure 2b). The ɛ4+ BDNFVal/Val group showed a moderate rate of cognitive decline. Consistent with our previous observations,15, 16, 17 BDNFVal66Met, such as APOE, did not moderate any cognitive variable in Aβ− individuals.
These results raise some important prognostic issues in managing preclinical AD and the implications for communicating these group data to individuals. The data demonstrates clearly that the rate of cognitive decline in preclinical AD is moderated by the combination of at least two genetic loci: ɛ4+ and BDNFMet. Aβ+ɛ4+ individuals showed significantly greater EM decline than Aβ+ɛ4− individuals and this difference became evident 18 months after enrolment. BDNFMet carriage increased the rate of memory decline related to Aβ and ɛ4, with differences in memory between Aβ+ɛ4+BDNFVal/Val and Aβ+ɛ4+BDNFMet groups becoming evident 36 months after enrolment (Supplementary Table 1). Another way of expressing these observations is to consider the length of time between establishing an individual's Aβ, ɛ4 and BDNF status and a criterion for clinically significant cognitive impairment (performance <1.5 s.d. below controls,27 dashed horizontal line in Figure 2). In the Aβ− group, irrespective of ɛ4 status, there was no evidence of decline over 4.5 years (Figure 2a). In contrast, extrapolation of the EM decline observed in the Aβ+ɛ4+group showed that clinically significant memory impairment would be met ~9 years after enrolment, while the Aβ+ɛ4− group would take ~27 years. Taking into consideration the additional effect of BDNF in Aβ+ individuals, the effect of BDNF is seen clearly in the ɛ4+ subgroup where BDNFVal/Val homozygotes would meet criteria for clinically significant impairment after ~10 years (similar rate based on ɛ4+ status alone) but the accelerated rate of memory decline in the BDNFMet group meant that they met this criteria after only 3 years (Figure 2b). Although there is some evidence in the model that even at baseline the Aβ+ɛ4+BDNFMet group performs worse than the other three groups, this difference was not statistically significant. One limitation of natural history cohorts is that the baseline performance of each individual is defined by their first visit, rather than symptom onset. As such, the data presented here can be interpreted as suggesting that the combination of Aβ+, ɛ4+ and BDNFMet does accelerate cognitive decline significantly such that even at the first assessment this decline is already evident. A diagnosis of mild cognitive impairment is typically made when objective evidence of clinically significant memory impairment is accompanied by individuals' acknowledgement of that impairment, usually corroborated by an informant.27 General cognitive function and functional activities are also typically preserved.27 Over the course of this study, relatively few healthy individuals were classified as meeting clinical criteria for mild cognitive impairment/AD (Figure 1). This indicates that although some individuals met criteria for clinically significant impairment, these individuals, or their caregivers, had not acknowledged any problems with cognition. We have reported previously that subjective memory impairment in the AIBL healthy cohort does not predict objectively defined cognitive impairment or Aβ levels.34 Presumably, individuals with clinically significant memory decline observed here will begin to report subjective memory complaints in the future.
An important caveat is that as three radioligands were used to measure Aβ, SUVr data could not be integrated to form a single continuous measure of Aβ burden. However, we found no relationship between the proportions of individuals who were high and low Aβ+ in the Aβ+ɛ4+and Aβ+ɛ4− groups, suggesting that the faster decline observed in Aβ+ɛ4+was not due to more advanced disease at enrolment. Second, although the large number of healthy individuals who have undergone Aβ PET neuroimaging in the AIBL cohort has allowed for this report to investigate the effects of APOE and BDNF on the clinical manifestation of high Aβ, the resultant sample sizes remain relatively small. Further, the AIBL study is also not a representative population sample. Healthy participants in the AIBL study were highly educated, were of Caucasian backgrounds and had few existing or untreated medical, neurological or psychiatric illnesses. Participants selected for neuroimaging were also enriched for APOE ɛ4 carriers. As such, these results need to be replicated in other more representative and ethnically diverse prospective cohorts of healthy individuals. Finally, APOE and BDNF are unlikely to be the only factors that moderate the clinical manifestation of Aβ rather, other co-morbidities (for example, cerebrovascular disease) and lifestyle and genetic factors will need to be considered in future work.
Acknowledgments
Alzheimer's Australia (Victoria and Western Australia) assisted with promotion of the study and the screening of telephone calls from volunteers. The AIBL team wishes to thank the clinicians who referred patients with AD to the study: Associate Professor Brian Chambers, Professor Edmond Chiu, Dr Roger Clarnette, Associate Professor David Darby, Dr Mary Davison, Dr John Drago, Dr Peter Drysdale, Dr Jacqueline Gilbert, Dr Kwang Lim, Professor Nicola Lautenschlager, Dr Dina LoGiudice, Dr Peter McCardle, Dr Steve McFarlane, Dr Alastair Mander, Dr John Merory, Professor Daniel O'Connor, Dr Ron Scholes, Dr Mathew Samuel, Dr Darshan Trivedi and Associate Professor Michael Woodward. We thank all those who participated in the study for their commitment and dedication to helping advance research into the early detection and causation of AD. Funding for the study was provided in part by the study partners (Commonwealth Scientific Industrial and research Organization (CSIRO), Edith Cowan University (ECU), Mental Health Research institute (MHRI), National Ageing Research Institute (NARI), Austin Health, CogState Ltd]. The study also received support from the National Health and Medical Research Council (NHMRC) and the Dementia Collaborative Research Centres program (DCRC2), as well as funding from the Science and Industry Endowment Fund (SIEF) and the Cooperative Research Centre for Mental Health (CRCMH).
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
CLM is an advisor to Prana Biotechnology Ltd and a consultant to Eli Lilly. RHP and PJS are scientific consultants to Cogstate Ltd. PM is a full-time employee of Cogstate Ltd. DA has served on scientific advisory boards for Novartis, Eli Lilly, Janssen and Pfizer Inc. RNM is a consultant to Alzhyme. CCR has served on scientific advisory boards for Bayer Pharma, Elan Corporation, GE Healthcare and AstraZeneca, has received speaker honoraria from Bayer Pharma and GE Healthcare and has received research support from Bayer Pharma, GE Healthcare, Piramal Lifesciences and Avid Radiopharmaceuticals. VLV served as a consultant for Bayer Pharma and has received research support from a NEDO grant from Japan. All other authors declare no conflict of interest.
Supplementary Material
References
- 1Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O et al. Amyloid β deposition, neurodegeneration and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol 2013; 12: 357–367. [DOI] [PubMed] [Google Scholar]
- 2Jedynak BM, Lang A, Liu B, Katz E, Zhang Y, Wyman BT et al. A computational neurodegenerative disease progression score: method and results with the Alzheimer's Disease Neuroimaging Initiative cohort. NeuroImage 2012; 63: 1478–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 2013; 9: 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4Fahnestock M. Brain-derived neurotrophic factor: the link between amyloid-β and memory loss. Future Neurol 2011; 6: 627–639. [Google Scholar]
- 5Lee CYD, Tse W, Smith JD, Landreth GE. Apolipoprotein E promotes β-amyloid trafficking and degradation by modulating microglial cholesterol levels. J Biol Chem 2012; 287: 2032–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Verghese PB, Castellano JM, Garai K, Wang Y, Jiang H, Shah A et al. ApoE influences amyloid-β (Aβ) clearance despite minimal apoE/Aβ association in physiological conditions. Proc Natl Acad Sci USA 2013; 110: 1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Hudry E, Dashkoff J, Roe AD, Takeda S, Koffie RM, Hashimoto T et al. Gene transfer of human Apoe isoform results in differential modulation of amyloid deposition and neurotoxicity in mouse brain. Sci Transl Med 2013; 5: 212ra161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8Zlokovic BV. Cerebrovascular effects of apolipoprotein E: implications for Alzheimer disease. JAMA Neurol 2013; 70: 440–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Mormino EC, Betensky RA, Hedden T, Schultz AP, Ward A, Huijbers W et al. Amyloid and APOE4 interact to influence short-term decline in preclinical Alzheimer's disease. Neurology 2014; 82: 1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL et al. Longitudinal modeling of age-related memory decline and the APOE ɛ4 effect. N Engl J Med 2009; 361: 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Cosentino S, Scarmeas N, Helzner E, Glymour MM, Brandt J, Albert M et al. APOE ɛ4 allele predicts faster cognitive decline in mild Alzheimer's disease. Neurology 2008; 70: 1842–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 2013; 14: 7–23. [DOI] [PubMed] [Google Scholar]
- 13Anastasia A, Deinhardt K, Chao MV, Will NE, Irmady K, Lee FS et al. Val66Met polymorphism of BDNF alters prodomain structure to induce neuronal growth cone retraction. Nat Commun 2013; 4: 2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14Ramser EM, Gan KJ, Decker H, Fan EY, Suzuki MM, Ferreira ST et al. Amyloid-β oligomers induce tau-independent disruption of BDNF axonal transport via calcineurin activation in cultured hippocampal neurons. Mol Biol Cell 2013; 24: 2494–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15Feng S, Sevigny J, Verma A, Bennett D, Lim YY, Maruff P. Genetic and image biomarkers associated with decline in cognitive measures and brain glucose metabolism in populations of early Alzheimer's disease. Alzheimer Dement 2013; 9: 178. [Google Scholar]
- 16Lim YY, Villemagne VL, Laws SM, Ames D, Pietrzak RH, Ellis KA et al. BDNF Val66Met moderates Aβ-related memory decline and hippocampal atrophy in prodromal Alzheimer's disease: A preliminary study. PLoS ONE 2014; 9: e86498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Lim YY, Villemagne VL, Laws SM, Ames D, Pietrzak RH, Ellis KA et al. BDNF Val66Met, Aβ amyloid and cognitive decline in preclinical Alzheimer's disease. Neurobiol Aging 2013; 34: 2457–2464. [DOI] [PubMed] [Google Scholar]
- 18Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging 2010; 31: 1275–1283. [DOI] [PubMed] [Google Scholar]
- 19Ellis KA, Bush AI, Darby D, De Fazio D, Foster J, Hudson P et al. The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: Methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer's disease. Int Psychogeriatr 2009; 21: 672–687. [DOI] [PubMed] [Google Scholar]
- 20Vandenberghe R, Van Laere K, Ivanoiu A, Salmon E, Bastin C, Triau E et al. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann Neurol 2010; 68: 319–329. [DOI] [PubMed] [Google Scholar]
- 21Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA et al. Use of florbetapir-PET for imaging beta-amyloid pathology. J Am Med Soc 2011; 305: 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Rowe CC, Bourgeat P, Ellis KA, Brown B, Lim YY, Mulligan R et al. Predicting Alzheimer disease with β-amyloid imaging: results from the Australian imaging, biomarkers, and lifestyle study of ageing. Ann Neurol 2014; 74: 905–913. [DOI] [PubMed] [Google Scholar]
- 23Lim YY, Maruff P, Pietrzak RH, Ames D, Ellis KA, Harrington K et al. Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer's disease. Brain 2014; 137: 221–231. [DOI] [PubMed] [Google Scholar]
- 24Harrington K, Lim YY, Ellis KA, Copolov C, Ames D, Darby D et al. The effect of Aβ amyloid and APOE ɛ4 on composite cognitive measures in healthy older adults and MCI. Int Psychogeriatr 2013; 25: 1667–1677. [DOI] [PubMed] [Google Scholar]
- 25Jack CR, Wiste HJ, Lesnick TG, Weigand SD, Knopman DS, Vemuri P et al. Brain β-amyloid load approaches a plateau. Neurology 2013; 80: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Cohen J. A power primer. Psychol Bull 1992; 112: 155–159. [DOI] [PubMed] [Google Scholar]
- 27Petersen RC. Mild cognitive impairment as a diagnostic entity. J Int Med 2004; 256: 183–194. [DOI] [PubMed] [Google Scholar]
- 28Lim YY, Ellis KA, Pietrzak RH, Ames D, Darby D, Harrington K et al. Stronger effect of amyloid load than APOE genotype on cognitive decline in healthy older adults. Neurology 2012; 79: 1645–1652. [DOI] [PubMed] [Google Scholar]
- 29Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA et al. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol 2013; 12: 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Doraiswamy PM, Sperling RA, Coleman RE, Johnson KA, Reiman EM, Davis MD et al. Amyloid-β assessed by florbetapir F 18 PET and 18-month cognitive decline. Neurology 2012; 79: 1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Stonnington CM, Chen K, Lee W, Locke DE, Dueck AC, Liu X et al. Fibrillar amyloid correlates of preclinical cognitive decline. Alzheimer Dement 2014; 10: e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32Irie F, Fitzpatrick AL, Lopez OL, Kuller LH, Peila R, Newman AB et al. Enhanced risk for Alzheimer disease in persons with type 2 diabetes and APOE epsilon4: The Cardiovascular Health Study Cognition Study. Arch Neurol 2008; 65: 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Roe CM, Fagan AM, Grant EA, Hassenstab J, Moulder KL, Maue Dreyfus D et al. Amyloid imaging and CSF biomarkers in predicting cognitive impairment up to 7.5 years later. Neurology 2013; 80: 1784–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34Buckley R, Saling MM, Ames D, Rowe CC, Lautenschlager NT, Macaulay SL et al. Factors affecting subjective memory complaints in the AIBL aging study: biomarkers, memory, affect, and age. Int Psychogeriatr 2013; 25: 1307–1315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.