Abstract
Hereditary forms of Wilms arise from developmentally arrested clones of renal progenitor cells with biallelic mutations of WT1; recently, it has been found that Wilms tumors may also be associated with biallelic mutations in DICER1 or DROSHA, crucial for miRNA biogenesis. We have previously shown that a critical role for WT1 during normal nephrogenesis is to suppress transcription of the Polycomb group protein, EZH2, thereby de-repressing genes in the differentiation cascade. Here we show that WT1 also suppresses translation of EZH2. All major WT1 isoforms induce an array of miRNAs, which target the 3′ UTR of EZH2 and other Polycomb-associated transcripts. We show that the WT1(+KTS) isoform binds to the 5′ UTR of EZH2 and interacts directly with the miRNA-containing RISC to enhance post-transcriptional inhibition. These observations suggest a novel mechanism through which WT1 regulates the transition from resting stem cell to activated progenitor cell during nephrogenesis. Our findings also offer a plausible explanation for the fact that Wilms tumors can arise either from loss of WT1 or loss of miRNA processing enzymes.
Keywords: Argonaute, dicer, mesenchymal stem cells (MSCs), microRNA (miRNA), polycomb, Enhancer of Zeste 2, kidney development, Wilms tumor 1
Introduction
In 1879, William Osler reported two pediatric patients from Montreal with massive kidney tumors containing bands of muscle-like tissue mixed with epithelial elements (35). Twenty years later, Max Wilms published his celebrated monograph recognizing the malignancy as a unique “mischgeschwulste der Niere” (mixed tumor of the kidney), composed of mixed stromal, epithelial, and undifferentiated mesenchymal cells. This “triphasic” histology suggests that Wilms tumors arise from developmentally arrested stem cells of the metanephric mesenchyme that occasionally exhibit abortive differentiation toward a stromal or an epithelial cell fate. It follows that a disturbance of molecular events governing the transition from renal progenitor cells into these differentiated lineages must be central to the pathogenesis of Wilms tumor.
Wilms tumor (WT)2 is the most common form of pediatric kidney cancer and affects about 1:10,000 children in North America (1). In a subset of these patients, a germline deletion of the transcription factor, WT1, is accompanied by a somatic mutation of the second WT1 allele, giving rise to clones of incompetent stem cells adjacent to the malignant tumor (2). These “nephrogenic rests” are thought to represent embryonic renal progenitors that have failed to respond to inductive WNT signals during embryogenesis. The pre-malignant cell clusters may persist within the normal kidney (3) until a constitutively activating mutation of the β-catenin gene (CTNNB1) (17, 18) bypasses the need for a normal WNT signal and drives un-regulated rapid cell growth (4). Wilms tumors retain a chromatin pattern resembling embryonic stem cells in which genes of the differentiation cascade are broadly silenced by tri-methylated lysine 27 residues in the H3 histone associated with their promoter regions (5). We recently showed that the WT1 isoforms lacking the three amino acid insertion lysine-threonine-serine between zinc fingers 3 and 4 (−KTS), inhibits transcription of the histone methyltransferase (Enhancer of Zeste Homolog 2, EZH2) that epigenetically silences β-catenin gene (CTNNB1) expression in mesenchymal stem cells and facilitates the canonical WNT/β-catenin pathway response to WNT9b (6).
Only about 20% of Wilms tumors are linked to WT1 mutations (7, 8). Recently, it was discovered that a subset of Wilms tumors with normal WT1 alleles are associated with mutations of either DICER1 (9) or DROSHA (10). These genes encode proteins that process microRNAs (miRNAs), implying that loss of either WT1 or miRNA biogenesis can lead to the same Wilms tumor phenotype. We reasoned that the crucial effects of WT1 during development might require both transcriptional and miRNA-mediated post-transcriptional inhibition of EZH2. Here we show that WT1 is evident both in the nucleus and cytoplasm of progenitor cells in embryonic mouse kidney nephrogenic mesenchyme. In mesenchymal stem cells isolated from human amniotic fluid, we show that WT1 induces expression of multiple miRNAs, including miRNA-26a (miR-26a) and miRNA-101 (miR-101). In fibroblasts, we show that the latter two miRNAs suppress EZH2 translation. Furthermore, we show that WT1 binds to the 5′ untranslated region (UTR) of EZH2 mRNA and cooperates with miRNA-containing RNA-induced silencing complexes (RISCs) to suppress EZH2 translation. These observations suggest a novel mechanism through which the WT1 transcription factor exerts post-transcriptional regulation in cooperation with miRNAs.
Experimental Procedures
Cell Culture
Human amniotic fluid-derived mesenchymal stem cells (amMSC) were grown in the presence of DMEM culture medium (Gibco, Life Technologies, Burlington, ON, Canada) with 15% fetal bovine serum (Wisent, Saint-Jean-Baptiste, QC, Canada) and 1% penicillin/streptomycin (Gibco). Human fibroblasts (MCH070) and mouse mesonephric kidney cells (M15) (11) were grown in DMEM culture medium (Gibco) with 10% fetal bovine serum (Wisent) and 1% penicillin/streptomycin (Gibco).
Viral Particle Transduction
amMSC were transduced with viral particles expressing the two principal subgroups of human WT1 isoforms containing exon 5 (17 amino acid sequence) with (+/+) or without (+/−) the KTS insertion in exon 9 or control GFP particles. The WT1 isoforms cDNA were cloned into the pWPI bicistronic vector (Dr. Trono Lab/Addgene). Each plasmid was co-transfected with pMD2.G, the envelope plasmid, and psPAX2, the packaging plasmid, into 293T cells. Viral particles were harvested and used for the transduction. Specific WT1 isoforms are denoted by plus or minus symbols; the first indicating the presence or absence of a 17-amino acid sequence in exon 5 and the second KTS between zinc fingers 3 and 4 status.
Immunoprecipitation
Cells were grown and harvested following standard techniques. Cell lysis buffer contained Tris (10 mm, pH 7.4), NaCl (10 mm), MgCl2 (3 mm), Nonidet P-40 (0.3%), and glycerol (10%). The immunoprecipitation was performed using Protein A/G-agarose beads. The following antibodies and titers were used: normal rabbit IgG (2729, 2 μg, Cell Signaling, Danvers, MA), WT1 C19 (sc192, 2 μg, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-Ago2/eIF2C2 (ab32381, 2 μg, Abcam, Toronto, ON, Canada), and anti-Dicer (ab14601, 2 μg, Abcam).
Immunoblotting
Following the immunoprecipitations, 6× loading dye was added to the Protein A/G-agarose beads, boiled at 95 °C for 8 min, loaded onto SDS-PAGE gel, and subjected to electrophoresis following standard immunoblotting techniques. The following primary antibodies and titers were used: WT1 C19 (sc192, 1/200, Santa Cruz Biotechnology, Inc.), anti-Ago2/eIF2C2 (ab32381, 1/500, Abcam), and anti-Dicer (ab4735, 1/500, Abcam). Immunoreactive bands were detected using species-specific horseradish peroxidase-conjugated secondary antibodies (1/2000, Cell Signaling) and visualized using GE Healthcare ECL Plus Western blotting Detection Reagents and the Storm Imager Scanner and software (GE Healthcare, Mississauga, ON, Canada).
Indirect Immunofluorescence Staining
Cryosections (5 μm) from embryonic mouse kidneys at 17.5 dpc stage were fixed with acetone at −20 °C for 7 min, incubated in 1% Triton X-100, PBS for 30 min, blocked with normal goat serum (10% in 1% BSA, 0.1% Triton X-100/PBS) for 1 h, and incubated with primary antibody WT1 C19 (sc192, 1/400, Santa Cruz Biotechnology, Inc.) and Alexa Fluor® 568-conjugated secondary antibodies (1/1000) at 4 °C overnight and room temperature for 1 h, respectively. The sections were also incubated with Dolichos biflorus agglutinin antibody (1/300, Vector Laboratories, Inc., Burlingame, CA) and the nuclei were counterstained with DAPI. Images were obtained with a laser scanning confocal microscope (LSM780) and the ZEN2010 software (Carl Zeiss Canada Ltd., Toronto, ON, Canada) at room temperature and processed by Adobe Photoshop and Illustrator software.
RNA Isolation and Real-time PCR Analysis
RNA, including miRNA, was purified using the miRNeasy mini kit according to the manufacturer's instructions (Qiagen, Toronto, ON, Canada). Reverse transcription for mRNA was done with the iScript cDNA synthesis kit (Bio-Rad), and for miRNA using the miScript II RT kit (Qiagen, Toronto, ON, Canada). Quantitative PCR for reverse transcribed mRNA was performed using with the SsoFast EvaGreen Supermix with Low ROX (Bio-Rad) and specific primer sets. Quantitative PCR for reverse transcribed miRNA was performed using the miScript SYBR Green PCR kit and specific primer assays for miRNA (Qiagen). Both PCR were performed in a LightCycler 480 II (Roche Applied Science, Laval, QC, Canada).
miRNA Expression Profile
RNA was extracted and sent for miRNA microarray and analysis (EXIQON, Vedbæk, Denmark). The samples were labeled using the miRCURY LNATM microRNA Hi-Power Labeling Kit, Hy3TM/Hy5TM, and hybridized on the miRCURY LNATM microRNA array (7th generation), following a dual color experimental design.
Transfection
Human fibroblasts (MCH070 cells) were transfected with pCMVSport6, pCMVSport6/hEZH2FL (insert size about 1.1 kb) (ATCC, MGC Human Clones, image ID: 3901250), WT1 cloned into a pcDNA3.1/Zeo(+) vector (kindly provided by Dr. P. Grundy, Calgary, AB, Canada), miScript miRNA mimics: Syn-hsa-miR-26a-5p, Syn-hsa-miR-101–3p, Syn-hsa-miR-93, and AllStars Negative Control siRNA, miScript miRNA inhibitor: anti-hsa-miR-26a-5p, anti-hsa-miR-101–3p, anti-hsa-miR-93, and miScript Inhibitor Negative Control (Qiagen) using Lipofectamine® 2000 transfection reagent according to the manufacturer's instructions (Invitrogen).
Electromobility Shift Assay
Synthetic oligonucleotides to the 5′ UTR of EZH2 were labeled by back filling with the Klenow fragment of DNA polymerase I using [α-32P]dCTP (3000 Ci/mmol) (PerkinElmer Life Science). WT1 protein was synthesized using a T7-coupled transcription/translation reticulocyte lysate system. The in vitro translated products were incubated with 32P-labeled probes at room temperature for 15 min in a solution containing 4% Ficoll, 1 mm EDTA (pH 8.0), 10 mm HEPES (pH 7.9), 1 mm dithiothreitol (DTT), and 1 μg of poly(dI)·poly(dC). For specificity tests, the protein preparation was preincubated with 1 μg of anti-WT1 (C-19) antibody for 20 min at room temperature and then incubated with the radiolabeled probe for 20 min at room temperature. For competition experiments, reaction mixtures were preincubated for 15 min at room temperature with unlabeled oligonucleotide prior to the addition of radiolabeled probes. Following the binding step, reaction mixtures were electrophoresed on a 6% polyacrylamide gel (acrylamide/bis-acrylamide ratio, 29:1) in 0.25× TBE buffer (22.25 mm Tris·HCl, 22.25 mm boric acid, and 1 mm EDTA) at 90 V at room temperature. The gels were dried and exposed to Kodak X-Omat film at room temperature.
RNA Immunoprecipitation Assay-qPCR
Cells were grown and harvested using 0.25% trypsin/EDTA (Gibco) following standard techniques and resuspended in RIP buffer (150 mm KCl, 25 mm Tris, pH 7.4, 5 mm EDTA, 0.5 mm DTT, 0.5% Nonidet P-40, 100 units/ml of RNase inhibitor and protease inhibitor). Embryonic mouse kidney at 17 dpc stage were flash frozen and pulverized using standard protocol and resuspended in RIP buffer. Sonication was performed on a Sonolab 7.1 (Covaris, Toronto, ON, Canada). The immunoprecipitation was performed using Protein A/G-agarose beads. The following antibodies and titers were used: normal rabbit IgG (2729, 2 μg, Cell Signaling), WT1 C19 (sc192, 2 μg, Santa Cruz Biotechnology, Inc.), and anti-Ago2/eIF2C2 (ab32381, 2 μg, Abcam). Purification of the RNA bound to the RNA-binding proteins was performed using the miRNeasy mini kit according to the manufacturer's instructions (Qiagen). cDNA synthesis was performed as mentioned before. Primers for the qPCR are miScript Primer Assays Hs_miR-26a_1, Hs_miR-101_3, and Hs_ miR-93 (Qiagen), as well as qPCR primers designed for the detection of the EZH2 gene (EZH2 forward: 5′-TTGTTGGCGGAAGCGTGTAAAATC and EZH2 reverse: 5′-TCCCTAGTCCCGCGCAATGAGC.
Statistical Analysis
Data are presented as mean ± S.E. of three or more independent results. Statistical significance was assessed using t test, one-way or two-way analysis of variance followed by comparison tests.
Results
WT1 Induces an Array of miRNAs in Mesenchymal Stem Cells
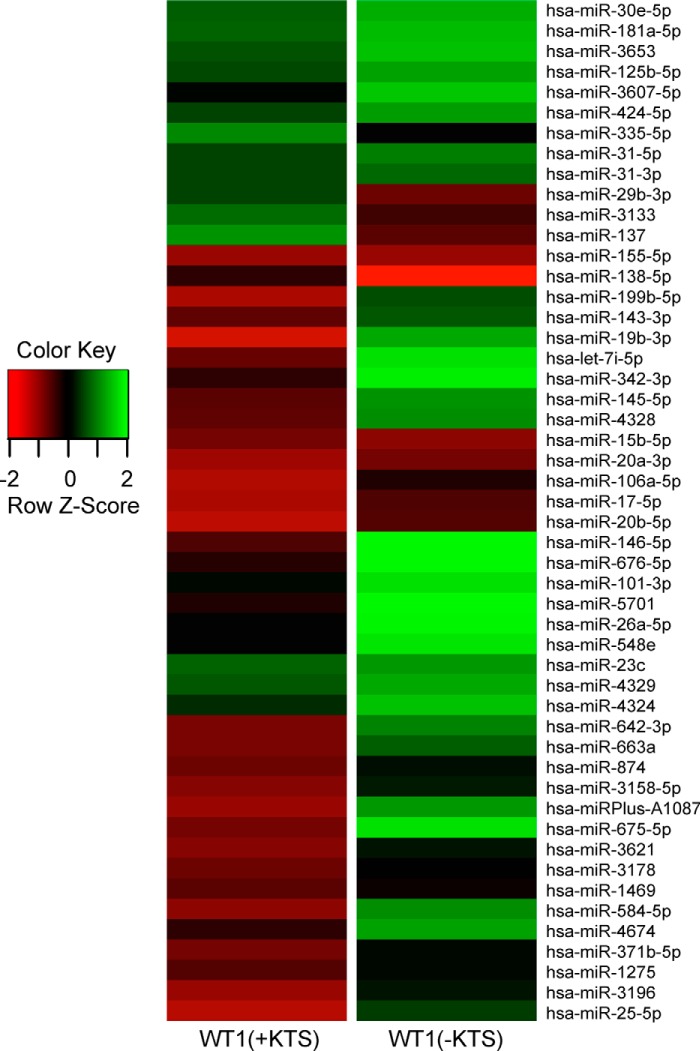
Because mutations of WT1 or DICER1 lead to a similar Wilms tumor phenotype, we reasoned that WT1 might normally induce key miRNAs in metanephric mesenchyme that are essential for differentiation. We isolated RNA from human mesenchymal stem cells (6) stably transduced with either a WT1(−KTS) or a WT1(+KTS) isoform. Fig. 1 shows the miRNA heat map, indicating up-regulation (>2-fold) of 12 miRNAs in the WT1(+KTS)-expressing cell line and28 miRNAs in the WT1(−KTS) cell line, compared with the mean for parental and empty vector-transfected controls. Eight of the WT1(+KTS) miRNAs overlapped with the WT1(−KTS) group, giving a total of 32 miRNAs induced by one isoform or the other. Because our previous studies suggest that down-regulation of EZH2 is a crucial event in de-repressing genes of the differentiation cascade (6), we screened the 3′ UTR of EZH2 for seed sequences for these 32 miRNAs. Interestingly, 16/32 miRNAs are predicted to target members of the Polycomb complex or associated methyltransferases, of these, 12/16 are predicted to target the EZH2 3′ UTR (Table 1). These 12 included miR26a and miR101, previously shown to suppress translation of EZH2 in myocytes, lung tissue, and breast epithelia, respectively (12–15). We decided to focus on these latter two miRNAs as representative of the putative concerted mechanism by which WT1 suppresses epigenetic silencing in stem cells (6). To validate these two miRNAs, we performed quantitative RT-PCR on mesenchymal stem cells stably transfected with WT1(−KTS) or WT1(+KTS) isoforms. As seen in Fig. 2, miR-26a and miR-101 levels were significantly increased in both cell lines expressing WT1.
FIGURE 1.
miRNA expression profile. The heat map shows the differential expression pattern of miRNAs in mesenchymal stem cells stably transfected with WT1 (+KTS) or WT1(−KTS) isoforms relative to the overall mean for both cell lines and an empty vector control. Each row represents a microRNA and its expression level relative to control. Red represents expression below the mean; green represents expression above the mean.
TABLE 1.
Up-regulated miRNAs targeting Polycomb group and associated proteins
16 of 32 WT1-induced miRNAs have putative seed sequences in the 3′ UTR of EZH2 and various Polycomb-associated proteins.
| EZH2 | EED | SUZ12 | DNMT3A | DNMT3B | DNMT1 | JARID | |
|---|---|---|---|---|---|---|---|
| hsa-let-7i-5p | X | ||||||
| hsa-miR-101–3p | X | X | X | X | X | X | |
| hsa-miR-125b-5p | X | X | X | X | X | X | X |
| hsa-miR106a-5p | X | X | X | X | X | X | X |
| hsa-miR-146b-5p | X | X | X | X | X | X | X |
| hsa-miR-17–5p | X | X | X | X | X | X | X |
| hsa-miR-181a-5p | X | X | X | X | X | X | X |
| hsa-miR-19c-3p | X | X | X | X | X | X | X |
| hsa-miR-25–5p | X | X | X | X | X | X | X |
| hsa-miR-26a-5p | X | ||||||
| hsa-miR-29b-3p | X | X | X | X | X | X | X |
| hsa-miR-31–5p | X | X | X | X | X | X | X |
| hsa-miR-335–5p | X | ||||||
| hsa-miR-4328 | X | X | |||||
| hsa-miR-4674 | X | X | |||||
| hsa-miR-584–5p | X | X |
FIGURE 2.
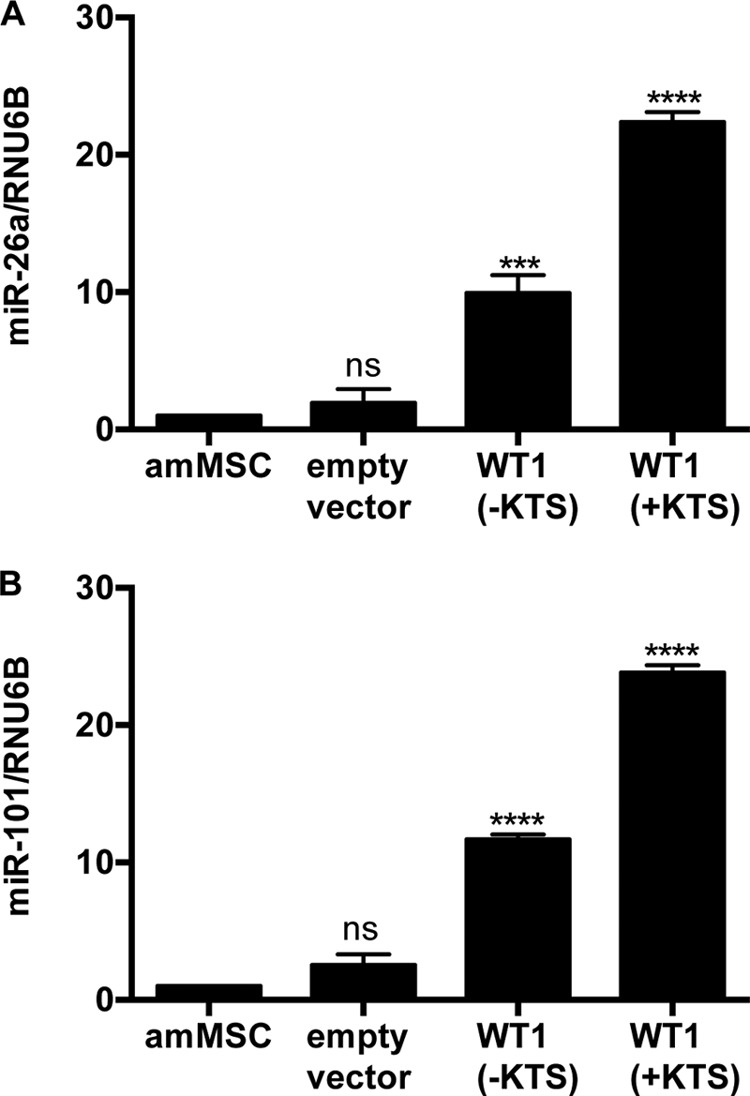
Validation of selected miRNAs. A, miR-26a levels were quantified (n = 3) by qRT-PCR with RNU6B as the reference miRNA transcript in various cell lines. B, miR-101 levels were quantified (n = 3), using RNU6B for normalization. One-way analysis of variance with Dunnett's multiple comparison tests versus parental amMSC line: p < 0.0001 (****), p < 0.001 (***), not significant (ns).
miR-26a and miR-101 Cooperate with WT1 to Suppress EZH2 Translation
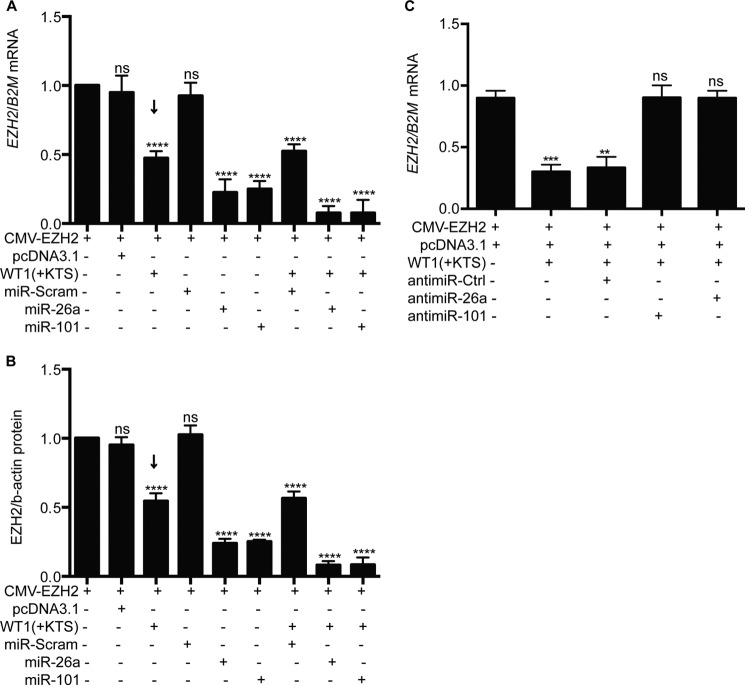
To confirm that miR-26a and miR-101 suppress EZH2 translation, we transfected human fibroblasts with an expression plasmid containing the full-length EZH2 mRNA driven by a CMV promoter; at baseline, fibroblasts had no detectable endogenous EZH2 or WT1. In the transfected cells, we confirmed high levels of exogenous EZH2 transcript (Fig. 3A). When fibroblasts were transfected with varying amounts of miR-26a or miR-101 mimics, we noted maximal suppression of EZH2 with 25 nm miR-26a vector and 20 nm miR-101 (Fig. 3B). In previous studies, we noted that the WT1(+KTS) isoforms suppress EZH2 protein levels in mesenchymal stem cells (6). If this effect is due to transcriptional inhibition, there should be no effect of WT1(+KTS) on the CMV-driven EZH2 expression in fibroblasts. However, when we transiently transfected fibroblasts with WT1(+KTS), we noted a 50% reduction (arrows) in exogenous EZH2 mRNA and protein levels (Fig. 4, A and B). When WT1(+KTS) was combined with miR-26a or miR-101 mimics (25 nm), EZH2 expression was further suppressed to 10–15% of baseline (Fig. 4, A and B).
FIGURE 3.
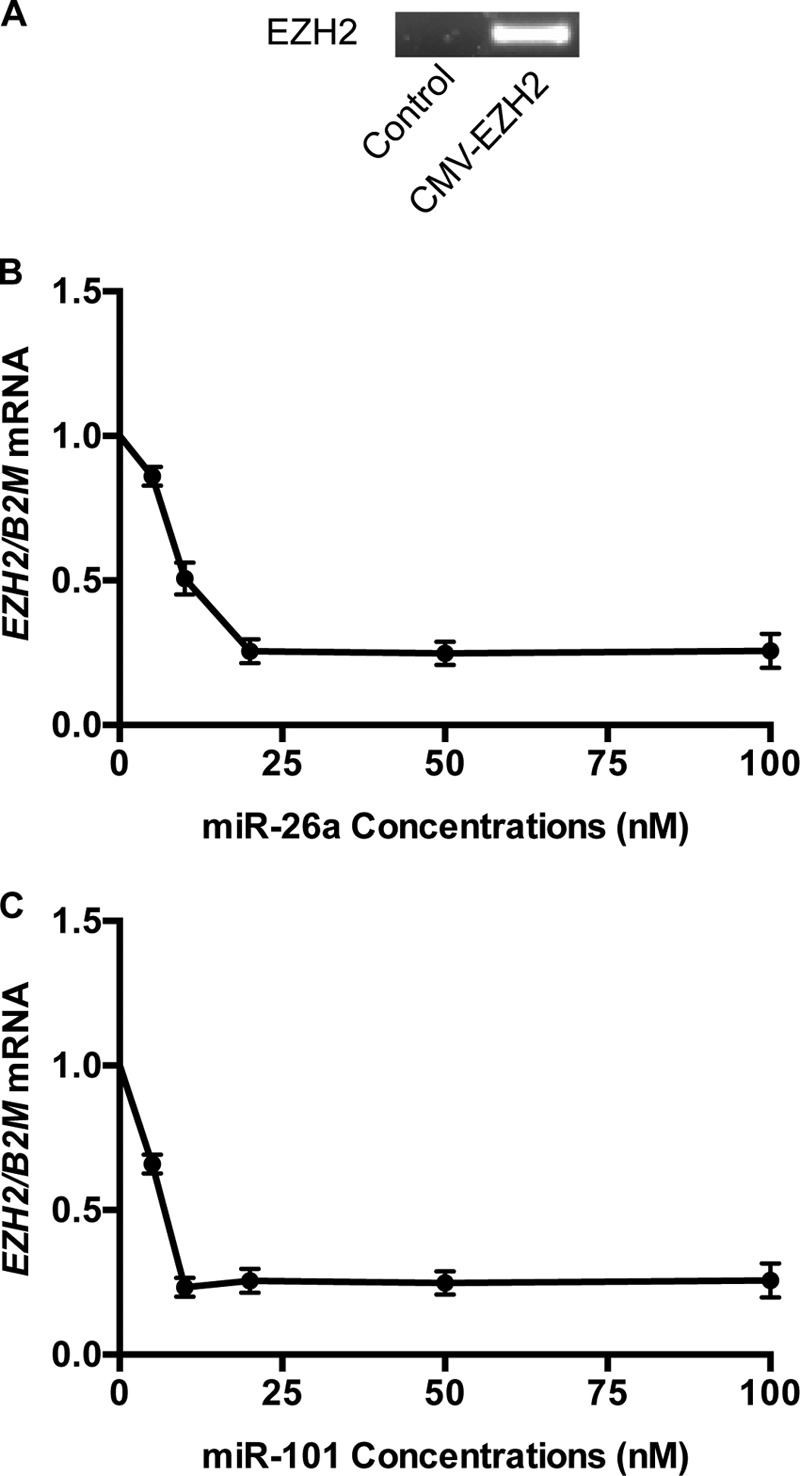
Effect of different miRNA concentrations on exogenous EZH2. A, absence of endogenous EZH2 transcript in control human fibroblasts versus fibroblasts transfected with CMV-EZH2 expression plasmid. B, dose-response curve showing the effect of various concentrations of miR-26a on the expression of exogenous EZH2 mRNA. The minimal dose with the maximum effect is 25 nm (n = 4). C, dose-response curve showing the effect of various concentrations of miR-101 on the expression of exogenous EZH2. The minimal dose with the maximum effect is 20 nm (n = 4).
FIGURE 4.
WT1 cooperates with miRNAs to inhibit EZH2 expression. A, levels of EZH2 mRNA were quantified by qRT-PCR (n = 4) and normalized to β2-microglobulin (B2M) in human fibroblasts transiently transfected with EZH2, WT1, miR-26a, miR-101, or their control plasmids. B, levels of EZH2 protein were quantified by densitometric analysis of Western immunoblots normalized for β-actin (n = 4) in human fibroblasts transiently transfected as above. C, levels of EZH2 mRNA were quantified by qRT-PCR (n = 4) and normalized to B2M in human fibroblasts transiently transfected with EZH2, WT1, anti-miR-26a, anti-miR-101, or their control plasmids. One-way analysis of variance with Dunnett's multiple comparison tests for each experimental cell line versus baseline cells transfected with EZH2 only: p < 0.0001 (****), p < 0.001 (***), not significant (ns).
To confirm that the effect of WT1(+KTS) on the exogenous EZH2 transcript is mediated by endogenous miRNAs, we preformed rescue experiments with antago-miRs specific for miR-101 and miR-26a. As seen in Fig. 4C, addition of either antago-miR blocks the effect of WT1 on the EZH2 transcript level.
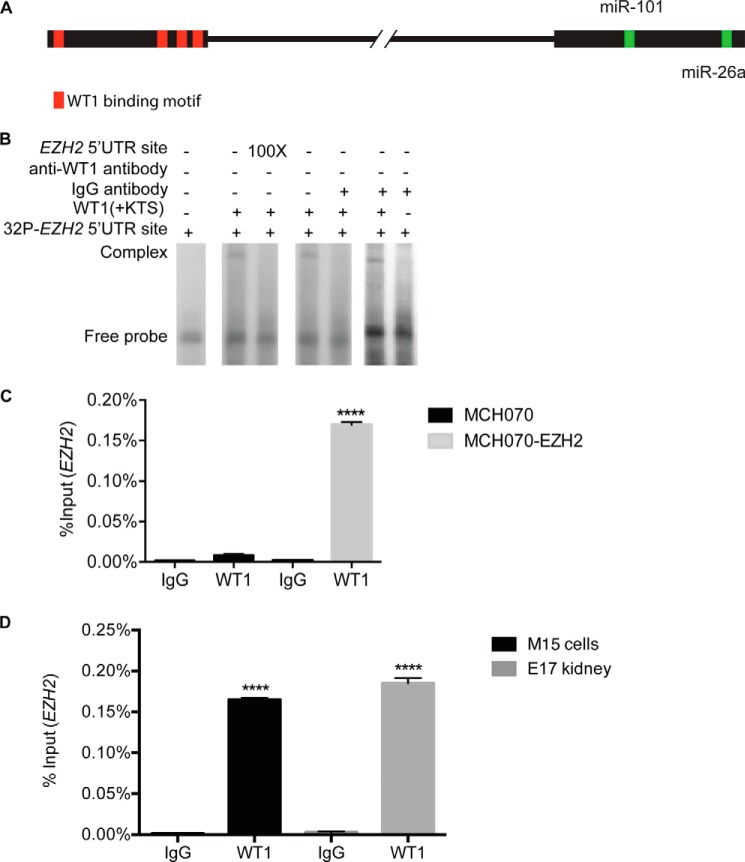
WT1(+KTS) Binds to a Recognition Motif in the EZH2 5′ UTR
Although it is known that the WT1(−KTS) isoforms regulate transcription of target genes in the nucleus, other investigators have noted that the WT1(+KTS) isoform can interact with RNA in the cytoplasm (16–22). This suggests that WT1(+KTS) may participate in the post-transcriptional regulation of targets such as EZH2. We screened the full-length EZH2 transcript for the consensus WT1 recognition motif (23) using MatInspector software. This identified a classical motif, located 5 base pairs upstream of the ATG translational start codon in the EZH2 5′ UTR (Fig. 5A). To confirm binding to this site, we incubated in vitro-translated WT1(+KTS) protein with a radiolabeled oligomer corresponding to the sequence of the 5′ UTR containing the putative recognition motif. In Fig. 5B, a high molecular weight complex is evident; interaction between the WT1(+KTS) protein and oligomer can be competed out with excess cold probe or blocked with WT1-specific antibody (Fig. 5B). To confirm that WT1 binds to EZH2 mRNA in whole cells, we extracted RNA from fibroblasts transfected with a CMV-EZH2 plasmid and incubated this with in vitro-translated WT1(+KTS). Following immunoprecipitation with anti-WT1 antibody, we quantified (qRT-PCR) EZH2 mRNA associated with the WT1 protein. As seen in Fig. 5C, the EZH2 transcript was detected in the anti-WT1 precipitate. To confirm that WT1 binds to the endogenous EZH2 transcript, we performed RNA-IP experiments followed by qRT/PCR. As seen in Fig. 5D, anti-WT1 antibody immunoprecipitates EZH2 mRNA in embryonic day E17 mouse kidneys and in a mouse mesonephric kidney cell line (M15) (11).
FIGURE 5.
WT1 binds the 5′ UTR of EZH2. A, line drawing of the human EZH2 gene sequence showing four putative WT1 recognition motifs (GXGXGGXG) in the 5′ UTR (red boxes), and the 8-nucleotide seed sequences for miR-26a (UACUUGAA) and miR-101 (GUACUGUA) in the 3′ UTR (green boxes). B, electromobility shift assay showing a high molecular weight complex containing the 5′ UTR site (18-mer) bound to in vitro translated WT1(+KTS) protein (lanes 2 and 4). This complex disappears in the presence of excess cold probe (lane 3) or specific antibody against WT1 (lane 5). The high molecular weight complex formed remains intact with IgG antibody (lane 6). C, mRNA associated with exogenous WT1 protein was immunoprecipitated from fibroblasts transfected with EZH2 plasmid (RIP) and EZH2 RNA levels were quantified by qRT-PCR; WT1-associated EZH2 mRNA levels are expressed as percent of total mRNA input. D, endogenous EZH2 mRNA was immunoprecipitated with anti-WT1 antibody from a mouse mesonephric kidney cell line (M15 cells, black bars) and from mouse embryonic E17 kidneys (gray bars) and quantified by qRT-PCR. Unpaired t test, p < 0.0001 (****).
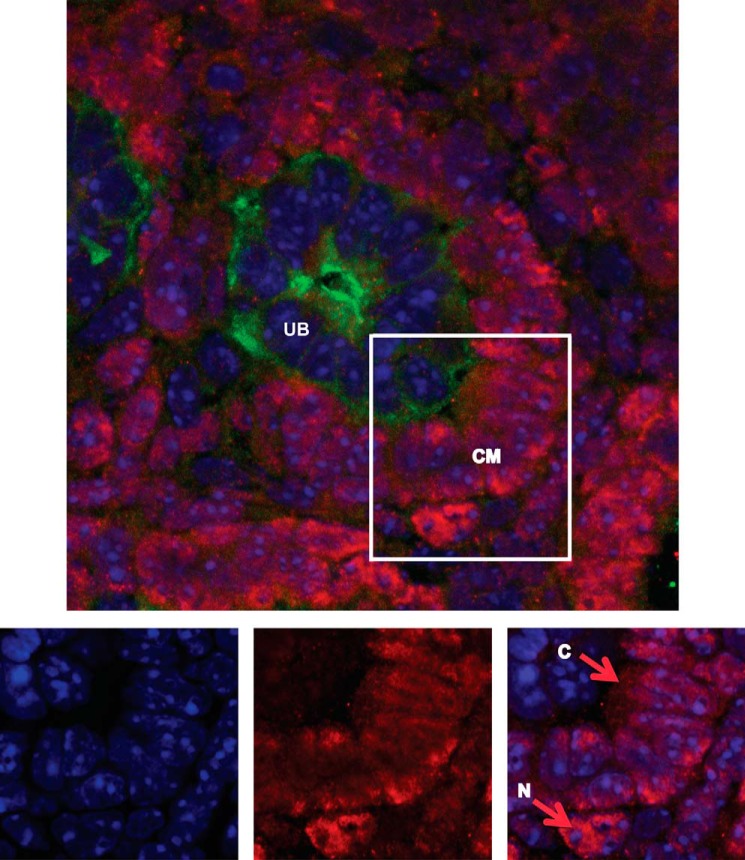
Cytoplasmic WT1 Protein Is Detected in Progenitor Cells of Mouse Embryonic Kidney Cap Mesenchyme
If WT1(+KTS) interacts with cytoplasmic EZH2 mRNA during normal kidney development, it should be detectable in the cytoplasm of progenitor cells in the cap mesenchyme of embryonic kidney. To confirm this, we performed immunofluorescent studies of embryonic day E17.5 mouse kidneys. WT1 protein was detected in both the nuclear and cytoplasmic compartments of cells in the cap mesenchyme (Fig. 6).
FIGURE 6.
Localization of WT1 within cap mesenchyme cells in embryonic mouse kidney. Representative immunofluorescent image of embryonic mouse kidney at 17.5 dpc stained for WT1 (red), D. biflorus agglutinin (DBA) (green), and DAPI (blue). WT1 protein is seen in crescent of cells forming the cap mesenchyme (CM) around the ureteric bud (UB) branch tips stained with DBA (top). The magnified CM region (lower panels) shows nuclear DAPI staining (bottom left) and WT1 staining (bottom middle). The merged image shows both cytoplasmic (C) and nuclear (N) staining for WT1 (bottom right), emphasized by the arrows.
WT1 Directly Interacts with miR-26a- and miR-101-containing RISC
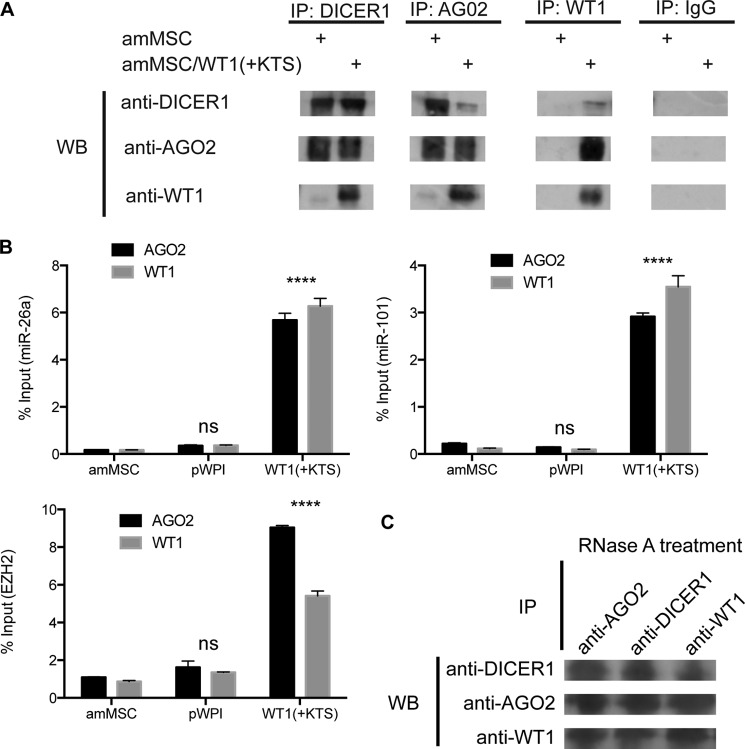
In Fig. 4, we noted that WT1(+KTS) and either miR26a or miR101 had a stronger inhibitory effect on fibroblast CMV-driven EZH2 expression than either molecule alone. We considered the possibility that this might involve direct interactions between WT1 at the EZH2 5′ UTR and the microRNA-containing RISC at the 3′ UTR. We lysed WT1(+KTS)-expressing amMSCs (or controls) and performed immunoprecipitation assays using antibodies specific for WT1, AGO2, DICER1, or IgG control (Fig. 7A). WT1 was precipitated with each member of the RISC. Conversely, when AGO2 or DICER1 were immunoprecipitated from the lysate, we were able to detect WT1 protein (Fig. 7A). To confirm that WT1 associates with the miRNA-containing RISC, we immunoprecipitated WT1 or AGO2 from cell lysates and screened for the presence of miR-26a, miR-101, and the EZH2 transcript by qRT-PCR. As seen in Fig. 7B, both miR-26a and miR-101 (but not miR-93, an unrelated miRNA lacking seed sequences in the transcript, not shown) were detectable in the WT1 and AGO2 precipitates. Similarly, we detected the EZH2 transcript by qRT-PCR in both anti-WT1 and anti-AGO2 precipitates from mesenchymal stem cells transfected with WT1(+KTS). To confirm that the association of these molecules was not due to their common interaction with EZH2 mRNA, we treated the cell lysates with RNase A after immunoprecipitating with anti-DICER1, WT1, or AGO2 antibodies. Although full-length EZH2 mRNA was no longer detectable by RT-PCR, we were still able to detect WT1 in association with each of its putative RISC partners (Fig. 7C).
FIGURE 7.
Interactions of WT1, miRNAs, and RISC components. A, representative Western blots (WB) probed with DICER, AGO2, or WT1 antibodies (left) of immunoprecipitates from amMSC versus amMSC/WT1(+KTS) using antibodies against DICER, AGO2, WT1, or control IgG (top). B, levels of EZH2 mRNA, miR-26a, and miR-101 were quantified by RIP-qPCR in amMSC expressing WT1(+KTS) (right bars) versus amMSC with empty vector (middle bars) or uninfected parental amMSC (left bars). Bar graphs show levels of miR26a (left upper), miR101 (right upper), and EZH2 (left lower) as percent of total RNA input. Two-way analysis of variance with Tukey's multiple comparison tests were used to compare WT1(+KTS) amMSC with empty vector control amMSC; p < 0.0001 (****), not significant (ns). C, representative Western blot probed with DICER1, AGO2, or WT1 antibodies (left) of RNase A-treated immunoprecipitates (top) from amMSC/WT1(+KTS) cells.
Discussion
Mammalian kidneys arise from stem cells of the intermediate mesoderm. A subset of these cells begin to express WT1 and then acquire properties of nephron progenitors capable of responding to a canonical WNT signal; another subset of WT1(−) cells form the epithelial nephric duct and its caudal offshoot, the ureteric bud. As the ureteric bud begins to arborize, nearby nephron progenitors form a cap of tightly clustered cells around the tip of each ureteric bud branch these cells express WT1. As we show here, WT1 can be visualized both in the nucleus and cytoplasm of nephron progenitors in cap mesenchyme. In response to WNT9b released by the ureteric bud, nephron progenitors undergo a dramatic mesenchyme-to-epithelium transition and proceed to form all segments of the nephron.
In the nucleus of renal progenitors, we previously found that the WT1(−KTS) isoform suppresses transcription of a Polycomb histone methyltransferase, EZH2 (6). This event is likely to be essential if the differentiation cascade is to be released by inductive WNT signals from ureteric bud. Here we show that transcriptional inhibition of EZH2 is paralleled by translational inhibition in the cytoplasm by the WT1(+KTS) isoform. WT1 was co-immunoprecipitated with endogenous EZH2 mRNA from embryonic kidney and a mesonephric cell line.
These findings support the observations of Hastie and co-workers (24), identifying a specific role for the WT1(+KTS) isoform in regulating RNA. We identified four classical WT1 recognition motifs in the 5′ untranslated end of the EZH2 transcript and studied one of these in detail, showing that it binds WT1(+KTS) protein with high affinity. All four putative WT1 binding sites are positioned amid sequences recognizing proteins involved in mRNA translation. Thus WT1(+KTS) binding to the EZH2 5′ UTR is likely to disturb the translation initiation complex assembly. Exogenous EZH2 expression from a CMV-driven plasmid was suppressed in vitro by WT1(+KTS).
Translation of EZH2 is also known to be controlled by specific microRNAs directed to seed sequences in its 3′ UTR. Two such miRNAs (miR-101 and miR-26a) suppress EZH2 translation in other cellular settings (myoblasts and cancer cells) (12–14). Interestingly, we observed that both major WT1 isoforms induce expression (>2fold) of an overlapping array of 32miRNAs in mesenchymal stem cells. These included miR-101 and miR-26a. About half of the 32 miRNAs have putative recognition motifs in the 5′ end of many other Polycomb protein transcripts. This suggests a concerted inhibitory mechanism by which WT1 isoforms broadly suppress expression of many Polycomb complex proteins that epigenetically silence differentiation cascade genes.
In this study, we show that both WT1(+KTS) and exogenous miRNAs inhibit EZH2 in human fibroblasts. Remarkably, we found that addition of miRNA antagonists completely block the effects of WT1. Thus, cytoplasmic WT1(+KTS) bound to the 5′ UTR appears to cooperate with endogenous RISC-associated miRNAs bound at the 3′ UTR to disrupt translation.
miRNAs are a class of single-stranded non-coding RNA molecules containing 22 nucleotides (25, 26). Primary miRNAs (pri-miRNA) are transcribed in the nucleus by RNA polymerase II and processed by the DROSHA-DGCR8 complex into a stem-loop structure called the precursor miRNA (pre-miRNA) (27). Exportin-5 (XPO-5) transports the pre-miRNA out of the nucleus (28). In the cytoplasm, DICER1 processes the RNA molecule into the miRNA duplex (29). The strands are separated, the mature miRNA is recognized and bound by an AGO protein at the 3′ end and is integrated into the RNA-induced silencing complex (30). Through complementarity, the miRNA binds the 3′ UTR of the target messenger RNA (mRNA) (31). Perfect base pair match leads to degradation of the mRNA, whereas imperfect pairing will lead to sequestration of the mRNA and inhibition of translation (32). miRNAs play a crucial role in the regulation of mammalian development, their expression is tissue-specific, and tightly regulated spatial-temporally (33, 34).
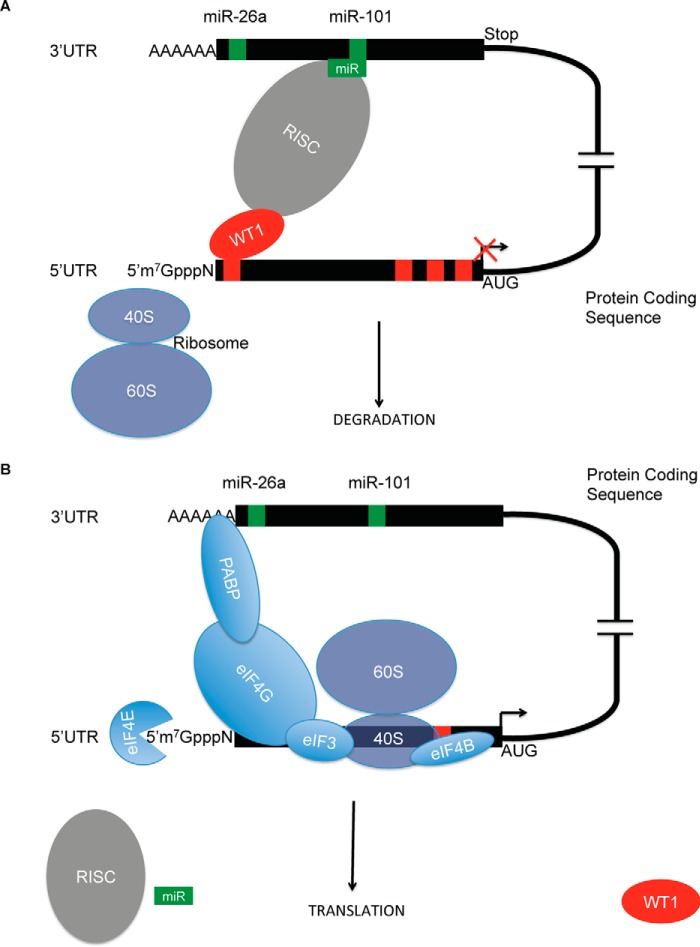
Our immunoprecipitation studies indicate direct interaction between the WT1(+KTS) protein and DICER1/DROSHA (members of the RISC complex that mediates post-transcriptional mRNA decay and mRNA translation). Furthermore, immunoprecipitates containing WT1 and DICER/DROSHA also contained miR26a and miR101. Association between WT1 and the RISC was unaffected by prior experimental RNase degradation of the EZH2 transcript. These observations prompt a model, shown as a schematic in Fig. 8, in which WT1(+KTS) binds to motifs in the EZH2 transcript 5′ UTR, which displace translation factors and inhibit translation; WT1 also interacts with RISC proteins targeted to the EZH2 3′ UTR by specific miRNAs and enhances their effects on transcript stability and protein translation.
FIGURE 8.
Schematic model of WT1 post-transcriptional regulation of EZH2. A, WT1(+KTS) (red) binds the 5′ UTR of the EZH2 transcript and the miRNA(green)-containing RISC (gray) binds to the 3′ UTR seed sequence (green) to block access of the translation machinery. B, in the absence of WT1(+KTS) and miRNA-containing RISC, the 40S ribosome and translation complexes bind to the EZH2 transcript 5′ UTR and promote protein translation.
In hereditary forms of Wilms tumor, biallelic loss of WT1 is thought to preclude the WNT response to WNT signals, resulting in a genetically unstable, developmentally arrested clone of progenitor cells; a second genetic “hit” then drives malignant transformation. Our observations demonstrate that the role of WT1 in de-repressing stem cell genes are, in part, dependent on specific microRNAs. We propose that this may explain why Wilms tumors may also arise from mutation of genes in miRNA processing.
Author Contributions
M. M. A. participated in experimental design, performed all in vitro experiments, analyzed the results, and participated in the preparation of the manuscript. L. L. C. performed the EMSA experiments. A. T. assisted with the qPCR validation. I. J. and L. H. assisted with the microRNA microarray analysis. E. T. provided WT1 immunofluorescent images in embryonic kidney. P. G. participated in experimental design, data analysis, and manuscript preparation. D. I. performed some of the experimentation, contributed to the design and analysis of other experiments, contributed to writing of the manuscript, and was co-supervisor for M. A. (first author, graduate student).
This work was supported in part by Operating Grant MOP-119571 from the Canadian Institutes of Health Research (CIHR) and an infrastructure support grant to the McGill University Health Center Research Institute from the Fonds de la Recherche en Santé du Québec (FRSQ). The authors declare that they have no conflicts of interest with the contents of this article.
- WT
- Wilms tumor
- KTS
- lysine-threonine-serine
- miRNA
- microRNA
- mir-26a
- microRNA-26a
- miR-101
- microRNA-101
- RISC
- RNA-induced silencing complex
- amMSC
- amniotic fluid-derived mesenchymal stem cell
- EZH2
- Enhancer of Zeste Homolog 2
- qPCR
- quantitative PCR.
References
- 1. Rivera M. N., and Haber D. A. (2005) Wilms' tumour: connecting tumorigenesis and organ development in the kidney. Nat. Rev. Cancer 5, 699–712 [DOI] [PubMed] [Google Scholar]
- 2. Call K. M., Glaser T., Ito C. Y., Buckler A. J., Pelletier J., Haber D. A., Rose E. A., Kral A., Yeger H., and Lewis W. H. (1990) Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms' tumor locus. Cell 60, 509–520 [DOI] [PubMed] [Google Scholar]
- 3. Fukuzawa R., Heathcott R. W., More H. E., and Reeve A. E. (2007) Sequential WT1 and CTNNB1 mutations and alterations of β-catenin localisation in intralobar nephrogenic rests and associated Wilms tumours: two case studies. J. Clin. Pathol. 60, 1013–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zirn B., Samans B., Wittmann S., Pietsch T., Leuschner I., Graf N., and Gessler M. (2006) Target genes of the WNT/β-catenin pathway in Wilms tumors. Genes Chromosomes Cancer 45, 565–574 [DOI] [PubMed] [Google Scholar]
- 5. Aiden A. P., Rivera M. N., Rheinbay E., Ku M., Coffman E. J., Truong T. T., Vargas S. O., Lander E. S., Haber D. A., and Bernstein B. E. (2010) Wilms tumor chromatin profiles highlight stem cell properties and a renal developmental network. Cell Stem Cell 6, 591–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Akpa M. M., Iglesias D. M., Chu L. L., Cybulsky M., Bravi C., and Goodyer P. R. (2015) Wilms tumor suppressor, WT1, suppresses epigenetic silencing of the β-catenin gene. J. Biol. Chem. 290, 2279–2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruteshouser E. C., Robinson S. M., and Huff V. (2008) Wilms tumor genetics: mutations in WT1, WTX, and CTNNB1 account for only about one-third of tumors. Genes Chromosomes Cancer 47, 461–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huff V., Miwa H., Haber D. A., Call K. M., Housman D., Strong L. C., and Saunders G. F. (1991) Evidence for WT1 as a Wilms tumor (WT) gene: intragenic germinal deletion in bilateral WT. Am. J. Hum. Genet. 48, 997–1003 [PMC free article] [PubMed] [Google Scholar]
- 9. Wu M. K., Sabbaghian N., Xu B., Addidou-Kalucki S., Bernard C., Zou D., Reeve A. E., Eccles M. R., Cole C., Choong C. S., Charles A., Tan T. Y., Iglesias D. M., Goodyer P. R., and Foulkes W. D. (2013) Biallelic DICER1 mutations occur in Wilms tumours. J. Pathol. 230, 154–164 [DOI] [PubMed] [Google Scholar]
- 10. Torrezan G. T., Ferreira E. N., Nakahata A. M., Barros B. D., Castro M. T., Correa B. R., Krepischi A. C., Olivieri E. H., Cunha I. W., Tabori U., Grundy P. E., Costa C. M., de Camargo B., Galante P. A., and Carraro D. M. (2014) Recurrent somatic mutation in DROSHA induces microRNA profile changes in Wilms tumour. Nat. Commun. 5, 4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Larsson S. H., Charlieu J. P., Miyagawa K., Engelkamp D., Rassoulzadegan M., Ross A., Cuzin F., van Heyningen V., and Hastie N. D. (1995) Subnuclear localization of WT1 in splicing or transcription factor domains is regulated by alternative splicing. Cell 81, 391–401 [DOI] [PubMed] [Google Scholar]
- 12. Wong C. F., and Tellam R. L. (2008) MicroRNA-26a targets the histone methyltransferase Enhancer of Zeste homolog 2 during myogenesis. J. Biol. Chem. 283, 9836–9843 [DOI] [PubMed] [Google Scholar]
- 13. Friedman J. M., Jones P. A., and Liang G. (2009) The tumor suppressor microRNA-101 becomes an epigenetic player by targeting the polycomb group protein EZH2 in cancer. Cell Cycle 8, 2313–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Friedman J. M., Liang G., Liu C. C., Wolff E. M., Tsai Y. C., Ye W., Zhou X., and Jones P. A. (2009) The putative tumor suppressor microRNA-101 modulates the cancer epigenome by repressing the polycomb group protein EZH2. Cancer Res. 69, 2623–2629 [DOI] [PubMed] [Google Scholar]
- 15. Varambally S., Cao Q., Mani R. S., Shankar S., Wang X., Ateeq B., Laxman B., Cao X., Jing X., Ramnarayanan K., Brenner J. C., Yu J., Kim J. H., Han B., Tan P., Kumar-Sinha C., Lonigro R. J., Palanisamy N., Maher C. A., and Chinnaiyan A. M. (2008) Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science 322, 1695–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Charlieu J. P., Larsson S., Miyagawa K., van Heyningen V., and Hastie N. D. (1995) Does the Wilms' tumour suppressor gene, WT1, play roles in both splicing and transcription? J. Cell Sci. Suppl. 19, 95–99 [DOI] [PubMed] [Google Scholar]
- 17. Niksic M., Slight J., Sanford J. R., Caceres J. F., and Hastie N. D. (2004) The Wilms' tumour protein (WT1) shuttles between nucleus and cytoplasm and is present in functional polysomes. Hum. Mol. Genet. 13, 463–471 [DOI] [PubMed] [Google Scholar]
- 18. Caricasole A., Duarte A., Larsson S. H., Hastie N. D., Little M., Holmes G., Todorov I., and Ward A. (1996) RNA binding by the Wilms tumor suppressor zinc finger proteins. Proc. Natl. Acad. Sci. U.S.A. 93, 7562–7566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhai G., Iskandar M., Barilla K., and Romaniuk P. J. (2001) Characterization of RNA aptamer binding by the Wilms' tumor suppressor protein WT1. Biochemistry 40, 2032–2040 [DOI] [PubMed] [Google Scholar]
- 20. Morrison A. A., Viney R. L., Saleem M. A., and Ladomery M. R. (2008) New insights into the function of the Wilms tumor suppressor gene WT1 in podocytes. Am. J. Physiol. Renal Physiol. 295, F12–17 [DOI] [PubMed] [Google Scholar]
- 21. Morrison A. A., Viney R. L., and Ladomery M. R. (2008) The post-transcriptional roles of WT1, a multifunctional zinc-finger protein. Biochim. Biophys. Acta 1785, 55–62 [DOI] [PubMed] [Google Scholar]
- 22. Morrison A. A., Venables J. P., Dellaire G., and Ladomery M. R. (2006) The Wilms tumour suppressor protein WT1 (+KTS isoform) binds α-actinin 1 mRNA via its zinc-finger domain. Biochem. Cell Biol. 84, 789–798 [DOI] [PubMed] [Google Scholar]
- 23. Nakagama H., Heinrich G., Pelletier J., and Housman D. E. (1995) Sequence and structural requirements for high-affinity DNA binding by the WT1 gene product. Mol. Cell. Biol. 15, 1489–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davies R., Moore A., Schedl A., Bratt E., Miyahawa K., Ladomery M., Miles C., Menke A., van Heyningen V., and Hastie N. (1999) Multiple roles for the Wilms' tumor suppressor, WT1. Cancer Res. 59, 1747–1750 [PubMed] [Google Scholar]
- 25. Krol J., and Krzyzosiak W. J. (2004) Structural aspects of microRNA biogenesis. IUBMB Life 56, 95–100 [DOI] [PubMed] [Google Scholar]
- 26. Winter J., Jung S., Keller S., Gregory R. I., and Diederichs S. (2009) Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat. Cell Biol. 11, 228–234 [DOI] [PubMed] [Google Scholar]
- 27. Lee Y., Ahn C., Han J., Choi H., Kim J., Yim J., Lee J., Provost P., Rådmark O., Kim S., and Kim V. N. (2003) The nuclear RNase III Drosha initiates microRNA processing. Nature 425, 415–419 [DOI] [PubMed] [Google Scholar]
- 28. Lee Y., Kim M., Han J., Yeom K. H., Lee S., Baek S. H., and Kim V. N. (2004) MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 23, 4051–4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim V. N., Han J., and Siomi M. C. (2009) Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 10, 126–139 [DOI] [PubMed] [Google Scholar]
- 30. Elbashir S. M., Lendeckel W., and Tuschl T. (2001) RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 15, 188–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gagnon K. T., and Corey D. R. (2012) Argonaute and the nuclear RNAs: new pathways for RNA-mediated control of gene expression. Nucleic Acid Ther. 22, 3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nelson P., Kiriakidou M., Sharma A., Maniataki E., and Mourelatos Z. (2003) The microRNA world: small is mighty. Trends Biochem. Sci. 28, 534–540 [DOI] [PubMed] [Google Scholar]
- 33. Zhao Y., and Srivastava D. (2007) A developmental view of microRNA function. Trends Biochem. Sci. 32, 189–197 [DOI] [PubMed] [Google Scholar]
- 34. O'Rourke J. R., Swanson M. S., and Harfe B. D. (2006) MicroRNAs in mammalian development and tumorigenesis. Birth Defects Res. C Embryo Today 78, 172–179 [DOI] [PubMed] [Google Scholar]
- 35. Cone T. E., Jr. (1970) Sir William writes about massive abdominal tumors in children. Pediatrics 46, 587. [PubMed] [Google Scholar]