Abstract
The imprinted oncofetal long non-coding RNA H19 has been reported to be involved in many kinds of human cancers. However, whether lncRNA H19 implicate in oncogenesis and cancer progression in gallbladder cancer remain largely unknown. In the present study, compared with adjacent normal tissues, the level of H19 was significantly upregulated in gallbladder cancer tissues and was positively associated with lymphatic metastasis and tumor size. The overall survival is shorter in those who had higher H19 expression among GBC patients. In vitro, both TGF-β1 and IL-6 treatment induced upregulation of H19, downregulated the protein level of E-cadherin while increased Vimentin, indicating an epithelial-mesenchymal transition (EMT) phenotype in GBC. The overexpression of H19 in GBC cells enhanced tumor invasion and promoted EMT by upregulated transcription factor Twist1. On the contrary, Loss of function studies indicated that H19 interference in GBC suppressed tumor cell invasion and promoted mesenchymal-epithelial transition (MET) via suppressing Twist expression. In vivo, the volume of the tumors in H19-inteference group was significantly decreased compared to those in the control group of nude mice. Both western-blot and immunohistochemistry confirmed that a MET phenotype existed in the H19 interference group when compared to control group. These results defined H19 as a novel prognostic factor for GBC, and indicated that it might play important regulatory roles in the EMT process.
Keywords: Gallbladder cancer, H19, epithelial-mesenchymal transition, tumor invasion
Introduction
Gallbladder cancer (GBC) is the most common biliary tract cancer, characterized by early lymph node invasion and distant metastases, and the fifth most common gastrointestinal malignancy worldwide [1]. Although encouraging progress in diagnosis and therapy in GBC has been achieved in the past decade, this tumor is a highly lethal disease with an overall 5-year survival of less than 5% and mean survival around 6 months [2]. Therefore, progression in mechanism research on GBC pathogenesis is urgently needed to pave the way for new therapeutic methods, as well as new biomarkers for early diagnosis.
EMT is a process of epithelial cells into mesenchymal cells trans-differentiation which is characterized by lost of cell-cell adhesion and acquired the traits of migratory and invasion. In the process of EMT, A number of key transcription factors, such as Twist1, Snail1, Snail2, were identified to potentiate EMT progression [3-5]. To adopt enhanced migratory and invasion characteristics, the expression of an epithelial marker E-cadherin is down-regulated, and the expression of mesenchymal markers fibronectin, Vimentin, and N-cadherin is up-regulated. Emerging evidence highlighted EMT-related molecules may act as novel markers for clinical prognosis and new targets for therapy in cancers [6].
Long non-coding RNAs (lncRNAs) are defined as non-coding RNAs of more than 200 nucleotides in length, and are characterized by the complexity and diversity of their sequences and mechanisms of action [7]. The human genome project suggested that only 1.2% of the mammalian genome encodes proteins, and most of the genome are transcribed to tens of thousands of long non-coding RNAs (ncRNA) [8]. An increasing number of ncRNAs have been found to play critical roles in cancer development and metastasis [9]. Kogo R and his colleagues reported a group of patients with high Hox antisense intergenic RNA (an lncRNA also named HOTAIR) expression in colorectal cancers had a relatively poorer prognosis [10]. The alternative good example was long non-coding MALAT-1 RNA indicated a poor prognosis in non-small cell lung cancer [11] Qin R and his associates found that lncRNA MEG3 displayed a tumor suppressing function in cervical cancer [12].
The long non-coding RNA (lncRNA) H19 gene is located on Chromosome 11 in human and is a maternally expressed imprinted gene that plays a vital role in mammalian development. Previous studies have shown that H19 is overexpressed in several malignancies, such as colorectal cancer [13], breast cancer [14], bladder cancer [15] and cervical carcinomas [16]. Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer [17]. Therefore, how H19 function in cancer had draw widely attention. A recently publication had proved that the lncRNA H19 promoted epithelial to mesenchymal transition by functioning as MiRNA sponges in colorectal cancer [18].
Overexpression of H19 in cancers highlighted its tumorigenic properties, while the detailed molecular mechanisms remain poorly understood in gallbladder cancer. In this study, compared with non-tumor adjacent tissues, the expression of H19 was significantly raised in gallbladder cancer tissues. Also, H19 expression level was upregulated in vitro EMT models induced by TGF-β1 or IL-6, the H19 expression level were upregulated by these two reagents separately. Subsequent results demonstrated that overexpression of H19 promoted epithelial to mesenchymal transition in vitro and in vivo.
Materials and methods
Patients and samples
Twenty-four GBC tissue samples and neighboring noncancerous gallbladder tissue samples (collected postoperatively from April 2009 to May 2011) used in this study were obtained from Eastern Hepatobiliary Surgery Hospital (Second Military Medical University, Shanghai, China). Upon removal of the surgical specimen, research personnel immediately transported the tissue to the surgical pathology lab. Each sample was snap-frozen in liquid nitrogen and stored at -80°C prior to RNA isolation and qRT-PCR analysis. Patients recruited to this study did not receive any pre-operative treatments. GBC patients were staged according to the tumor node metastasis (TNM) staging system (the 7th edition) of the American Joint Committee on Cancer (AJCC) staging system. All patients provided written informed consent. Complete clinic-pathological follow-up data of the GBC patients whose specimens were collected are available. This study was approved by the Human Ethics Committee of Xinhua Hospital at Shanghai Jiao tong University (Shanghai, China).
Cell culture
Four human GBC cell lines (NOZ, GBC-SD, SGC-996, and EH-GB1) were used in this study. NOZ was purchased from the Health Science Research Resources Bank (Osaka, Japan). GBC-SD and SGC-996 were purchased from Cell Bank of the Chinese Academy of Science (Shanghai, China). EH-GB1 was a generous gift from Eastern Hepatobiliary Surgical Hospital and Institute, The Second Military University, Shanghai, China. The cell lines were cultured in Dulbecco’s modified Eagle’s medium (Gibco BRL, Grand Island, NY, USA), supplemented with 10% fetal bovine serum (FBS, HyClone, Invitrogen, Camarillo, CA, USA), and 100 ug/ml penicillin and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA, USA). Cells were incubated at 37°C with 5% CO2.
Recombinant interleukin-6 treatment
Recombinant human IL-6 was purchased from PeproTech Inc. (Rocky Hill, NJ). GBC-SD and Noz cells were cultured in plates to 60% confluence. After cells had attached, media were replenished with DMEM without serum. Cells were incubated for an additional 24 h to synchronized cells in a non-activating and non-proliferating phase. The cells were stimulated with IL-6 (10 ng/ml and 100 ng/ml) for 72 h.
RNA extraction, reverse transcription and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA from tissue and cells was extracted using Trizol reagent (TaKaRa, Dalian, China). RNA was reversed transcribed into cDNA using the Primer-Script one step RT-PCR kit (TaKaRa, Dalian, China). The cDNA template was amplified by real-time RT-PCR using the SYBR Premix Dimmer Eraser kit (TaKaRa, Dalian, China). Gene expression in each sample was normalized to GADPH expression. The primer sequences used were as follows: for GAPDH-F, 5’-CGGAGTCAACGGATTTGGTCGTAT-3’, GAPDH-R, 5’-AGCCTTCTCCATGGTGGTGAAGAC-3’; H19-F, 5’-TTCAAAGCCTCCACGACTCT-3’; H19-R, 5’-GCTCACACTCACGCACACTC-3’. Real-time RT-PCR reactions were performed by the ABI7500 system (Applied Biosystems, Carlsbad, CA, USA). Real-time PCRs was performed in triplicate. The relative expression fold change of mRNAs was calculated by the 2-ΔΔCt method.
Cell transfection
The siRNAs specifically targeting H19 were synthesized by Shanghai Gene Pharma Co, Ltd. The siRNA sequences for H19 were si-H19-1, 5’-CCAACAUCAAAGACACCAUdTdT-3’; si-H19-2, 5’-UAAGUCAUUUGCACUGGUUdTdT-3’. The pLV-CMV-Not/BamHI-GFP-puro-H19 (pLV-CMV-H19) was synthesized for the overexpression of H19 in GBC-SD cells (data not published). Plasmids were transfected into cells using Lipofectamine TM 2000 (Invitrogen, Carlsbad, CA, USA) and were incubated for 48 h according to the manufacturer’s instructions.
Transwell invasion assay
Transwell invasion assay was performed using the Matrigel-coated (BD, Franklin Lakes, NJ, USA) filters in 24-well plates. Cells were trypsinized and seeded onto the upper chambers of the transwells (1 × 105 cells/well) in serum-free DMEM medium. The lower chambers were filled with the DMEM medium (including 10% fetal bovine serum). The chambers were incubated at 37°C with 5% CO2 for 24 h. At the end of incubation, cells on the upper surface of the filter were removed by wiping with a cotton swab. Cells migrating through the filter to the lower surface were fixed with 4% paraformaldehyde for 10 min and stained with 0.1% crystal violet for 10 min. After washed in PBS for 3 times, cells were viewed and photographed under aphase-contrast microscope (Olympus, Tokyo, Japan) and counted from randomly chosen fields.
Protein extraction and western blot analysis
Cells were washed three times with ice-cold phosphate-buffered saline (PBS) and lysed in RIPA buffer (50 mM Tris (pH 7.4), 150 mM NaCl, 0.5% sodium deoxycholate, 1 mM EDTA, 1% Triton X-100) containing fresh protease and phosphatase inhibitor cocktails (Sigma) by incubating at 4°C for 20 min. The protein concentration was determined using the Bio-Rad assay system (Bio-Rad, Hercules, CA, USA). Equal amount of protein extracts (50 μg) was subjected to 10% sodium dodecyl sulfate-polyacrylamide (SDS-PAGE) gel electrophoresis and transferred to polyvinylidenedifluoride (PVDF) membranes (Millipore, Billerica, MA) and sequentially incubated with indicated primary antibodies against E-cadherin, Vimentin (1:1000, Santa Cruz Biotechnology Inc), Twist1 (Abcam, USA) and GAPDH, (1:1000, Cell Signaling Technology), incubated at 4°C overnight on a shaker. Blots were incubated in Horseradish peroxidase (HRP)-conjugated secondary antibody (1:2000, Abcam) at room temperature for 2 h. Blots were developed using enhanced chemiluminescence detection reagents and scanned with a Molecular Imager system (Bio-Rad).
Immunofluorescence analysis
Cells were fixed in a solution of 4% formaldehyde/PBS for 4 h, washed in PBS/0.1% Triton X-100 (PBST) for 15 min, and blocked in 5% goat serum/PBS for 1 h. Cells were then incubated in primary antibodies overnight at 4°C with the following dilutions: E-cadherin and Vimentin (1:1000, Santa Cruz Biotechnology, Inc), Twist1 (1:1000, Abcam, USA). Cells were washed and incubated in secondary antibody (Abcam Co. Cambridge, MA) for one and half hours at 37°C. Immunofluorescence was analyzed using fluorescence microscopy.
Immunohistochemistry
Tumor specimens from nude mice were fixed in 4% paraformaldehyde and then embedded in paraffin. Sections were used for the analysis of E-cadherin (Santa Cruz Biotechnology, Santa Cruz, CA) and Vimentin (Santa Cruz Biotechnology, Santa Cruz, CA). The samples were then incubated at 4°C overnight with primary antibodies against E-cadherin (1:100) and Vimentin (1:150). The sections were treated with secondary antibody for 30 min at room temperature and stained with diaminobenzidine (DAB) until brown granules appeared. Sections were blindly evaluated by two pathologists with light microscopy.
Xenograft mouse model
Noz cells (1 × 106) stably expressing control shRNA or shRNA-H19 were subcutaneously injected into either side of the flank area of 4-week-old male athymic nude mice (n = 3 mice per group). Tumor volumes were measured (0.5 × length × width2) in mice on a weekly basis. After 4 weeks, the nude mice were sacrificed and the tumor tissues were excised and fixed in 4% Paraformaldehyde solution for further study. All animal experiments were performed in animal laboratory center of Xinhua Hospital and in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication number 85-23, revised 1996). The study protocol was approved by the Animal Care and Use committee of Xinhua Hospital.
Statistical analysis
Derived values were presented as the means ± SD. Comparisons between two groups were conducted using two-tail Student’s T-test or Student-Newman-Keuls (SNK test, ANOVA) analyses were conducted using SPSS 13.0 software. Kaplan-Meier survival analysis and log-rank tests using patient postoperative survival were conducted to further evaluate the correlation between the expression level of lncRNA-H19 and the prognosis of GBC patients. P < 0.05 was considered as statistically significant.
Results
H19 was overexpressed in GBC compared with normal tissues and associated with lymphatic metastasis, tumor size and overall survival
In the current study, H19 expression in the 24 GBC samples was significantly higher when compared to the adjacent normal tissues (Figure 1A). It was significantly correlated with tumor sizes (P = 0.040) and tumor status, lymph node metastasis (P = 0.017) (Figure 1B and 1C; Table 1). However, H19 expression level was not correlated with other parameters, such as patient’s gender or age (Table 1). According to the median ratio of relative H19 expression (2.80) in tumor tissues, the 24 GBC patients were classified into two groups: high-H19 group (n = 13, H19 expression ratio ≥ median ratio) and low-H19 group (n = 11, H19 expression ratio < median ratio). High-H19 group associated with shorter overall survival time in GBC patients (Figure 1D).
Figure 1.
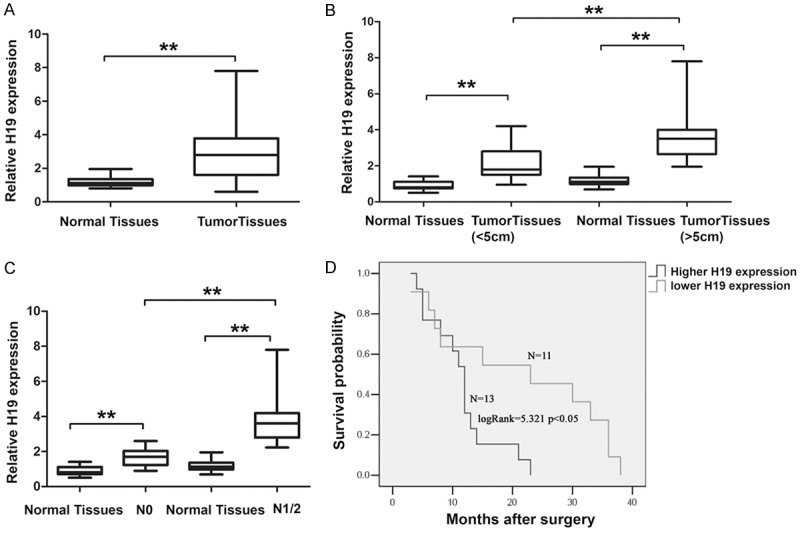
Relative H19 expression in gallbladder cancer tissues and its clinical significance. A. Relative expression of H19 in gallbladder cancer tissues in comparison with corresponding non-tumor normal tissues. H19 expression was examined by qRT-PCR and normalized to GAPDH expression. Data was presented as fold-change in tumor tissues relative to normal tissues. B. Relationship between H19 expression and primary tumor size (P < 0.05). C. Relationship between H19 expression and lymph node metastasis (P < 0.05). D. Kaplan-Meier overall survival curves according to H19 expression level. The overall survival of the High-H19 group (n = 13; H19 expression ratio ≥ median ratio) was significantly lower than that of Low-H19 group (n = 11; H19 expression ratio ≤ median ratio; P < 0.05, log-rank test). **P < 0.05.
Table 1.
The association of H19 expression in gallbladder cancer tissues with clinical characteristics
| H19 expression | ||||
|---|---|---|---|---|
|
|
||||
| Characteristics | Case number | Low | High | P-value |
| Gender | 0.41 | |||
| Male | 6 | 2 | 4 | |
| Female | 18 | 9 | 9 | |
| Age | 0.115 | |||
| ≤ 60 | 13 | 4 | 9 | |
| > 60 | 11 | 7 | 4 | |
| tumor size | 0.04** | |||
| < 5 | 10 | 2 | 8 | |
| > 5 | 14 | 9 | 5 | |
| Histological grade | ||||
| Well and morderately | 10 | 6 | 4 | 0.223 |
| Poorly and others | 14 | 5 | 9 | |
| N status | ||||
| N0 | 13 | 9 | 4 | 0.017** |
| N1/2 | 11 | 2 | 9 | |
| Clinical stage | 0.1 | |||
| I-II | 12 | 8 | 4 | |
| III-IV | 12 | 3 | 9 | |
P < 0.05.
To further explore the function of H19 in GBC, the expression level of H19 was examined using qRT-PCR in four GBC cell lines (SGC-996, GBC-SD, EHGB-1, Noz) (Figure 2A). The pLv-CMV-H19 was transfected into GBC-SD cells for gain of function studies and siRNA-H19 was transfected into NoZ cells for loss of function analyses, respectively. QRT-PCR analyses of H19 levels were performed after 48 h transfection and revealed that H19 expression was increased 12-fold in GBC-SD cells when compared with control cells. However, in NOZ cells, the expression of H19 was effectively knocked down by si-H19-1 (55%) or si-H19-2 (75%), respectively. Both siRNA and pLv-CMV-H19 of the results were the representatives of three independent experiments (Figure 2B and 2C). The si-H19-2 was used as the representative for the following experiments for efficient knockdown for H19.
Figure 2.
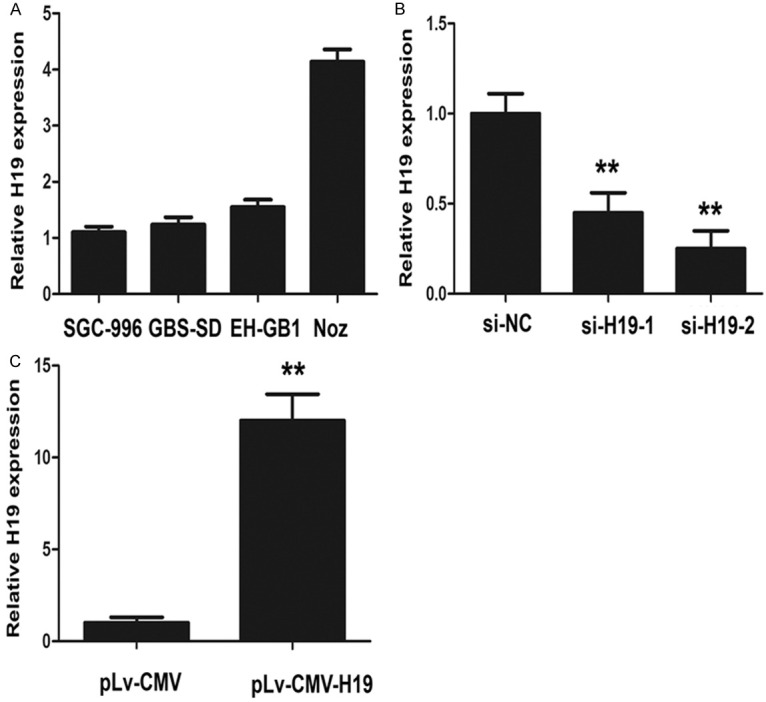
Expression levels of H19 in GBC cell lines. A. Expression levels of H19 in four GBC cell lines (SGC-996, GBC-SD, EH-GB1 and NOZ). B. H19-specific siRNA-1 and siRNA-2 reduced the endogenous H19 mRNA level in NOZ cells. C. The pLv-CMV-H19 overexpressed the mRNA level of H19 in GBC-SDcells. The statistical differences between groups were analyzed using independent samples t-test. Error bars represent the mean ± S.D. of triplicate experiments, **P < 0.05.
H19 was elevated in TGF-β1 and IL-6 Induced EMT model in GBC
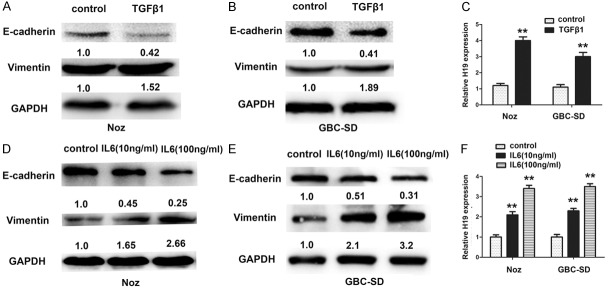
To assess whether H19 is involved in the EMT of GBC, a well-characterized EMT inducer transforming growth factor-β1 (TGF-β1) was used to establish in vitro EMT models in Noz and GBC-SD cells. Decrease E-cadherin and increase of Vimentin was displayed in the protein level in both cells (Figure 3A and 3B), suggesting that the EMT models were successfully established. Meanwhile, the H19 level was dramatically upregulated after cells were treated by TGF-β1 (Figure 3C). Clinical studies have shown that the levels of the pro-inflammatory cytokine IL-6 are frequently elevated in some tumors and correlated with EMT [19,20]. GBC-SD and Noz cells were treated with recombinant IL-6 with concentration of 10 ng/mL and 100 ng/mL seperately, the protein level of E-cadherin were decreased by a dose independent maner and vimentin were elevated by a dose indepent maner too (Figure 3D and 3E), and H19 level was also upregulated by IL-6 (Figure 3F). Collectively, these findings supported that H19 may be involved in EMT progression in gallbladder cancer cells.
Figure 3.
Upregulation of lncRNA H19 was observed in gallbladder cancer cells after subsequently treated with TGF-β1 or IL-6. A, B. Protein expression levels of E-cadherin and Vimentin were monitored in Noz and GBC-SD cells to evaluate the effect after subsequently treated with TGF-β1 for 72 hours. C. QRT-PCR was performed to measure the expression level of H19 after TGF-β1 treatment for 72 hours, **P < 0.05. D, E. Protein expression levels of E-cadherin and Vimentin were monitored in Noz and GBC-SD cells to evaluate the effect after subsequently treated with IL-6 (10 ng/ml and 100 ng/ml) for 72 hours. F. QRT-PCR was performed to measure the expression level of H19 after IL-6 (10 ng/ml and 100 ng/ml) treatment for 72 hours, **P < 0.05.
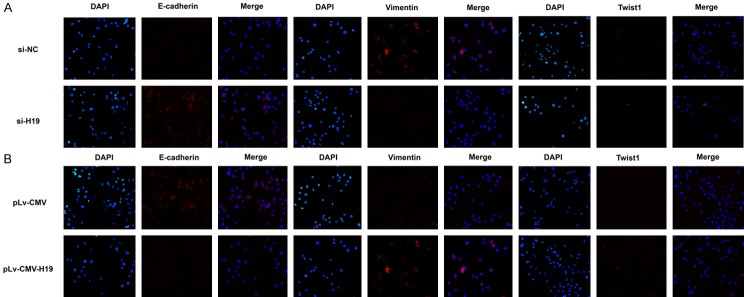
To elucidate the function of H19 involved in EMT of gallbladder cancer, H19 was knocked down in Noz cells and overexpressed in GBC-SD cells. Transwell assay showed that Noz cell invasion significantly decreased when H19 was knocked down with H19-siRNA, whereas GBC-SD cell invasion was increased significantly with transfection of pLv-CMV-H19 (Figure 4A and 4B). Furthermore, Western-blot results indicated that knockdown of H19 by transfection of H19-siRNA led to downregulation of key transcription factor Twist1, upregulation of epithelial marker E-cadherin and downregulation of mesenchymal marker Vimentin in Noz cells (Figure 4C). Overexpression of H19 led to upregulation of E-cadherin and downregulation of Vimentin and Twist1 in GBC-SD cells after transfection of pLv-CMV-H19 (Figure 4D). To further confirm the role of H19 in EMT, after transfection with H19-siRNA or pLv-CMV-H19, the immunofluorescence method was used to evaluate the expression of EMT marker. E-cadherin was increased in the Noz cell membrane, but Twist1 and Vimentin were decreased in cells after H19-siRNA transfection. On the contrary, overexpression of H19 in GBC-SD cell decreased the expression of E-cadherin in the membrane and increased the expression of Twist1 and Vimentin (Figure 5A and 5B). These findings indicated that H19 might potentiate the transdifferentiation from epithelial phenotype to mesenchymal phenotype in GBC cells.
Figure 4.
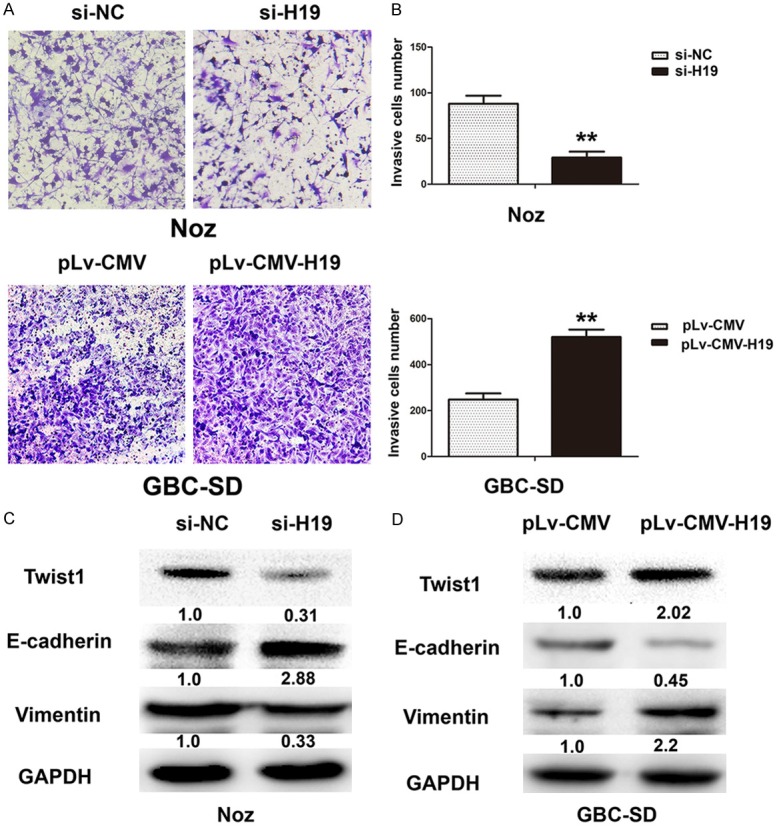
Ectopic expression of H19 promoted epithelial to mesenchymal transition (EMT) in gallbladder cancer cells. A. The cell invasion capacity were evaluated by transwell invasion assay by silencing H19 expression or ectopic H19 expression in Noz and GBC-SD cells. B. The numbers of cells invasion were assessed by transwell invasion assay in Noz and GBC-SD cells, **P < 0.05. C. The expression of EMT key transcription factors Twist1 and marker genes E-cadherin and Vimentin were evaluated in silencing H19 expression in Noz cells. D. The expression of EMT key transcription factors Twist1 and marker genes E-cadherin and Vimentin were evaluated in overexpression H19 in GBC-SD cells.
Figure 5.
The expression of Twist1 and E-cadherin and Vimentin were detected by immunofluorescence. A. The expression of EMT key transcription factors Twist1 and marker genes E-cadherin and Vimentin were evaluated in silencing H19 expression in Noz. B. The expression of EMT key transcription factors Twist1 and marker genes E-cadherin and Vimentin were evaluated in overexpression H19 GBC-SD cells.
H19 promoted the invasion of gallbladder cancer cells in vivo
Cells stably expressing control shRNA or H19 shRNA by lenti-virus vectors were subcutaneously injected into either side of the flank area of 4-week-old male nude mice (n = 3 mice per group). Four weeks after injection, the mice were sacrificed and the tumors were excised. Compared with control groups, Lv-shRNA-H19 cells generated significantly smaller tumors (Figure 6A and 6B). Thereafter, the expression patterns of E-cadherin and Vimentin were obtained by immunohistochemical and Western-blot in these tumor samples. In accordance with in vitro results, the expression of E-cadherin increased, and the expression of Vimentin decreased in Lv-shRNA-H19 group (Figure 6C-E), suggesting an oncogenic effect of H19 in GBC.
Figure 6.
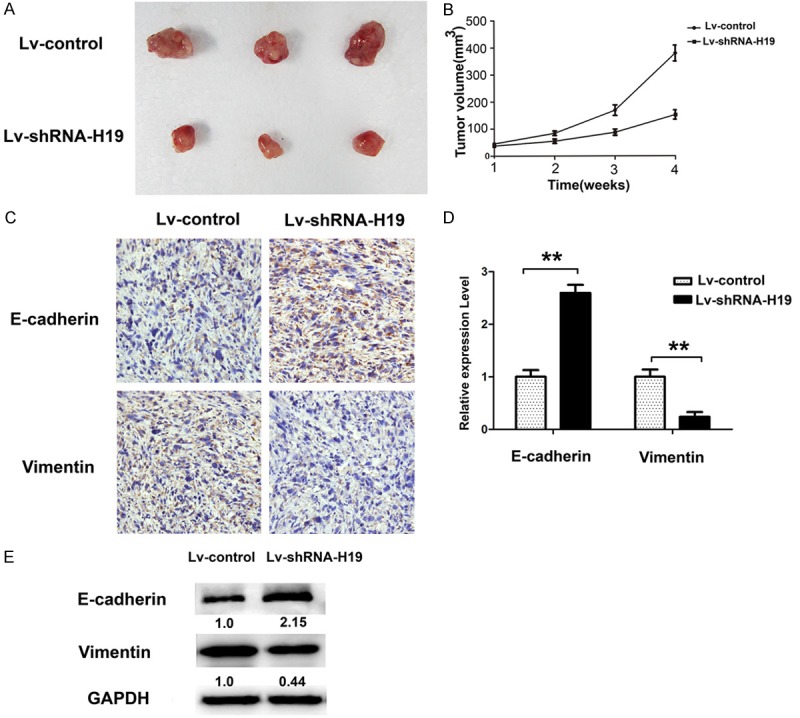
H19 promotes tumor growth in vivo. A. The representative tumor tissues from nude mice were showed the Lv-shRNA-H19 and Lv-control transfected stable cells were subcutaneously injected into each side of nude mice for 4 weeks. B. Tumor volumes were measured and calculated. C, D. Immunohistochemical staining of E-cadherin and Vimentin demonstrated that H19 silencing inhibited the aggressive phenotype of gallbladder cancer cells in vivo, as indicated by the expression of E-cadherin and Vimentin-positive cells number, **P < 0.05. E. The expression of EMT marker genes E-cadherin and Vimentin were evaluated in Lv-shRNA-H19 and the control group in tumor tissues by Western-blot, representative pictures were captured and showed.
Discussion
EMT is essential for cancer invasion and metastasis, and is regarded as a major mechanism inducing metastasis in cancer. Since most of human malignancies arise from the epithelial tissues, the investigations on EMT will be beneficial for cancer treatment. Recently reported data suggested that lncRNA is related to EMT in different kinds of cancers [21-23]. H19 was under the control of both raf and Rif genes, these two unlinked genes also transact to determine the mRNA level of α-fetoprotein (AFP). Similar to AFP, H19 is high in embryonic stage but experienced a sharp reduction in adulthood. Re-expression of H19 in adults is often linked to tumorigenesis [24]. Therefore, whether H19 can be used as a prognostic factor in cancer has gained much attention.
There were an increasing number of reports regarding the role of H19 in promoting tumor progression. A recent study reported that lncRNA H19 promoted pancreatic cancer cell invasion and migration at least partially by increasing HMGA2-mediated EMT through antagonizing let-7 [22]. Imad J. Matouk and his associates reported that H19 induction of Slug was miR-675 dependent and was accompanied by ablation of E-cadherin [25]. It is worth noting that contrary results have been report by L. Zhang and his colleagues, whose study showed that H19 suppressed hepatocellular carcinoma progression metastasis and promoted mesenchymal to epithelial transition via altered miR-200 pathway [26]. Another study reported that H19 was identified as an oncogene in colorectal cancer functioning as a competing endogenous RNA (ceRNA) for miR-138 and miR-200a, antagonizing their functions and leading to the de-repression of their endogenous targets Vimentin, ZEB1, and ZEB2, all of which were core marker genes for mesenchymal cells [17]. However, there is no known report about the role of H19 in tumor formation and progression in GBC so far.
In the present study, we explored whether H19 was correlated with poor prognosis of GBC by analyzing the clinical data via indicated statistical approach. H19 RNA expression levels were significantly higher in gallbladder tumor tissues than in corresponding adjacent normal tissues. High H19 expression group had poor prognosis in gallbladder cancer patients. Moreover, H19 expression was also positively associated with lymphatic metastasis and tumor size in GBC. Yadav A reported that IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition [19]. Imad J. Matouk and his colleague found that slug might be the key transcription factor which modulated by H19 and lead to EMT in breast cancer cell model and this TGF-β dependent induction of H19 had been recently corroborated in an experimental model of colorectal cancer [17,25]. Our subsequent study revealed that H19 was upregulated by TGF-β1 or IL-6 which also observed the EMT phenotype in GBC. Further study confirmed that ectopic expression of H19 led to decreased the expression of E-cadherin (epithelial marker), increased the expression of Vimentin (mesenchymal markers) and Twist1 (EMT-related transcription factor) in GBC-SD cell line. The invasion ability was enhanced in GBC-SD cells when H19 was overexpressed. The downregulation of H19 by RNAi had the opposite effect (MET phenotype and suppression of cell invasion) in NOZ cell line. In vivo, the results also demonstrated that knockdown of H19 displayed significantly decreased in the tumor size and inhibited EMT in nude mice. There is also a large amount of in vivo evidence supporting the pro-metastatic nature of H19. Sub-colonies of a mammary gland tumor cell line differ not only in their ability to migrate to the lung and colonialize there, but also by their relative H19 expression levels [25,27]. Functional and mechanistic studies all indicated that H19 might play important role in modulated EMT process, suggesting that H19 may promote gallbladder cancer cells invasion by inducing EMT.
In conclusion, we found H19 was highly expressed in gallbladder cancer tissues which hinted poor outcome. H19 promoted cell invasion and EMT in GBC cells, suggesting that H19 might function as a gallbladder cancer oncogene. Thus, H19 might be a potential prognostic marker and a therapeutic target for gallbladder cancer.
Acknowledgements
We thank the Eastern Hepatobiliary Surgical Hospital and Institute, The Second Military University, Shanghai, for their generous help. This work was supported by the National Natural Science Foundation of China (Grant number 81272747) and Doctorial innovation fund of Shanghai Jiao tong University School of Medicine.
Disclosure of conflict of interest
None.
References
- 1.Zhu AX, Hong TS, Hezel AF, Kooby DA. Current management of gallbladder carcinoma. Oncologist. 2010;15:168–181. doi: 10.1634/theoncologist.2009-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rakic M, Patrlj L, Kopljar M, Klicek R, Kolovrat M, Loncar B, Busic Z. Gallbladder cancer. Hepatobiliary Surg Nutr. 2014;3:221–226. doi: 10.3978/j.issn.2304-3881.2014.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang P, Hu P, Shen H, Yu J, Liu Q, Du J. Prognostic role of Twist or Snail in various carcinomas: a systematic review and meta-analysis. Eur J Clin Invest. 2014;44:1072–1094. doi: 10.1111/eci.12343. [DOI] [PubMed] [Google Scholar]
- 4.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 5.Zhou JP, Gao ZL, Zhou ML, He MY, Xu XH, Tao DT, Yang CC, Liu LK. Snail interacts with Id2 in the regulation of TNF-alpha-induced cancer cell invasion and migration in OSCC. Am J Cancer Res. 2015;5:1680–1691. [PMC free article] [PubMed] [Google Scholar]
- 6.Mitra A, Mishra L, Li S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget. 2015;6:10697–10711. doi: 10.18632/oncotarget.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Spizzo R, Almeida MI, Colombatti A, Calin GA. Long non-coding RNAs and cancer: a new frontier of translational research? Oncogene. 2012;31:4577–4587. doi: 10.1038/onc.2011.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15:7–21. doi: 10.1038/nrg3606. [DOI] [PubMed] [Google Scholar]
- 10.Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, Miyano S, Mori M. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320–6326. doi: 10.1158/0008-5472.CAN-11-1021. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt LH, Spieker T, Koschmieder S, Schaffers S, Humberg J, Jungen D, Bulk E, Hascher A, Wittmer D, Marra A, Hillejan L, Wiebe K, Berdel WE, Wiewrodt R, Muller-Tidow C. The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth. J Thorac Oncol. 2011;6:1984–1992. doi: 10.1097/JTO.0b013e3182307eac. [DOI] [PubMed] [Google Scholar]
- 12.Qin R, Chen Z, Ding Y, Hao J, Hu J, Guo F. Long non-coding RNA MEG3 inhibits the proliferation of cervical carcinoma cells through the induction of cell cycle arrest and apoptosis. Neoplasma. 2013;60:486–492. doi: 10.4149/neo_2013_063. [DOI] [PubMed] [Google Scholar]
- 13.Tsang WP, Ng EK, Ng SS, Jin H, Yu J, Sung JJ, Kwok TT. Oncofetal H19-derived miR-675 regulates tumor suppressor RB in human colorectal cancer. Carcinogenesis. 2010;31:350–358. doi: 10.1093/carcin/bgp181. [DOI] [PubMed] [Google Scholar]
- 14.Berteaux N, Lottin S, Monte D, Pinte S, Quatannens B, Coll J, Hondermarck H, Curgy JJ, Dugimont T, Adriaenssens E. H19 mRNA-like noncoding RNA promotes breast cancer cell proliferation through positive control by E2F1. J Biol Chem. 2005;280:29625–29636. doi: 10.1074/jbc.M504033200. [DOI] [PubMed] [Google Scholar]
- 15.Byun HM, Wong HL, Birnstein EA, Wolff EM, Liang G, Yang AS. Examination of IGF2 and H19 loss of imprinting in bladder cancer. Cancer Res. 2007;67:10753–10758. doi: 10.1158/0008-5472.CAN-07-0329. [DOI] [PubMed] [Google Scholar]
- 16.Kim SJ, Park SE, Lee C, Lee SY, Jo JH, Kim JM, Oh YK. Alterations in promoter usage and expression levels of insulin-like growth factor-II and H19 genes in cervical carcinoma exhibiting biallelic expression of IGF-II. Biochim Biophys Acta. 2002;1586:307–315. doi: 10.1016/s0925-4439(01)00109-0. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Yu B, Li J, Su L, Yan M, Zhu Z, Liu B. Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer. Oncotarget. 2014;5:2318–2329. doi: 10.18632/oncotarget.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang WC, Fu WM, Wong CW, Wang Y, Wang WM, Hu GX, Zhang L, Xiao LJ, Wan DC, Zhang JF, Waye MM. The lncRNA H19 promotes epithelial to mesenchymal transition by functioning as miRNA sponges in colorectal cancer. Oncotarget. 2015;6:22513–22525. doi: 10.18632/oncotarget.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yadav A, Kumar B, Datta J, Teknos TN, Kumar P. IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Mol Cancer Res. 2011;9:1658–1667. doi: 10.1158/1541-7786.MCR-11-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H, Ren G, Wang T, Chen Y, Gong C, Bai Y, Wang B, Qi H, Shen J, Zhu L, Qian C, Lai M, Shao J. Aberrantly expressed Fra-1 by IL-6/STAT3 transactivation promotes colorectal cancer aggressiveness through epithelial-mesenchymal transition. Carcinogenesis. 2015;36:459–468. doi: 10.1093/carcin/bgv017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou P, Zhao Y, Li Z, Yao R, Ma M, Gao Y, Zhao L, Zhang Y, Huang B, Lu J. LincRNA-ROR induces epithelial-to-mesenchymal transition and contributes to breast cancer tumorigenesis and metastasis. Cell Death Dis. 2014;5:e1287. doi: 10.1038/cddis.2014.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma C, Nong K, Zhu H, Wang W, Huang X, Yuan Z, Ai K. H19 promotes pancreatic cancer metastasis by derepressing let-7’s suppression on its target HMGA2-mediated EMT. Tumour Biol. 2014;35:9163–9169. doi: 10.1007/s13277-014-2185-5. [DOI] [PubMed] [Google Scholar]
- 23.Zhao J, Liu Y, Huang G, Cui P, Zhang W, Zhang Y. Long non-coding RNAs in gastric cancer: versatile mechanisms and potential for clinical translation. Am J Cancer Res. 2015;5:907–927. [PMC free article] [PubMed] [Google Scholar]
- 24.Pachnis V, Belayew A, Tilghman SM. Locus unlinked to alpha-fetoprotein under the control of the murine raf and Rif genes. Proc Natl Acad Sci U S A. 1984;81:5523–5527. doi: 10.1073/pnas.81.17.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matouk IJ, Raveh E, Abu-lail R, Mezan S, Gilon M, Gershtain E, Birman T, Gallula J, Schneider T, Barkali M, Richler C, Fellig Y, Sorin V, Hubert A, Hochberg A, Czerniak A. Oncofetal H19 RNA promotes tumor metastasis. Biochim Biophys Acta. 2014;1843:1414–1426. doi: 10.1016/j.bbamcr.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Yang F, Yuan JH, Yuan SX, Zhou WP, Huo XS, Xu D, Bi HS, Wang F, Sun SH. Epigenetic activation of the MiR-200 family contributes to H19-mediated metastasis suppression in hepatocellular carcinoma. Carcinogenesis. 2013;34:577–586. doi: 10.1093/carcin/bgs381. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]