Abstract
Background
Atrial fibrillation (AF) is associated with cardiac fibrosis, which can now be measured noninvasively using T1-mapping with cardiac magnetic resonance imaging (CMRI). This study aimed to assess the impact of AF on ventricular T1 at the time of CMRI.
Methods
Subjects with AF scheduled for AF ablation underwent CMRI with standard electrocardiography gating and breath-hold protocols on a 1.5 T scanner with post-contrast ventricular T1 recorded from 6 regions of interest at the mid-ventricle. Baseline demographic, clinical, and imaging characteristics were examined using univariate and multivariable linear regression modeling for an association with myocardial T1.
Results
One hundred fifty-seven patients were studied (32% women; median age, 61 years [interquartile range {IQR}, 55–67], 50% persistent AF [episodes>7 days or requiring electrical or pharmacologic cardioversion], 30% in AF at the time of the CMRI). The median global T1 was 404 ms (IQR, 381–428). AF at the time of CMRI was associated with a 4.4% shorter T1 (p=0.000) compared to sinus rhythm when adjusted for age, sex, persistent AF, body mass index, congestive heart failure, and renal dysfunction (estimated glomerular filtration rate<60). A post-hoc multivariate model adjusted for heart rate suggested that heart rate elevation (p=0.009) contributes to the reduction in T1 observed in patients with AF at the time of CMRI. No association between ventricular T1 and AF recurrence after ablation was demonstrated.
Conclusion
AF at the time of CMRI was associated with lower post-contrast ventricular T1 compared with sinus rhythm. This effect was at least partly due to elevated heart rate. T1 was not associated with the recurrence of AF after ablation.
Keywords: Atrial fibrillation, Myocardial T1, Cardiac MRI, Ventricular T1, Atrial fibrillation ablation
1. Introduction
Atrial fibrillation (AF) is a common disease that has been identified as both a cause and consequence of adverse cardiac electrical and structural remodeling [1], [2]. Structural remodeling in the form of cardiac fibrosis occurs in both the atria and ventricles, and it is known to contribute to the development of AF [3], [4], [5]. Diffuse myocardial fibrosis can now be measured using cardiac magnetic resonance imaging (CMRI) post-contrast T1 relaxation (T1) time. Gadolinium-based contrast accumulates in areas of increased extracellular space, resulting in a shorter T1. The degree to which contrast shortens T1 is, therefore, a reflection of the extent of diffuse myocardial fibrosis. A shorter T1 in both the atria and ventricles predicts AF presence and severity, and it may predict the clinical response to AF therapies such as catheter-based AF ablation [5], [6].
Given the increasing interest in exploring the potential clinical applications of T1, it is important to understand the factors that influence its measurement. Experimental evidence shows that at the time of CMRI, cardiac rhythm abnormalities such as tachycardia or irregularity may lead to an underestimation of T1 [7], [8]. Tachycardia and irregularity are characteristic of AF; however, the effect of AF on myocardial T1 in humans has not been previously investigated. Accordingly, the primary goal of this study was to test the hypothesis that AF at the time of CMRI is an independent predictor of shortened ventricular T1 in patients with AF referred for catheter-based ablation. As a secondary goal, given previous reports of ventricular T1 predicting AF recurrence after ablation [6], we sought to provide independent validation of that finding.
2. Material and methods
2.1. Study population
Subjects were enrolled in the Vanderbilt AF Ablation Registry and provided written informed consent. The study protocol was approved by the Vanderbilt Institutional Review Board. Patients with data that met the following eligibility criteria were included in our analysis: age>18 years, AF ablation after 1/1/2008, a pre-ablation CMRI performed at Vanderbilt University, and no prior history of catheter-based or surgical ablation for AF. To explore the association with AF recurrence, only subjects with at least 6 months of clinical follow-up after AF ablation were included.
2.2. CMRI protocol
As part of routine clinical care, a CMRI study was performed prior to AF ablation to provide detailed assessment of cardiac and PV anatomy and to generate a three-dimensional (3D) electroanatomic map. Imaging was performed using a 1.5-T Siemens Avanto (Siemens Healthcare, Erlangen, Germany) with an 8-channel cardiac coil. Ventricular function was assessed using breath-hold, electrocardiogram-gated, serial short-axis steady-state free precession cine images as previously described [9]. Intravenous Gd-DTPA contrast (gadopentate dimeglumine, Magnevist®, Bayer Healthcare Pharmaceuticals, Wayne, NJ, USA) was administered at a dose of 0.2 mmol/kg. The size of the left atrium was measured in the anterior–posterior orientation on axial half-Fourier acquisition turbo spin-echo images. A Look-Locker sequence was obtained in a short-axis plane at the level of the papillary muscles 10 min after contrast administration with these imaging parameters: field of view, 275–400×340–400 mm; matrix, 66–96×192; slice thickness, 8 mm; flip angle, 30°; and no parallel imaging. Standard Look-Locker imaging included 15–35 images acquired every other R-R interval with phase intervals of approximately 30 ms. The images were electrocardiography gated and acquired using a segmented k-space during breath-holds. For the left ventricular (LV) volumes, ejection fraction, and mass measurements, epicardial and endocardial contours were drawn in end-diastole and end-systole on a Leonardo Workstation (Siemens Healthcare, Erlangen, Germany).
2.3. Quantification of myocardial T1
The Look-Locker images were analyzed utilizing an open source software program (MRMap version 1.3 [http://mrmap.sourceforge.net]) [10], [11]. The T1 map generated was saved as a Digital Imaging and Communications in Medicine image and imported into MatLab (MathWorks, Natick, MA, USA). Six regions of interest were manually drawn in the midventricular segment corresponding to the anterior, anteroseptal, inferoseptal, inferior, inferolateral, and anterolateral walls in short-axis orientation. The myocardial T1 values for each voxel in each of the 6 regions of interest were then exported for averaging and analysis. A global T1 value was calculated by averaging the T1 from the 6 regions of interest.
2.4. Catheter ablation
In brief, AF ablation was performed with the patient under general anesthesia with continuous invasive monitoring of blood pressure and oxygen saturation. A 3D mapping system (Carto, Biosense-Webser, Inc., Diamond Bar, CA, USA) was used for non-fluoroscopic catheter navigation, computed tomographic, or magnetic resonance image integration as well as tagging of ablation sites. Trans-septal access was obtained using fluoroscopy and intracardiac or transesophageal echocardiographic guidance. An irrigated-tip ablation catheter was used. Circumferential left atrial (LA) ablation lines were placed around the antrum of the ipsilateral pulmonary veins, and the demonstration of pulmonary vein (PV) isolation was the major procedural endpoint. PV potentials were recorded using a circular mapping catheter placed in each PV to test for the absence of signals conducting into the PV during LA pacing (entrance block) or into the LA from the PVs during PV pacing (exit block). Additional ablation was performed until PV isolation was achieved. Empiric linear lesions to the LA roof, basal posterior wall, and mitral isthmus and/or ablation of complex fractionated electrograms were placed based on operator discretion. Anticoagulation with heparin was used in an attempt to maintain an activated clotting time>300 s during LA access.
2.5. Follow-up
Patients were seen in clinical follow-up at 1, 3, 6, and 12 months. Ambulatory 48-h Holter (3-month) or 7-day auto-triggered event monitoring (6- and 12-month) was performed to assess for asymptomatic AF recurrence. Recurrence was defined as >30 s of AF, atrial tachycardia, or atrial flutter (AF/AT/AFL). As is standard for AF ablation reporting, a 3-month blanking period was used such that recurrences during that period were not counted toward the arrhythmia recurrence endpoint [12].
2.6. Statistical analysis
Baseline patient characteristics are reported as the frequency and percentage for categorical variables and as the median and interquartile range (IQR) for continuous variables. Groups were compared using a Wilcoxon sum rank test for continuous variables and a Chi-square test or Fisher’s exact test for categorical variables. The analysis for predictors of global T1 was performed using multivariable linear regression. To avoid over-fitting our multivariable linear regression model, we calculated the number of covariates based on a ratio of >15 subjects per degree of freedom. Age and sex were pre-specified covariates for our final model, and additional covariates were selected if the p value on univariate analysis was <0.10. Covariates in the regression models were evaluated for multicollinearity by calculation of the variance inflation factor (VIF), and covariates were excluded when VIF values were >2.5. Continuous variables were graphically assessed for normality and log-transformed to improve the residuals. A complete case analysis was performed, and records with a missing value were excluded from the final analysis. As a secondary analysis, a Cox proportional hazards model was used to test the ability of T1 to predict time to first occurrence of any atrial tachyarrhythmia after ablation. A 10:1 ratio of degrees of freedom to events was used for the Cox proportional hazards model. The assumptions of the Cox proportional hazards model were met including: (1) censoring was non-informative; and (2) the assumption of proportional hazards was examined using log–log plots that demonstrated parallel curves with proportional separation. Age, sex, and global T1 were pre-specified covariates, and additional covariates were selected based on their significance on univariate analysis. Finally, a post-hoc analysis was performed using multivariable linear regression to test for an association between heart rate during CMRI, heart rhythm during CMRI, and global T1. Two-sided p values≤0.05 were considered statistically significant in all of the analyses. Analysis was performed using SPSS version 21 (IBM Corporation, Armonk, NY, USA).
3. Results
3.1. Patient characteristics
A total of 196 patients met the inclusion criteria; of them, 39 were excluded due to poor image quality of the myocardial T1 map generated. Among the excluded patients, AF at the time of CMRI was more common (61%, n=22/36, p=0.001; 3 patients had indeterminate rhythm). The final cohort comprised 157 patients (mean age, 61±6 years; 68% men; 59% hypertensive; mean body mass index [BMI], 29 kg/m2). Fifty percent (78/157) of the patients were classified as having persistent AF (episodes>7 days or requiring electrical or pharmacologic cardioversion). A complete list of patient characteristics is presented in Table 1.
Table 1.
Patient characteristics.
| Eligible patients | ||
| Age (years) | 157 | 61 (55–67) |
| Sex (female) | 157 | 50 (32%) |
| Atrial fibrillation | 157 | 157 (100%) |
| Paroxysmal atrial fibrillation | 157 | 79 (50%) |
| Time since atrial fibrillation diagnosis (months) | 157 | 51 (20–102) |
| Lone atrial fibrillation | 157 | 16 (10%) |
| Stroke | 146 | 7 (5%) |
| Body mass index (kg/m²) | 157 | 29 (26–34) |
| Hypertension | 146 | 93 (59%) |
| Coronary artery disease | 156 | 28 (18%) |
| Diabetes mellitus | 146 | 25 (16%) |
| Congestive heart failure | 145 | 12 (8%) |
| Preserved ejection fraction (LVEF≥50%) | 12 | 8 (67%) |
| Renal dysfunction (eGFR<60) | 147 | 30 (19%) |
Continuous data are expressed as median (interquartile range) or number (percentage). Data for every parameter were not available for every patient. LVEF, left ventricular ejection fraction; eGFR, estimated glomerular filtration rate.
3.2. Cardiac chambers, ejection fraction, and myocardial T1
Morphological and functional measurements as well as myocardial T1 data are presented in Table 2. Average left atrial and ventricular chamber sizes were within normal limits. The overall LV ejection fraction was also normal. Thirty percent of patients (n=47) were in AF at the time of the CMRI study (8 of 78 patients with paroxysmal AF, 39 of 77 patients with persistent AF). The average global T1 for all patients was 404 ms. T1 was not measurable in 0.9% of segments (10/1099).
Table 2.
Magnetic resonance imaging data.
| Eligible Patients | ||
| Left atrial size (mm) | 157 | 37 (33–43) |
| Left ventricular ejection fraction (%) | 157 | 64 (56–72) |
| Left ventricular diastolic diameter (mm) | 157 | 48 (43–52) |
| Atrial fibrillation during CMRI | 155 | 47 (30%) |
| Interventricular septum thickness (mm) | 155 | 9 (8–10) |
| Left ventricular posterior wall thickness (mm) | 154 | 7 (6–8) |
| Global T1 (ms) | 157 | 404 (381–428) |
| Anterior T1 (ms) | 156 | 420 (392–448) |
| Anteroseptal T1 (ms) | 157 | 395 (372–420) |
| Anterolateral T1 (ms) | 155 | 407 (387–436) |
| Inferior T1 (ms) | 154 | 391 (371–419) |
| Inferoseptal T1 (ms) | 157 | 405 (376–431) |
| Inferolateral T1 (ms) | 153 | 397 (367–424) |
Continuous data are expressed as median (interquartile range). CMRI, cardiac magnetic resonance imaging; T1, post-contrast T1 relaxation time.
3.3. Univariate and multivariate predictors of myocardial T1
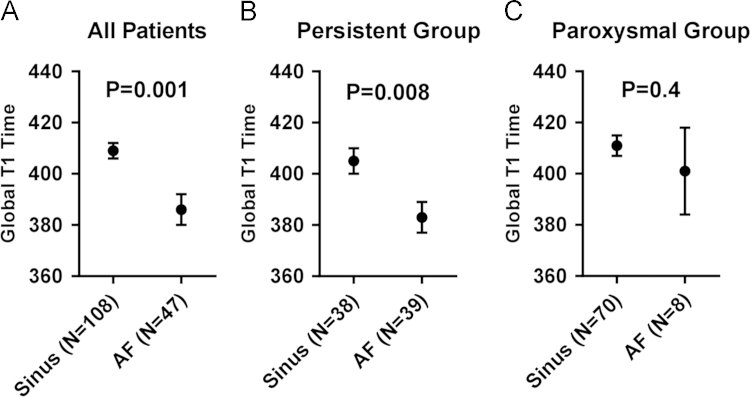
The univariate analysis results of patient characteristics, comorbidities, and imaging parameters are shown in Table 3. On univariate analysis, sex, paroxysmal AF, BMI, congestive heart failure (CHF), renal dysfunction, and rhythm during CMRI were significantly associated with global T1. The majority of patients in AF at the time of CMRI were classified as having persistent AF (83%, 39/47, p=0.000). AF (as opposed to sinus rhythm) at the time of CMRI remained a significant predictor of reduced T1 in the persistent AF subgroup but was not significant in the paroxysmal subgroup, although only 8 patients in this group presented in AF (Fig. 1). The mean global T1 was shorter for patients with persistent vs. paroxysmal AF (394±33.6 ms vs. 410±36.5 ms, respectively).
Table 3.
Univariate Predictors of Global T1.
| Β-coefficienta | Pvalue | |
| AF during CMRI | −22 (−35, −11) | 0.000 |
| Age (per decade) | −4 (−9, 2) | 0.21 |
| Sex (female) | −28 (−39, −17) | 0.000 |
| Paroxysmal AFb | 16 (5, 27) | 0.005 |
| Time since AF diagnosis (per 12 months) | 0.5 (−0.4, 1.3) | 0.26 |
| Lone AF | 14 (−4, 33) | 0.13 |
| Stroke | 14 (−14, 42) | 0.32 |
| Body mass index (per 10 kg/m²) | −11, (−21, −2) | 0.02 |
| Hypertension | 2 (−11,14) | 0.80 |
| Coronary artery disease | 1 (−13,16) | 0.85 |
| Diabetes mellitus | −3, (−19, 13) | 0.72 |
| Congestive heart failure | 26 (5, 47) | 0.016 |
| Renal dysfunction (eGFR<60) | −14 (−29, 1) | 0.06 |
| Left atrial size (per 10 mm) | −2 (−9, 5) | 0.64 |
| LV ejection fraction (per 10%) | 4, (−2, 9) | 0.16 |
| LV diastolic diameter (per 10 mm) | 8 (−1, 16) | 0.08 |
| LV interventricular septum thickness (mm) | 0.8 (−2.2, 3.7) | 0.61 |
| LV posterior wall thickness (mm) | 2.2 (−1.2, 5.7) | 0.20 |
The B-coefficient is the variation from the mean of the global T1 in milliseconds given the specified change in the independent variable.g
Persistent AF is the reference group. AF, atrial fibrillation; CMRI, cardiac magnetic resonance imaging; eGFR, estimated glomerular filtration rate; LV, left ventricular; T1, post-contrast T1 relaxation time.
Fig. 1.
Cardiac rhythm as a predictor of ventricular T1. Cardiac rhythm (AF as opposed to sinus rhythm) was associated with reduced ventricular T1 in (a) all patients, (b) persistent AF patients, and (c) paroxysmal AF patients, although this association missed statistical significance in the paroxysmal AF group. AF, atrial fibrillation; T1, post-contrast T1 relaxation time.
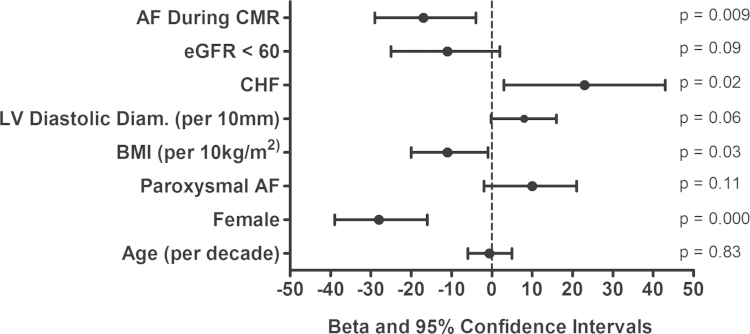
To investigate the finding of shorter T1 in patients with persistent AF, a limited multivariable regression analysis including terms for paroxysmal AF status and rhythm during CMRI was performed. The association between paroxysmal AF and T1 was no longer statistically significant (paroxysmal: β-coefficient=9 [95% confidence interval (CI), −3 to 21] p=0.13) when adjusted for rhythm during CMRI (AF: β=−19 [95% CI, −32 to −5] p=0.006). Clinical covariates included in our final multivariable model were age, sex, rhythm during CMRI, paroxysmal AF, BMI, CHF, renal dysfunction (estimated glomerular filtration rate<60 ml/min/1.75 m2), and LV diastolic diameter (Table 3). In our fully adjusted model, AF at the time of CMRI was found to statistically significantly shorten the global T1 by 17 ms (95% CI, −29 to −4 ms, p=0.009). Female sex was also associated with a 28-ms reduction in T1 (95% CI, −39 to −16, p=0.000). A 10 kg/m2 increase in BMI was associated with an 11 ms reduction in T1 (95% CI, −20 to −1, p=0.03). CHF was associated with a 23-ms increase in T1 (95% CI, 3–43, p=0.02). Age (p=0.83), paroxysmal status (p=0.11), LV diastolic diameter (p=0.06), and renal dysfunction (p=0.09) did not significantly affect T1 after multivariate adjustment (Fig. 2).
Fig. 2.
Multivariate analysis of global T1. After multivariate adjustment, female sex, higher BMI, and atrial fibrillation at the time of CMRI were associated with a reduced myocardial T1 (n=145). The clinical diagnosis of CHF was associated with an elevated myocardial T1. AF, atrial fibrillation; BMI, body mass index; CMRI, cardiac magnetic resonance imaging; eGFR, estimated glomerular filtration rate; T1, post-contrast T1 relaxation time.
To assess for other differences between patients with and without AF at the time of CMRI, we ran a univariate analysis between rhythm during CMRI (sinus rhythm vs. AF) and the variables in Table 1. As expected, patients in sinus rhythm at the time of CMRI were more likely to be paroxysmal (65% vs. 17%, p<0.001), have lone AF (12% vs. 2%, p=0.048), and were less likely to have CHF (5% vs. 15%, p=0.028) or a prior stroke (2% vs. 11%, p=0.015) compared to patients in AF at the time of CMRI. Furthermore, patients in sinus rhythm at the time of CMRI had smaller left atria (mean±SD, 3.7±0.7 cm vs. 4.1±0.9, p=0.005) and higher LVEF (66±8% vs. 56±13%, p<0.001). Patients in sinus rhythm showed a non-significant trend toward being less likely to have CAD (15% vs. 26%, p=0.097). Sex, hypertension, diabetes, and renal dysfunction showed no association with rhythm at the time of CMRI.
In our post-hoc analysis performed to examine the influence of heart rate during CMRI on the association observed between AF during CMRI and global T1, patients in AF during CMRI had a statistically significantly higher heart rate than patients in normal sinus rhythm (median, 64 bpm [IQR, 55–70] vs. 80 bpm [IQR, 73–100] p=0.000). On univariate analysis, heart rate during CMRI decreased global T1 by 6 ms per 10 bpm increase in heart rate (95% CI, −9 to −3 ms, p=0.000). On multivariate analysis, heart rate during CMRI and heart rhythm during CMRI were both associated with a reduction in global T1. The association between heart rate during CMRI and ventricular T1 was highly statistically significant (β=−4 ms per 10 bpm [95% CI, −8 to −1 ms], p=0.009). However, heart rhythm during CMRI missed statistical significance (β=−13 ms [95% CI, −26 to 1 ms], p=0.075). Within the subgroup of patients in sinus rhythm at the time of CMRI, the correlation between heart rate and ventricular T1 remained statistically significant (β=−7 ms per 10 bpm [95% CI, −12 to −2 ms], p=0.004). However, in the subgroup in AF at the time of CMRI, this correlation showed the same trend but was not significant (β=−3 ms per 10 bpm [95% CI, −8 to 2 ms], p=0.299).
3.4. Predictors of AF recurrence
AF/AT/AFL recurrence was observed in 47% of patients (74/157). The mean time to recurrence was 168±82 days. Results from the univariate analysis of predictors of AF recurrence after catheter ablation are shown in Supplemental Table 1. Global T1 was not a statistically significant predictor of recurrence. Age, the only significant predictor of recurrence on univariate analysis, increased the risk of recurrence by 30% per decade (hazard ratio, 1.3 [range, 1.0–1.7], p=0.03). Covariates included in the multivariable analysis included age, sex, global T1, rhythm during CMRI, LVEF, paroxysmal AF, and CHF. No covariates were statistically significantly associated with AF recurrence on multivariate analysis (Supplemental Table 1).
4. Discussion
In this study, we found that being in AF at the time of CMRI imaging was a significant predictor of a shorter ventricular T1 independent of other clinically relevant covariates. Recent reports have demonstrated the clinical utility of T1 measurements in populations with AF but have not accounted for cardiac rhythm at the time of CMRI or excluded patients in AF [13], [14]. Our study adds to the existing literature by demonstrating a strong association between cardiac rhythm at the time of CMRI and T1, and it highlights the need to understand whether this association results from the identification of a subset of patients with AF and an increased amount of diffuse ventricular fibrosis or technical limitations involved with the image acquisition and analysis.
Several studies have examined the clinical utility of T1 mapping as a noninvasive surrogate for diffuse myocardial fibrosis in subjects with AF. Patients with a history of AF have shorter ventricular T1 than controls without AF [15]. Evidence that this may result from an increase in diffuse ventricular fibrosis due to episodes of tachycardia is suggested by the finding that patients with a history of focal atrial tachycardia have shortened ventricular T1 that normalizes after successful ablation [16]. In this study, we expected to find that subjects with persistent AF had a shorter T1 than those with paroxysmal AF due to a greater AF episode frequency and duration. Although an association between paroxysmal/persistent AF status was present in univariate analysis, it was no longer significant when adjusted for the presence of AF at the time of CMRI. We interpret this finding to suggest that either: (1) AF at the time of CMRI better identified a subset of patients with a higher AF burden (and, therefore, higher diffuse fibrosis) than the classification of paroxysmal vs. persistent AF status; or (2) the relative irregularity and faster cardiac rhythm associated with AF at the time of CMRI resulted in artifactual shortening of T1.
Experimental evidence exists to support the possibility that AF adversely affects myocardial T1 measurement accuracy. Specifically, a commonly used method for assessing myocardial T1 values, the modified Look-Locker Inversion recovery, has been shown to underestimate T1 in the presence of tachycardia or an irregular cardiac rhythm, which are both characteristic of AF [7], [8]. Our data showed that patients in AF at the time of CMRI were significantly more tachycardic compared to patients in sinus rhythm. Our post-hoc analysis suggested that tachycardia appears to be a key factor underlying the reduction in global T1 for patients in AF during CMRI. The cardiac irregularity could not be quantified, but given the strong trend toward an association between AF during CMRI and global T1 (p=0.075) when adjusted for heart rate, the remaining contribution may be accounted for by irregularity.
4.1. Clinical implications
Our finding that AF during CMRI affects T1 measurement is potentially an important finding because recent studies examined the predictive power of T1 in AF populations [5], [6]. In these studies, AF at the time of CMRI was either not adjusted for or used as an exclusion criterion [13], [14]. If AF during CMRI shortens T1 measurement irrespective of the degree of fibrosis, the results of these studies may reflect an association with factors other than diffuse fibrosis.
As previously reported, our study demonstrated that female sex was a strong independent predictor of shorter myocardial T1 [17], [18]. It has not been determined histologically whether this finding is truly due to a greater degree of diffuse fibrosis in women or some other variable, such as a thinner ventricular septum, lower packed cell volume of the blood pool, or overall difference in cardiac or body size [18].
Our results showed a seemingly paradoxical longer myocardial T1 in patients with a clinical diagnosis of CHF. These results contradict prior reports showing that heart failure and increased myocardial stiffness are associated with shorter myocardial T1 [19], [20], [21]. Given that only 8% (12/145) of our patients had a CHF diagnosis and the expected finding that CHF would be associated with increased diffuse fibrosis, we suspect that the longer T1 in CHF patients was due to a chance association.
Future directions of this study should aim to understand the mechanism of the association between T1 and rhythm at CMRI. Paired analysis of T1 from CMRI images during AF and normal sinus rhythm may be informative. While repeating the CMRI 3–6 months after catheter-based AF ablation is common [12], at our institution, follow-up CMRI studies were performed without a repeat Look-Locker sequence, which precluded the measurement of ventricular T1 post-ablation. Finally, our study failed to demonstrate an association between T1 and arrhythmia recurrence after AF ablation, which was observed in other studies. It is possible that a smaller effect exists, which our study was unable to detect; however, our study was larger than previous reports of a positive association [6]. The same is true of paroxysmal versus persistent AF status, as paroxysmal AF showed only a non-significant trend toward decreased recurrence in our study.
5. Conclusions
In a population of patients undergoing catheter-based ablation for AF, the presence of AF at the time of CMRI was an independent predictor of shortened ventricular T1. This appears to be partly related to higher resting heart rates with patients in AF. As the clinical utility of T1 is further explored, additional research will be needed to determine if AF at the time of CMRI reflects an increased level of diffuse fibrosis or simply a technical artifact.
Conflict of interest
All authors contributed to and approve of this work. All authors declare no conflicts of interest related to this study.
Footnotes
Supplementary data associated with this article can be found in the online version at 10.1016/j.joa.2015.08.005.
Appendix A. Supplementary material
Supplementary material
References
- 1.Wijffels M.C., Kirchhof C.J., Dorland R. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 2.Nattel S., Harada M. Atrial remodeling and atrial fibrillation: recent advances and translational perspectives. J Am Coll Cardiol. 2014;63:2335–2345. doi: 10.1016/j.jacc.2014.02.555. [DOI] [PubMed] [Google Scholar]
- 3.Platonov P.G., Mitrofanova L.B., Orshanskaya V. Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age. J Am Coll Cardiol. 2011;58:2225–2232. doi: 10.1016/j.jacc.2011.05.061. [DOI] [PubMed] [Google Scholar]
- 4.Burstein B., Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 5.Ling L.H., Kistler P.M., Ellims A.H. Diffuse ventricular fibrosis in atrial fibrillation: noninvasive evaluation and relationships with aging and systolic dysfunction. J Am Coll Cardiol. 2012;60:2402–2408. doi: 10.1016/j.jacc.2012.07.065. [DOI] [PubMed] [Google Scholar]
- 6.McLellan A.J., Ling L.H., Azzopardi S. Diffuse ventricular fibrosis measured by T1 mapping on cardiac MRI predicts success of catheter ablation for atrial fibrillation. Circ Arrhyth Electrophysiol. 2014;7:834–840. doi: 10.1161/CIRCEP.114.001479. [DOI] [PubMed] [Google Scholar]
- 7.Gai N.D., Stehning C., Nacif M. Modified Look-Locker T1 evaluation using Bloch simulations: human and phantom validation. Mag Res Med. 2013;69:329–336. doi: 10.1002/mrm.24251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitts M., Breton E., Kholmovski E.G. Arrhythmia insensitive rapid cardiac T1 mapping pulse sequence. Mag Res Med. 2013;70:1274–1282. doi: 10.1002/mrm.24586. [DOI] [PubMed] [Google Scholar]
- 9.Schulz-Menger J., Bluemke D.A., Bremerich J. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMRI) Board of Trustees Task Force on Standardized Post Processing. J Cardiovasc Magn Reson. 2013;15:35. doi: 10.1186/1532-429X-15-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nacif M.S., Turkbey E.B., Gai N. Myocardial T1 mapping with MRI: comparison of look-locker and MOLLI sequences. J Magn Reson Imaging. 2011;34:1367–1373. doi: 10.1002/jmri.22753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Messroghli D.R., Rudolph A., Abdel-Aty H. An open-source software tool for the generation of relaxation time maps in magnetic resonance imaging. BMC Med Imaging. 2010;10:16. doi: 10.1186/1471-2342-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calkins H., Kuck K.H., Cappato R. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;2012(9):632–696. doi: 10.1016/j.hrthm.2011.12.016. (e21) [DOI] [PubMed] [Google Scholar]
- 13.Ling L.H., McLellan A.J., Taylor A.J. Magnetic resonance post-contrast T1 mapping in the human atrium: validation and impact on clinical outcome after catheter ablation for atrial fibrillation. Heart Rhythm. 2014;11:1551–1559. doi: 10.1016/j.hrthm.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Neilan T.G., Mongeon F.P., Shah R.V. Myocardial extracellular volume expansion and the risk of recurrent atrial fibrillation after pulmonary vein isolation. JACC Cardiovasc Imaging. 2014;7:1–11. doi: 10.1016/j.jcmg.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ling L.H.K.P., Ellims A.H., Iles L.M. Diffuse ventricular fibrosis in atrial fibrillation: noninvasive evaluation and relationships with aging and systolic dysfunction. J Am Coll Cardiol. 2012;60:2402–2408. doi: 10.1016/j.jacc.2012.07.065. [DOI] [PubMed] [Google Scholar]
- 16.Ling L.H., Kalman J.M., Ellims A.H. Diffuse ventricular fibrosis is a late outcome of tachycardia-mediated cardiomyopathy after successful ablation. Circ Arryth Electrophysiol. 2013;6:697–704. doi: 10.1161/CIRCEP.113.000681. [DOI] [PubMed] [Google Scholar]
- 17.Liu C.Y., Liu Y.C., Wu C. Evaluation of age-related age-related interstitial myocardial fibrosis with cardiac magnetic resonance contrast-enhanced T1 mapping: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2013;62:1280–1287. doi: 10.1016/j.jacc.2013.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sado D.M., Flett A.S., Banypersad S.M. Cardiovascular magnetic resonance measurement of myocardial extracellular volume in health and disease. Heart. 2012;98:1436–1441. doi: 10.1136/heartjnl-2012-302346. [DOI] [PubMed] [Google Scholar]
- 19.Ellims A.H., Shaw J.A., Stub D. Diffuse myocardial fibrosis evaluated by post-contrast t1 mapping correlates with left ventricular stiffness. J Am Coll Cardiol. 2014;63:1112–1118. doi: 10.1016/j.jacc.2013.10.084. [DOI] [PubMed] [Google Scholar]
- 20.Iles L., Pfluger H., Phrommintikul A. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol. 2008;52:1574–1580. doi: 10.1016/j.jacc.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 21.Sibley C.T., Noureldin R.A., Gai N. T1 Mapping in cardiomyopathy at cardiac MR: comparison with endomyocardial biopsy. Radiology. 2012;265:724–732. doi: 10.1148/radiol.12112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material