Abstract
Protein modification by small ubiquitin-related modifiers (SUMOs) is essential and conserved in the malaria parasite, Plasmodium falciparum. We have previously shown that interactions between the SUMO E1-activating and E2-conjugating enzyme in P. falciparum are distinct compared with human, suggesting a potential target for development of parasite-specific inhibitors of SUMOylation. The parasite asexual trophozoite stage is susceptible to iron-induced oxidative stress and is subsequently a target for many of the current anti-malarial drugs. Here, we provide evidence that SUMOylation plays a role in the parasite response to oxidative stress during red blood cell stages, indicative of a protective role seen in other organisms. Using x-ray crystallography, we solved the structure of the human SUMO E1 ubiquitin fold domain in complex with the E2, Ubc9. The interface defined in this structure guided in silico modeling, mutagenesis, and in vitro biochemical studies of the P. falciparum SUMO E1 and E2 enzymes, resulting in the identification of surface residues that explain species-specific interactions. Our findings suggest that parasite-specific inhibitors of SUMOylation could be developed and used in combination therapies with drugs that induce oxidative stress.
Keywords: crystallography, malaria, oxidative stress, plasmodium, small ubiquitin-like modifier (SUMO), ubiquitin-conjugating enzyme (E2 enzyme), ubiquitin fold domain (Ufd)
Introduction
Malaria is a mosquito-borne infectious disease caused by protozoan parasites of the genus, Plasmodium. There are five species of Plasmodium that infect humans, with Plasmodium falciparum being the most deadly. During 2013, there were 200 million cases of malaria worldwide, leading to >500,000 deaths (1). Because of the complex nature of malaria infection, control measures require integrated approaches that span vector control, vaccine development, and anti-malarial drug treatments. Current drug therapy regimens rely on artemisinin-based combination therapy; however, artemisinin resistance has begun to spread throughout southeast Asia (1). The development of resistance to first line anti-malarial drugs has accelerated the need for new drugs with novel targets for effective malaria treatment.
The life cycle of the Plasmodium parasite requires infection of an Anopheles mosquito and subsequent transmission to a human host (2). Infected mosquitoes inject motile sporozoites that travel to the liver through the bloodstream, where they proliferate in hepatocytes. After multiple rounds of syncytial division, or schizogony, merozoites are released into the bloodstream where they invade erythrocytes and undergo asexual replication. The intraerythrocyte developmental cycle consists of three distinct stages: single invasion of a red blood cell producing a ring stage parasite, a trophozoite stage characterized by host hemoglobin digestion, and successive rounds of mitosis to produce a multinucleated schizont. Ruptured erythrocytes release merozoites for subsequent invasion into new red blood cells. Sexual gametes are also produced during the intraerythrocyte developmental cycle and are taken up by mosquitoes during a blood meal, completing the life cycle of the parasite in the mosquito midgut.
The complex life cycle of Plasmodium requires tight regulation to coordinate the stage specific functions and remodeling that must occur to maintain infectivity. Post-translational modifications (PTMs)2 provide one method for spatial and temporal control of cellular activities. Several PTMs have been described in Plasmodium, including: SUMOylation, ubiquitylation, neddylation, phosphorylation, acetylation, nitrosylation, lipidation, and methylation (3–6). Because of the important role of PTMs in cell division, regulating protein localization and activity, as well as protein-protein interactions, several efforts have focused on disrupting these pathways (7). The reversible covalent attachment to proteins mediated by these PTMs requires the action of specific conjugation and deconjugation machinery. Because these pathways are essential and functionally conserved in the parasite, thorough characterization must be performed to determine differences between Plasmodium and human proteins that could be targeted for parasite-specific inhibition.
Protein modification by small ubiquitin-like modifiers (SUMOs) is essential for normal cellular function, including stress response, transcription, cell division, DNA replication and repair, and ion transport and utilizes a conserved cascade of enzymes (8). There are four SUMO paralogs in humans, whereas P. falciparum encodes only one. SUMO is expressed as an inactive precursor that is processed by a SUMO-specific protease, revealing a C-terminal diglycine motif. A heterodimeric E1-activating enzyme (Aos1/Uba2) adenylates the C terminus of mature SUMO and then transfers SUMO to the catalytic cysteine of Uba2, forming a covalent thioester linkage. The sole SUMO E2-conjugating enzyme Ubc9 interacts with the ubiquitin fold domain (Ufd) of the E1 Uba2 subunit to promote transfer of SUMO and formation of a thioester linkage with the E2 catalytic cysteine. Modification of target proteins occurs upon Ubc9 recognition of a consensus motif, ΨKXE, where Ψ is a hydrophobic residue, K is the acceptor lysine, and X is any amino acid, found on substrates. The covalent attachment to the ϵ-amino group of a substrate lysine residue can be mediated in either an E3 ligase-independent or -dependent manner. The removal of SUMO is catalyzed by SUMO specific isopeptidases, leading to a dynamic balance of SUMO conjugation and deconjugation.
Protective roles for SUMOylation in the oxidative stress response have been described previously in organisms from yeast to human, but whether it plays a role during the parasite stress response remains largely uncharacterized (9–11). We previously showed that SUMOylation levels peak during the trophozoite stage of the intraerythrocyte developmental cycle, which is characterized by enhanced oxidative stress caused by parasite digestion of host hemoglobin (12). Notably, anti-malarials, such as chloroquine and artemisinin, are thought to kill parasites at least in part by overloading oxidative stress response pathways (13, 14). Here, we determine that SUMOylation is modulated in response to levels of oxidative stress, providing further evidence for a role in the parasite stress response. The development of parasite-specific SUMOylation inhibitors could provide a novel synergistic therapy, as resistance to the first line anti-malarial, artemisinin, begins to spread.
The SUMO conjugation machinery is conserved in P. falciparum; however, we have previously determined that the surface residues important for interactions between the E1-activating and E2-conjugating enzymes have diverged significantly from humans (12). Analysis of human and P. falciparum Ubc9 chimeric proteins revealed that residues comprising the α1 helix and β1-β2 loop are critical for functional and also species-specific interactions with cognate E1-activating enzymes. These residues are predicted to make direct contacts with the Uba2 Ufd domain. However, the specific contribution that individual residues within the α1 helix and β1-β2 loop of Ubc9 play in E1 binding remained unknown. To more fully define the molecular basis for species-specific E1-E2 interactions, we solved the structure of the human Uba2Ufd-Ubc9 complex. We also used molecular modeling to guide mutagenesis of the predicted Plasmodium Uba2Ufd-Ubc9 interaction surface and analyzed effects of specific mutations on binding and conjugation in vitro. Our findings identified residues involved in Uba2Ufd-Ubc9 binding that underlie parasite-specific E1-E2 interactions and that could aid in develop of parasite-specific inhibitors of SUMOylation.
Experimental Procedures
P. falciparum Cell Culture and Synchronization
Parasites were cultured using a modified standard procedure in human O+ erythrocytes at 2% hematocrit in 3% CO2, 3% O2, 94% N2 atmosphere at 37 °C (15). Complete malaria culture media consisted of RPMI 1640 (Invitrogen) supplemented with 25 mm HEPES, 0.2% sodium bicarbonate (Invitrogen), 12.5 μg/ml hypoxanthine (Sigma), and 5 mg/ml AlbuMAX II (Life Technologies).
Dd2attB strain parasites were grown as described above; synchronized with one cycle of sorbitol treatment at the ring stage, as described; and allowed to mature to schizonts (12). A further round of synchronization was performed using a MACS column (16).
Drug Treatment, FACS Analysis, Harvesting, and Lysate Preparation
Purified trophozoites were pooled into two sample sets for flow cytometry and Western blot analysis, respectively. Samples were treated the same except Western blot samples were incubated in malaria culture media while the flow samples were being incubated in 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA). For flow cytometry, parasites were labeled with 5 μm H2DCFDA for 30 min in the dark at 37 °C and washed with culture media. Parasites were treated with artemisinin (100, 250, and 500 nm) for 2 h at 37 °C. Samples were washed and harvested for Western blot analysis at this point. Samples for flow cytometry were further labeled with 0.5 μm SYTO61 for 45 min at room temperature. The samples were washed in malaria culture media and diluted to 0.025% hematocrit. Flow cytometry measurements were performed on a BD Biosciences FACSCalibur. Samples were excited at 488 nm (DCF; 530/30 nm bandpass filter) and 633 nm (SYTO61; 660/20 nm bandpass filter). 250,000 events were recorded based on forward versus side scatter plots. Analysis was performed using FlowJo, and trophozoites were gated based on SYTO61 signal. The median DCF signal was reported.
For Western blot analysis, treated parasites were harvested via saponin lysis, as previously described (12). Parasite pellets were resuspended in PBS lysis buffer containing 200 mm NaCl, 1% Nonidet P-40, 0.1% SDS, 0.05% sodium deoxycholate, 100 μm leupeptin, 1 mm PMSF, 5 mm N-ethylmaleimide, 20 μg/ml aprotinin, and 1 μg/ml pepstatin and lysed using a Biorupter water bath (Diagenode) for five rounds of 30 s on, 30 s with ice. The cells were pelleted at 14,000 rpm at 4 °C for 1 h. Equal amounts of supernatants were added to SDS sample buffer for gel analysis.
Samples were heated at 72 °C for 10 min, resolved by SDS-PAGE, and transferred to 0.45 μm PVDF. Membranes were probed with 1:1000 mAb Pf SUMO 7E11 and 1:15,000 HRP-conjugated GαM (Jackson) (12). Signals were detected by ECL Prime chemiluminescent substrate (GE Healthcare).
HsUba2Ufd-HsUbc9 Structure Determination
HsUba2Ufd (residues 444–561) and HsUbc9 were purified as described and mixed in equimolar amounts (12). The HsUba2Ufd-HsUbc9 complex was purified using a Superdex 75 size exclusion column (GE Healthcare) and concentrated to 10 mg/ml. Crystallization screening was performed using a Mosquito Crystallization Robot (TTP Labtech, Cambridge, MA). Diffraction quality crystals were grown at 20 °C using the sitting drop method, with each drop containing a mixture of 200 nl of complex (10 mg/ml), 20 nl of seed crystals from 200 mm sodium formate, 20% PEG3350, and 200 nl of buffer (100 mm Tris, pH 8.0, 20% PEG 550 monomethyl ether, 200 mm potassium formate, 20% glycerol) from the 60 μl of buffer well. Crystals were mounted onto nylon loops and flash frozen in liquid nitrogen. Crystals were shipped to SSRL Beamline 11-1, and data sets were collected under cryogenic conditions, diffracting to 2.4 Å resolution. XDS was used for processing and indexing, with a 2.5 Å cutoff, and the structure was solved in the C121 space group through molecular replacement with human Ubc9 (Protein Data Bank code 1U9B) and Saccharomyces cerevisiae Uba2Ufd (Protein Data Bank code 3ONG) using CCP4 MOLREP (17–19). Model building and refinement was performed using Coot, REFMAC, and PHENIX suites (20–22). The final structure was analyzed with validation tools in Coot as well as MolProbity, with 0 Ramachandran outliers (23).
Mutant Plasmid Construction, Protein Expression, and Purification
Amino acid substitutions of P. falciparum Uba2 ubiquitin fold domain (PfUba2Ufd residues 508–625) and PfUbc9 in pGex6p1 expression vectors were produced with mutant oligonucleotide primers using the QuikChange site-directed mutagenesis kit (Stratagene). All constructs were verified by DNA sequence analysis. Proteins were expressed and purified as previously described (12). Briefly, cell pellets were resuspended in lysis buffer (50 mm Tris, pH 7.5, 150 mm NaCl, 1 mm DTT, 0.1% Triton X-100, 100 μm leupeptin, 1 μg/ml pepstatin, 20 μg/ml aprotinin, 10 units/ml benzonase, 1 mm PMSF) and lysed via sonication. The soluble sample was passed over glutathione agarose beads (Pierce). Uba2Ufd samples were eluted with 20 mm glutathione, and GST tags were cleaved from Ubc9 samples using on-column cleavage with Prescission Protease. Samples were further purified on a Superdex 75 size exclusion column.
Circular Dichroism
The folding of mutants with reduced binding and conjugation activity was assessed by CD spectroscopy. CD spectra were recorded on a Jasco J-810 spectropolarimeter provided with a PFD-425S temperature controller and a thermostatic cell compartment. The Spectra Manager software J-800 was used for signal averaging and processing. The spectra were registered at 25 °C in the range of 185–260 nm with a scanning speed of 100 nm min−1 in 50 mm HEPES, 150 mm NaCl, and 1 mm DTT. The average of three scans was reported.
GST Binding Assays
Glutathione beads (Pierce) were washed with assay buffer (20 mm HEPES, pH 7.3, 110 mm potassium acetate, 2 mm magnesium acetate, 0.05% Tween 20) and incubated with 33 μg of GST-Uba2Ufd (total protein) for 2 h at 4 °C. The beads were blocked in assay buffer containing 2% BSA for 1 h at 25 °C and washed twice with assay buffer. The E1-E2 binding reaction was performed in 500 μl of assay buffer with 9.9 μg of untagged E2 and rocked for 2 h at room temperature. The samples were washed four times and resuspended in SDS sample buffer. Samples were analyzed by SDS-PAGE and Coomassie staining.
In Vitro SUMOylation Assays
Modification assays using recombinant RanGAP1 were performed in 20-μl reactions containing 1 μm GST-RanGAP1 (residues 419–589), 5 μm PfSUMO, 50 nm PfE1, 100 nm PfE2, 10 mm phosphocreatine, 0.6 unit/ml inorganic pyrophosphatase, 0.6 unit/ml creatine phosphokinase, 20 mm HEPES, pH 7.4, 2 mm Mg(Ac)2, 110 mm KAc, 5 mm ATP. (We have observed that the 2.5-fold excess of ATP over Mg2+ in our reaction does not significantly inhibit conjugation.) The reactions were incubated at 30 °C, and aliquots were combined with sample buffer for analysis by SDS-PAGE, followed by immunoblot analysis. Membranes were probed with 1:500 mAb GST for 1 h at room temperature (Santa Cruz Biotechnology Inc., Santa Cruz, CA). HRP-conjugated secondary antibody (1:15,000; goat-anti mouse; Jackson ImmunoResearch) was detected by ECL Prime (GE Healthcare). The reactions were linear during the first 15 min of the assay, as assessed by ImageJ analysis of band intensities.
Results
SUMOylation Levels Are Modulated in Response to Oxidative Stress
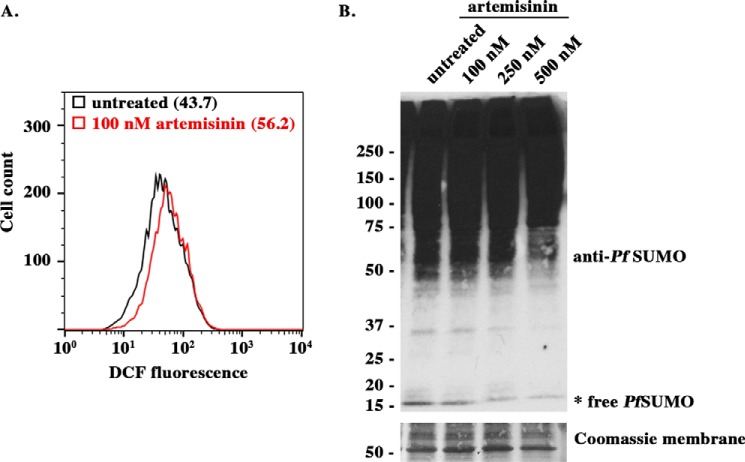
SUMOylation levels peak during the intraerythrocyte developmental cycle trophozoite stage, concomitant with increased oxidative stress caused by hemoglobin degradation (12). To more systematically investigate how SUMOylation levels respond to changes in oxidative stress, we synchronized Dd2attB parasites to enrich for trophozoites and treated cultures with a pro-oxidant anti-malarial drug, artemisinin, for 2 h. To assess the effects of artemisinin treatments on parasite oxidative stress levels, we used the cell-permeable reactive oxygen species reporter dye H2DCFDA in culture during our treatments. Reactive oxygen species production converts the nonfluorescent H2DCFDA molecule into a highly fluorescent product, 2′,7′-dichlorofluorescein (DCF). Treated parasites were stained with the DNA-binding dye, SYTO-61, to distinguish trophozoite stage parasites and analyzed by flow cytometry. Trophozoite stage parasites showed a 30% increase in DCF fluorescence following artemisinin treatment, consistent with predicted increases in oxidative stress and comparable to changes in DCF signal previously reported in P. falciparum (Fig. 1A) (24).
FIGURE 1.
SUMO levels are modulated by oxidative stress. A, trophozoites were incubated with the oxidative stress reporter dye H2DCFDA and treated with the pro-oxidant artemisinin. Fluorescent DCF signal was analyzed by flow cytometry. A representative histogram is shown for the gated infected red blood cells, representing the trophozoite population based on SYTO-61 signal. Median DCF fluorescence is indicated in parentheses. B, trophozoites treated with artemisinin were lysed and diluted in SDS sample buffer. The samples were analyzed by SDS-PAGE and immunoblotting with PfSUMO antibodies. Both low and high exposures are shown for comparison. Equivalent lysate loading is demonstrated by a Coomassie-stained membrane.
To investigate how SUMOylation is affected by changes in oxidative stress, we next performed immunoblot analysis on trophozoite stage parasites purified from untreated control cultures and cultures treated with increasing doses of artemisinin. Immunoblot analysis with a monoclonal PfSUMO antibody revealed a smear of high molecular mass conjugates in untreated control parasites and minor levels of free SUMO, as previously reported (Fig. 1B) (12). In contrast, we detected a dose-dependent reduction in free SUMO and a concomitant shift to higher molecular mass conjugates in lysates prepared from parasites treated with artemisinin.
Structure of a Human Uba2Ufd-Ubc9 Complex
We previously determined that human and P. falciparum SUMO-conjugating enzymes are biochemically unique as a consequence of divergent E1-E2 interactions (12). Our findings were based on functional assays and preliminary structural analysis of predicted molecular complexes of Uba2Ufd and Ubc9 from human and P. falciparum. To assess in greater detail the structural differences mediating species-specific E1-E2 interactions, a crystal structure of the human Uba2Ufd (HsUba2Ufd), corresponding to residues 444–561, in complex with human Ubc9 (HsUbc9), was determined at 2.5 Å resolution (Table 1). Molecular replacement using previous individually solved structures of HsUbc9 (Protein Data Bank code 1U9B) and S. cerevisiae Uba2Ufd (ScUba2Ufd) (Protein Data Bank code 3ONG) was used to obtain initial phases (17, 18). There was one heterodimeric complex per asymmetric unit. The complex was refined using PHENIX to 2.5 Å resolution with an Rwork of 22.1% and Rfree of 28.3% (20).
TABLE 1.
Data collection, phasing, and refinement statistics
| Hs Uba2Ufd-Ubc9 | |
|---|---|
| Data collection | SSRL |
| Space group | C 1 2 1 |
| Cell dimensions | |
| a, b, c (Å) | 161.27, 35.27, 58.67 |
| α, β, γ (°) | 90, 96.69, 90 |
| Wavelength | 0.9795 |
| Resolution (Å) | 44.7 − 2.5 (2.58 − 2.5) |
| Rmerge | 0.1215 (3.829) |
| Rmeas | 0.1438 |
| CC1/2 | 0.997 (0.223) |
| CCa | 0.999 (0.604) |
| I/σI | 9.32 (0.33) |
| Completeness (%) | 91.12 (76.05) |
| Multiplicity | 3.5 (3.5) |
| Wilson B (A2) | 80.68 |
| Total reflections | 37,753 (3795) |
| Unique reflections | 10,654 (1081) |
| Reflections used for Rfree | 553 |
| Rwork/Rfree | 0.2211 (0.4514)/0.2828 (0.4619) |
| No. of non-hydrogen atoms | 2179 |
| Macromolecules | 2095 |
| Ligand/ion | 10 |
| Water | 74 |
| Protein residues | 264 |
| B-factors (Å2) | |
| Protein | 80.5 |
| Ligand/ion | 98.2 |
| Water | 75.2 |
| Root mean square deviations | |
| Bond lengths (Å) | 0.002 |
| Bond angles (°) | 0.50 |
| Ramachandran favored (%) | 95 |
| Ramachandran outliers (%) | 0 |
| Clashscore (MolProbity) | 5.70 |
a The highest resolution shell is shown in parentheses.
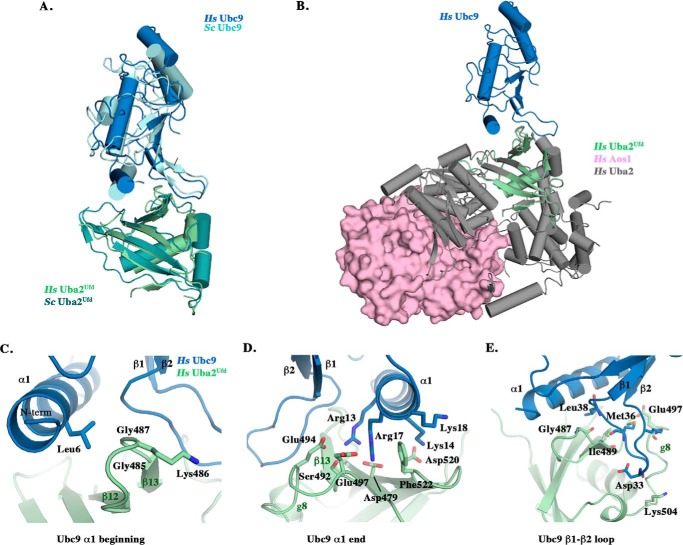
The HsUba2Ufd-HsUbc9 complex displays an E1-E2 interaction mediated by the α1 helix and β1-β2 loop of Ubc9 and the β-grasp fold of the Uba2Ufd domain. The structure superimposes with the prior structure of ScUba2Ufd-ScUbc9 with 0.8 Å root mean square deviation over all alpha carbons, with a notable ∼13° twist in the orientation of HsUbc9 across the HsUba2Ufd β-grasp fold upon alignment of the Ufd domains (Fig. 2A) (25). The HsUba2Ufd-HsUbc9 interface buries 1464 Å2 of surface area, comparable with that reported for the S. cerevisiae complex and representative of interactions found in similarly transient heterodimeric complexes (25–27).
FIGURE 2.
Structural conservation of a human Uba2Ufd-Ubc9 complex. A, ribbon representation of the human Uba2Ufd-Ubc9 complex (light green and dark blue) superimposed with the previously solved S. cerevisiae Uba2Ufd-Ubc9 structure (teal and light blue) (Protein Data Bank code 3ONG) (25). B, superposition of the human complex onto the previously solved Ufd domain of a human Aos1/Uba2-SUMO1 adenylate structure (pink and gray) (Protein Data Bank code 3KYC) (18). C–E, residues involved in the human Uba2Ufd-Ubc9 interaction are highlighted, organized by binding region: beginning and end of HsUbc9 α1 helix and β1-β2 loop.
Superposition of a previously solved structure containing HsUba2 with HsUba2Ufd in our complex reveals a high degree of structural similarity (0.57 Å root mean square deviation over all alpha carbons) (Fig. 2B) (18). The orientation of HsUbc9 in this superposition places the end of the HsUbc9 α1 helix in close proximity to the α4-helix of HsUba2, a region outside of the Ufd domain, potentiating additional interactions. Overall, the HsUbc9 observed in our structure also shows a high degree of similarity with a prior HsUbc9 structure (0.51 Å root mean square deviation over all alpha carbons), with a slight shift in the β1-β2 loop to accommodate the surface of HsUba2Ufd (17).
Similar to contacts seen in the S. cerevisiae structure, the HsUbc9 contacts in the HsUba2Ufd-HsUbc9 interface are comprised of three predominant sites consisting of the residues near the beginning and end of the α1 helix and within the β1-β2 loop. Interactions mediated by residues at the beginning of the HsUbc9 α1 helix lack the Ufd interstrand hydrophobic patch described in S. cerevisiae; however, the twisted orientation positions HsUbc9 Leu6 between Gly485 and Gly487 of the HsUba2Ufd β12-β13 loop (Fig. 2C) (25). Electrostatic interactions predominate near the end of α1 helix, with Arg13 and Arg17 nestling within a pocket formed by a HsUba2Ufd acidic loop comprised of Asp479, Glu497, Ser492, and Glu494. HsUbc9 Lys14 interacts with HsUba2Ufd Asp520, on the opposing face of the Ufd Phe522 wedge (Fig. 2D).
The HsUbc9 β1-β2 loop and α1 helix are separated by two HsUba2Ufd ridges formed by β12-β13 and β13-g8. The cavity formed by these ridges lacks the electrostatic contacts seen in the S. cerevisiae Uba2Ufd-Ubc9 structure; consequently, the human β1-β2 loop anchors itself exclusively through hydrogen bonding (Fig. 2E). Several of the interacting residues at the HsUba2Ufd-HsUbc9 interface have diverged between human and P. falciparum, including HsUbc9 Leu6, Ser7, Lys14, and Ala15 in the α1 helix and Lys30, Asn31, Pro32, and Thr35 in the β1-β2 loop, suggesting unique structural features that play a role in the species-specific interactions of Uba2Ufd-Ubc9.
Structural Analysis of the P. falciparum (Pf) Uba2Ufd-Ubc9 Interface
The specific recognition between different ubiquitin-like (Ubl) pathway enzymes is dictated by distinct charge distributions on the E1Ufd-E2 interfaces (28). Many ubiquitin E2 enzymes contain basic residues in the α1 helix (corresponding to residues Leu6 and Ser7 of HsUbc9) that interact with a negatively charged Ufd (28, 29). Notably, Ubc1 Lys5 (equivalent to PfUbc9 Lys6) is critical for E1-E2 ubiquitin transthiolation (28). Although these residues are replaced by corresponding hydrophobic interactions in human and S. cerevisiae, it is evident from sequence alignment that PfUbc9 maintains a basic N terminus, suggesting that the Plasmodium SUMO E1-E2 interaction may use contacts reminiscent of ubiquitin E2s (Fig. 3A).
FIGURE 3.
P. falciparum has a divergent Uba2Ufd-Ubc9 interface. A, multiple sequence alignment of Ubc9 α1 helix (positions 1–21) from human (Hs), S. cerevisiae (Sc), S. pombe (Sp), P. falciparum (Pf), P. vivax (Pv), P.œnowlesi (Pk), and P. berghei (Pb) ubiquitin E2s, Ubc1, Ubc2, and Ubc4, respectively. Ubc9 α1 helix residues important for human and Sc Ubc9-Ufd binding are denoted by Δ. Additional residues mediating Ubc2-Ufd interactions are denoted by •. Conserved basic N-terminal residues are highlighted with red shading. B, ribbon representation of the PfUbc9 (yellow) (4JUE) docked with the predicted PfUba2Ufd structure (orange) (iTasser), superimposed on the Hs Uba2Ufd-Ubc9 complex by alignment of the Ufd domains. C–E, residues involved in the PfUba2Ufd-Ubc9 interaction based on the docked structure are highlighted, organized by binding region: beginning and end of Ubc9 α1 helix and β1-β2 loop. Interactions denoted by • and Δ from A are specified above the residue label.
To gain further insight into the basis for Plasmodium specific E1-E2 interactions, we attempted to obtain crystallographic structures of a PfUba2Ufd-PfUbc9 complex but were unsuccessful. To identify residues in Pf Uba2Ufd and PfUbc9 that mediate their unique interactions, we utilized an iTasser server predicted PfUba2Ufd model and performed rigid body local docking with our previously solved PfUbc9 structure (Protein Data Bank code 4JUE) aligned to the HsUba2Ufd-HsUbc9 complex (Protein Data Bank code 4W5V), using the Rosetta Dock server with default parameters (Fig. 3B) (12, 30–33).
Simulated docking places PfUbc9 across the PfUba2Ufd in an orientation similar to that found in the S. cerevisiae and human structures. Also as seen in these structures, PfUbc9 contacts in the predicted PfUba2Ufd-PfUbc9 interface are comprised of three predominant sites consisting of the residues near the beginning and end of the α1 helix and within the β1-β2 loop (Fig. 3, C–E). Unlike human and S. cerevisiae, the beginning of the PfUbc9 α1 helix is highly basic. Based on our predicted PfUba2Ufd-PfUbc9 complex, PfUbc9 Lys5 is anchored by an acidic patch containing Asp544, Asp546, and Asp547 on PfUba2Ufd, whereas PfUbc9 Lys6 is predicted to form hydrogen bonds with the functional group oxygen of PfUba2Ufd Asn591 (Fig. 3C). PfUbc9 Ala9 is placed above an interstrand hydrophobic patch on PfUba2Ufd. As in the human and S. cerevisiae structures, basic residues near the end of the PfUbc9 α1 helix make electrostatic contacts with a Ufd acidic patch in the β3-α2 loop (equivalent to HsUba2 β13-g8) including residues Glu558, Asp560, and Asp561 (Fig. 3D). Additionally, the extended β1-β2 loop, characteristic of most Ubc9 enzymes, is further elongated in PfUbc9 by an additional residue, Lys34, which is predicted to hydrogen bond to a Ufd backbone carbonyl (Fig. 3E). Finally, our docked complex also predicts that PfUbc9 Glu66 may bind PfUba2Ufd Arg548 (Fig. 3C), a residue important for binding S. cerevisiae Ubc9 Asp32 in the β1-β2 loop.
Biochemical Analysis of the PfUba2Ufd-PfUbc9 Interface
To more directly assess the role of individual residues in facilitating PfUbc9 and PfUba2Ufd interactions, we evaluated the effects of charge reversal and neutralization substitutions, spanning the predicted interaction surface, using purified recombinant proteins and in vitro binding assays. GST-tagged wild type or mutant PfUba2Ufd was bound to glutathione beads and incubated with wild type or mutant PfUbc9. Single mutations in both the α1 helix and β1-β2 loop of PfUbc9 were sufficient to interfere with binding (Fig. 4A). Both Lys5 and Lys6 at the beginning of PfUbc9 α1 helix showed reduced binding to PfUba2Ufd upon charge reversal. Lys5 is predicted to interact with an acidic patch containing Asp544, Asp546, and Asp547, on PfUba2Ufd, and whereas mutations in Asp544 and Asp547 decreased binding, alanine substitution of Asp546 showed no affect on binding (Fig. 4B). Alanine substitutions of Arg12 and Arg16, near the end of the α1 helix, also decreased binding to PfUba2Ufd, and binding was further reduced to undetectable levels with Arg12 charge reversals (Fig. 4A). Alanine substitutions in the PfUba2Ufd β3-α2 acidic loop (Glu558, Asp560, Asp561, and Asp562) showed no significant decrease in Ubc9 binding, suggesting that individual residues within the highly charged loop may be able to compensate for loss of local charge (Fig. 4B). Based on our predicted P. falciparum complex, PfUbc9 Lys26 is positioned above the acidic β3-α2 loop of PfUba2Ufd. Whereas Ala substitution of Lys26 showed no reduction in binding, charge reversals led to reduced binding levels, suggesting that in concert with Arg12 and Arg16, Lys26 may play a role in stabilizing the PfUba2Ufd acidic β3-α2 loop interaction (Fig. 4A).
FIGURE 4.
Single mutations throughout the PfUba2Ufd-Ubc9 interface are sufficient to disrupt binding. Noncovalent complexes between 33 μg of wild type GST-PfUba2Ufd and 9.9 μg of mutant PfUbc9 (A) and mutant GST-PfUba2Ufd and wild type PfUbc9 (B) were formed at room temperature for 2 h and resuspended in SDS sample buffer. The samples were analyzed by SDS-PAGE and Coomassie staining. Mutants are organized by Ubc9 binding regions.
We previously showed that the β1-β2 loop in PfUbc9 was critical for functional interactions with the Plasmodium E1-activating enzyme (12). The PfUbc9 β1-β2 loop contains several residues not conserved with S. cerevisiae or human, as well as the addition of Lys34, creating an extended loop. Lys34 charge reversal reduced PfUba2Ufd binding, consistent with a role in binding as suggested by the docked structure. Moreover, although Asp32 in the β1-β2 loop is conserved between S. cerevisiae, human, and Plasmodium, neither alanine substitution nor charge reversal affected PfUba2Ufd binding (Fig. 4A). In S. cerevisiae, Asp32 makes a salt bridge with ScUba2Ufd Arg484. Mutation of the corresponding residue in PfUba2Ufd, Arg548, led to reduced PfUbc9 binding (Fig. 4B). The modest E1 binding defects of single mutations in the β1-β2 loop are consistent with the dispersal of low energy binding interactions predicted between the PfUbc9 β1-β2 loop and Ufd in the docked structure, suggesting that several residues may work collectively to increase the affinity of the interaction.
Given that individual mutations within PfUbc9 α1 helix and β1-β2 loop were sufficient to disrupt binding, we further assessed their effects on activity through in vitro SUMO modification reactions using mammalian RanGAP1 as a model substrate. All PfUbc9 mutants that showed reduced Uba2Ufd interaction in our binding assay also showed impaired rates of RanGAP1 conjugation (Fig. 5). Proper folding of individual mutants was confirmed by CD spectroscopy (Fig. 6). Notably, double alanine mutations at PfUbc9 Arg12 and Arg16 were sufficient to disrupt RanGAP1 conjugation, consistent with predicted roles in forming salt bridges with residues in Uba2Ufd (25). Several Ubc9 mutations, including at residues Lys5, Arg16, and Lys26, showed reduced Uba2Ufd binding but had only moderate effects on RanGAP1 conjugation rates. In addition, individual charge reversals of α1 helix residues Lys6 and Arg12 were sufficient to inhibit conjugation, indicative of particularly critical roles in E1 binding.
FIGURE 5.
Mutations in PfUbc9 α1 helix and β1-β2 loop disrupt RanGAP1 conjugation. 1 μm GST-RanGAP1 was incubated with 50 nm PfE1, 5 μm PfSUMO, 5 mm ATP, and 100 nm of the indicated PfUbc9 mutant. The reactions were stopped with the addition of SDS sample buffer at the indicated time points. The samples were separated by SDS-PAGE and analyzed by immunoblotting with GST antibodies. Panels are organized by binding region: beginning and end of Ubc9 α1 helix and β1-β2 loop. R1 and R1∼S denote unmodified and SUMO-modified RanGAP1, respectively.
FIGURE 6.

PfUbc9 charge reversal mutants are properly folded. CD spectra of wild type PfUbc9 and charge reversal mutants (K5E, K6E, R12E, R16E, K26E, D32K, and K34E) show characteristic bands representative of structured proteins. The averages of three scans are reported, and values are given in terms of ellipticity, measured in millidegrees (mdeg).
In summary, this work reveals a selection of residues that are involved in Plasmodium Uba2Ufd-E2 binding and subsequently activity. The mapping of critical mutants on the E1 and E2 surface are consistent with an interaction mediated by the PfUbc9 α1 helix and β1-β2 loop and PfUba2Ufd β2-β3 and β3-α2 loops (Fig. 7A). By mapping the site-specific conservation of surface residues, it can be seen that residues critical for PfUbc9 and PfUba2Ufd interaction are located in regions of the E1-E2 interface that have diverged between human and Plasmodium, suggesting the potential for developing parasite-specific inhibitors for this interaction (Fig. 7B).
FIGURE 7.
Select residues mediate Plasmodium specific Uba2Ufd-Ubc9 binding and conjugation activity. A, ribbon representation of the PfUba2Ufd-Ubc9 docked structure with open book rotation of the interface. Surface residues important for binding are colored light blue to dark blue based on increasing importance. E2 residues important for conjugation activity are scored by asterisks (ranging from least important (*) to most important (****)). B, surface residue conservation map of the Uba2Ufd-Ubc9 interface between P. falciparum and human, colored from gray (identical amino acids) to light pink (similar amino acids) to dark pink (nonconservative substitution). Residues were clustered by similar chemical characteristics (GAVLI, FYW, CM, ST, KRH, DENQ, and P), with nonconservative substitutions deviating from these groups (46).
Discussion
Specificity in E1-E2 Interactions
Interventions to disrupt ubiquitin-like post-translational modifications have become increasingly important as their essential biological roles in diseases, such as cancer and neurodegeneration, become clearer (34). Protein-protein interactions have been widely characterized among Ubl conjugation cascades, and the presence of a single E1 and limited number of E2 enzymes makes E1-E2 interactions a promising step in the pathway to explore for selective therapeutic drug design. Importantly, whereas many of the Ubl E1 and E2 enzymes share common structural scaffolds across pathways, they have also diverged significantly, allowing for selective interactions between cognate E1 and E2s that guide the coordinated transfer of specific Ubls (25, 29, 35, 36). Previously, we extended this observation of unique E1-E2 interactions by identifying selective interactions between E1 and E2 enzymes of the SUMO pathway in divergent organisms, namely human and P. falciparum (12). Here, we have extended our initial findings, providing new insights into SUMO functions in P. falciparum and the molecular basis for species-specific interactions between SUMO E1 and E2 enzymes.
Essential Roles for SUMOylation in the Oxidative Stress Response
We have provided further evidence for the role of SUMO in the clinically relevant P. falciparum stress response by demonstrating that SUMOylation levels are modulated in response to oxidative stress treatment. Importantly, SUMOylation plays a protective role during oxidative stress in other organisms, suggesting a similar essential function in P. falciparum (9–11). Consistent with SUMOylation being essential during the parasite red blood cell life cycle, prior studies have shown that inhibitors of SUMO proteases inhibit parasite replication during mid to late red blood cell stages (37). The mechanisms by which SUMOylation promotes stress survival remain unclear but may involve effects on gene expression and protein quality control (38). Because relatively few SUMOylated proteins have been identified in P. falciparum to date, further studies are needed to explore possible stress-related functions and underlying mechanisms of action (3). Nonetheless, because many anti-malarial drugs are thought to act by overloading the parasite oxidative stress response, our findings indicate that inhibitors of SUMOylation may be effective in combination therapies with such drugs (13, 14).
Molecular Basis for Human and P. falciparum SUMO E1-E2 Interactions
Previous studies investigating yeast and human enzymes have established that two distinct domains within the SUMO E1 are critical for functional interactions with Ubc9: the conserved Uba2 catalytic Cys domain (Uba2Cys) and the Uba2Ufd domain (25, 39–41). Structural analysis of E1-catalyzed Ubl activation and E2 recruitment suggests that Ubl adenylation and E1 thiolation induce significant E1 conformational changes that lead to the reorientation of the Uba2Ufd domain, promoting bipartite binding of the E2 to the Ufd and Cys domains for transthiolation catalysis (25, 29, 40–43). In vitro assays have calculated dissociation constants of 87 μm for Uba2Cys-Ubc9 complexes and 1.2 μm for Uba2Ufd-Ubc9 complexes, consistent with the Uba2Ufd domain playing a major role in initial E2 binding and Uba2Cys interactions playing a role in the subsequent transfer of SUMO from E1 to Ubc9 (12, 41). Within the SUMO pathway, the Uba2Ufd domain has been shown to be essential for transthiolation and substrate modification in vitro (40). Consistent with an essential role in Ubc9 interactions, deletion of the Ufd domain from ScUba2 is lethal in vivo (40). Structural and biochemical studies also demonstrate that the Ufd domain of the ubiquitin E1-activating enzyme is important for E1-E2 binding and transthiolation, demonstrating conservation between Ubl pathways (29, 36). However, the Ufd domain of the Nedd8 E1 is dispensable, presumably because of a unique N-terminal extension in the cognate E2 that provides compensatory interactions (35, 42, 44).
Based on structural analysis and biochemical studies of yeast enzymes, it has been established that multiple residues within the N-terminal α1 helix and the β1-β2 loop of Ubc9 mediate Uba2Ufd interactions (25, 39). We previously established that multiple residues in these same regions of P. falciparum Ubc9 are also critical for functional E1 interactions and play a determining role in species-specific E1-E2 association (12). Mutational analysis of both yeast and P. falciparum enzymes confirms that multiple residues across the α1 helix and β1-β2 loop contribute collectively to stable E1 interactions required for transthiolation and substrate modification. A detailed analysis of interactions between human E1 and E2 enzymes within the SUMO pathway has not been previously been reported.
Our current studies were designed to more precisely define the Uba2Ufd-Ubc9 interfaces of P. falciparum and human enzymes and identify the residues responsible for species-specific interactions. Our crystal structure of the human Uba2Ufd-Ubc9 complex revealed a binding surface highly conserved with the previously determined S. cerevisiae enzyme interface, consistent with the finding that human Ubc9 complements S. cerevisiae Ubc9 deletion to maintain viability (25, 45). In contrast, structural and biochemical analysis of the P. falciparum Uba2Ufd-Ubc9 interface revealed significant divergence, and we identified multiple residues on PfUba2Ufd and PfUbc9 important for selective Plasmodium interactions. Most notably, the N terminus of the PfUbc9 α1 helix was found to represent both a divergent and a functionally critical surface. This surface is hydrophobic in nature in both S. cerevisiae and human enzymes but contains two charged lysine residues in PfUbc9. Based on our modeled complex, we predicted that PfUbc9 Lys5 would contribute significant electrostatic contacts to the binding interface, yet whereas mutation of Lys5 significantly affected PfUba2Ufd binding, it had only modest effects on RanGAP1 conjugation. This likely reflects a limitation of our in vitro binding assay to detect reduced, but still functionally sufficient, binding activity. Surprisingly, mutation of the neighboring Lys6 was sufficient to suppress PfUba2Ufd binding and RanGAP1 conjugation, even though our predicted structure does not reveal obvious E1 contacts. Further analysis will be necessary to determine what role PfUbc9 Lys6 plays in promoting productive E1-E2 interactions.
Additional residues within the C-terminal end of the α1 helix and within the β1-β2 loop of PfUbc9 were also found to be important for functional interaction with PfE1, consistent with E2 residues contributing collectively to E1 binding. Notably, combined alanine mutations at conserved arginine residues (Arg12 and Arg16) within the α1 helix were sufficient to disrupt RanGAP1 conjugation. In addition, combined mutations in the PfUbc9 α1 helix and β1-β2 loop led to conjugation inhibition, as shown previously with humanized PfUbc9 chimeras and studies examining the E1-Ubc9 interaction in yeast (12, 39). Together, the three predominant Uba2Ufd interaction sites on Ubc9, consisting of the residues near the beginning and end of the α1 helix and within the β1-β2 loop, are linked by regions with low conservation between human and Plasmodium and are predicted to interact with a predominantly nonconserved surface of the Ufd domain (Fig. 7B). This suggests a novel surface for the development of parasite-specific inhibitors that could disrupt functional E1-E2 interactions. Further structural characterization will be necessary to determine how PfUbc9 interacts with the PfE1 for functional thioester transfer, perhaps revealing additional interactions unique to Plasmodium.
Author Contributions
K. H. R. and M. J. M. designed the study and wrote the paper. A. R. optimized the parasite treatment and flow cytometry conditions. X. X. optimized the E1-E2 binding conditions. L. E. B. and J. B. aided in structure determination. All authors analyzed the results and approved the final version of the manuscript.
Acknowledgments
We thank members of the Matunis and Bosch laboratories and Dr. Sean Prigge and Dr. Scott Bailey for helpful discussions and assistance during the course of the studies. We also thank the beamline scientists at SSRL Beamline 11-1 for help and support during data collection.
This work was funded in part by National Institutes of Health Grant GM060980 (to M. J. M.), the Sommer Scholar's Program (to K. H. R.), the Bloomberg Family Foundation (to J. B.), the National Institutes of Health Chemistry-Biology Interface Training Grant T32GM080189, and a predoctoral Fellowship from Johns Hopkins Malaria Research Institute (to L. E. B.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The atomic coordinates and structure factors (code 4W5V) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- PTM
- post-translational modification
- SUMO
- small ubiquitin-related modifier
- Ufd
- ubiquitin fold domain
- H2DCFDA
- 2′,7′-dichlorodihydrofluorescein diacetate
- DCF
- 2′,7′-dichlorofluorescein
- Ubl
- ubiquitin-like.
References
- 1. World Health Organization (2014) World Malaria Report 2014, World Health Organization, Geneva, Switzerland [Google Scholar]
- 2. Miller L. H., Ackerman H. C., Su X. Z., and Wellems T. E. (2013) Malaria biology and disease pathogenesis: insights for new treatments. Nat. Med. 19, 156–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Issar N., Roux E., Mattei D., and Scherf A. (2008) Identification of a novel post-translational modification in Plasmodium falciparum: protein sumoylation in different cellular compartments. Cell Microbiol. 10, 1999–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Artavanis-Tsakonas K., Misaghi S., Comeaux C. A., Catic A., Spooner E., Duraisingh M. T., and Ploegh H. L. (2006) Identification by functional proteomics of a deubiquitinating/deNeddylating enzyme in Plasmodium falciparum. Mol. Microbiol. 61, 1187–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chung D. W., Ponts N., Cervantes S., and Le Roch K. G. (2009) Post-translational modifications in Plasmodium: more than you think!. Mol. Biochem. Parasitol. 168, 123–134 [DOI] [PubMed] [Google Scholar]
- 6. Doerig C., Rayner J. C., Scherf A., and Tobin A. B. (2015) Post-translational protein modifications in malaria parasites. Nat. Rev. Microbiol. 13, 160–172 [DOI] [PubMed] [Google Scholar]
- 7. Karve T. M., and Cheema A. K. (2011) Small changes huge impact: the role of protein posttranslational modifications in cellular homeostasis and disease. J. Amino Acids 2011, 207691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Geiss-Friedlander R., and Melchior F. (2007) Concepts in sumoylation: a decade on. Nat. Rev. Mol. Cell Biol. 8, 947–956 [DOI] [PubMed] [Google Scholar]
- 9. Conti L., Kioumourtzoglou D., O'Donnell E., Dominy P., and Sadanandom A. (2009) OTS1 and OTS2 SUMO proteases link plant development and survival under salt stress. Plant Signal. Behav. 4, 225–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Golebiowski F., Matic I., Tatham M. H., Cole C., Yin Y., Nakamura A., Cox J., Barton G. J., Mann M., and Hay R. T. (2009) System-wide changes to SUMO modifications in response to heat shock. Sci. Signal. 2, ra24. [DOI] [PubMed] [Google Scholar]
- 11. Lee Y. J., Miyake S., Wakita H., McMullen D. C., Azuma Y., Auh S., and Hallenbeck J. M. (2007) Protein SUMOylation is massively increased in hibernation torpor and is critical for the cytoprotection provided by ischemic preconditioning and hypothermia in SHSY5Y cells. J. Cereb. Blood Flow Metab. 27, 950–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reiter K., Mukhopadhyay D., Zhang H., Boucher L. E., Kumar N., Bosch J., and Matunis M. J. (2013) Identification of biochemically distinct properties of the small ubiquitin-related modifier (SUMO) conjugation pathway in Plasmodium falciparum. J. Biol. Chem. 288, 27724–27736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meshnick S. R., Thomas A., Ranz A., Xu C. M., and Pan H. Z. (1991) Artemisinin (qinghaosu): the role of intracellular hemin in its mechanism of antimalarial action. Mol. Biochem. Parasitol. 49, 181–189 [DOI] [PubMed] [Google Scholar]
- 14. Sullivan D. J. Jr., Gluzman I. Y., Russell D. G., and Goldberg D. E. (1996) On the molecular mechanism of chloroquine's antimalarial action. Proc. Natl. Acad. Sci. U.S.A. 93, 11865–11870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trager W., and Jensen J. B. (1976) Human malaria parasites in continuous culture. Science 193, 673–675 [DOI] [PubMed] [Google Scholar]
- 16. Ribaut C., Berry A., Chevalley S., Reybier K., Morlais I., Parzy D., Nepveu F., Benoit-Vical F., and Valentin A. (2008) Concentration and purification by magnetic separation of the erythrocytic stages of all human Plasmodium species. Malar. J. 7, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Giraud M. F., Desterro J. M., and Naismith J. H. (1998) Structure of ubiquitin-conjugating enzyme 9 displays significant differences with other ubiquitin-conjugating enzymes which may reflect its specificity for sumo rather than ubiquitin. Acta Crystallogr. D Biol. Crystallogr. 54, 891–898 [DOI] [PubMed] [Google Scholar]
- 18. Olsen S. K., Capili A. D., Lu X., Tan D. S., and Lima C. D. (2010) Active site remodelling accompanies thioester bond formation in the SUMO E1. Nature 463, 906–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vagin A., and Teplyakov A. (2010) Molecular replacement with MOLREP. Acta Crystallogr. D Biol. Crystallogr. 66, 22–25 [DOI] [PubMed] [Google Scholar]
- 20. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., Richardson J. S., Terwilliger T. C., and Zwart P. H. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Emsley P., and Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 22. Vagin A. A., Steiner R. A., Lebedev A. A., Potterton L., McNicholas S., Long F., and Murshudov G. N. (2004) REFMAC5 dictionary: organization of prior chemical knowledge and guidelines for its use. Acta Crystallogr. D Biol. Crystallogr. 60, 2184–2195 [DOI] [PubMed] [Google Scholar]
- 23. Chen V. B., Arendall W. B. 3rd, Headd J. J., Keedy D. A., Immormino R. M., Kapral G. J., Murray L. W., Richardson J. S., and Richardson D. C. (2010) MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klonis N., Crespo-Ortiz M. P., Bottova I., Abu-Bakar N., Kenny S., Rosenthal P. J., and Tilley L. (2011) Artemisinin activity against Plasmodium falciparum requires hemoglobin uptake and digestion. Proc. Natl. Acad. Sci. U.S.A. 108, 11405–11410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang J., Taherbhoy A. M., Hunt H. W., Seyedin S. N., Miller D. W., Miller D. J., Huang D. T., and Schulman B. A. (2010) Crystal structure of UBA2(ufd)-Ubc9: insights into E1-E2 interactions in Sumo pathways. PLoS One 5, e15805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krissinel E., and Henrick K. (2007) Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 [DOI] [PubMed] [Google Scholar]
- 27. Nooren I. M., and Thornton J. M. (2003) Structural characterisation and functional significance of transient protein-protein interactions. J. Mol. Biol. 325, 991–1018 [DOI] [PubMed] [Google Scholar]
- 28. Lee I., and Schindelin H. (2008) Structural insights into E1-catalyzed ubiquitin activation and transfer to conjugating enzymes. Cell 134, 268–278 [DOI] [PubMed] [Google Scholar]
- 29. Olsen S. K., and Lima C. D. (2013) Structure of a ubiquitin E1-E2 complex: insights to E1-E2 thioester transfer. Mol. Cell 49, 884–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lyskov S., Chou F. C., Conchúir S. Ó., Der B. S., Drew K., Kuroda D., Xu J., Weitzner B. D., Renfrew P. D., Sripakdeevong P., Borgo B., Havranek J. J., Kuhlman B., Kortemme T., Bonneau R., Gray J. J., and Das R. (2013) Serverification of molecular modeling applications: the Rosetta Online Server that Includes Everyone (ROSIE). PLoS One 8, e63906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lyskov S., and Gray J. J. (2008) The RosettaDock server for local protein-protein docking. Nucleic Acids Res. 36, W233–W238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chaudhury S., Berrondo M., Weitzner B. D., Muthu P., Bergman H., and Gray J. J. (2011) Benchmarking and analysis of protein docking performance in Rosetta v3.2. PLoS One 6, e22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang J., Yan R., Roy A., Xu D., Poisson J., and Zhang Y. (2015) The I-TASSER Suite: protein structure and function prediction. Nat. Methods 12, 7–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bedford L., Lowe J., Dick L. R., Mayer R. J., and Brownell J. E. (2011) Ubiquitin-like protein conjugation and the ubiquitin-proteasome system as drug targets. Nat. Rev. Drug. Discov. 10, 29–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang D. T., Miller D. W., Mathew R., Cassell R., Holton J. M., Roussel M. F., and Schulman B. A. (2004) A unique E1-E2 interaction required for optimal conjugation of the ubiquitin-like protein NEDD8. Nat. Struct. Mol. Biol. 11, 927–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tokgöz Z., Siepmann T. J., Streich F. Jr., Kumar B., Klein J. M., and Haas A. L. (2012) E1-E2 interactions in ubiquitin and Nedd8 ligation pathways. J. Biol. Chem. 287, 311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ponder E. L., Albrow V. E., Leader B. A., Békés M., Mikolajczyk J., Fonović U. P., Shen A., Drag M., Xiao J., Deu E., Campbell A. J., Powers J. C., Salvesen G. S., and Bogyo M. (2011) Functional characterization of a SUMO deconjugating protease of Plasmodium falciparum using newly identified small molecule inhibitors. Chem. Biol. 18, 711–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Enserink J. M. (2015) Sumo and the cellular stress response. Cell Div. 10, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bencsath K. P., Podgorski M. S., Pagala V. R., Slaughter C. A., and Schulman B. A. (2002) Identification of a multifunctional binding site on Ubc9p required for Smt3p conjugation. J. Biol. Chem. 277, 47938–47945 [DOI] [PubMed] [Google Scholar]
- 40. Lois L. M., and Lima C. D. (2005) Structures of the SUMO E1 provide mechanistic insights into SUMO activation and E2 recruitment to E1. EMBO J. 24, 439–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang J., Hu W., Cai S., Lee B., Song J., and Chen Y. (2007) The intrinsic affinity between E2 and the Cys domain of E1 in ubiquitin-like modifications. Mol. Cell 27, 228–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang D. T., Paydar A., Zhuang M., Waddell M. B., Holton J. M., and Schulman B. A. (2005) Structural basis for recruitment of Ubc12 by an E2 binding domain in NEDD8's E1. Mol. Cell 17, 341–350 [DOI] [PubMed] [Google Scholar]
- 43. Huang D. T., Hunt H. W., Zhuang M., Ohi M. D., Holton J. M., and Schulman B. A. (2007) Basis for a ubiquitin-like protein thioester switch toggling E1-E2 affinity. Nature 445, 394–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Walden H., Podgorski M. S., and Schulman B. A. (2003) Insights into the ubiquitin transfer cascade from the structure of the activating enzyme for NEDD8. Nature 422, 330–334 [DOI] [PubMed] [Google Scholar]
- 45. van Waardenburg R. C., Duda D. M., Lancaster C. S., Schulman B. A., and Bjornsti M. A. (2006) Distinct functional domains of Ubc9 dictate cell survival and resistance to genotoxic stress. Mol. Cell Biol. 26, 4958–4969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stothard P. (2000) The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. BioTechniques 28, 1102, 1104 [DOI] [PubMed] [Google Scholar]