Abstract
Angiotensin II (Ang II) is a vasopressive hormone but is also a potent activator of cellular migration. We have previously shown that it can promote the activation of the GTPase ARF6 in a heterologous overexpressing system. The molecular mechanisms by which receptors control the activation of this small G protein remain, however, largely unknown. Furthermore, how ARF6 coordinates the activation of complex cellular responses needs to be further elucidated. In this study, we demonstrate that Ang II receptors engage β-arrestin, but not Gq, to mediate ARF6 activation in HEK 293 cells. To further confirm the key role of β-arrestin proteins, we overexpressed β-arrestin2-(1–320), a dominant negative mutant known to block receptor endocytosis. We show that expression of this truncated construct does not support the activation of the GTPase nor cell migration. Interestingly, β-arrestin2 can interact with the ARF guanine nucleotide exchange factor ARNO, although the C-terminally lacking mutant does not. We finally examined whether receptor endocytosis controlled ARF6 activation and cell migration. Although the clathrin inhibitor PitStop2 did not impact the ability of Ang II to activate ARF6, cell migration was markedly impaired. To further show that ARF activation regulates key signaling events leading to migration, we also examined MAPK activation. We demonstrate that this signaling axis is relevant in smooth muscle cells of the vasculature. Altogether, our findings show for the first time that Ang II receptor signaling to β-arrestin regulates ARF6 activation. These proteins together control receptor endocytosis and ultimately cell migration.
Keywords: 7-helix receptor, ADP ribosylation factor (ARF), angiotensin II, arrestin, cell migration, vascular smooth muscle cells
Introduction
Angiotensin II (Ang II)2 is a peptidic hormone that acts primarily through its angiotensin II type 1 receptor (AT1R) to promote a broad variety of biological effects. In the vasculature, its main target is the vascular smooth muscle cell (VSMC). In addition to promoting vasoconstriction through increased intracellular calcium levels, Ang II may promote enhancement of oxidative stress (1, 2), hypertrophy (3), and cell migration (4, 5). Misregulation of AT1R function can be deleterious and therefore contribute to the development of pathological conditions (6). Because of its ability to promote activation of numerous signaling events as well as the development of different ligands to modulate receptor activation, AT1Rs have been broadly studied in heterologous recombinant cellular systems. However, their native roles in VSMCs remain understudied. Abnormal function of these cells is critically involved in disease development, i.e. abnormal migration is associated with atherosclerosis processes (7). To develop new tools effective in treating complex vascular diseases, we must elucidate the mechanisms controlling Ang II-mediated VSMC responses such as migration.
Stimulation of the AT1R leads to the classical activation of heterotrimeric G proteins to produce intracellular accumulation of second messengers. Upon sustained activation, receptors become desensitized by the recruitment of β-arrestin (8, 9). Over the years, the role of these proteins as signaling molecules has emerged from the studies reporting that they can interact with numerous partners (10, 11). The first example that β-arrestin not only acts to terminate receptor-mediated second messenger production but actively contributes to control the fate of receptors following their stimulation came from the demonstration that these directly bind components of the clathrin-coated vesicles (12–14). Furthermore, their ability to interact with the different components of the mitogen-activated protein kinase (MAPK) pathway, leading to activation of extracellular signal-regulated kinase 1/2 (ERK1/2) (15, 16), c-Jun N-terminal kinase 3 (JNK3) (17), or p38 MAPK (18) has further demonstrated that β-arrestins can act as scaffold proteins.
Signaling through β-arrestin has been shown to be important for the receptor-mediated increase in cellular motility. For instance, β-arrestin expression is required for cell migration stimulated by protease-activated-2 receptor (PAR-2) (19). Furthermore, leukocyte chemotaxis promoted by CXC chemokine receptor type-4 (CXCR4) activation was found to be defective in β-arrestin2 knock-out mice (20), and knockdown of β-arrestin2, by siRNA, reduced Ang II-mediated cell migration (4). Numerous studies have reported that β-arrestin regulates small GTP-binding protein activation. β-Arrestin1 was shown to activate RhoA in coordination with Gq (21), through a mechanism whereby β-arrestin1 acts to inhibit deactivation of the GTPase by modulating the function of its GTPase-activating proteins (22). Our previous work has demonstrated that stimulation of the β2-adrenergic receptor can lead to the association of β-arrestin isoforms and ARF6 in HEK 293 cells (23). This and further studies have also shown that this small GTPase mediates G protein-coupled receptor endocytosis (24). ARF proteins are small GTPases of the Ras superfamily, and six isoforms have been identified (ARF1–6). ARF proteins also act to promote remodeling of membrane lipids (25, 26), vesicular trafficking and adhesion (27, 28), as well as reorganization of the actin cytoskeleton (29). Like all GTPases, ARF cycles between a GDP- and a GTP-bound form. This is regulated by guanine nucleotide exchange factors (GEF) and GTPase-activating proteins (30). We have demonstrated, in heterologous recombinant cellular systems, that Ang II stimulation leads to the activation of ARF6 and ultimately impacts the Rac signaling pathway leading to cellular ruffling (31). In addition, we and others showed that both ARF1 and ARF6 are key regulators of migration and invasion of breast cancer cells (32, 33) further supporting a role for ARF GTPases in mediating receptor-dependent cellular behavior associated with pathophysiology. Using an in vitro approach, we have demonstrated that β-arrestin facilitates activation of ARF6 (23). However, the molecular details driving ARF6 activation remain to be defined, in particular in physiologically relevant cell models. Numerous reports demonstrate that activation of Ras-related GTPases can be mediated by different molecular pathways. For instance, the thromboxane A2 β-type receptor requires the Gq protein to activate ARF6 (34). In contrast, both G proteins and β-arrestin are necessary to drive GTP loading of Rho (21).
Here, we have studied the nature of the proximal events in the Ang II-mediated activation of ARF6 and defined key molecular pathways regulating cell migration. We have validated our findings in both HEK 293 cells and VSMCs. Using biochemical inhibitors, a biased ligand, RNA interference, and a β-arrestin mutant, we demonstrate for the first time that activation of this ARF isoform is mediated by β-arrestin and is independent of G protein activation. We further show that the β-arrestin C-terminal tail is responsible for scaffolding the ARF GEF ARNO, therefore defining the molecular mechanism responsible for β-arrestin-mediated ARF6 activation. Finally, we report that receptor endocytosis contributes to Ang II-stimulated cell migration. Altogether, we provide a molecular mechanism by which the AT1R can promote migration of VSMCs, a process that contributes namely to atherosclerosis and cardiovascular diseases. We show that by controlling both receptor internalization and actin remodeling, ARF6 regulates cellular migration in these cells.
Experimental Procedures
Reagents and Antibodies
l-Threonine, (3R)-N-acetyl-3-hydroxy-l-leucyl-(aR)-a-hydroxybenzenepropanoyl 2,3-idehydro-N-methylalanyl-l-alanyl-N-methyl-l-alanyl-(3R)-3-[[(2S,3R)-3-hydroxy-4methyl-1-oxo-2-[(1-oxopropyl)amino]pentyl] oxy]-l-leucyl-N,O-dimethyl-(7→1) lactone (9CI)) (UBO-QIC) (35), also known as FR900359 (36), was procured from the Institute of Pharmaceutical Biology of Bonn (Bonn, Germany). The polyclonal 3978 antibody is directed against a C-terminal epitope of β-arrestin1 and -2 (37). Anti-HA antibodies (3F10 and 12CA5) were purchased from Roche Applied Science (Cambridge, MA). Anti-pan-actin and anti-phospho-ERK1/2 were from Cell Signaling Technology (Danvers, MA). Protein G PLUS-agarose beads, anti-ARF6 (3A-1), anti-β-arrestin2 (H-9), and anti-Gq/11 (C-19) were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). PitStop2 was purchased from Abcam Biochemicals (Cambridge, MA). Lipofectamine 2000 was purchased from Invitrogen. Coelenterazine h was purchased from Nanolight® Technology (Pinetop, AZ). Polyornithine and all other products were from Sigma.
DNA Plasmids and siRNA
GST-GGA3, GST-ARNO, and HA-ARNO were gifts from Dr. J.-L. Parent (Université de Sherbrooke, Quebec, Canada). Double-stranded small interfering RNA (siRNA) targeting rat ARF6 (5′-AAUCCUCAUCUUCGCCAACAA-3′), human β-arrestin1 (5′-AAAGCCUUCUGCGCGGAGAAU-3′), and β-arrestin2 (5′-AAGGACCGCAAAGUGUUUGUG-3′) were previously described (24, 38). On-TARGET non-targeting siRNA was used as a control. All siRNAs were synthesized by GE Healthcare Dharmacon. Full-length and 1–320 β-arrestin2 constructs were previously characterized (39) and are a generous gift from Dr. J. L. Benovic (Thomas Jefferson University, Philadelphia).
Cell Culture and Transfection
HEK 293 cells stably expressing the HA-tagged AT1R were characterized previously (40) and were cultured in Eagle's minimal essential medium. Aortic VSMCs, isolated from Wistar rats by explant, were provided by Dr. Marc Servant (Université de Montréal, Montreal, Canada) and used at passages 7–10. These cells were characterized and shown to be responsive to Ang II as well as to express AT1R until passage 16 (41–44). Here, experiments were performed and repeated in cells from passages 7–16 to ensure reproducibility of the results in all assays. Cells were also monitored for their cuboidal morphology and sensitivity to Ang II throughout the different passages. VSMCs were maintained in Dulbecco's modified Eagle's medium. For both HEK 293 and VSMCs, culture media were supplemented with 10% fetal bovine serum (Wisent, St-Bruno, Quebec, Canada). All cells were maintained at 37 °C in 5% CO2 and used for no more than six other passages. All cells were transfected with siRNA using Lipofectamine 2000 according to the manufacturer's instructions. DNA constructs were transfected using a calcium phosphate mix (HBS 2×: 50 mm HEPES, pH 7.1, 280 mm NaCl, 1.5 mm Na2HPO4, mixed with 2.5 m CaCl2).
ARF6 Activation Assay
Cells were stimulated with Ang II (100 nm to 1 μm) at 37 °C for the indicated times. They were then lysed in 200 μl of lysis buffer E (pH 7.4, 50 mm Tris-HCl, 1% Nonidet P-40, 137 mm NaCl, 10% glycerol, 5 mm MgCl2, 20 mm NaF, 1 mm NaPPi, 1 mm Na3VO4, and protease inhibitors). Samples were spun for 10 min at 10,000 × g at 4 °C. GST-GGA3 fusion protein coupled to glutathione-Sepharose 4B beads was added to each tube, and samples were rotated at 4 °C for 1 h. Proteins were eluted into 20 μl of SDS sample buffer containing 5% β-mercaptoethanol by heating to 65 °C for 15 min, resolved on 14% SDS-PAGE, and detected by immunoblot using a specific anti-ARF6 antibody.
BRET Measurements
Cells were transfected with AT1R-YFP (3 μg) along with βarr2-RlucII (100 ng). The day following transfection, cells were detached and replated onto polyornithine-coated white 96-well plates at a density of ∼25,000 cells per well. The next day, cells were washed once with pre-warmed Tyrode's buffer (140 mm NaCl, 2.7 mm KCl, 1 mm CaCl2, 12 mm NaHCO3, 5.6 mm d-glucose, 0.5 mm MgCl2, 0.37 mm NaH2PO4, 25 mm HEPES, pH 7.4) and then incubated in the absence (DMSO) or presence of PitStop2 (10 μm) for 30 min at 37 °C. Then the cells were stimulated with either Ang II (final 1 μm) or Tyrode's (control), and BRET values were measured every minute. The cell-permeable substrate, coelenterazine h, was added at a final concentration of 5 μm in Tyrode's buffer 3 min before Ang II stimulation. Measurements were performed using a VictorTM X Light Luminescence plate reader (PerkinElmer Life Sciences) with a filter set of 460/40 and 535/25 nm for detecting the RlucII Renilla luciferase (donor) and YFP (acceptor) light emissions, respectively. The BRET signal was determined by calculating the ratio of the light intensity emitted by the YFP over the light intensity emitted by the RlucII. All BRET measurements were performed in triplicate at 37 °C.
Boyden Chamber Assay
Transfected cells were serum-starved and seeded into Boyden chambers (24-well inserts with 8-μm pore collagen-coated membranes). One hour after plating, cells were stimulated with Ang II (100 nm) or left untreated. After 4 h, cells were fixed using paraformaldehyde (4%) for 20 min and incubated with crystal violet (0.1% in 20% MeOH, overnight). Membranes were washed three times in distilled H2O, and cells were removed from the upper chamber, leaving those that migrated through the membrane in the lower chamber. Pictures of four different fields were taken, and cell migration was quantified using ImageJ. The average number of migrating cells was determined for each condition.
Wound Healing Assay
VSMCs were grown in 35-mm plates until they reached 100% confluence and were serum-starved overnight. Small linear scratches were performed using a 10-μl pipette tip. Cells were then rinsed several times with serum-free media to remove dislodged cells and were allowed to continue growing at 37 °C in media alone or with the addition of the agonist to the media, in a humidified incubator with 5% CO2. Cells were then fixed with paraformaldehyde (4%, 15 min) at designated time points (0 and 24 h), and pictures of five different fields were taken. A representative picture is presented for each condition.
Immunoprecipitation Experiments
Transfected HEK 293 cells were lysed in 200 μl of TGH buffer (50 mm NaCl, 50 mm HEPES, pH 7.3, 5 mm EDTA, 10% glycerol, 1% Triton X-100, supplemented with protease inhibitors and 1 mm Na3VO4). Samples were spun for 10 min at 10,000 × g at 4 °C. Equal amounts of protein were immunoprecipitated using the 3F10 HA antibody (2 h at 4 °C), and protein G-PLUS-agarose beads were subsequently added for 2 h. The bead-bound complexes were pelleted, washed several times with TGH lysis buffer, and proteins eluted with SDS sample buffer containing 5% β-mercaptoethanol by heating to 65 °C for 5 min.
GST Pulldown Assay
Transfected HEK 293 cells were lysed in 200 μl of lysis buffer E (pH 7.4, 50 mm Tris-HCl, 1% Nonidet P-40, 137 mm NaCl, 10% glycerol, 5 mm MgCl2, 20 mm NaF, 1 mm NaPPi, 1 mm Na3VO4, and protease inhibitors). Samples were spun for 10 min at 10,000 × g at 4 °C. GST-ARNO fusion protein coupled to glutathione-Sepharose 4B beads was added to each tube, and samples were rotated at 4 °C for 1 h. Proteins were eluted into 20 μl of SDS sample buffer containing 5% β-mercaptoethanol by heating to 65 °C for 15 min and resolved on 14% SDS-PAGE.
Western Blotting
Cells were harvested, and total soluble proteins were run on polyacrylamide gels and transferred onto nitrocellulose membranes. The membranes were blotted for relevant proteins using specific primary antibodies (as described for each experiment). Secondary antibodies were FITC- or HRP-conjugated, and fluorescence was detected using a Typhoon 9410 scanner (GE Healthcare) or with ECL detection reagent. Quantification of the digital images obtained was performed using ImageJ.
Statistical Analysis
Statistical analyses were performed using a one-way analysis of variance followed by Bonferroni's or Dunnett's multiple comparison tests (version 4.0a; San Diego).
Results
β-Arrestin Controls AT1R-mediated Activation of ARF6 in HEK 293 Cells
We first aimed to define the molecular mechanism by which AT1R promoted activation of ARF6. To investigate the role of endogenous Gq, we used a novel chemical inhibitor, UBO-QIC (35, 45, 46). This compound directly binds the G protein to inhibit GDP release (47). As illustrated in Fig. 1, Ang II stimulation enhanced ARF6 activation in HEK 293 cells stably overexpressing the AT1R. Pretreatment of the cells with UBO-QIC had no effects on the ability of Ang II to promote activation of this GTPase (Fig. 1). However, UBO-QIC was effective in inhibiting ERK1/2 phosphorylation, a process that appears to be mainly mediated by Gq in our HEK 293 cells.
FIGURE 1.

Ang II-dependent ARF6 activation is unaffected by Gq inhibitor UBO-QIC. HA-AT1R-expressing HEK 293 cells were pretreated with DMSO (0.1%) or Gq inhibitor UBO-QIC (100 nm) for 30 min. They were stimulated afterward with Ang II (1 μm) for 2 min. Cells were lysed, and ARF6 activity was assessed in a GTPase activation assay using GST-GGA3. ARF6 levels were assessed by Western blotting using specific antibodies against ARF6. Quantifications are the mean ± S.E. of three independent experiments. *, p < 0.05 are values compared with the basal ARF6 activation level of control (ctl) cells. IB, immunoblot.
We next examined the role of β-arrestin in Ang II-mediated ARF6 activation. Because there are no biochemical inhibitors yet effective in blocking β-arrestin proteins, we used RNAi to knock down endogenous expression of the two β-arrestin isoforms. As illustrated in Fig. 2A, transfection of the β-arrestin siRNA (48 h), into HEK 293 cells stably expressing the HA-AT1R (40), reduced by 66% expression of β-arrestin1 and -2 compared with the control condition. Ang II stimulation of control cells (transfected with a scrambled siRNA) promoted a 2.3-fold increase in ARF6 activation. In contrast, in conditions where expression of the two β-arrestin isoforms was reduced, Ang II stimulation was ineffective in promoting GTP loading of this ARF protein (Fig. 2A). To complement these data, we overexpressed a truncated β-arrestin2 lacking the C-terminal tail (βarr2-(1–320)) and examined Ang II-mediated ARF6 activation. This truncated β-arrestin mutant is known to have a dominant negative effect on receptor endocytosis (39) because of the deletion of both the clathrin and the β-adaptin (AP-2)-binding sites (12, 14). Although transfection of full-length β-arrestin2 had no effect on ARF6 activation, overexpression of the β-arrestin2(1–320) construct impaired the ability of this ligand to promote ARF6 activation (Fig. 2B). These data demonstrate that impaired β-arrestin function greatly impacts the ability of the AT1R to activate ARF6. Altogether, these results suggest that Ang II-mediated activation of this ARF isoform is a process requiring receptor signaling to β-arrestin but not activation of Gq.
FIGURE 2.

β-Arrestin depletion and truncated mutant expression inhibits Ang II-dependent ARF6 activation. A, HA-AT1R-expressing HEK 293 cells were transfected with control (ctl) or a mixture of β-arrestin1 and -2 siRNA for 48 h. They were then stimulated with Ang II (1 μm) for 2 min. Cells were lysed, and ARF6 activity was assessed using GST-GGA3. B, HA-AT1R expressing HEK 293 were transfected with an empty vector, full-length β-arrestin2, or a truncated β-arrestin2-(1–320) mutant for 24 h. They were stimulated afterward with Ang II (1 μm) for 2 min. Cells were lysed, and ARF6 activity was assessed. IB, immunoblot. Quantifications are the mean ± S.E. of three independent experiments. *, p < 0.05.
Full-length but Not Truncated β-Arrestin2 Interacts with the ARF GEF ARNO to Facilitate ARF6 Activation in HEK 293 Cells
Because of their ability to act as scaffolds, β-arrestin can interact with many different proteins. First, we demonstrate that Ang II stimulation promoted the association of β-arrestin and ARF6 in a time-dependent fashion with an early peak after 3 min of agonist stimulation and a later peak at 15 min (Fig. 3A). To further understand how β-arrestin can regulate GTP-loading of ARF6, we examined whether the C-terminal truncated mutant could still interact, or be found in complex, with either ARF6 or ARNO. In a co-immunoprecipitation experiment, we observed that ARF6 could interact with β-arrestin2-(1–320), when HEK 293 cells were in complete medium (Fig. 3B). In contrast, the truncated β-arrestin2 mutant form was unable to associate with the ARF GEF (Fig. 3C). To confirm our findings, we used an alternative approach and attempted to pull down overexpressed β-arrestin from cell lysates using purified GST-ARNO. As illustrated in Fig. 3, D and E, truncated β-arrestin2 showed much lower association with the ARF GEF, as GST-ARNO precipitated only 2% of the β-arrestin2-(1–320), whereas 31% of wild type β-arrestin2 was pulled down by the same fusion protein. In sum, these data suggest that β-arrestin acts as a scaffold to facilitate activation of ARF6 by interacting with its GEF.
FIGURE 3.
Interaction of β-arrestin with ARF6 and ARNO. A, HA-AT1R-expressing HEK 293 cells were stimulated for the indicated times with Ang II (1 μm). Cells were lysed, and endogenous ARF6 was immunoprecipitated (IP) using an anti-ARF6 antibody. Interacting β-arrestin2 levels were assessed by Western blotting using specific antibodies against β-arrestin2. Quantifications are the mean ± S.E. of four independent experiments. B and C, HEK 293 cells were transfected with HA-ARF6 (B) or HA-ARNO (C) and either full-length or truncated β-arrestin2-(1–320) for 24 h. Cells were lysed, and HA-ARF6 (B) or HA-ARNO (C) was immunoprecipitated using an anti-HA antibody. Interacting β-arrestin2 levels were assessed by Western blotting. Blots shown are representative of three experiments. D, HEK 293 cells were transfected with an empty vector, full-length, or truncated β-arrestin2-(1–320) for 24 h. Cells were lysed, and β-arrestin2 was precipitated using GST-ARNO. Interacting β-arrestin2 levels were assessed by Western blotting using specific antibodies against β-arrestin2. Blots shown are representative of three experiments. E, quantifications of results obtained in D. Percentages were determined by establishing the ratio between the intensity of the bands of pulled down wild type or mutant β-arrestin2 and the intensity of their corresponding input bands. Quantifications are the mean ± S.E. of four independent experiments. ***, p < 0.001; **, p < 0.01; *, p < 0.05. IB, immunoblot.
Because β-arrestin is a key regulator of clathrin-mediated endocytosis of receptors and that, as mentioned previously, its C-terminal truncated form cannot support this process, we next examined whether blocking internalization could impair the ability of Ang II to stimulate ARF6. For these experiments, we used PitStop2, a potent inhibitor of clathrin-mediated endocytosis (48). As observed in Fig. 4A, pretreatment of the cells with PitStop2 did not significantly affect Ang II-dependent ARF6 activation nor did it affect the ability of β-arrestin2 to interact with ARF6 in cells (Fig. 4B). Furthermore, inhibition of endocytosis using PitStop2 did not affect the ability of β-arrestin2 to be recruited by receptors following Ang II stimulation in a BRET-based assay (Fig. 4C). This observation was confirmed when we examined the distribution of β-arrestin2 by imaging. As illustrated in Fig. 4D, β-arrestin2-GFP was mainly distributed in the cytosol of both control and PitStop2-treated cells. Ang II stimulation led to the translocation of β-arrestin2-GFP at the plasma membrane and subsequent trafficking into endosomes (90 and 240 s, respectively) in control cells (top right panel, arrows in the inset). Although PitStop2 treatment did not prevent the ability of β-arrestin2-GFP to relocalize to the membrane upon Ang II stimulation, it blocked internalization of the receptor·β-arrestin2 complex. Indeed, β-arrestin2-GFP remained mostly in clusters trapped at the plasma membrane (Fig. 4D, lower right panel, inset). Altogether, these results demonstrate that receptor internalization is not a key event regulating GTP loading of the ARF.
FIGURE 4.
Effect of PitStop2 on ARF6 activation and β-arrestin recruitment to the AT1R. A, HA-AT1R-expressing HEK 293 cells were pretreated with DMSO (0.1%) or PitStop2 (10 μm) for 30 min. They were stimulated afterward with Ang II (1 μm) for 2 min. Cells were lysed, and ARF6 activity was assessed by Western blotting. Quantifications are the mean ± S.E. of three independent experiments. B, HEK 293 cells were transfected with HA-ARF6 and full-length β-arrestin2 for 24 h. Cells were pretreated with DMSO or PitStop2 (10 μm) for 30 min and then were lysed, and HA-ARF6 was immunoprecipitated (IP) using an anti-HA antibody. Interacting β-arrestin2 levels were assessed by Western blotting. Blots shown are representative of three experiments. C, time course of the β-arrestin recruitment to AT1R. HEK293 cells expressing AT1R-YFP and β-arrestin2-RLucII were incubated in the absence (DMSO) or presence of 10 μm PitStop2 for 30 min before addition of Ang II (final concentration of 1 μm) and BRET measurements. Data are expressed as ΔBRET by subtracting the basal BRET ratios observed in the control (no Ang II) group. Data represent means ± S.E. of four independent experiments. D, confocal microscopy of the β-arrestin2 trafficking upon AT1R activation. Cells transiently expressing AT1R and β-arrestin2-YFP were incubated in the absence (DMSO, top panels) or presence of 10 μm PitStop2 (bottom panels) for 30 min before Ang II (final concentration of 200 nm) stimulation for up to 4 min. Dashed boxes represent blown-up section of cells shown in insets. Arrows depict endosomes containing β-arrestin2 inside the cells. Scale bars, 10 μm. *, p < 0.05. IB, immunoblot.
HEK 293 Cell Migration Is Regulated by β-Arrestin, ARF6, and Receptor Endocytosis
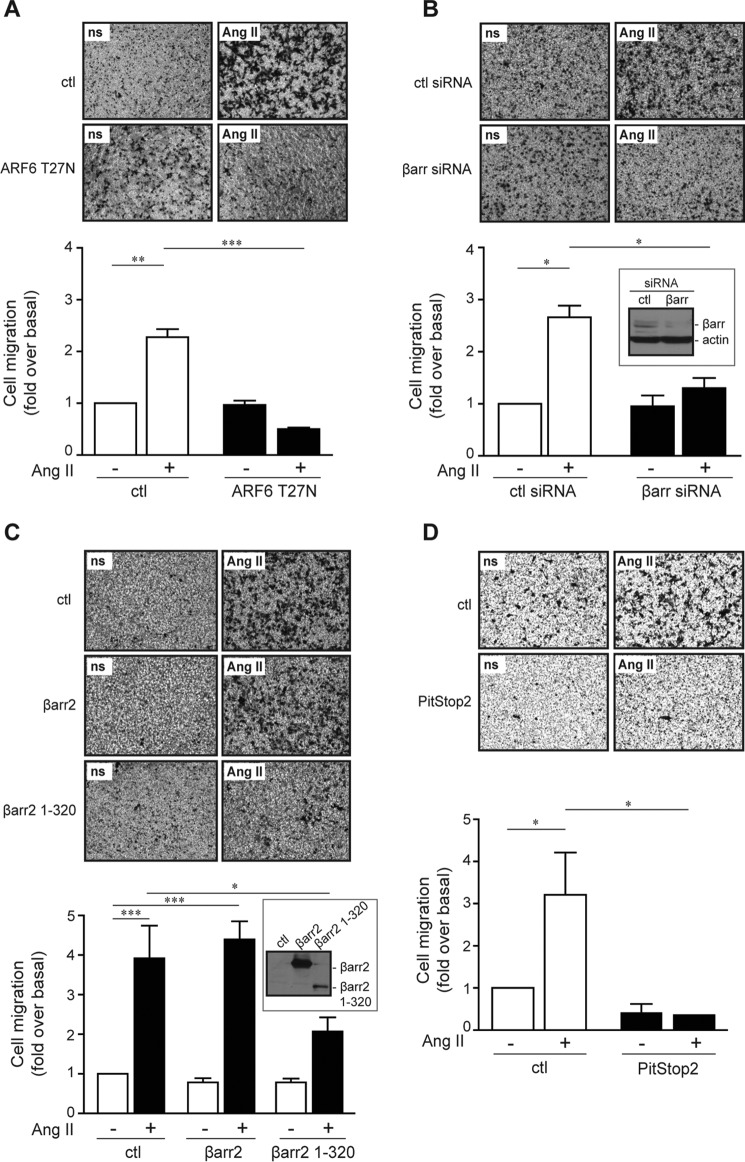
One of the most important and best-studied Ang II-dependent cellular responses is migration. Here, we therefore examined the role of ARF6 using the dominant negative mutant, ARF6 T27N. We used this approach instead of completely knocking down expression of the GTPase because we have shown previously that limiting ARF6 expression results in high Rac1 activation and spontaneous membrane ruffling, a process unrelated with GTP loading of this ARF protein in HEK 293 cells (31). As illustrated in Fig. 5A, expression of the dominant negative ARF6 mutant did not affect basal cell migration but completely blocked the ability of Ang II to promote this response. To confirm that the key proteins we have identified in the activation process of this GTPase also regulate ARF6-mediated effects, we examined whether β-arrestin could modulate this response. As shown in Fig. 5B, knockdown of β-arrestin effectively reduced migration of AT1R-expressing HEK 293 cells following a 4-h Ang II treatment when using the Boyden chamber assay. In contrast, overexpression of wild type β-arrestin2 had no effect on Ang II-stimulated cell migration suggesting that β-arrestin expression is not limiting in these cells. Interestingly, expression of the β-arrestin2 mutant defective in ARF6 activation and unable to support receptor endocytosis inhibited Ang II-stimulated cell migration by 47% compared with the control condition and 53% compared with the β-arrestin2-overexpressing condition (Fig. 5C). Because this β-arrestin as well as dominant negative ARF6 are both unable to effectively support receptor internalization, we examined whether treatment with PitStop2 could also modulate Ang II-mediated cell migration. We observe that treatment of AT1R-expressing HEK 293 cells with this clathrin inhibitor effectively blocked Ang II-stimulated cell migration (Fig. 5D). To confirm this finding, we used hyperosmotic sucrose treatment as an alternative approach to block clathrin-mediated endocytosis. As illustrated in Fig. 6A, this treatment also prevented Ang II-dependent cell migration, while having no impact on ARF6 activation (Fig. 6B) as well as on the interaction between β-arrestin2 and the small GTPase (Fig. 6C). In sum, these observations confirm the role of β-arrestin in Ang II-stimulated cell migration, but they also highlight the importance of receptor endocytosis in this important physiological response.
FIGURE 5.
Dominant negative ARF expression, β-arrestin depletion, and truncated mutant expression inhibit Ang II-dependent cell migration. A–C, HA-AT1R-expressing HEK 293 cells were transfected with ARF6 T27N for 24 h (A), with control (ctl) or a mixture of β-arrestin1 and -2 siRNA for 48 h (B), or with an empty vector, full-length β-arrestin2, or a truncated mutant (β-arrestin2-(1–320)) for 24 h (C). Cells were trypsinized and reseeded into Boyden chambers where they were left for 1 h. One set of cells was stimulated with Ang II (100 nm), and the other was left untreated. Migration to the lower chamber was evaluated after 4 h for all conditions. D, HA-AT1R-expressing HEK 293 cells were trypsinized and reseeded in Boyden chambers where they were pretreated with DMSO or PitStop2 (10 μm) for 30 min. One set of cells was stimulated with Ang II (100 nm), and the other was left untreated. Migration to the lower chamber was evaluated after 4 h for all conditions. For all experiments, quantifications are the mean ± S.E. of three independent experiments. ***, p < 0.001; **, p < 0.01; *, p < 0.05. ns, nonstimulated.
FIGURE 6.

Effect of sucrose on cell migration and ARF6 activation. A, HA-AT1R-expressing HEK 293 cells were trypsinized and reseeded in Boyden chambers where they were pretreated or not with sucrose (0.45 m) for 30 min. One set of cells was stimulated with Ang II (100 nm), and the other was left untreated. Migration to the lower chamber was evaluated after 4 h for all conditions. Quantifications are the mean ± S.E. of three independent experiments. B, HA-AT1R-expressing HEK 293 cells were pretreated or not with sucrose (0.45 m) for 30 min. They were stimulated afterward with Ang II (1 μm) for 2 min. Cells were lysed, and ARF6 activity was assessed by Western blotting. Quantifications are the mean ± S.E. of three independent experiments. C, HEK 293 cells were transfected with HA-ARF6 and full-length β-arrestin2 for 24 h. Cells were pretreated or not with sucrose (0.45 m) for 30 min and then were lysed, and HA-ARF6 was immunoprecipitated using an anti-HA antibody. Interacting β-arrestin2 levels were assessed by Western blotting. Blots shown are representative of three experiments. ***, p < 0.001; **, p < 0.01; *, p < 0.05. IB, immunoblot.
Because endocytosis partially controls Ang II signaling to mitogenic pathways, we further investigated whether the key signaling events we have identified also controls ERK1/2 activation. As illustrated in Fig. 7A, expression of the ARF6 dominant negative mutant, ARF6T27N, reduced ERK1/2 phosphorylation by 32%. Treatment of the cells with PitStop2 had similar effects (24%) (Fig. 7B). However, inhibition of Gq using UBO-QIC reduced by 71% ERK phosphorylation (Fig. 7C). These findings suggest that in our HEK 293 cells, activation of the MAPK pathway is mainly regulated by Gq. ARF6 and endocytosis do however also play a role. Finally, we examined whether ERK activation was important for cell migration. As illustrated in Fig. 7D, treatment of cells with the MEK inhibitor PD98059 did not significantly block Ang II-stimulated ERK1/2 activation, underscoring the lack of role of this cellular event in cell migration.
FIGURE 7.

Effect of ARF6 inhibition, PitStop2 treatment, and UBO-QIC treatment on AT1R-dependent ERK activation. HA-AT1R-expressing HEK 293 cells were either transfected with ARF6 T27N for 24 h (A) or pretreated with DMSO or PitStop2 (B) or UBO-QIC (C). They were stimulated afterward with Ang II (1 μm) for the indicated times. Cells were lysed, and ERK1/2 activity was assessed by Western blotting. D, HA-AT1R expressing HEK 293 cells were trypsinized and reseeded in Boyden chambers where they were pretreated with DMSO (ctl) or PD98059 (50 μm) for 30 min. One set of cells was stimulated with Ang II (100 nm), and the other was left untreated. Migration to the lower chamber was evaluated after 4 h for all conditions. Quantifications are the mean ± S.E. of three independent experiments. ***, p < 0.001; **, p < 0.01; *, p < 0.05. IB, immunoblot.
Ang II Stimulation Promotes ARF6 Activation, an Event Required for VSMC Migration
Because few studies have explored the role of ARF6 proteins in primary cells expressing endogenous levels of receptors and effectors, we aimed at defining whether stimulation of the AT1R, in VSMCs, led to the activation of this ARF isoform and involved the molecular mechanisms identified in HEK 293 cells. VSMCs were isolated from rat aorta as described under “Experimental Procedures.” We and others have shown that these cells, when cultured in the conditions described, retained the expression of AT1R and that Ang II stimulation resulted in the activation of numerous cellular events, which could be blocked by selective AT1R antagonists (42–44, 49). As illustrated in Fig. 8A, Ang II treatment resulted in a rapid and transient increase of ARF6-GTP levels, which were maximal after 5 min of stimulation and returned to basal levels 15 min post-treatment. Because selective engagement of the β-arrestin-dependent signaling events can be achieved by using biased ligands, we stimulated cells with SII, known to promote β-arrestin recruitment to the receptor, but not receptor coupling to heterotrimeric G proteins (50, 51). As illustrated in Fig. 8B, stimulation of VSMCs with SII promoted a rapid and transient activation of ARF6, similar to what we observed when cells were stimulated with Ang II with the peak of ARF6-GTP detected after 5 min of stimulation, and a return to basal levels after 10 min.
FIGURE 8.
Ang II and SII stimulation of VSMCs leads to ARF6 activation. VSMCs were stimulated with Ang II (100 nm) (A) or SII (10 μm) (B) for the indicated durations. Cells were lysed; ARF6 activity was assessed by Western blotting. Quantifications are the mean ± S.E. of four independent experiments performed in cells at different passages (between 7 and 16). ***, p < 0.001; **, p < 0.01 are values compared with the basal activation level. IB, immunoblot.
We next examined the ability of Ang II and SII to promote migration of VSMCs in a wound healing assay, an approach regularly used to assess cell motility. After 24 h of treatment, wound healing was found to be on average 29% in control conditions demonstrating the intrinsic capacity of those cells to migrate and close the wounded area (Fig. 9A). Ang II stimulation enhanced motility and healing of the wound by 55%. Interestingly, SII was as potent and resulted in an average 60% closure of the wound suggesting that AT1R engagement of β-arrestin is sufficient to promote VSMC migration. To demonstrate that in this cell line Gq activation is not a key event regulating this cellular response, we examined the consequence of inhibiting this G protein. UBO-QIC treatment did not modulate the ability of Ang II to promote cell migration (Fig. 9B).
FIGURE 9.

Ang II and SII induce VSMC migration, an event that requires ARF6 expression. A, VSMCs were grown to confluence, and scratches were performed. These cells were stimulated with either Ang II (100 nm) or SII (10 μm) or were left untreated. Cells were fixed after 24 h, and six to eight pictures were taken for each condition. The images shown here are representative of four experiments, and quantifications are the mean ± S.E. of all four independent experiments. B, VSMCs were trypsinized and reseeded in Boyden chambers where they were pretreated with DMSO (ctl) or UBO-QIC (100 nm) for 30 min. One set of cells was stimulated with Ang II (100 nm), and the other was left untreated. Migration to the lower chamber was evaluated after 4 h for all conditions. C, VSMCs were transfected with ARF6 siRNA for 48 h. They were serum-starved for 16 h, then trypsinized, and reseeded into Boyden chambers where they were left for 1 h. One set of cells was stimulated with Ang II (100 nm), and the other was left untreated. Migration to the lower chamber was evaluated after 4 h for all conditions. Quantifications are the mean ± S.E. of three independent experiments. All experiments were performed in cells at different passages (between 7 and 16). ***, p < 0.001; **, p < 0.01; *, p < 0.05. IB, immunoblot. ns, nonstimulated.
To confirm the importance of ARF6 in the migratory response of these cells, we next knocked down expression of this ARF isoform. First, stimulation of VSMCs for 4 h with Ang II enhanced the migratory capacities of these cells by 2.3-fold (Fig. 9C). Our previous findings have shown that this process is dependent upon the presence of β-arrestin proteins because their depletion effectively blocks migration in this cell type (43). In these conditions, our ARF6 siRNA was effective in reducing ARF6 expression by 93%. As illustrated in Fig. 9C, depletion of this ARF isoform markedly abolished Ang II-promoted migration of cells. In sum, these data demonstrate that engagement of ARF6 is a key event in the signaling cascade leading to VSMC migration following Ang II treatment.
Altogether, these findings suggest that Ang II-mediated migration of VSMCs depends on the ability of the endogenously expressed AT1R to engage β-arrestin without the need to activate Gq, and this process also requires ARF6.
Receptor Endocytosis Contributes to Ang II-mediated Cell Migration
Because we have observed that receptor endocytosis is a key event in migration of HEK 293 cells, and because growing evidence suggests that receptor endocytosis can drive cell motility (52), we next examined the consequence of blocking clathrin-coated pit formation with PitStop2 in VSMCs. As illustrated in Fig. 10A, treatment of these cells with the biochemical inhibitor significantly inhibited their ability to migrate upon Ang II stimulation. Contrary to HEK 293 cells, pretreatment of VSMCs with the MEK inhibitor PD98059 blocked Ang II-dependent migration (Fig. 10B), confirming the importance of the ERK pathway in this event. We also observed that PitStop2 was effective at reducing AT1R-mediated ERK activation following either Ang II or SII stimulation of cells (Fig. 10, C and D). Consistent with the role of ARF6 in regulating AT1R internalization and the need of receptor endocytosis for MAPK in VSMCs, we also confirmed that this small GTPase regulated β-arrestin-dependent ERK1/2 activation. In VSMCs, ERK1/2 phosphorylation peaked at 5 min following Ang II stimulation, and ARF6 knockdown markedly reduced this signaling event (Fig. 11A). The β-arrestin-biased ligand SII, although less potent, was also able to promote ERK1/2 activation (Fig. 11B), a process also dependent upon ARF6 GTPase.
FIGURE 10.
Inhibition of clathrin-mediated endocytosis inhibits VSMC migration, as well as Ang II- and SII-dependent ERK activation. A and B, VSMCs were trypsinized and reseeded in Boyden chambers where they were pretreated with DMSO (ctl), PitStop2 (10 μm) (A) or PD98059 (50 μm) (B) for 30 min. One set of cells was stimulated with Ang II (100 nm), and the other was left untreated. Migration to the lower chamber was evaluated after 4 h for all conditions. C and D, VSMCs were pretreated with DMSO (0.1%) or PitStop2 (10 μm) for 30 min. They were stimulated afterward with Ang II (100 nm) (C) or SII (10 μm) (D) for the indicated durations. Cells were lysed, and ERK1/2 activation was assayed by Western blotting. Quantifications are the mean ± S.E. of three independent experiments. All experiments were performed in cells at different passages (between 7 and 16). ***, p < 0.001; **, p < 0.01; *, p < 0.05. ns, nonstimulated.
FIGURE 11.
ARF6 depletion inhibits Ang II- and SII-dependent ERK activation. A and B, VSMCs were transfected for 48 h with either control or ARF6 siRNA. Cells were stimulated with Ang II (100 nm) (A) or SII (10 μm) (B) for the indicated durations. Cells were lysed, and ERK1/2 activation was assayed by Western blotting. Quantifications are the mean ± S.E. of three independent experiments. All experiments were performed in cells at different passages (between 7 and 16). **, p < 0.01; *, p < 0.05. IB, immunoblot.
In summary, these findings reveal that receptor signaling via β-arrestin regulate activation of the small GTPase ARF6 to control key cellular responses and to ultimately promote cellular migration. For the first time, we show that the coordinated regulation of receptor endocytosis and MAPK activation by both β-arrestin and ARF6 are key events regulating migration of VSMCs.
Discussion
In this study, we have initially used HEK 293 cells to delineate the molecular mechanisms by which AT1R leads to the activation of ARF6 and ultimately cell migration. Using VSMCs, we have confirmed that AT1R engages β-arrestin to activate ARF6, and through the regulation of endocytosis, these events contribute to control cell migration.
The AT1R has been used as a model receptor to show that G protein-coupled receptors can engage cellular responses through coupling either to G proteins or β-arrestin. Activation of the GTPase Rho by the AT1R was shown to require Gq and β-arrestin (21). Alternatively, thromboxane A2 β-type receptor was reported to activate ARF6 via coupling to Gq (34). Although we show that in HEK 293 cells we can effectively block Gq activation by using a novel biochemical inhibitor, UBO-QIC, this G protein does not mediate activation of ARF6. In contrast, inhibition of β-arrestin expression or function greatly impacts the ability of Ang II to promote ARF6 activation. The ability of G protein-coupled receptors to activate a key molecular switch such as ARF6 resides in the intrinsic properties of those receptors to engage, with different efficacy, their effectors. Although it remains to be fully documented, the thromboxane A2 β-type receptor and the AT1R may have distinct propensity to activate Gq versus β-arrestin in HEK 293 cells and therefore utilize differently these effectors to activate common downstream signaling proteins.
The use of a truncated form of β-arrestin (β-arrestin-(1–320)) construct that lacked the C-terminal domain allowed us to demonstrate that this adaptor protein serves to scaffold the ARF GEF ARNO, a process necessary for activation of ARF6. This mutant β-arrestin construct does not have a nuclear export signal domain but can still be recruited to receptors (39, 53). It has been reported that when in complex with the receptor, β-arrestin undergoes a conformational rearrangement. The C-terminal tail of β-arrestin is displaced and thus free to interact with other proteins (54). This model was supported by elucidation of the structure of the C terminus lacking the p44 β-arrestin1 splice variant (55) as well as by elucidation of the structure of β-arrestin1 with the C-terminal tail of the vasopressin receptor (56). Together, this evidence suggests that a β-arrestin mutant lacking its C terminus would be able to bind an activated receptor but would have reduced affinity for some of its effectors/interacting partners, like ARNO. This ARF GEF belongs to the cytohesin family, whose autoinhibition can be relieved by interacting with other proteins (57). Binding of β-arrestin to ARNO could serve to bring the GEF to a more activated state. Interestingly, cytohesins can also bind activated ARF6 to reach a more active state, suggesting that GTP loading on ARF6 molecules could be stimulated by a positive feedback loop (58). Moreover, it has been shown that EFA6, another ARF6 GEF, interacts with β-arrestin through its C-terminal end (59). It was proposed that both GEFs (cytohesin and EFA6) successively mediate ARF6 activation during endocytic events (60).
In addition, the β-arrestin2-(1–320) construct lacks the AP-2 and the clathrin-binding sites (14), enabling it to support receptor endocytosis. Our findings using this tool led us to examine the role of clathrin-mediated internalization in ARF6 activation and cell migration. Using inhibitors such as PitStop2 and sucrose, we observed that this cellular response is not important for activation of ARF6 but that receptor trafficking does contribute to more complex cellular responses such as migration. Previous studies have revealed the importance of clathrin-mediated internalization in both agonist-dependent (52) or -independent (61) cell migration. When we inhibited clathrin-mediated endocytosis, we observed that Ang II-dependent cell migration was blocked. Considering that this observation has also been reported for the platelet-derived growth factor receptor (52), it suggests that this event may not be limited to receptors of the G protein-coupled family.
Because sequestration of receptors from the cell surface has been shown to contribute to the continuation of signaling inside cells such as MAPK activation (15, 17), we also considered whether the engagement of such signaling pathways contributed to the regulation of Ang II-dependent cell migration. In our HEK 293 cells, inhibiting MAPK activation did not affect cell migration. Similar observations have also been reported before (4). In contrast, activation of AT1R, by a β-arrestin biased ligand, which promotes receptor internalization and MAPK activation, resulted in VSMC migration (43). Moreover, inhibition of either MAPK activation or receptor endocytosis greatly impeded Ang II-dependent VSMC migration, potentially linking these two events for cell migration regulation. Our findings also suggest that endocytosis and β-arrestin contribute to AT1R-mediated migration of HEK 293 cells. However, it is still unclear how internalization and whether other signaling events dependent on β-arrestin contribute to regulating migration in these cells. Our findings underscore the importance of studying such complex cellular responses in different cell types, in particular relevant physiological ones like VSMCs. Despite VSMCs expressing AT1R and being responsive to Ang II (even in late passage tissue culture) (42–44, 62–64) for MAPK activation, the signaling pathway leading to cell migration differs from HEK 293 cells as observed here. A detailed analysis in multiple cell lines may indeed reveal that signaling pathways engaged by receptors to promote a specific cellular response can vary between cell types but also for the same cell type depending on its origin as shown previously for MAPK in HEK 293 cells (65). Our results thus suggest that depending on the molecular mechanisms by which a signaling intermediate such as MAPK is activated, its role in the cell migration response may vary.
Altogether, our study has contributed to elucidate the molecular mechanisms by which the AT1R activate ARF6 and ultimately cell migration (Fig. 12). It is the ability of this receptor to engage β-arrestin-dependent signaling events that serve to control activation of this small GTP-binding protein by ARNO and possibly other ARF GEF. GTP-bound ARF6, in turn, activates its numerous effectors. It was shown that this ARF remained present at the plasma membrane (66), although it can be found in a specific subset of endosomes (67). Upon Ang II treatment, ARF6 controls the recruitment of clathrin and AP-2 to the receptor·β-arrestin complex to promote internalization, in addition to the remodeling of the actin cytoskeleton (29, 68). With better knowledge of the mechanisms regulating activation of ARF6, we can better understand how receptors control physiological responses such as cell migration. Proteins such as ARF could thus serve as molecular targets for the design of new therapeutics to treat diseases of the cardiovascular system where such responses are deregulated.
FIGURE 12.
Visual summary of the pathways demonstrated in this study. This figure summarizes the early events, which lead to Ang II-mediated cell migration. First, after stimulation of the AT1R, β-arrestins were recruited to the activated receptor while scaffolding an ARF GEF to promote ARF6 activation at the membrane. Second, β-arrestins and ARF6 recruit clathrin, which promotes the formation of clathrin-coated pits and enables receptor endocytosis. Finally, β-arrestins, which are still in a stable complex with the AT1R, can scaffold elements of the MAPK cascade leading to ERK activation.
Author Contributions
R. C., S. A. L., and A. C. designed the experimental plan and wrote the paper. R. C., Y. N., and M. C. performed the research.
Acknowledgments
We thank Dr. M. Bouvier (Institut de Recherche en Immunologie et en Cancérologie, Université de Montréal) for providing PitStop2. We also thank Dr. J. L. Benovic (Thomas Jefferson University) for the β-arrestin constructs as well as Dr. M. Servant (Université de Montréal) for providing VSMCs.
This work was supported in part by the Canadian Institutes of Health Research Grants MOP-79470 (to A. C.) and MOP-74603 (to S. A. L.) and the Heart and Stroke Foundation of Canada (to A. C.). The authors declare that they have no conflicts of interest with the contents of this article.
- Ang II
- angiotensin II
- ARF
- ADP ribosylation factor
- AT1R
- angiotensin II type 1 receptor
- VSMC
- vascular smooth muscle cell
- UBO-QIC
- l-threonine, (3R)-N-acetyl-3-hydroxy-l-leucyl-(aR)-a-hydroxybenzenepropanoyl 2,3-idehydro-N-methylalanyl-l-alanyl-N-methyl-l-alanyl-(3R)-3-[[(2S,3R)-3-hydroxy-4methyl-1-oxo-2-[(1-oxopropyl)amino]pentyl]oxy]-l-leucyl-N,O-dimethyl-(7→1) lactone (9CI)
- GEF
- guanine nucleotide exchange factor
- BRET
- bioluminescence resonance energy transfer.
References
- 1. Rajagopalan S., Kurz S., Münzel T., Tarpey M., Freeman B. A., Griendling K. K., and Harrison D. G. (1996) Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Invest. 97, 1916–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laursen J. B., Rajagopalan S., Galis Z., Tarpey M., Freeman B. A., and Harrison D. G. (1997) Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation 95, 588–593 [DOI] [PubMed] [Google Scholar]
- 3. Ushio-Fukai M., Zafari A. M., Fukui T., Ishizaka N., and Griendling K. K. (1996) p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J. Biol. Chem. 271, 23317–23321 [DOI] [PubMed] [Google Scholar]
- 4. Hunton D. L., Barnes W. G., Kim J., Ren X. R., Violin J. D., Reiter E., Milligan G., Patel D. D., and Lefkowitz R. J. (2005) β-Arrestin 2-dependent angiotensin II type 1A receptor-mediated pathway of chemotaxis. Mol. Pharmacol. 67, 1229–1236 [DOI] [PubMed] [Google Scholar]
- 5. Lee H. M., Lee C. K., Lee S. H., Roh H. Y., Bae Y. M., Lee K. Y., Lim J., Park P. J., Park T. K., Lee Y. L., Won K. J., and Kim B. (2007) p38 mitogen-activated protein kinase contributes to angiotensin II-stimulated migration of rat aortic smooth muscle cells. J. Pharmacol. Sci. 105, 74–81 [DOI] [PubMed] [Google Scholar]
- 6. Touyz R. M., and Schiffrin E. L. (2000) Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol. Rev. 52, 639–672 [PubMed] [Google Scholar]
- 7. Louis S. F., and Zahradka P. (2010) Vascular smooth muscle cell motility: from migration to invasion. Exp. Clin. Cardiol. 15, e75–85 [PMC free article] [PubMed] [Google Scholar]
- 8. Lohse M. J., Benovic J. L., Codina J., Caron M. G., and Lefkowitz R. J. (1990) β-Arrestin: a protein that regulates β-adrenergic receptor function. Science 248, 1547–1550 [DOI] [PubMed] [Google Scholar]
- 9. Attramadal H., Arriza J. L., Aoki C., Dawson T. M., Codina J., Kwatra M. M., Snyder S. H., Caron M. G., and Lefkowitz R. J. (1992) β-Arrestin2, a novel member of the arrestin/β-arrestin gene family. J. Biol. Chem. 267, 17882–17890 [PubMed] [Google Scholar]
- 10. DeWire S. M., Ahn S., Lefkowitz R. J., and Shenoy S. K. (2007) β-Arrestins and cell signaling. Annu. Rev. Physiol. 69, 483–510 [DOI] [PubMed] [Google Scholar]
- 11. Xiao K., McClatchy D. B., Shukla A. K., Zhao Y., Chen M., Shenoy S. K., Yates J. R. 3rd, and Lefkowitz R. J. (2007) Functional specialization of β-arrestin interactions revealed by proteomic analysis. Proc. Natl. Acad. Sci. U.S.A. 104, 12011–12016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krupnick J. G., Goodman O. B. Jr., Keen J. H., and Benovic J. L. (1997) Arrestin/clathrin interaction. Localization of the clathrin binding domain of nonvisual arrestins to the carboxy terminus. J. Biol. Chem. 272, 15011–15016 [DOI] [PubMed] [Google Scholar]
- 13. Goodman O. B. Jr., Krupnick J. G., Santini F., Gurevich V. V., Penn R. B., Gagnon A. W., Keen J. H., and Benovic J. L. (1996) β-Arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature 383, 447–450 [DOI] [PubMed] [Google Scholar]
- 14. Laporte S. A., Oakley R. H., Zhang J., Holt J. A., Ferguson S. S., Caron M. G., and Barak L. S. (1999) The β2-adrenergic receptor/βarrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc. Natl. Acad. Sci. U.S.A. 96, 3712–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DeFea K. A., Zalevsky J., Thoma M. S., Déry O., Mullins R. D., and Bunnett N. W. (2000) β-Arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J. Cell Biol. 148, 1267–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luttrell L. M., Roudabush F. L., Choy E. W., Miller W. E., Field M. E., Pierce K. L., and Lefkowitz R. J. (2001) Activation and targeting of extracellular signal-regulated kinases by β-arrestin scaffolds. Proc. Natl. Acad. Sci. U.S.A. 98, 2449–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McDonald P. H., Chow C. W., Miller W. E., Laporte S. A., Field M. E., Lin F. T., Davis R. J., and Lefkowitz R. J. (2000) β-Arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science 290, 1574–1577 [DOI] [PubMed] [Google Scholar]
- 18. Sun Y., Cheng Z., Ma L., and Pei G. (2002) β-Arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J. Biol. Chem. 277, 49212–49219 [DOI] [PubMed] [Google Scholar]
- 19. Ge L., Shenoy S. K., Lefkowitz R. J., and DeFea K. (2004) Constitutive protease-activated receptor-2-mediated migration of MDA MB-231 breast cancer cells requires both β-arrestin-1 and -2. J. Biol. Chem. 279, 55419–55424 [DOI] [PubMed] [Google Scholar]
- 20. Fong A. M., Premont R. T., Richardson R. M., Yu Y. R., Lefkowitz R. J., and Patel D. D. (2002) Defective lymphocyte chemotaxis in β-arrestin2- and GRK6-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 99, 7478–7483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barnes W. G., Reiter E., Violin J. D., Ren X. R., Milligan G., and Lefkowitz R. J. (2005) β-Arrestin 1 and Gαq/11 coordinately activate RhoA and stress fiber formation following receptor stimulation. J. Biol. Chem. 280, 8041–8050 [DOI] [PubMed] [Google Scholar]
- 22. Anthony D. F., Sin Y. Y., Vadrevu S., Advant N., Day J. P., Byrne A. M., Lynch M. J., Milligan G., Houslay M. D., and Baillie G. S. (2011) β-Arrestin 1 inhibits the GTPase-activating protein function of ARHGAP21, promoting activation of RhoA following angiotensin II type 1A receptor stimulation. Mol. Cell. Biol. 31, 1066–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Claing A., Chen W., Miller W. E., Vitale N., Moss J., Premont R. T., and Lefkowitz R. J. (2001) β-Arrestin-mediated ADP-ribosylation factor 6 activation and β2-adrenergic receptor endocytosis. J. Biol. Chem. 276, 42509–42513 [DOI] [PubMed] [Google Scholar]
- 24. Houndolo T., Boulay P. L., and Claing A. (2005) G protein-coupled receptor endocytosis in ADP-ribosylation factor 6-depleted cells. J. Biol. Chem. 280, 5598–5604 [DOI] [PubMed] [Google Scholar]
- 25. Brown F. D., Rozelle A. L., Yin H. L., Balla T., and Donaldson J. G. (2001) Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J. Cell Biol. 154, 1007–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Honda A., Nogami M., Yokozeki T., Yamazaki M., Nakamura H., Watanabe H., Kawamoto K., Nakayama K., Morris A. J., Frohman M. A., and Kanaho Y. (1999) Phosphatidylinositol 4-phosphate 5-kinase α is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell 99, 521–532 [DOI] [PubMed] [Google Scholar]
- 27. Eva R., Crisp S., Marland J. R., Norman J. C., Kanamarlapudi V., ffrench-Constant C., and Fawcett J. W. (2012) ARF6 directs axon transport and traffic of integrins and regulates axon growth in adult DRG neurons. J. Neurosci. 32, 10352–10364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen P. W., Luo R., Jian X., and Randazzo P. A. (2014) The Arf6 GTPase-activating proteins ARAP2 and ACAP1 define distinct endosomal compartments that regulate integrin α5β1 traffic. J. Biol. Chem. 289, 30237–30248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Myers K. R., and Casanova J. E. (2008) Regulation of actin cytoskeleton dynamics by Arf-family GTPases. Trends Cell Biol. 18, 184–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heasman S. J., and Ridley A. J. (2008) Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 9, 690–701 [DOI] [PubMed] [Google Scholar]
- 31. Cotton M., Boulay P. L., Houndolo T., Vitale N., Pitcher J. A., and Claing A. (2007) Endogenous ARF6 interacts with Rac1 upon angiotensin II stimulation to regulate membrane ruffling and cell migration. Mol. Biol. Cell 18, 501–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sabe H. (2003) Requirement for Arf6 in cell adhesion, migration, and cancer cell invasion. J. Biochem. 134, 485–489 [DOI] [PubMed] [Google Scholar]
- 33. Boulay P. L., Cotton M., Melançon P., and Claing A. (2008) ADP-ribosylation factor 1 controls the activation of the phosphatidylinositol 3-kinase pathway to regulate epidermal growth factor-dependent growth and migration of breast cancer cells. J. Biol. Chem. 283, 36425–36434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Giguère P., Rochdi M. D., Laroche G., Dupré E., Whorton M. R., Sunahara R. K., Claing A., Dupuis G., and Parent J. L. (2006) ARF6 activation by Gαq signaling: Gαq forms molecular complexes with ARNO and ARF6. Cell. Signal. 18, 1988–1994 [DOI] [PubMed] [Google Scholar]
- 35. Karpinsky-Semper D., Volmar C. H., Brothers S. P., and Slepak V. Z. (2014) Differential effects of the Gβ5-RGS7 complex on muscarinic M3 receptor-induced Ca2+ influx and release. Mol. Pharmacol. 85, 758–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schrage R., Schmitz A. L., Gaffal E., Annala S., Kehraus S., Wenzel D., Büllesbach K. M., Bald T., Inoue A., Shinjo Y., Galandrin S., Shridhar N., Hesse M., Grundmann M., Merten N., et al. (2015) The experimental power of FR900359 to study Gq-regulated biological processes. Nat. Commun. 6, 10156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zimmerman B., Simaan M., Lee M. H., Luttrell L. M., and Laporte S. A. (2009) c-Src-mediated phosphorylation of AP-2 reveals a general mechanism for receptors internalizing through the clathrin pathway. Cell. Signal. 21, 103–110 [DOI] [PubMed] [Google Scholar]
- 38. Ahn S., Nelson C. D., Garrison T. R., Miller W. E., and Lefkowitz R. J. (2003) Desensitization, internalization, and signaling functions of β-arrestins demonstrated by RNA interference. Proc. Natl. Acad. Sci. U.S.A. 100, 1740–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Orsini M. J., and Benovic J. L. (1998) Characterization of dominant negative arrestins that inhibit β2-adrenergic receptor internalization by distinct mechanisms. J. Biol. Chem. 273, 34616–34622 [DOI] [PubMed] [Google Scholar]
- 40. Fessart D., Simaan M., and Laporte S. A. (2005) c-Src regulates clathrin adapter protein 2 interaction with β-arrestin and the angiotensin II type 1 receptor during clathrin-mediated internalization. Mol. Endocrinol. 19, 491–503 [DOI] [PubMed] [Google Scholar]
- 41. Douillette A., Bibeau-Poirier A., Gravel S. P., Clément J. F., Chénard V., Moreau P., and Servant M. J. (2006) The proinflammatory actions of angiotensin II are dependent on p65 phosphorylation by the IκB kinase complex. J. Biol. Chem. 281, 13275–13284 [DOI] [PubMed] [Google Scholar]
- 42. Doyon P., van Zuylen W. J., and Servant M. J. (2013) Role of IκB kinase-β in the growth-promoting effects of angiotensin II in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 33, 2850–2857 [DOI] [PubMed] [Google Scholar]
- 43. Zimmerman B., Beautrait A., Aguila B., Charles R., Escher E., Claing A., Bouvier M., and Laporte S. A. (2012) Differential β-arrestin-dependent conformational signaling and cellular responses revealed by angiotensin analogs. Sci. Signal. 5, ra33. [DOI] [PubMed] [Google Scholar]
- 44. Goupil E., Fillion D., Clément S., Luo X., Devost D., Sleno R., Pétrin D., Saragovi H. U., Thorin É., Laporte S. A., and Hébert T. E. (2015) Angiotensin II type I and prostaglandin F2α receptors cooperatively modulate signaling in vascular smooth muscle cells. J. Biol. Chem. 290, 3137–3148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zaima K., Deguchi J., Matsuno Y., Kaneda T., Hirasawa Y., and Morita H. (2013) Vasorelaxant effect of FR900359 from Ardisia crenata on rat aortic artery. J. Nat. Med. 67, 196–201 [DOI] [PubMed] [Google Scholar]
- 46. Tilley D. G., Zhu W., Myers V. D., Barr L. A., Gao E., Li X., Song J., Carter R. L., Makarewich C. A., Yu D., Troupes C. D., Grisanti L. A., Coleman R. C., Koch W. J., Houser S. R., Cheung J. Y., and Feldman A. M. (2014) β-Adrenergic receptor-mediated cardiac contractility is inhibited via vasopressin type 1A-receptor-dependent signaling. Circulation 130, 1800–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Takasaki J., Saito T., Taniguchi M., Kawasaki T., Moritani Y., Hayashi K., and Kobori M. (2004) A novel Gαq/11-selective inhibitor. J. Biol. Chem. 279, 47438–47445 [DOI] [PubMed] [Google Scholar]
- 48. von Kleist L., Stahlschmidt W., Bulut H., Gromova K., Puchkov D., Robertson M. J., MacGregor K. A., Tomlin N., Pechstein A., Chau N., Chircop M., Sakoff J., von Kries J. P., Saenger W., Kräusslich H. G., et al. (2011) Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell 146, 471–484 [DOI] [PubMed] [Google Scholar]
- 49. Doyon P., and Servant M. J. (2010) Tumor necrosis factor receptor-associated factor-6 and ribosomal S6 kinase intracellular pathways link the angiotensin II AT1 receptor to the phosphorylation and activation of the IκB kinase complex in vascular smooth muscle cells. J. Biol. Chem. 285, 30708–30718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Holloway A. C., Qian H., Pipolo L., Ziogas J., Miura S., Karnik S., Southwell B. R., Lew M. J., and Thomas W. G. (2002) Side-chain substitutions within angiotensin II reveal different requirements for signaling, internalization, and phosphorylation of type 1A angiotensin receptors. Mol. Pharmacol. 61, 768–777 [DOI] [PubMed] [Google Scholar]
- 51. Wei H., Ahn S., Shenoy S. K., Karnik S. S., Hunyady L., Luttrell L. M., and Lefkowitz R. J. (2003) Independent β-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc. Natl. Acad. Sci. U.S.A. 100, 10782–10787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kawada K., Upadhyay G., Ferandon S., Janarthanan S., Hall M., Vilardaga J. P., and Yajnik V. (2009) Cell migration is regulated by platelet-derived growth factor receptor endocytosis. Mol. Cell. Biol. 29, 4508–4518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Scott M. G., Le Rouzic E., Périanin A., Pierotti V., Enslen H., Benichou S., Marullo S., and Benmerah A. (2002) Differential nucleocytoplasmic shuttling of β-arrestins. Characterization of a leucine-rich nuclear export signal in β-arrestin2. J. Biol. Chem. 277, 37693–37701 [DOI] [PubMed] [Google Scholar]
- 54. Xiao K., Shenoy S. K., Nobles K., and Lefkowitz R. J. (2004) Activation-dependent conformational changes in β-arrestin 2. J. Biol. Chem. 279, 55744–55753 [DOI] [PubMed] [Google Scholar]
- 55. Kim Y. J., Hofmann K. P., Ernst O. P., Scheerer P., Choe H. W., and Sommer M. E. (2013) Crystal structure of pre-activated arrestin p44. Nature 497, 142–146 [DOI] [PubMed] [Google Scholar]
- 56. Shukla A. K., Manglik A., Kruse A. C., Xiao K., Reis R. I., Tseng W. C., Staus D. P., Hilger D., Uysal S., Huang L. Y., Paduch M., Tripathi-Shukla P., Koide A., Koide S., Weis W. I., et al. (2013) Structure of active β-arrestin-1 bound to a G-protein-coupled receptor phosphopeptide. Nature 497, 137–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. DiNitto J. P., Delprato A., Gabe Lee M. T., Cronin T. C., Huang S., Guilherme A., Czech M. P., and Lambright D. G. (2007) Structural basis and mechanism of autoregulation in 3-phosphoinositide-dependent Grp1 family Arf GTPase exchange factors. Mol. Cell 28, 569–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cohen L. A., Honda A., Varnai P., Brown F. D., Balla T., and Donaldson J. G. (2007) Active Arf6 recruits ARNO/cytohesin GEFs to the PM by binding their PH domains. Mol. Biol. Cell 18, 2244–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Macia E., Partisani M., Paleotti O., Luton F., and Franco M. (2012) Arf6 negatively controls the rapid recycling of the β2 adrenergic receptor. J. Cell Sci. 125, 4026–4035 [DOI] [PubMed] [Google Scholar]
- 60. Padovani D., Folly-Klan M., Labarde A., Boulakirba S., Campanacci V., Franco M., Zeghouf M., and Cherfils J. (2014) EFA6 controls Arf1 and Arf6 activation through a negative feedback loop. Proc. Natl. Acad. Sci. U.S.A. 111, 12378–12383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rappoport J. Z., and Simon S. M. (2003) Real-time analysis of clathrin-mediated endocytosis during cell migration. J. Cell Sci. 116, 847–855 [DOI] [PubMed] [Google Scholar]
- 62. Berk B. C., and Rao G. N. (1993) Angiotensin II-induced vascular smooth muscle cell hypertrophy: PDGF A-chain mediates the increase in cell size. J. Cell Physiol. 154, 368–380 [DOI] [PubMed] [Google Scholar]
- 63. Muniz P., Fortuno A., Zalba G., Fortuno M. A., and Diez J. (2001) Effects of loop diuretics on angiotensin II-stimulated vascular smooth muscle cell growth. Nephrol. Dial. Transplant. 16, 14–17 [DOI] [PubMed] [Google Scholar]
- 64. Li F., and Malik K. U. (2005) Angiotensin II-induced Akt activation through the epidermal growth factor receptor in vascular smooth muscle cells is mediated by phospholipid metabolites derived by activation of phospholipase D. J. Pharmacol. Exp. Ther. 312, 1043–1054 [DOI] [PubMed] [Google Scholar]
- 65. Lefkowitz R. J., Pierce K. L., and Luttrell L. M. (2002) Dancing with different partners: protein kinase a phosphorylation of seven membrane-spanning receptors regulates their G protein-coupling specificity. Mol. Pharmacol. 62, 971–974 [DOI] [PubMed] [Google Scholar]
- 66. Cavenagh M. M., Whitney J. A., Carroll K., Zhang Cj., Boman A. L., Rosenwald A. G., Mellman I., and Kahn R. A. (1996) Intracellular distribution of Arf proteins in mammalian cells. Arf6 is uniquely localized to the plasma membrane. J. Biol. Chem. 271, 21767–21774 [DOI] [PubMed] [Google Scholar]
- 67. Donaldson J. G., and Jackson C. L. (2011) ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat. Rev. Mol. Cell Biol. 12, 362–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Poupart M. E., Fessart D., Cotton M., Laporte S. A., and Claing A. (2007) ARF6 regulates angiotensin II type 1 receptor endocytosis by controlling the recruitment of AP-2 and clathrin. Cell. Signal. 19, 2370–2378 [DOI] [PubMed] [Google Scholar]