Abstract
B7-H3, a newly identified B7 family member, has functional duality as a co-stimulator and co-inhibitor that fine-tunes T cell-mediated immune responses. Given that B7-H3 expression on human monocytes and dendritic cells is enhanced by inflammatory cytokines, its potential inmmunoregulatory role at sites of inflammation has been suggested. Further, monocytes play crucial roles in the pathophysiology of various inflammatory disorders including autoimmune diseases; however, the immunological role of B7-H3 in rheumatoid arthritis (RA) has not been defined. Thus, we aimed to investigate the possible roles of monocyte B7-H3 in the pathogenesis of RA. Synovial monocytes, but not peripheral monocytes, in RA patients predominantly express surface B7-H3. The 4Ig isoform of B7-H3 is exclusively induced on the cell surface, whereas the 2Ig B7-H3 isoform is constitutively expressed in the intracytoplasmic region of both peripheral and synovial monocytes. B7-H3 knockdown experiments reveal that surface B7-H3 has an inhibitory effect on IFN-γ production in CD4 memory cells. Moreover, surface B7-H3 expression on synovial monocytes inversely correlates with RA clinical parameters. Our findings demonstrate that activation-induced B7-H3 expression on synovial monocytes has the potential to inhibit Th1-mediated immune responses and immunomodulatory roles affecting RA pathogenesis.
Keywords: autoimmune disease, cellular immune response, immunoglobulin-like domain, inflammation, monocyte, B7-H3, interferon-gamma, rheumatoid arthritis, synovial fluid
Introduction
Rheumatoid arthritis (RA)3 is a systemic autoimmune disorder characterized by chronic inflammatory responses that primarily attack synovial membranes (1, 2). The synovial environment in RA is comprised of a complex mix of cell types including T cells, B cells, neutrophils, monocytes/macrophages, and fibroblast-like synoviocytes (3). The interplay among these cell types is known to contribute significantly to disease pathophysiology (4). Furthermore, a growing body of evidence has revealed that the interactions between T cells and a variety of infiltrating immune cells and structural cells in the synovial environment play central roles in the pathogenesis of RA (4). In this context, it has recently been shown that in vivo activated monocytes derived from the synovial fluid of active RA patients specifically promote Th17 responses, largely in a cell contact-dependent manner. On the other hand, they also enhance cytokine production and suppressive activity of regulatory T cells by monocyte-derived cytokines (5). These findings suggest that synovial monocytes provide signals to T cells through their distinct surface molecules and cytokines and thus are responsible for modulating T cell responses (6).
An optimal T cell response is accomplished through the integration of signals downstream of the antigen-specific T cell receptor (TCR) and from a set of auxiliary signals, which can be either stimulatory or inhibitory, generated through co-signaling molecules (7, 8). The B7 family members are among the most intensively studied co-signaling molecules and play a pivotal role in the regulation of T cell responses by providing auxiliary signals. The prototypical B7 family members, CD80 (B7-1) and CD86 (B7-2), deliver co-stimulatory signals through ligation of CD28 on T cells. In contrast, binding of CD80 or CD86 with CTLA-4, a homolog of CD28, inhibits T cell responses by delivering a negative signal. Besides CD80 and CD86, there were several newly identified B7 family molecules, including PD-L1 (B7-H1), PD-L2 (B7-DC), B7-H3 (CD276), B7-H4, and ICOS ligand (B7-H2), that can deliver positive or negative signals to effector cells. The dynamic control of B7 family molecules and their receptors, expressed on antigen-presenting cells (APCs) and T cells, respectively, is essential for fine-tuning T cell responses. Therefore, aberrant expression of co-signaling molecules has been implicated in the pathogenesis of many immune disorders (9, 10).
B7-H3 (CD276) is a recently identified member of the B7 family expressed in humans and mice. Similar to the other B7 family members, B7-H3 is a type I transmembrane protein with typical extracellular IgV- and IgC-like domains and 20–30% amino acid sequence homology to CD80 and CD86 (11). However, B7-H3 is quite unique in that different isoforms exist between human and mouse (12–14). In humans, the B7-H3 gene has four Ig-like repeats that can be alternatively spliced to yield a protein containing either four Ig-like domains (4Ig) or two Ig-like domains (2Ig), whereas the mouse gene only contains two Ig-like repeats, and thus, only one form (2Ig) is generated (12). Systemic genomic analysis suggested that the 4Ig form, with two copies of IgV-IgC domain, was generated from tandem exon duplication during evolution of the immune system (12). Unlike CD80 and CD86, human B7-H3 is broadly expressed at low levels in multiple organs and markedly overexpressed on several cancers (15, 16). In immune cells, B7-H3 expression is induced in human monocytes and dendritic cells by inflammatory cytokines and augmented by synovial monocytes of RA patients (11, 17), indicating potential immunoregulatory function at sites of inflammation (7).
The precise immunological function of B7-H3 is still relatively unclear. Human B7-H3 was initially discovered as a co-stimulatory molecule for T cell responses. Ligation of its putative receptor on activated T cells leads to augment proliferation and cytotoxic T lymphocyte generation and enhanced production of IFN-γ (11). On the other hand, several studies have indicated that B7-H3 may exert co-inhibitory functions upon T cell responses. Recombinant human or mouse B7-H3 protein inhibits T cell proliferation, effector cytokine production, and activation of transcription factors such as NFAT, NF-κB, and AP-1 (18, 19). The mechanisms underlying these contrasting roles of B7-H3 during immune responses are currently unknown; however, they are likely dependent on the expression pattern of the two distinct B7-H3 isoforms, as well as their putative receptors (15, 20, 21).
Although B7-H3 has been known to possess functional duality as a T cell co-stimulator or co-inhibitor for modulating T cell-mediated immune responses (18), few studies have documented the impact of B7-H3 on T cell responses in human diseases. B7-H3 is expressed by human monocytes following inflammatory cytokine stimulation (18). Moreover, an increase in inflammatory monocytes within the synovial fluid inflammatory cytokine milieu is implicated in the pathogenesis of RA (22, 23). Therefore, we hypothesized that B7-H3 might be expressed on synovial monocytes in RA and furthermore that B7-H3 might exert an immunomodulatory role on T cell responses and thus affect RA pathogenesis.
In the present study, we provide evidence that in RA patients, synovial monocytes, but not peripheral monocytes, predominantly induce B7-H3 expression on their cell surface. Induced B7-H3 on synovial monocytes is exclusively the 4Ig isoform, whereas the 2Ig isoform is constitutively expressed in the intracytoplasmic region of both peripheral and synovial monocytes. Furthermore, gene silencing experiments provide evidence that the surface B7-H3 has an inhibitory effect on IFN-γ production by CD4 memory cells. Moreover, the expression level of B7-H3 on synovial monocytes from RA patients inversely correlates with clinical parameters such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and disease activity score in 28 joints (DAS28), suggesting that that the expression of B7-H3 is related to inflammatory conditions.
Experimental Procedures
Cell Preparation
The study protocols were reviewed and approved by the institutional review board of Chungnam National University Hospital (institutional review board no. 2012-01-024) and the institutional review board of Seoul National University Hospital (institutional review board no. 1109-055-378). All RA patients and healthy volunteers provided their written informed consent to participate in this study. Peripheral blood and synovial fluid of RA patients were collected after obtaining written informed consent at the Department of Internal Medicine of Chungnam National University Hospital. The patient characteristics of RA patients enrolled in this study are summarized in Table 1. Peripheral blood of healthy volunteers was drawn after obtaining written informed consent at Seoul National University College of Medicine. Mononuclear cells were isolated from peripheral blood and synovial fluid by density gradient centrifugation (Bicoll separating solution; BIOCHROM Inc., Cambridge, UK). Monocytes were positively separated from peripheral blood mononuclear cells (PBMCs) and synovial fluid mononuclear cells (SFMCs) with anti-human CD14 microbeads (Miltenyi Biotec Inc., Auburn, CA). CD4 memory cells were negatively isolated from PBMCs with a human memory CD4+ T cell enrichment kit (EasySep negative selection; Stemcell Technologies Inc., Vancouver, Canada).
TABLE 1.
The characteristics of RA patients
| Patient characteristics | Value | Medication | No. (%) |
|---|---|---|---|
| Age (years), means ± S.D. | 59.42 ± 15.05 | Methotrexate | 16/19 (84.2) |
| Sex (female/male) | 13/6 | Prednisolone | 17/19 (89.5) |
| Rheumatoid factor, no. of positive (%) | 15/19 (78.9) | Hydroxychloroquine | 14/19 (73.7) |
| Rheumatoid factor titer (IU/ml), means ± S.D.a | 97.04 ± 76.67 | Sulfasalazine | 5/19 (26.3) |
| Anti-citrullinated protein antibody, no. of positive (%) | 15/16 (93.8%) | ||
| Anti-citrullinated protein antibody titer (units/ml), means ± S.D.b | 177.23 ± 166.27 | ||
| Disease duration (months), means ± S.D. | 37.26 ± 36.89 | ||
| ESR (mm/h), means ± S.D.c | 70.21 ± 29.71 | ||
| CRP (mg/dL), means ± S.D. | 3.34 ± 3.22 | ||
| DAS28, means ± S.D. | 4.76 ± 1.07 |
a Rheumatoid factor titer normal range was 0–18.
b Anti-citrullinated protein antibody titer normal range was <17.0.
c ESR normal range was 0–20.
d CRP normal range was 0–0.5.
Flow Cytometric Analysis
PBMCs and SFMCs were stained for 30 min at 4 °C degree with mouse anti-human monoclonal antibodies (Abs) to CD14, HLA-DR (both from BD Bioscience, San Jose, CA), and B7-H3 (clone 7-517; eBiosciences, San Diego, CA). Stained cells were acquired by a BD LSRII or LSRFortessa (BD Bioscience) and analyzed using FlowJo software (Tree Star, Ashland, OR).
Cell Culture
Purified monocytes and CD4 memory T cells were cultured in RPMI 1640 medium supplemented with 10% FBS, 1% penicillin/streptomycin, and 1% l-glutamine. Purified monocytes were stimulated for 18 h with LPS (10 ng/ml; Sigma-Aldrich), R848 and Pam3Csk4 (50 ng/ml and 1 μg/ml, respectively; both from InvivoGen, San Diego, CA), TNF-α (25 ng/ml; ProSepc, East Brunswick, NJ), IL-6 and IL-1β (25 ng/ml of each; both from R&D Systems, Minneapolis, MN), GM-CSF (60 ng/ml; R&D Systems), and IFN-γ (25 ng/ml; eBioscience) as indicated. After an 18-h treatment, monocytes were stained with APC-conjugated anti-B7-H3 Ab (clone 7-517) and acquired by a BD LSRII or LSRFortessa (BD Bioscience).
Enzyme-linked Immunosorbent Assay
The amount of IL-17A and IFN-γ in co-culture supernatant was quantified using commercial ELISA kits (eBioscience for IL-17A and BioLegend for IFN-γ). The measurement of optical density was performed using the Infinite 200 Pro Multimode microplate reader (Tecan Group Ltd., Seestrasse, Switzerland).
RT-PCR
Total RNA was extracted from freshly isolated or co-cultured cells using TRIzol reagent (Life Technologies), and cDNA was synthesized by GoScript reverse transcription system (Promega, Madison, WI). Real time quantitative RT-PCR was performed in triplicate on a 7500 PCR system (Applied Biosystems, Grand Island, NY) using the following primers: B7-H3, 5′-ACCATCACACCCCAGAGAAG-3′ and 5′-GCCAGATGAGGTTGAGCTGT-3′; and β-actin, 5′-GGACTTCGAGCAAGAGATGG-3′ and 5′-AGCACTGTGTTGGCGTACAG-3′. The levels of gene expression were normalized to the expression of β-actin. The comparative CT method (ΔΔCT) was used for the quantification of gene expression. To identify two distinct isoforms of B7-H3 (2Ig and 4Ig), RT-PCR was performed using AccuPower® PCR premix (Bioneer, Daejeon, Republic of Korea) with the following primers: B7-H3, 5′-GTCCCTGAGTCCCAGAGT-3′ and 5′-GGTCCTCAGCTCCTGCATT-3′. This primer set allows us to discriminate between the two isoforms based on the sizes of the amplified PCR products.
Gene Expression Arrays
Total RNA was purified from synovial and peripheral monocytes from the same RA patients and peripheral monocytes from healthy controls using the RNeasy mini kit (Qiagen). Microarray analysis was done with human whole genome expression 44K (Agilent Technologies, Santa Clara, CA).
Immunoblotting
Monocytes and cell lines were washed with ice-cold PBS and lysed in lysis buffer containing 5 mm iodoacetamide, 10 mm Tris-HCl, 100 mm NaCl, and 1% Triton X-100 or 1% saponin. Lysate buffer also contained protease and phosphatase inhibitor mixture (Cell Signaling, Danvers, MA). Cell lysates were run on a 10% SDS-PAGE gel and transferred onto a PVDF membrane (Bio-Rad), which was then blocked for 1 h with 5% BSA in Tris-buffered saline solution, containing 0.1% Tween 200. The membrane was then incubated overnight at 4 °C with the goat anti-human B7-H3 polyclonal Ab (R&D Systems), washed, and incubated for 1 h at room temperature with the HRP-conjugated secondary Ab. Recombinant human 4Ig and 2Ig proteins (extracellular region of each proteins with a C-terminal 10× His tag), which are expressed in NS0 mouse myeloma cell line, were purchased from R&D Systems. The membranes were developed by chemiluminescence ECL (Millipore, Billerica, MA). Total cellular membrane proteins/cytosolic fractions were isolated using the plasma membrane protein extraction kit (Biovision, Milpita, CA). Cells were collected and washed with ice-cold PBS and then homogenized using a Dounce homogenizer. Fractionation was performed according to the manufacturer's protocol. Anti-Na/K-ATPase Ab and anti-α-tubulin Ab (both from Cell Signaling) were used for verifying successful fractionation of total cellular membrane and cytosolic fraction, respectively.
B7-H3 Silencing and Co-culture with T Cells
THP-1 cells were transfected with 100 μm of premade human B7-H3-specific siRNA (SIRNA no. 1027894) or 100 μm of control siRNA (both from Bioneer) by the Neon transfection system (Life Technologies). After resting for 24 h before use, knockdown efficiencies were monitored by flow cytometric analysis with anti-human B7-H3 Ab (clone 7-517). For co-culture with T cells, the transfected THP-1 cells were seeded at 5 × 103 into each well of a U-bottomed 96-well plate and incubated with soluble anti-CD3 and anti-CD28 antibodies. After incubation for 1 h, 2.5 × 104 carboxyfluorescein succinimidyl ester-labeled or nonlabeled CD4+ memory T cells were added into each well and co-cultured with THP-1 cells for another 7 days. On day 3 after co-culture, the cells were harvested and stained with APC-conjugated anti-CD4 and 7-aminoactinomycin D, and carboxyfluorescein succinimidyl ester dilution in viable CD4 T cells was assessed using an LSR Fortessa (BD Biosciences). On day 7 after co-culture, the amount of IL-17A and IFN-γ in the supernatant was quantified by commercial ELISA kits (eBioscience for IL-17A and BioLegend for IFN-γ).
Statistical Analysis
Two-tailed paired t test or unpaired t test was done to analyze data using Prism 5 software (GraphPad Software Inc., La Jolla, CA) as indicated in the figure legends. p values of less than 0.05 were considered statistically significant.
Results
B7-H3 Expression Is Markedly Elevated on Synovial Monocytes from RA Patients
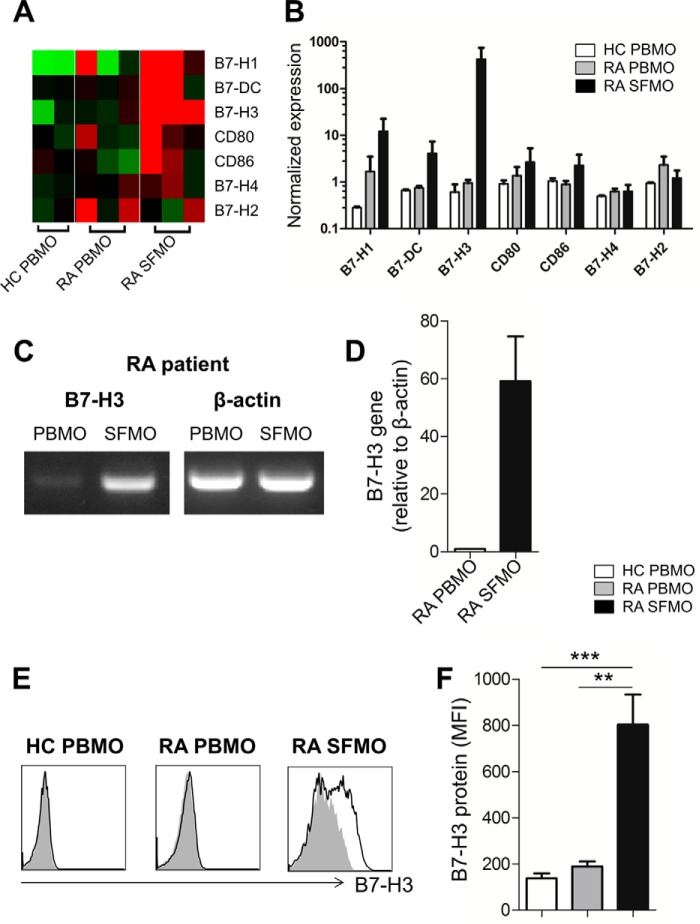
Consistent with our previous finding, a pilot microarray assay identified a striking up-regulation of B7-H3 expression on synovial monocytes among B7 family members when compared with peripheral monocytes of RA patients (Fig. 1, A and B) (17). To confirm our microarray data in a secondary cohort, CD14+ monocytes were purified from PBMCs and SFMCs, which were prepared simultaneously from the same RA patients, and B7-H3 expression was analyzed using RT-PCR and flow cytometry. As seen in Fig. 1C, RT-PCR results clearly revealed that synovial monocytes, but not peripheral monocytes, express B7-H3 mRNA. Furthermore, the level of B7-H3 mRNA was dramatically up-regulated in purified synovial monocytes (mean ± S.E., 59.12 ± 15.57-fold) when compared with peripheral monocytes from RA patients (Fig. 1D). This finding was confirmed by flow cytometric analysis. B7-H3 expression on synovial monocytes in RA patients was found to be markedly enhanced at the protein level when compared with that on peripheral monocytes in RA patients or healthy controls (Fig. 1, E and F; p < 0.05). These findings demonstrate that the enhanced expression of surface B7-H3 is a feature unique to synovial monocytes in RA patients.
FIGURE 1.
RA patient synovial monocytes have markedly elevated expression of B7-H3. A, microarray heat map analysis on B7 family members. CD14+ monocytes were purified from PBMCs and SFMCs, which were prepared simultaneously from the same RA patients (n = 3). CD14+ monocytes derived from peripheral blood of healthy controls (HC) were used as control (n = 2). B, normalized expression values of B7 family members were compared among different monocyte groups. PBMO and SFMO indicate peripheral blood monocytes and synovial fluid monocytes, respectively. C and D, representative results of RT-PCR (C) and quantitative PCR analysis of B7-H3 gene expression (D) between PBMO and SFMO freshly purified from the same RA patient (n = 6). Expression was normalized to β-actin, and the comparative CT method (ΔΔCT) was used for the quantification of gene expression. E, representative flow cytometry histogram plots of B7-H3 expression by CD14+HLA-DR+ monocytes in peripheral blood of RA patients and healthy controls and in the synovial fluid of RA patients. The black line indicates B7-H3 staining, and the gray shading indicates isotype staining as negative control. F, comparison of B7-H3 expression among three different samples: PBMO (n = 8) of healthy donors and PBMO (n = 7) and SFMO (n = 9) of RA patients. The mean fluorescence intensity (MFI) is indicated. Horizontal bars and error bars show the means ± S.E. *, p < 0.05 by unpaired t test in F.
Expression of Surface B7-H3 on Monocytes Is Affected by Treatment with TLR Ligands and Cytokines
In humans, B7-H3 is up-regulated in monocyte and dendritic cells in response to stimulation, such as with cytokines (7), suggesting that the synovial fluid inflammatory milieu may cause the de novo induction of B7-H3 on monocytes. To test this possibility, purified CD14+ monocytes were exposed to various stimuli, and B7-H3 expression was evaluated by flow cytometric analysis. As seen in Fig. 2, B7-H3 expression was significantly enhanced following stimulation with the TLR4 ligand LPS for 18 h. The stimulation with other TLR ligands such as R848 and Pam3Csk4 also increased B7-H3 expression, although the difference did not reach statistical significance. Among the cytokines assessed, we found that only GM-CSF clearly induced up-regulation of B7-H3 expression on monocytes (Fig. 2, A and B; p < 0.05). Further, no significant effect of the monocyte-derived proinflammatory cytokines TNF-α, IL-1β, or IL-6 when utilized individually or in combination was observed on B7-H3 expression in these experiments (Fig. 2, A and B). IFN-γ was previously reported to enhance B7-H3 expression on dendritic cells (11); however, there did not appear to be any significant synergistic effect of IFN-γ on GM-CSF-mediated induction of B7-H3 in monocytes (Fig. 2B).
FIGURE 2.
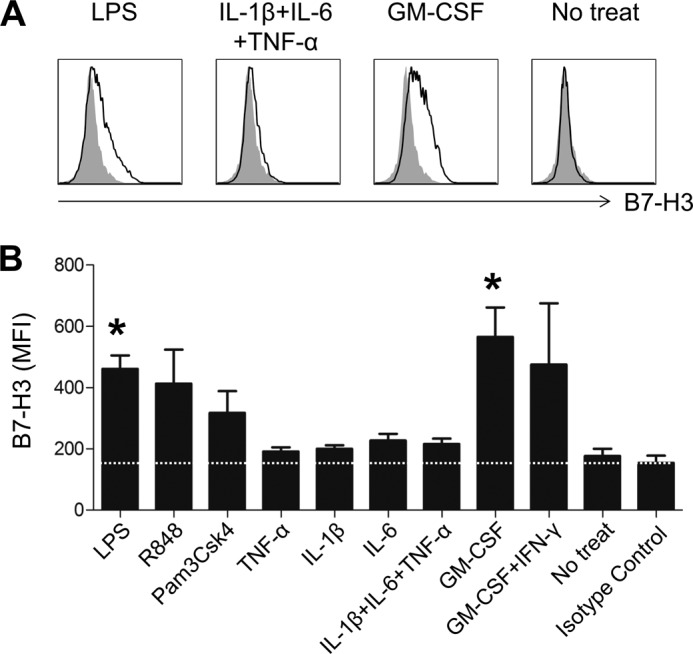
Expression of B7-H3 on monocytes is altered by treatment with TLR ligands and cytokines. PBMCs derived from healthy controls were stimulated for 18 h with the indicated TLR agonists or cytokines, and B7-H3 expression was analyzed by flow cytometry. A, representative flow cytometric analysis of the change in B7-H3 expression on CD14+HLA-DR+ cells after the indicated stimulations. B, bar graph showing the change in B7-H3 expression by monocytes treated with the indicated TLR agonists and proinflammatory cytokines (LPS (10 ng/ml), R848 (50 ng/ml), Pam3Csk4 (1 μg/ml), TNF-α (25 ng/ml), IL-6 (25 ng/ml), IL-1β (25 ng/ml), GM-CSF (60 ng/ml), and IFN-γ (25 ng/ml)) (n = 4). The mean fluorescence intensity (MFI) is indicated. Horizontal bars and error bars show the means ± S.E. *, p < 0.05 compared with the no treatment group by paired t test in B.
RA Patient Synovial Monocyte-induced B7-H3 Is Exclusively the 4Ig Isoform
Unlike murine B7-H3, human B7-H3 can be expressed as two different isoforms (4Ig B7-H3 and 2Ig B7-H3) through alternative splicing (13). Notably, a recent study suggested that the two distinct isoforms of B7-H3 might have differing structures that confer opposite regulatory roles in T cells (14). To investigate which B7-H3 isoform is induced by synovial monocytes in RA patients, we first designed a primer set specific for the leader sequence and spanning the sequence between the transmembrane and cytoplasmic regions of B7-H3, respectively (Fig. 3A). These primers allowed us to discriminate between the two isoforms based on the size of RT-PCR-amplified products. As shown in Fig. 3B, synovial monocytes very strongly enhanced the express of 4Ig B7-H3 mRNA, whereas 2Ig B7-H3 mRNA was expressed in both synovial and peripheral monocytes in RA patients. To assess expression at the protein level, whole cell lysates from purified peripheral and synovial monocytes were subject to immunoblotting using an antibody against B7-H3. This antibody successfully recognized both isoforms of B7-H3, detecting recombinant proteins of extracellular region of 4Ig B7-H3 and 2Ig B7-H3 at ∼95 kDa and slightly less than 55 kDa, respectively (Fig. 3C) (3). Recombinant 2Ig B7-H3 protein was observed as a doublet in Fig. 3C, suggesting potential glycosylation of this protein. In fact, several potential N-linked glycosylation sites are predicted in the sequence of Ig-like domains (UniProt accession number Q5ZPR3).
FIGURE 3.
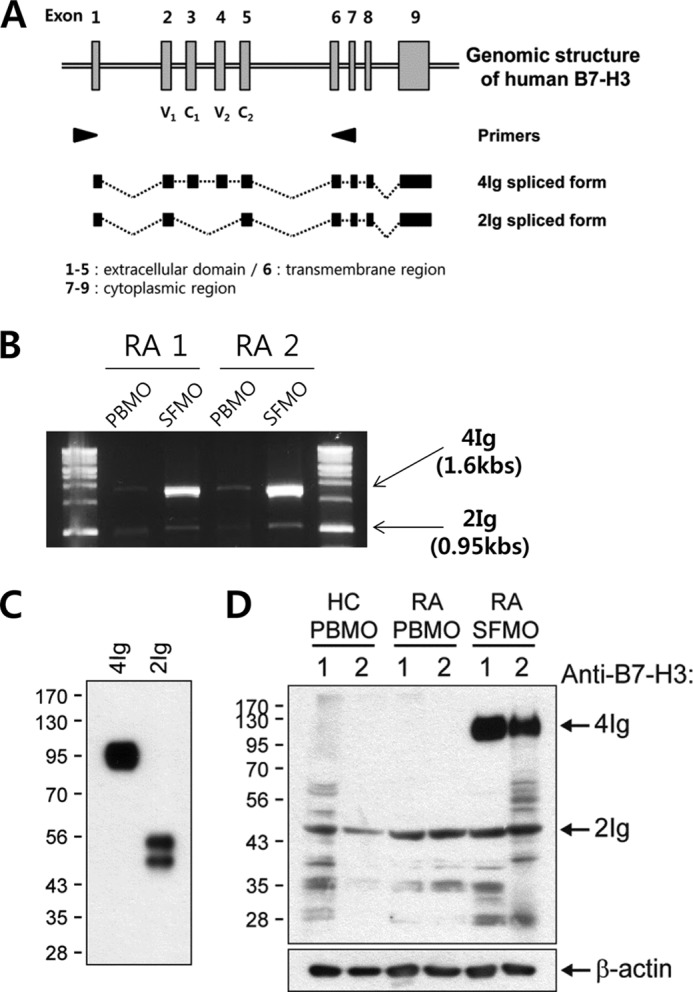
B7-H3 induced on synovial monocytes from RA patients is preferentially the 4Ig isoform. A, genomic structure and alternatively spliced isoforms of the human B7-H3 gene. PCR primers (arrowheads) common to both 2Ig and 4Ig sequences were designed. The amino acid-coding exons are presented as gray boxes. These primers allow for discrimination between the two isoforms using RT-PCR based on the sized of the amplified products. B, RT-PCR analysis of the two distinct B7-H3 gene isoforms in PBMO and SFMO from two patients using the primer sets described in A. 4Ig gives a 1.6-kb PCR product, whereas 2Ig gives a 0.95-kb PCR product. C, goat anti-human B7-H3 polyclonal Ab used in immunoblotting can detect recombinant 4Ig and 2Ig proteins of human B7-H3. D, expression pattern of the two isoforms of B7-H3 in whole cell lysates prepared from freshly purified PBMO of healthy donors and PBMO and SFMO of RA patients. The samples loaded in all lanes were derived from each individual RA patient or healthy control except RA SFMO sample 1, which was pooled from two different RA patients. β-Actin was used as the protein loading control. HC, healthy control.
Supporting our RT-PCR data, freshly isolated peripheral monocytes constitutively expressed 2Ig B7-H3 isoform. However, induced B7-H3 in synovial monocytes was predominantly the 4Ig isoform, running at 110 kDa as reported (13) (likely because of transmembrane domain and cytoplasmic tail), whereas the 2Ig isoform was expressed at a level comparable to that seen in peripheral monocytes of RA patients (Fig. 3D).
The Human B7-H3 Isoforms Reside in Different Monocyte Cellular Compartments
It has been reported that several human tumor cells noticeably overexpress B7-H3 (11, 24–26). Thus, we analyzed the expression of B7-H3 in the human monocytic cell line THP-1. As seen in Fig. 4A, there is a very strong band at 110 kDa in THP-1 cells, and upon further exposure a weak band at 50 kDa is seen in both peripheral monocytes and THP-1 cells (Fig. 4A). Flow cytometric analysis revealed B7-H3 expression on the surface of THP-1 cells but not on peripheral monocytes (Fig. 4B). Because surface FACS analysis revealed that THP-1 cells possessing 4Ig B7-H3 isoform expressed B7-H3 on their surface and no B7-H3 expressing cells were observed in peripheral monocytes having 2Ig B7-H3 (Fig. 4B), it is reasonable to assume that the two distinct isoforms are localized within different cellular compartments. To test this idea, whole cell lysates from THP-1 cells and synovial monocytes were fractionized into total cellular membrane proteins and cytosol fractions to assess the localization of 4Ig B7-H3 and 2Ig B7-H3 isoforms. As seen in Fig. 4C, the 2Ig B7-H3 isoform selectively localized to the cytosolic faction, whereas the 4Ig B7-H3 isoform was most strongly detected in the total cellular membrane fraction of synovial monocytes and THP-1 cells. Taken together, our data demonstrate that the constitutively expressed 2Ig B7-H3 isoform is largely localized to the cytosolic fraction and minimally expressed in the plasma membrane fraction, whereas 4Ig B7-H3 is the major isoform expressed on the surface of monocytes following stimulation.
FIGURE 4.

Human 2Ig B7-H3 and 4Ig B7-H3 differentially localized to unique monocyte cellular compartments. A, B7-H3 expression in whole lysates prepared from peripheral monocytes of a healthy donor or from THP-1 cells was analyzed using immunoblotting with a short (10 s) or long (5 min) exposure. β-Actin was used as a loading control. B, representative flow cytometric histogram of surface B7-H3 expression on monocyte and THP-1 cells. C, differential localization of 2Ig B7-H3 and 4Ig B7-H3 in fractionized total cellular membrane proteins (TCM) and in the cytosolic fraction of THP-1 cells (left) and synovial monocytes freshly purified from RA patient (right) (short exposure, 5 s; long exposure, 3 min). Successful fractionation was verified by the presence of Na/K-ATPase in total cellular membrane and the presence of α-tubulin in cytosol, respectively.
Inhibitory Role of the 4Ig B7-H3 Isoform on CD4 T Cell Responses
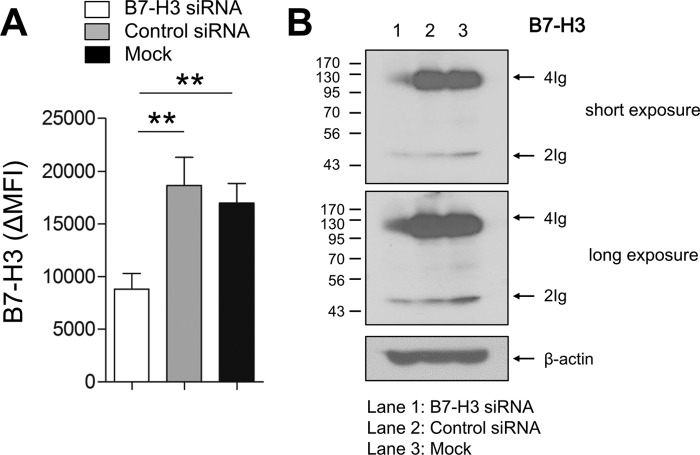
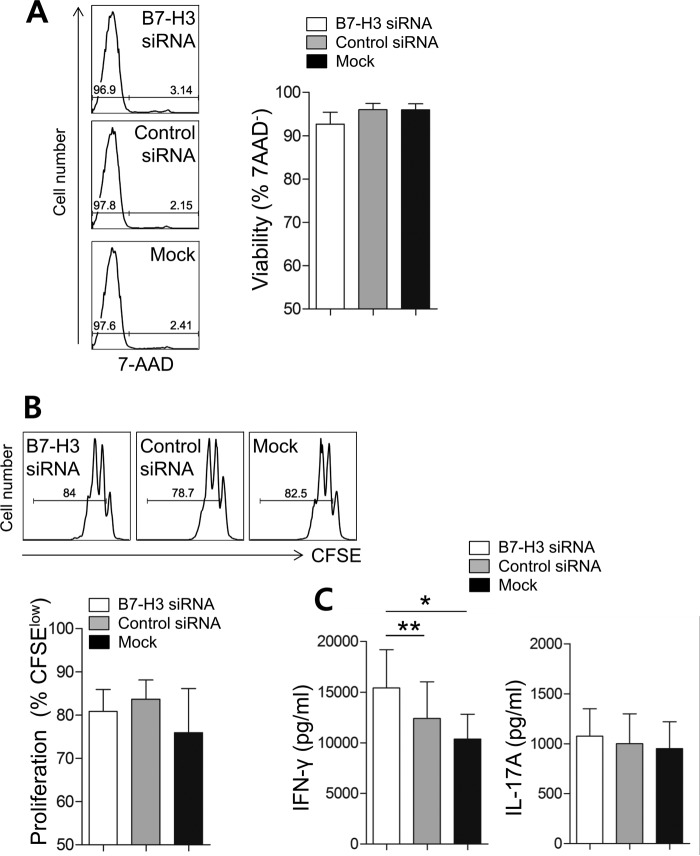
To investigate a co-signaling role for the induced 4Ig B7-H3 isoform during T cell responses, human monocytic THP-1 cells, which constitutively overexpress 4Ig B7-H3 on their surface, were utilized for CD4 T cell co-culture experiments. To repress B7-H3 expression, THP-1 cells were transfected with siRNA before co-culture, whereas control THP-1 cells were transfected with scrambled siRNA. As seen in Fig. 5A, the surface expression of B7-H3 was reduced by ∼50%, and this reduction persisted for at least 3 days post-transfection (data not shown). Although both forms of B7-H3 share the siRNA target site, our immunoblotting data clearly showed that the 4Ig B7-H3 isoform of THP-1 cells was more efficiently silenced by the siRNA than 2Ig B7-H3 isoform (Fig. 5B). The function of B7-H3 has previously been evaluated by T cell proliferation assays and analysis of cytokine production profiles (13, 27). Given that CD4 memory T cells are the abundant T cell subset in RA patient synovial fluid (data not shown), freshly purified CD4 memory T cells were used for co-culture with B7-H3-silenced THP-1 cells and control THP-1 cells for 7 days. No significant effect of B7-H3 silencing on cell death or proliferation of CD4 T cells was observed on day 3 after co-culture (Fig. 6, A and B); however, knockdown of B7-H3 had functional consequences on cytokine production by CD4 T memory cells. Thus, co-culture of CD4 memory T cells with B7-H3-silenced THP-1 cells showed significantly increased levels of IFN-γ production, but not IL-17A production, when compared with mock transfection, as well as control siRNA transfected THP-1 cells (Fig. 6C). This suggests that the 4Ig B7-H3 isoform has an inhibitory effect on Th1 cytokine production by CD4 memory T cells.
FIGURE 5.
Knockdown efficiency and specificity of human B7-H3 siRNA. A, knockdown efficiency of B7-H3. B7-H3-expressing THP-1 cells were transfected with human B7-H3-specific or control siRNA (100 μm of both siRNAs), and the surface expression of B7-H3 was analyzed at 48 h after transfection using flow cytometry. B, differential knockdown effect of human B7-H3 siRNA on 2Ig and 4Ig B7-H3 isoforms. B7-H3 expression in whole lysates was analyzed using immunoblotting at 48 h after siRNA transfection. Horizontal bars and error bars show the means ± S.E. **, p < 0.01 by paired t test in A. MFI, mean fluorescence intensity.
FIGURE 6.
Surface B7-H3 exerts a negative effect on Th1-mediated immune responses. B7-H3-expressing THP-1 cells were transfected with B7-H3-specific siRNA and co-cultured in the presence of soluble anti-CD3 and anti-CD28 antibodies (1 μg/ml of both) with CD4 memory T cells purified from healthy controls for 7 days (n = 10). A, the viability of co-cultured CD4 memory T cells was determined by the frequency of 7-aminoactinomycin D (7-AAD−) cells on day 3 after co-culture. B, the proliferation of CD4 memory T cells was analyzed with carboxyfluorescein succinimidyl ester (CFSE) dilution assay on day 3 after co-culture. C, on day 7, the culture supernatant was harvested, and IFN-γ and IL-17A in the supernatant were quantified using commercial ELISA kits. Each pair represents an independent experiment (n = 10). Horizontal bars and error bars show the mean ± S.E. *, p < 0.05; **, p < 0.01 by paired t test in C.
Clinical Relevance of Overexpressed B7-H3 in RA Patients
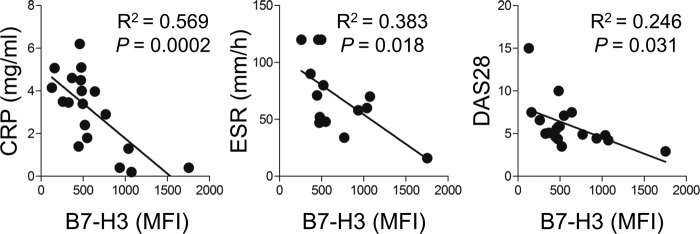
In vitro data obtained by us and others suggest that the induced 4Ig B7-H3 isoform exerts co-inhibitory functions on inflammatory T cell responses. Given the enhanced expression of B7-H3 on synovial monocytes, we next asked whether this increased expression is associated with clinical parameters and disease severity of RA patients. As seen in Fig. 7, the expression level of B7-H3 on synovial monocytes inversely correlates with the serum level of CR and the ESR, suggesting that the expression of B7-H3 is closely related to inflammatory conditions (p < 0.0005 and p < 0.02, respectively). More importantly, there was a significant inverse correlation of B7-H3 expression on synovial monocytes with RA disease severity as measured by DAS28 (Fig. 7; R2 = 0.246, p = 0.031), suggesting that B7-H3 may participate in the regulation of inflammatory responses in RA.
FIGURE 7.
Clinical relevance of enhanced B7-H3 surface expression on synovial monocytes in RA patients. Correlation of surface B7-H3 expression of synovial monocytes with RA clinical parameters (n = 19). The mean fluorescence intensity (MFI) of surface B7-H3 was plotted against serum CRP, ESR, and DAS28. p values were obtained using the Pearson correlation analysis.
Discussion
Our studies demonstrate that the expression of B7-H3, a recently identified B7 family member, is distinctively up-regulated on the cell surface of synovial monocytes from RA patients compared with both their peripheral monocytes and healthy controls. Furthermore, surface B7-H3 on synovial monocytes is prevailingly presented as the 4Ig isoform, whereas the shorter 2Ig B7-H3 isoform is constitutively expressed in the intracytoplasmic region of monocytes irrespective of their activation status. Knockdown experiments revealed that the 4Ig B7-H3 isoform functions as a co-inhibitory molecule for IFN-γ production by CD4 memory cells following TCR ligation. Moreover, the expression level of surface B7-H3 on synovial monocytes from RA patients inversely correlates with clinical parameters, suggesting that B7-H3 may participate in the regulation of inflammatory responses during RA.
Monocytes function as essential innate effectors for the efficient control and elimination of various pathogens; however, in many clinical settings, activated monocytes can also be involved in the pathogenesis of chronic inflammatory disorders including autoimmune diseases (28). A massive influx of activated monocytes/macrophages is seen in the inflamed joints of RA patients, and thus, these cells have become novel targets around which to design immunotherapeutic approaches. All B7 family molecules hitherto identified are constitutively or inducibly expressed in APCs (29, 30). Considering that synovial monocytes play key roles as APCs, B7 family molecules expressed by these cells may be crucial for delivery of co-stimulatory or co-inhibitory signals to modulate T cell responses at sites of inflammation during RA (6).
The expression pattern of B7-H3 is closely linked to activation and differentiation of monocytes and dendritic cells (11, 31, 32). Thus, extensive research has been done to determine the immunological role of this molecule; however, findings concerning the biological function of B7-H3 have been conflicting (11, 15, 18–21). A novel finding of our study is that the B7-H3 induced in synovial monocytes and in cytokine- or TLR-activated monocytes is exclusively the 4Ig isoform. In contrast, the 2Ig isoform of B7-H3 is constitutively expressed by resting monocytes. More importantly, 2Ig and 4Ig isoforms preferentially localize to intracytoplasmic regions and cellular membranes of monocytes, respectively (Figs. 3 and 4), implying differing functional roles. Recent sequencing analysis revealed that the 4Ig B7-H3 isoform has a novel conserved region between the first IgC and the second IgV domain, which is absent in 2Ig B7-H3. This difference might be produced by the splicing of donor sites during exon duplication (14). Although this conserved region is relative short, only six amino acids, this region is thought to make a pivotal contribution to structural changes in 4Ig B7-H3, likely causing important functional consequences (14).
Like other B7 family members, a soluble form of human B7-H3 (sB7-H3) has been identified (18, 33–35). A recent study suggested that release of sB7-H3 from the cell surface might be mediated by proteolytic shedding. Notably, it has been shown that the novel conserved region in 4Ig B7-H3 interfered with matrix metalloproteinase cleavage by causing structural changes, and thus, only the 2Ig isoform could be presented in the soluble form (14). On the other hand, another study suggested that the production of soluble B7-H3 in hepatocellular carcinoma cells occurs by alternative splicing of mRNA, which lacks a transmembrane domain (36). It is possible that there are several spliced forms of B7-H3 in humans. Our findings clearly show that human monocytes have a spliced 2Ig mRNA that includes the exon 6 for transmembrane region (Fig. 2, A and B), and 2Ig B7-H3 is predominantly detected in the intracytoplasmic fraction and minimally expressed on the surface of monocytes (Fig. 4) compared with 4Ig B7-H3. Therefore, this suggests that proteolysis cleavage might be a critical step for releasing human 2Ig B7-H3 in immune cells. Given the considerable level (∼3 ng/ml) of plasma sB7-H3 in healthy controls and its significant elevation in various disease conditions, the underlying mechanisms for the alternative splicing and trafficking of sB7-H3 form needs to be understood (18, 33).
It has been previously shown by us, as well as others, that 4Ig B7-H3 is the dominant isoform induced on immune cells and tumor cells in humans (31) (Figs. 3 and 4). Therefore, more effort has been put toward investigation of the immunoregulatory roles of 4Ig B7-H3. However, it is impractical to establish the 4Ig B7-H3 KO mouse model for study of B7-H3 because of the absence of thisisoform in mice (15, 37). Therefore, most studies have been conducted using plate-bound 4Ig Fc fusion proteins or nonimmune cell lines transfected with 4Ig expression vectors to test the immunoregulatory roles of this molecule on T cell responses.
In this study, we found that human monocytic cells, THP-1 cells, constitutively overexpress the 4Ig isoform on their surface in manner similar to synovial monocytes. Thus, THP-1 cells were utilized for delivering 4Ig B7-H3-mediated signals to co-cultured CD4 memory T cells in the presence of TCR stimulation (Fig. 6). Moreover, knockdown of surface B7-H3 in THP-1 cells using siRNA technology significantly enhanced IFN-γ production from TCR-stimulated CD4 memory T cells but had no substantial effect on T cell proliferation or cell death in our experimental system. This suggests a selective negative role for this molecule in Th1-mediated immune responses (Fig. 6C). It is intriguing that only synovial monocytes, but not peripheral monocytes, of RA patients induce expression of the 4Ig B7-H3 isoform on their cell surface. Differential expression patterns between mRNA and protein have been reported for this molecule, suggesting that the expression of B7-H3 is controlled by post-transcriptional regulation (15, 38). As seen in Fig. 3, it is likely that stimulation by cytokines, specifically GM-CSF, or by TLR ligands is linked to the post-transcriptional regulation of B7-H3.
Previous studies have demonstrated that synovial monocytes of RA patients have significantly enhanced expression of HLA-DR and CD80/86(B7-1/B7-2), indicating that they are in an activated state, presumably because of the proinflammatory milieu of the synovial fluid. As previously suggested, induction of the 4Ig isoform could be a part of the differentiation program from monocytes to dendritic cells (32) to counterbalance the augmented T cell responses caused by enhanced expression of HLA-DR and CD80/86 (B7-1/B7-2) in synovial monocytes.
We previously reported that synovial monocytes possess the unique capability to promote Th1 and Th17 responses of peripheral CD4 T cells (17). Although the higher expression of surface B7-H3 was described as one of major features of synovial monocytes in the studies, we also showed the enhanced expressions of HLA-DR and CD80, which delivers “signal 1” and “signal 2” to T cells, respectively. Thus, synovial monocytes could have immunostimulatory effects on T cell responses at the site of inflammation (17). In addition, inflammatory CD14+CD16+ monocytes, which greatly produce TNF-α and IL-1β upon stimulation, are more expanded in synovial fluid (17, 39). Presumably, the inhibitory effect of surface B7-H3 on CD4 T cells is not sufficient to overcome the stimulatory signals from co-stimulatory molecules, as well as proinflammatory cytokines in many RA patients. Moreover, the synovial and peripheral monocytes in the previous study were stimulated with LPS before in vitro co-culture with CD4 T cells. As shown in Fig. 2, surface B7-H3 expression is significantly enhanced even by peripheral monocytes derived from healthy donors following stimulation with LPS. Therefore, the co-culture system with LPS-treated monocyte might not be suitable for evaluating the sole effect of B7-H3. In present study, THP-1 cells for siRNA experiments were not stimulated with LPS to examine the sole effect of B7-H3 on T cell function (Fig. 5).
Furthermore, we found that the surface B7-H3 expression level on synovial monocytes inversely correlates with clinical parameters including CRP level, ESR, and more interestingly, DAS28 (Fig. 7). These findings provide the first evidence that surface B7-H3 induced in synovial monocytes is related to inhibitory regulation of inflammatory responses in RA.
TLT-2 (triggering receptor expressed on myeloid cells-like transcript 2) was recently identified as a counter-receptor for murine B7-H3 (20). TLT-2 is expressed on T cells, and its interaction with B7-H3 preferentially enhances T cell responses (20). On the contrary, other group immediately reported that TLT-2 did not serve as receptor for murine B7-H3 and T cell-B7-H3 interaction in mouse model potently and consistently mediated an inhibitory T cell response (7, 12, 19, 27). A functional role of TLT-2 in humans is also still controversial (20, 27). As discussed above, duplicate isoforms of human B7-H3 have different structures and therefore might bind to other receptors on activated T cells (14), and the contrasting roles for B7-H3 can likely be attributed to different receptors on activated T cells (38). In this context, it should be noted that the human 2Ig isoform, possibly a soluble form, acts as positive co-stimulatory signal, whereas the 4Ig isoform delivers negative co-inhibitory signal to T cells (14, 18). Identification of putative receptors for 2Ig and 4Ig on T cells will be required to achieve a further understanding of the diverse roles of human B7-H3 on the modulation of T cell responses in disease conditions like RA.
Here we provide evidence of a role for 4Ig B7-H3 in synovial monocytes during the pathogenesis of RA, and we demonstrate the distinctive up-regulation of 4Ig B7-H3 on the surface of synovial monocytes, but not peripheral monocytes, in patients with RA. Further, an inhibitory function of this molecule on IFN-γ production by CD4 memory cells in the presence of TCR signaling was shown through B7-H3-silencing experiments. In RA patients, the expression of surface B7-H3 on synovial monocytes inversely correlates with RA clinical parameters, suggesting that B7-H3 may participate in the regulation of inflammatory responses in RA.
Author Contributions
B. R. Y. and Y.-H. C.: participated in the design of the study, performed most of the experiments and data collection and analysis, and drafted the manuscript. K. K. performed experiments, data collection, and analysis. S.-J. Y., J. K., and I. S. Y. participated in the design of the study, collected patient samples and data analysis, and revised the manuscript. C.-G. P. conceived of the study and participated in its design. S. W. K. conceived of the study, participated in its design, performed data analysis, and drafted the manuscript. W.-W. L. conceived of the study, participated in its design and coordination, performed data analysis and writing of manuscript, and has full access to all the data in this study and financial support. All authors have read and approved the final manuscript.
This work was supported in part by Grant HI11C1705 (A111752 to W.-W. L.) from the Korea Healthcare Technology R&D Project, Ministry for Health & Welfare Affairs, Republic of Korea and by Grant 0420100540 (2010-1078 to W.-W. L.) from the Seoul National University Hospital Research Fund, Republic of Korea. The authors declare that they have no conflicts of interest with the contents of this article.
- RA
- rheumatoid arthritis
- APC
- antigen-presenting cell
- CRP
- C-reactive protein
- DAS28
- disease activity score in 28 joints
- ESR
- erythrocyte sedimentation rate
- GM-CSF
- granulocyte macrophage colony-stimulating factor
- PBMC
- peripheral blood mononuclear cells
- PBMO
- peripheral blood monocytes
- sB7-H3
- soluble form of B7-H3
- SFMC
- synovial fluid mononuclear cells
- SFMO
- synovial fluid monocytes
- TCR
- T cell receptor
- Ab
- antibody.
References
- 1. McInnes I. B., and O'Dell J. R. (2010) State-of-the-art: rheumatoid arthritis. Ann. Rheum. Dis. 69, 1898–1906 [DOI] [PubMed] [Google Scholar]
- 2. McInnes I. B., and Schett G. (2011) The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365, 2205–2219 [DOI] [PubMed] [Google Scholar]
- 3. Tran C. N., Thacker S. G., Louie D. M., Oliver J., White P. T., Endres J. L., Urquhart A. G., Chung K. C., and Fox D. A. (2008) Interactions of T cells with fibroblast-like synoviocytes: role of the B7 family costimulatory ligand B7-H3. J. Immunol. 180, 2989–2998 [DOI] [PubMed] [Google Scholar]
- 4. Fox D. A., Gizinski A., Morgan R., and Lundy S. K. (2010) Cell-cell interactions in rheumatoid arthritis synovium. Rheum. Dis. Clin. North Am. 36, 311–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walter G. J., Evans H. G., Menon B., Gullick N. J., Kirkham B. W., Cope A. P., Geissmann F., and Taams L. S. (2013) Interaction with activated monocytes enhances cytokine expression and suppressive activity of human CD4+CD45ro+CD25+CD127(low) regulatory T cells. Arthritis Rheum. 65, 627–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Evans H. G., Gullick N. J., Kelly S., Pitzalis C., Lord G. M., Kirkham B. W., and Taams L. S. (2009) In vivo activated monocytes from the site of inflammation in humans specifically promote Th17 responses. Proc. Natl. Acad. Sci. U.S.A. 106, 6232–6237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suh W. K., Gajewska B. U., Okada H., Gronski M. A., Bertram E. M., Dawicki W., Duncan G. S., Bukczynski J., Plyte S., Elia A., Wakeham A., Itie A., Chung S., Da Costa J., Arya S., Horan T., Campbell P., Gaida K., Ohashi P. S., Watts T. H., Yoshinaga S. K., Bray M. R., Jordana M., and Mak T. W. (2003) The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat. Immunol. 4, 899–906 [DOI] [PubMed] [Google Scholar]
- 8. Vigdorovich V., Ramagopal U. A., Lázár-Molnár E., Sylvestre E., Lee J. S., Hofmeyer K. A., Zang X., Nathenson S. G., and Almo S. C. (2013) Structure and T cell inhibition properties of B7 family member, B7-H3. Structure 21, 707–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Katsiari C. G., Liossis S. N., Souliotis V. L., Dimopoulos A. M., Manoussakis M. N., and Sfikakis P. P. (2002) Aberrant expression of the costimulatory molecule CD40 ligand on monocytes from patients with systemic lupus erythematosus. Clin. Immunol. 103, 54–62 [DOI] [PubMed] [Google Scholar]
- 10. Bijl M., Horst G., Limburg P. C., and Kallenberg C. G. (2001) Expression of costimulatory molecules on peripheral blood lymphocytes of patients with systemic lupus erythematosus. Ann. Rheum. Dis. 60, 523–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chapoval A. I., Ni J., Lau J. S., Wilcox R. A., Flies D. B., Liu D., Dong H., Sica G. L., Zhu G., Tamada K., and Chen L. (2001) B7-H3: a costimulatory molecule for T cell activation and IFN-γ production. Nat. Immunol. 2, 269–274 [DOI] [PubMed] [Google Scholar]
- 12. Ling V., Wu P. W., Spaulding V., Kieleczawa J., Luxenberg D., Carreno B. M., and Collins M. (2003) Duplication of primate and rodent B7-H3 immunoglobulin V- and C-like domains: divergent history of functional redundancy and exon loss. Genomics 82, 365–377 [DOI] [PubMed] [Google Scholar]
- 13. Steinberger P., Majdic O., Derdak S. V., Pfistershammer K., Kirchberger S., Klauser C., Zlabinger G., Pickl W. F., Stöckl J., and Knapp W. (2004) Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J. Immunol. 172, 2352–2359 [DOI] [PubMed] [Google Scholar]
- 14. Sun J., Fu F., Gu W., Yan R., Zhang G., Shen Z., Zhou Y., Wang H., Shen B., and Zhang X. (2011) Origination of new immunological functions in the costimulatory molecule B7-H3: the role of exon duplication in evolution of the immune system. PLoS One 6, e24751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yi K. H., and Chen L. (2009) Fine tuning the immune response through B7-H3 and B7-H4. Immunol. Rev. 229, 145–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zang X., and Allison J. P. (2007) The B7 family and cancer therapy: costimulation and coinhibition. Clin Cancer Res. 13, 5271–5279 [DOI] [PubMed] [Google Scholar]
- 17. Yoon B. R., Yoo S. J., Choi Y. H., Chung Y. H., Kim J., Yoo I. S., Kang S. W., and Lee W. W. (2014) Functional phenotype of synovial monocytes modulating inflammatory T-cell responses in rheumatoid arthritis (RA). PLoS One 9, e109775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang G., Wang J., Kelly J., Gu G., Hou J., Zhou Y., Redmond H. P., Wang J. H., and Zhang X. (2010) B7-H3 augments the inflammatory response and is associated with human sepsis. J. Immunol. 185, 3677–3684 [DOI] [PubMed] [Google Scholar]
- 19. Prasad D. V., Nguyen T., Li Z., Yang Y., Duong J., Wang Y., and Dong C. (2004) Murine B7-H3 is a negative regulator of T cells. J. Immunol. 173, 2500–2506 [DOI] [PubMed] [Google Scholar]
- 20. Hashiguchi M. (2012) Human B7-H3 binds to triggering receptor expressed on myeloid cells-like transcript 2 (TLT-2) and enhances T cell responses. Open J. Immunol. 02, 9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jiang J., Jiang J., Liu C., Zhang G., Gao L., Chen Y., Zhu R., Wang T., Wang F., Zhang X., and Xue Q. (2013) Enhancement of membrane B7-H3 costimulatory molecule but reduction of its soluble form in multiple sclerosis. J. Clin. Immunol. 33, 118–126 [DOI] [PubMed] [Google Scholar]
- 22. Brennan F. M., and McInnes I. B. (2008) Evidence that cytokines play a role in rheumatoid arthritis. J. Clin. Invest. 118, 3537–3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McInnes I. B., and Schett G. (2007) Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 7, 429–442 [DOI] [PubMed] [Google Scholar]
- 24. Castriconi R., Dondero A., Augugliaro R., Cantoni C., Carnemolla B., Sementa A. R., Negri F., Conte R., Corrias M. V., Moretta L., Moretta A., and Bottino C. (2004) Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc. Natl. Acad. Sci. U.S.A. 101, 12640–12645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zang X., Thompson R. H., Al-Ahmadie H. A., Serio A. M., Reuter V. E., Eastham J. A., Scardino P. T., Sharma P., and Allison J. P. (2007) B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc. Natl. Acad. Sci. U.S.A. 104, 19458–19463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crispen P. L., Sheinin Y., Roth T. J., Lohse C. M., Kuntz S. M., Frigola X., Thompson R. H., Boorjian S. A., Dong H., Leibovich B. C., Blute M. L., and Kwon E. D. (2008) Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin. Cancer Res. 14, 5150–5157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leitner J., Klauser C., Pickl W. F., Stöckl J., Majdic O., Bardet A. F., Kreil D. P., Dong C., Yamazaki T., Zlabinger G., Pfistershammer K., and Steinberger P. (2009) B7-H3 is a potent inhibitor of human T-cell activation: No evidence for B7-H3 and TREML2 interaction. Eur. J. Immunol. 39, 1754–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shi C., and Pamer E. G. (2011) Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 11, 762–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Collins M., Ling V., and Carreno B. M. (2005) The B7 family of immune-regulatory ligands. Genome Biol. 6, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Greenwald R. J., Freeman G. J., and Sharpe A. H. (2005) The B7 family revisited. Annu. Rev. Immunol. 23, 515–548 [DOI] [PubMed] [Google Scholar]
- 31. Zhou Y. H., Chen Y. J., Ma Z. Y., Xu L., Wang Q., Zhang G. B., Xie F., Ge Y., Wang X. F., and Zhang X. G. (2007) 4IgB7-H3 is the major isoform expressed on immunocytes as well as malignant cells. Tissue Antigens 70, 96–104 [DOI] [PubMed] [Google Scholar]
- 32. Zhang G., Dong Q., Xu Y., Yu G., and Zhang X. (2005) B7-H3: another molecule marker for Mo-DCs? Cell. Mol. Immunol. 2, 307–311 [PubMed] [Google Scholar]
- 33. Zhang G., Hou J., Shi J., Yu G., Lu B., and Zhang X. (2008) Soluble CD276 (B7-H3) is released from monocytes, dendritic cells and activated T cells and is detectable in normal human serum. Immunology 123, 538–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jeannin P., Magistrelli G., Aubry J. P., Caron G., Gauchat J. F., Renno T., Herbault N., Goetsch L., Blaecke A., Dietrich P. Y., Bonnefoy J. Y., and Delneste Y. (2000) Soluble CD86 is a costimulatory molecule for human T lymphocytes. Immunity 13, 303–312 [DOI] [PubMed] [Google Scholar]
- 35. Wong C. K., Lit L. C., Tam L. S., Li E. K., and Lam C. W. (2005) Aberrant production of soluble costimulatory molecules CTLA-4, CD28, CD80 and CD86 in patients with systemic lupus erythematosus. Rheumatology 44, 989–994 [DOI] [PubMed] [Google Scholar]
- 36. Chen W., Liu P., Wang Y., Nie W., Li Z., Xu W., Li F., Zhou Z., Zhao M., and Liu H. (2013) Characterization of a soluble B7-H3 (sB7-H3) spliced from the intron and analysis of sB7-H3 in the sera of patients with hepatocellular carcinoma. PLoS One 8, e76965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sun M., Richards S., Prasad D. V., Mai X. M., Rudensky A., and Dong C. (2002) Characterization of mouse and human B7-H3 genes. J. Immunol. 168, 6294–6297 [DOI] [PubMed] [Google Scholar]
- 38. Hofmeyer K. A., Ray A., and Zang X. (2008) The contrasting role of B7-H3. Proc. Natl. Acad. Sci. U.S.A. 105, 10277–10278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rossol M., Kraus S., Pierer M., Baerwald C., and Wagner U. (2012) The CD14(bright) CD16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum. 64, 671–677 [DOI] [PubMed] [Google Scholar]