Abstract
Emergency granulopoiesis occurs in response to infectious or inflammatory challenge and is a component of the innate immune response. Some molecular events involved in initiating emergency granulopoiesis are known, but termination of this process is less well defined. In this study, we found that the interferon consensus sequence binding protein (Icsbp/Irf8) was required to terminate emergency granulopoiesis. Icsbp is an interferon regulatory transcription factor with leukemia suppressor activity. Expression of Icsbp is decreased in chronic myeloid leukemia, and Icsbp−/− mice exhibit progressive granulocytosis with evolution to blast crisis, similar to the course of human chronic myeloid leukemia. In this study, we found aberrantly sustained granulocyte production in Icsbp−/− mice after stimulation of an emergency granulopoiesis response. Icsbp represses transcription of the genes encoding Fas-associated phosphatase 1 (Fap1) and growth arrest-specific 2 (Gas2) and activates genes encoding Fanconi C and F. After stimulation of emergency granulopoiesis, we found increased and sustained expression of Fap1 and Gas2 in bone marrow myeloid progenitor cells from Icsbp−/− mice in comparison with the wild type. This was associated with resistance to Fas-induced apoptosis and increased β-catenin activity in these cells. We also found that repeated episodes of emergency granulopoiesis accelerated progression to acute myeloid leukemia in Icsbp−/− mice. This was associated with impaired Fanconi C and F expression and increased sensitivity to DNA damage in bone marrow myeloid progenitors. Our results suggest that impaired Icsbp expression enhances leukemogenesis by deregulating processes that normally limit granulocyte expansion during the innate immune response.
Keywords: calpain, CD95 (APO-1/Fas), gene expression, hematopoiesis, innate immunity
Introduction
Steady-state granulopoiesis is a continuous homeostatic process for replacing granulocytes that are lost to normal programmed cell death. Studies in murine models determined that steady-state granulopoiesis requires the transcription factors PU.1 and C/EBPα and is enhanced by the cytokines GM-CSF2 and G-CSF (1–4). In contrast, emergency (or stress) granulopoiesis is an episodic process for producing granulocytes in response to inflammatory or infectious challenge as part of the innate immune response (5, 6). Studies in murine models found that emergency granulopoiesis requires IL1β, C/EBPβ, and Stat3 and is enhanced by an IL1β-dependent increase in G-CSF (5, 7–10).
Emergency granulopoiesis has four phases: immediate release of mature granulocytes from the bone marrow, expansion of the common granulocyte/monocyte progenitor pool, accelerated differentiation, and termination of the response. Granulocyte release is dependent on CXCR proteins and their ligands (11). S phase shortening during progenitor expansion requires activation of the Fanconi DNA repair pathway to maintain genomic integrity (12). Also, both expansion and differentiation of progenitors require the transcription factors Stat3 and C/EBPβ (7, 8). In contrast, relatively little is known about molecular events that terminate emergency granulopoiesis.
In this study, we hypothesized that Icsbp (also known as interferon regulatory factor 8 (IRF8)) plays a role in regulating emergency granulopoiesis. Icsbp is expressed in hematopoietic stem cells, and expression increases during granulopoiesis and monopoiesis (13–16). Activity of this transcription factor is also modulated by cytokine-induced tyrosine phosphorylation, which is regulated by Jak2 and Shp1/2 (17–19).
Less Icsbp is expressed in CML bone marrow in comparison with normal bone marrow, and a leukemia suppressor role for Icsbp has been suggested by murine models (20–25). In one model, mice were transplanted with bone marrow that was transduced with vectors to express both Bcr-abl and Icsbp or Bcr-abl alone or with 32D cells transduced with these vectors (24, 25). Recipients of co-transduced cells developed CML more slowly than recipients of cells expressing Bcr-abl alone (24, 25). Another model involved disruption of the IRF8 gene. These mice developed granulocytosis that progressed to acute myeloid leukemia (AML), resembling chronic phase to blast crisis progression in human CML (17, 26).
The first identified Icsbp target genes encoded proteins involved in the effector functions of granulocytes, monocytes, or both (e.g. NADPH oxidase proteins, Toll-like receptors, lysosome-related genes, and MHC class I proteins) (13, 15, 16, 27, 28). Additional studies have identified other Icsbp target genes that fall into several functional categories, including DNA repair proteins (e.g. Fanconi C and F), regulators of proliferation/survival (e.g. Neurofibromin1, Bcl2, and Klf4), and co-regulators of Fas and βcatenin (e.g. Fap1 and Gas2) (12, 18, 21, 22, 25, 29).
Studies of bone marrow from mice with IRF8 gene disruption have indicated that loss of this transcription factor expands the common granulocyte/monocyte progenitor population, impairs terminal differentiation/activation of granulocytes, impairs monocyte activation, and prevents monocytes from differentiating into macrophages or dendritic cells (14, 17–19, 26, 28, 30, 31). In vitro, Icsbp−/− myeloid progenitor cells exhibited enhanced proliferation in response to hematopoietic cytokines, including GM-CSF (14, 18). Under long-term culture conditions in GM-CSF, bone marrow cells from IRF8 knockout mice exhibited granulocyte over monocyte commitment (14, 28, 31). Re-expression of Icsbp in these cells facilitated macrophage-like differentiation but impaired granulocyte differentiation, perhaps because of interaction between Icsbp and C/EBPα (31). In other studies, re-expression of Icsbp in Bcr-abl-transduced 32D cells facilitated the expression of markers characteristic of granulocyte differentiation, including G-CSF receptor and C/EBPα (25). Also, a dominant negative C/EBP-mutant that inhibits C/EBPα, β, and ϵ impaired the production of granulocytes and monocytes in both WT and Icsbp−/− bone marrow (31, 32). Therefore, Icsbp exerts multiple effects on granulocyte and monocyte differentiation and activation in a context-dependent manner.
We found that impaired expression of Gas2 and Fap1 contributes to the leukemia suppression effect of Icsbp. Icsbp represses the GAS2 and PTPN13 (encoding Fap1) promoters, and expression of these genes is increased in human CML (20–23). Gas2 inhibits the serine protease activity of calpain, and β-catenin is a calpain substrate in myeloid progenitors (33). We found stabilization of β-catenin protein in Bcr-abl+ or Icsbp−/− bone marrow progenitors in a Gas2/calpain-dependent manner, increasing proliferation (via cyclin D1 and c-myc), and decreasing apoptosis (via survivin) (22).
Fap1 interacts with and dephosphorylates Fas, impairing apoptosis (34). We found Fap1-dependent Fas resistance in Bcr-abl+ and Icsbp−/− bone marrow progenitors (21, 23). Fap1 also interacts with adenomatous polyposis coli protein, facilitating Gsk3β inactivation (dephosphorylation) by Fap1 (35). Ubiquitination and proteasomal degradation of β-catenin is enhanced by Gsk3β-induced phosphorylation, and β-catenin is stabilized in Bcr-abl+ or Icsbp−/− cells via this mechanism (35). In vitro, the effects of Fap1 were inhibited by a Fas-C-terminal tripeptide that blocks a Fap1 protein/protein interaction domain (SLV peptide) (21, 23, 35).
Although these prior studies have implicated Fap1 and Gas2 in leukemogenesis, the role of these proteins in normal granulopoiesis was unknown. In this study, we found that Icsbp contributes to the termination of emergency granulopoiesis by repressing genes encoding Fap1 and Gas2. This antagonizes mechanisms of progenitor expansion that are stimulated during emergency granulopoiesis (i.e. Fas resistance and β-catenin activation). Termination of granulocytosis during the innate immune response is a novel role for an interferon regulatory factor. Our results identified an intersection between innate immunity and leukemogenesis involving Icsbp. This connection is also implied by the observed increase in C/EBPβ-expression in human CML and the association of Stat3 activation with AML in severe congenital neutropenia (36, 37).
Experimental Procedures
Mice and Emergency Granulopoiesis Assay
The study was approved by the Animal Care and Use Committees of Jesse Brown Veterans Affairs Medical Center and Northwestern University. Icsbp+/− mice were obtained from Dr. K. Ozato (National Institutes of Health, Bethesda, MD) and used as a source of Icsbp−/− and Icsbp+/+ (wild-type littermates) (26). The mice were housed in a low-pathogen “barrier” environment or “standard” housing, as specified under “Results.” In this context, a barrier environment was defined as housing animals with a specified pathogen-free status in a tightly regulated environment that included control of the flow of animals, equipment, and personnel; use of micro-isolator cages; and use of husbandry procedures to minimize pathogen exposure and disease outbreak.
WT or Icsbp−/− mice (20–25 weeks of age) were injected intraperitoneally every 4 weeks with an ovalbumin/alum mixture (referred to as Alum in this study) or saline control (10 mice/group). Alum was prepared as described previously (38), and a volume of 0.5 ml was injected. Individual Icsbp−/− mice were chosen so that the average number of circulating granulocytes at the beginning of the experiment was similar in the two treatment groups. Mice were raised and maintained under low-pathogen barrier conditions except when indicated otherwise.
This age group of mice was chosen because they exhibited greater homogeneity of blood counts in comparison with younger mice, and fewer mice of this age exhibited excess myeloid blasts in the circulation or bone marrow in comparison with older mice. The results of Alum injection in a cohort of mice 8–15 weeks old (6 mice/group) were similar to the 20- 25-week-old cohort, but no mice in the younger, control saline-injected group progressed to AML during the study period (consistent with prior work). The younger cohorts were reported separately to prevent skewing in favor of a difference with Icsbp knockout.
In other studies, WT or Icsbp−/− mice were injected intraperitoneally with purified, sterile SLV or VLS peptide (500 μg in 500 μl of sterile PBS). Injections were performed 3 times per week. Mice were enrolled in the study at 8 weeks of age and maintained in a low-pathogen barrier environment.
Peripheral blood was obtained from the tail vein of each mouse, and complete blood counts were determined using an automated cell counter. Myeloid blast counts were verified by hand-counting May-Grünewald-Giemsa-stained peripheral blood smears (blinded for the automated differential, 300 cells/slide). Mice developing leukocytosis (>100,000 cells/mm3) or hemoglobin (≤ 6.0 g/dl) were sacrificed. Images of peripheral blood were captured by light microscopy (×40 magnification).
Studies of Murine Bone Marrow and Other Tissues
Slides made from decalcified sternal bone marrow and paraffin-embedded lung tissues were stained using hematoxylin and eosin by the Pathology Core Facility of the Robert H. Lurie Comprehensive Cancer Center. Light microscopy was performed, and digital images were captured (×40 magnification).
Murine bone marrow mononuclear cells were obtained the femora of mice by standard procedures. Briefly, dissected femora were flushed repeatedly with Hanks' balanced salt solution until no additional cells were obtained. Washed cells were treated with ammonium-chloride-potassium buffer to lyse red blood cells and then washed extensively. Cells from the two femora were combined and counted for each individual mouse. This technique was highly reproducible in our laboratory (12, 17, 22). In some studies, CD34+ cells (myeloid progenitors) were isolated using the Miltenyi magnetic bead affinity technique (Miltenyi Biotech, San Diego, CA).
Flow Cytometry
Murine bone marrow cells were washed with PBS, counted, and labeled with anti-mouse FITC-conjugated antibodies to Sca1 (Ly-6A/E), c-kit, CD34, Mac1 (CD11b), F4/80, Gr1, Ly-6G, or Ly-6C (eBioscience, San Diego, CA). Apoptosis was assessed using the Annexin V-PE apoptosis detection kit I according to the instructions of the manufacturer (BD Biosciences). Bone marrow from four different mice per cohort were examined, and data were reported as the mean ± S.D. from triplicate samples.
Serum IL1β and G-CSF Determination
Serum obtained from the tail veins of WT or Icsbp−/− mice was analyzed by ELISA for IL1β or G-CSF using a commercially available kit (R&D Systems). Cytokine concentrations were calculated according to the instructions of the manufacturer.
Quantitative Real-time PCR
RNA was isolated using TRIzol reagent (Gibco-BRL) and tested for integrity by denaturing gel electrophoresis. Primers were designed with Applied Biosystems software, and real-time PCR was performed using SYBR green by the “standard curve” method (cDNA from WT cells cultured in GM-CSF; IL3 and stem cell factor was used for the standard curve). At least three independent samples were evaluated in triplicate. The results for mRNA expression were normalized to 18S and actin.
Calpain Assays
Calpain activity assays were performed using a kit from Biovision (Mountain View, CA). Lysates of cells from murine bone marrow were assayed for calpain activity by flurometric change and normalized for the percent of apoptotic cells.
DNA Damage Assays
Bone marrow was harvested from WT or Icsbp−/− mice between 20–26 weeks of age. Icsbp−/− mice with less than 5% myeloid blasts in the peripheral blood were selected. Mononuclear cells were cultured in GM-CSF, IL3, and stem cell factor for 24 h, followed by 24 h in G-CSF. Cells were treated with mitomycin C (20 ng/ml) for the final 24 h. Chromatin spreads were made as described previously (29) and analyzed microscopically for breaks and radials.
Statistical Analysis
Statistical significance was determined by unpaired two tailed Student's t test (comparing two conditions), analysis of variance (for more than two conditions) or log-rank analysis (for survival curves) using SigmaPlot and SigmaStat software. p < 0.02 was considered statistically significant. In all graphs, error bars represent the mean ± S.E.
Results
Icsbp−/− Mice Exhibited a Sustained Emergency Granulopoiesis Response
In a low-pathogen environment (i.e. barrier housing), young Icsbp−/− mice exhibit mild granulocytosis that increases with age. Under these conditions, we found an average number of circulating granulocytes (or polymorphonuclear leukocytes PMNs) of 1.1 + 0.6 (× 103 cells/mm3) in mice younger than 10 weeks, rising to 2.8 + 0.4 by 20 weeks of age (p < 0.01, n = 6). However, we observed accelerated granulocytosis in Icsbp−/− mice maintained in standard housing (i.e. non-barrier). The average number of circulating granulocytes at 20 weeks in Icsbp−/− mice in this environment was 11.3 + 0.5 (p < 0.0001, n = 6 in comparison with barrier housing). Because pathogen exposure would not influence steady-state granulopoiesis, we considered the possibility that Icsbp regulates emergency granulopoiesis.
Emergency granulopoiesis is studied in mice by intraperitoneal injection of either pathogens (bacterial/fungal) or an ovalbumin/alum mixture (referred to as Alum) (9, 10, 12, 38). In either model, mice develop an IL1β-dependent response that includes immediate release of granulocytes from the bone marrow, increased G-CSF in the serum and bone marrow, increased granulopoiesis, and circulating granulocytosis (maximal at 10–14 days) (9, 12, 38). Because we were interested in studying multiple episodes of emergency granulopoiesis, we chose an Alum injection because it does not result in death or chronic infection in WT mice. Specifically, WT mice easily tolerate six episodes of Alum-induced emergency granulopoiesis, 4 weeks apart, without death or debility (12). Circulating granulocytes return to baseline between injections in WT mice (12).
We first studied the involvement of Icsbp in the granulocyte release phase of emergency granulopoiesis by injecting Icsbp−/− mice and WT littermates with Alum or saline (as a control for steady-state granulopoiesis). Mice were age-matched (20–25 weeks), and 10 mice were assigned to each group. Peripheral white blood cell counts were determined 12, 24, and 48 h after injection.
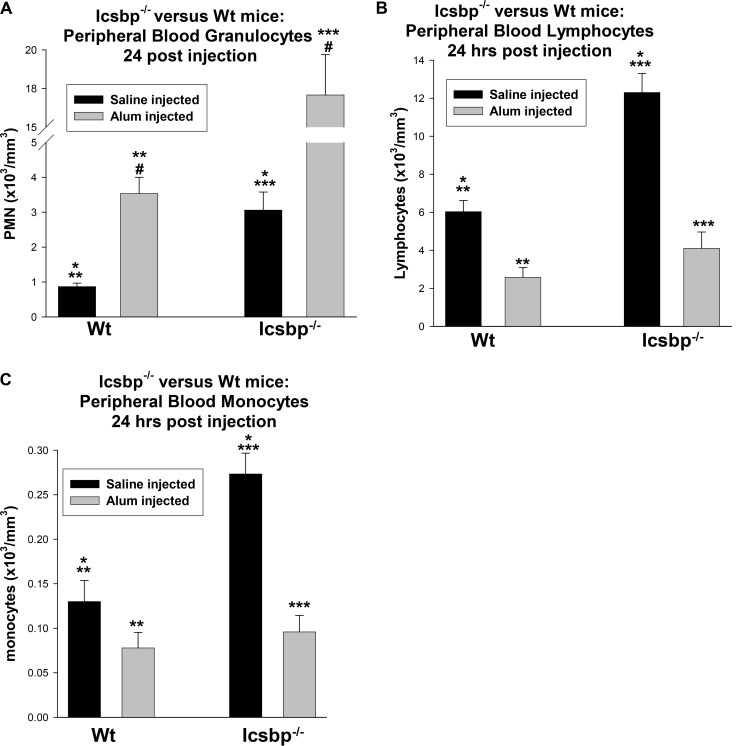
We found that circulating PMNs peaked 24 h after Alum injection in WT or Icsbp−/− mice (Fig. 1A). The baseline value was greater in Icsbp−/− mice (p < 0.0001, n = 10), and the relative increase in circulating PMNs with Alum injection was also greater (3.6-fold for WT versus 6.7-fold for Icsbp−/− mice). Circulating PMNs returned to baseline 48 h after Alum injection in all mice. Relative lymphopenia (Fig. 1B) and monocytopenia (Fig. 1C) occurred 24 h after Alum injection in WT or Icsbp−/− mice, as expected (38). Baseline circulating lymphocytes were higher in Icsbp−/− mice relative to WT mice (p < 0.0001, n = 10), but the relative decrease was similar (2.4-fold decrease in WT versus 3.3-fold in Icsbp−/− mice). Circulating monocytes were greater at steady state in Icsbp−/− mice, but their decrease was relatively greater upon Alum stimulation (2.5-fold decrease versus 1.7-fold).
FIGURE 1.
Icsbp was not required for granulocyte release from the bone marrow during the first phase of emergency granulopoiesis. Icsbp−/− and wild-type mice were injected with Alum to stimulate emergency granulopoiesis or saline as a steady-state granulopoiesis control. Peripheral blood counts were determined 24 h later. A, granulocytes (PMNs) increased significantly in Icsbp−/− and WT mice after Alum injection. Statistically significant differences are indicated by *, **, ***, and #. B and C, lymphocytes (B) or monocytes (C) decreased significantly in Icsbp−/− and WT mice 24 h after Alum injection. Statistically significant differences are indicated by *, **, and ***. p < 0.02 was considered statistically significant.
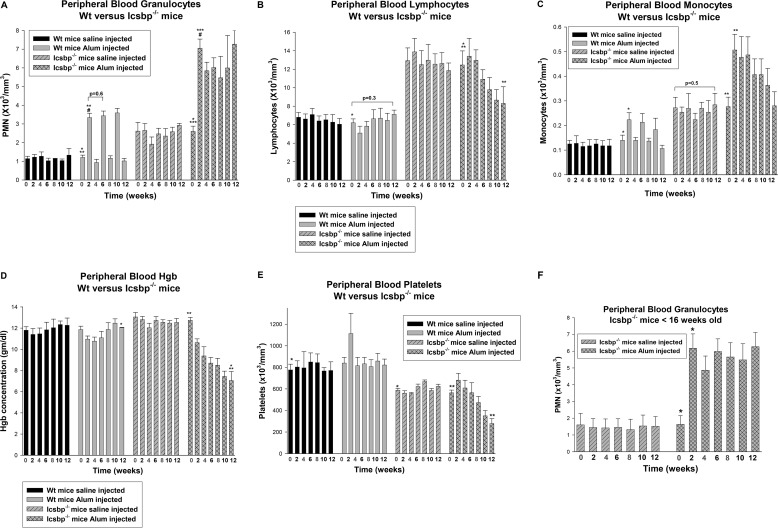
In WT mice, the number of circulating PMNs increased an average of 2.7 + 0.25-fold 2 weeks after Alum injection (12). Icsbp−/− mice started with more circulating PMNs, but we observed a comparable relative increase 2 weeks after Alum injection (2.4 + 0.34-fold, p = 0.3, n = 10 for comparison with the WT) (Fig. 2A). In WT mice, circulating PMNs returned to baseline 4 weeks after Alum injection but remained an average of 1.8 + 0.28-fold above baseline in Icsbp−/− mice (Fig. 2A).
FIGURE 2.
Icsbp−/− mice developed sustained granulocytosis with progressive anemia and thrombocytopenia during multiple episodes of emergency granulopoiesis. Icsbp−/− and wild-type mice were injected every 4 weeks (red numbers) with Alum to stimulate emergency granulopoiesis or saline as a steady-state control. Peripheral blood counts were determined every 2 weeks. A, PMNs did not return to baseline after stimulation of emergency granulopoiesis in Icsbp−/− mice, but emergency granulopoiesis resolved by 4 weeks in WT mice. Statistically significant differences are indicated by *, **, ***, and #. B, multiple episodes of emergency granulopoiesis induced relative lymphopenia in Icsbp−/− mice. Statistically significant differences are indicated by * and **. C, monocytosis after Alum injection in Icsbp−/− and WT mice resolved in the latter but was initially sustained in the former. Statistically significant differences are indicated by * and **. D, multiple episodes of emergency granulopoiesis induced progressive anemia in Icsbp−/− mice but only transient anemia in WT mice. Statistically significant differences are indicated by * and **. Hgb, hemoglobin. E, multiple episodes of emergency granulopoiesis resulted in progressive thrombocytopenia in Icsbp−/− mice but not WT mice. Statistically significant differences are indicated by * and **. F, a young cohort of Icsbp−/− mice has the same PMN response to multiple cycles of emergency granulopoiesis as older mice. Statistically significant difference is indicated by *. p < 0.02 was considered statistically significant.
To investigate the consequences of Icsbp loss during multiple episodes of emergency granulopoiesis, injections were repeated every 4 weeks. In WT mice, the pattern of increased PMNs followed by recovery was replicated for each Alum injection cycle (Fig. 2A). In contrast, circulating PMNs never returned to baseline after the first Alum injection in Icsbp−/− mice (Fig. 2A). A further increase in circulating granulocytes was not seen with subsequent Alum injections in Icsbp−/− mice. One possibility explanation for this observation is that the additional PMNs were accumulating in the bone marrow or tissues. Alternatively, granulocyte production might be sustained at maximal levels in Icsbp−/− bone marrow after the first Alum injection (i.e. no further increase possible), or key factors required for progenitor expansion and granulocyte production might be depleted after the first injection. These alternatives are considered further below.
The numbers of circulating lymphocytes were not altered by multiple cycles of Alum injection in WT mice but decreased during the experiment in Icsbp−/− mice (p = 0.002, n = 6) (Fig. 2B). Circulating monocytes were increased 2-fold 2 weeks after Alum injection in both WT and Icsbp−/− mice (Fig. 2C). Monocytosis resolved by 4 weeks after Alum injection in WT mice but was sustained in Icsbp−/− mice with a gradual decrease over time.
Hemoglobin concentration was not significantly different at baseline in Icsbp−/− or WT mice (p = 0.05, n = 10). Mild anemia developed, and subsequently resolved, in WT mice during the first Alum injection cycles (Fig. 2D). In contrast, Icsbp−/− mice developed progressive anemia during three Alum injection cycles (p < 0.0001, n = 10) (Fig. 2D). Some WT mice exhibited mild thrombocytosis with the first cycle of Alum injection that subsequently resolved (Fig. 2E). At baseline, platelet counts were lower in Icsbp−/− mice versus WT mice (p < 0.001, n = 10), and thrombocytopenia was progressive in Icsbp−/− mice upon repeated cycles of Alum injection (Fig. 2E) (p < 0.001, n = 10).
We performed similar studies with a cohort of Icsbp−/− mice between 8–15 weeks of age (6 mice/group). The younger mice exhibited similar responses to the 20- to 25-week-old cohort during three injection cycles. These mice had fewer circulating granulocytes at treatment initiation but exhibited a similar -fold increase in response to the first Alum injection with sustained granulocytosis over subsequent injections (Fig. 2F). The emergency granulopoiesis response was not influenced by age in WT mice (data not shown).
Icsbp Influenced Bone Marrow Progenitor Expansion during Emergency Granulopoiesis
To determine whether similar mechanisms were involved in emergency granulopoiesis in WT versus Icsbp−/− mice, we first investigated the effects on various bone marrow populations. We analyzed bone marrow from WT or Icsbp−/− mice by flow cytometry 2 weeks after injection with Alum or saline (representing emergency and steady-state granulopoiesis, respectively).
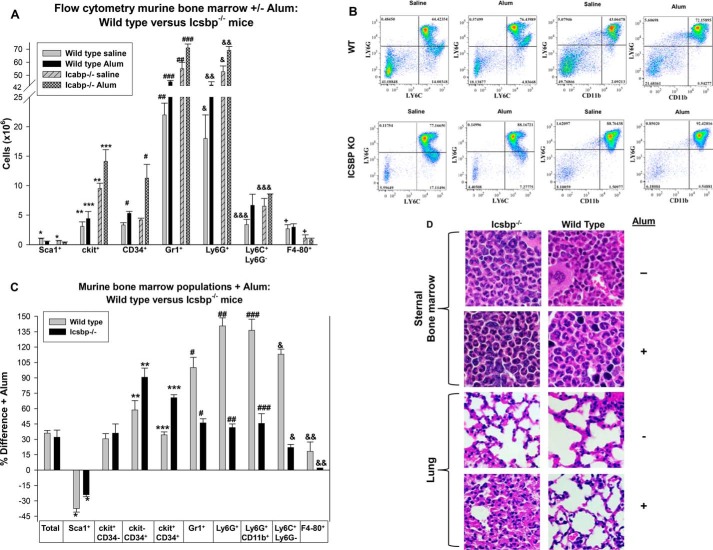
There were twice as many total bone marrow mononuclear cells in Icsbp−/− mice versus WT mice during both steady-state and emergency granulopoiesis (average of 82.5 + 3.5 × 106 versus 37.4 + 6.5 × 106 bone marrow cells, two femora per mouse counted) (p < 0.001, n = 3). However, the number of Sca1+ bone marrow cells was significantly less in Icsbp−/− mice in comparison with WT mice under both conditions (p < 0.01, n = 3) (Fig. 3A). Conversely, the number of differentiating and mature granulocytes (Gr1+ or Ly6G+ cells) was ∼2.5-fold greater in Icsbp−/− bone marrow in comparison with WT bone marrow at steady state (p < 0.0001, n = 3) but significantly less than 2-fold greater during emergency granulopoiesis (Fig. 3, A and B). Early and committed myeloid progenitors (c-kit+ and CD34+ cells, respectively) were more than 2-fold greater in Icsbp−/− versus WT mice after Alum injection (p < 0.001, n = 3). At steady state, CD34+ cells were equivalent in WT and Icsbp−/− mice, but the c-kit+ population was relatively expanded in Icsbp−/− bone marrow (Fig. 3A).
FIGURE 3.
Different bone marrow populations expanded during emergency granulopoiesis in wild-type versus Icsbp−/− mice. Mice were injected with Alum to stimulate emergency granulopoiesis or saline as a steady-state control and sacrificed 2 weeks later. A, during steady-state granulopoiesis, populations of Gr1+, Ly6G+, Ly6C+Ly6G−, and c-kit+ bone marrow cells were greater in Icsbp−/− versus WT mice, and, during emergency granulopoiesis, the CD34+ population was also greater in Icsbp−/− mice. Bone marrow was evaluated by flow cytometry, and cell numbers were calculated. Statistically significant differences are indicated by *, **, ***, #, ##, ###, &, &&, &&&, and +. B, Ly6C+Ly6G+ and Ly6G+CD11b+ cells increased more in the bone marrow of WT mice in comparison with Icsbp−/− mice 2 weeks after emergency granulopoiesis stimulation. The study was repeated three times, and representative histograms are shown. C, during emergency granulopoiesis, Icsbp−/− mice had a relatively greater increase in CD34+ cell populations, but WT mice had a greater relative increase in granulocyte and monocyte populations. The data above were analyzed for percent change. Statistically significant differences are indicated by *, **, ***, #, ##, ###, &, and &&. D, sternal bone marrow from Alum-injected WT or Icsbp−/− mice showed abundant mature granulocytes, but only Alum-injected Icsbp−/− mice demonstrated pulmonary infiltration with granulocytes. Tissues were stained with H&E and examined by light microscopy at ×40 magnification. p < 0.02 was considered statistically significant.
Icsbp is involved in terminal differentiation/activation of granulocytes, and prior studies have determined that monocyte activation or differentiation to macrophage/dendritic cells is impaired in Icsbp−/− murine bone marrow (17, 26). Therefore, we also investigated the expression of Ly6C+Ly6G− (mature but not activated monocytes) and F4–80 (tissue macrophages) in the bone marrow during emergency granulopoiesis. We found that the abundance of Ly6C+Ly6G− monocytes was approximately twice as great in Icsbp−/− bone marrow versus WT bone marrow at steady state (p < 0.001, n = 3) (Fig. 3A). Similar results were seen with CD11b+Ly6G− cells (data not shown). After Alum injection, the abundance of monocytes increased in the bone marrow of both WT and Icsbp−/− mice (Fig. 3A). In contrast, the abundance of F4–80+ macrophages in Icsbp−/− bone marrow was low in comparison with WT bone marrow (∼2-fold less) and did not change during emergency granulopoiesis (Fig. 3A).
The number of total bone marrow cells increased proportionately in WT and Icsbp−/− mice after Alum injection (36.4% + 0.9% versus 39.5% + 1.6%, respectively) (Fig. 3C). The relative abundance of Sca1+ bone marrow cells decreased 2 weeks after Alum injection in both WT and Icsbp−/− mice (p < 0.001, n = 3), but the decrease was less in Icsbp−/− mice (Fig. 3C). In contrast, the increase in c-kit−CD34+ or c-kit+CD34+ cells in the bone marrow of Icsbp−/− mice was 90% and 75%, respectively, during emergency granulopoiesis (p < 0.001, n = 3) but significantly less in WT mice (p < 0.01, n = 3 for comparisons between WT and Icsbp−/− mice) (Fig. 3C).
We found that CD34+ cells were the population with the greatest expansion during emergency granulopoiesis in Icsbp−/− mice. In contrast, the populations with the relatively greatest expansion in WT mice were Gr1+ or Ly6G+CD11b+ (granulocytes) and Ly6C+Ly6G− (monocytes). In WT mice, relative expansion of these populations was more than 2-fold the expansion in Icsbp−/− mice (Fig. 3C). This suggested that Icsbp limits myeloid progenitor expansion during emergency granulopoiesis but enhances expansion of differentiating/mature granulocytes. In Icsbp−/− mice, total CD34+ cells averaged 25.3% + 0.2% of total bone marrow mononuclear cells during steady-state granulopoiesis but 37.0% + 0.3% after Alum injection (p < 0.01, n = 3). The latter was similar to the relative abundance of CD34+ cells in the bone marrow of Icsbp−/− mice older than 20 weeks in standard (not barrier) housing (35.2% + 0.4%, p = 0.2, n = 3).
Histological examination of sternal bone marrow sections confirmed the increase in mature PMNs in Icsbp−/− bone marrow relative to WT bone marrow at steady state (Fig. 3D). In WT mice, bone marrow PMNs increased dramatically after Alum injection (Fig. 3D). We found PMN infiltration of pulmonary alveoli in Alum-injected Icsbp−/− mice but not in Alum-injected WT mice or saline-injected WT or Icsbp−/− mice (Fig. 3D).
Expression of Icsbp Target Genes Was Modulated during Emergency Granulopoiesis
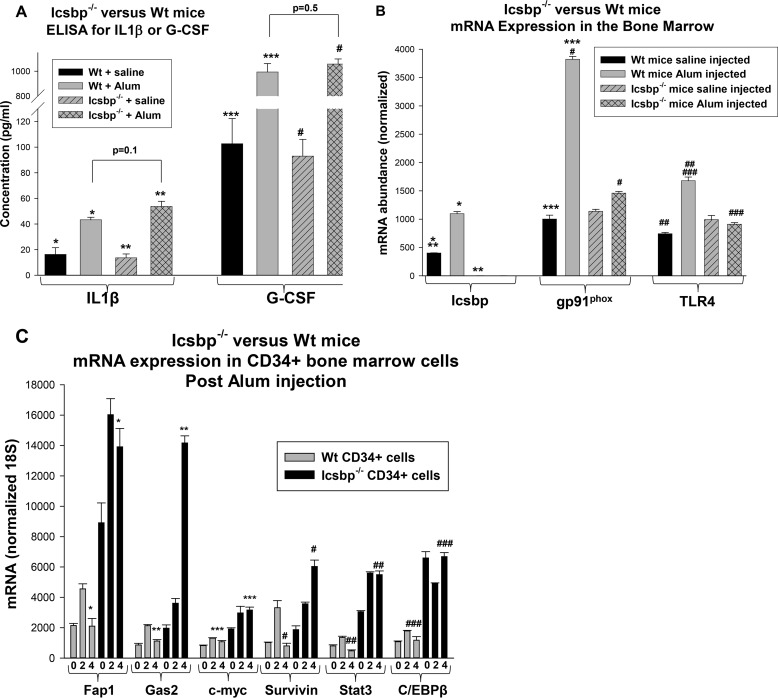
To investigate the contribution of Icsbp to emergency granulopoiesis at the molecular level, we first examined expression of IL1β and G-CSF. This was important because Icsbp is implicated in regulating the IL1β gene, although it is redundant with other interferon regulatory factors for this function (39). We measured IL1β and G-CSF in the serum of Icsbp−/− or WT mice 2 weeks after Alum or saline injection. We found a significant increase in serum levels of both cytokines after Alum injection, with or without Icsbp (Fig. 4A). IL1β and G-CSF were not significantly different in Icsbp−/− versus WT mice during steady-state or emergency granulopoiesis (p > 0.2, n = 4).
FIGURE 4.
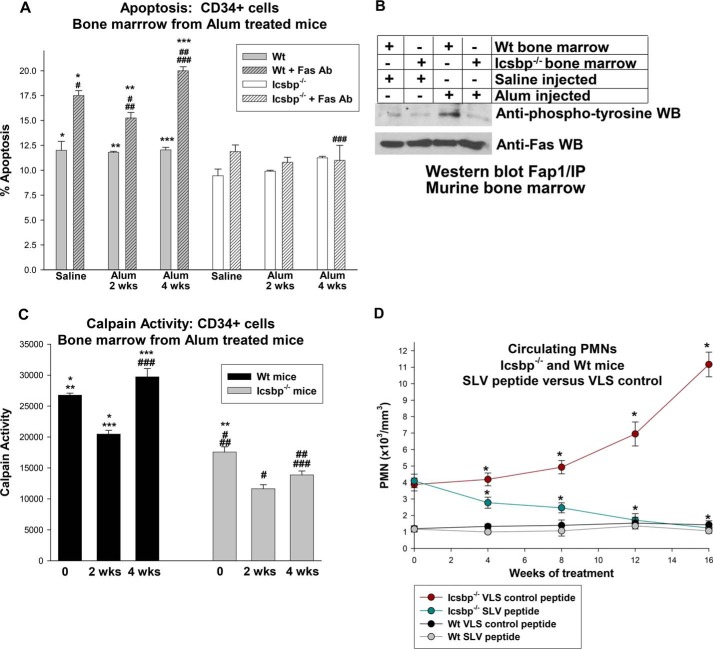
Icsbp target genes were differentially regulated in Icsbp−/− mice versus wild-type mice during emergency granulopoiesis. A, serum levels of IL1β and G-CSF were not significantly different in WT versus Icsbp−/− mice during steady-state or emergency granulopoiesis. Mice were injected with Alum to induce emergency granulopoiesis or saline control and sacrificed after 2 weeks. Serum IL1β and G-CSF levels were determined by ELISA. Statistically significant differences are indicated by *, **, ***, and #. B, expression of phagocyte effector Icsbp target-genes increased in the bone marrow of WT mice but not Icsbp−/− mice during emergency granulopoiesis. Bone marrow from the mice described above was analyzed for gene expression by real-time PCR. Statistically significant differences are indicated by *, **, ***, #, ##, and ###. C, expression of Icsbp target genes that influence proliferation/survival (Fap1 and Gas2), β-catenin target genes (c-myc and survivin), and genes required for emergency granulopoiesis (Stat3 and C/EBPβ) increased initially during emergency granulopoiesis in WT mice and returned to baseline at 4 weeks, but increased expression was sustained in Icsbp−/− mice. Mice were sacrificed before or 2–4 weeks after Alum injection, and gene expression in CD34+ bone marrow cells was determined. Statistically significant differences are indicated by *, **, ***, #, ##, and ##. p < 0.02 was considered statistically significant.
We also investigated the effect of Icsbp on the expression of phagocyte effector target genes, including gp91phox (a rate-limiting NADPH oxidase protein) and Toll-like receptor 4 (TLR4). We found that the expression of gp91phox and TLR4 mRNA in the bone marrow of WT mice increased significantly 2 weeks after Alum injection in comparison with the steady state (p < 0.001, n = 4) (Fig. 4B). Steady-state expression of these genes was not significantly different in Icsbp−/− bone marrow versus WT bone marrow, but Alum-induced expression was impaired without Icsbp (Fig. 4B). This was observed in the bone marrow of Alum-treated Icsbp−/− versus WT mice despite the greater prevalence of granulocytes in the former (the relevant population for expression of these differentiation stage-specific genes).
Icsbp represses genes encoding Gas2 and Fap1 (21, 22, 35). We hypothesized that increased Gas2 and Fap1 expression early in emergency granulopoiesis facilitates myeloid progenitor expansion and that repression of these genes by Icsbp terminates the process. We also examined C/EBPβ and Stat3 expression, transcription factors required for the initiation of emergency granulopoiesis. We quantified the expression of these genes, and β-catenin target genes, by real-time PCR in CD34+ myeloid progenitor cells from WT or Icsbp−/− bone marrow at steady state and 2 or 4 weeks after Alum injection.
In WT mice, we found a significant increase in expression of Fap1 or Gas2 2 weeks after Alum injection (p < 0.001, n = 3) that returned to baseline by 4 weeks (p > 0.4, n = 3) (Fig. 4C). This would favor transient expansion of bone marrow populations by decreasing Fas sensitivity and increasing β-catenin activity. In the bone marrow of Icsbp−/− mice, Fap1 and Gas2 also increased significantly after Alum injection (p < 0.001, n = 3) but did not return to steady-state levels at 4 weeks (p < 0.0001, n = 3) (Fig. 4C). Indeed, expression of Gas2 continued to increase between 2 and 4 weeks after Alum injection in these mice (p < 0.0001, n = 3). Similarly, expression of β-catenin target genes (c-myc and survivin) was increased in CD34+ bone marrow cells from WT or Icsbp−/− mice 2 weeks after Alum injection (p < 0.001, n = 3) (Fig. 4C). Expression of these genes returned to baseline by 4 weeks in WT mice, but c-myc expression remained elevated, and survivin continued to increase in Icsbp−/− murine bone marrow (Fig. 4C).
Both Stat3 and C/EBPβ increased in expression in CD34+ WT bone marrow cells 2 weeks after Alum injection (p < 0.001, n = 3), consistent with roles of these transcription factors in sustaining emergency granulopoiesis, and expression returned to baseline by 4 weeks (Fig. 4C). Interestingly, expression of Stat3 and C/EBPβ was significantly greater in Icsbp−/− CD34+ cells in comparison with WT cells at baseline (p < 0.0001, n = 3). Stat3 increased significantly 2 weeks after Alum injection, and this increase was sustained in Icsbp−/− mice (Fig. 4C). However, C/EBPβ expression actually fell slightly 2 weeks after Alum in these mice, but the level recovered to baseline by 4 weeks.
Because Fap1 inhibits Fas, we investigated the effect of Icsbp on sensitivity to Fas-induced apoptosis during emergency granulopoiesis. CD34+ bone marrow cells from Icsbp−/− or WT mice were analyzed by flow cytometry for Annexin V staining 2 or 4 weeks after Alum or saline injection. In WT cells, baseline apoptosis was not altered by Alum injection. Fas-induced apoptosis of these cells was significantly less 2 weeks after injection compared with the steady state (p < 0.02, n = 3) but was greater than the steady state at 4 weeks (p < 0.01, n = 3) (Fig. 5A). Icsbp−/− CD34+ cells were resistant to Fas-induced apoptosis in comparison with WT cells during steady-state and emergency granulopoiesis (p < 0.01, n = 3) (Fig. 5A). In contrast to WT bone marrow CD34+ cells, Fas resistance increased slightly at the 4-week time point in Icsbp−/− cells. Consistent with this, Fas phosphorylation was relatively less in bone marrow progenitor cells from Alum-injected Icsbp−/− mice compared with WT cells (Fig. 5B).
FIGURE 5.
Sustained Fas resistance and calpain-repression were observed in bone marrow progenitor cells from Icsbp−/− mice after stimulation of emergency granulopoiesis. Icsbp−/− or wild-type mice were injected with Alum to induce emergency granulopoiesis or saline as a steady-state granulopoiesis control, and bone marrow was obtained at various time points. A, Fas-induced apoptosis was significantly greater in CD34+ bone marrow cells from Alum-treated WT mice than in Icsbp−/− mice. Statistically significant differences are indicated by *, **, ***, #, ##, and ###. B, Fas phosphorylation was decreased in bone marrow progenitor cells from Icsbp−/− mice in comparison with WT bone marrow. CD34+ cells from Icsbp−/− or WT murine bone marrow were analyzed with or without G-CSF stimulation for Fas phosphorylation by immunoprecipitation/Western blotting (WB). C, calpain activity in WT bone marrow progenitor cells was increased relative to baseline 4 weeks after stimulation of emergency granulopoiesis but remained decreased in Icsbp−/− bone marrow. CD34+ cells were analyzed by calpain activity assay. Statistically significant differences are indicated by *, **, ***, #, ##, and ###. D, treatment with Fap1-blocking SLV peptide prevented progressive granulocytosis in Icsbp−/− mice but had no effect on WT mice. Mice were injected three times per week with SLV or VLS (control) peptide. Time points with statistically significant differences are indicated by *. p < 0.02 was considered statistically significant.
Because Gas2 is a calpain inhibitor, we considered the effect of emergency granulopoiesis on calpain activity in WT or Icsbp−/− mice using bone marrow as described above. Calpain activity was significantly less in CD34+ bone marrow cells from Icsbp−/− mice versus WT cells at baseline, as anticipated (21). After Alum injection, calpain activity in WT bone marrow CD34+ cells decreased significantly at 2 weeks but was greater than the steady state by 4 weeks (Fig. 5C). This correlated with changes in Gas2 expression and β-catenin activity in these cells. Calpain activity in Icsbp−/− bone marrow also decreased significantly 2 weeks after Alum injection but did not recover to baseline by 4 weeks (Fig. 5C).
Interaction of Fap1 with Fas or adenomatous polyposis coli protein is blocked by a tripeptide representing the Fas C terminus (SLV) (21, 23, 35). We employed this peptide to investigate the functional consequences of increased Fap1 expression in Icsbp−/− mice. For these studies, Icsbp−/− and WT control littermates were treated with SLV or scrambled control peptide (VLS) by intraperitoneal injection three times per week. Treatment began at 30 weeks of age, when Icsbp−/− mice exhibited peripheral granulocytosis but circulating myeloid blasts were less than 5%.
We found an increase in PMNs over time in Icsbp−/− mice treated with control peptide (Fig. 5D). In contrast, circulating PMNs fell during treatment with SLV peptide and were significantly less in comparison with control peptide-treated Icsbp−/− mice (p < 0.01, n = 6). Neither SLV nor VLS peptide altered circulating PMNs in age-matched control WT mice (Fig. 5D).
Repeated Episodes of Emergency Granulopoiesis Induced Excess Mortality and AML in Icsbp−/− Mice
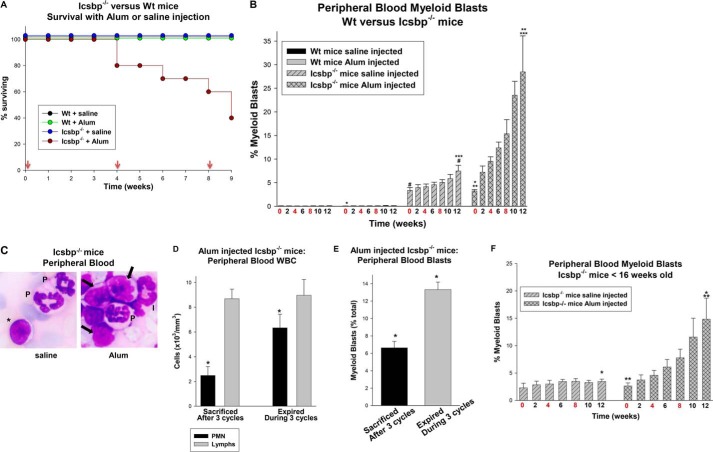
Icsbp−/− mice tolerated one episode of emergency granulopoiesis without excess mortality, but survival was compromised significantly during subsequent episodes. Only 60% of Icsbp−/− mice survived a second episode and 40% a third (Fig. 6A). In contrast, there was no morality during this time in WT mice treated with Alum or in saline-injected WT or Icsbp−/− mice (Fig. 6A).
FIGURE 6.
Icsbp−/− mice exhibited impaired tolerance for repeated episodes of emergency granulopoiesis. Icsbp−/− or wild-type mice were injected every 4 weeks with Alum to induce emergency granulopoiesis or saline as a steady-state control. A, Icsbp−/− mice exhibited excess mortality during multiple episodes of emergency granulopoiesis. Red arrows indicate weeks with injections. B, the number of circulating myeloid blasts was relatively greater in Icsbp−/− mice undergoing multiple episodes of emergency granulopoiesis versus steady-state granulopoiesis. Statistically significant differences are indicated by *, **, ***, and #. C, myeloid blasts appeared in the circulation of Icsbp−/− mice after three episodes of emergency granulopoiesis. Peripheral blood smears from Icsbp−/− mice after three cycles of Alum or saline control injection (×40 magnification). Granulocytes are indicated by PMN, a lymphocyte by the asterisk, myeloid cells of intermediate differentiation by I, and myeloid blasts by arrows. D and E, Icsbp−/− mice expiring during multiple cycles of emergency granulopoiesis exhibited increased circulating PMNs (D) and myeloid blasts (E) relative to surviving mice. Statistically significant differences are indicated by *. F, circulating myeloid blasts increased in a cohort of younger Icsbp−/− mice undergoing multiple cycles of emergency granulopoiesis in comparison with the steady state. Circulating blasts did not increase in the younger, steady-state Icsbp−/− cohort during the experiment. Statistically significant differences are indicated by * and **. p < 0.02 was considered statistically significant.
Further examination of peripheral blood counts identified a significant increase in myeloid blasts in Icsbp−/− mice during the three cycles of emergency granulopoiesis (Fig. 6B). This was significantly greater than the increase in myeloid blasts observed during steady-state granulopoiesis in saline-injected Icsbp−/− mice over the same period (p < 0.001, n = 10) (Fig. 6B). Acute leukemia (AML, as indicated by >10% circulating myeloid blasts) occurred in 17% of the saline-injected Icsbp−/− mice by the end of the third cycle, but 100% of Alum-injected Icsbp−/− mice developed AML by the start of the third cycle. The increase in circulating blasts in Alum-injected Icsbp−/− mice (Fig. 6C) correlated with decreased production of other lineages (Fig. 2, B–E). The subset of Alum-injected Icsbp−/− mice that expired during the study had elevated circulating PMNs (Fig. 6D) and myeloid blasts (Fig. 6E) in comparison with mice that survived the three episodes of emergency granulopoiesis (p < 0.0001, n = 4). This associated increased mortality with progression to AML.
A younger cohort of Icsbp−/− mice (8–15 weeks old) also demonstrated a significant increase in circulating myeloid blasts during three cycles of Alum injection (Fig. 6F). In contrast, none of the control, steady-state, saline-injected mice in this younger cohort developed AML during the treatment period (Fig. 6F).
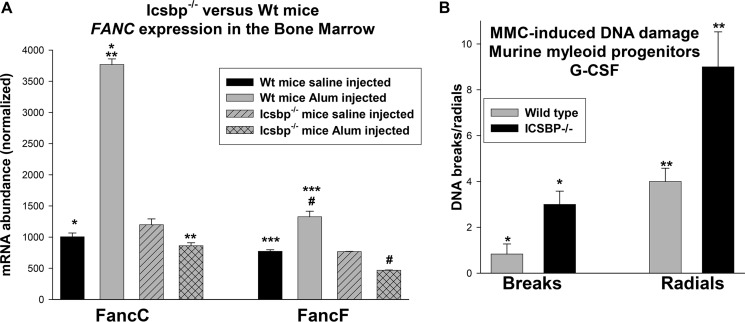
The observation that Icsbp−/− mice are more susceptible to development of AML over time suggests that loss of Icsbp predisposes to acquisition of mutations leading to differentiation block. Impaired expression of Fanconi proteins C and F (FancC and FancF) is one possible expression for ongoing mutation in Icsbp−/− mice (12, 29). We determined that expression of FancC and FancF in the bone marrow was not significantly different in WT versus Icsbp−/− mice during steady-state granulopoiesis (p = 0.6, n = 4) (Fig. 7A). Although expression of FancC and FancF increased significantly in the bone marrow of WT mice 2 weeks after Alum injection (p < 0.001, n = 4), expression of these genes did not increase after Alum injection in Icsbp−/− mice but decreased slightly (p < 0.02, n = 4) (Fig. 7A). To investigate the significance of this for DNA repair, we treated bone marrow myeloid progenitor cells from WT or Icsbp−/− mice with G-CSF (at a dose consistent with emergency granulopoiesis) and mitomycin C (to generate DNA cross-links). We found a significant increase in DNA breaks and radials in chromatin spread with Icsbp−/− versus control bone marrow (p < 0.0001, n = 3) (Fig. 7B).
FIGURE 7.
Expression of Fanconi C and F was impaired in the bone marrow of Icsbp−/− mice during emergency granulopoiesis, and susceptibility to DNA damage was increased. A, expression of the Icsbp target genes FANCC and FANCF in the bone marrow during emergency granulopoiesis was decreased in Icsbp−/− mice versus WT mice. Icsbp−/− and WT mice were injected with Alum to induce emergency granulopoiesis or saline control and sacrificed 2 weeks later. Bone marrow was analyzed by real-time PCR for expression of Fanconi C or F mRNA. Statistically significant differences are indicated by *, **, ***, and #. B, Icsbp−/− bone marrow exhibited increased DNA breaks and radials in comparison with WT bone marrow after G-CSF stimulation. CD34+ bone marrow cells from Icsbp−/− or WT mice were treated with G-CSF and analyzed for mitomycin C-induced DNA breaks or radials in chromatin spreads. Statistically significant differences are indicated by * and **. p < 0.02 was considered statistically significant.
Discussion
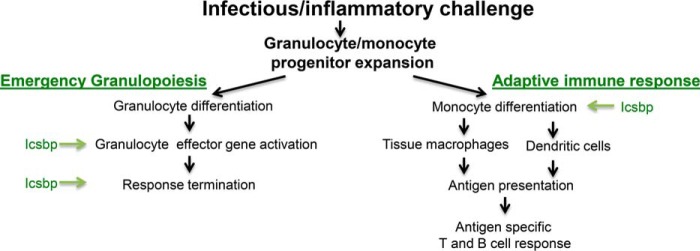
Phagocytes and B cells from Icsbp−/− mice are functionally impaired, and the first identified Icsbp target genes encoded immune effector proteins (13–15, 28). Mice with homozygous loss of Icsbp exhibit granulocytosis with functionally incompetent cells, suggesting that Icsbp is required for terminal differentiation and function of granulocytes (17). Prior studies have similarly documented a decrease in activated circulating monocytes in Icsbp−/− mice and loss of bone marrow macrophages and dendritic cells (26, 28, 31). Taken together, these prior works and this study implicate Icsbp in regulating the short-term, nonspecific granulocyte response to inflammation/infection (i.e. emergency granulopoiesis) and also the more durable, antigen-specific macrophage/dendritic cell arm of innate immunity (Fig. 8). In addition, Icsbp activates genes encoding Fanconi C and F, and increased activity of the Fanconi DNA repair pathway protects bone marrow stem and progenitor cells from DNA damage during emergency granulopoiesis (12, 29). These results led to our initial hypothesis that Icsbp is required to initiate emergency granulopoiesis, similar to Stat3 and C/EBPβ (7, 8).
FIGURE 8.
Icsbp coordinates multiple aspects of the innate immune response. Green arrows indicate events facilitated by the presence of Icsbp in the bone marrow.
Consistent with this hypothesis, we found that emergency granulopoiesis in Icsbp−/− mice was characterized by impaired expression of target genes involved in phagocyte effector functions. However, in contrast to our hypothesis, we also found a sustained increase in granulocyte production upon stimulation of emergency granulopoiesis in Icsbp−/− mice. This was characterized by sustained expression of Fap1, Gas2, and β-catenin target genes in bone marrow progenitor cells, impaired Fas-induced apoptosis, and a sustained decrease in calpain activity. Our results identified a previously unrecognized function of Icsbp: limiting myeloid progenitor expansion during the emergency granulopoiesis phase of the innate immune response. This was mechanistically pleasing because it suggests that Icsbp terminates the nonspecific emergency granulopoiesis response while simultaneously activating antigen-specific macrophage/dendritic responses to infectious challenge.
The early hours of emergency granulopoiesis are characterized by release of granulocytes from the bone marrow. The percent increase in circulating granulocytes in Icsbp−/− mice 24 h after stimulation with Alum was greater than in WT mice. This reflected the larger percentage of Gr1+ granulocytes in the bone marrow of Icsbp−/− mice in comparison with WT bone marrow at steady state and indicated that Icsbp was not required for granulocyte transit from the bone marrow.
Two weeks after stimulation of emergency granulopoiesis, circulating PMNs were increased in both WT and Icsbp−/− mice. Although Icsbp−/− mice had a higher starting baseline, the percent PMN increase during emergency granulopoiesis was not different from WT mice. However, different bone marrow populations expanded during this process in Icsbp−/− versus WT mice. For both, the relative abundance of Sca1+ cells fell as progenitors committed to differentiation, although the effect was less in Icsbp−/− mice. The bone marrow populations with the greatest relative increase during emergency granulopoiesis in Icsbp−/− mice were c-kit−CD34+ and c-kit+CD34+ myeloid progenitors, but, in WT mice, the Gr1+ and Ly6G+ granulocyte populations and Ly6C+Ly6G− monocyte population underwent the greatest relative expansion. These results suggest that Icsbp inhibits progenitor expansion and facilitates granulocyte differentiation in this context.
By 4 weeks after stimulation of emergency granulopoiesis, circulating PMNs returned to baseline in WT mice, associated with decreased expression of genes that enhance proliferation and survival of bone marrow progenitor cells (Fap1, Gas2, and β-catenin target genes). In contrast, the number of circulating PMNs was sustained in Icsbp−/− mice. This was associated with sustained expression of genes involved in Fas resistance and β-catenin stabilization in the bone marrow progenitor cells of these mice versus return to baseline. Consistent with this, inhibition of Fap1 with a tripeptide normalized granulocyte production in Icsbp−/− mice.
Circulating granulocytes did not increase further in Icsbp−/− mice with additional cycles of Alum injections and began to fall as the bone marrow accumulated myeloid blasts at the expense of other lineages. This suggested that the first Alum challenge resulted in a maximal and sustained response in Icsbp−/− mice. One possible mechanism for limiting additional expansion with subsequent stimuli is internalization and degradation of receptors on progenitor cells that mediate the emergency granulopoiesis response. Such receptor degradation controls megakaryocyte or macrophage cell masses in the bone marrow but has not been demonstrated explicitly for granulopoiesis (40, 41). Alternatively, it is possible that loss of Icsbp facilitates some aspect of emergency granulopoiesis, such as Stat3 or C/EBPβ expression, which is maximal after the first cycle. Additional studies will clarify this issue.
Although activated monocytes and macrophages and dendritic cells are decreased in Icsbp−/− mice, the steady-state level of circulating monocytes was increased relative to WT mice. This may represent the decreased ability of Icsbp−/− monocytes to move to the tissues and differentiate to macrophages and other antigen-presenting cells. The contribution of monocytes to emergency granulopoiesis has not been well defined. Studies by other investigators indicate an increase in circulating monocytes during the proliferative phase of emergency granulopoiesis, and monopoiesis is stimulated to some extent by G-CSF (9, 38, 43).
In some contexts, direct interaction with granulocytes contributes to activation of CD11c+ dendritic cells, and cytokines produced by these cells may influence granulocyte function (44). Additional work will be required to determine the interplay between activated granulocytes and monocytes, or cells derived from monocytes, during innate immunity. In this work, we focused on granulocyte expansion and did not investigate monocyte activation, nor did we investigate dendritic cell differentiation/activation. Use of the Icsbp−/− model will facilitate such studies, including determining the extent to which the latter cells are involved in granulocyte dysfunction in Icsbp−/− mice.
S phase is shortened in myeloid progenitor cells during emergency granulopoiesis, and a major role of the Fanconi anemia DNA repair pathway is to rescue collapsed and stalled replication forks (45–47). We found bone marrow failure during emergency granulopoiesis in FancC−/− mice because of apoptosis of hematopoietic stem and progenitor cells (12). It was of interest that repeated emergency granulopoiesis in Icsbp−/− mice induced acute myeloid leukemia. These results suggested that the combination of apoptosis resistance and decreased Fanconi pathway activity in Icsbp−/− bone marrow was especially unfortunate during this process.
Icsbp expression is decreased in human CML, increases with Bcr-abl inhibition, but falls again with drug resistance or blast crisis (20). We have found previously that increased expression of Fap1 and Gas2 in Bcr-abl+ myeloid progenitor cells, because of decreased Icsbp, resulted in Fas resistance and β-catenin activation (21–23, 35). This study implicates the same pathways in sustained granulocyte production during emergency granulopoiesis in the absence of Icsbp. Expression of C/EBPβ is also increased in CML and in the bone marrow of Icsbp−/− mice (36). Regulation of CEBPB by Icsbp is a topic of ongoing study in the laboratory.
This work implicates pathways that terminate the emergency granulopoiesis response during innate immunity in the pathogenesis of CML. This is consistent with clinically observed, exaggerated granulocytosis during bacterial infections in subjects with CML or chronic myelomonocytic leukemia. The latter is characterized by Icsbp inhibition because of aberrant interaction with the Tel-PDGFRα fusion protein (42). These results contradict the classic paradigm in which Bcr-abl enhances steady-state granulopoiesis and suggests that episodic, signal-dependent events may promote leukemogenesis. Our findings also suggested that therapeutic targeting of Fap1 or Gas2/calpain may address functionally relevant pathways that drive leukemia stem cell expansion in CML.
We found that Icsbp is essential to protect the bone marrow from adverse consequences of emergency granulopoiesis and to conclude this aspect of the innate immune response. However, Icsbp is also required for activation of phagocyte effector genes, granulocyte functional competence, monocyte activation, and macrophage/dendritic cell production. This suggests that clinical situations with decreased Icsbp activity would include sustained production of functionally compromised granulocytes, consistent with the observed phenotype in human CML.
Author Contributions
L. H. performed the majority of the experiments, assisted with the design, and suggested directions for the work. W. H. assisted with the design and performance of the experiments involving murine models and apoptosis. E. E. H. and L. B. assisted with the performance of the experiments involving murine models. L. C. P. provided input regarding the design of the experiments involving signal transduction. E. A. E. directed the overall project, determined the experiments for the manuscript, identified the goals of the study, and wrote the manuscript.
This work was supported by National Institutes of Health Grants R01-DK098812 and R01-CA174205 (to E. A. E.) and R01-CA77816 and CA155566 (to L. C. P.) and Veterans Affairs Grant IBX002067 (to E. A. E.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- GM-CSF
- granulocyte-macrophage colony-stimulating factor
- Icsbp
- interferon consensus sequence binding protein
- CML
- chronic myeloid leukemia
- AML
- acute myeloid leukemia
- PMN
- polymorphonuclear leukocyte
- C/EBPα
- Creb enhancer binding protein α
- G-CSF
- granulocyte colony stimulating factor.
References
- 1. Lieschke G. J., Grail D., Hodgson G., Metcalf D., Stanley E., Cheers C., Fowler K. J., Basu S., Zhan Y. F., and Dunn A. R. (1994) Mice lacking G-CSF have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency and impaired neutrophil mobilization. Blood 84, 1737–1746 [PubMed] [Google Scholar]
- 2. Panopoulos A. D., and Watowich S. S. (2008) Granulocyte colony stimulating factor: molecular mechanisms of activation during steady state and emergency hematopoiesis. Cytokine 42, 277–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DeKoter R. P., Walsh J. C., and Singh H. (1998) PU.1 regulates both cytokine-dependent proliferation and differentiation of granulocyte/macrophage progenitors. EMBO J. 17, 4456–4468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang D. E., Zhang P., Wang N. D., Hetherington C. J., Darlington G. J., and Tenen D. G. (1997) Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein α-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 94, 569–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ueda Y., Cain D. W., Kuraoka M., Kondo M., and Kelsoe G. (2009) IL1R type I dependent hematopoietic stem cell proliferation is necessary for inflammatory granulopoiesis and reactive neutrophilia. J. Immunol. 182, 6477–6484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cain D. W., Snowden P. B., Sempowski G. D., and Kelsoe G. (2011) Inflammation triggers emergency granulopoiesis through a density-dependent feedback mechanism. PLoS ONE 6, e19957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hirai H., Zhang P., Dayaram T., Hetherington C. J., Mizuno S., Imanishi J., Akashi K., and Tenen D. G. (2006) C/EBPβ is required for “emergency” granulopoiesis. Nat. Immunol. 7, 732–739 [DOI] [PubMed] [Google Scholar]
- 8. Panopoulos A. D., Zhang L., Snow J. W., Jones D. M., Smith A. M., El Kasmi K. C., Liu F., Goldsmith M. A., Link D. C., Murray P. J., and Watowich S. S. (2006) Stat3 governs distinct pathways in emergency granulopoiesis and mature neutrophils. Blood 108, 3682–3690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhan Y., Lieschke G. J., Grail D., Dunn A. R., and Cheers C. (1998) Essential roles for GM-CSF and G-CSF in the sustained hematopoietic response of Listeria monocytogenes infected mice. Blood 91, 863–869 [PubMed] [Google Scholar]
- 10. Basu S., Hodgson G., Zhang H. H., Katz M., Quilici C., and Dunn A. R. (2000) Emergency granulopoiesis in G-CSF deficient mice in response to Candida albicans infection. Blood 95, 3725–3733 [PubMed] [Google Scholar]
- 11. Eash K. J., Means J. M., White D. W., and Link D. C. (2009) CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood 113, 4711–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hu L., Huang W., Hjort E., and Eklund E. A. (2013) Increased Fanconi C expression contributes to the emergency granulopoiesis response. J. Clin. Invest. 123, 3952–3966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Driggers P. H., Ennist D. L., Gleason S. L., Mak W. H., Marks M. S., Levi B. Z., Flanagan J. R., Appella E., and Ozato K. (1990) An interferon γ-regulated protein that binds the interferon-inducible enhancer element of major histocompatibility complex class I genes. Proc. Natl. Acad. Sci. U.S.A. 87, 3743–3747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scheller M., Foerster J., Heyworth C. M., Waring J. F., Löhler J., Gilmore G. L., Shadduck R. K., Dexter T. M., and Horak I. (1999) Altered development and cytokine responses of myeloid progenitors in the absence of transcription factor, interferon consensus sequence binding protein. Blood 94, 3764–3771 [PubMed] [Google Scholar]
- 15. Eklund E. A., Jalava A., and Kakar R. (1998) PU.1, interferon regulatory factor 1, and interferon consensus sequence binding protein cooperate to increase gp91phox expression. J. Biol. Chem. 273, 13957–13965 [DOI] [PubMed] [Google Scholar]
- 16. Eklund E. A., and Kakar R. (1999) Recruitment of CBP by PU.1, IRF1 and ICSBP is necessary for gp91phox and p67phox expression. J. Immunol. 163, 6095–6105 [PubMed] [Google Scholar]
- 17. Konieczna I., Horvath E., Wang H., Lindsey S., Saberwal G., Bei L., Huang W., Platanias L., and Eklund E. A. (2008) Constitutive activation of SHP2 cooperates with ICSBP-deficiency to accelerate progression to acute myeloid leukemia. J. Clin. Invest. 118, 853–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang W., Saberwal G., Horvath E., Zhu C., Lindsey S., and Eklund E. A. (2006) Leukemia associated, constitutively active mutants of SHP2 protein tyrosine phosphatase inhibit NF1-transcriptional activation by the interferon consensus sequence binding protein. Mol. Cell Biol. 26, 6311–6332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kakar R., Kautz B., and Eklund E. A. (2005) JAK2 is necessary and sufficient for interferon γ-induced transcription of the gene encoding gp91PHOX. J. Leukocyte Biol. 77, 120–127 [DOI] [PubMed] [Google Scholar]
- 20. Radich J. P., Dai H., Mao M., Oehler V., Schelter J., Druker B., Sawyers C., Shah N., Stock W., Willman C. L., Friend S., and Linsley P. S. (2006) Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc. Natl. Acad. Sci. U.S.A. 103, 2794–2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang W., Zhu C., Wang H., Horvath E., and Eklund E. A. (2008) The interferon consensus sequence binding protein (ICSBP/IRF8) represses PTPN13 gene transcription in differentiating myeloid cells. J. Biol. Chem. 283, 7921–7935 [DOI] [PubMed] [Google Scholar]
- 22. Huang W., Zhou W., Saberwal G., Konieczna I., Horvath E., Katsoulidis E., Platanias L. C., and Eklund E. A. (2010) The interferon consensus sequence binding protein (ICSBP) decreases Bcatenin-activity in myeloid cells by repressing GAS2 transcription. Mol. Cell Biol. 30, 4575–4594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang W., Bei L., and Eklund E. A. (2013) Fas-associated phosphatase 1 (Fap1) mediates Fas-resistance in myeloid progenitor cells expressing the Bcr-abl oncogene. Leuk. Lymphoma 54, 619–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hao S. X., and Ren R. (2000) Expression of interferon consensus sequence binding protein (ICSBP) is downregulated in Bcr-Abl-induced murine chronic myelogenous leukemia-like disease, and forced coexpression of ICSBP inhibits Bcr-Abl-induced myeloproliferative disorder. Mol. Cell. Biol. 20, 1149–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burchert A., Cai D., Hofbauer L. C., Samuelsson M. K., Slater E. P., Duyster J., Ritter M., Hochhaus A., Müller R., Eilers M., Schmidt M., and Neubauer A. (2004) Interferon consensus sequence binding protein (ICSBP; IRF-8) antagonizes BCR/ABL and down-regulates bcl-2. Blood 103, 3480–3489 [DOI] [PubMed] [Google Scholar]
- 26. Holtschke T., Löhler J., Kanno Y., Fehr T., Giese N., Rosenbauer F., Lou J., Knobeloch K.-P., Gabriele L., Waring J. F., Bachmann M. F., Zinkernagel R. M., Morse H. C. 3rd, Ozato K., and Horak I. (1996) Immuno-deficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell 87, 307–317 [DOI] [PubMed] [Google Scholar]
- 27. Rehli M., Poltorak A., Schwarzfischer L., Krause S. W., Andreesen R., and Beutler B. (2000) PU.1 and interferon consensus sequence-binding protein regulate the myeloid expression of the human Toll-like receptor 4 gene. J. Biol. Chem. 275, 9773–9781 [DOI] [PubMed] [Google Scholar]
- 28. Kurotaki D., Osato N., Nishiyama A., Yamamoto M., Ban T., Sato H., Nakabayashi J., Umehara M., Miyake N., Matsumoto N., Nakazawa M., Ozato K., and Tamura T. (2013) Essential role of the IRF8-KLF4 transcription factor cascade in murine monocyte differentiation. Blood 121, 1839–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saberwal G., Horvath E., Hu L., Zhu C., Hjort E., and Eklund E. A. (2009) The interferon consensus sequence binding protein (ICSBP/IRF8) activates transcription of the FANCF gene during myeloid differentiation. J. Biol. Chem. 284, 33242–33254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tamura T., Nagamura-Inoue T., Shmeltzer Z., Kuwata T., and Ozato K. (2000) ICSBP directs bipotential myeloid progenitor cells to differentiate into mature macrophages. Immunity 13, 155–165 [DOI] [PubMed] [Google Scholar]
- 31. Kurotaki D., Yamamoto M., Nishiyama A., Uno K., Ban T., Ichino M., Sasaki H., Matsunaga S., Yoshinari M., Ryo A., Nakazawa M., Ozato K., and Tamura T. (2014) IRF8 inhibits C/EBPa activity to restrain mononuclear phagocyte progenitors from differentiating into neutrophils. Nat. Commun. 19, 4978. [DOI] [PubMed] [Google Scholar]
- 32. Iwama A., Osawa M., Hirasawa R., Uchiyama N., Kaneko S., Onodera M., Shibuya K., Shibuya A., Vinson C., Tenen D. G., and Nakauchi H. (2002) Reciprocal roles for CCAAT/enhancer binding protein (C/EBP) and PU.1 transcription actors in Langerhans cell commitment. J. Exp. Med. 195, 547–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benetti R., Copetti T., Dell'Orso S., Melloni E., Brancolini C., Monte M., and Schneider C. (2005) The calpain system is involved in the constitutive regulation of β-catenin signaling functions. J. Biol. Chem. 280, 22070–22080 [DOI] [PubMed] [Google Scholar]
- 34. Yanagisawa J., Takahashi M., Kanki H., Yano-Yanagisawa H., Tazunoki T., Sawa E., Nishitoba T., Kamishohara M., Kobayashi E., Kataoka S., and Sato T. (1997) The molecular interaction of Fas and FAP-1: a tripeptide blocker of human Fas interaction with FAP-1 promotes Fas-induced apoptosis. J. Biol. Chem. 272, 8539–8545 [DOI] [PubMed] [Google Scholar]
- 35. Huang W., Bei L., and Eklund E. A. (2013) Fas associated phosphatase 1 (Fap1) influences βcatenin activity in myeloid progenitor cells expressing the Bcr-abl oncogene. J. Biol. Chem. 288, 12766–12776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zheng C., Li L., Haak M., Brors B., Frank O., Giehl M., Fabarius A., Schatz M., Weisser A., Lorentz C., Gretz N., Hehlmann R., Hochhaus A., and Seifarth W. (2006) Gene expression profiling of CD34+ cells identifies a molecular signature of chronic myeloid leukemia blast crisis. Leukemia 20, 1028–1034 [DOI] [PubMed] [Google Scholar]
- 37. Ward A. C., van Aesch Y. M., Schelen A. M., and Touw I. P. (1999) Defective internalization and sustained activation of truncated granulocyte colony-stimulating factor receptor found in severe congenital neutropenia/acute myeloid leukemia. Blood. 93, 447–458 [PubMed] [Google Scholar]
- 38. Kool M., Pétrilli V., De Smedt T., Rolaz A., Hammad H., van Nimwegen M., Bergen I. M., Castillo R., Lambrecht B. N., and Tschopp J. (2008) Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J. Immunol. 181, 3755–3759 [DOI] [PubMed] [Google Scholar]
- 39. Kim Y. M., Kang H. S., Paik S. G., Pyun K. H., Anderson K. L., Torbett B. E., and Choi I. (1999) Roles of IFN consensus sequence binding protein and PU.1 in regulating IL-18 gene expression. J. Immunol. 163, 2000–2007 [PubMed] [Google Scholar]
- 40. Stoffel R., Wiestner A., and Skoda R. C. (1996) Thrombopoietin in thrombocytopenic mice: evidence against regulation at the mRNA level and for a direct regulatory role for platelets. Blood 87, 567–573 [PubMed] [Google Scholar]
- 41. Salto Y., Boddupalli C. S., Borsotti C, and Manz M. C. (2013) Dendritic cell homeostasis is maintained by non-hematopoietic and T-cell produced Flt3-ligand in steady state and during the immune responses. Eur. J. Immunol. 43, 1651–1658 [DOI] [PubMed] [Google Scholar]
- 42. Huang W., Hu L., Bei L., Hjort E., and Eklund E. A. (2012) The leukemia-associated fusion-protein Tel-platelet derived growth factor receptor B (Tel-PdgfRB) inhibits transcriptional repression of the PTPN13 gene by the interferon consensus sequence binding protein (Icsbp). J. Biol. Chem. 287, 8110–8125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lord B. I., Molineux G., Pojda Z., Souza L. M., Mermod J.-J., and Dexter T. M. (1991) Myeloid cell kinetics in mice treated with recombinant interleukin-3, granulocyte colony-stimulating factor (CSF), or granulocyte-macrophage CSF in vivo. Blood 77, 2154–2159 [PubMed] [Google Scholar]
- 44. Kreisel D., Sugimoto S., Zhu J., Nava R., Li W., Okazaki M., Yamamoto S., Ibrahim M., Huang H. J., Toth K. A., Ritter J. H., Krupnick A. S., Miller M. J., and Gelman A. E. (2011) Emergency granulopoiesis promoter neutrophil-dendritic cell encounters that prevent mouse lung allograft acceptance. Blood 118, 6172–6182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schlacher K., Wu H., and Jasin M. (2012) A distinct replication fork protection pathway connects Fanconi anemia tumor suppressors to Rad51-BrCA1/2. Cancer Cell 22, 106–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang L.C., Stone S., Hoatlin M.E., and Gautier J. (2008) Fanconi anemia proteins stabilize replication forks. DNA Repair 7, 1973–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Taniguchi T., Garcia-Higuera I., Andreassen P. R., Gregory R. C., Grompe M., and D'Andrea A. D. (2002) S-phase specific interaction of the Fanconi anemia protein, FANCD2, with BRCA1 and RAD51. Blood 100, 2414–2420 [DOI] [PubMed] [Google Scholar]