Abstract
OBJECTIVES:
Functional gastrointestinal disorders occur more frequently among deployed veterans, although studies evaluating the relative impact of risk factors, including stress and antecedent infectious gastroenteritis (IGE), are limited. We examined risk factors for new-onset irritable bowel syndrome (IBS) among active duty participants in the military's Millennium Cohort Study.
METHODS:
Medical encounter data from 2001 to 2009, limited to Cohort members on active duty, were used to identify incident IBS cases (any and highly probable). IGE was identified using medical encounter or self-report. Covariate data were obtained from the Millennium Cohort Study surveys and analyzed using Cox proportional hazards methods.
RESULTS:
Overall, 41,175 Cohort members met the eligibility criteria for inclusion and 314 new-onset cases of IBS were identified among these. Significant risk factors (adjusted hazard ratio, 95% confidence interval) included antecedent IGE (2.05, 1.53–2.75), female gender (1.96, 1.53–2.52), number of life stressors (1: 1.82, 1.37–2.41; 2: 2.86, 2.01–4.06; 3+: 6.69, 4.59–9.77), and anxiety syndrome (1.74, 1.17–2.58). Limited to highly probable IBS, a stronger association with antecedent IGE was observed, particularly when based on medical encounter records (any IGE: 2.20, 1.10–4.43; medical encounter IGE only: 2.84, 1.33–6.09). Precedent anxiety or depression and IGE interacted with increased IBS risk compared with IGE alone.
CONCLUSIONS:
These results confirm previous studies on the association between sociodemographic or life stressors and IBS. IGE was significantly associated with IBS risk. Whether deployed or not, US service members often encounter repeated exposure to high levels of stress, which, combined with other environmental factors such as IGE, may result in long-term debilitating functional gastrointestinal disorders.
INTRODUCTION
Functional gastrointestinal disorders, including irritable bowel syndrome (IBS), represent a significant burden of disease in the United States and globally, with an IBS prevalence estimate of 14% (ref. 1). A recent systematic review found direct medical costs related to IBS of $1,562–$7,547, and indirect costs of $791–$7,737 per patient per year (2). In addition to increased direct medical care costs, IBS negatively impacts a patient's quality of life, resulting in increased fatigue, limitations in physical capabilities, and an overall lower perception of general health compared with the general population (3). Although data are emerging on the complex and varied disease mechanisms of IBS, epidemiological studies remain important to further elucidate relationships between risk factors and disease development (4). Previous studies have described IBS incidence and risk factors among the US military members using the Department of Defense medical encounter databases, confirming other civilian population-based studies identifying gender and antecedent gastrointestinal infection as risk factors (5). However, these previous reports, which relied on existing administrative databases containing medical encounter and demographic data, lacked information on many confounders such as life stressors and health behaviors, which are likely important in understanding risk and underlying causal mechanisms for this condition. To further explore incidence and risk factors for IBS in the US military, we used data from the Millennium Cohort Study, a large prospective study of military service members, to better understand associations between multiple exposures and risk of developing IBS.
METHODS
Study population
The Millennium Cohort Study is a 21-year longitudinal study initiated in 2001 to prospectively follow the US military personnel from all service branches to evaluate the impact of military service, including deployment, on short- and long-term health. The methodology has previously been described (6, 7, 8). In brief, it is a large, population-based cohort representing all military service branches and includes regular active duty, Reserve, and National Guard personnel. Since the first wave of invitations in 2001, over 200,000 participants have been enrolled as part of four separate accession panels: panel 1 (July 2001–June 2003), N=77,047; panel 2 (June 2004–February 2006), N=31,110; panel 3 (June 2007–December 2008), N=43,439; and panel 4 (April 2011–April 2013), N=50,052. More than 70% of the first two panels have submitted at least one follow-up questionnaire. Panel 1 was drawn from a population-based random sample of the US military in October 2000, with oversampling of Reserve/Guard personnel, women, and those with previous deployment experience in Bosnia, Kosovo, or Southwest Asia. Panels 2 and 3 sampled new accessions only, those with 1–3 years of military service, and oversampled for Marines and women. Study participants have completed a baseline survey, and they will continue to receive follow-up questionnaires approximately every 3 years.
The population for this study included Millennium Cohort Study participants who completed a baseline and at least one follow-up questionnaire between 2001 and 2009, who had not left active military service at the time of completing their first survey, and were without IBS or inflammatory bowel disease (IBD) at baseline. Excluded were subjects who endorsed every provider-based illness diagnosis on any survey, as well as those missing any data for covariates of interest. This latter exclusion contributes to slight differences in study populations for any IBS and highly probable IBS. For the any IBS model, all cases were censored at the date of diagnosis, and data on covariates were captured from the most recent survey completed before diagnosis. Depending on whether there were incomplete or missing data on that survey, the participant may or may not have been included in the final any IBS model. For the highly probable IBS model, cases that did not meet the stricter definition of highly probable IBS were assigned a new censoring date, therefore covariate data could have been chosen from a different survey than for the any IBS model. Depending on the status of missing data, this resulted in participants being included in the any IBS model but excluded from the highly probable IBS model or vice versa. The number of participants affected by this change in censoring date and survey assignment for covariates was small (any IBS: n=13; highly probable IBS: n=17) and did not affect the interpretation of any of the final models.
Participants were enrolled in the Millennium Cohort Study after providing full informed consent. The IBS study protocol was approved by the institutional review boards at the Uniformed Services University of the Health Sciences and the Naval Health Research Center (Protocol NHRC.2000.0007). Both studies were conducted in compliance with all applicable federal regulations governing the protection of human subjects in research.
Incident IBS
Incident cases of IBS were identified using the military medical encounter data from July 2001 to December 2009, received from the TRICARE Management Activity. Any person with at least two medical encounters occurring within a 365-day period and containing the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) code 564.1 (IBS) in any diagnosis field was considered an incident case of IBS and defined as any IBS. In addition, a more specific definition of highly probable IBS was used if an endoscopy was documented via procedure codes (451, 4511–4516, 4519, 4523, 4524) or Current Procedural Technology code (45330–45334, 45338, 45378, 45379, 45380, 45382, 45384, 45385) between the two medical encounters, and no evidence of a diagnosis of IBD between the two IBS medical encounters. IBD was defined as either self-report of provider-based IBD diagnosis on the Millennium Cohort survey or two IBD-related medical encounters (ICD-9-CM codes 555.0, 555.1, 555.9, and all 556 subgroups) in a 365-day period.
Baseline IBS and IBD
Assessment of baseline IBS and IBD cases based on medical encounter data were similar to incident IBS and IBD cases excluding the 365-day period. If IBS or IBD case definitions were met between 1 June 1998, and completion of the first survey for Panel 1 participants or between 1 June 2001, and completion of the first survey for Panel 2 participants, those Cohort members were excluded. Participants who self-reported being diagnosed with IBD by a medical provider on the baseline survey were considered baseline IBD cases and were similarly excluded.
Antecedent infectious gastroenteritis
Antecedent infectious gastroenteritis (IGE) data were collected from medical encounter records and self-reported post-deployment health assessments. Only those events occurring between completion of the baseline survey and IBS diagnosis or censure were considered. Any medical encounter containing a relevant ICD-9-CM code for IGE (all 001 subgroups, 003.0, 003.9, all 004 subgroups, 005.4, all 008.0 subgroups, 008.43, 008.44, 008.47, 008.49, 008.5, 009.0–009.3, all 005.8 subgroups, 005.9, 006.0–006.2, 006.9, all 007 subgroups, all 008.6 subgroups, and 008.8) in any diagnosis field was defined as an IGE episode. In addition, participants were deemed to have IGE if they self-reported diarrhea during or after deployment on a post-deployment health assessment. Subjects with no medical encounters or self-report of IGE were considered to not have IGE.
Other covariates
Demographic and military characteristics, including deployment, were obtained from Defense Manpower Data Center records. Missing Defense Manpower Data Center data were supplemented with information from Millennium Cohort surveys when available. All behavioral and mental health characteristics were assessed using data collected from the Millennium Cohort surveys. Body mass index was calculated from self-reported height and weight. Never smokers were defined as those who had smoked <100 cigarettes in their lifetime. Among smokers, those who reported having successfully quit smoking were defined as former smokers, and all others were categorized as current smokers. Non-drinkers were defined as those who reported no drinking in a typical week or those who had <12 drinks in the past year. Moderate female drinkers reported having between 1 and 7 drinks in a typical week (or on average ≤1 drink/day), whereas heavy female drinkers reported having >7 drinks in a typical week (or, on average, >1 drink/day). Moderate male drinkers reported having between 1 and 14 drinks in a typical week (or, on average, ≤2 drinks/day), whereas heavy male drinkers reported having >14 drinks in a typical week (or, on average, >2 drinks/day).
Survey questions related to life stressors were categorized using a modified version of the Holmes–Rahe Social Readjustment Scale (9). Stress related to divorce/separation, major financial problems, sexual assault, sexual harassment, physical assault, illness or death of a loved one, and a disabling illness or injury was assessed at baseline (ever experienced) and in each follow-up survey (preceding 3 years). Because only a small number of stressors were available total number of stressors was counted rather than assigning a weight to each.
Mental health data were collected using standardized survey instruments embedded in the Millennium Cohort survey, including the Patient Health Questionnaire (PHQ) and the post-traumatic stress disorder (PTSD) Checklist-Civilian Version. Using defined criteria for the PHQ-9 items that constitute the depression module, (10, 11) depression was identified if participants reported experiencing over the last 2 weeks either a depressed mood (little interest or pleasure in doing things) or anhedonia (feeling down, depressed, or hopeless), in addition to responding to experiencing a total of 5 or more depression items “more than half the days” or “nearly every day.” Anxiety was identified by a positive screen for either panic syndrome or other anxiety syndrome on the PHQ. Panic syndrome was assessed using a 15-item module that asked whether the participants had experienced an anxiety attack in the last month and what symptoms they may have experienced. If participants endorsed all 4 questions regarding anxiety attacks and 4 of 11 symptoms listed, they were considered positive for panic syndrome. In addition, other anxiety syndromes were identified using criteria defined by the PHQ for a 7-item module; if participants endorsed experiencing “feeling nervous, anxious, on edge or worrying a lot about different things” in the prior 4 weeks and endorsed experiencing 3 or more of the remaining 6 anxiety symptoms on “more than half the days”, (11, 12, 13) they were identified as screening positive for anxiety. The survey also included a question from the PHQ regarding current medication use for anxiety, depression, or stress. PTSD was assessed using the Checklist-Civilian Version-, a standardized 17-item survey instrument that inquires about PTSD symptoms experienced in the past month (14). For this study, PTSD was identified using the sensitive criteria, as defined by the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, only. All covariates were assessed at the time of the most recent survey prior to the censoring date for each participant, regardless of data source.
Time censoring calculations
For each member of the study population, start time for the period of observation was defined as the date of completion of first survey. Time was censored at the earliest of 5 dates: date of IBS diagnosis, date of IBD diagnosis, date of separation from military service, date of death, or end of study.
Statistical analysis
Survival analyses were performed using the PHREG procedure in SAS, Version 9.3 (SAS Institute, Cary, NC) for any IBS and highly probable IBS, modeled separately. The primary exposure of interest, antecedent IGE, was modeled as a time-varying covariate. Antecedent IGE was forced into the model, and then forward selection was used to add the remaining variables to the model, with a P<0.05 threshold for entry into the model. In addition, confounders of the IGE–IBS relationship were included in the final model if they changed the IGE parameter estimate by 10% or more. Interactions between each covariate in the model and the main exposure variable were examined. If an interaction term was significant at P<0.05, Akaike information criterion was used to select between models with and without the interaction term. If Akaike information criterion difference between 2 models was >4, the model without an interaction term was selected for model parsimony and interpretability (15). Two methods, Martingale residuals and interaction with time, were used to assess the proportional hazards assumption. Covariates that violated the assumption (P<0.05) using both methods included marital status in any IBS and all source IGE model, and marital status along with race/ethnicity in any IBS and medical encounter IGE model. A stratified Cox model was used when a violation of the proportional hazards assumption was observed. These analyses were repeated, restricting IGE to only those events identified using medical encounter data.
RESULTS
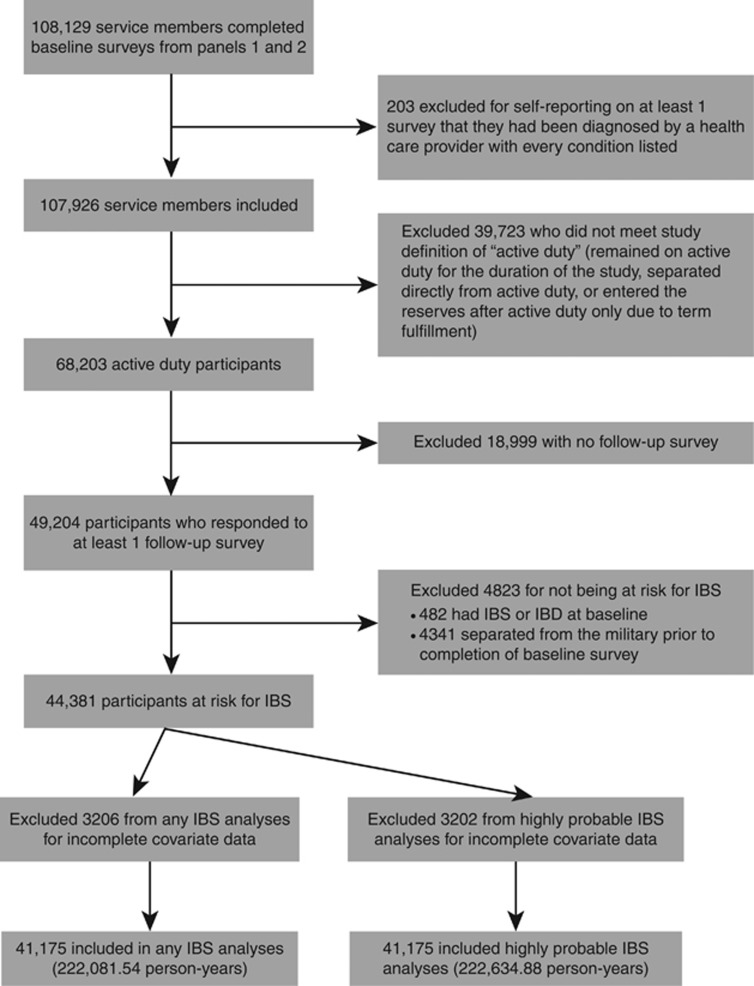
Of the 108,129 participants from Panels 1 and 2 who completed a baseline questionnaire, 203 were excluded for endorsing every provider-based diagnoses on at least 1 survey (pan-endorser). Participants were also excluded if they did not meet this study's definition of “active duty,” meaning they remained on active duty for the duration of the study, separated directly from active duty, or entered the reserves after active duty only owing to term fulfillment, leaving 68,203 participants. In addition, Millennium Cohort Study participants were excluded because they had not completed a follow-up survey (n=18,999), reporting IBS or IBD at baseline (n=482), having separated from the military before completion of their baseline survey (n=4,341),and missing data on covariates of interest (n=3206).This resulted in 41,175 participants with 222,081.54 person-years of follow-up available for analyses for any IBS and 41,179 participants with 222,634.88 person-years of follow-up for highly probable IBS (see Figure 1).
Figure 1.
Inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) inclusion and exclusion criteria for final study population.
Demographic, military, and behavioral health characteristics of cohort members included for any IBS are presented in Tables 1 and 2. Women represented slightly more than a quarter of participants, with the majority (66.2%) of participants being of white, non-Hispanic race/ethnicity. Smoking history, either former or current, was found in ~42%. A majority of subjects (56.3%) reported one or more life stressors, whereas relative few reported that they were taking medication for a mental health issue (6.9%). Nearly 60% had one or more deployments. Descriptive characteristics of cohort members in the highly probable IBS population had similar distributions to cohort members for any IBS (data not shown).
Table 1. Overall descriptive characteristics of active duty Millennium Cohort participantsa.
|
Active duty (N =41,175) |
||
|---|---|---|
| n | % | |
| Sex | ||
| Male | 30,023 | 72.9 |
| Female | 11,152 | 27.1 |
| Birth year | ||
| Pre-1960 | 3621 | 8.8 |
| 1960–1969 | 13,539 | 32.9 |
| 1970–1979 | 15,950 | 38.7 |
| 1980-present | 8065 | 19.6 |
| Race/ethnicity | ||
| White, non-Hispanic | 27,260 | 66.2 |
| Black, non-Hispanic | 5540 | 13.5 |
| Other | 8375 | 20.3 |
| Marital status | ||
| Single | 9503 | 23.1 |
| Married | 29,070 | 70.6 |
| Divorced/widowed/legally separated | 2602 | 6.3 |
| Education | ||
| Less than a bachelor's degree | 28,790 | 69.9 |
| Bachelor's degree or higher | 12,385 | 30.1 |
| Service branch | ||
| Army | 16,647 | 40.4 |
| Navy/Coast Guard | 9171 | 22.3 |
| Marine Corps | 2604 | 6.3 |
| Air Force | 12,753 | 30.1 |
| Rank | ||
| Enlisted | 30,347 | 73.7 |
| Officer | 10,828 | 26.3 |
| Occupation | ||
| Combat specialist | 7839 | 19.0 |
| Functional support | 8443 | 20.5 |
| Service and supply | 3471 | 8.4 |
| Health care | 4602 | 11.2 |
| Electrical/mechanical equipment repair | 5755 | 14.0 |
| Other | 11,065 | 26.9 |
Excludes participants who endorsed every provider-based illness diagnosis on at least one survey, and anyone who did not complete at least one follow-up survey.
Table 2. Health risk behaviors, stress and comorbid conditions among study participants.
|
Active duty (N =41,175) |
||
|---|---|---|
| n | % | |
| Smokinga | ||
| Never | 23,817 | 57.9 |
| Former | 10,411 | 25.3 |
| Current | 6947 | 16.9 |
| Alcohol consumptionb | ||
| Abstainer/light | 16,735 | 40.6 |
| Moderate | 21,282 | 51.7 |
| Heavy | 3158 | 7.7 |
| BMIc | ||
| Normal/underweight | 13,668 | 33.2 |
| Overweight | 21,323 | 51.8 |
| Obese | 6184 | 15.0 |
| Number of life stressorsd | ||
| 0 | 17,590 | 42.8 |
| 1 | 16,222 | 39.4 |
| 2 | 5188 | 12.6 |
| 3+ | 2175 | 5.2 |
| Depressione | ||
| Never | 39,444 | 95.8 |
| Ever | 1731 | 4.2 |
| Anxiety and panice | ||
| Never | 39,464 | 95.8 |
| Ever | 1711 | 4.2 |
| PTSD f | ||
| Never | 38,834 | 94.3 |
| Ever | 2341 | 5.7 |
| Multiple deployments | ||
| 0 | 16,737 | 40.6 |
| 1 | 12,268 | 29.8 |
| 2+ | 12,170 | 29.6 |
BMI, body mass index; PTSD, post-traumatic stress disorder.
Never=self-reported smoking <100 cigarettes in their lifetime, former=self-reported successful smoking cessation, current=reported never trying to quit or unsuccessful at quitting.
No/light=self-reported 0 drinks on a typical week, moderate=self-reported an average of 1–7 drinks per week for women and 1–14 per week for men, heavy=self-reported an average of >7 drinks per week for women and >14 per week for men.
Normal/underweight, <25; overweight, 25–29.9; obese, 30+.
Assessed using the social readjustment rating scale.
Assessed using responses to the patient health questionnaire.
Assessed using responses to the PTSD checklist-civilian version.
New-onset IBS was identified in 314 participants, with an estimated incidence of 141.39/100,000 person-years, whereas the incidence of highly probable IBS was significantly lower at 27.40/100,000 person-years for a total of 61 incidence cases. IGE was associated with an increased risk of IBS in all models; however, when limited to medical encounters for IGE and highly probable IBS, the largest effect estimate was observed (univariate hazard ratio (HR), 3.80; 95% confidence interval (CI), 1.79–8.08; Table 3). Additional univariate analyses are also detailed in Table 3. In general, restricting analysis to the highly probable IBS definition and/or documented medical encounter IGE demonstrated consistency in the direction of effect estimates, although bias toward the null or loss of statistical significance due to smaller cell sizes was also observed.
Table 3 Table 3. Univariate hazard ratios in the Millennium Cohort, 2001–2009aContinued.
|
Any IBS |
Highly probable IBS |
|||
| N | Univariate HR (95% CI) | N | Univariate HR (95% CI) | |
| Total | 314 | 61 | ||
| Antecedent IGE, any | ||||
| None | 250 | Ref | 50 | Ref |
| Any | 64 | 1.81 (1.36–2.40) | 11 | 1.61 (0.82–3.14) |
| Antecedent IGE, medical encounter | ||||
| None | 281 | Ref | 50 | Ref |
| Medical encounter | 33 | 2.87 (1.99–4.13) | 8 | 3.80 (1.79–8.08) |
| Sex | ||||
| Male | 148 | Ref | 31 | Ref |
| Female | 166 | 3.29 (2.63–4.10) | 30 | 2.80 (1.70–4.63) |
| Birth year | ||||
| Pre-1960 | 14 | 0.66 (0.38–1.15) | 6 | 1.53 (0.62–3.77) |
| 1960–1969 | 113 | 1.08 (0.84–1.39) | 24 | 1.25 (0.71–2.22) |
| 1970–1979 | 126 | Ref | 23 | Ref |
| 1980-present | 61 | 1.38 (1.01–1.87) | 8 | 0.95 (0.42–2.13) |
| Race/ethnicity | ||||
| White non-Hispanic | 218 | Ref | 38 | Ref |
| Black non-Hispanic | 48 | 1.05 (0.77–1.44) | 13 | 1.64 (0.87–3.08) |
| Other | 48 | 0.66 (0.48–0.90) | 10 | 0.79 (0.40–1.60) |
| Marital status | ||||
| Single | 93 | Ref | 14 | Ref |
| Married | 194 | 0.49 (0.38–0.63) | 42 | 0.72 (0.39–1.32) |
| Divorced/widowed/legally separated | 27 | 0.74 (0.48–1.13) | 5 | 0.92 (0.33–2.57) |
| Education | ||||
| Less than a bachelor's degree | 230 | 1.42 (1.10–1.82) | 40 | 0.98 (0.58–1.66) |
| Bachelor's degree or higher | 84 | Ref | 21 | Ref |
| Panel | ||||
| 2001–2003 | 234 | Ref | 53 | Ref |
| 2004–2006 | 80 | 1.47 (1.13–1.91) | 8 | 0.59 (0.28–1.26) |
| Service branch | ||||
| Army | 107 | 0.73 (0.56–0.95) | 21 | 0.76 (0.42–1.38) |
| Navy/Coast Guard | 80 | 1.03 (0.78–1.37) | 16 | 1.09 (0.57–2.08) |
| Marine Corps | 11 | 0.56 (0.3–1.04) | 2 | 0.54 (0.13–2.30) |
| Air Force | 116 | Ref | 22 | Ref |
| Rank | ||||
| Enlisted | 238 | Ref | 44 | Ref |
| Officer | 76 | 1.38 (1.06–1.78) | 17 | 1.13 (0.64–1.98) |
| Occupation | ||||
| Combat specialist | 37 | Ref | 9 | Ref |
| Functional support | 90 | 2.25 (1.54–3.30) | 20 | 2.04 (0.93–4.48) |
| Service and supply | 29 | 1.86 (1.15–3.03) | 4 | 1.05 (0.32–3.41) |
| Health care | 40 | 1.84 (1.18–2.87) | 7 | 1.31 (0.49–3.52) |
| Electrical/mechanical equipment repair | 30 | 1.21 (0.75–1.96) | 10 | 1.64 (0.67–4.05) |
| Other | 88 | 1.78 (1.21–2.62) | 11 | 0.91 (0.38–2.19) |
| Smokingb | ||||
| Never | 173 | Ref | 35 | Ref |
| Former | 81 | 1.12 (0.86–1.46) | 15 | 1.02 (0.56–1.87) |
| Current | 60 | 1.38 (1.03–1.85) | 11 | 1.24 (0.63–2.44) |
| Alcohol consumptionc | ||||
| No/light | 163 | Ref | 29 | Ref |
| Moderate | 130 | 0.59 (0.47–0.75) | 31 | 0.80 (0.48–1.33) |
| Heavy | 21 | 0.76 (0.48–1.20) | 1 | 0.20 (0.03–1.49) |
| BMId | ||||
| Normal/underweight | 141 | Ref | 23 | Ref |
| Overweight | 135 | 0.56 (0.45–0.72) | 26 | 0.67 (0.38–1.18) |
| Obese | 38 | 0.54 (0.38–0.77) | 12 | 1.05 (0.52–2.12) |
| Number of life stressorse | ||||
| 0 | 80 | Ref | 18 | Ref |
| 1 | 129 | 1.93 (1.46–2.55) | 23 | 1.52 (0.82–2.82) |
| 2 | 55 | 3.29 (2.33–4.64) | 7 | 1.84 (0.77–4.41) |
| 3+ | 50 | 9.56 (6.70–13.64) | 13 | 10.97 (5.36–22.47) |
| Depression syndromef | ||||
| No | 289 | Ref | 54 | Ref |
| Yes | 25 | 2.41 (1.60–3.62) | 7 | 3.59 (1.63–7.89) |
| Anxiety syndromef | ||||
| No | 284 | Ref | 57 | Ref |
| Yes | 30 | 2.86 (1.97–4.17) | 4 | 1.88 (0.68–5.17) |
| PTSDg | ||||
| No | 288 | Ref | 55 | Ref |
| Yes | 26 | 1.77 (1.18–2.64) | 6 | 2.12 (0.91–4.92) |
| Self-reported prescribed medication for mental health | ||||
| No | 282 | Ref | 55 | Ref |
| Yes | 32 | 2.17 (1.51–3.13) | 6 | 2.06 (0.89–4.78) |
| Deployments | ||||
| 0 | 159 | Ref | 41 | Ref |
| 1 | 83 | 0.58 (0.44–0.75) | 11 | 0.30 (0.15–0.58) |
| 2+ | 72 | 0.45 (0.34–0.60) | 9 | 0.22 (0.11–0.46) |
BMI, body mass index; IBS, irritable bowel syndrome; IGE, infectious gastroenteritis; PTSD, post-traumatic stress disorder.
Note: N=41,175 and 41,179 for any IBS and highly probably IBS, respectively.
Excludes subjects who endorsed every provider-based illness diagnosis on any survey, and anyone who did not complete at least 1 follow-up survey.
Never=self-reported smoking <100 cigarettes in their lifetime, former=self-reported successful smoking cessation, current=self-reported never trying to quit or unsuccessful at quitting.
No/light=self-reported 0 drinks on a typical week, moderate=self-reported an average of 1–7 drinks per week for women and 1–14 per week for men, heavy=self-reported an average of >7 drinks per week for women and >14 per week for men.
Normal/underweight, <25; overweight, 25–29.9; obese, 30+.
Assessed using the social readjustment rating scale.
Assessed using responses to the patient health questionnaire.
Assessed using responses to the PTSD checklist-civilian version.
The significantly increased risk of IBS associated with antecedent IGE persisted in the adjusted models for all source IGE (adjusted HR=2.05; 95% CI, 1.53–2.75) and for medical encounter IGE (aHR, 2.07; 95% CI,1.44–3.01) (Table 4). Models of highly probable IBS showed a similar increased risk following any IGE (aHR, 2.20; 95% CI, 1.10–4.43) and medical encounter-based IGE (aHR, 2.84; 95% CI, 1.33–6.09). Female gender (aHR=1.96), Army service (aHR=0.67), moderate alcohol consumption (aHR=0.68), body mass index (overweight: aHR=0.77; obese: aHR=0.67), number of life stressors (1: aHR=1.82; 2: aHR=2.86; 3+: aHR=6.69), anxiety syndrome (aHR=1.74), and number of deployments (1: aHR=0.61; 2+: aHR=0.52) remained significant in the adjusted Cox model for any IBS and all source IGE, whereas female gender (aHR=1.79), 3+ life stressors (aHR=6.80), depression syndrome (aHR=2.29), and deployments (1 deployment: aHR=0.29; 2+ deployments: aHR=0.22) remained associated when restricting to highly probable IBS and all source IGE. Similar HRs for covariates were found when restricting analysis to documented medical encounter IGE, with only Army service branch no longer significant in the any IBS model.
Table 4. Adjusted hazard ratios for the association between IGEa and IBS in the Millennium Cohort, 2001–2009b.
|
Any IGE |
Medical encounter IGE |
|||
|---|---|---|---|---|
| Any IBS (95% CI)c (Stratified Cox model) | Highly probable IBS (95% CI) (Cox PH model) | Any IBS (95% CI)c (Stratified Cox model) | Highly probable IBS (95% CI) (Cox PH model) | |
| Antecedent IGE | ||||
| None | Ref | Ref | Ref | Ref |
| Any | 2.05 (1.53–2.75) | 2.20 (1.10–4.43) | 2.07 (1.44–3.01) | 2.84 (1.33–6.09) |
| Sex | ||||
| Male | Ref | Ref | Ref | Ref |
| Female | 1.96 (1.53–2.52) | 1.79 (1.06–3.03) | 2.03 (1.58–2.61) | 1.75 (1.04–2.96) |
| Service branch | ||||
| Army | 0.67 (0.51–0.87) | |||
| Navy/Coast Guard | 0.99 (0.74–1.32) | |||
| Marine Corps | 0.66 (0.35–1.23) | |||
| Air Force | Ref | |||
| Alcohol consumptiond | ||||
| No/light | Ref | Ref | ||
| Moderate | 0.68 (0.54–0.86) | 0.69 (0.54–0.87) | ||
| Heavy | 0.68 (0.43–1.08) | 0.64 (0.4–1.02) | ||
| BMIe | ||||
| Normal/underweight | Ref | Ref | ||
| Overweight | 0.77 (0.61–0.99) | 0.78 (0.61–1.00) | ||
| Obese | 0.67 (0.46–0.97) | 0.68 (0.47–0.98) | ||
| Number of life stressorsf | ||||
| 0 | Ref | Ref | Ref | Ref |
| 1 | 1.82 (1.37–2.41) | 1.40 (0.76–2.60) | 1.81 (1.37–2.39) | 1.41 (0.76–2.61) |
| 2 | 2.86 (2.01–4.06) | 1.48 (0.61–3.59) | 2.79 (1.96–3.96) | 1.49 (0.61–3.60) |
| 3+ | 6.69 (4.59–9.77) | 6.80 (3.18–14.53) | 6.44 (4.42–9.38) | 6.81 (3.19–14.54) |
| Depression syndromeg | ||||
| No | Ref | Ref | ||
| Yes | 2.29 (1.01–5.19) | 2.36 (1.04–5.34) | ||
| Anxiety syndromeg | ||||
| No | Ref | Ref | ||
| Yes | 1.74 (1.17–2.58) | 1.68 (1.14–2.49) | ||
| Deployments | ||||
| 0 | Ref | Ref | Ref | Ref |
| 1 | 0.61 (0.47–0.80) | 0.29 (0.15–0.56) | 0.64 (0.49–0.84) | 0.32 (0.16–0.62) |
| 2+ | 0.52 (0.39–0.70) | 0.22 (0.10–0.47) | 0.57 (0.43–0.76) | 0.26 (0.13–0.55) |
BMI, body mass index; IBS, irritable bowel syndrome; IGE, infectious gastroenteritis.
NOTE. N=41,175 and 41,179 for any IBS and highly probably IBS, respectively.
From all sources or from medical encounter data only.
Excludes subjects who endorsed every provider-based illness diagnosis on any survey, and anyone who did not complete at least 1 follow-up survey.
Stratified on marriage and race/ethnicity.
No/light=self-reported 0 drinks on a typical week, moderate=self-reported an average of 1–7 drinks per week for women and 1–14 per week for men, heavy=self-reported an average of >7 drinks per week for women and >14 per week for men.
Normal/underweight, <25; overweight, 25–29.9; obese, 30+.
Assessed using the social readjustment rating scale.
Assessed using responses to the patient health questionnaire.
In addition to the primary effects, interactions between IGE and other covariates (e.g., smoking, stress, deployment, anxiety, PTSD, and depression) were explored. Consistent interactions among highly probable IBS and both IGE exposure categories were found for both depression and anxiety. In these interaction models, the combination of medical encounter IGE and either anxiety or depression and the combination of all source IGE and depression resulted in a differential risk of highly probable IBS compared with stratification by IGE or mental health condition alone, although the numbers were small (Tables 5 and 6).
Table 5. Interaction between IGE and depression for risk of highly probable IBS.
| Any IGE | No IBS | IBS | OR (95% CI) |
|---|---|---|---|
| No IGE and no depression | 32,611 | 47 | 1.00 |
| IGE and no depression | 6786 | 7 | 1.55 (0.62–3.33) |
| No IGE and depression | 1324 | 3 | 1.18 (0.28–3.30) |
| IGE and depression | 397 | 4 | 12.93 (3.69–34.77) |
| Medical encounter IGE | |||
|---|---|---|---|
| No IGE and no depression | 36,917 | 49 | 1.00 |
| IGE and no depression | 2480 | 5 | 1.88 (0.65–4.37) |
| No IGE and depression | 1574 | 4 | 1.45 (0.43–3.68) |
| IGE and depression | 147 | 3 | 22.26 (5.30–63.07) |
CI, confidence interval; IBS, irritable bowel syndrome; IGE, infectious gastroenteritis, OR, odds ratio.
Note: both controlled for sex, stress, and deployment.
Table 6. Interaction between IGE and anxiety for risk of highly probable IBS.
| Any IGE | No IBS | IBS | OR (95% CI) |
|---|---|---|---|
| No IGE and no anxiety | 32,615 | 49 | 1.00 |
| IGE and no anxiety | 6789 | 8 | 1.68 (0.71–3.49) |
| No IGE and anxiety | 1320 | 1 | 0.17 (0.01–0.93) |
| IGE and anxiety | 394 | 3 | 4.08 (0.86–14.08) |
| Medical encounter IGE | |||
|---|---|---|---|
| No IGE and no anxiety | 36,919 | 52 | 1.00 |
| IGE and no anxiety | 2485 | 5 | 1.76 (0.61–4.07) |
| No IGE and anxiety | 1572 | 1 | 0.15 (0.01–0.80) |
| IGE and anxiety | 142 | 3 | 9.59 (2.13–30.55) |
CI, confidence interval; IBS, irritable bowel syndrome; IGE, infectious gastroenteritis, OR, odds ratio.
Note: both controlled for sex, stress, and deployment.
DISCUSSION
Overall IBS incidence (any IBS definition) was estimated at 141.39 (89.38 male, 293.81 female) per 100,000 person-years in this study population, which is lower than 2 previous studies in civilian populations reporting incidence between 200 and 260 cases per 100,000 person-years (16, 17). However, our rates for women are similar to those reported by Locke et al. (16) for similar age strata from Olmsted County, Minnesota. A nested case-control study using a Dutch national medical encounter data system estimated a 1-year incidence of ~300 per 100,000 person-years in individuals not reporting an antecedent acute IGE episode (18), which is also higher than our estimated incidence. Some possible explanations for our lower rates are the “healthy worker effect” among active duty military personnel, under-representation of female gender, and/or potential differences in health care-seeking behavior of individuals in the military compared with civilian populations.
Consistent with prior studies, we found a 2–3-fold increase in IBS risk after IGE, with higher effect estimates when IBS and IGE were more precisely defined. Although these point estimates are lower than pooled effect estimates reported in recent systematic reviews, (19, 20) they do support a positive association between acute enteric infection and IBS and are consistent with individual effect estimates recently reported in a medical encounter system population-based Dutch study (relative risk, 4.85; 95% CI, 2.02–11.63), as well as a study among returning UK veterans from Iraq (self-reported IGE: odds ratio, 2.59, 95% CI, 1.83–2.67; medical encounter IGE: OR, 4.34, 95% CI, 2.55–7.39) (18, 21). It is logical that study designs based on medical encounter data may result in lower effect estimates compared with active surveillance studies because of differences in non-differential misclassification of exposure and/or outcomes, as well as population-unique effects with an active duty healthier population that may be less susceptible to developing IBS.
Interestingly, this study identified other associations of particular interest in a military population. A consistently identified increase in IBS risk with PTSD has been described in studies of women veterans (22, 23). A recent study by Maguen et al. of a population of over 600,000 Iraq and Afghanistan war veterans found that for both men and women, IBS was three times more likely to be present among those with PTSD than those without PTSD (24). Our finding of increased risk of incident IBS among those with PTSD in univariate analyses is consistent with these prior reports. There are biological and psychological explanations that might explain this association, such as the reported effect of PTSD and depression on systemic inflammation (25) and abnormalities in brain function, that could affect pain perception and awareness of visceral stimuli (26). However, significantly increased risk was not observed in our adjusted model; this could be attributed to insufficient power or the use of the most proximal survey data to assess PTSD. Additional studies focused on PTSD and chronic functional gastrointestinal disorders that consider the timing of association, as well as potential common pathological pathways are warranted. Another military-specific association was observed between decreased IBS risk and multiple deployments. Additional measures of deployment (i.e., cumulative time deployed, deployment before Operation Enduring Freedom/Operation Iraqi Freedom, self-report of combat exposure while deployed) were assessed in univariate analyses, and the same pattern of reduced risk was observed (data not shown). This may be indicative of a healthy worker effect as has been previously described (27). Interestingly, when a more specific definition of IBS (highly probable IBS) was used, this protective effect became more pronounced.
As in prior studies, a number of risk factors were found that continue to support a biopsychosocial model of disease. As described in other studies, we found that the number of life stressor events increased the risk of IBS (28, 29). Furthermore, we found that self-reported anxiety and depression were independently associated with increased risk of IBS (univariate analyses only), similar to other studies (28, 29). However, the joint effect of these conditions, in combination with IGE exposure, resulted in an increased IBS risk greater than the risk from either of these exposures alone (Tables 5 and 6). Our understanding of the importance of the two-way “cross-talk” between the brain and the gut continues to grow, particularly how gut–brain interactions with the microbiome might result in dysbiosis and development of disease in the presence of nervous system disturbances. Multiple potential direct and indirect pathways exist through which altered gut microbiota can modulate the gut–brain axis (30). Under stressful conditions, the hypothalamus–pituitary–adrenal axis regulates cortisol, which can alter the immune system, gut permeability, and barrier function, and result in a change in gut microbiota composition. This has been described recently in military training settings where Singaporean Army rapid response troops were monitored for psychological and gastrointestinal symptoms, as well as immunological and intestinal biomarkers during intensive combat training (31). Interestingly, the anticipated increases in stress, anxiety, and depression were associated with gastrointestinal symptoms (noninfectious) and markers of pro-inflammatory immune activation and increased intestinal permeability. Although human participant studies have shown that risk of functional gastrointestinal disorders are associated with psychiatric comorbidities (32, 33, 34), it has also been described in animal models where microbiome changes (perhaps as a consequence of acute infection) can change behavior and brain neurochemistry (35). Thus, it is quite plausible that the combination of acute enteric infection and psychological stress interactions could prove to be the pathophysiological basis of some chronic gastrointestinal sequelae (36, 37). A relevant mouse model, which has independently and concomitantly evaluated stress and Citrobacter rodentium infection, has been developed and provides support for this mechanism (38). Our emerging understanding of these concepts provides a framework to further investigate gut–brain axis relationships and ultimately identify potential solutions for chronic gastroenterological health conditions that are being observed among service members who are frequently exposed to both infection and stress during training and deployment situations (4, 27).
This study had several limitations. Data were obtained from existing self-report questionnaires; however, the survey instruments used validated questions and were administered consistently (39). Furthermore, clinical examinations for confirmation of both self-reported symptoms and conditions could not be assumed, and ICD-9-CM-based medical encounter data on IGE exposure and IBS outcomes are also potentially susceptible to error or misclassification. Specifically, with respect to our primary outcome of IBS, misclassification for an ill-defined complex of unexplained GI symptoms may have been ascribed without the application of Rome criteria. Furthermore, whereas we excluded from eligibility those with pre-existing IBD, we did not exclude those who may have had other mimic conditions, such as celiac disease, tropical sprue, or intestinal malabsorption. It is possible that cohort subjects could have had these pre-existing diagnoses which were subsequently properly diagnosed or misclassified as IBS. However, the clinical incidence of these conditions is very low in this population and thus would unlikely have had a large bias on our results (40, 41). Because it was necessary to use ICD-9-CM-based outcome diagnoses contained in medical encounter data (IBS is not among the health conditions on the survey), a more strict definition which required concomitant colonoscopy visit codes was used, and the cohort population was limited to individuals on active duty who received care at military treatment facilities. Such factors could explain both the relatively low IBS incidence and the effect estimates. Furthermore, the exclusion of those not meeting the active duty definition (~37%) may also limit generalizability and application of these results to reserve and guard populations. Prospective studies using validated and active functional gastrointestinal outcome surveillance among both deployed and garrison populations, as well reserve and guard components would be of value.
This study also had several strengths. Its population-based, prospective cohort design allowed for baseline and follow-up assessments (using time-varying covariates when appropriate) among individuals. In addition, the design enabled estimates of IBS incidence and strong statistical power for assessing primary effects, whereas controlling for multiple confounders and exploring novel-risk factors.
In summary, these findings represent additional data that contribute to an accumulating body of evidence linking acute gastrointestinal infections and chronic gastrointestinal sequelae. In addition to important findings from mechanistic studies also being reported, our findings add to the belief that this observed phenomenon is not exaggerated (42). A coordinated and resourced research agenda is needed to understand the heterogeneity in observed risk and disease outcomes based on host, agent, and environmental differences, including pathoetiological mechanisms (4). More urgently needed are potential measures to mitigate the burdensome consequences of acute gastroenteritis, which are known to have substantial impact on medical care costs and quality of life among millions of at-risk travelers and deployed military personnel.
Study Highlights

Acknowledgments
We thank Ashleigh Peoples and Kelly A. Jones from the Naval Health Research Center for statistical support, and The Millennium Cohort Study Team.
DISCLAIMER
The views expressed in this research are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, Department of Veterans Affairs, or the United States Government. Approved for public release; distribution is unlimited.
COPYRIGHT
Some authors are employees of the United States Government and military service members. This work was prepared as part of official duties. Title 17 U.S.C. §105 provides that “Copyright protection under this title is not available for any work of the United States Government”. Title 17 U.S.C. §101 defines a United States Government work as a work prepared by a military service member or employee of the United States Government as part of that person's official duties.
HUMAN SUBJECTS RESEARCH
Human participants were enrolled in the Millennium Cohort Study after providing full informed consent. The IBS study protocol was approved by the institutional review boards at the Uniformed Services University of the Health Sciences and the Naval Health Research Center (Protocol NHRC.2000.0007). Both studies were conducted in compliance with all applicable federal regulations governing the protection of human subjects in research.
Guarantor of the article: Tomoko I. Hooper, MD, MPH, PhD.
Specific author contributions: Conception and design of the study: Mark S. Riddle, Chad K. Porter, Tomoko I. Hooper, and Edward J. Boyoko; generation, collection, assembly, analysis and/or interpretation of data: Marleen Welsh, Chiping Neih, Mark S. Riddle, and Chad K. Porter; drafting or revision of the manuscript: all authors; approval of the final version of the manuscript: all authors.
Financial support: This work was supported by the Military Operational Medicine Research Program, US Army Medical Research and Materiel Command (Fort Detrick, Maryland) and Uniformed Services University of the Health Sciences intramural grant R0879D. Resources from the VA Puget Sound Health Care System supported Dr Boyko's involvement in this research.
Potential competing interests: None.
References
- 1Hungin AP, Chang L, Locke GR et al. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharmacol Ther 2005;21:1365–1375. [DOI] [PubMed] [Google Scholar]
- 2Nellesen D, Yee K, Chawla A et al. A systematic review of the economic and humanistic burden of illness in irritable bowel syndrome and chronic constipation. J Manag Care Pharm 2013;19:755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Gralnek IM, Hays RD, Kilbourne A et al. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology 2000;119:654–660. [DOI] [PubMed] [Google Scholar]
- 4Deising A, Gutierrez RL, Porter CK et al. Postinfectious functional gastrointestinal disorders: a focus on epidemiology and research agendas. Gastroenterol Hepatol (N Y) 2013;9:145–157. [PMC free article] [PubMed] [Google Scholar]
- 5Porter CK, Choi D, Cash B et al. Pathogen-specific risk of chronic gastrointestinal disorders following bacterial causes of foodborne illness. BMC Gastroenterol 2013;13:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6Gray GC, Chesbrough KB, Ryan MA et al. The millennium Cohort Study: a 21-year prospective cohort study of 140,000 military personnel. Mil Med 2002;167:483–488. [PubMed] [Google Scholar]
- 7Ryan MA, Smith TC, Smith B et al. Millennium Cohort: enrollment begins a 21-year contribution to understanding the impact of military service. J Clin Epidemiol 2007;60:181–191. [DOI] [PubMed] [Google Scholar]
- 8Smith TC, Jacobson IG, Hooper TI et al. Health impact of US military service in a large population-based military cohort: findings of the Millennium Cohort Study, 2001-2008. BMC Public Health 2011;11:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9Holmes TH, Rahe RH. The social readjustment rating scale. J Psychosom Res 1967;11:213–218. [DOI] [PubMed] [Google Scholar]
- 10Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. Jama 1999;282:1737–1744. [DOI] [PubMed] [Google Scholar]
- 12Spitzer RL, Williams JB, Kroenke K et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. Jama 1994;272:1749–1756. [PubMed] [Google Scholar]
- 13Spitzer RL, Williams JB, Kroenke K et al. Validity and utility of the PRIME-MD patient health questionnaire in assessment of 3000 obstetric-gynecologic patients: the PRIME-MD Patient Health Questionnaire Obstetrics-Gynecology Study. Am J Obstet Gynecol 2000;183:759–769. [DOI] [PubMed] [Google Scholar]
- 14Forbes D, Creamer M, Biddle D. The validity of the PTSD checklist as a measure of symptomatic change in combat-related PTSD. Behav Res Ther 2001;39:977–986. [DOI] [PubMed] [Google Scholar]
- 15Burnham K, Anderson D. Multimodel inference: understanding AIC and BIC in model selection. Socio Meth Res 2004;33:44. [Google Scholar]
- 16Locke GR 3rd, Yawn BP, Wollan PC et al. Incidence of a clinical diagnosis of the irritable bowel syndrome in a United States population. Aliment Pharmacol Ther 2004;19:1025–1031. [DOI] [PubMed] [Google Scholar]
- 17Garcia Rodriguez LA, Ruigomez A, Wallander MA et al. Detection of colorectal tumor and inflammatory bowel disease during follow-up of patients with initial diagnosis of irritable bowel syndrome. Scand J Gastroenterol 2000;35:306–311. [DOI] [PubMed] [Google Scholar]
- 18Kowalcyk BK, Smeets HM, Succop PA et al. Relative risk of irritable bowel syndrome following acute gastroenteritis and associated risk factors. Epidemiol Infect 2014;142:1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Thabane M, Kottachchi DT, Marshall JK. Systematic review and meta-analysis: the incidence and prognosis of post-infectious irritable bowel syndrome. Aliment Pharmacol Ther 2007;26:535–544. [DOI] [PubMed] [Google Scholar]
- 20Halvorson HA, Schlett CD, Riddle MS. Postinfectious irritable bowel syndrome-a meta-analysis. Am JGastroenterol 2006;101:1894–1899. [DOI] [PubMed] [Google Scholar]
- 21Goodwin L, Bourke JH, Forbes H et al. Irritable bowel syndrome in the UK military after deployment to Iraq: what are the risk factors? Soc Psychiatry Psychiatr Epidemiol 2013;48:1755–1765. [DOI] [PubMed] [Google Scholar]
- 22White DL, Savas LS, Daci K et al. Trauma history and risk of the irritable bowel syndrome in women veterans. Aliment Pharmacol Ther 2010;32:551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23Savas LS, White DL, Wieman M et al. Irritable bowel syndrome and dyspepsia among women veterans: prevalence and association with psychological distress. Aliment Pharmacol Ther 2009;29:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24Maguen S, Madden E, Cohen B et al. Association of Mental health problems with gastrointestinal disorders in iraq and afghanistan veterans. Depress Anxiety 2014;31:160–165. [DOI] [PubMed] [Google Scholar]
- 25von Kanel R, Hepp U, Kraemer B et al. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res 2007;41:744–752. [DOI] [PubMed] [Google Scholar]
- 26Mayer EA, Naliboff BD, Chang L. Evolving pathophysiological model of functional gastrointestinal disorders: implications for treatment. Gastroenterology 2002;68 (Suppl 587) 3–9. [PubMed] [Google Scholar]
- 27Porter CK, Gloor K, Cash BD et al. Risk of functional gastrointestinal disorders in U.S. military following self-reported diarrhea and vomiting during deployment. Dig Dis Sci 2011;56:3262–3269. [DOI] [PubMed] [Google Scholar]
- 28Videlock EJ, Adeyemo M, Licudine A et al. Childhood trauma is associated with hypothalamic-pituitary-adrenal axis responsiveness in irritable bowel syndrome. Gastroenterology 2009;137:1954–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Bennett EJ, Tennant CC, Piesse C et al. Level of chronic life stress predicts clinical outcome in irritable bowel syndrome. Gut 1998;43:256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012;13:701–712. [DOI] [PubMed] [Google Scholar]
- 31Li X, Kan EM, Lu J et al. Combat-training increases intestinal permeability, immune activation and gastrointestinal symptoms in soldiers. Aliment Pharmacol Ther 2013;37:799–809. [DOI] [PubMed] [Google Scholar]
- 32Whitehead WE, Palsson OS, Levy RR et al. Comorbidity in irritable bowel syndrome. Am J Gastroenterol 2007;102:2767–2776. [DOI] [PubMed] [Google Scholar]
- 33Lydiard RB. Increased prevalence of functional gastrointestinal disorders in panic disorder: clinical and theoretical implications. CNS Spectr 2005;10:899–908. [DOI] [PubMed] [Google Scholar]
- 34Fullwood A, Drossman DA. The relationship of psychiatric illness with gastrointestinal disease. Annu Rev Med 1995;46:483–496. [DOI] [PubMed] [Google Scholar]
- 35Bercik P, Denou E, Collins J et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011;141:599–609. [DOI] [PubMed] [Google Scholar]
- 36Lyte M, Vulchanova L, Brown DR. Stress at the intestinal surface: catecholamines and mucosa-bacteria interactions. Cell Tissue Res 2011;343:23–32. [DOI] [PubMed] [Google Scholar]
- 37O'Malley D, Quigley EM, Dinan TG et al. Do interactions between stress and immune responses lead to symptom exacerbations in irritable bowel syndrome? Brain Behav Immun 2011;25:1333–1341. [DOI] [PubMed] [Google Scholar]
- 38Spreadbury I, Ochoa-Cortes F, Ibeakanma C et al. Concurrent psychological stress and infectious colitis is key to sustaining enhanced peripheral sensory signaling. Neurogastroenterol Motil 2015;27:347–355. [DOI] [PubMed] [Google Scholar]
- 39Smith TC, Smith B, Jacobson IG et al. Reliability of standard health assessment instruments in a large, population-based cohort study. Ann Epidemiol 2007;17:525–532. [DOI] [PubMed] [Google Scholar]
- 40McCarroll MG, Riddle MS, Gutierrez RL et al. Infectious gastroenteritis as a risk factor for tropical sprue and malabsorption: a case-control study. Dig Dis Sci 2015;60:3379–3385. [DOI] [PubMed] [Google Scholar]
- 41Riddle MS, Murray JA, Porter CK. The incidence and risk of celiac disease in a healthy US adult population. Am J Gastroenterol 2012;107:1248–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42Verdu EF, Riddle MS. Chronic gastrointestinal consequences of acute infectious diarrhea: evolving concepts in epidemiology and pathogenesis. Am J Gastroenterol 2012;107:981–989. [DOI] [PubMed] [Google Scholar]