Abstract
Background
Although patients taking non-vitamin K antagonist oral anticoagulants (NOACs) do not require routine coagulation monitoring, high-risk patients require monitoring to assess pharmacodynamics.
Methods
We measured (1) anti-factor Xa activity (AXA), using chromogenic assay with the HemosIL Liquid Heparin kit, (2) prothrombin time (PT), and (3) activated partial thromboplastin time (aPTT) in 188 blood samples from 70 patients with non-valvular atrial fibrillation, of whom 36 received rivaroxaban once daily and 34 received apixaban twice daily.
Results
After the rivaroxaban therapy, AXA ranged from 0 to 3.65 IU/mL; PT, from 9.6 to 44.5 s; and APTT, from 19.3 to 69.7 s. After the apixaban therapy, AXA ranged from 0.02 to 3.18 IU/mL; PT, from 10.2 to 20.8 s; and APTT, from 21.8 to 59.8 s. At peak time, the AXA of patients who received rivaroxaban and apixaban were almost the same (2.08±0.91 IU/mL vs. 1.71±0.57 IU/mL), but the PT and APTT of patients who received rivaroxaban were more prolonged than those of patients who received apixaban (18.1±5.6 s vs. 13.8±0.9 s, p<0.001 and 40.9±7.3 s vs. 35.5±7.5 s, p<0.01, respectively). At trough time, the AXA and PT of patients who received rivaroxaban were respectively lower and shorter than those of patients who received apixaban (0.28±0.31 IU/mL vs. 1.04±0.72 IU/mL, p<0.001 and 11.9±2.0 s vs. 13.7±2.4 s, p<0.01, respectively), but the APTT of patients who received rivaroxaban and apixaban did not significantly differ (32.3±4.3 s vs. 34.3±3.8 s).
Conclusions
Measurement of AXA might be useful to assess the pharmacodynamics of high-risk patients, such as high age, low body weight, and/or low renal function, and to assess the intensity of anticoagulation by using different methods of administration, such as crushed tablet via the nasogastric tube.
Keywords: Atrial fibrillation, Direct factor Xa inhibitor, Anti-factor Xa activity, Chromogenic assay
1. Introduction
Administration of non-vitamin K antagonist oral anticoagulants (NOAC) is convenient, as they do not require routine coagulation monitoring. However, in patients with low renal function or old age, monitoring to assess pharmacodynamics and to avoid intracranial hemorrhage or major bleeding due to overdose is needed. Anti-factor Xa chromogenic assay has been proved to have excellent linearity with the plasma concentration of direct anti-factor Xa inhibitors [1], [2], [3], [4], [5]. It has been used for measurement of the intensity of anticoagulation and for drug quantification in patients receiving direct anti-factor Xa inhibitors [6], [7], [8]. The HemosIL Liquid Heparin kit (Instrumentation Laboratory, Bedford, Massachusetts, USA) is an Xa-dependent chromogenic assay and can measure unfractionated and low-molecular-weight heparins, and direct and indirect Xa inhibitors [9], [10]. Because it is approved for the measurement of blood heparin concentration by the Public Medical Insurance, the HemosIL Liquid Heparin kit is more accessible than other anti-factor Xa assay kits in Japan.
Large clinical trials [11], [12], [13], [14] were performed to assess the efficacy and safety of NOACs, but the percentage of patients with low renal function in these studies was relatively smaller than that in real-world data. The aim of this study was to examine the implication of monitoring the direct factor Xa inhibitors rivaroxaban [11], [12] and apixaban [13], [14] by performing an anti-factor Xa assay using the HemosIL Liquid Heparin kit in a clinical population of elderly patients with low renal function.
2. Materials and methods
2.1. Subjects
The subjects were 70 patients with non-valvular atrial fibrillation, of whom 36 received rivaroxaban once daily and 34 received apixaban twice daily. Either rivaroxaban or apixaban was chosen according to the physician's decision. Written informed consent was obtained from the patients or their next of kin. This study was approved by the institutional review board of Shinoda General Hospital (approval no. 20120725-4) on July 25, 2012, and conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
The rivaroxaban dosage was chosen according to the recommended regimen in Japan, which is 15 mg once daily for patients with creatinine clearance (CCr) ≥50 mL/min and 10 mg once daily for patients with CCr<50 mL/min. Rivaroxaban was contraindicated in patients with CCr<15 mL/min. The apixaban dosage was also chosen according to the recommended regimen, which is 5 mg twice daily for patients with 1 of the following 3 characteristics or lower values than those indicated in the following: age ≥80 years, body weight ≤60 kg, and serum creatinine level ≥ than 1.5 mg/dL, and 2.5 mg twice daily for patients with at least 2 of the 3 characteristics. Apixaban was contraindicated in patients with CCr<15 mL/min.
2.2. Measurements of anti-factor Xa activity, PT, and APTT
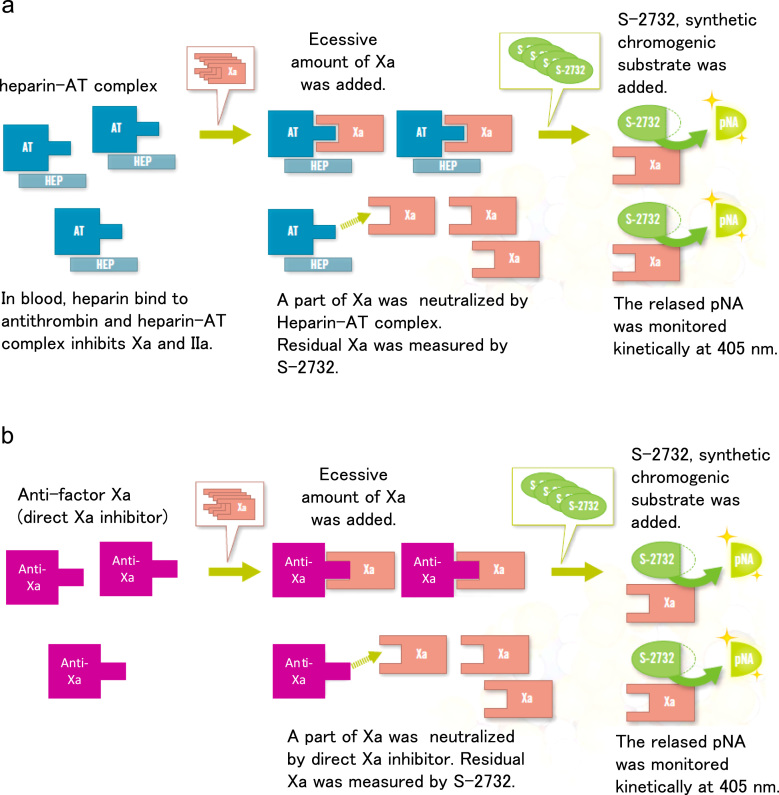
We measured anti-factor Xa activity by performing a chromogenic assay by using the HemosIL Liquid Heparin kit [6], [7] (Instrumentation Laboratory), PT (RecombiPlasTin; HemosIL, Instrumentation Laboratory), and aPTT (SynthASil; HemosIL, Instrumentation Laboratory) in 188 blood samples from the 70 subjects. The blood samples were collected in 3.2% sodium citrate tubes. Anti-Xa activity, PT, and APTT were measured by using the analyzer system ACL TOP 500 CTS (Instrumentation Laboratory). The principle of the anti-factor Xa assay is shown in Fig. 1. The anti-factor Xa activity measurement was expressed as heparin activity (IU/mL). The plasma concentration of rivaroxaban was also estimated by using the following formula: 1 IU/mL=225 ng/mL [10].
Fig. 1.
Principles of anti-factor Xa assay using the HemosIL Liquid Heparin kit. Comparison between heparin assay (a) and anti-factor Xa assay (b). Revised with permission from the pamphlet of HemosIL Liquid Heparin kit in Japanese. (a) Principles of Heparin Assay. and ,(b) Principles of anti-factor Xa Assay.
2.3. Statistical analysis
Continuous variables were expressed as mean±SD. Categorical variables were presented as numbers. A statistical comparison was performed by using the Student t test or Wilcoxon signed-rank test for the continuous variables, the chi-square test or Fisher exact test for the categorical variables, and the Pearson product-moment correlation analysis. Two-sided p<0.05 was considered statistically significant.
3. Results
3.1. Clinical characteristics of the subjects
The clinical characteristics of the patients are shown in Table 1. The 36 patients who received rivaroxaban once daily were 35–97 years old and had CCr of 33.0–134.3 mL/min. The 34 patients who received apixaban twice daily were 61–97 years old and had CCr of 16.2–95.4 mL/min. The patients who received apixaban were older, had lower body weights, lower body mass indexes, lower CCr, higher CHADS2 and CHA2DS2-VASc scores, and higher percentage of patients with reduced dose than those who received rivaroxaban, indicating significant differences. No significant differences were observed between the rivaroxaban and apixaban groups in the use of angiotensin II receptor blockers, angiotensin-converting enzyme inhibitor, antiarrhythmic drugs, Na channel blockers, amiodarone, beta blockers, statin, antiplatelets, P glycoprotein inhibitors, and past use of warfarin, as well as NOACs. Only the use of aldosterone blockers was significantly higher in the patients who received apixaban (32%) than in those who received rivaroxaban (8%).
Table 1.
Clinical characteristics of the subjects.
| Rivaroxaban | Apixaban | p Value | |
|---|---|---|---|
| Patients | 36 | 34 | |
| Male/female | 22/14 | 13/21 | NS |
| Paroxysmal/chronic | 16/20 | 16/18 | NS |
| Age (years), range (mean±SD) | 35–97 (74.1±13.9) | 61–97 (82.9±10.3) | <0.01 |
| Body weight (kg) | 34.5–82.0 (60.3±11.7) | 34.0–73.2 (49.1±8.7) | <0.001 |
| Body mass index (kg/m2) | 17.6–28.8 (23.6±2.5) | 16.5–26.6 (21.1±2.7) | <0.001 |
| Cr (mg/dL) | 0.45–1.48 (0.82±0.27) | 0.45–1.39 (0.87±0.22) | NS |
| CCr (mL/min) | 33.0–134.3 (69.0±26.6) | 16.2–95.4 (44.5±19.9) | <0.001 |
| CHADS2 score | 0–5 (2.1±1.3) | 1–5 (3.2±1.1) | <0.001 |
| CHA2DS2-VASc score | 0–7 (3.4±1.9) | 1–7 (4.9±1.4) | <0.001 |
| Normal dose/reduced dose | 22/14 | 9/25 | <0.01 |
| Medication | |||
| ARB | 16 (44%) | 16 (44%) | NS |
| ACE-I | 5 (14%) | 8 (24%) | NS |
| Aldosterone blockers | 3 (8%) | 11 (32%) | <0.05 |
| Antiarrhythmic drugs | 10 (28%) | 7 (21%) | NS |
| Na channel blockers | 9 (25%) | 3 (9%) | NS |
| Amiodarone | 1 (3%) | 4 (12%) | NS |
| Beta blockers | 19 (53%) | 18 (53%) | NS |
| Statin | 4 (11%) | 8 (24%) | NS |
| Antiplatelets | 1 (3%) | 3 (9%) | NS |
| P glycoprotein inhibitors | 5 (14%) | 4 (12%) | NS |
| Past use of warfarin | 13 (36%) | 9 (27%) | NS |
| Past use of other NOACs | 8 (22%) | 4 (12%) | NS |
| Blood samples | 98 | 90 | |
3.2. Anti-factor Xa activity, PT, and APTT
Blood sample measurements were performed 7–911 days (mean±SD: 229±231 days) after the first administration of rivaroxaban, and 7–378 days (83±90 days) after the first administration of apixaban. The measured parameters are summarized in Table 2a. In the patients who received rivaroxaban, the anti-factor Xa activity ranged from 0 to 3.65 IU/mL; the estimated plasma concentration of rivaroxaban, from 0 to 821.3 ng/mL (269.6±237.3 ng/mL); PT, from 9.6 to 44.5 s; and APTT, from 19.3 to 69.7 s. In the patients who received apixaban, the anti-factor Xa activity ranged from 0.02 to 3.18 IU/mL; PT, from 10.2 to 20.8 s; and APTT, from 21.8 to 59.8 s. The mean values of anti-factor Xa activity did not significantly differ between ribaroxaban and apixaban (1.20±1.05 IU/mL vs. 1.12±0.79 IU/mL). However, PT and APTT were significantly longer in the rivaroxaban group than in the apixaban group (PT: 15.5±4.7 s vs. 13.1±2.2 s, p<0.001; APTT: 39.0±7.9 s vs. 34.4±5.1 s, p<0.001).
Table 2a.
Anti-factor Xa activity, PT, and APTT of all data.
| Rivaroxaban | Apixaban | p Value | |
|---|---|---|---|
| Samples | 98 | 90 | |
| Anti-factor Xa activity (IU/mL) | 0.00–3.65 (1.20±1.05) | 0.02–3.18 (1.12±0.79) | NS |
| Rivaroxaban concentration (ng/mL) | 0.0–821.3 (269.6±237.3) | ||
| PT (s) | 9.6–44.6 (15.5±4.7) | 10.2–20.8 (13.1±2.2) | <0.001 |
| APTT (s) | 19.3–69.7 (39.0±7.9) | 21.8–59.8 (34.4±5.1) | <0.001 |
| (mean±SD) |
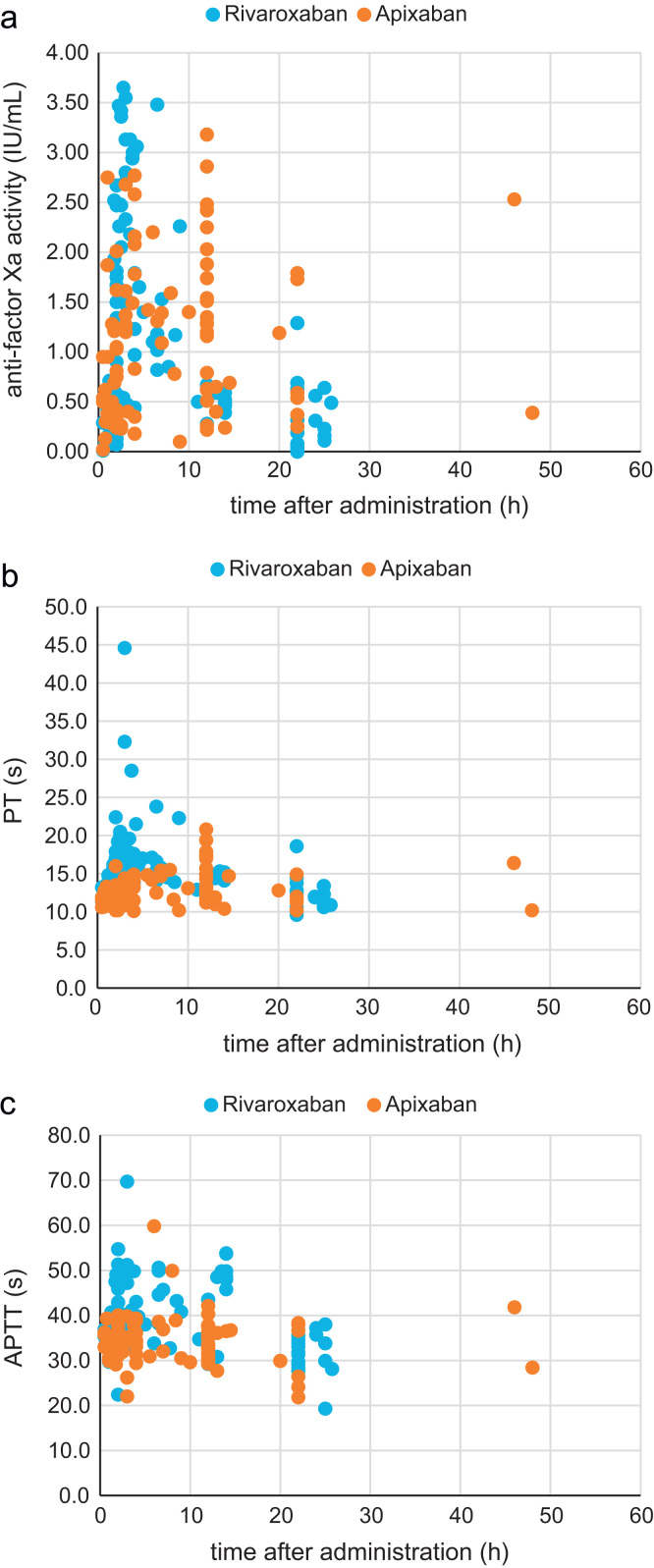
Fig. 2 shows the plot for anti-factor Xa activity, and PT and APTT against the times after the administration of rivaroxaban and apixaban. For both drugs, the peak of the anti-factor Xa was observed around 2–5 h after administration. For rivaroxaban, small PT and APTT peaks were also observed. However, for apixaban, peaks were not clear both in PT and in APTT.
Fig. 2.
Anti-factor Xa activity (a), prothrombin time (b), and activated partial thromboplastin time (c) in relation to the time after administration of rivaroxaban and apixaban. The blue points indicate the values for rivaroxaban, and the red points indicate the values for apixaban.
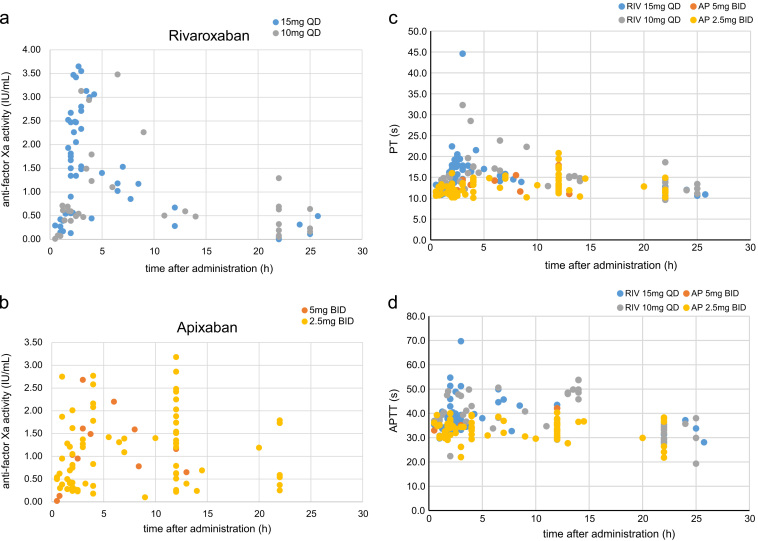
Fig. 3 shows the plot for anti-factor Xa activity, and PT and APTT against the times after the administration of rivaroxaban and apixaban for normal and reduced doses. For both drugs, the reduced dose was chosen according to the recommended regimen in Japan. In the patients who received rivaroxaban, the anti-factor Xa activities of the normal dose (15 mg once daily) and reduced dose (10 mg once daily) seemed to be on the same time-activity curve. Also, in the patients who received apixaban, the anti-factor Xa activities of the normal dose (5 mg twice daily) and reduced dose (2.5 mg twice daily) seemed to be on the same time-activity curve. Relatively high anti-factor Xa activity was observed at trough time in several patients who received 2.5-mg apixaban twice daily. Relatively high anti-factor Xa activity was observed in patients with CCr<30 mL/min. Anti-factor Xa activity >2.5 IU/mL at trough time was observed in patients with CCr<20 mL/min. Apparent differences in PT and APTT were not found between the normal and reduced doses of both drugs.
Fig. 3.
Effects of normal and reduced doses on anti-factor Xa activity in rivaroxaban (a) and apixaban (b), and on PT (c) and APTT (d). For rivaroxaban, the blue points indicate the normal dose (15 mg once daily) and the gray points indicate the reduced dose (10 mg once daily). For apixaban, the red points indicate the normal dose (5 mg twice daily) and the yellow points indicate the reduced dose (2.5 mg twice daily). The figure shows the values obtained within 30 h after the administration of the drugs (rivaroxaban, 98 samples; apixaban, 88 samples), 229±231 days after the first administration of rivaroxaban, and 83±91 days after the first administration of apixaban. For rivaroxaban and apixaban, the anti-factor Xa activity of the normal and reduced doses seemed to be on the same time–activity curve. Apparent differences in PT and APTT were not found between the normal and reduced doses of both drugs.
Table 2b shows the anti-factor Xa activity, PT, and APTT at peak time, 2–5 h after the administration of both drugs, trough time, 20–26 h after the administration of rivaroxaban, and 10–14 h after the administration of apixaban. In the patients who received rivaroxaban, anti-factor Xa activity, rivaroxaban concentration, PT, and APTT at peak time were significantly greater than those at trough time (p<0.001, p<0.001, p<0.001, and p<0.001, respectively). However, in the patients who received apixaban, the anti-factor Xa activity at peak time was significantly greater than that at trough time (p<0.01), and the PT and APTT at peak time were not significantly prolonged than those at trough time.
Table 2b.
Anti-factor Xa activity, PT, and APTT at peak and trough times.
| Rivaroxaban |
Apixaban |
|||
|---|---|---|---|---|
| Peak time (2–5 h) | Trough time (20–26 h) | Peak time (2–5 h) | Trough time (10–14 h) | |
| Samples | 42 | 23 | 19 | 19 |
| Anti-factor Xa activity (IU/mL) | 0.44–3.65 (2.08±0.91**) | 0.00–1.29 (0.28±0.31##) | 0.83–2.77 (1.71±0.57*) | 0.22–2.86 (1.04±0.72) |
| Rivaroxaban concentration (ng/mL) | 99.0–821.3 (467.3±204.2**) | 0.0–290.3 (62.5±70.6) | ||
| PT (s) | 12.2–44.6 (18.1±5.6**##) | 10.1–18.6 (11.9±2.0#) | 11.9–15.4 (13.8±0.9) | 11.0–17.9 (13.7±2.4) |
| APTT (s) | 32.1–69.7 (40.9±7.3**#) | 19.3–38.0 (32.3±4.3) | 22.0–59.8 (35.5±7.5) | 27.7–42.1 (34.3±3.8) |
| (mean±SD) | ||||
p<0.01, vs. trough time.
p<0.001, vs. trough time.
p<0.01, vs. apixaban.
p<0.001, vs. apixaban.
At peak time, the anti-factor Xa activities of rivaroxaban and apixaban were almost the same (2.08±0.91 IU/mL vs. 1.71±0.57 IU/mL), but the PT and APTT of rivaroxaban were more prolonged than those of apixaban (PT: 18.1±5.6 s vs. 13.8±0.9 s, p<0.001; APTT: 40.9±7.3 s vs. 35.5±7.5 s, p<0.01). At trough time, the anti-factor Xa activity of rivaroxaban was lower than that of apixaban (0.28±0.31 IU/mL vs. 1.04±0.72 IU/mL, p<0.001). The PT of rivaroxaban was shorter than that of apixaban (11.9±2.0 s vs. 13.7±2.4 s, p<0.01). However, the APTTs of rivaroxaban and apixaban were not significantly different (32.3±4.3 s vs. 34.3±3.8 s).
3.3. Relationship of anti-factor Xa activity to PT or APTT
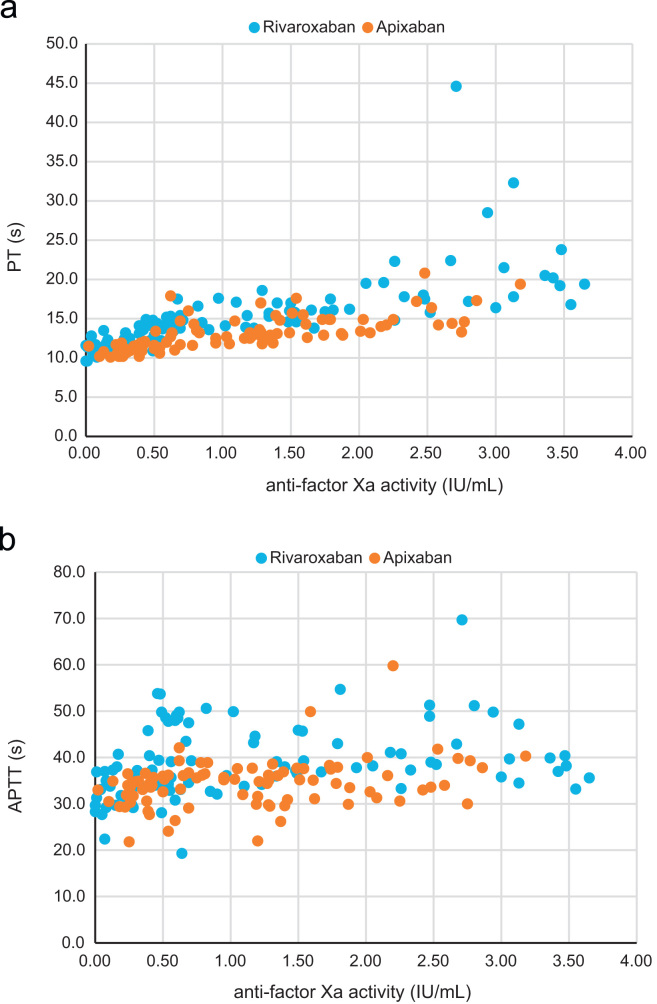
Fig. 4 shows the plot for PT or APTT against the anti-factor Xa activities of rivaroxaban and apixaban. In the patients who received rivaroxaban, PT was prolonged in proportion to the increase in anti-Xa activity, but the change in PT was small and much overlapped. In the patients who received apixaban, PT was slightly prolonged in proportion to the increase in anti-Xa activity, but significant prolongation of PT was observed only when the anti-Xa activity was very high.
Fig. 4.
The relationship of PT (a) and APTT (b) to the anti-factor Xa activity in rivaroxaban and apixaban. The blue points indicate the values for rivaroxaban, and the red points indicate the values for apixaban.
In the patients who received rivaroxaban, APTT tended to be prolonged as the anti-Xa activity increased but much more overlapped than PT. In the patients who received apixaban, APTT tended to be slightly prolonged as the anti-Xa activity increased but much more overlapped than in that in patients who received rivaroxaban.
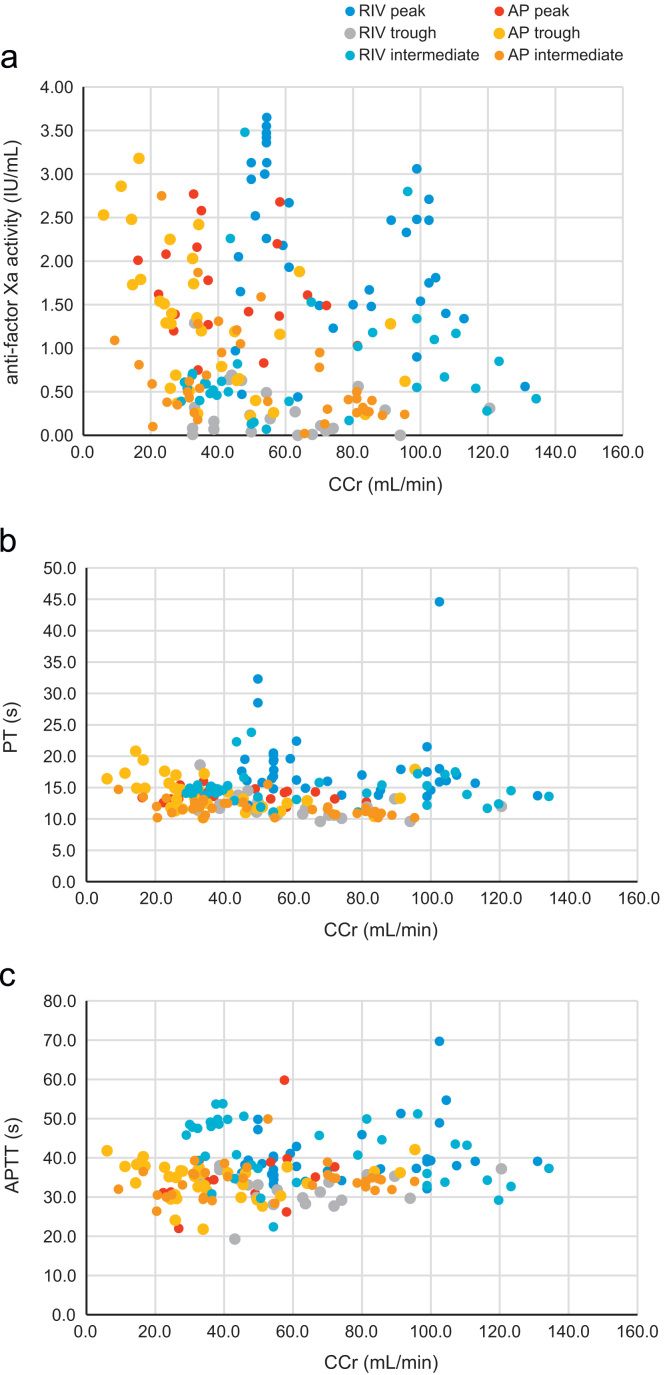
3.4. Relationship of anti-factor Xa activity, PT, and APTT to CCr
Fig. 5a shows the relationship between the anti-factor Xa activity and CCr of rivaroxaban and apixaban. The relationship of PT or APTT to CCr are shown in Fig. 5b and c. Data were plotted in different colors according to blood sampling time as follows: peak time, 2–5 h after the administration of both drugs; trough time, 20 to 26 h after the administration of rivaroxaban and 10–14 h after the administration of apixaban; and intermediate time, other than peak or trough time. The graph in Fig. 5 demonstrates the influence of time after administration on the relationship of CCr to anti-factor Xa activity, PT, or APTT.
Fig. 5.
The relationship of anti-factor Xa activity (a), PT (b), and APTT (c) to CCr. The blue, cobalt, and gray points indicate the values for rivaroxaban at peak, intermediate, and trough times, respectively. The red, orange, and yellow points indicate the values for apixaban at peak, intermediate, and trough times, respectively.
In the patients who received rivaroxaban, the anti-factor Xa activity at peak time tended to increase as the CCr decreased, but that at trough time did not increase as the CCr decreased. Thus, no significant correlation was found between anti-factor Xa activity and CCr (r=0.089, NS), between PT and CCr (r=0.031, NS), nor between APTT and CCr (r=−0.026, NS). In the patients who received apixaban, the anti-factor Xa activity at peak time tended to increase as the CCr decreased, and that at trough time also increased when the CCr decreased severely to <20 mL/min. A significant correlation was found between anti-factor Xa activity and CCr (r=−0.439, p<0.01), and between PT and CCr (r=−0.427, p<0.01), but not between APTT and CCr (r=0.101, NS).
Except at trough time, the anti-factor Xa activity of rivaroxaban tended to be higher than that of apixaban, in the comparison between the patients with same degree of CCr. Anti-factor Xa activity >2.5 IU/mL was frequently observed in the rivaroxaban patients with CCr<50 mL/min and in the apixaban patients with CCr<30 mL/min. Anti-factor Xa activity was >3.0 IU/mL in 5 patients (4 patients taking rivaroxaban and 1 patient taking apixaban), in 3 patients at peak time (all taking rivaroxaban), and in 2 patients after peak time (1 taking rivaroxaban and 1 taking apixaban). The 2 patients with very high anti-factor Xa activity after peak time (cases A and B) were quite elderly.
3.5. Case presentations
3.5.1. Cases with high anti-factor Xa activity
Case A (Table 3a) was a 97-year-old female patient who had been taking 10-mg rivaroxaban once daily, had paroxysmal atrial fibrillation, and had artificial cardiac pacemaker implantation for a second-degree AV block. Her body weight was 42.5 kg; CCr, 47.9 mL/min; CHADS2 score, 3; and CHA2DS2-VASc score, 5. The anti-factor Xa activity at 6.5 h after administration was 3.48 IU/mL. The rivaroxaban concentration was 783 ng/mL, more than 3 times higher than the normal curve [15]. Although no clinical sign of hemorrhage was observed, rivaroxaban was changed to 2.5-mg apixaban twice daily according to the physician's decision. After the change to apixaban, the anti-factor Xa activity was 1.42 IU/mL at 5.5 h after the administration of apixaban and CCr was 49.0 mL/min.
Table 3a.
Clinical course of case A.
| CCr (mL/min) | Rivaroxaban (ng/mL) | Anti-factor Xa activity (IU/mL) | PT (s) | APTT (s) | |
|---|---|---|---|---|---|
| Rivaroxaban | |||||
| 10 mg once daily (6.5 h after) | 47.9 | 783.0 | 3.48 | 23.8 | 38.2 |
| Apixaban | |||||
| 2.5 mg twice daily (5.5 h after) | 49.0 | – | 1.42 | 14.8 | 30.9 |
Case B (Table 3b) was a 90-year-old female patient who had been taking 2.5-mg apixaban twice daily, had paroxysmal atrial fibrillation, and had histories of myocardial infarction, congestive heart failure, and chronic renal failure. Before treatment with apixaban, CCr was 24.4 mL/min. After treatment with 2.5-mg apixaban twice daily, anti-factor Xa activity was 3.18 IU/mL at 12 h after administration and the CCr decreased to 16.5 mL/min. After dose adjustment to 2.5 mg of apixaban once daily, the anti-factor Xa activity at trough time was 1.79 IU/mL. At 1.5 months after, the CCr decreased to 6.0 mL/min because of decreased renal function from the congestive heart failure. Apixaban was given at 2.5 mg once every other day, and the anti-factor Xa activity at trough time was 2.53 IU/mL. As the CCr decreased under contraindication level, apixaban administration was discontinued. For cases of severe chronic renal failure such as those with CCr of 15–20 mL/min, 2.5-mg apixaban once daily, not 2.5-mg apixaban twice daily, might be considered as one of the treatment option.
Table 3b.
Clinical course of case B.
| CCr (mL/min) | Anti-factor Xa activity (IU/mL) | PT (s) | APTT (s) | |
|---|---|---|---|---|
| Before | 24.4 | – | 11.0 | 33.3 |
| Apixaban (trough) | ||||
| (14th day) 2.5 mg twice daily | 16.5 | 3.18 | 19.4 | 40.3 |
| (22nd day) 2.5 mg once daily | 17.0 | 1.79 | 14.9 | 37.9 |
| (36th day) 2.5 mg once daily | 15.1 | 1.73 | 14.9 | 38.3 |
| (53rd day) 2.5 mg once for every other day | 6.0 | 2.53 | 16.4 | 41.8 |
Case C (Table 3c) was a 94-year old female patient who had been taking 2.5-mg apixaban twice daily, had chronic atrial fibrillation, chronic cardiac failure from hypertensive heart disease, and chronic renal failure. Her body weight was 48.2 kg, CCr was 28.1 mL/min, CHADS2 score was 3, and CHA2DS2-VASc score was 5. On the 14th day of treatment, anti-factor Xa activity was 2.77 IU/mL at 4 h after administration (peak) and 1.74 IU/mL at 12 h after administration (trough time). At peak time, PT (14.6 s) and APTT (39.3 s) were mildly prolonged. At trough time, PT (12.9 s) and APTT (37.5 s) were not prolonged and CCr was 32.7 mL/min. The apixaban dosage was changed to 2.5-mg once daily.
Table 3c.
Clinical course of case C.
| CCr (mL/min) | Anti-factor Xa activity (IU/mL) | PT (s) | APTT (s) | |
|---|---|---|---|---|
| Before | 28.1 | – | 10.3 | 34.2 |
| Apixaban | ||||
| (14th day) 2.5 mg twice daily peak | 32.7 | 2.77 | 14.6 | 39.3 |
| 2.5 mg twice daily trough | 1.74 | 12.9 | 37.5 | |
| (28th day) 2.5 mg once daily peak | 27.9 | 0.35 | 11.5 | 33.1 |
| 2.5 mg once daily trough | 0.37 | 11.6 | 36.6 | |
| (42nd day) 2.5 mg twice daily peak | 33.7 | 2.16 | 14.0 | 36.1 |
| 2.5 mg twice daily trough | 1.35 | 12.9 | 36.1 | |
Two weeks after, anti-factor Xa activity was 0.35 IU/mL at 4 h after administration (peak time) and 0.37 IU/mL at 22 h after administration (trough time). The apixaban dose was returned to 2.5 mg twice daily. Two weeks after, anti-factor Xa activity was 2.16 IU/mL at 4 h after administration (peak time) and 1.35 IU/mL at 22 h after administration (trough time). In this case of slightly severe renal failure, with CCr of 25–35 mL/min, 2.5-mg apixaban twice daily was needed to maintain anti-factor Xa activity.
3.5.2. A case of administration of crushed tablet via a nasogastric tube
Anti-factor Xa assay might be also useful to assess the intensity of anticoagulation by using different methods of administration such as crushed tablet via a nasogastric tube. Case D (Table 3d) was an 83-year-old male patient who had been taking 10 mg of crushed rivaroxaban tablet once daily via a nasogastric tube, and had chronic atrial fibrillation and cardiogenic cerebral embolism. His body weight was 39.7 kg, CCr was 48.3 mL/min, CHADS2 score was 4, and CHA2DS2-VASc score was 5. At 4 h after administration, anti-factor Xa activity was 0.71 IU/mL, and the rivaroxaban concentration was 159.75 ng/mL, about half of the normal curve [12]. At 22 h after administration, anti-factor Xa activity was 0 IU/mL and the rivaroxaban concentration was 0 ng/mL. The altered method of administration of crushed tablet via a nasogastric tube might have affected drug absorption.
Table 3d.
Clinical course of case D.
| Rivaroxaban concentration (ng/mL) | Anti-factor Xa activity (IU/mL) | PT (s) | APTT (s) | |
|---|---|---|---|---|
| Rivaroxaban | ||||
| 10 mg of crushed tablet | ||||
| 4 h after | 159.75 | 0.71 | 21.9 | 42.8 |
| 22 h after | 0.00 | 0.00 | 14.7 | 36.0 |
4. Discussion
4.1. Anti-factor Xa activity of rivaroxaban and apixaban
We measured anti-factor Xa activity, PT, and aPTT in 188 blood samples from 70 patients with non-valvular atrial fibrillation, of whom 36 received rivaroxaban once daily and 34 received apixaban twice daily. The study subjects were older than the subjects of large clinical trials [11], [12], [13], [14].
In this study, anti-factor Xa activity measurements were expressed as heparin activity (IU/mL) and converted to plasma concentration of rivaroxaban by using the conversion formula [10]. Estimation of plasma drug concentration was convenient in the assessment of pharmacodynamics in individual patients, as in cases A and D. The plasma concentration of apixaban could also be estimated by using other chromogenic assay kits [5], [6]. The conversion formula for apixaban in the HemosIL Liquid Heparin kit must be established.
Meanwhile, anti-factor Xa activity expressed in IU/mL was useful in the comparison between the treatment effects of rivaroxaban and apixaban [6]. Thus, we mainly used anti-factor Xa activity (IU/mL) in this study. For both drugs, the peak of the anti-factor Xa was observed around 2–5 h after administration. The Tmax of rivaroxaban on repetitive administration in healthy aged Japanese men and women was reported to be 1.0–4.0 h (median for 15 mg once daily, 3.5 h) and 1.5– 4.0 h (median for 10 mg once daily 3.0 h) [15]. The Tmax of apixaban on repetitive administration in healthy Japanese men was reported to be 3.0–4.0 h (median for 5 mg twice daily, 3.5 h) and 1.0–4.0 h (median for 2.5 mg twice daily, 2.0 h) [16]. Our data were almost comparable with these data.
In most patients, the anti-factor Xa activities of plasma rivaroxaban and apixaban were within the acceptable range of anticoagulation. Both for rivaroxaban and apixaban, the anti-factor Xa activities of the normal and reduced doses seemed to be on the same time-activity curve. The dosage regimens of 15 mg once daily or 10 mg once daily of rivaroxaban, and 5 mg twice daily or 2.5 mg twice daily of apixaban were thought to be acceptable based on our study results.
The differences in anti-factor Xa activity between the peak and trough times were greater in rivaroxaban than in apixaban, corresponding to the difference in dosage, that is, once daily to twice daily [6]. At peak time, the PT of rivaroxaban was more prolonged than that of apixaban (18.1±5.6 s vs. 13.8±0.9 s), although anti-Xa activity was almost the same (2.08±0.91 IU/mL vs. 1.71±0.57 IU/mL).
4.2. PT and APTT
In the patients who received rivaroxaban, the PT at peak time was longer than that at trough time (18.1±5.6 s vs. 11.9±2.0 s). The PT was prolonged in proportion to the increase in anti-Xa activity, but the change in PT was small and much overlapped. In the patients who received apixaban, PT was slightly prolonged in proportion to the increase in anti-Xa activity but significantly prolonged only when anti-Xa activity was very high.
In both drugs, the prolongation of PT was too small to be used as a clinical index to assume the intensity of anticoagulation. Barrett el al. [7] demonstrated that an anti-factor Xa assay was preferable to PT. In the patients who received rivaroxaban and apixaban, APTT tended to be prolonged but much overlapped. APTT was not suitable for use as a clinical marker of anticoagulation of direct factor Xa inhibitors [1], [4], [5].
Our results coincide well with the results of the recent systematical review by Cuker et al. [17]. They reviewed studies that assessed relationships of drug levels of dabigatran, rivaroxaban, and apixaban to coagulation assay results. They concluded that for rivaroxaban and apixaban, anti-Xa activity was linear over a wide range of drug levels and may be used for drug quantification. However, PT was less sensitive, especially for apixaban. A normal PT may not exclude clinically relevant levels. APTT demonstrated insufficient sensitivity and linearity for quantification [17].
4.3. CCr and anti-factor Xa activity
In the patients who received apixaban, anti-factor Xa activity tended to be higher as CCr decreased. A significant correlation was found between anti-factor Xa activity and CCr. It must be mentioned that in the patients who received apixaban, the anti-factor Xa activity at trough time might have increased with the severe decrease in CCr to <20 mL/min. However, in the patients who received rivaroxaban, a significant correlation was not found between anti-factor Xa activity and CCr. This difference might be related to the greater decrease in anti-factor Xa activity at trough time in the patients who received rivaroxaban than in the patients who received apixaban.
In comparison of the patients with the same degree of CCr, the anti-factor Xa activity of rivaroxaban, except at trough time, tended to be higher than that of apixaban. Anti-factor Xa activity>2.5 IU/mL was frequently observed in the patients who received rivaroxaban and had CCr<50 mL/min, and in the patients who received apixaban and had CCr<30 mL/min. Monitoring anti-factor Xa activity might be beneficial to patients who receive rivaroxaban and have CCr<50 mL/min and to patients who receive apixaban and have CCr<30 mL/min.
As for apixaban, in the patients with slightly severe renal failure with CCr of 25–35 mL/min, 2.5 mg twice daily was needed to maintain anti-factor Xa activity. However, in the patients with more severe renal failure with CCr of 15–20 mL/min, 2.5 mg once daily, not 2.5 mg twice daily, might be considered as a treatment option. In the patients with super-high age, low body weight, and severe renal dysfunction, the clinical safety and benefit of NOACs have not been established yet. In such patients, warfarin may still be safer than NOACs. However, Jun et al. [18] reported that among older adults with atrial fibrillation starting warfarin administration, those with reduced kidney function with an estimated glomerular filtration rate <15 mL/min/1.73 m2 were associated with 3.5-fold greater gastrointestinal bleeding during the first 30 days of treatment.
It is possible that with the use of anti-factor Xa assay, direct factor Xa inhibitors can be used more safely in patients with super-high age, low body weight, and/or low renal function. The therapeutic range of anti-factor Xa activity must be established in patients receiving rivaroxaban and apixaban.
4.4. Assessment of the intensity of anticoagulation in the altered method of administration
Anti-factor Xa activity was lower in the patients who received crushed rivaroxaban tablet via nasogastric tube. The altered method of administration might have affected drug absorption. A previous study reported that the Cmax of rivaroxaban decreased to 56% when rivaroxaban granulate was released in the proximal small intestine [19]. Okada et al. demonstrated that the estimated plasma concentration of rivaroxaban by anti-factor Xa assay in patients who received crushed tablets via a nasogastric tube was 72% lower at 4 h and 70% lower at 9 h [8].
4.5. Limitations of this study
The study patients were not randomized to receive either rivaroxaban or apixaban. Because the drug selection depended on the physician's decision, the patients who received apixaban were significantly older, and had lower body weight, lower CCr, higher CHADS2 score, and CHA2DS2-VASc score than the patients who received rivaroxaban. Blood sampling was performed in clinical situations. We did not perform multiple serial sampling after administration. Hence, the accurate peak time of anti-factor Xa activity or the peak value of anti-factor Xa activity could not be determined from this study. The patient populations were also small. A larger multicenter study must be conducted to establish the therapeutic range of the anti-factor Xa activities of rivaroxaban or apixaban.
5. Conclusion
Measurement of anti-factor Xa activity by using the HemosIL Liquid Heparin kit might be useful to assess the pharmacodynamics of high-risk patients, such as those with high age, low body weight, and/or low renal function, and to assess the intensity of anticoagulation by using different methods of administration, such as crushed tablet via a nasogastric tube.
Conflict of interest
All authors declare no conflict of interest related to this study.
Funding information
There was no funding source related to this study.
Acknowledgments
We thank Mr. Akihiro Hosoya and Mr. Satoshi Yuki from the Clinical Laboratory of Shinoda General Hospital and Mr. Hiroshi Kishi from the Clinical Laboratory of Chitose Shinoda Hospital for the anti-factor Xa assay.
References
- 1.Samama M.M., Martinoli J.L., LeFlem L. Assessment of laboratory assays to measure rivaroxaban – an oral, direct factor Xa inhibitor. Thromb Haemost. 2010;103:815–825. doi: 10.1160/TH09-03-0176. [DOI] [PubMed] [Google Scholar]
- 2.Samama M.M., Amiral J., Guinet C. An optimized, rapid chromogenic assay, specific for measuring direct factor Xa inhibitors (rivaroxaban) in plasma. Thromb Haemost. 2010;104:1078–1079. doi: 10.1160/TH10-03-0204. [DOI] [PubMed] [Google Scholar]
- 3.Harenberg J., Krämer R., Giese C. Determination of rivaroxaban by different factor Xa specific chromogenic substrate assays: reduction of interassay variability. J Thromb Thrombol. 2011;32:267–271. doi: 10.1007/s11239-011-0622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Douxfils J., Mullier F., Loosen C. Assessment of the impact of rivaroxaban on coagulation assays: laboratory recommendations for the monitoring of rivaroxaban and review of the literature. Thromb Res. 2012;130:956–966. doi: 10.1016/j.thromres.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Douxfils J., Chatelain C., Chatelain B. Impact of apixaban on routine and specific coagulation assays: a practical laboratory guide. Thromb Haemost. 2013;110:283–294. doi: 10.1160/TH12-12-0898. [DOI] [PubMed] [Google Scholar]
- 6.Frost C., Song Y., Barrett Y.C. A randomized direct comparison of the pharmacokinetics and pharmacodynamics of apixaban and rivaroxaban. Clin Pharmacol. 2014;6:179–187. doi: 10.2147/CPAA.S61131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrett Y.C., Wang Z., Frost C. Clinical Laboratory measurement of direct factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost. 2010;104:1263–1271. doi: 10.1160/TH10-05-0328. [DOI] [PubMed] [Google Scholar]
- 8.Okada T., Toyoda K., Okamoto A. Anticoagulation intensity of rivaroxaban for stroke patients at a special low dosage in Japan. PLoS One. 2014;9(11):e113641. doi: 10.1371/journal.pone.0113641. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabbeta J, Krougliak V, Quiazon E, et al. Liquid Heparin assay: rapid monitoring of clinically used heparins. In: XXI Congress of the International Society on Thrombosis and Haemostasis; July 2007.
- 10.Krougliak V, Gabetta J, Kung C, et al. Monitoring direct and indirect factor Xa inhibitors with a new Liquid Heparin assay. In: The 21st International Congress on Thrombosis; July 2010.
- 11.Patel M.R., Mahaffey K.W., Garg J. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 12.Hori M., Matsumoto M., Tanahashi N. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation: the J-ROCKET AF study. Circ J. 2012;76:2104–2111. doi: 10.1253/circj.cj-12-0454. [DOI] [PubMed] [Google Scholar]
- 13.Granger C.B., Alexander J.H., McMurray J.J.V. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 14.Owgawa S., Shinohara Y., Kanmuri K. Safety and efficacy of the oral direct factor Xa inhibitor apixaban in Japanese patients with non-valvular atrial fibrillation: the ARISTOTLE-J study. Circ J. 2011;75:1852–1859. doi: 10.1253/circj.cj-10-1183. [DOI] [PubMed] [Google Scholar]
- 15.Xarelto gaiyo. 2014 March version. Osaka: Bayer Yakuhin, Ltd.; 2014, 33–4. 〈http://www.bayer-hv.jp/hv/files/pdf.php/140311_XAR-14_gaiyo_201403.pdf?id=1039e591f60472d3542ebbe71e3a0fc〉.
- 16.Eliquis interview form. Tokyo: Bristol-Myers Squibb, and Tokyo: Pfizer Japan Inc., 2015; 37–9. 〈http://file.bmshealthcare.jp/bmshealthcare/pdf/interview/IF_EQ1502.pdf〉.
- 17.Cuker A., Siegal D.M., Crowther M.A. Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants. J Am Coll Cardiol. 2014;64:1128–1139. doi: 10.1016/j.jacc.2014.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jun M., James M.T., Manns B.J. The association between kidney function and major bleeding in older adults with atrial fibrillation starting warfarin treatment: population based observational study. Br Med J. 2015;350:h246. doi: 10.1136/bmj.h246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xarelto (Rivaroxaban Film-Coated Oral Tablets) Drug Information. RxList: 〈http://www.rxlist.com/script/main/hp.asp〉.